Introduction
Malignant gliomas, especially glioblastomas,
represent the most frequent and most lethal primary tumors of the
central nervous system. The median survival time and overall
survival rate at 5 years of patients with glioblastomas are 14.6
months and only 10%, respectively, despite multimodality treatments
including extensive tumor resection, radiotherapy, and chemotherapy
(1,2). On the other hand, some cancers have
been reported to harbor small cell populations possessing growth
sustaining and tumorigenetic abilities. Such cells, termed cancer
stem cells (CSCs) or cancer initiating cells, have been identified
in certain kinds of tumors including gliomas (2,3).
Glioma stem-like cells (GSCs or glioma initiating cells) maintain
some properties of cancer stem cells, express genes characteristic
of neural stem cells and differentiate into phenotypically diverse
populations, including neuronal, astrocytic, and oligodendroglial
cells (2,4), and have been reported to contribute
to the radioresistance and chemoresistance of gliomas (5,6).
GSCs may thus play an important role in tumorigenesis, treatment
resistance, and tumor recurrence. Although research on CSCs has
consequently received much attention, few ultrastructures of GSCs
have, to our knowledge, been adequately described, despite the fact
that certain molecular alterations and ultrastructural features of
the corresponding tumor cells have been investigated in detail
(7,8).
In this study, we provide further data for the
biological characterization and morphological description of GSCs
using immunocytochemical analysis and transmission electronic
microscopy, which may not only provide insight into the oncogenesis
of glioblastomas/gliomas but also help in the development of
therapies that are suitable for brain CSCs as the target.
Materials and methods
Glioma stem-like cells
Two neurosphere-like tumor cell lines (consisting of
glioma stem-like cells, glioma initiating cells, brain-tumor stem
cells, or glioblastoma stem cells), #0125 and #0222, were provided
from Nagoya University School of Medicine, Japan.
The cell lines satisfied the following criteria: a)
the cells could be maintained in neurobasal media with N2
(Invitrogen) and B27 supplements (Invitrogen), human recombinant
basic fibroblast growth factor (bFGF; R&D Systems), and
epidermal growth factor (EGF; R&D Systems) (20 ng/ml each) for
3 months (minimum), and b) 103 of the cells could form
tumors in the brain of nonobese diabetic mice with severe combined
immunodeficiency disease (NOD/SCID mice), but 105 of the
cells after being subcultured with DMEM media (Invitrogen)
containing 10% fetal bovine serum could not. These cells were
subcultured monthly (minimum) by dissociating the spheres with
NeuroCult (StemCell Technologies) (2,9).
Immunocytochemical staining of GSCs
Immunofluorescence staining for the cancer stem cell
markers, CD133 and CD15, was performed to evaluate the
characteristics of the GSCs isolated from the human glioblastomas
(#0125 and #0222). CD133 is the most accredited marker for CSCs in
various organs including glioma, while CD15 is one of the most
recently highlighted neural stem cell markers and is also employed
to identify CSCs in human brain tumors (3).
Cells were incubated with antibodies against CD133/1
(1:100; 130-080-801, mouse monoclonal IgG1; Milteny Biotec) and
CD15 (1:50; MMA, mouse monoclonal; Dako) overnight at 4°C.
Appropriate secondary antibodies, anti-mouse immunoglobulins/HRP,
Alexa Fluor 594 goat anti-mouse IgM, or Alexa Fluor 488 donkey
anti-mouse IgG (following established procedures), were used.
Furthermore, for the characterization of GSCs, immunofluorescence
was performed with primary antibodies, GFAP for astrocytes (1:100;
Millipore: MAB360), Oligo2 for oligodendrocytes (1:100; Product
Description: 13999-1-AP), NeuN for neurons (1:100; Millipore:
MAB377), and CD34 for endothelial cells (1:100; Immunotech:
IM1185). Expression of these cell markers was detected with a laser
scanning confocal microscope (Carl Zeiss), and the resultant images
were captured on a color CCD at specific magnifications.
