Introduction
Between 70 and 80% of bladder tumor cases are
non-muscle-invasive tumors confined to the mucosa. Patients with
this type of tumor undergo conservative treatment consisting of
transurethral resection of the bladder tumor (TURBT) followed by
intravesical adjuvant treatment (1,2).
Intravesical immunotherapy with bacillus Calmette-Guerin (BCG) is
the most effective adjuvant therapy for intermediate or high risk
non-muscle-invasive bladder cancer (NMIBC) (3). However, the recurrence rate of NMIBCs
is 60–70%, and 30% of recurrent tumors eventually progress to
muscle invasive tumors (3).
Moreover, the intravesical instillation of BCG can lead to
non-specific inflammation in the bladder and sometimes result in
side effects of sufficient severity to cause the treatment to be
discontinued (4). Thus, new
strategies and therapeutic agents with superior antitumor effects
to those of conventional intravesical therapy with BCG are highly
desirable for the treatment of NMIBC.
The phosphatidylinositol 3-kinase
(PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway
has been linked to tumorigenesis in many tissues (5,6). The
PI3K/Akt/mTOR pathway controls processes involved in tumor cell
growth, proliferation and survival after DNA damage (7). The kinase mTOR consists of mTOR
complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which are two
functionally distinct multiprotein complexes (8). mTORC1 inhibitors, such as RAD001, are
known to have potent preclinical activities with respect to a wide
variety of cancers, and RAD001 has been clinically approved as a
treatment for renal cancer (9,10).
In urothelial carcinoma (UC), preclinical studies have shown that
mTORC1 inhibitors exhibit antitumor effects both in vitro
and in vivo (11–14).
NVP-BEZ235 is a dual PI3K and mTORC1/2 inhibitor,
the antitumor activity of which is expected to be higher than that
of mTORC1 inhibitors in various human cancers because it inhibits
the upregulation of phospho-Akt (pAkt) through mTORC2 (15). Preclinical studies demonstrated the
antitumor activity of NVP-BEZ235 in various models (16,17).
However, the efficacy of dual PI3K/mTORC1/2 inhibitors has not yet
been examined in bladder cancer in vivo.
The orthotopic bladder cancer model is effective for
the evaluation of new intravesical agents against bladder cancer.
In our model, a high incidence of bladder cancer was observed after
the simple instillation of MBT-2 cell suspensions into the bladders
of syngeneic hosts (18). IL-2,
IL-12 and IL-15 intravesical gene therapies have previously been
reported by our group using this model (19–21).
We here examined the cytotoxic effects of the dual
PI3K and mTORC1/2 inhibitor NVP-BEZ235 in vitro and
determined whether intravesically administered NVP-BEZ235 exhibited
therapeutic effects in a MBT-2 murine orthotopic bladder cancer
model through inhibition of the PI3K/Akt/mTOR pathway.
Materials and methods
Reagents
Rabbit monoclonal antibodies for pAkt (Ser473),
phospho-S6 ribosomal protein (pS6) (Ser235/236) (D57.2.2E), and
phospho-4E-BP1 (p4EBP1) (Thr37/46) were obtained from Cell
Signaling Technology (Boston, MA, USA), and a mouse monoclonal
antibody for β-actin was purchased from Sigma-Aldrich (St. Louis,
MO, USA). NVP-BEZ235 was kindly provided by Novartis (Basel,
Switzerland) via a material transfer agreement.
Cell line and animals
The MBT-2 murine bladder cancer cell line was
provided by Dr T. Ratliff (University of Iowa, Iowa City, IA, USA).
MBT-2 was established from
N-[4-(5-nitro-2-furyl)-2-thiazolyl]-formamide-induced urothelial
carcinoma of the bladder removed from a female C3H/He mouse. Cells
were maintained in Roswell Park Memorial Institute (RPMI)-1640
medium containing 10% heat-inactivated fetal bovine serum at 37°C
in a humidified 5% CO2 atmosphere. Female C3H/He mice (8
weeks old) were purchased from Sankyo Laboratory Service Co.
(Tokyo, Japan). The Animal Care Committee of Keio University
approved all the procedures in this study involving animals and
their care in accordance with institutional and Japanese government
guidelines for animal experiments.
Cytotoxic assay in vitro
MBT-2 cells were seeded on 96-well plates in a
volume of 1×104 cells/100 μl/well culture medium and
incubated overnight at 37°C under 5% CO2 in a humidified
incubator. MBT-2 cells were treated with selected concentrations of
NVP-BEZ235 for 12, 24 or 48 h. At the end of the predetermined
incubation periods, the cells in each well were counted in
triplicate with a Premix water-soluble tetrazolium (WST)-1 Cell
Proliferation Assay System (Kyoto, Japan). Each well was treated
with Premix WST-1 (10 μl/well) and the plates were further
incubated for 60 min. A microplate reader was then used to measure
absorbance at 450 nm with a reference wavelength of 650 nm. Data
were expressed as the percentage of surviving cells relative to
that of a control.
Cell extraction and western blot
analysis
To obtain whole-cell extracts, we used RIPA buffer
consisting of 50 mM Tris-HCl (pH 7.5), protease inhibitors, 1%
NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, and 150 mM
NaCl. In western blotting, 50 μg of total protein was separated
using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on
a 12.5% acrylamide gel and then transferred to a nitrocellulose
membrane. The membrane was blocked for 1 h in Tris-buffered saline
(TBS) containing 5% PhosphoBlocker™ Blocking Reagent (Cell Biolabs,
Inc. San Diego, CA, USA) and 0.2% Tween-20. To detect pAkt, pS6,
p4EBP1, and β-actin, anti-pAkt (Ser473, Cell Signaling Technology),
anti-pS6 (Ser235/236, D57.2.2E, Cell Signaling Technology),
anti-p4EBP1 (Thr37/46, Cell Signaling Technology), and anti-β-actin
(Sigma-Aldrich) antibodies were incubated with the membranes
overnight at 4°C. The membranes were then washed for 3–5 min in TBS
with 0.2% Tween-20 and then incubated in a peroxidase-labeled
secondary antibody (Dako, Glostrup, Denmark) for 1 h. Membranes
were then washed again and analyzed after visualization by enhanced
chemiluminescence reagents with the ECL Plus™ Western Blotting
Detection System (GE Healthcare UK Ltd., Buckinghamshire, UK). The
intensities of the signals were quantified using the LAS 4000
system (Fujifilm, Tokyo, Japan).
