Introduction
Normal blood vessels are organized in a hierarchy of
evenly distributed arteries, capillaries and veins. The vessels are
covered by pericytes to maintain vascular integrity (1). Unlike normal blood vessels, tumor
vessels are structurally and functionally abnormal. Tumor blood
vessels are absent in pericyte coverage, and are highly permeable
to plasma and plasma proteins. An imbalance of pro- and
anti-angiogenic factors causes endothelial cell migration and
proliferation (2). It is well
known that vascular endothelial growth factor (VEGF) plays an
important role in tumor neovascularization.
Tumor-associated macrophages (TAMs) are abundant
immunosuppressive cells recruited into the tumor microenvironment
by cytokines such as macrophage colony-stimulating factor (M-CSF).
The relevance of TAMs to tumor progression and metastasis is well
established, and they promote angiogenesis, tissue remodeling and
repair (3,4). TAMs have the potential to release
angiogenic growth factors such as VEGF and thereby enhance the
formation of tumor vasculature (5,6).
Therefore, TAMs are potential targets for anticancer and
anti-angiogenic therapy.
Bisphosphonates such as clodronic acid and
zoledronic acid (ZOL) are compounds used to prevent or inhibit the
development of bone metastasis or excessive bone resorption and for
the therapy of inflammatory diseases such as rheumatoid arthritis
and osteoarthritis (7,8). ZOL is a highly charged hydrophilic
molecule that does not readily cross the plasma cell membrane, and
it reaches pharmacologically active concentrations only in cells
that exhibit marked fluid-phase endocytosis, such as osteoclasts
and macrophages. Therefore, ZOL is an efficient reagent for the
selective depletion of macrophages. The use of ZOL as an
anti-angiogenic agent has been found to suppress solid tumor growth
(9).
Anti-angiogenesis effects are known to change the
tumor vasculature. Preclinical studies have shown that anti-VEGF
therapy changes the tumor vasculature toward a more mature or
normal phenotype (10).
Normalization of disorganized tumor vasculature using therapeutics,
rather than the blockage or disruption of tumor blood vessels,
reduces tumor hypoxia, interstitial fluid pressure (IFP) and
hyper-permeability and facilitates the delivery of exogenous
therapeutics. Therefore, tumor vascular normalization has become a
complementary therapeutic paradigm for cancer (1,2). The
anti-angiogenesis effect has already been applied in combination
therapy. Bevacizumab, an anti-VEGF antibody, was developed for
blocking angiogenesis, and it is used clinically with other drugs
to improve the efficiency of conventional chemotherapy.
Previously, we found that intravenous injections of
ZOL solution into tumor-bearing mice induced changes of vascular
structure in the tumor (11);
however, the effect of ZOL on the tumor microenvironment was not
clear. Polyethylene glycol (PEG)-modified liposomes are long-lived
in the circulation and accumulate passively in tumors. The tumor
accumulation of the liposomes in tumor tissues is due to leakiness
of tumor vessels to the macromolecular agents [enhanced
permeability and retention (EPR) effect]. It has been reported that
transforming growth factor (TGF)-β type I receptor inhibitors were
able to increase the antitumor effect of liposomal DXR or micelle
DXR by changing the microenvironment of the vasculature (12,13).
Therefore, in this study, we examined whether ZOL treatments could
facilitate the delivery of liposomal DXR (Doxil) by changing the
microenvironment of the vasculature and increase therapeutic
efficacy in vivo.
Materials and methods
Materials
Zoledronic acid (ZOL) was obtained from Enzo Life
Sciences (Farmingdale, NY, USA). Doxorubicin hydrochloride (DXR)
was purchased from Wako Pure Chemical Industries Inc. (Osaka,
Japan). Liposomal DXR, Doxil, was obtained from Janssen
Pharmaceutical K.K. (Tokyo, Japan). All other chemicals were of the
finest grade available.
Cell culture
Murine Lewis lung carcinoma LLC was obtained from
the Cell Resource Center for Biomedical Research, Tohoku University
(Miyagi, Japan). Murine macrophage RAW264.7 was obtained from the
European Collection of Cell Cultures (ECACC, Wiltshire, UK). LLC
and RAW264.7 cells were cultured in RPMI-1640 medium with 10%
heat-inactivated fetal bovine serum (FBS) and kanamycin (100 μg/ml)
in a humidified atmosphere containing 5% CO2 at
37°C.
Tumor model
All animal experiments were performed with approval
from the Institutional Animal Care and Use Committee of Hoshi
University. For the generation of LLC tumors, 1×106
cells suspended in 100 μl of PBS were inoculated subcutaneously
into the flank of female C57BL/6N mice (Sankyo Lab. Service Corp.).
The tumor volume was calculated using the following formula: tumor
volume = 0.5 × a × b2, where a and b are the larger and
smaller diameters, respectively.
Immunohistochemical analysis
To examine the anti-angiogenic effect of ZOL on
tumor, we intravenously injected ZOL solution at a dose of 5, 20 or
40 μg of ZOL/mouse per day for one, two or three consecutive days
into mice bearing an LLC tumor when the tumor volume reached ~200
mm3. The tumors 24 h after the final injection of ZOL
solution were frozen on dry ice and sliced at 16 μm. Their sections
were incubated with rat anti-mouse CD31 (PECAM-1) monoclonal
antibody (Clone MEC 13.3, BD Pharmingen, San Diego, CA, USA) for
the detection of mouse endothelial cells, and subsequently
incubated with goat anti-rat IgG conjugated to Alexa Fluor 488
(Invitrogen, Carlsbad, CA, USA) as a secondary antibody. In the
detection of mouse pericytes, the sections were further incubated
with Cy3-conjugated rabbit anti-smooth muscle α-actin (α-SMA)
antibody (Sigma-Aldrich, MO, USA).
To examine the effect of ZOL on macrophages in tumor
and liver, we intravenously injected ZOL solution at a dose of 40
μg of ZOL/mouse per day for three consecutive days into mice
bearing an LLC tumor. The sections of tumor and liver 24 h after
the final injection of ZOL solution were incubated with rat
anti-mouse F4/80 monoclonal antibody (Clone CI:A3-1, AbD Serotec,
Oxford, UK) for the detection of mouse macrophages, and
subsequently incubated with goat anti-rat IgG conjugated to Alexa
Fluor 488 as a secondary antibody. Immunofluorescence was examined
microscopically using an Eclipse TS100-F microscope (Nikon, Tokyo,
Japan).
IFP measurement in tumors
When the tumor volume reached ~150 mm3,
the LLC tumor-bearing mice were intravenously injected with ZOL
solution at a dose of 5, 20 or 40 μg of ZOL/mouse per day for three
consecutive days. Twenty-four hours after the final injection of
ZOL solution, the mice were anesthetized with isoflurane, and then
interstitial fluid pressure (IFP) of tumors was measured with a
needle probe pressure monitor, fitted with an 18-gauge side-ported
needle (Intra-Compartmental Pressure Monitor System; Stryker,
Kalamazoo, MI, USA) connected to a syringe filled with 0.9% saline,
as previously reported (14). The
needle probe was inserted into the center of the tumor or normal
muscle, and IFP was recorded. The IFP in tumors was normalized to
that in muscle [normalized IFP = IFP (mmHg) of tumor/IFP (mmHg) of
muscle].