Quantification of MGMT mRNA and protein
expression of MGMT on GSCs
Quantitative values for MGMT
(O6-methylguanine-DNA methyltransferase) mRNA were
estimated in GSCs from the human glioblastomas (#0125 and #0222),
since the DNA repair enzyme MGMT has been widely considered to be
involved in one of the most prominent resistance mechanisms for
alkylating anticancer drugs including temozolomide
(3-methyl-4-oxo-3,4-dihydroimidazo[5,1-d]
[1,2,3,5]tetrazine-8-carboxamide; TMZ), a standard therapeutic
agent for malignant gliomas (1).
The quantification of the MGMT gene expression was performed
by the real-time quantitative reverse transcription-PCR (RT-PCR)
method, as described previously (10).
Furthermore, the expression of MGMT protein in the
GSCs (#0125 and #0222) was determined by immunohistochemistry using
mouse monoclonal anti-MGMT antibody (MAB16200, clone MT3.1, 1:100;
Millipore). Prior to incubation of the primary antibody, a
heat-mediated antigen retrieval technique and blocking of
endogenous peroxidase activity were carried out. Incubation of the
primary antibody was performed for 1 h at 4°C. Diaminobenzidine
(DAB) was used for the detection as described previously (11). Nuclei were counterstained with
Mayer’s hematoxylin. A negative control was undertaken by omission
of the primary antibody.
Transmission electron microscope
examination of the GSC ultrastructure
GSCs from the human glioblastomas were fixed in 1%
glutaraldehyde and 0.1 M phosphate buffer for 15 min at 4°C. The
cells were washed in phosphate buffer twice for 15 min each.
Postfixation was performed in 1% osmium tetroxide for 1 h at 4°C,
followed by another two 15-min washes in the same buffer. After
dehydration, the material was embedded in Quetol 812 (Nisshin EM)
diluted in propylene oxide (1:1) and incubated at room temperature
for 24 h. The pellet was then transferred to pure Quetol 812 resin
and incubated at 60°C for 72 h, until completely polymerized.
Semithin and ultrathin sections were obtained with
the aid of an ultramicrotome. The semithin sections were stained
with 1% toluidine blue. The ultrathin sections (100 nm) were placed
on copper grids and stained with uranyl acetate and lead citrate.
The grids were examined and photographed under a Hitachi H7000
electron microscope.
Results
Characterization of GSCs by
immunocytochemistry
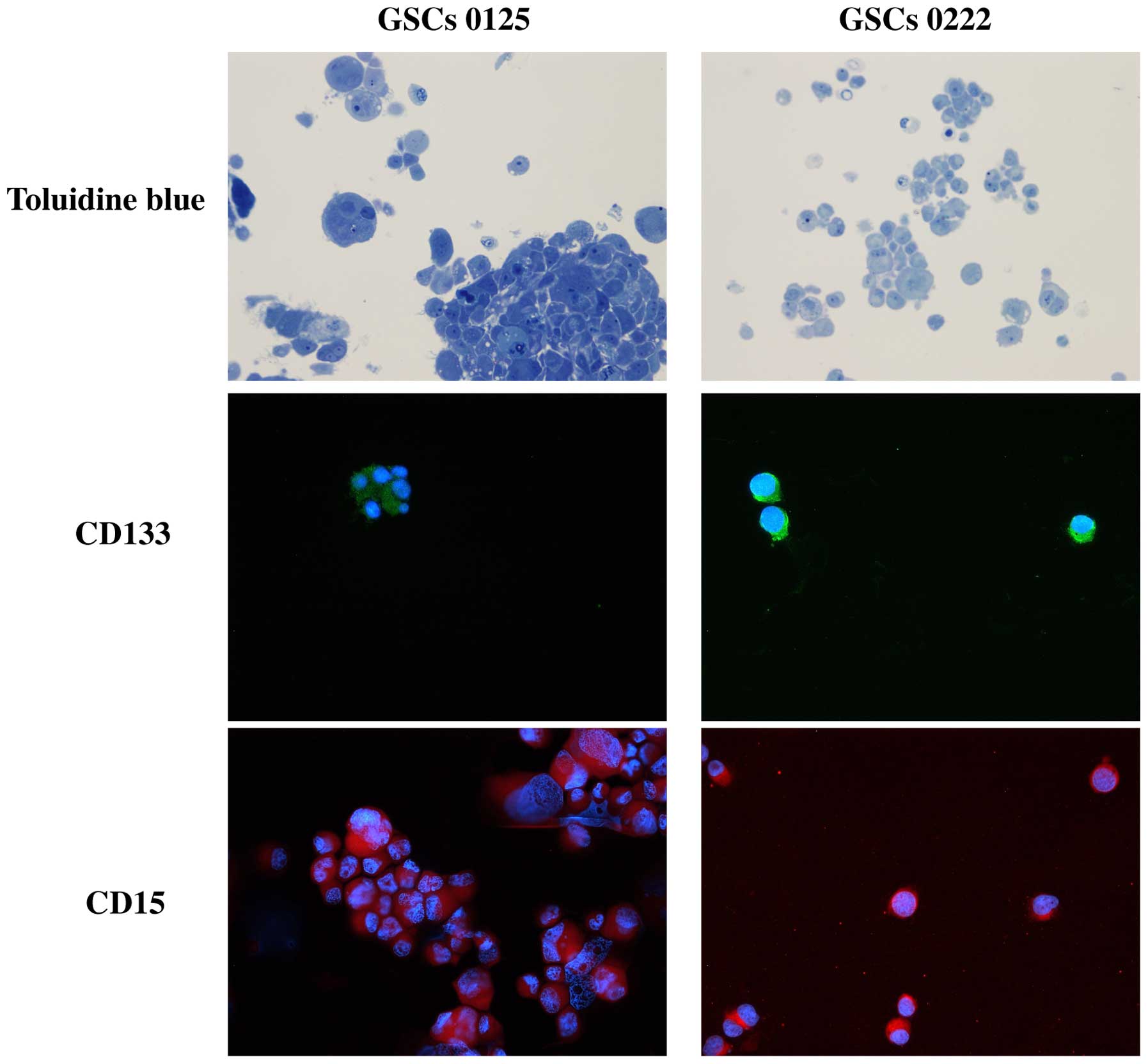
Immunofluorescence staining demonstrated that most
cells of GSCs 0125 and 0222 expressed the stem cell surface markers