Establishment of an orthotopic MBT-2
bladder cancer model
An orthotopic bladder cancer model was created by
the intravesical implantation of MBT-2 cells, as previously
described (18). Briefly, mice
were anesthetized with an intraperitoneal injection of
pentobarbital sodium (1.5 mg/200 μl) and mild pressure was applied
to the abdomen to obtain urine samples evacuated from the bladder.
MBT-2 cells (5×105 in a 50-μl suspension of serum-free
RPMI-1640 medium) were injected into the bladder through a 24-gauge
Teflon-coated catheter that had been introduced into the lumen of
the bladder through the urethra. The catheter was then removed,
and, to prevent the voiding of MBT-2 cells, the urethra was tied
with 4-0 silk thread for 2 h. We previously investigated serial
tumor growth in this orthotopic MBT-2 bladder tumor model and found
that very small superficial tumors had been established 3 days
after the implantation of MBT-2 cell suspensions and gradually grew
into visible masses (18). All the
procedures were tolerated well, and none of the mice died
postoperatively.
Intravesical NVP-BEZ235 therapy in an
orthotopic bladder cancer model
Regarding intravesical administration, 100 μl of PBS
or 40 μM (18.78 mg/l) of NVP-BEZ235 in 100 μl of PBS was instilled
transurethrally (n=15 mice per group). The NVP-BEZ235 and PBS
instillation experiments were started 5 days after tumor
implantation and were carried out 5 times at 3-day intervals (i.e.,
on days 5, 8, 11, 14 and 17). Transurethral injections were carried
out under anesthesia induced by an intraperitoneal injection of
pentobarbital sodium. All the surviving mice were sacrificed on day
21 and necropsied. To assess the growth of bladder tumors, the
weights of the bladders were measured. Additionally, to assess the
potential toxicity of intravesical NVP-BEZ235 therapy, we examined
the histological appearances of the major organs, including the
heart, lung, liver, and kidney. The serum concentrations of
albumin, total bilirubin, creatinine, and alanine aminotransferase
were also determined.
Analysis of MBT-2 tumor extracts from
mice treated with NVP-BEZ235
A second set of in vivo experiments was
performed to evaluate changes in the PI3K/Akt/mTOR pathway after
the intravesical administration of NVP-BEZ235 in tumors. Mice
treated with NVP-BEZ235 (40 μM, 18.78 mg/l) or vehicle control were
sacrificed 12 h after the last dose (on day 18) (n=3 mice per
group), whole tumor extracts in most parts of the bladder were
obtained using RIPA buffer, and the protein expression of pAkt, pS6
and p4EBP1 was evaluated by western blotting, as described above.
Furthermore, some of the tumors in the bladders of mice treated
with NVP-BEZ235 (40 μM, 18.78 mg/l) or vehicle control were
immunohistochemically evaluated for the expression of the pS6
protein. Formalin-fixed, paraffin-embedded sections (4 μm) were
analyzed. Antigen retrieval with citric acid (pH 6.0) was carried
out, and then endogenous peroxidase activity was blocked by a
20-min treatment with 0.3% hydrogen peroxide. Sections were
incubated for 15 min in a blocking solution (Protein Block
Serum-Free Ready-to-use, Dako) and were then incubated with an
antibody specific for pS6 (dilution 1:100) (Ser235/236, D57.2.2E,
Cell Signaling Technology) for 2 h. Slides were incubated for 30
min with secondary antibodies conjugated to a peroxidase-labeled
dextran polymer. Visualization of the immunoreaction was achieved
using diaminobenzidine followed by counterstaining with 10%
hematoxylin.
Statistical analysis
All values are presented as the mean ± standard
deviation (SD). The significance of differences between groups was
assessed using ANOVA and/or the Student’s t-test, as appropriate.
Significance was determined as P<0.05. These analyses were
performed using IBM® SPSS® Statistics Version
21 (International Business Machines Corp., Armonk, NY, USA).