Determination of serum cytokine
levels
When the tumor volume reached ~150 mm3,
LLC tumor-bearing mice were intravenously injected with ZOL
solution at a dose of 40 μg of ZOL/mouse per day for three
consecutive days. Twenty-four hours after the final injection of
ZOL solution, serum was prepared by separation of the coagulated
whole blood. Serum cytokine levels, including interleukin (IL)-10
and -12 (p70), granulocyte-macrophage colony-stimulating factor
(GM-CSF) and tumor necrosis factor (TNF)-α, were determined using
mouse cytokine Th1/Th2 Panel (Bio-Rad, Hercules, CA, USA) and
Bio-Plex 200 system (Bio-Rad). Normal values were determined using
blood obtained from age-matched, normal mice without an LLC
tumor.
Quantitative real-time PCR
When the tumor volume reached ~200 mm3,
the LLC tumor-bearing mice were intravenously injected with ZOL
solution at a dose of 40 μg of ZOL/mouse per day for three
consecutive days. For the expression level of vascular endothelial
growth factor (VEGF) mRNA in tumor tissues, the tumors were excised
from LLC tumor-bearing mice 24 h after the final injection of ZOL
solution, and then total RNA was isolated from the tumors using the
TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA).
RNA yield and purity were checked by spectrometric measurements at
260 and 280 nm. cDNA was synthesized from total RNA by using the
PrimeScript RT Reagent kit with gDNA Eraser (Takara Bio Inc.,
Shiga, Japan). Quantitative real-time PCR was performed with the
Takara Thermal Cycler Dice (Takara Bio Inc.) and TaqMan Gene
expression assays (vegfa: Mm00437306_m1, gapdh: Mm99999915_g1;
Applied Biosystems, CA, USA). Samples were run in triplicate and
the expression levels of VEGF mRNA were normalized for the amount
of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in the
same sample, and analyzed using the comparative Ct method.
Cytotoxicity
LLC and RAW264.7 cells were seeded separately at a
density of 1×104 cells per well in 96-well plates and
maintained in RPMI-1640 medium supplemented with 10% FBS for 24 h
before treatment. To examine cytotoxicity for ZOL, LLC and RAW
264.7 cells were treated with medium containing a range of 2.5 to
40 μM ZOL, and they were then incubated for 48 h. To examine the
effect of ZOL on the cytotoxicity of DXR, LLC and RAW 264.7 cells
were treated with medium containing a range of 0.125 to 2 μM DXR in
the presence or absence of 20 μM ZOL and they were then incubated
for 48 h. The cell number was determined with Cell Counting Kit-8
(Dojindo Laboratories, Kumamoto, Japan). Cell viability is
expressed relative to the absorbance at 450 nm of untreated
cells.
In vivo therapeutic studies
When the average volume of the tumors reached
100–200 mm3 in mice bearing LLC tumors, ZOL solution was
intravenously administered via lateral tail veins at a dose of 40
μg of ZOL/mouse on days 0, 1 and 2, and then Doxil was
intravenously administered at a dose of 5 mg of DXR/kg on day 3.
Tumor volume and body weight were measured for individual
animals.
Biodistribution of DXR
When the average volume of the tumors reached 150
mm3 in mice bearing LLC tumors, ZOL solution was
intravenously administered via lateral tail veins at a dose of 40
μg of ZOL/mouse on days 0, 1 and 2, and then Doxil was
intravenously administered at a dose of 5 mg of DXR/kg on day 3.
The tumors and organs were excised 24 h after the injection of
Doxil, and then homogenized in 0.1 M
NH4Cl/NH3 buffer (pH 9.0). DXR was extracted
with chloroform/methanol (2:1 v/v) and analyzed by HPLC, as
previously described (13).
Statistical analysis
The statistical significance of differences between
mean values was determined by Student’s t-test. Multiple
measurement comparisons were performed by analysis of variance
followed by one-way analysis of variance on ranks with post
hoc Tukey-Kramer’s test. A p-value of ≤0.05 was considered
significant.
Results
Vascular structure of tumor after
treatment with ZOL
Previously, we reported that the change of vascular
structure in tumor was observed when ZOL solution was intravenously
injected into tumor-bearing mice (11); however, the change of tumor
environment upon ZOL treatments was not clear. In this study, we
investigated whether ZOL treatments could improve the tumor
environment via change of tumor vasculature and enhance the
antitumor efficacy of liposomal DXR, Doxil.
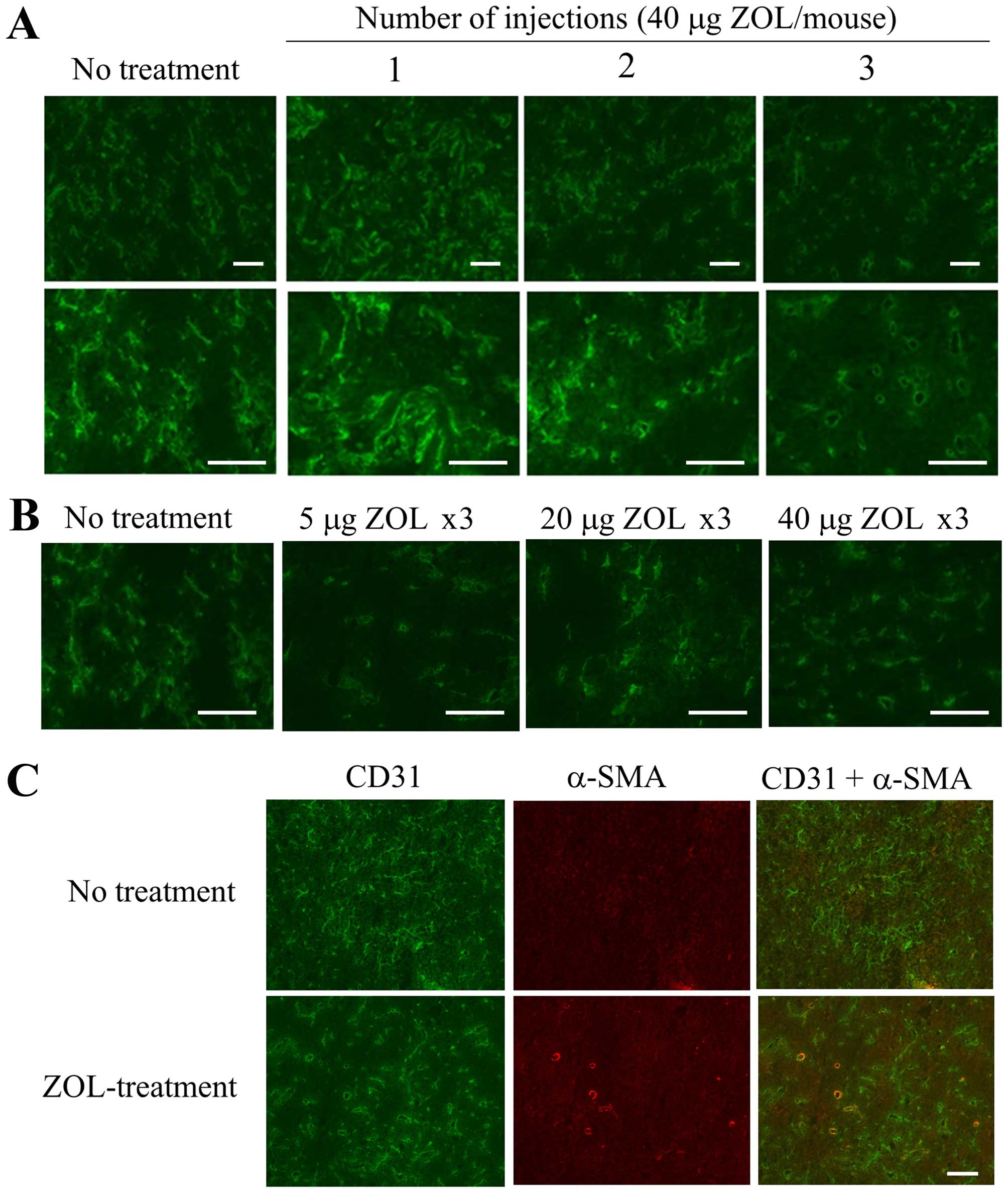
First, to examine the frequency of ZOL
administration and dosage amount (μg) of ZOL required to change the
vascular structure in LLC tumor, we intravenously injected ZOL
solution at a dose of 40 μg of ZOL/mouse per day for one, two or
three consecutive days into mice bearing an LLC tumor. When ZOL was
injected for three consecutive days, apparent changes of vascular
structure in the tumor were observed by immunostaining for CD31,
which is a marker for endothelial cells, compared with those after
one or two administrations (Fig.