CD133 and CD15 (Fig. 1).
As described above, immunocytochemistry was also
performed on GSCs 0125 and 0222 using the following antibodies:
GFAP (for astrocytes), Oligo2 (for oligodendrocytes), NeuN (for
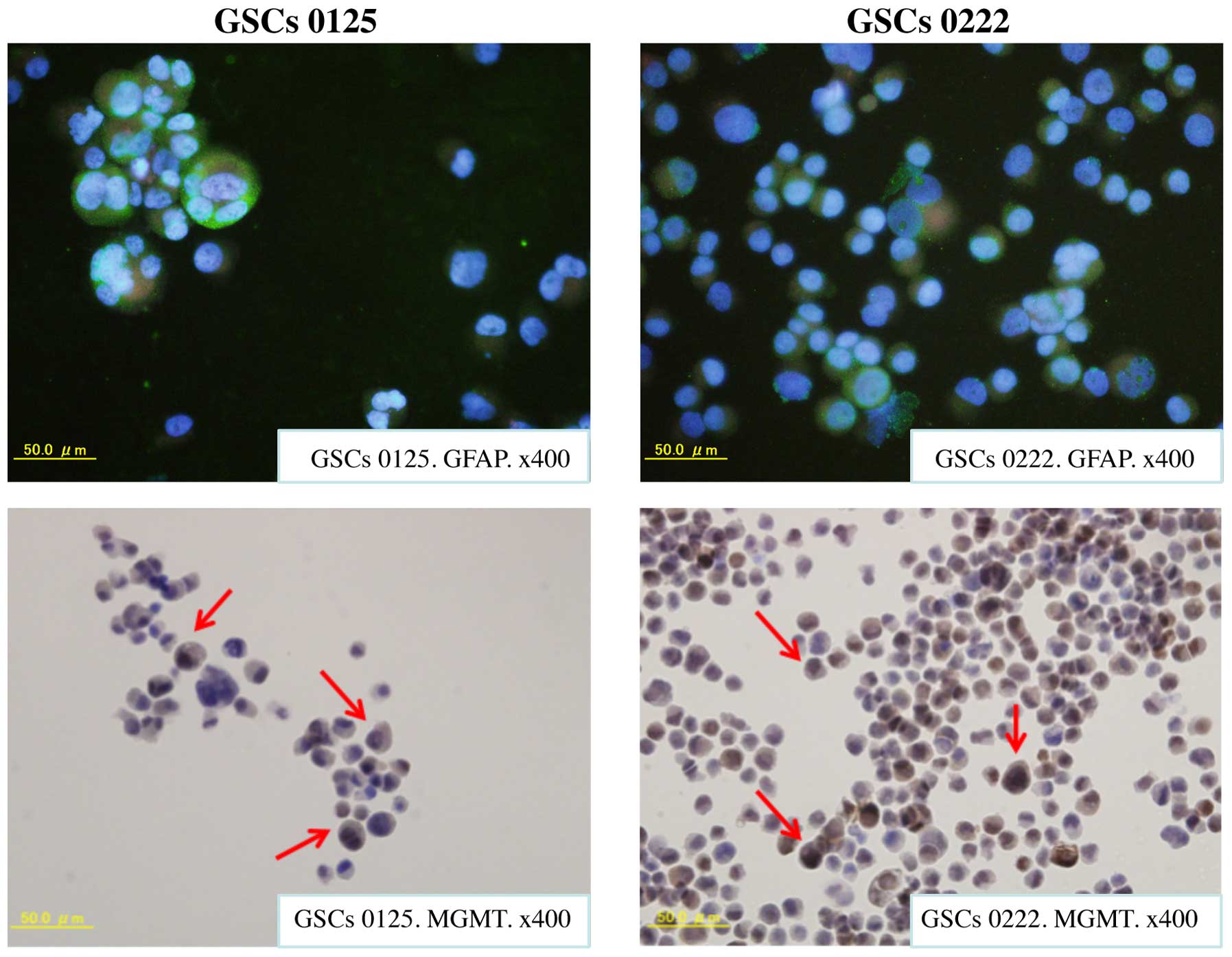
neurons), and CD34 (for endothelial cells). Most cells of GSCs 0125
and 0222 were stained for GFAP (Fig.
2). However, a few GSCs of 0125 and 0222 were immunopositive
for Oligo2, NeuN, and CD34. These experiments demonstrated that the
GSCs studied here expressed stem cell markers and differentiated
mainly astrocytes.
Quantitation of MGMT mRNA and protein
expression of MGMT on GSCs
The absolute values of MGMT mRNA normalized
to the level of GAPDH in GSCs 0125 and 0222 were 3.8×103
and 3.1×103, and 5.1×103 and
7.5×103 copies/μg RNA, respectively. These absolute
values for MGMT mRNA were almost equivalent to those of
TMZ-resistant cell lines (10).
Furthermore, high expression of MGMT protein was detected in the
cell nuclei and cytoplasm of both GSCs 0125 and 0222 (Fig. 2). These findings suggest that the
resistance of these cells to alkylating anticancer drugs including
TMZ (data for the resistance of these cells to alkylating drugs are
not shown here) is probably related to MGMT expression.