Results
Cytotoxic effects of NVP-BEZ235 on MBT-2
cells in vitro
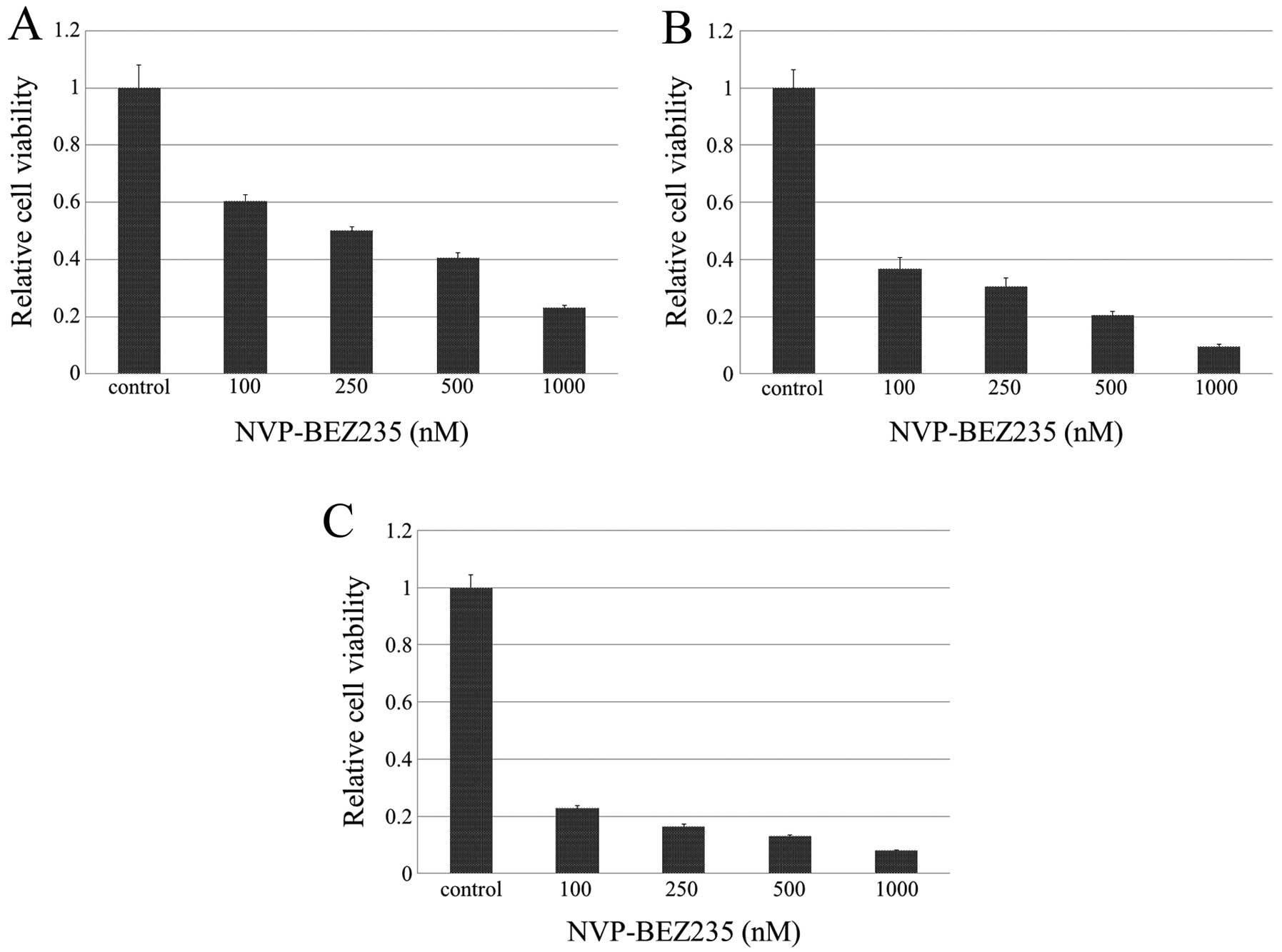
We examined the cytotoxic effects of NVP-BEZ235 on
MBT-2 cells in vitro (Fig.
1). After being exposed to NVP-BEZ235 at concentrations of 100,
250, 500 and 1000 nM for 12 h, the mean relative cell viabilities
to vehicle were 60.4±9.1%, 50.0±5.3%, 40.4±7.5%, and 23.0±3.5%,
respectively. The inhibition of cell growth was significantly
greater in cells treated at any concentration of NVP-BEZ235 than in
those treated with vehicle control after 12 h of exposure to
NVP-BEZ235 in a dose-dependent manner. Equivalent or stronger
cytotoxic effects were observed after 24 and 48 h of exposure to
NVP-BEZ235.
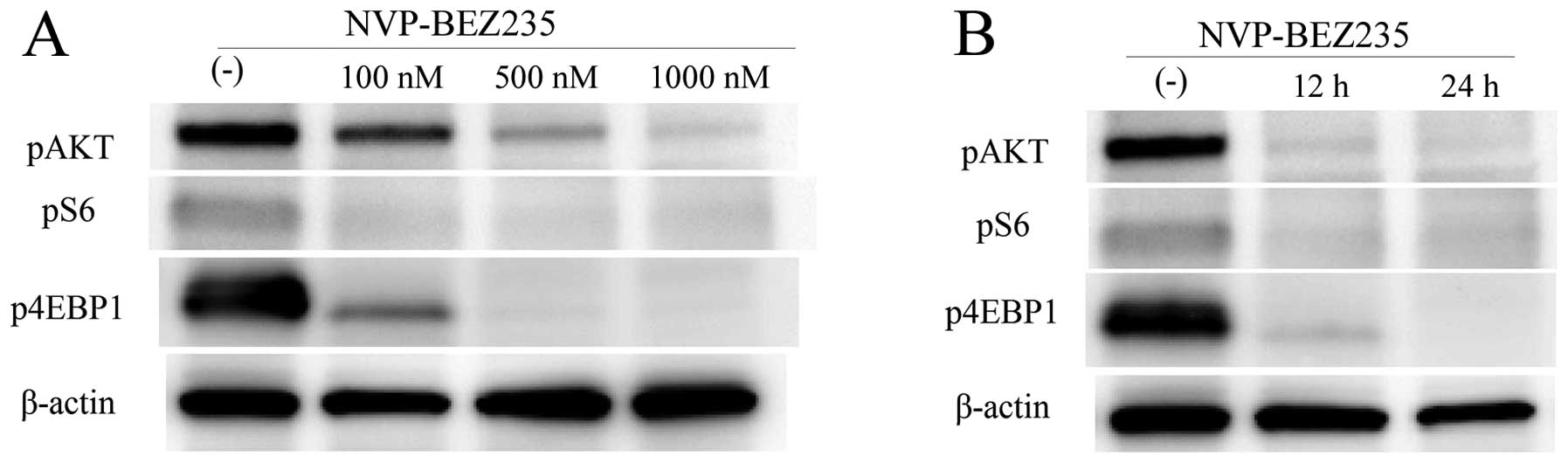
NVP-BEZ235 inhibited pAkt, pS6, and
p4EBP1 in MBT-2 cells in vitro
To assess the inhibitory effect of NVP-BEZ235 on the
PI3K/Akt/mTOR signaling pathway, we evaluated the protein
expression of pAkt, pS6, and p4EBP1 in MBT-2 cells treated with or
without NVP-BEZ235 by western blotting. The expression of pS6 in
MBT-2 cells was significantly inhibited by NVP-BEZ235 at a dose of
100 nM or higher (Fig. 2A). The
expression of pAkt and p4EBP1 was also significantly inhibited by
NVP-BEZ235 in a dose-dependent manner (Fig. 2A). The expression of pS6, pAkt, and
p4EBP1 was inhibited in cells exposed to NVP-BEZ235 for 12 h, and
these inhibitory effects persisted for 24 h (Fig. 2B).