1A). Regarding dosage amount, changes of vascular structure in
the tumor were observed upon ZOL injection at 5, 20 and 40 μg of
ZOL/mouse per day for three consecutive days (Fig. 1B). ZOL treatments reduced narrow
vessels in tumor and increased open vessels, indicating that blood
flow in the tumor might be improved by the change of vasculature
structure. Furthermore, some CD31-positive endothelial cells were
covered with α-SMA-positive pericytes in tumor section treated at
40 μg of ZOL/mouse for three consecutive days, although most of the
CD31-positive endothelial cells in tumor section of untreated mouse
were not covered with α-SMA-positive pericytes (Fig. 1C), suggesting that ZOL treatments
did not markedly affect pericyte coverage in tumor vessels. This
histological change of tumor vasculature after ZOL treatment seemed
to be similar to the phenomenon called normalization of the tumor
vasculature (10).
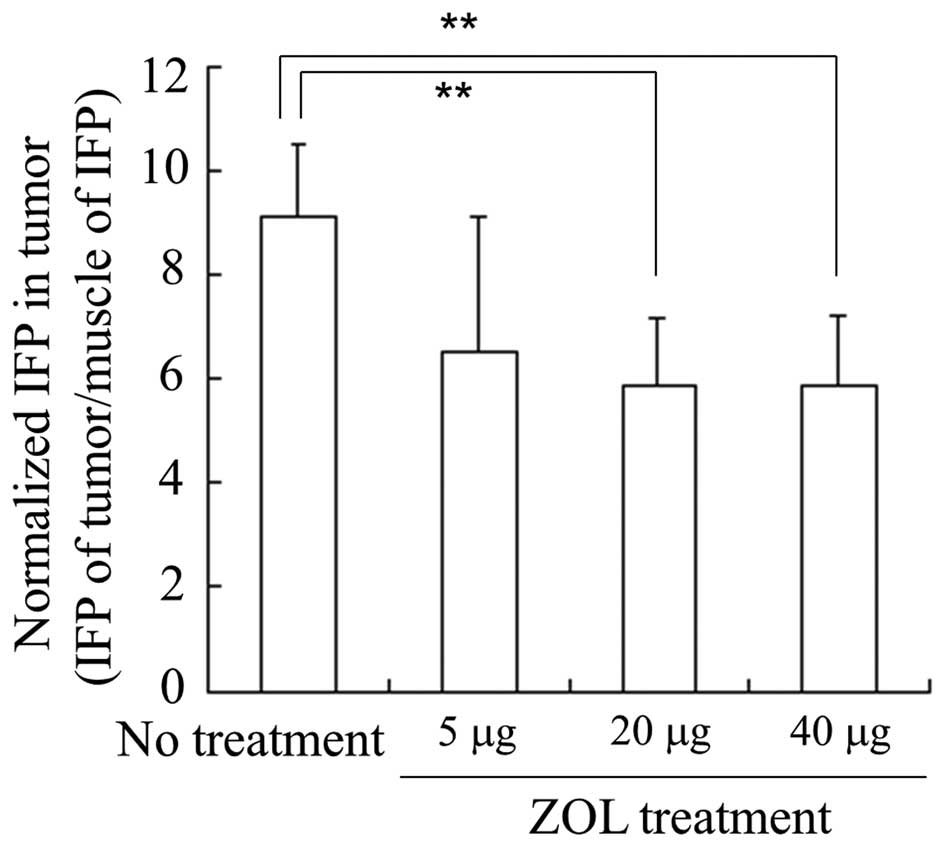
Change of IFP
To examine the effect of ZOL on IFP in tumors, we
measured IFP of tumors and muscles 24 h after intravenous
injections of ZOL, and normalized the IFP of tumors by that of
muscles. When ZOL solution was injected for three consecutive days,
normalized IFP in LLC tumors was significantly decreased by
injections of ZOL solution at 20 and 40 μg of ZOL/mouse per day
(5.8±1.3 and 5.9±1.3 in normalized IFP, respectively) compared with
no treatment (9.1±1.4 in normalized IFP), but not by 5 μg of
ZOL/mouse per day (6.6±2.6 in normalized IFP) (Fig. 2). This indicated that the injection
of 20 or 40 μg of ZOL could decrease the IFP of the tumor by
changing the tumor vasculature. Therefore, in subsequent
experiments, we performed injections of 40 μg of ZOL/mouse per day
for three consecutive days.
It has been reported that Colon 26 and LLC tumors
have well- and poorly vascularized blood vessels, respectively
(15). Previously, we reported
that LLC tumors showed higher IFP than Colon 26 tumors (14). When ZOL solution was injected into
mice bearing Colon 26 tumor for three consecutive days, no decrease
of IFP in the tumor was observed (2.4±1.0 and 3.7±1.2 of normalized
IFP in Colon 26 tumor with no treatment and ZOL treatment,
respectively) (data not shown). These findings suggest that
reduction of IFP in tumor by ZOL treatments might be effective for
tumors having high IFP.
