Introduction
Colon cancer is the most common gastrointestinal
cancer worldwide, with the incidence increasing in most countries
over the past 20 years (1). Colon
cancer is often diagnosed at an advanced stage, leading to a poor
prognosis (2–5). As the current clinical procedures
utilized for disease diagnosis are invasive, unpleasant, and
inconvenient, the development of simple blood tests that can be
used for early detection would be beneficial for ultimately
controlling and preventing colon cancer (2,4,5).
Serum tumor markers, such as carcinoembryonic antigen (CEA) and
carbohydrate antigen 199 (CA19-9), greatly improve diagnosis.
However, their application is limited to surveillance postsurgery,
and they are not suitable for the early detection of colon cancer,
as their sensitivity and specificity are very low (6–8).
Therefore, it is vital to identify new clues to understand the
pathogenesis of colon cancer and to explore effective therapeutic
strategies.
Cortactin protein was first identified in chicken
cells transformed by the src oncogene. Human cortactin, encoded by
the CTTN/EMS1 gene, is a v-Src substrate localized with cortical
actin at the plasma membrane (9),
cortactin is enriched in cortical structures such as membrane
ruffles and lamellipodia, and plays key roles in the
microfilament-membrane interactions as well in transducing signals
from the cell surface to the cytoskeleton (10,11).
Cortactin overexpression results from the 11q11.3 chromosomal
region amplification in various cancers, such as head and neck
squamous carcinoma, hepatocellular carcinoma, breast and bladder
cancer, and correlates with poor patient prognosis and decreased
survival (12–15). Clark et al demonstrated that
cortactin expression levels in HNSCC cells correlate with the
formation of invadopodia and associated with the invasive potential
of tumor cells (16). In addition,
Wei et al revealed that cortactin overexpression promoted
EGFR expression and confers a more malignant phenotype to gastric
cancer cells (17).
Despite the many previous studies that were carried
out, cotactin expression in colon cncer is still not completely
known. Therefore, the present study examined the role of cortactin
in colon cancer development, and our results indicated that
cortactin is markedly upregulated in colon cancer and can
significantly promote colon cancer cell proliferation both in
vitro and in vivo.
These data may suggest that cortactin acts as a
promoter of colon cancer cell growth and may constitute a potential
therapeutic target in colon cancer.
Materials and methods
Tissue specimens
To examine the expression of cortactin in human
colon cancers, sixty surgical resected colon cancers and adjacent
non-tumor specimens were obtained from Department of General
Surgery, Affiliated Hospital of Nantong University. Both colon
cancers and adjacent non-tumor tissues were confirmed by
pathological examination, and immediately stored in liquid nitrogen
after surgery. The study protocols for the investigations involving
human tissues were approved by the Ethics Committee of Nantong
University.
Colon cancer cell lines
The human colon cancer cell lines (HCT116, HT29,
DLD1, LS180, SW480 and SW620) and normal human colon epithelial
cells (NCM460) were obtained from American Type Culture Collection
(Manassas, VA, USA). HCT116 cells were cultured in McCoy's 5A
medium supplemented with 10% fetal bovine serum, all the other cell
lines were cultured in DMEM medium supplemented with 10% fetal
bovine serum. The cell lines were propagated in a 37°C-humidified
incubator with 5% CO2.
Quantitative real-time PCR
Total RNA from tissue samples and cultured cells was
extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
then reverse transcribed into cDNA using the PrimeScript RT Reagent
kit (Takara, Japan). Quantitative real-time PCR (qRT-PCR) assays
were carried out to detect mRNA expression using SYBR Premix Ex Taq
(Takara, Japan) according to the manufacturer's instructions.
Cortactin primers used in qRT-PCR were as follows: forward primer,
5′-TCCCATGGCTATGGAGGGAA-3′; reverse primer,
5′-CGACTGATATTCGTGGCCGA-3′. β-actin was used as an internal control
and amplified with forward primer, 5′-AGAGC CTCGCCTTTGCCGATCC-3′;
and reverse primer, 5′-CTGGG CCTCGTCGCCCACATA-3′.
Immunohistochemical staining
All tissues were paraffin-embedded and were obtained
from the Department of Pathology, Affiliated Hospital of Nantong
University. The paraffin-embedded tissues were cut into 4-μm
sections, and then incubated with rabbit anti-cortactin polyclonal
antibody (dilution 1:200; Abcam, Cambridge, UK) at 4°C overnight,
SP-9000 HistostainTM Plus kits (ZSGB-BIO) were used
according to the manufacturer's protocol. Tumor slices were
examined in a blinded manner. Ten fields were selected for
examination of the cell-staining intensity and proportion of
positive cells. Immunohistochemical staining was assessed according
to the immunoreactive score (IRS) that evaluated the staining
intensity and the proportion of positive cells. The staining
intensity was graded as 0 (no staining), 1 (weak), 2 (moderate) and
3 (strong). The proportion of positive cells was scored as 0
(negative), 1 (<10%), 2 (10–50%) and 3 (>50%). Both of the
scores were multiplied and the IRS was determined: values ≥3 were
defined as cytoplasmic expression positive, and values <3 were
regarded as negative.
shRNA preparation
SiRNA against cortactin was designed according to
the Invitrogen Co. and chemically synthesized by Shanghai
GenePharma Co. (Shanghai, China). The sequence of siRNA-410 was:
CCUUAAGGAGAAGGAAC UUdTdT (sense) and AAGUUCCUUCUCCUUAAGGdTdT
(antisense). Negative control (NC) siRNA synthesized by Shanghai
GenePharma Co. was used as a control. The sequence of si-NC was as
follows: UUCUCCGAACGUGUCACGUTT (sense) and ACGUGACACGUUCGGAGAATT
(antisense). ShRNA duplexes against cortactin was designed
according to the Invitrogen Co. and synthesized by GenePharma Co.