Characterization of GSCs by light and
electron microscopy
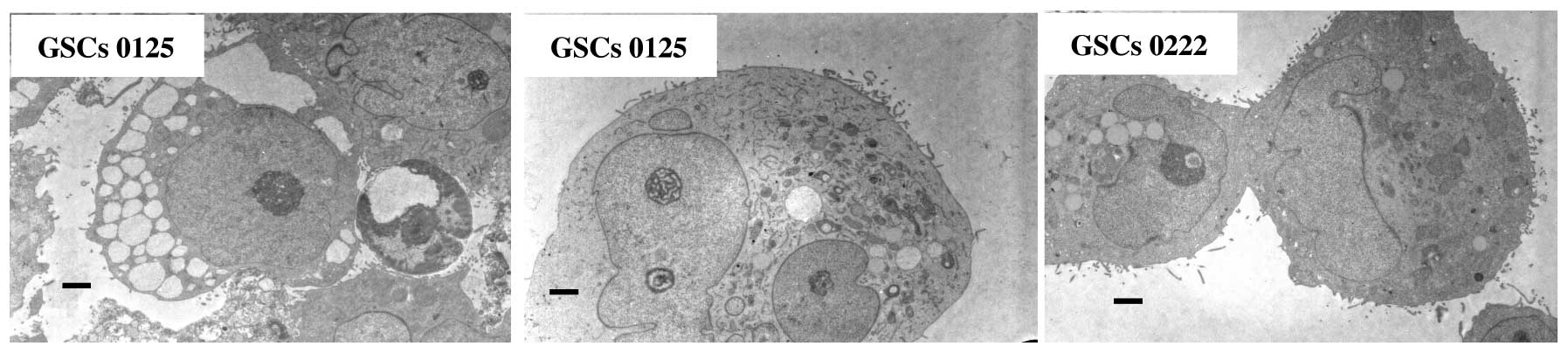
We employed light microscopy and transmission
electron microscopy to observe the morphology of the GSCs. There
were no large structural differences between GSCs 0222 and
0125.
Neurosphere-like clusters (tumor spheres), formed by
variable numbers of cells, were frequently seen in the GSCs, with
no typical organization (Figs. 1
and 3). They exhibited a variable
appearance in both their size and morphology, but none of the cells
we studied demonstrated typical features of neurons, ependymal
cells, or vessels. The GSCs had many microvilli (like cell surface
extensions) that spread throughout the intercellular space. The
nuclear-cytoplasmic ratio was generally from high to moderate
degree, and the GSCs sometimes had multiple nuclei that were
occasionally cleaved (deep indentations) and irregular in shape.
The nucleolus was generally prominent but sometimes obscure.
Cellular organelles were generally abundant in the form of
mitochondria, rough endoplasmic reticulum, and numerous coated
vesicles could be seen. Infrequently, cells at division phases and
different phases of the apoptotic process were observed (Fig. 3), but typical autophagosomes could
not be detected in either a single GSC or cells within the tumor
spheres.
Discussion
The CSC hypothesis suggests that neoplasms are
significantly due to a small fraction of cells with stem cell
properties (8). There are five
main characteristics of CSCs: i) a self-renewal ability, ii)
differentiation potential, iii) high tumorigenicity, iv) drug
resistance, and v) radioresistance (8,12,13).
The above hypothesis must therefore have crucial implications
regarding the process of tumorigenesis and choice of therapeutic
targets for improving the survival time of cancer patients
including glioblastoma patients (6,14).
Furthermore, it may help to explain why the present standard
treatment can reduce the tumor but often cannot eradicate it
resulting in eventual recurrence (6,15,16).
CD133, which was originally detected in
neuroepithelial stem cells of mice, is a cell surface marker
expressed on human neural stem cells (NSCs) (3,4,17),
hematopoietic stem cells (18),
and endothelial progenitor cells (18). It is most frequently used as a
representative CSC marker, including in gliomas (14,17).
CD133-positive (CD133+) CSCs have demonstrated a
capacity for unlimited self-renewal, as well as an ability to
initiate and drive tumor progression in animal models (6,19),
and a close correlation has been observed between the expression of
CD133 and chemo-resistance and survival in gliomas (6,20,21).
However, recent studies have indicated that there are
CD133-negative (CD133-) GSCs (22), and that the expression of CD133 may
reflect the environmental conditions and stress responses such as
hypoxia as well as mitochondrial dysfunction (14,17,22).