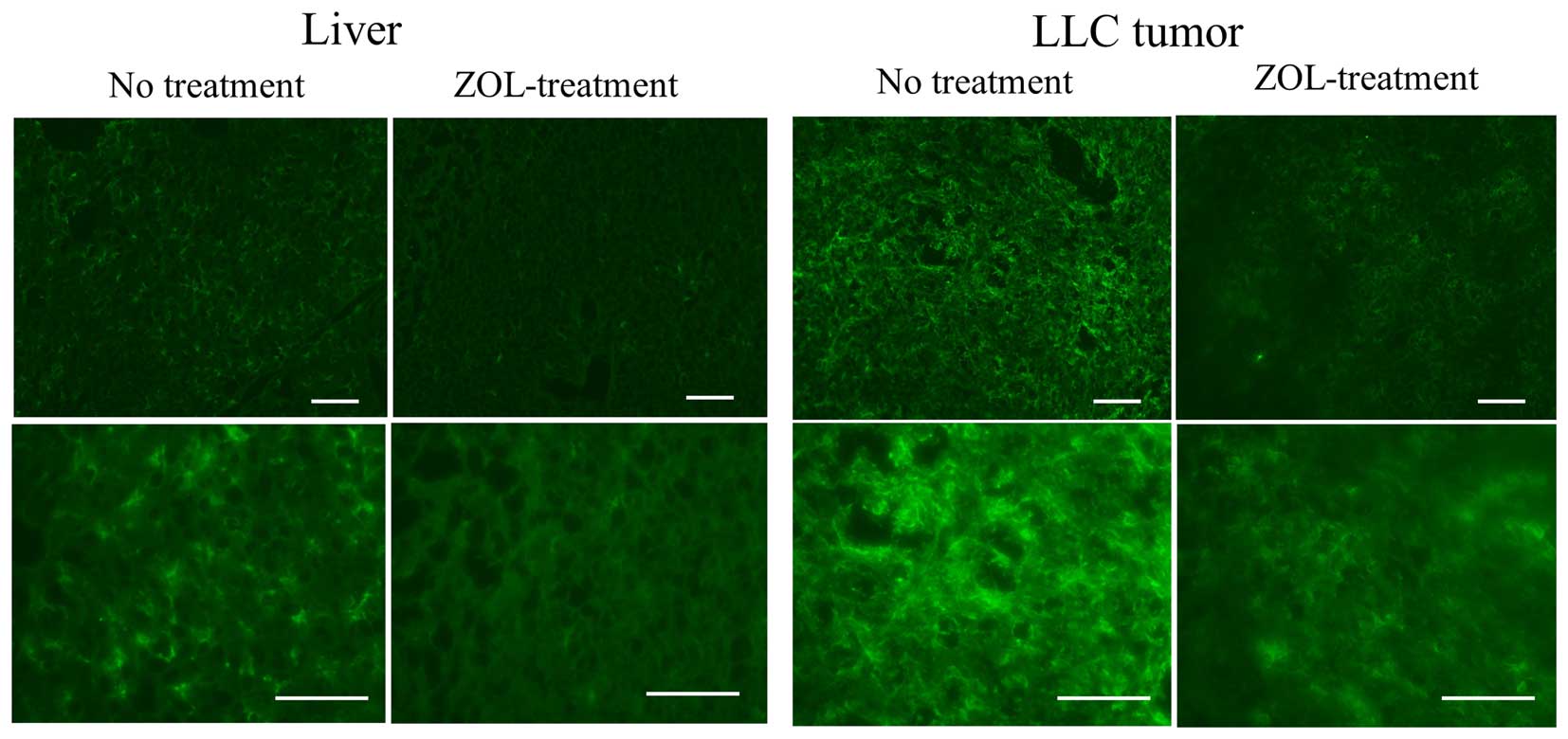
Change of macrophages in tumor and
cytokine levels in serum after ZOL treatments
Bisphosphonates are internalized into cells by
fluid-phase endocytosis, and then endosomal acidification causes
the release of the bisphosphonates into the cytosol (16). Highly phagocytic cells such as
macrophages have the ability to internalize bisphosphonates, which
makes them an ideal target for these drugs. Therefore, we examined
the effect of ZOL on macrophages in tumor and liver. In untreated
mice, a large number of macrophages in the livers and tumors was
detected by immunostaining with F4/80 antibody; however, in ZOL
treatments, the number of macrophages in the tumors and livers was
markedly decreased (Fig. 3).
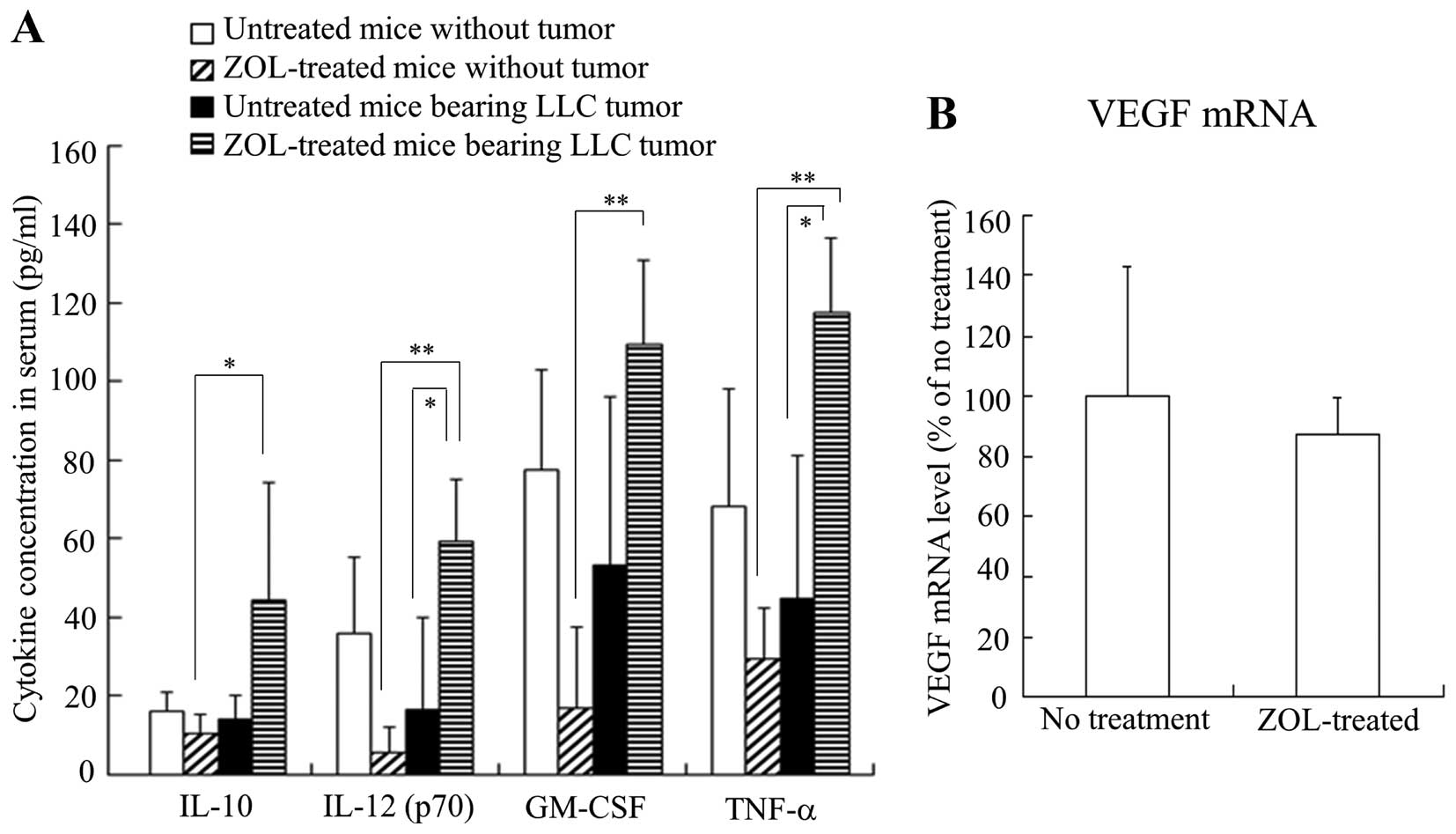
To examine the effect of ZOL treatments on the
inflammatory cytokines in serum, we measured IL-10, IL-12 (p70),
GM-CSF and TNF-α levels in serum after ZOL treatment in mice with
or without LLC tumors. The ZOL injections into mice without tumor
decreased IL-12, GM-CSF and TNF-α levels compared with those in
untreated mice without tumor, although their levels were not
significantly different (Fig. 4A).