The sequences were incorporated into the Vector p-SUPER
(OligoEngine, USA) to generate p-SUPER-shRNA-cortactin. The
sequence of shRNA-410 was as follows: GATCCCCCCTT
AAGGAGAAGGAACTTTTCAAGAGAAAGTTCCTTCTC CTTAAGGTTTTTGGAAA (sense) and
AGCTTTTCCAAA AACCTTAAGGAGAAGGAACTTTCTCTTGAAAAGTTC CTTCTCCTTAAGGGGG
(antisense). The sequence of sh-NC was as follows: GATCCCCTTCT
CCGAACGTGTCACGTTT CAAGAGAACGTGACACGTTCGGAGAATTTTTGGAAA (sense) and
AGCTTTTCCAAAAATTCTCCGAACGTGT CACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG
(antisense). The constructs were verified by sequencing.
Construction of cortactin expression
vector
The cortactin open reading frame (ORF) was amplified
from the human colon cDNA library (Genbank: NM_001184740.1) using
Prime Star PCR and constructed into the expression vector pcDNA1.1B
to generate pcDNA1.1B-cortactin. The sequence of the forward primer
was as follows: 5′-EcoRI-AGAGAATTCATGTGGA AAGCTTCAGCA-3′ and
reverse primer' 5′-BamHI-AGA GGATCCTCACGGGCACTCCGGGAC-3′.
The construct was verified by sequencing.
Cell transfection
Both the shRNA and cortactin expression vector were
transfected using Lipofectamine 2000 (Invitrogen) according to the
manufacturer's instructions.
Cell proliferation assay
Cells were seeded at a density of 2,000–5,000
cells/well in 100 μl complete medium in 96-well plates. The Cell
Counting Kit-8 (Dojindo Labs) was used to measure cell viability
according to the manufacturer's instructions. Each experiment was
repeated at least 3 times.
Colony formation assay
To examine the effect of upregulated or
downregulated cortactin expression on proliferation of colon cancer
cell lines, cells transfected with different plasmids were used for
the colony formation assay. Each type of cell was seeded into 10-cm
plates (50,000 cells/well) and cultured for 3 weeks in medium
containing 1,000 μg/ml G418. These cultures were stained with 0.4%
crystal violet. Clones >2 mm were counted and the number of
clones per well was averaged from three wells for each experiment.
Each experiment was repeated at least 3 times.
Soft agar colony formation assay
Cells transfected with different plasmids were
suspended in 0.5 ml 1% low melting point agarose with complete
culture medium, and then layered on top of 0.5 ml 2% low melting
agarose in 24-well plates. Cell numbers varied from 2,000 to 5,000
for different cell lines. The plates were incubated in a 5%
CO2, 37°C-humidified incubator for 2 weeks. Colonies in
at least 6 random microscopic fields were counted and photographed.
All experiments were repeated 3 times.
Tumorigenicity assay in nude mice
Male BALB/c nude mice (3–4-week-old) were purchased
from the Department of Laboratory Animal Center, Nantong
University. Cells with differential cortactin expression were
injected subcutaneously into the lateral root of the anterior limb
of nude mice (500×106 cells/mouse, 4 mice in each
experimental group). Tumor size was measured every third day after
injection. Three weeks after injection, the mice were sacrificed
and photographed. Tumor weight was then calculated. Care of
experimental animals was in accordance with institutional animal
care and use committee guidelines.
Western blot analysis
Cell lysates were prepared using cold lysis buffer
containing 25 mmol/l Tris-Cl (pH 7.5), 5 mmol/l EDTA, 1% SDS and
protease inhibitor cocktail (Sigma, USA). After boiling for 5 min,
samples were subjected to electrophoresis in 10% SDS-PAGE and
transferred onto a polyvinylidene difluoride (PVDF) membrane, and
blocked for 1 h at room temperature with 5% blocking buffer. The
membranes were washed three times with 0.1% Tris-buffered saline
with Tween-20 (TBST) and incubated with the primary antibody
overnight at 4°C. The membranes were washed again and then
incubated with the secondary antibody at room temperature for 1 h.
Primary antibodies used in this study included: rabbit
anti-cortactin polyclonal antibody (1:1,000; Abcam), rabbit
anti-EGFR polyclonal antibody (1:200; Abcam), rabbit anti-ERK
polyclonal antibody (1:1,000; Abcam), rabbit anti-pERK polyclonal
antibody (1:500; Abcam), rabbit anti-cyclin D1 polyclonal antibody
(1:1,000; Abcam) and rabbit anti-β-actin polyclonal antibody
(1:1,000; Abcam). Proteins were detected using an ECL Western
blotting detection system (Pierce) by enhanced
chemiluminescence.
Patient follow-up
The total of 60 patients underwent resection of
colon cancer with curative intent at the Department of General
Surgery, Affiliated Hospital of Nantong University between March
2009 and February 2010. Curative resection was considered as
complete resection of tumor mass, with the tumor margins free, and
resection of the regional lymph nodes. Patients with distant
metastasis were eliminated from this study. The patients were
followed from March 2010 to February 2015. The detailed
clinicopathological characteristics of the 60 patients with colon
cancer are listed in Table I.
 | Table IExpression of cortactin in human colon
cancer according to clinicopathological features of patients. |
Table I
Expression of cortactin in human colon
cancer according to clinicopathological features of patients.
| Cortactin
expression | | |
|---|
|
| | |
|---|
| Clinicopathological
features | Strong (+)
n=34 | Weak (−)
n=26 | χ2
value | P-value |
|---|
| Age (years) |
| <50 | 15 | 11 | | |
| ≥50 | 19 | 15 | 0.020 | 0.889 |
| Gender |
| Male | 18 | 14 | | |
| Female | 16 | 12 | 0.005 | 0.944 |
| Depth | | | | |
| Localized in
subserosa | 11 | 8 | | |
| Beyond
subserosa | 23 | 18 | 0.017 | 0.896 |
| Tumor size |
| <3 | 8 | 13 | | |
| ≥3 | 26 | 13 | 4.538 | 0.033 |
| Stage |
| I/II | 6 | 12 | | |
| III/IV | 28 | 14 | 5.701 | 0.017 |
| Lymphatic
invasion |
| Absent | 5 | 13 | | |
| Present | 29 | 13 | 8.740 | 0.003 |
Statistical analysis
Statistical analysis was performed using SPSS 18.0
and Graphpad Prism 5.0 software. Quantitative data were recorded as
mean ± SD. Differences between two groups were assessed by
Student's t-test (two-tailed). Categorical data were evaluated by
the χ2 test. Survival rates were assessed by the
Kaplan-Meier method. The log-rank test was used to compare
significance. P<0.05 was considered statistically
significant.