Therapeutic effects of intravesical
NVP-BEZ235 in an orthotopic bladder cancer model
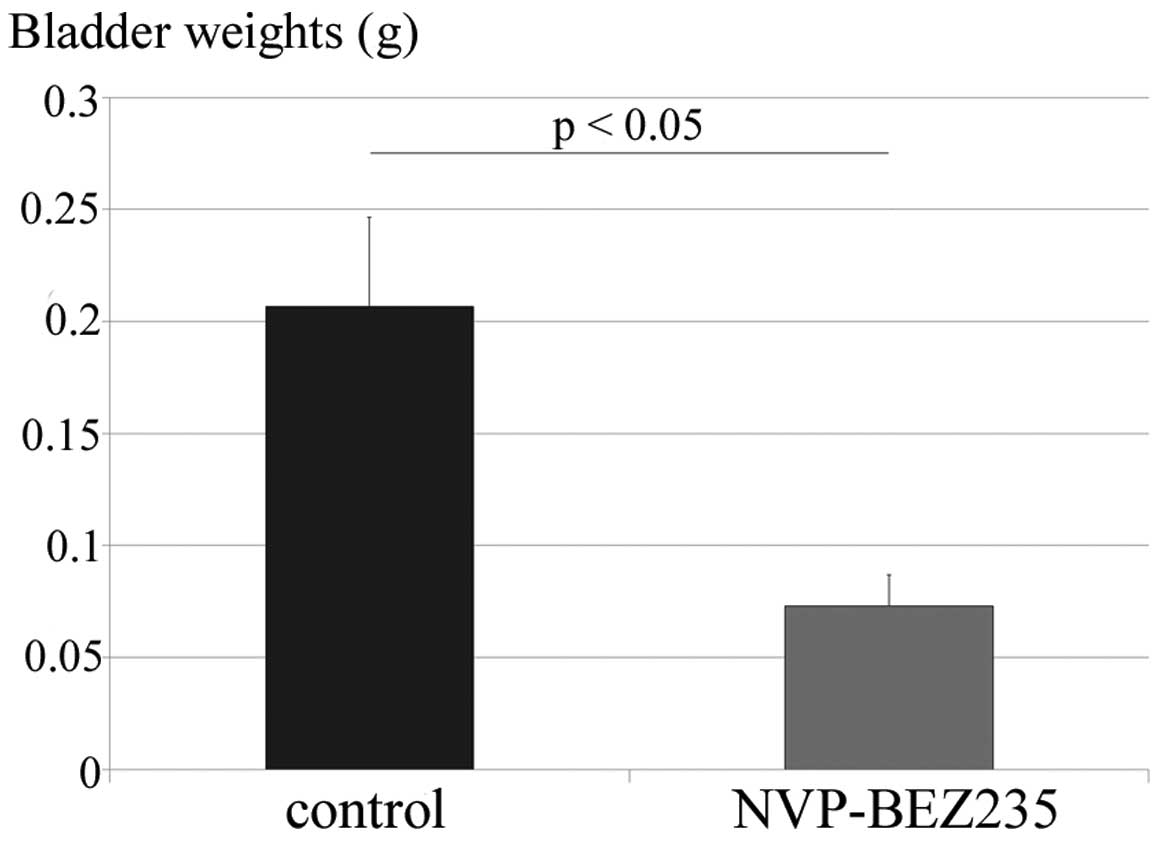
Five days after the intravesical implantation of
MBT-2 cells, PBS (vehicle control) or NVP-BEZ235 (40 μM, 18.78
mg/l) was administered intravesically into mouse bladders on days
5, 8, 11, 14 and 17. All mice tolerated the 5 instillations of PBS
or NVP-BEZ235 well. The mice were sacrificed on day 21, and the
weights of their bladders were ascertained to assess the growth of
bladder tumors (n=15 in each group). Fig. 3 shows that bladder weights were
significantly lower in the intravesical NVP-BEZ235 therapy in
situ group (72.8±54.5 mg) than in the control group
(206.6±154.9 mg; P=0.0038).
The serum concentrations of albumin, total
bilirubin, creatinine, and alanine aminotransferase on day 21 were
all within normal ranges in the 2 groups, which indicated that
intravesical NVP-BEZ235 therapy did not result in any systemic side
effects. We also analyzed the histological appearances of the major