In contrast, the ZOL injections into LLC tumor-bearing mice
significantly increased IL-12 and TNF-α levels compared with those
of untreated mice bearing LLC tumors. These findings suggest that
ZOL injections may affect tumor cells or TAMs in tumor tissues and
induce inflammatory responses.
The vascular endothelial growth factor (VEGF)
protein is a prominent cytokine, which promotes endothelial cell
proliferation during angiogenesis. Therefore, we investigated
whether ZOL treatments could affect the expression level of VEGF
mRNA in the tumor by quantitative RT-PCR analysis. Surprisingly,
VEGF mRNA level was not changed by ZOL treatments (Fig. 4B), indicating that the change of
vascular structure might be caused in a VEGF-independent
manner.
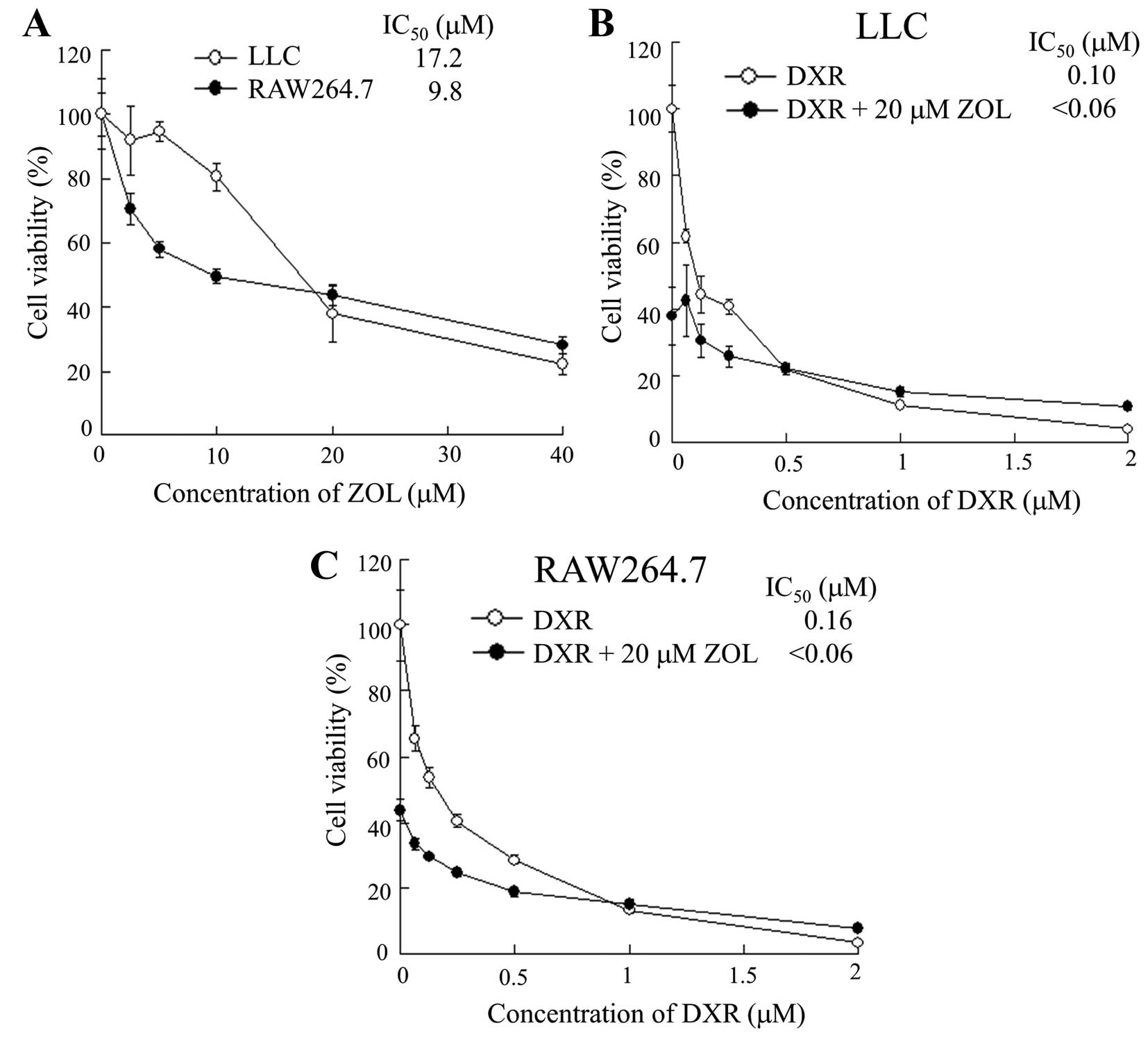
In vitro antitumor effect
To confirm whether ZOL was taken up by tumor cells
or macrophages, and was able to induce cytotoxic effects, we
examined the cytotoxicity for LLC or RAW264.7 cells by ZOL. ZOL
treatment showed higher cytotoxicity for RAW 264.7 cells than for
LLC cells (Fig. 5A), indicating
that this cytotoxicity by ZOL might be due to uptake by fluid-phase
endocytosis in macrophage cells.
To examine the effect of ZOL on cytotoxicity by DXR,
we examined the cytotoxicity for LLC or RAW264.7 cells by DXR in
the presence of 20 μM ZOL. ZOL showed additive cytotoxic effects
for RAW 264.7 and LLC cells, rather than synergistic effects
(Fig. 5B and C), suggesting that
ZOL could not increase chemosensitivity by DXR for macrophages or
LLC tumors.