Results
Cortactin expression in colon cancer
tissues and colon cancer cell lines
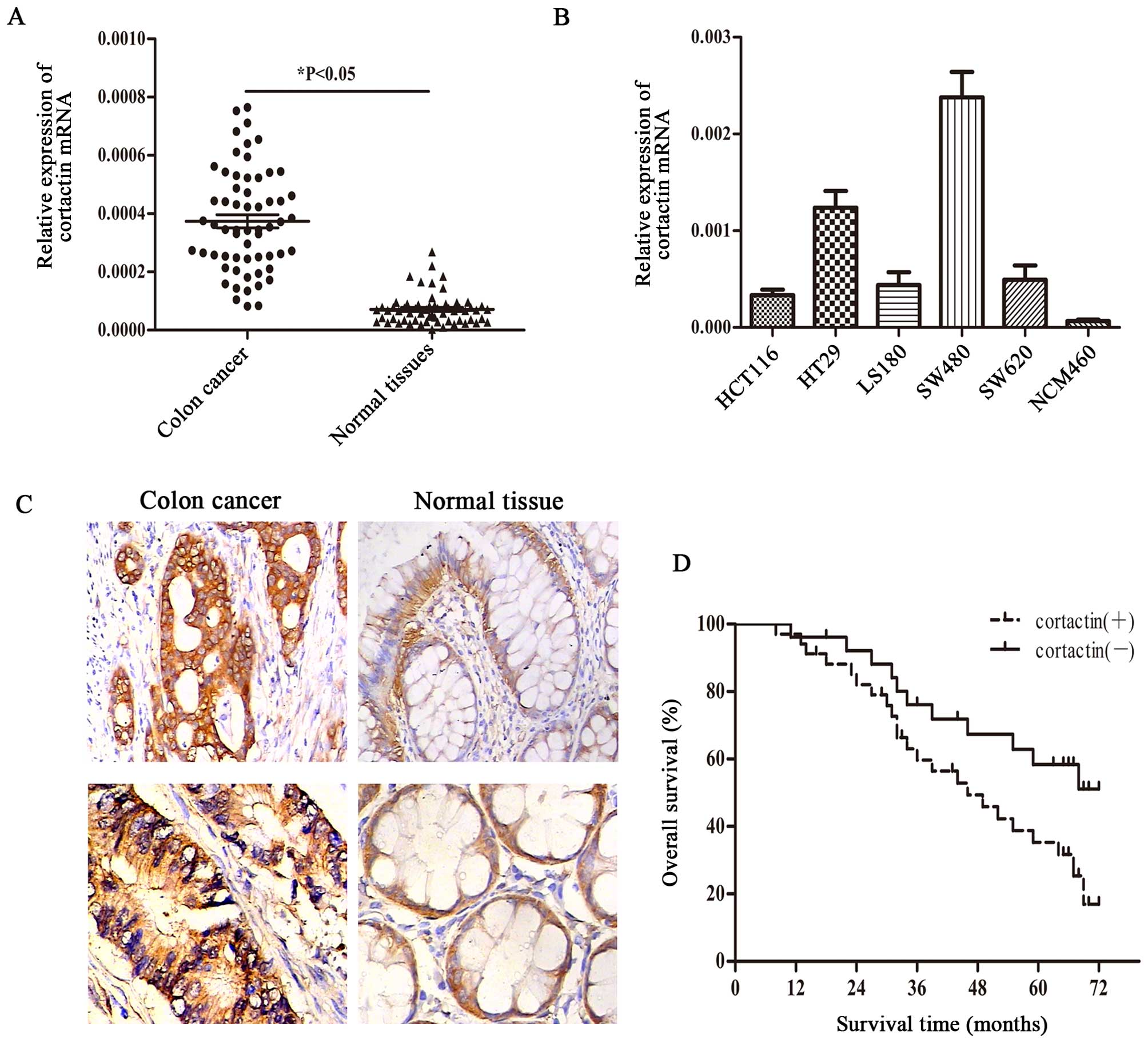
Quantitative real-time PCR was performed to measure
the cortactin mRNA expression levels in colon cancer and adjacent
non-cancerous tissues from 60 patients. The results indicated that
cortactin mRNA was increased in 45 (75%) of 60 colon cancer
specimens compared with the matched normal colon tissues (Fig. 1A). We performed
immunohistochemistry (IHC) to evaluate cortactin protein expression
in colon cancer specimens and paired normal colon tissues in the
same 60 matched samples. Of these specimens, 42/60 (70.0%) of
cancerous specimens showed positive staining, whereas 18/60 (30.0%)
of normal colon tissues showed positive staining (Fig. 1C). Furthermore, we evaluated the
expression of cortactin in 6 colon cancer cell lines using
quantitative real-time PCR. Cortactin mRNA was significantly
increased in all colon cancer derived cell lines (HCT116, HT29,
DLD1, LS180, SW480 and SW620) compared with normal human colon
epithelial cells (NCM460) (Fig.
1B). In summary, the collective data showed that cortactin is
increased in colon cancer.
Correlation of cortactin expression with
the patient clinicopathological features
To investigate whether the increased expression of
cortactin was associated with the clinicopathological features such
as age, gender, depth, tumor size, tumor stages and lymphatic
invasion, we classified the patients into two groups on the basis
of our qRT-PCR results for cortactin: weak expression (−) and
strong expression (+). As shown in Table I, those patients with tumor size
(≥3 cm) had a significantly higher expression of cortactin compared
with those patients with tumor size (<3 cm). In addition, in
stages III/IV, expression of cortactin was dramatically higher than
stages I/II. Furthermore, lymphatic invasion was significantly
correlated with a strong cortactin expression, whereas no
significant difference was observed in terms of the patient age,
gender and tumor depth. In summary, cortactin functions as an
oncogene accelerator in colon cancer.
Increased expression of cortactin
predicts poor prognosis in patients with colon cancer
Out of the 60 cases, there were 52 patients with
definite follow-ups. A total of 34 patients died, of whom 23 showed
strong expression of cortactin, 11 showed weak expression of
cortactin. The 5-year OS rates of patients with strong cortactin
expression were 16.9%, which were lower than those of patients with
weak cortactin expression (51.1%) (Fig. 1D).