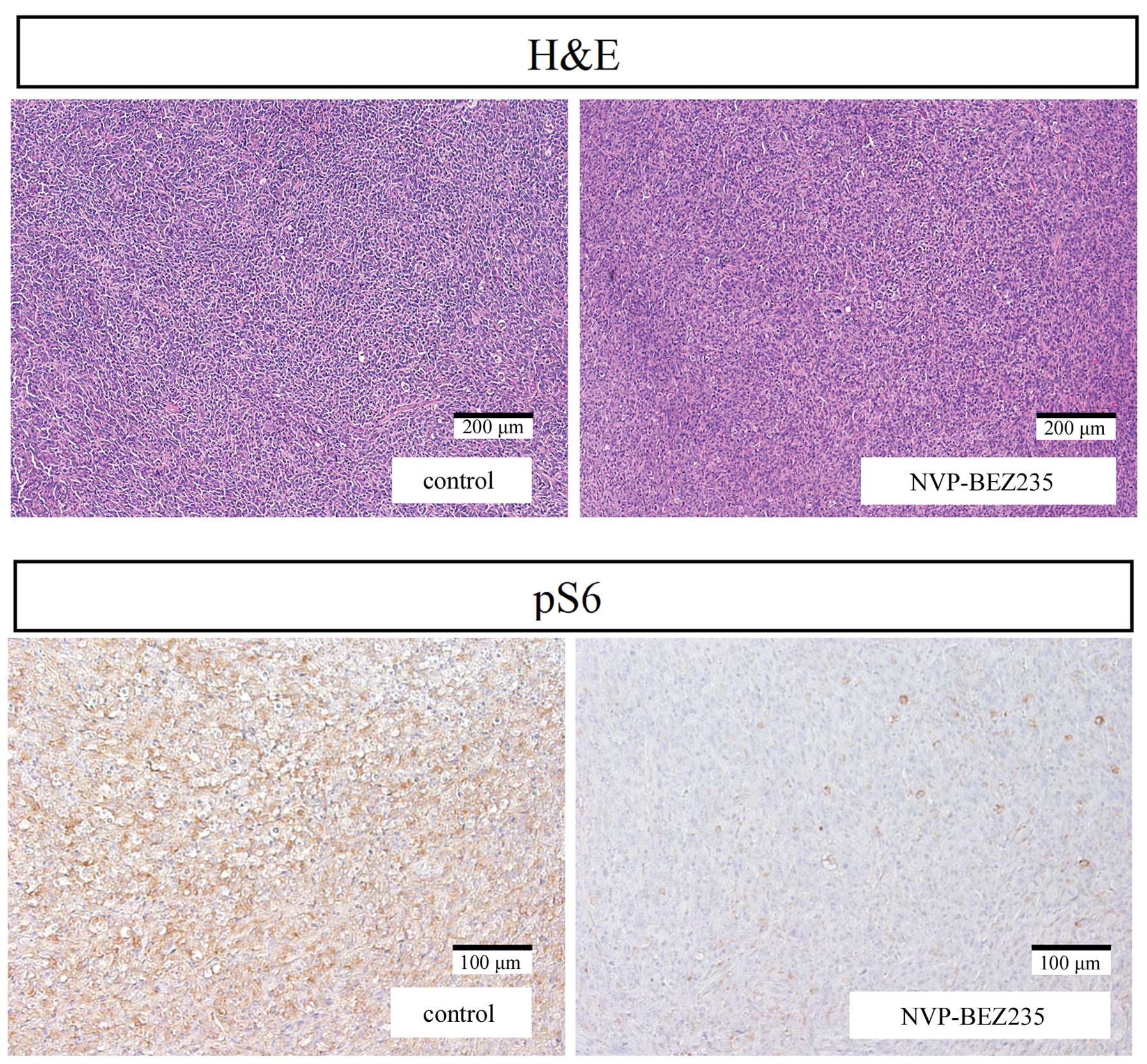
organs, including the liver, kidneys, heart and lungs. Staining
with hematoxylin and eosin showed that there were no significant
differences in the histological appearances of or damage to the
major organs of the control or NVP-BEZ235-treated mice.
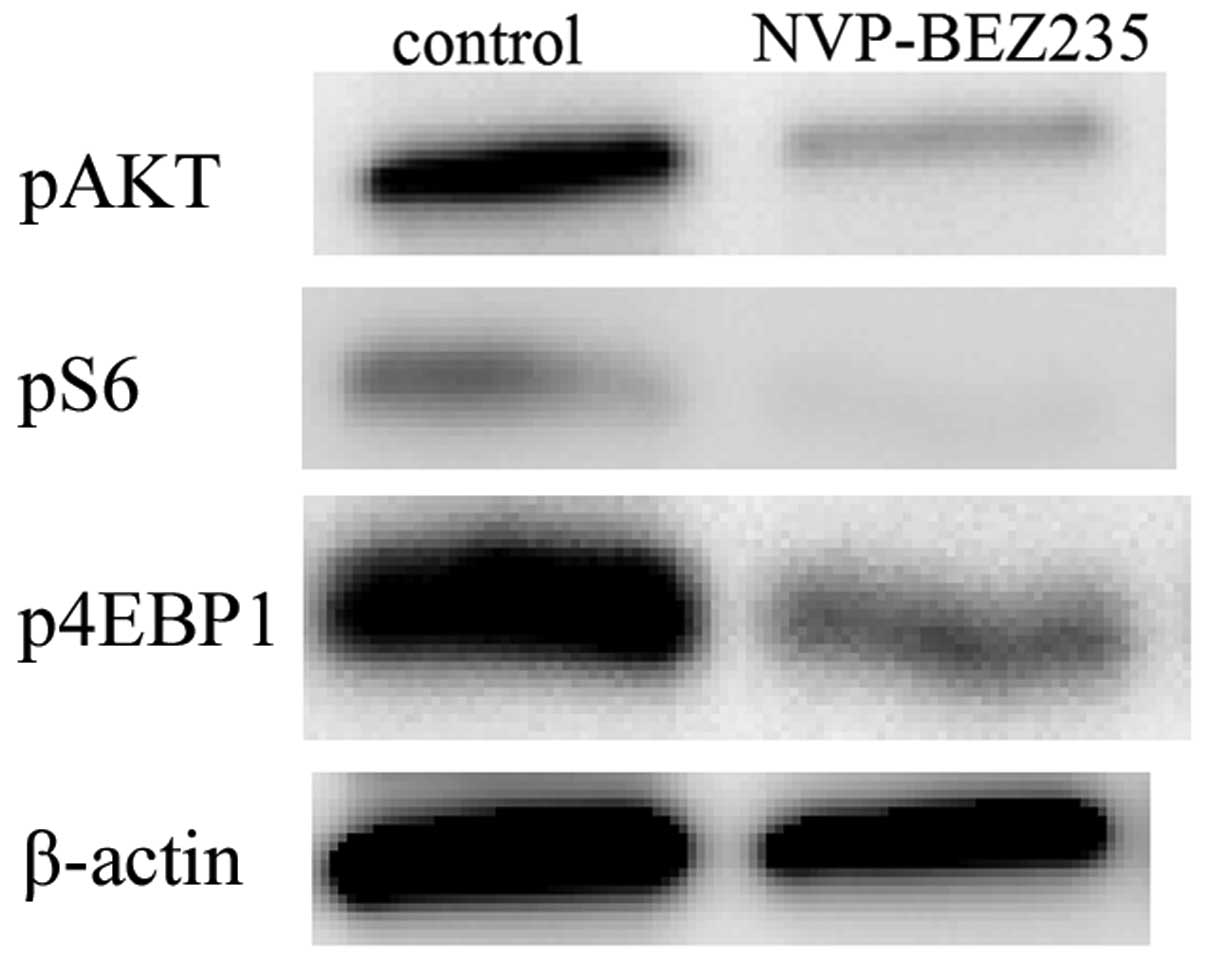
Analysis of orthotopic MBT-2 tumor
tissues by western blotting
Mice treated with vehicle control or NVP-BEZ235 were
sacrificed 12 h after the last dose. The tumors were then removed
and analyzed using western blotting for pAkt, pS6, and p4EBP1. The
analysis of MBT-2 tumors indicated that pAkt, pS6, and p4EBP1
signaling was markedly lower in tumors treated with NVP-BEZ235 than
in those treated with vehicle control (Fig. 4). This result demonstrated that, in
the orthotopic bladder cancer model, the intravesical NVP-BEZ235
treatment inhibited elevations in pAkt and downstream targets (pS6
and p4EBP1) in bladder cancer tissues.