On the other hand, CD15, trisaccharide
3-fucosyl-N-acetyllactosamine, which is known as stagespecific
embryonic antigen 1, is strongly expressed in many types of
pluripotent stem cells and NSCs in the adult brain (17,23).
Furthermore, CD15 was recently proposed to be a marker of stem-like
cells derived from brain tumors (14,17).
The tumor spheres studied here indicated the existence of
CD133+ and CD15+ GSCs. The data could imply
intrinsic relationships between NSCs and GSCs, and suggested that
GSCs might retain some characteristics of NSCs (22,24).
On the other hand, most cells studied here were CD15-, CD133-, and
GFAP-positive, while a few fractions were Oligo2, NeuN or
CD34-positive. These findings may imply that the GSCs in the
present study were primitively different as compared to
undifferentiated NSCs (25). Mao
et al have previously suggested that the available data have
tended to complicate the issue of the source of GSCs, suggesting
that GSCs may also contain different types of stem cells which
probably originated from different types of NSCs or progenitors
(22). However, since normal
tissue stem cells and CSCs could well have some similar properties,
further studies should focus on the differences, including the
molecular genetics and epigenetics, between CD15+ and/or
CD133+ normal tissue stem cells and CD15+
and/or CD133+ GSCs. Understanding such differences may
help to facilitate elucidation of the tumorigenesis and
establishment of useful therapies for glioblastomas.
The actual postoperative standard protocol for the
treatment of glioblastomas consists of radiotherapy and concomitant
TMZ (1). In addition, MGMT
hypermethylation (epigenetical silencing of the promoter and coding
regions of the MGMT gene) is considered to be one of the principal
mechanisms contributing to the TMZ sensitivity of glioblastomas.
MGMT removes alkylating adducts from the O6 position of
guanine and protects cells from cytotoxic and mutagenic effects,
conferring a resistance of the tumor cells to alkylating agent
chemotherapy including TMZ (10).
More recently, the results of the EORTC-NCIC trial have established
a predictive value for MGMT methylation status for the
benefit from TMZ treatment achieved by patients with glioblastoma
(1). Currently available data
suggest that resistance of GSCs to chemotherapy may be
significantly linked to MGMT (6),
which is consistent with the results showing higher MGMT
mRNA and protein expression of GSCs in the present study.
The observed ultrastructural features were similar
in both GSCs 0125 and 0222. The nuclear-cytoplasmic ratio was
generally from high to moderate degree, and the GSCs sometimes had
multiple nuclei that were occasionally cleaved (deep indentations)
and irregular in shape. Cellular organelles were generally abundant
in the form of mitochondria, rough endoplasmic reticulum, and
numerous coated vesicles could be seen. These ultrastructures also
implied that the GSCs were primitively differentiated as compared
to undifferentiated NSCs (26).
Thus, the ultrastructural features observed in this study were
approximately in agreement with those described in previous reports
(7,8). Atypia of the cell nucleus was
apparent in the GSCs, indicating that the degree of malignancy of
these cells including their invasive ability tends to be high
(8). Rough endoplasmic reticulum
was rich in the cytoplasm of the GSCs. It has been suggested that
rough endoplasmic reticulum may play an important role after
exposure to radiotherapy/chemotherapy in preferentially activating
protein synthesis and repair of cell damage, because it is covered
with ribosomes which contribute to mRNA translation into proteins
(8). Microvilli were also rich in
the presently studied GSCs. Cell surface expression of microvilli
may help to protect GSCs from the immune system, cytotoxic effector
cells, so that they survive more easily than common/usual glioma
cells (8,27,28).
Microenvironmental cues and cell-cell interactions in the adult
brain participate in the regulation of stem cell quiescence and
proliferation, and of the neurogenetic or gliogenetic lineage
(17). The microvilli of GSCs are
also considered to play an important role in the receipt of signals
from the microenvironment and cell-cell interactions related to the
maintenance of GSCs.