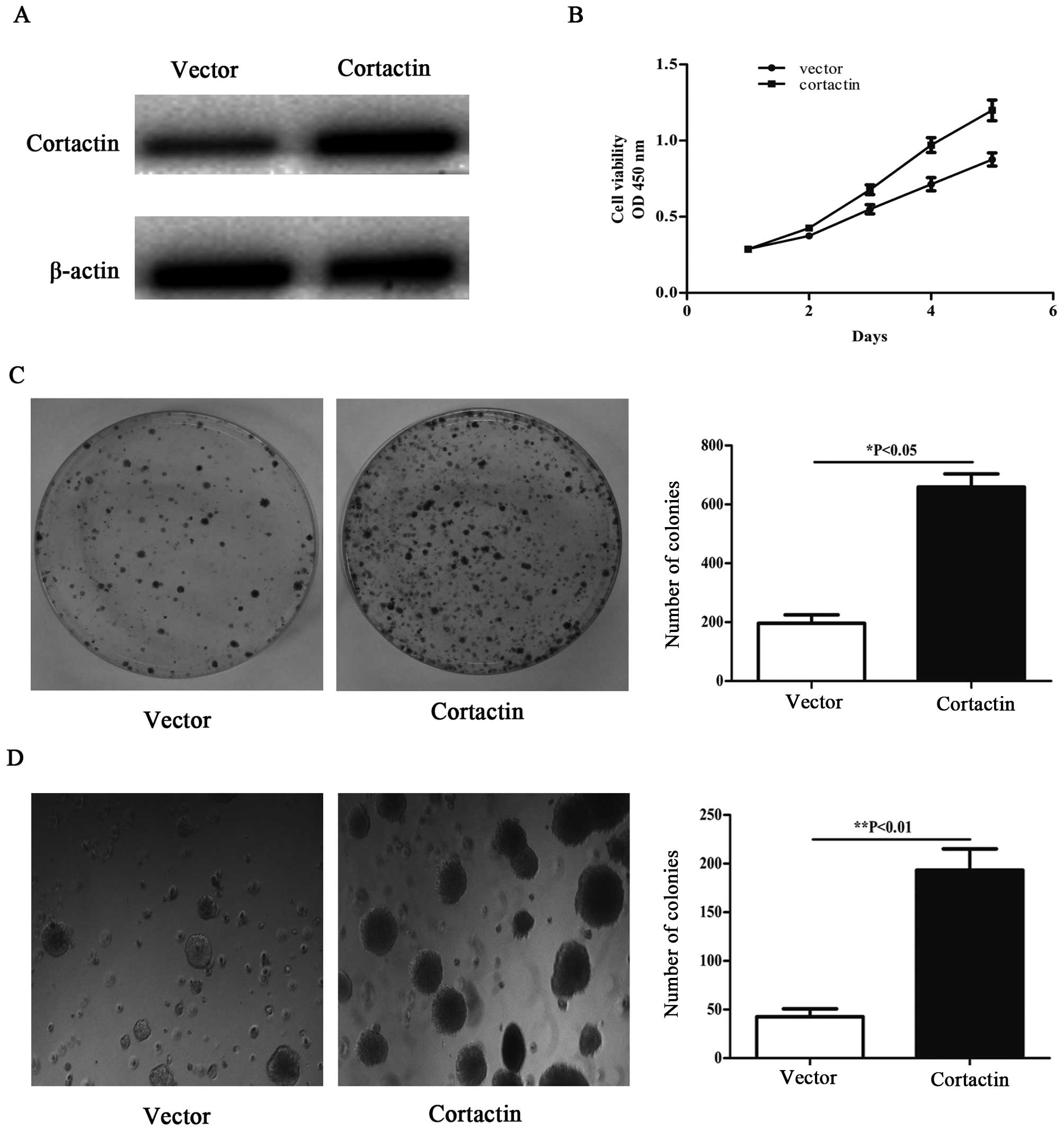
Overexpression of cortactin promotes
proliferation and colony formation in HCT116 cells
To overexpress cortactin, the recombinant
pcDNA1.1B-cortactin was transfected into the HCT116 cells. We
performed western blotting to evaluate cortactin protein expression
in HCT116 cells transfected with pcDNA1.1B-cortactin 48 h
post-transfection. Cortactin protein expression in
pcDNA1.1B-cortactin transfected cell was significantly higher than
in pcDNA1.1B transfected cell (Fig.
2A). To investigate the promotive effects in
pcDNA1.1B-cortactin transfected cell, cellular growth was monitored
for 5 days. The pcDNA1.1B-cortactin transfected HCT116 cells showed
a significant increase in cellular growth compared with pcDNA1.1B
transfected cells (Fig. 2B).
HCT116 cells with upregulated cortactin expression were subjected
to colony formation assay. As shown in Fig. 2C, overexpression of cortactin in
HCT116 cells resulted in significant promotion of colony formation
as compared with HCT116 cells transfected with pcDNA1.1B, and the
majority of clones were larger than those of control cells. We then
used a soft agar assay for colony formation, which is the most
stringent assay for detecting the proliferative ability of cells.
We observed enhanced formation of colonies in soft agar (Fig. 2D) that had been seeded with HCT116
cells transfected with pcDNA1.1B-cortactin compared with pcDNA1.1B
transfected cells.