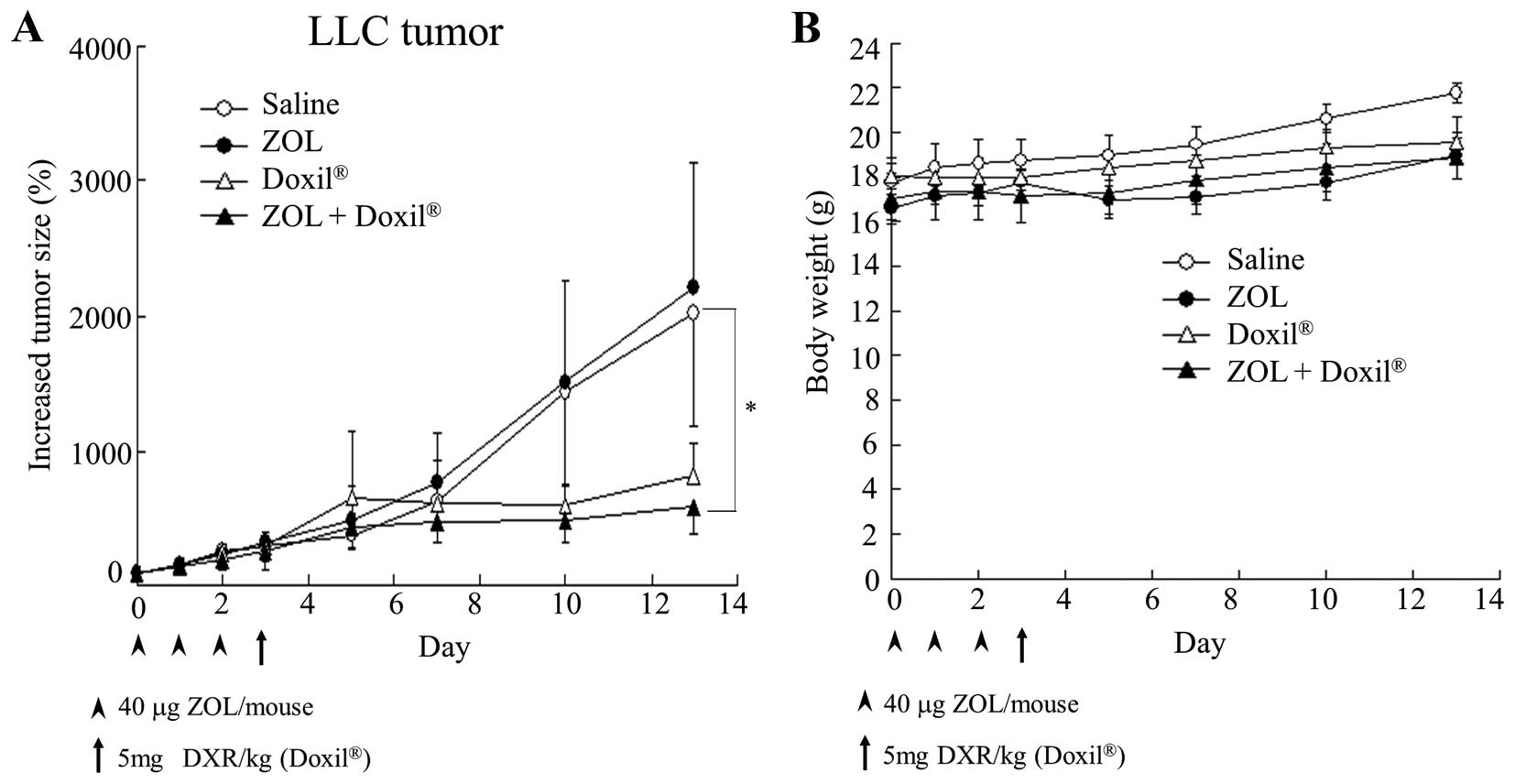
Antitumor effect on LLC tumor-bearing
mice
To examine whether ZOL injections could increase the
antitumor effect of Doxil by change of the tumor microenvironment,
we evaluated the antitumor effect of Doxil after three intravenous
injections of ZOL into LLC tumor-bearing mice. ZOL solution was
intravenously administered on days 0, 1 and 2, and then Doxil was
on day 3. Three injections of ZOL solution did not show antitumor
activity for the tumors (Fig. 6A),
although they had an anti-angiogenic effect (Fig. 1). Single injection of Doxil showed
a large antitumor effect. Furthermore, injections of ZOL increased
the antitumor activity by Doxil. There were no remarkable
differences in mouse body weight changes after the administration
of ZOL and/or Doxil (Fig. 6B).
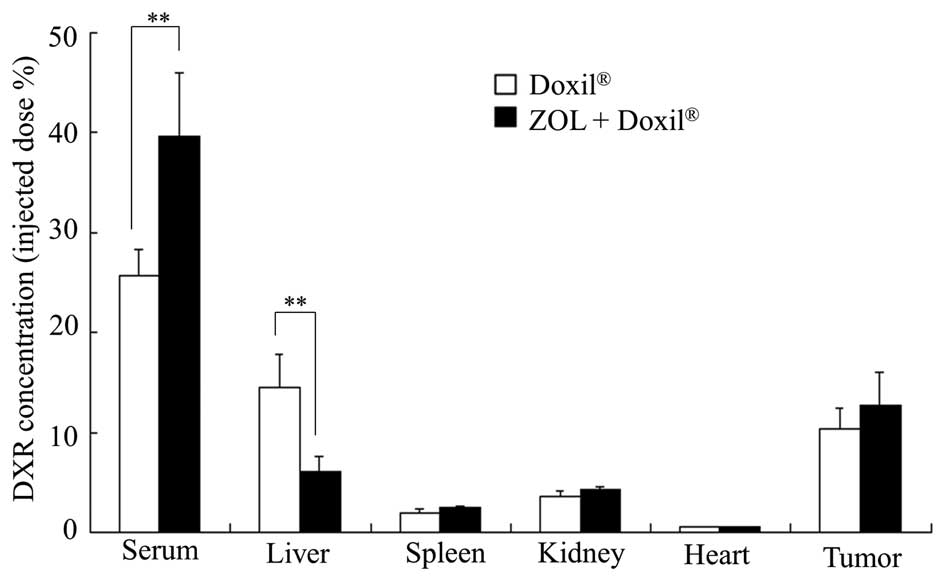
Accumulation of DXR liposomes in the
tumor
Finally, we examined whether ZOL treatments affected
the biodistribution of DXR in mice bearing LLC tumors after the
injection of Doxil. ZOL injections significantly increased the
blood concentration of DXR after the injection of Doxil and
decreased the accumulation of DXR in the liver (Fig. 7). The change of DXR accumulation in
the liver may have been due to the depletion of Kupffer cells.
However, the accumulation of DXR in the tumor was not significantly
different between untreated and ZOL-treated tumors. These findings
indicate that an increase of the antitumor effect of Doxil upon ZOL
injections might be explained by an increased blood circulation
time of Doxil and/or wide distribution of DXR in the tumor by a
change of the tumor microenvironment.