Immunohistochemical analysis of tumor
tissues
pS6 immunostaining on tumor tissue was evaluated in
mice treated with NVP-BEZ235 or vehicle control. The expression of
the pS6 protein was significantly lower in bladder tumor tissue
treated with NVP-BEZ235 than in the tissue treated with vehicle
control (Fig. 5).
Discussion
In the present study, the cytotoxic effects of
NVP-BEZ235 were observed in MBT-2 tumor cells in vitro
through the inhibition of pAkt protein expression, as well as the
downstream targets of pS6 and p4EBP1. Furthermore, the intravesical
NVP-BEZ235 therapy had significant inhibitory effects on tumor
growth in the MBT-2 orthotopic bladder cancer model. An analysis of
tumor tissues by western blotting revealed that the NVP-BEZ235
treatment strongly reduced the expression of pAkt, pS6, and p4EBP1.
An immunohistochemical analysis revealed that the expression of pS6
was significantly inhibited by the intravesical instillation of
NVP-BEZ235. These results indicated that targeting the
PI3K/Akt/mTOR pathway with NVP-BEZ235 had a strong cytotoxic effect
on bladder tumors; therefore, the intravesical administration of
NVP-BEZ235 may represent a novel therapeutic modality for
NMIBC.
The PI3K/Akt/mTOR pathway has attracted significant
interest among UC researchers because the clinical importance of
members of the PI3K/Akt/mTOR signaling pathway in UC has been
demonstrated in previous studies (22–26).
The expression of various PI3K/Akt/mTOR signaling pathway members
including p-mTOR, pAkt and p4EBP1 was shown to be higher in UCs
than in normal urothelia (24,25).
Korkolopoulou et al (24)
reported that the cytoplasmic expression levels of p-mTOR and pAkt
in UCs were higher than those in normal urothelia. Several studies
have also shown that the expression of various PI3K/Akt/mTOR
signaling pathway members (p-mTOR, pS6 and pAkt) correlated
positively with higher grades and/or stages (23,25,26).
Sun et al (26)
demonstrated that the expression of pS6 and pAkt correlated
positively with higher grades in 887 cases of bladder cancer.
Furthermore, recent studies reported that the expression levels of
p-mTOR, pS6, and p4EBP1 were independent prognostic factors of
bladder cancer-specific survival (CSS) (23–26).
The strong expression of pS6 was previously shown to predict
shorter recurrence-free survival, especially in patients with NMIBC
(22,25). These findings indicated that
upregulating the PI3K/Akt/mTOR pathway may be strongly associated
with tumor aggressiveness, invasion and survival in patients with
UC.
Previous preclinical studies reported that rapamycin
and its derivative RAD001, reduced cancer cell viability and
decreased the expression of PI3K/Akt/mTOR signaling pathway members
such as p-mTOR, pS6, p4EBP1 and p-p70S6K in bladder cancer cell
lines (11–14). The effects of inhibiting mTORC1 on
bladder tumor growth have also been reported in a xenograft mouse
model (11,13,14).
Only one previous study on intravesical mTOR inhibitor therapy used
the mTORC1 inhibitor rapamycin (27). They demonstrated that this therapy
successfully suppressed bladder tumorigenesis, and subsequently
concluded that the inhibition of mTOR signaling may have potential
therapeutic benefits for patients at high risk of developing
invasive bladder cancer. However, Vasconcelos-Nóbrega et al
(28) recently reported that
RAD001 only exerted modest effects in a chemically-induced UC
animal model. Furthermore, clinical phase II trials on metastatic
UC using temsirolimus, an inhibitor of mTORC1, failed to
demonstrate any clinical activity (29). These controversial findings for
mTORC1 inhibitors suggest the existence of mechanisms that result
in rapamycin resistance. mTORC2 is considered to be regulated by
rapamycin and activates the Akt signal pathway. This Akt-driven
feedback loop has been identified as one of the mechanisms by which
resistance to mTORC1 inhibitors develops (30,31).
Thus, the inhibition of not only mTORC1, but also mTORC2 may be
required to achieve a significant clinical effect (17).
mTORC2 is known to control processes that involve
cytoskeletal remodeling, such as cell spreading and migration, in
several cancers including UC (32). One study demonstrated that
increased mTORC2 activity was associated with bladder cancer
invasion that was primarily mediated through Rac1 as a major
downstream target of mTORC2 (32).
In the present study, we speculated that the dual inhibition of
PI3K and mTORC1/2 may have strong therapeutic effects on bladder
tumors in vivo and, thus, investigated the inhibitory
effects of NVP-BEZ235, which is a novel dual PI3K and mTORC1/2
inhibitor, on orthotopic bladder tumors through an intravesical
administration protocol. Several preclinical studies have reported
promising activities for NVP-BEZ235 against various tumors
(16,17), and various phase 1/2 clinical
trials on NVP-BEZ235 as a breast cancer treatment are now being
performed in different countries. We demonstrated that NVP-BEZ235
effectively blocked the PI3K/Akt/mTOR pathway and reduced tumor
growth in the MBT-2 orthotopic bladder cancer model. To the best of
our knowledge, intravesical dual PI3K and mTORC1/2 inhibitor
therapy has not previously been assessed using an orthotopic
bladder cancer model. Although no clinical trial has examined the
effects of NVP-BEZ235 in UC patients, the experimental data
generated in the present study strongly support the potential
clinical application of this strategy in the future.