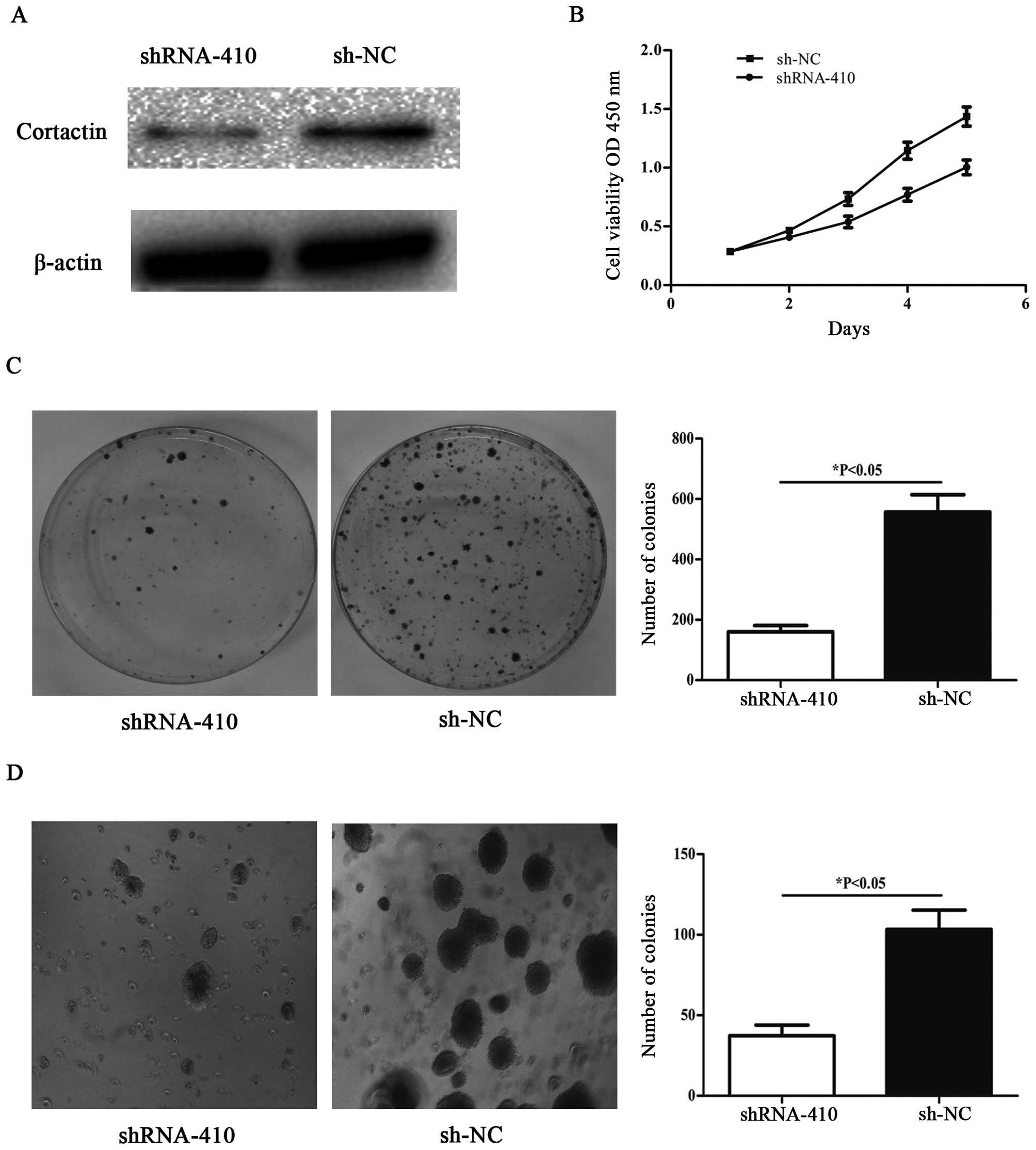
Knockdown of cortactin inhibits
proliferation and colony formation in SW480 cell line
To knockdown cortactin, the recombinant
p-SUPER-shRNA-cortactin was transfected into SW480 cells. Western
blot analyses were performed to assess the efficiency of cortactin
knockdown in SW480 cells transfected with p-SUPER-shRNA-cortactin
48 h post-transfection. Cortactin protein expression in
p-SUPER-shRNA-cortactin transfected cells was significantly lower
than that in sh-NC transfected cells (Fig. 3A). The p-SUPER-shRNA-cortactin
transfected SW480 cells showed significant suppression of cellular
growth compared with sh-NC transfected cells (Fig. 3B). SW480 cells with downregulated
cortactin expression were subjected to colony formation assay. As
shown in Fig. 3C, decreased
expression of cortactin in SW480 cells resulted in significant
suppression of colony formation as compared with cells transfected
with sh-NC. We also observed inhibited formation of colonies in
soft agar (Fig. 3D) when seeded
with SW480 cells transfected with p-SUPER-shRNA-cortactin compared
with sh-NC transfected cells.
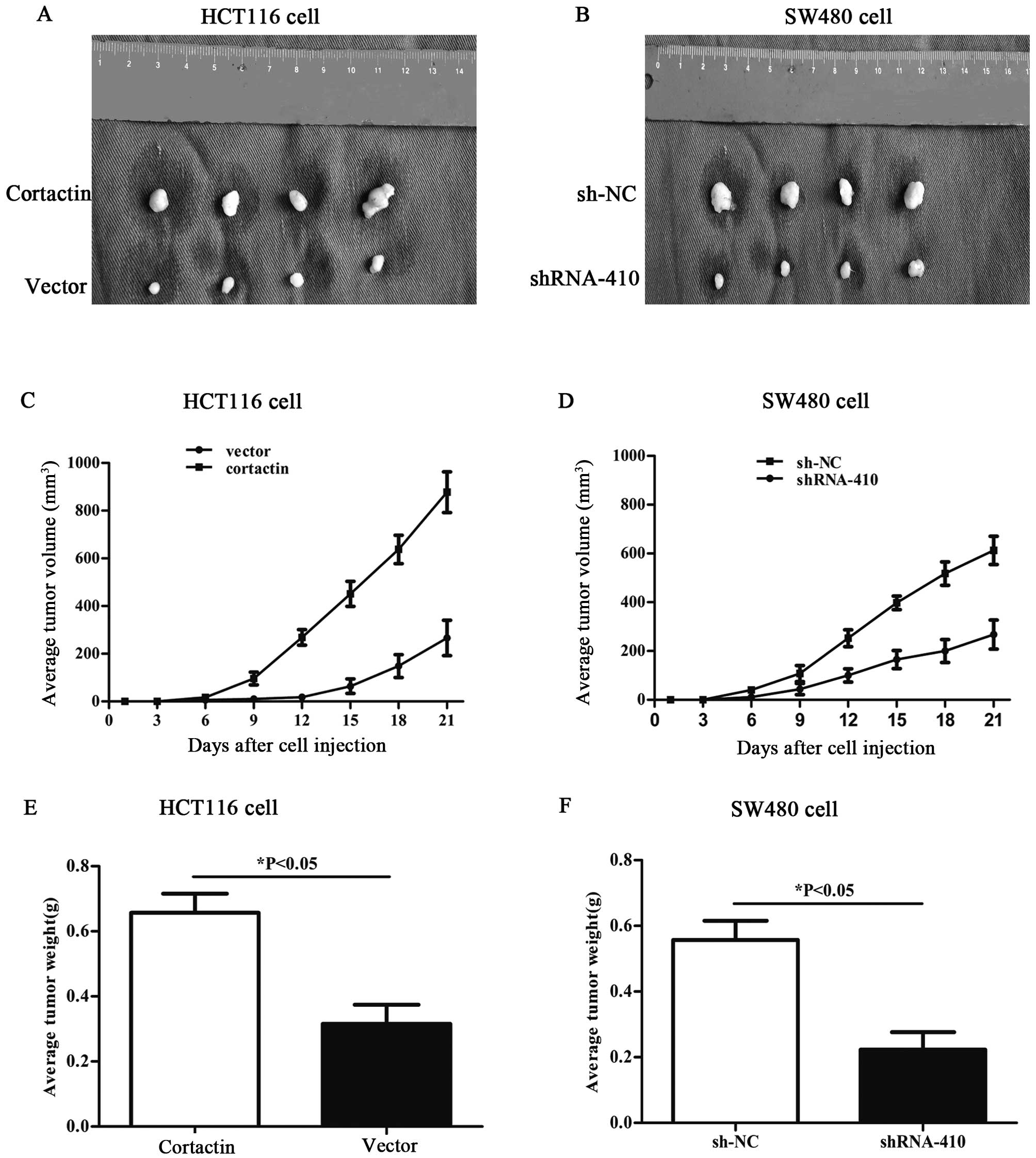
Differential expression of cortactin
influences tumorigenesis in nude mice
The effects of differential cortactin expression on
the tumorigenic potential of colon cancer cells in vivo were
also evaluated. HCT116 cells overexpressing cortactin and SW480
with downregulated cortactin expression were injected
subcutaneously into BALB/c nude mice (500×106
cells/mouse, 4 mice in each experimental group). Tumor size was
measured every third day after injection. After 3 weeks, mice were