On the other hand, some EGF-expanded free-floating
neurosphere cells derived from rat fetal striatum possess a single
cilium typical of early neural precursors (26). Furthermore, it has been
demonstrated that ependymal cells are capable of forming spherical
clones, and some of the cells within these spheres maintain the
ependymal phenotype as indicated by extensive ciliation (30–50 per
cell) (26). In the present study,
we did not observe a single cilium or multiple cilia in the GSCs.
These findings seem reasonable because most of the GSCs in the
present study were stained for CD15, which has been described as a
marker for NSCs that do not contain ependymal cells (22). Moreover, it has been suggested that
ependymal cells or astrocytes may originate from multi-potent adult
NSCs (26,29). Such considerations complicate our
understanding of the source of GSCs, which probably originate from
different types of NSCs or progenitors.
Most normal stem cells are considered to be at
G0 stages of their cell cycles, but a few GSCs at
division phases were observed in the present study, which was
consistent with the proliferation capabilities of GSCs. Further,
the apoptotic process could be detected, but typical autophagosomes
could not be observed in either a single GSC or cells within the
tumor spheres. Zhao et al have reported that apoptosis
bodies and autophagosomes could barely be found in their
ultrastructural studies on glioma stem cells/progenitor cells, and
presumed that deficiencies of apoptosis may originate in
deficiencies of autophagy (7). Kim
et al (14) and Eramo et
al (30) suggested that drug resistance observed in GSCs might
depend on abnormalities of the cell death pathway, such as
overexpression of anti-apoptotic factors or silencing of key death
effectors. The discrepancies regarding apoptosis in GSCs thus
require further examination. On the other hand, there is growing
evidence to support the participation of autophagy in processes
such as cellular differentiation (7). It may thus be reasonable that
autophagosomes were not found in the GSCs, because the GSCs in the
present study mainly contained CD133+ and
CD15+ cells indicating that these cells were
immature.
In conclusion, although further confirmative studies
need to be undertaken, the antigenic characteristics and
ultrastructural features of the GSCs isolated from human
glioblastomas, could imply that GSCs have certain relations with
NSCs but are primitively different from undifferentiated NSCs. The
data may support the GSC hypothesis suggesting an important role in
tumorigenesis, treatment resistance, and tumor recurrence, and also
facilitate the development of future glioblastoma therapies
targeting GSCs.
Acknowledgements
This study was financially supported in part by a
grant from the Health Sciences Research Institute, Inc., Yokohama,
Japan for Division of Companion Diagnostics, Department of
Pathology of Microbiology, Nihon University School of Medicine. We
wish to thank Dr Hiroyuki Suzuki (Nihon University School of
Medicine), Mr. Nobuo Miyazaki (Toray Industries Inc.) and Mr.
Hiroyuki Satake (Toray Industries Inc.) for their valuable
advice.
References
|
1
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al; European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group. Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuki K, Natsume A, Yokoyama H, Kondo Y,
Ohno M, Kato T, Chansakul P, Ito M, Kim SU and Wakabayashi T:
Induction of oligodendrogenesis in glioblastoma-initiating cells by
IFN-mediated activation of STAT3 signaling. Cancer Lett. 284:71–79.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
4
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar
|
|
7
|
Zhao Y, Huang Q, Zhang T, Dong J, Wang A,
Lan Q, Gu X and Qin Z: Ultrastructural studies of glioma stem
cells/progenitor cells. Ultrastruct Pathol. 32:241–245. 2008.
View Article : Google Scholar
|
|
8
|
Yang B, Wang Y, Yang C, Ouyang W, Zhou F,
Zhou Y and Xie C: The ultrastructural difference between
CD133-positive U251 glioma stem cells and normal U251 glioma cells.
Ultrastruct Pathol. 36:404–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Natsume A, Kato T, Kinjo S, Enomoto A,
Toda H, Shimato S, Ohka F, Motomura K, Kondo Y, Miyata T, et al:
Girdin maintains the stemness of glioblastoma stem cells. Oncogene.