Discussion
Anti-angiogenesis effects are known to change the
tumor vasculature. In this study, we found that ZOL treatment
decreased IFP in tumor via the inhibition of tumor
neovascularization (Figs. 1 and
2). Santini et al reported
that single treatment of ZOL reduced circulating VEGF levels in
cancer patients (17). However, in
our study, a reduction of VEGF mRNA was not observed in tumors
after ZOL treatments (Fig. 4B).
Ogawara et al reported that VEGF did not play a major role
in the angiogenesis in LLC tumors, suggesting that other
proangiogenic factors except for VEGF might trigger angiogenesis in
LLC tumors (18). Giraudo et
al reported that ZOL suppressed the expression of matrix
metalloproteinase-9 (MMP-9) by infiltrating macrophages and
inhibited metalloproteinase activity, reducing the association of
VEGF with its receptor on angiogenic endothelial cells (19). From these findings, the depletion
of TAMs in the tumor by ZOL treatments might affect tumor
neovascularization via inhibition of the association of VEGF and
its receptor. However, it has also been reported that ZOL inhibited
anti-angiogenesis through an apoptotic effect on endothelial cells
in tumor and the tumor microenvironment (20,21).
ZOL exerts an inhibitory effect on endothelial cell adhesion and
migration via the modulation of adhesion molecules (22). The mechanism by which ZOL
treatments changed the vascular structures in the tumors was not
clear, but ZOL treatments decreased IFP in the tumor via the
inhibition of tumor neovascularization.
The most common adverse event associated with
bisphosphonate therapy is transient fever (23). It has been shown that treatment
with intravenous nitrogen-containing bisphosphonates such as ZOL
caused systemic acute-phase responses (APRs) characterized by
fever, pain, nausea and fatigue in up to 50% of all patients within
48 h after administration (24).
These flu-like symptoms are typically transient, resolve
spontaneously, and are accompanied by decreased lymphocyte counts
and elevated levels of pro-inflammatory cytokines such as IL-6,
IFN-γ and TNF-α (23,25). In our study, we observed elevated
levels of IL-10 and -12, GM-CSF and TNF-α after injections of ZOL
solution into mice bearing a tumor (Fig. 4A); however, in normal mice without
tumor, ZOL injections did not affect the level of inflammatory
cytokines in serum. Although it was not clear why the ZOL
treatments increased the levels of the inflammatory cytokines in
tumor-bearing mice, these cytokines might be released from tumor
tissues by ZOL treatments and cause inhibition of tumor
neovascularization.
Polyethylene glycol (PEG)-modified liposomes are
long-lived in the circulation and accumulate in the tumors. The
TGF-β type I receptor inhibitors were reported to increase the
antitumor effect of DXR encapsulated in PEGylated liposomes or
micelles by changing the microenvironment of the vasculature
(12,13). Therefore, we examined whether ZOL
treatments could increase the accumulation of Doxil in tumors and
enhance the antitumor effect. As a result, ZOL treatments increased
the antitumor effect of Doxil (Fig.
6); however, did not increase the accumulation of DXR in the
tumor 24 h after the injection of Doxil (Fig. 7). ZOL is known as a specific
inhibitor of farnesyl pyrophosphate synthase in the mevalonate
pathway and exerts pleiotropic effects in tumor and non-tumor cells
(26,27). Riganti et al reported that
ZOL restored the chemosensitivity of DXR in multidrug-resistant
cancer cells (28). However, in
our study, ZOL treatments alone did not induce an antitumor effect
in LLC tumors (Fig. 6), and did
not show enhancement of cytotoxicity by DXR in LLC cells (Fig. 5B). Yoshizawa et al reported
that pre-treatment with a V EGF receptor-2 inhibitor, SU5416,
changed vascular structures in tumor but did not significantly
increase the tumor accumulation of paclitaxel after the injection
of PEGylated liposomal paclitaxel, compared with the untreated mice
(29). However, they concluded
that the treatment increased the distribution of PEGylated
liposomal paclitaxel in the core region of the tumor, as well as
conversely decreasing the ratio of its peripheral distribution.
Therefore, we speculate that the enhanced antitumor effect observed
in an in vivo experiment might be due to the improvement of
DXR distribution in tumor, not an increase of DXR chemosensitivity
in tumor cells. To prove this hypothesis, we observed the
localizations of DXR in the tumor after ZOL treatments by
fluorescent microscopy, but the localization was not well detected
due to the weak intensity of DXR fluorescence (data not shown).
Further study should be performed to investigate the distribution
of DXR in tumor after ZOL treatments.
Resident macrophages in the liver called Kupffer
cells comprise the major population of the reticuloendothelial
system (RES). Doxil can avoid RES uptake by PEG modification;
however, the effectiveness for the prevention of RES uptake is
still incomplete. Previously, it was reported that the depletion of
Kupffer cells by clodronic acid-entrapped liposomes (clodrolip)
inhibited RES uptake in the liver and increased the plasma
concentration of DXR after the injection of Doxil, resulting in
enhancement of antitumor effects in a xenograft model (30). In our study, depletion of Kupffer
cells (macrophages) in the liver was observed after the injection
of ZOL (Fig. 3), and exhibited
extended blood circulation of DXR and reduced its accumulation in
the liver (Fig. 7). This depletion
might be one of the reasons why the combination of ZOL and Doxil
was able to enhance therapeutic efficacy.
Ottewell et al reported that the inhibition
of tumor growth was observed by sequential injection with DXR and
ZOL in a mouse model of breast and mammary tumor (31,32).
They concluded that sequential treatment with DXR followed by ZOL
elicited substantial antitumor effects in vivo, but ZOL
followed by DXR did not (31). The
discrepancy between our results and previous reports might be
caused by the schedule of administration of ZOL and DXR. In
sequential treatment with ZOL followed by DXR, DXR was injected
into the mice 24 h after the injection of ZOL; however, the tumors
after ZOL treatment displayed no obvious differences in terms of
the degree of vascularization compared with the saline control
(31). In our experiments, no
change of vascular structure in LLC tumors was observed 24 h after
single injection of ZOL (Fig. 1A).
These results might indicate that the repeated injections of ZOL
were needed to increase the antitumor effect of DXR by the change
of vascular structure.
In this study, we found that ZOL treatments
decreased IFP in tumor via a change of tumor vasculature and
enhanced the antitumor efficacy of liposomal doxorubicin (Doxil).
ZOL treatment can be an alternative approach to increase the
antitumor effect by liposomal drugs.
Acknowledgements
We thank Ms. Akira Kiyota for assistance in the
experimental work. This study was supported in part by a
Grant-in-Aid for Scientific Research (C) from the Japan Society for
the Promotion of Science (KAKENHI grant no. 26460046).
