Over the past decade there has been a growing need
to understand more about the mechanisms involved in the progression
of early (pre-invasive) breast cancer [ductal carcinoma in
situ (DCIS)] to invasive breast cancer [invasive ductal
carcinoma (IDC)]. The breast cancer screening programme is
detecting more DCIS than before. Understanding how DCIS progresses
will help to tailor therapy and avoid overtreatment, because only
50% of detected DCIS cases will develop into invasive cancer
(1–3).
A significant body of work has focused on
unravelling the genetic alterations that occur in cancer cells in
the hope of identifying new molecular targets. A study carried out
on breast cancer examining normal, atypical ductal hyperplasia
(ADH), DCIS and IDC (the traditional view of progression) attempted
to show the changes in genetic abnormalities during the development
of breast cancer. However, the results demonstrated surprisingly
few changes at the various stages, with the greatest number of
genetic alterations seen in ADH, which were maintained in DCIS and
IDC. The study indicated that the changes to gene expression that
occur early in the disease largely reflect those of advanced breast
cancer (4). Studies by others have
gone on to confirm this hypothesis (5–7). The
caveat to this study is that it reflects low-grade disease and
therefore may not be relevant to high grade DCIS, HER2+
and basal types, which have the worst prognosis. Furthermore, the
advent of Next Generation Sequencing may reveal more subtle genetic
changes associated with disease progression.
This apparent lack of genetic evolution between DCIS
and invasive breast cancer has centred attention on the
microenvironment in mediating the transition to invasion. This
review will focus on the role of the inflammatory cells in
progression of DCIS to invasive disease (Table I), for a review of other
environmental factors at play see the review by Allen and Jones
(8).
Since Virchow first observed leukocytes in the
stroma of neoplastic tissue in 1863 the immune system has been
known to play a role in cancer. The modern paradigm of the role of
the tumour microenvironment was further elaborated by Bissell et
al (9) and there has since
been an explosion of interest, especially in the role of
inflammation.
In the 1990's several studies outlined the use of
the inflammatory infiltrate in breast cancer as a prognostic
marker. Aaltomaa et al examined 489 breast cancer patients
with up to 10 years follow-up. They found lymphocyte infiltrate
(LI) positively correlated to axillary lymph node status, tumour
diameter and histomorphological variables. Multivariate analysis
showed that LI was independently related to axillary lymph node
status and was able to predict recurrence free survival as well as
breast cancer related survival, however this analysis required the
proliferation rate to be used in the categorisation of the tumours
to be significant (10).
This study was corroborated by the group of Adrian
Harris, albeit in breast cancer, not DCIS. This study demonstrated
a significant positive correlation between angiogenesis and
macrophages in breast tumours, with highly vascular tumours having
higher numbers of macrophages. They went on to show that high focal
macrophage infiltration was predictive of worse outcome, reduced
relapse free and reduced overall survival albeit in invasive breast
carcinoma (15).
The advent of cancer therapies to reactivate
anticancer immune responses (mainly via alterations to the T-cell
population such as PD-1 inhibition) in lung cancer and melanoma,
and studies described below (among others), have focused attention
on the predictive importance of tumour-infiltrating lymphocytes
(TILs) in breast cancer. In December 2013 a TIL Working Group
(16) was convened to establish
consensus methodological recommendations for TIL evaluation to aid
standardisation across clinical trial design and translation
research. The authors suggest this study may lead to the
establishment of an immunological grade for breast cancer, which
reflects a patient's own antitumour immune response.
Tumour associated macrophages (TAMs) are generated
predominantly from peripheral monocytes that traffic to the nascent
tumour site (e.g. DCIS) where the factors released by the tumours
influence the monocytes to differentiate into macrophages.
Macrophages have a plastic phenotype that is highly dependent on
the prevalent cytokines and growth factors found in the
microenvironment, and the phenotype can change in response to
changes in the microenvironment. The scientific community have
attempted to define the types of macrophage by classifying them on
a spectrum ranging from M1 to M2. Classically activated macrophages
(M1) are characterised as pro-inflammatory, secrete cytokines like
IL-12 and TNF-α and have tumouricidal activity. Alternative
activated macrophages (M2) are characterised as immunosuppressive
and express cytokines such as IL-10 and TGF-β (17). TAMs exhibit a range of
subpopulations, the most well documented are those with an M2
phenotype which express tumour promoting cytokines and growth
factors that drive angiogenesis (VEGF), matrix remodelling (MMP2
and MMP9) and immune evasion (TGF-β) (18).
In breast cancer macrophages are one of the most
abundant immune cell types and have been shown to be critical to
the development of mammary tumours in mouse models (21). The mouse model MMTV-PyMT has been
observed to exhibit an increase in macrophages in premalignant
tissue prior to the angiogenic switch (22). Following on from this, the
importance of macrophages in the progression of breast cancer was
reported by Scholl et al who demonstrated that tumours with
high levels of nuclear CSF-1 (a macrophage growth and recruitment
factor) had more frequent metastases and this correlated with poor
survival (23). Lin et al
were able to mutate the CSF-1 gene in PyMT mice, this had the
effect of reducing the macrophage infiltrate at the tumour site and
inhibited the angiogenic switch, delaying tumour progression
(24). By overexpressing CSF-1 in
a transgenic model they were able to demonstrate robust
angiogenesis, even at premalignant stages, due to premature
macrophage infiltration (13).