31:2715–2724. 2012. View Article : Google Scholar
|
|
10
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E,
et al: Gene expression profiling predicts response to temozolomide
in malignant gliomas. Int J Oncol. 36:1367–1377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He J, Shan Z, Li L, Liu F, Liu Z, Song M
and Zhu H: Expression of glioma stem cell marker CD133 and
O6-methylguanine-DNA methyltransferase is associated
with resistance to radiotherapy in gliomas. Oncol Rep.
26:1305–1313. 2011.PubMed/NCBI
|
|
12
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KJ, Lee KH, Kim HS, Moon KS, Jung TY,
Jung S and Lee MC: The presence of stem cell marker-expressing
cells is not prognostically significant in glioblastomas.
Neuropathology. 31:494–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villalva C, Cortes U, Wager M, Tourani JM,
Rivet P, Marquant C, Martin S, Turhan AG and Karayan-Tapon L:
O6-Methylguanine-methyltransferase (MGMT) promoter
methylation status in glioma stem-like cells is correlated to
temozolomide sensitivity under differentiation-promoting
conditions. Int J Mol Sci. 13:6983–6994. 2012. View Article : Google Scholar
|
|
17
|
Dimov I, Tasić-Dimov D, Conić I and
Stefanovic V: Glioblastoma multiforme stem cells. Sci World J.
11:930–958. 2011. View Article : Google Scholar
|
|
18
|
Jin X, Jin X, Jung JE, Beck S and Kim H:
Cell surface Nestin is a biomarker for glioma stem cells. Biochem
Biophys Res Commun. 433:496–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133(+) and CD133(-) glioblastoma-derived cancer stem cells
show differential growth characteristics and molecular profiles.
Cancer Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeppernick F, Ahmadi R, Campos B, Dictus
C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B and
Herold-Mende CC: Stem cell marker CD133 affects clinical outcome in
glioma patients. Clin Cancer Res. 14:123–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pavon LF, Marti LC, Sibov TT, Malheiros
SM, Oliveira DM, Guilhen DD, Camargo-Mathias MI, Amaro Junior E and
Gamarra LF: The ultrastructural study of tumorigenic cells using
nanobiomarkers. Cancer Biother Radiopharm. 25:289–298. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao XG, Zhang X, Xue XY, Guo G, Wang P,
Zhang W, Fei Z, Zhen HN, You SW and Yang H: Brain tumor stem-like
cells identified by neural stem cell marker CD15. Transl Oncol.
2:247–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Capela A and Temple S: LeX/ssea-1 is
expressed by adult mouse CNS stem cells, identifying them as
nonependymal. Neuron. 35:865–875. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Son MJ, Woolard K, Nam DH, Lee J and Fine
HA: SSEA-1 is an enrichment marker for tumor-initiating cells in
human glioblastoma. Cell Stem Cell. 4:440–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang QB, Ji XY, Huang Q, Dong J, Zhu YD
and Lan Q: Differentiation profile of brain tumor stem cells: A
comparative study with neural stem cells. Cell Res. 16:909–915.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lobo MV, Alonso FJ, Redondo C,
López-Toledano MA, Caso E, Herranz AS, Paíno CL, Reimers D and
Bazán E: Cellular characterization of epidermal growth
factor-expanded free-floating neurospheres. J Histochem Cytochem.
51:89–103. 2003. View Article : Google Scholar
|
|
27
|
Arum CJ, Anderssen E, Viset T, Kodama Y,
Lundgren S, Chen D and Zhao CM: Cancer immunoediting from
immunosurveillance to tumor escape in microvillus-formed niche: A
study of syngeneic orthotopic rat bladder cancer model in
comparison with human bladder cancer. Neoplasia. 12:434–442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zaguia F and Schneider R: Microvilli
expressed on glioma cells keep cytotoxic cells at a distance.
Cancer Biol Ther. 11:1–3. 2011. View Article : Google Scholar
|
|
29
|
Gritti A, Vescovi AL and Galli R: Adult
neural stem cells: Plasticity and developmental potential. J
Physiol Paris. 96:81–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eramo A, Ricci-Vitiani L, Zeuner A,
Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C and
De Maria R: Chemotherapy resistance of glioblastoma stem cells.
Cell Death Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|