References
|
1
|
Shang B, Cao Z and Zhou Q: Progress in
tumor vascular normalization for anticancer therapy: Challenges and
perspectives. Front Med. 6:67–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi N, Miyoshi S, Mikami T, Koyama
H, Kitazawa M, Takeoka M, Sano K, Amano J, Isogai Z, Niida S, et
al: Hyaluronan deficiency in tumor stroma impairs macrophage
trafficking and tumor neovascularization. Cancer Res. 70:7073–7083.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang
PY, Xu HX, Kong LQ, Wang L, Wu WZ and Tang ZY: Depletion of
tumor-associated macrophages enhances the effect of sorafenib in
metastatic liver cancer models by antimetastatic and antiangiogenic
effects. Clin Cancer Res. 16:3420–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rogers MJ, Gordon S, Benford HL, Coxon FP,
Luckman SP, Monkkonen J and Frith JC: Cellular and molecular
mechanisms of action of bisphosphonates. Cancer. 88(Suppl):
2961–2978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ross JR, Saunders Y, Edmonds PM, Patel S,
Wonderling D, Normand C and Broadley K: A systematic review of the
role of bisphosphonates in metastatic disease. Health Technol
Assess. 8:1–176. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bäckman U, Svensson A, Christofferson RH
and Azarbayjani F: The bisphosphonate, zoledronic acid reduces
experimental neuroblastoma growth by interfering with tumor
angiogenesis. Anticancer Res. 28A:1551–1557. 2008.
|
|
10
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hattori Y, Yamashita J, Sakaida C, Kawano
K and Yonemochi E: Evaluation of antitumor effect of zoledronic
acid entrapped in folate-linked liposome for targeting to
tumor-associated macrophages. J Liposome Res. Sep 9–2014.(Epub
ahead of print). View Article : Google Scholar
|
|
12
|
Kano MR, Bae Y, Iwata C, Morishita Y,
Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, et al:
Improvement of cancer-targeting therapy, using nanocarriers for
intractable solid tumors by inhibition of TGF-beta signaling. Proc
Natl Acad Sci USA. 104:3460–3465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taniguchi Y, Kawano K, Minowa T, Sugino T,
Shimojo Y and Maitani Y: Enhanced antitumor efficacy of
folate-linked liposomal doxorubicin with TGF-β type I receptor
inhibitor. Cancer Sci. 101:2207–2213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kato M, Hattori Y, Kubo M and Maitani Y:
Collagenase-1 injection improved tumor distribution and gene
expression of cationic lipoplex. Int J Pharm. 423:428–434. 2012.
View Article : Google Scholar
|
|
15
|
Ogawara K, Un K, Minato K, Tanaka K,
Higaki K and Kimura T: Determinants for in vivo anti-tumor effects
of PEG liposomal doxorubicin: Importance of vascular permeability
within tumors. Int J Pharm. 359:234–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson K, Rogers MJ, Coxon FP and
Crockett JC: Cytosolic entry of bisphosphonate drugs requires
acidification of vesicles after fluid-phase endocytosis. Mol
Pharmacol. 69:1624–1632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santini D1, Vincenzi B, Dicuonzo G,
Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L,
Tirindelli MC, Altomare V, et al: Zoledronic acid induces
significant and long-lasting modifications of circulating
angiogenic factors in cancer patients. Clin Cancer Res.
9:2893–2897. 2003.PubMed/NCBI
|
|
18
|
Ogawara K, Abe S, Un K, Yoshizawa Y,
Kimura T and Higaki K: Determinants for in vivo antitumor effect of
angiogenesis inhibitor SU5416 formulated in PEGylated emulsion. J
Pharm Sci. 103:2464–2469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giraudo E, Inoue M and Hanahan D: An
amino-bisphosphonate targets MMP-9-expressing macrophages and
angiogenesis to impair cervical carcinogenesis. J Clin Invest.
114:623–633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wood J, Bonjean K, Ruetz S, Bellahcène A,
Devy L, Foidart JM, Castronovo V and Green JR: Novel antiangiogenic
effects of the bisphosphonate compound zoledronic acid. J Pharmacol
Exp Ther. 302:1055–1061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Corso A, Ferretti E and Lazzarino M:
Zoledronic acid exerts its antitumor effect in multiple myeloma
interfering with the bone marrow microenvironment. Hematology.
10:215–224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bezzi M, Hasmim M, Bieler G, Dormond O and
Rüegg C: Zoledronate sensitizes endothelial cells to tumor necrosis
factorinduced programmed cell death: Evidence for the suppression
of sustained activation of focal adhesion kinase and protein kinase
B/Akt. J Biol Chem. 278:43603–43614. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dicuonzo G, Vincenzi B, Santini D,
Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola
R and Tonini G: Fever after zoledronic acid administration is due
to increase in TNF-alpha and IL-6. J Interferon Cytokine Res.
23:649–654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanvetyanon T and Stiff PJ: Management of
the adverse effects associated with intravenous bisphosphonates.
Ann Oncol. 17:897–907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reid IR, Gamble GD, Mesenbrink P, Lakatos
P and Black DM: Characterization of and risk factors for the
acute-phase response after zoledronic acid. J Clin Endocrinol
Metab. 95:4380–4387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clézardin P and Massaia M:
Nitrogen-containing bisphosphonates and cancer immunotherapy. Curr
Pharm Des. 16:3007–2014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coscia M, Quaglino E, Iezzi M, Curcio C,
Pantaleoni F, Riganti C, Holen I, Mönkkönen H, Boccadoro M, Forni
G, et al: Zoledronic acid repolarizes tumour-associated macrophages
and inhibits mammary carcinogenesis by targeting the mevalonate
pathway. J Cell Mol Med. 14:2803–2815. 2010. View Article : Google Scholar
|
|
28
|
Riganti C, Castella B, Kopecka J, Campia
I, Coscia M, Pescarmona G, Bosia A, Ghigo D and Massaia M:
Zoledronic acid restores doxorubicin chemosensitivity and
immunogenic cell death in multidrug-resistant human cancer cells.
PLoS One. 8:e609752013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshizawa Y, Ogawara K, Fushimi A, Abe S,
Ishikawa K, Araki T, Molema G, Kimura T and Higaki K: Deeper
penetration into tumor tissues and enhanced in vivo antitumor
activity of liposomal paclitaxel by pretreatment with angiogenesis
inhibitor SU5416. Mol Pharm. 9:3486–3494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohara Y, Oda T, Yamada K, Hashimoto S,
Akashi Y, Miyamoto R, Kobayashi A, Fukunaga K, Sasaki R and
Ohkohchi N: Effective delivery of chemotherapeutic nanoparticles by
depleting host Kupffer cells. Int J Cancer. 131:2402–2410. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ottewell PD, Mönkkönen H, Jones M, Lefley
DV, Coleman RE and Holen I: Antitumor effects of doxorubicin
followed by zoledronic acid in a mouse model of breast cancer. J
Natl Cancer Inst. 100:1167–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ottewell PD, Brown HK, Jones M, Rogers TL,
Cross SS, Brown NJ, Coleman RE and Holen I: Combination therapy
inhibits development and progression of mammary tumours in
immunocompetent mice. Breast Cancer Res Treat. 133:523–536. 2012.
View Article : Google Scholar
|