More recently DeNardo et al have demonstrated that blocking
the CSF-1 receptor (with a CSF1 mAb or PLX3397, a CSF-1R ATP
inhibitor) in combination with paclitaxel treatment improved
survival in mammary tumour-bearing mice. The mice exhibited slowed
primary tumour development and reduced development of high grade
carcinomas and pulmonary metastasis. This was attributed to the
reduced macrophage infiltration and angiogenesis as well as
increased CD8 T-cells (25).
Another prominent set of chemokines involved in
recruiting TAMs to breast cancer are CCL2 and CCL5. These two
chemokines stimulate the migration of monocytes and T-cells and are
not normally expressed in breast epithelia. In breast cancer
increased expression of CCL2 and CCL5 has been observed and has
been correlated with advanced disease and progression (26). Both CCL2 and CCL5 have been shown
to stimulate monocyte/macrophage cells to secrete MMP9 and uPA
which drive matrix remodelling (27). CCL2 expression in primary breast
cancer was shown to have significant prognostic value for relapse
free survival and correlated with tumour grade and lack of estrogen
and progesterone receptor expression (indicative of poor prognosis)
(28,29). Additional smaller studies have
shown associations between CCL2 and poor prognosis (30,31).
Because CCL2 is a secreted chemokine the possibility
of using it as a serum biomarker for prognostic purposes has been
investigated. Several studies have been carried out with varying
results, significant differences in the patient cohorts used makes
interpreting between the studies difficult. However, some of the
studies did detect increased CCL2 serum levels in breast cancer
patients with advanced stage cancer (32–34).
Study by Qian et al demonstrated that targeting the
CCL2/CCR2 axis in tumour cells reduces tumour growth and metastases
(35).
High incidence and intensity of CCL5 expression in
breast tumour cells correlate with advanced stages of disease
(stage II/III vs. stage I) (36)
and CCL5 levels in patients with progression, relapse and/or
metastasis have been found to be higher than those in remission.
CCL5 is also a serum biomarker that is elevated in breast cancer
patients when compared to healthy individuals and may correlate
with stage (37). It has been
shown that CCL5 is a significant predictor of disease progression
in stage II patients and using CCL5+/ER−
together improves the prediction of disease progression compared to
using them alone (38).
CCL5 may potentially be a causative tumour promoting
factor in breast cancer, a murine model of breast cancer using 4T1
tumour cells expressing CCL5 antisense exhibited supressed tumour
growth and reduced metastasis (39). This is supported by two further
studies using CCR5 receptor antagonists, a group from Frances
Balkwill's laboratory demonstrated reduced tumour growth of 410.4
murine mammary carcinoma cells with met-CCL5 (40). More recently Velasco-Velazquez
et al were able to show the CCR5 receptor antagonists
Maraviroc and Vicriviroc reduced in vitro invasion of basal
breast cancer cells. In addition Maraviroc decreased pulmonary
metastasis of MDA-MB-231 in a mouse model (41).
Multiple clinical studies have supported TAM
measurement in pretreatment biopsies for the prediction of outcome
in human breast cancer. Adrian Harris' group have carried out two
studies which have demonstrated that increased CD68 levels
associate with increased vascularity and nodal metastasis as well
as decreased recurrence free and overall survival in human breast
cancer (15,44). While Tsutsui et al were able
to show that patients with increased TAM density have worse disease
free survival (45). More recently
Mahmoud et al found that higher total macrophage number was
associated with higher tumour grade, ER and PgR negativity, HER-2
positivity and basal phenotype. In univariate survival analysis,
higher numbers of CD68 macrophages were significantly associated
with worse breast cancer-specific survival (p<0.001) and shorter
disease-free interval (p=0.004). However in multivariate model
analysis, the CD68 macrophage count was not an independent
prognostic marker. The authors point out that the subsets of
macrophages may still be of prognostic use, as these were not
determined in their study (46). A
study carried out by Jin et al has also demonstrated
increased CD68+ cells in invasive breast cancer and
linked this to increased IL-1β expression, which they suggest is
(in part) produced by the increased numbers of macrophages. This
increase in IL-1β was linked to markers of more aggressive breast
cancer. A small increase in IL-1β was seen in DCIS compared to
normal tissue but it was not significantly different to benign
disease (47).
There is strong evidence that in some cancers
immune-surveillance plays an integral role in initiation, growth
and response to therapy. The present paradigm is that tumour cells
are held in check by the immune system and evasion is required to
establish the primary tumour (48). One way tumour cells can evade the
immune system is to downregulate their immunogenicity by reducing
expression of immunogenic antigens. Another is recruitment of
immune suppressor cells, e.g. myeloid derived suppressor cells
(MDSC).