sacrificed and photographed, and the tumors were removed and
weighed. In comparison with the mice injected with HCT116 cells
transfected with pcDNA1.1B, the mice injected with HCT116 cells
transfected with pcDNA1.1B-cortactin displayed larger tumors during
the same time period, and the average tumor volumes and weights
were significantly higher than those in the control group (Fig. 4A, C and E). Compared with the mice
injected with SW480 cells transfected with p-SUPER-sh-NC, the mice
injected with SW480 cells transfected with p-SUPER-shRNA-cortactin
showed an obvious decreased capacity for tumorigenesis (Fig. 4B, D and F). Taken together, these
results strongly suggest that cortactin acts as an promotor of
tumor cell growth and tumorigenicity in vivo.
Differential expression of cortactin
influences the EGFR-ERK signaling pathway related proteins
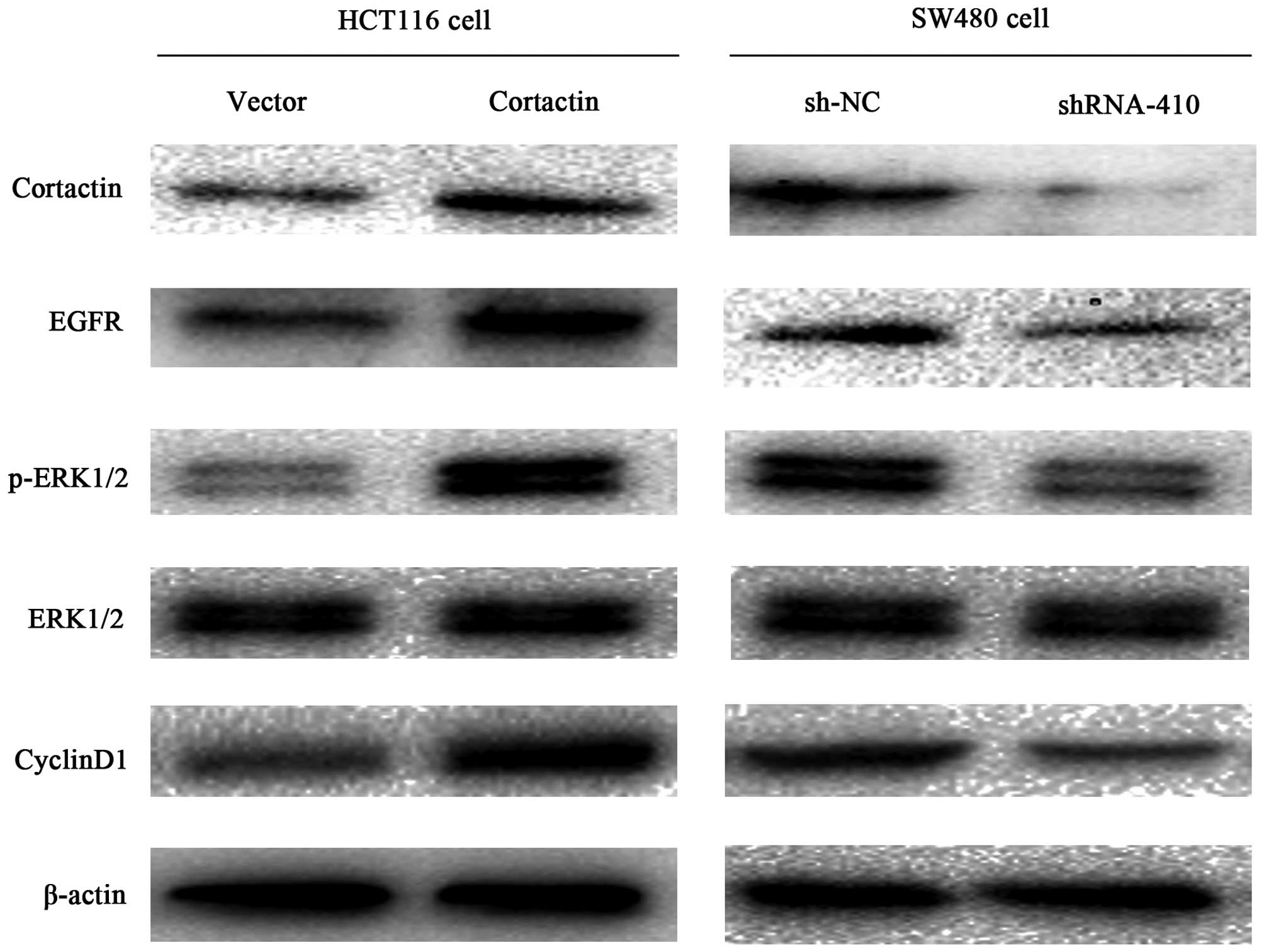
To study the mechanism by which cortactin enhances
the growth of colon cancer, the EGFR, ERK, p-ERK, cyclin D1 protein
levels were detected by western blotting. As shown in Fig. 5, the levels of EGFR, p-ERK and
cyclin D1 were increased in the pcDNA1.1B-cortactin transfected
HCT116 cells compared with the pcDNA1.1B transfected HCT116 cells,
and no difference in the tERK protein levels were observed between
the two cell groups. The levels of EGFR, p-ERK, cyclin D1 were
decreased in the p-SUPER-shRNA-cortactin transfected SW480 cells
compared with the sh-NC transfected SW480 cells. Similarly, the
expression of tERK was not different between the cell groups. These
results indicate that cortactin overexpression enhances the EGFR
expression and in turn constitutively activates the EGFR-ERK
signaling pathway.