It is important to recognize that when tumor cells
are removed from their natural milieu, they behave differently from
those in the organ of origin (33,34).
The orthotopic bladder cancer model used in the present study
closely mimics the clinical situation, and has consequently helped
to shed light on the mechanisms involved, which is a prerequisite
for drawing meaningful conclusions (35). Various orthotopic bladder cancer
models have been described; however, limitations have been
associated with all these models, including low rates of tumor
implantation and damage to the urothelium as a result of
electrocautery or the application of chemical agents to facilitate
tumor uptake, which induces the tumors to become invasive (36,37).
The incidence of tumor establishment was high in our model
following the simple instillation of MBT-2 cell suspensions into
the bladders of syngeneic hosts (18). Our model was easily established by
different investigators working in different institutions, is
highly reproducible, and avoids the need for any open surgical
procedures for implantation. The model can also be used to evaluate
new intravesical agents against bladder tumors in situ.
IL-2, IL-12 and IL-15 intravesical gene therapies have previously
been examined by our group using this model (19–21).
Although the toxicity of NVP-BEZ235 remains unknown,
the systemic absorption of agents rarely occurs through
intravesical administration. The intravesical instillation of
biological and chemotherapeutic agents facilitates the direct
access of these agents to the bladder mucosa and tumor (38). Therefore, a distinct benefit of
this localized approach is that agents can be used at high doses
with a minimal risk of systemic adverse effects (39). The intravesical NVP-BEZ235 therapy
did not cause any systemic side effects in our study.
In conclusion, we herein demonstrated that
intravesically administered NVP-BEZ235 therapy exhibited
therapeutic effects in the MBT-2 orthotopic bladder cancer model by
inhibiting the PI3K/Akt/mTOR signaling pathway. This administration
route is unique. The results obtained in the present study provide
molecular-level evidence that supports the clinical use of a dual
PI3K and mTORC1/2 inhibitor and may indicate that this is a useful
approach for treating patients with bladder cancer.
Acknowledgements
The study was supported in part by Grants-in Aid for
Scientific Research (KAKENHI, no. 25861450) from the Ministry of
Education, Science, Sports, Culture and Technology of Japan.
Abbreviations:
|
TURBT
|
transurethral resection of the bladder
tumor
|
|
BCG
|
bacillus Calmette-Guerin
|
|
NMIBC
|
non-muscle-invasive bladder cancer
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
mTORC1
|
mTOR complex 1
|
|
mTORC2
|
mTOR complex 2
|
|
UC
|
urothelial carcinoma
|
|
pAkt
|
phospho-Akt
|
|
pS6
|
phospho-S6 ribosomal protein
|
|
p4EBP1
|
phospho-4E-BP1
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
WST
|
water-soluble tetrazolium
|
|
TBS
|
Tris-buffered saline
|
|
CSS
|
cancer-specific survival
|
References
|
1
|
Hsieh JT, Dinney CP and Chung LW: The
potential role of gene therapy in the treatment of bladder cancer.
Urol Clin North Am. 27:103–113. ix2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein JP and Skinner DG: Radical
cystectomy for invasive bladder cancer: Long-term results of a
standard procedure. World J Urol. 24:296–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Böhle A and Brandau S: Immune mechanisms
in bacillus Calmette-Guerin immunotherapy for superficial bladder
cancer. J Urol. 170:964–969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alexandroff AB, Jackson AM, O’Donnell MA
and James K: BCG immunotherapy of bladder cancer: 20 years on.
Lancet. 353:1689–1694. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai X and Jiang Y: Key factors in mTOR
regulation. Cell Mol Life Sci. 67:239–253. 2010. View Article : Google Scholar
|
|
6
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seeliger H, Guba M, Kleespies A, Jauch KW
and Bruns CJ: Role of mTOR in solid tumor systems: A therapeutical
target against primary tumor growth, metastases, and angiogenesis.
Cancer Metastasis Rev. 26:611–621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe H and Kamai T: Recent advances in the
treatment of metastatic renal cell carcinoma. Int J Urol.
20:944–955. 2013.PubMed/NCBI
|
|
10
|
Wada Y, Takahashi W, Kawano Y and Eto M:
Current status of pharmacotherapy against metastatic renal cell
carcinoma in Japan. Int J Urol. 19:284–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiong E, Lee IL, Dadbin A, Sabichi AL,
Harris L, Urbauer D, McConkey DJ, Dickstein RJ, Cheng T and
Grossman HB: Effects of mTOR inhibitor everolimus (RAD001) on
bladder cancer cells. Clin Cancer Res. 17:2863–2873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fechner G, Classen K, Schmidt D, Hauser S
and Müller SC: Rapamycin inhibits in vitro growth and release of
angiogenetic factors in human bladder cancer. Urology. 73:665–668;
discussion 668–669. 2009. View Article : Google Scholar
|
|
13
|
Makhlin I, Zhang J, Long CJ, Devarajan K,
Zhou Y, Klein-Szanto AJ, Huang M, Chernoff J and Boorjian SA: The
mTOR pathway affects proliferation and chemosensitivity of
urothelial carcinoma cells and is upregulated in a subset of human
bladder cancers. BJU Int. 108:E84–E90. 2011. View Article : Google Scholar :
|
|
14
|
Mansure JJ, Nassim R, Chevalier S, Rocha
J, Scarlata E and Kassouf W: Inhibition of mammalian target of
rapamycin as a therapeutic strategy in the management of bladder
cancer. Cancer Biol Ther. 8:2339–2347. 2009. View Article : Google Scholar
|
|
15
|
Serra V, Markman B, Scaltriti M, Eichhorn
PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S,
et al: NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K
signaling and inhibits the growth of cancer cells with activating
PI3K mutations. Cancer Res. 68:8022–8030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho DC, Cohen MB, Panka DJ, Collins M,
Ghebremichael M, Atkins MB, Signoretti S and Mier JW: The efficacy
of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared
with rapamycin in renal cell carcinoma. Clin Cancer Res.