Discussion
Recurrence and metastasis remain the major causes of
treatment failure and poor prognosis in patients with colon cancer,
and it is a multistage process that involves the motility and
migration of cells and proliferation in a new site (18). Cortactin is a multidomain protein
consisting of an NH2-terminal acidic region, which binds the actin
related protein (Arp)2/3 complex, a repeat region, which associates
with filamentous actin, and a COOH-terminal src homology (SH)3
domain, which recruits a variety of cellular proteins (19). One cellular role for cortactin is
to bridge the endocytic machinery with components and regulators of
the actin cytoskeleton (20). This
interaction has recently been shown to participate in
receptor-mediated endocytosis (20–22).
The gene encoding cortactin, EMS1, localizes to chromosomal locus
11q13, a region commonly amplified in breast cancers and head and
neck squamous cell carcinoma (23,24).
Since cortactin overexpression increases the motility of
fibroblasts and endothelial cells (25,26),
enhances bone metastasis of MDA-MB-231 breast cancer cells in a
nude mouse model (27) and
promotes gastric cancer cell SGC-7901 proliferation (17).
In the present study, we first determined the
expression of cortactin in colon cancer, and further investigated
its role in colon cancer cell proliferation and tumorigenicity both
in vitro and in vivo.
EGFR is frequently implicated in cancer cell
proliferation, inhibition of apoptosis and tumor-induced
neovascularization via the activation of downstream signaling
pathways. EGFR is often overexpressed in various types of tumors,
such as salivary gland carcinomas, non-small cell lung cancer,
biliary tract cancer, and gastric cancer (28–32).
One of the most frequent targets downstream of EGFR and other
receptor tyrosine kinases is the extracellular signal-regulated
kinase (ERK) kinase signal transduction pathway. This pathway is
involved in proliferation, differentiation, apoptosis, and
angiogenesis (33). It is
constitutively active in a variety of solid tumor models, including
lung, pancreas, and breast (33).
Elevated levels of activated ERK are frequently seen in carcinoma
cell lines (34,35). Activation of the ERK pathway
regulates the activity of a number of substrates through
phosphorylation (36).
In this study, we first determined the expression of
EGFR and ERK in colon cancer cells, it revealed that cortactin
enhances EGFR expression and ERK phosphorylation. Sustained ERK
activity can upregulate cyclin D1 expression.
In conclusion, our findings show that cortactin is
significantly upregulated in colon cancer, while ectopic cortactin
expression promotes cell proliferation, colony formation, soft agar
colony formation and tumorigenicity. Further investigation revealed
that cortactin stimulates the EGFR-ERK signaling pathway. This
study is the first to discover that cortactin expression plays an
important role in colon cancer progression, but further studies are
required to identify the detailed interaction between cortactin and
EGFR-ERK signaling pathway in colon cancer, and whether cortactin
could act as a therapeutic agent for colon cancer patients should
be investigated.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Fan NJ, Kang R, Ge XY, Li M, Liu Y, Chen
HM and Gao CF: Identification alpha-2-HS-glycoprotein precursor and
tubulin beta chain as serology diagnosis biomarker of colorectal
cancer. Diagn Pathol. 9:532014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomson DMP, Krupey J, Freedman SO and
Gold P: The radio-immunoassay of circulating carcinoembryonic
antigen of the human digestive system. Proc Natl Acad Sci USA.
64:161–167. 1969. View Article : Google Scholar
|
|
4
|
Duffy MJ: Role of tumor markers in
patients with solid cancers: A critical review. Eur J Intern Med.
18:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roulston JE: Limitations of tumour markers
in screening. Br J Surg. 77:961–962. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagaria B, Sood S, Sharma R and Lalwani S:
Comparative study of CEA and CA19-9 in esophageal, gastric and
colon cancers individually and in combination (ROC curve analysis).
Cancer Biol Med. 10:148–157. 2013.
|
|
7
|
Tan E, Gouvas N, Nicholls RJ, Ziprin P,
Xynos E and Tekkis PP: Diagnostic precision of carcinoembryonic
antigen in the detection of recurrence of colorectal cancer. Surg
Oncol. 18:15–24. 2009. View Article : Google Scholar
|
|
8
|
Flamini E, Mercatali L, Nanni O, Calistri
D, Nunziatini R, Zoli W, Rosetti P, Gardini N, Lattuneddu A,
Verdecchia GM, et al: Free DNA and carcinoembryonic antigen serum
levels: An important combination for diagnosis of colorectal
cancer. Clin Cancer Res. 12:6985–6988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu H, Reynolds AB, Kanner SB, Vines RR and
Parsons JT: Identification and characterization of a novel
cytoskeleton-associated pp60src substrate. Mol Cell Biol.
11:5113–5124. 1991.PubMed/NCBI
|
|
10
|
Wu H and Parsons JT: Cortactin, an
80/85-kilodalton pp60src substrate, is a filamentous actin-binding
protein enriched in the cell cortex. J Cell Biol. 120:1417–1426.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weed SA and Parsons JT: Cortactin:
Coupling membrane dynamics to cortical actin assembly. Oncogene.
20:6418–6434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bringuier PP, Tamimi Y, Schuuring E and
Schalken J: Expression of cyclin D1 and EMS1 in bladder tumours;
relationship with chromosome 11q13 amplification. Oncogene.
12:1747–1753. 1996.PubMed/NCBI
|
|
13
|
Yuan BZ, Zhou X, Zimonjic DB, Durkin ME
and Popescu NC: Amplification and overexpression of the EMS 1
oncogene, a possible prognostic marker, in human hepatocellular
carcinoma. J Mol Diagn. 5:48–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rothschild BL, Shim AH, Ammer AG, Kelley
LC, Irby KB, Head JA, Chen L, Varella-Garcia M, Sacks PG, Frederick
B, et al: Cortactin overexpression regulates actin-related protein
2/3 complex activity, motility, and invasion in carcinomas with
chromosome 11q13 amplification. Cancer Res. 66:8017–8025. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hofman P, Butori C, Havet K, Hofman V,
Selva E, Guevara N, Santini J and Van Obberghen-Schilling E:
Prognostic significance of cortactin levels in head and neck
squamous cell carcinoma: Comparison with epidermal growth factor
receptor status. Br J Cancer. 98:956–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clark ES, Whigham AS, Yarbrough WG and
Weaver AM: Cortactin is an essential regulator of matrix
metalloproteinase secretion and extracellular matrix degradation in
invadopodia. Cancer Res. 67:4227–4235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei J, Zhao Z-X, Li Y, Zhou Z-Q and You
T-G: Cortactin expression confers a more malignant phenotype to
gastric cancer SGC-7901 cells. World J Gastroenterol. 20:3287–3300.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang YY, Chen B and Ding YQ:
Metastasis-associated factors facilitating the progression of
colorectal cancer. Asian Pac J Cancer Prev. 13:2437–2444. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daly RJ: Cortactin signalling and dynamic
actin networks. Biochem J. 382:13–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McNiven MA, Kim L, Krueger EW, Orth JD,
Cao H and Wong TW: Regulated interactions between dynamin and the
actin-binding protein cortactin modulate cell shape. J Cell Biol.
151:187–198. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao H, Orth JD, Chen J, Weller SG, Heuser
JE and McNiven MA: Cortactin is a component of clathrin-coated pits
and participates in receptor-mediated endocytosis. Mol Cell Biol.
23:2162–2170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engqvist-Goldstein AE, Zhang CX, Carreno
S, Barroso C, Heuser JE and Drubin DG: RNAi-mediated Hip1R
silencing results in stable association between the endocytic
machinery and the actin assembly machinery. Mol Biol Cell.
15:1666–1679. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hui R, Campbell DH, Lee CS, McCaul K,
Horsfall DJ, Musgrove EA, Daly RJ, Seshadri R and Sutherland RL:
EMS1 amplification can occur independently of CCND1 or INT-2
amplification at 11q13 and may identify different phenotypes in
primary breast cancer. Oncogene. 15:1617–1623. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodrigo JP, García LA, Ramos S, Lazo PS
and Suárez C: EMS1 gene amplification correlates with poor
prognosis in squamous cell carcinomas of the head and neck. Clin
Cancer Res. 6:3177–3182. 2000.PubMed/NCBI
|
|
25
|
Patel AS, Schechter GL, Wasilenko WJ and
Somers KD: Overexpression of EMS1/cortactin in NIH3T3 fibroblasts
causes increased cell motility and invasion in vitro. Oncogene.
16:3227–3232. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang C, Liu J, Haudenschild CC and Zhan
X: The role of tyrosine phosphorylation of cortactin in the
locomotion of endothelial cells. J Biol Chem. 273:25770–25776.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Tondravi M, Liu J, Smith E,
Haudenschild CC, Kaczmarek M and Zhan X: Cortactin potentiates bone
metastasis of breast cancer cells. Cancer Res. 61:6906–6911.
2001.PubMed/NCBI
|
|
28
|
Clauditz TS, Gontarewicz A, Lebok P,
Tsourlakis MC, Grob TJ, Münscher A, Sauter G, Bokemeyer C, Knecht R
and Wilczak W: Epidermal growth factor receptor (EGFR) in salivary
gland carcinomas: Potentials as therapeutic target. Oral Oncol.
48:991–996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gazdar AF: Epidermal growth factor
receptor inhibition in lung cancer: The evolving role of
individualized therapy. Cancer Metastasis Rev. 29:37–48. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pirker R, Pereira JR, von Pawel J,
Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE,
Paz-Ares L, Störkel S, et al: EGFR expression as a predictor of
survival for first-line chemotherapy plus cetuximab in patients
with advanced non-small-cell lung cancer: Analysis of data from the
phase 3 FLEX study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar
|
|
31
|
Harder J, Waiz O, Otto F, Geissler M,
Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A and Opitz OG:
EGFR and HER2 expression in advanced biliary tract cancer. World J
Gastroenterol. 15:4511–4517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galizia G, Lieto E, Orditura M, Castellano
P, Mura AL, Imperatore V, Pinto M, Zamboli A, De Vita F and
Ferraraccio F: Epidermal growth factor receptor (EGFR) expression
is associated with a worse prognosis in gastric cancer patients
undergoing curative surgery. World J Surg. 31:1458–1468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoshino R, Chatani Y, Yamori T, Tsuruo T,
Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, et al:
Constitutive activation of the 41-/43-kDa mitogen-activated protein
kinase signaling pathway in human tumors. Oncogene. 18:813–822.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amundadottir LT and Leder P: Signal
transduction pathways activated and required for mammary
carcinogenesis in response to specific oncogenes. Oncogene.
16:737–746. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alessi DR, Cuenda A, Cohen P, Dudley DT
and Saltiel AR: PD 098059 is a specific inhibitor of the activation
of mitogen-activated protein kinase kinase in vitro and in vivo. J
Biol Chem. 270:27489–27494. 1995. View Article : Google Scholar : PubMed/NCBI
|