16:3628–3638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maira SM, Stauffer F, Brueggen J, Furet P,
Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker
K, et al: Identification and characterization of NVP-BEZ235, a new
orally available dual phosphatidylinositol 3-kinase/mammalian
target of rapamycin inhibitor with potent in vivo antitumor
activity. Mol Cancer Ther. 7:1851–1863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horiguchi Y, Kikuchi E, Ozu C, Nishiyama
T, Oyama M, Horinaga M, Yoshioka K and Tachibana M: Establishment
of orthotopic mouse superficial bladder tumor model for studies on
intravesical treatments. Hum Cell. 21:57–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horiguchi Y, Larchian WA, Kaplinsky R,
Fair WR and Heston WD: Intravesical liposome-mediated interleukin-2
gene therapy in orthotopic murine bladder cancer model. Gene Ther.
7:844–851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horinaga M, Harsch KM, Fukuyama R, Heston
W and Larchian W: Intravesical interleukin-12 gene therapy in an
orthotopic bladder cancer model. Urology. 66:461–466. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumoto K, Kikuchi E, Horinaga M, Takeda
T, Miyajima A, Nakagawa K and Oya M: Intravesical interleukin-15
gene therapy in an orthotopic bladder cancer model. Hum Gene Ther.
22:1423–1432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fahmy M, Mansure JJ, Brimo F, Yafi FA,
Segal R, Althunayan A, Hicks J, Meeker A, Netto G and Kassouf W:
Relevance of the mammalian target of rapamycin pathway in the
prognosis of patients with high-risk non-muscle invasive bladder
cancer. Hum Pathol. 44:1766–1772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansel DE, Platt E, Orloff M, Harwalker J,
Sethu S, Hicks JL, De Marzo A, Steinle RE, Hsi ED, Theodorescu D,
et al: Mammalian target of rapamycin (mTOR) regulates cellular
proliferation and tumor growth in urothelial carcinoma. Am J
Pathol. 176:3062–3072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Korkolopoulou P, Levidou G, Trigka EA,
Prekete N, Karlou M, Thymara I, Sakellariou S, Fragkou P, Isaiadis
D, Pavlopoulos P, et al: A comprehensive immunohistochemical and
molecular approach to the PI3K/AKT/mTOR (phosphoinositide
3-kinase/v-akt murine thymoma viral oncogene/mammalian target of
rapamycin) pathway in bladder urothelial carcinoma. BJU Int.
110:E1237–E1248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SJ, Lee TJ and Chang IH: Role of the
mTOR pathway in the progression and recurrence of bladder cancer:
An immunohistochemical tissue microarray study. Korean J Urol.
52:466–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seager CM, Puzio-Kuter AM, Patel T, Jain
S, Cordon-Cardo C, McKiernan J and Abate-Shen C: Intravesical
delivery of rapamycin suppresses tumorigenesis in a mouse model of
progressive bladder cancer. Cancer Prev Res (Phila). 2:1008–1014.
2009. View Article : Google Scholar :
|
|
28
|
Vasconcelos-Nóbrega C, Pinto-Leite R,
Arantes-Rodrigues R, Ferreira R, Brochado P, Cardoso ML, Palmeira
C, Salvador A, Guedes-Teixeira CI, Colaço A, et al: In vivo and in
vitro effects of RAD001 on bladder cancer. Urol Oncol.
31:1212–1221. 2013. View Article : Google Scholar
|
|
29
|
Gerullis H, Eimer C, Ecke TH, Georgas E,
Freitas C, Kastenholz S, Arndt C, Heusch C and Otto T: A phase II
trial of temsirolimus in second-line metastatic urothelial cancer.
Med Oncol. 29:2870–2876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carracedo A, Ma L, Teruya-Feldstein J,
Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma
SC, et al: Inhibition of mTORC1 leads to MAPK pathway activation
through a PI3K-dependent feedback loop in human cancer. J Clin
Invest. 118:3065–3074. 2008.PubMed/NCBI
|
|
31
|
O’Reilly KE, Rojo F, She QB, Solit D,
Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al:
mTOR inhibition induces upstream receptor tyrosine kinase signaling
and activates Akt. Cancer Res. 66:1500–1508. 2006. View Article : Google Scholar
|
|
32
|
Gupta S, Hau AM, Beach JR, Harwalker J,
Mantuano E, Gonias SL, Egelhoff TT and Hansel DE: Mammalian target
of rapamycin complex 2 (mTORC2) is a critical determinant of
bladder cancer invasion. PLoS One. 8:e810812013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Connor J, Bannerji R, Saito S, Heston W,
Fair W and Gilboa E: Regression of bladder tumors in mice treated
with interleukin 2 gene-modified tumor cells. J Exp Med.
177:1127–1134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fidler IJ: Critical factors in the biology
of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial
award lecture. Cancer Res. 50:6130–6138. 1990.PubMed/NCBI
|
|
35
|
Ratliff TL: Role of animal models in
understanding intravesical therapy with bacille Calmette-Guérin.
Clin Infect Dis. 31(Suppl 3): S106–S108. 2000. View Article : Google Scholar
|
|
36
|
Chin J, Kadhim S, Garcia B, Kim YS and
Karlik S: Magnetic resonance imaging for detecting and treatment
monitoring of orthotopic murine bladder tumor implants. J Urol.
145:1297–1301. 1991.PubMed/NCBI
|
|
37
|
Weldon TE and Soloway MS: Susceptibility
of urothelium to neoplastic cellular implantation. Urology.
5:824–827. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsu CC, Chuang YC and Chancellor MB:
Intravesical drug delivery for dysfunctional bladder. Int J Urol.
20:552–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dalbagni G, Russo P, Sheinfeld J, Mazumdar
M, Tong W, Rabbani F, Donat MS, Herr HW, Sogani P, dePalma D, et
al: Phase I trial of intravesical gemcitabine in bacillus
Calmette-Guérin-refractory transitional-cell carcinoma of the
bladder. J Clin Oncol. 20:3193–3198. 2002. View Article : Google Scholar : PubMed/NCBI
|