MDSCs are a heterogeneous population of bone marrow
derived cells (BDMC) including immature macrophages, monocytes,
neutrophils and dendritic cells. They function as potent inhibitors
of natural killer (NK) cells and T-cells and are critical for the
immune escape of tumours. A study with the 4T1 mammary tumour model
showed accumulation of granulocytic MDSC correlates with metastatic
progression. The MDSCs were found to be potent suppressors of in
vitro T-cell proliferation (49). Another study using the 4T1 model by
Yang et al demonstrated that MDSC may not only supress the
immune response, but actively drive invasion by secreting MMPs at
the invasive front (50). Blocking
MDSC with zoledronic acid reduces tumour growth and improves
antitumour responses in a HER2 breast murine model (51). Additionally, CCL5 has been shown to
be important in generation of MDSCs in mice and in humans, CCL5
blocking antibodies have been shown to inhibit MDSCs and drive
increased T-cell proliferation (52). In breast cancer patients
circulating MDSCs correlate with cancer stage, the highest
abundance being found in stage IV patients with metastatic disease
(53). MDSCs isolated from breast
cancer tissues have been shown to supress T-cell responses via
indoleamine 2,3-dioxygenase (IDO) (54).
The extent of T-cell infiltration into invasive
breast cancer has been reported to range from 1–45% of the tumour
mass (55). In rapidly
proliferating tumours T-cell infiltration correlates with good
prognosis, clear auxiliary nodes, smaller tumours, lower grade and
better relapse free survival (10). But the type of T-cells present
affects progression. A tumour directed immune response involves
CD8+ cytolytic T-cells (Th1) and NK cells, which is
protective against the development and progression of a cancer. If
the immune response involves the humoral immune response and/or a
CD4+ (Th2) T-cell population the likely outcome is
tumour progression (56). The
percentage of CD4+ T-cells in breast cancer positively
correlates with markers of disease progression, including
metastatic spread to sentinel nodes and increase primary tumour
size (55,57).
In the 4T1 model lung metastasis requires T-reg
mediated NK cell inhibition, while depletion of the T-regs reduces
lung metastasis (58). Tumour cell
expression of galectin-1 is associated with increased T-reg
frequency and increased lung metastasis (59). Similarly to MDSC, T-regs have also
been show to play an active role in driving invasion, independent
of their immunosuppressive function. Gavin et al
demonstrated that T-regs can promote lung metastasis of RANK
expressing mammary tumours via production of RANK ligand (60).
The levels of T-regs in the peripheral blood of
breast cancer patients has been found to be high and tumour
infiltrating T-regs levels are higher in breast cancer tissue than
normal breast. These higher levels of T-regs correlate with poor
prognosis (61). T-regs enriched
in FOXP3, GITR and CTLA4 exert the potential to supress effector
T-cells in the periphery and are found at high levels in cancer
patients, depletion or blockade of this subset can enhance immune
protection (56). Gupta et
al examined intratumoral expression of FOXP3 in invasive breast
cancer compared to DCIS and adjacent tissue; they were able to
demonstrate a linear association of intra-tumoural FOXP3 as a
marker of progression and metastasis (62). High levels of T-regs have been
found to identify breast cancer patients at higher risk of relapse
or recurrence and analysis of breast tumours has demonstrated a
link between FOXP3 expression and progression of breast cancer
(63,64).
Most of the research into the role of T-cells in
breast cancer has focused on the immune-suppressive functions,
however CD8+ lymphocytes are a known crucial component
of cell-mediated immunity. Mahmoud et al set out to
determine the prognostic value of tumour-infiltrating
CD8+ cytotoxic lymphocytes in breast cancer. They
examined 1,334 unselected breast tumours and found that the total
number of CD8+ cells was positively correlated with
tumour grade and inversely correlated with patient's age at
diagnosis, ER-α and PgR expression. Total number and distant
stromal CD8+ lymphocytes were associated with better
patient survival. In a multivariate analysis, total CD8+
T-cell count was an independent prognostic factor for better
patient survival. These results suggest that tumour-infiltrating
CD8+ T lymphocytes have antitumour activity due to the
effect on patient survival (65).
In breast cancer B-cells are found in draining lymph
nodes and the tumour stroma, the sentinel nodes are enriched with
IgG positive, proliferating B-cells. Studies have shown that the
presence of B-cells in the sentinel and auxiliary nodes correlates
with disease stage and tumour burden (66). Analysis of the axillary nodes from
breast cancers found the presence of IgG positive follicles and/or
IgM positive lymphoid cells were statistically related to breast
tumours of high grade and >3 lymph node metastasis (67). The occurrence of auto-antibodies
(to smooth muscle or p53) in the serum of cancer patients has been
show to correlate with poor prognosis (68). However, more recently Mahmoud et
al used immunohistochemistry to investigate the density and
localisation of B lymphocytes infiltrating 1,470 breast tumours to
identify any prognostic significance and relationship to various
clinicopathological factors. There was a positive correlation
between higher numbers of total CD20+ B-cells and higher
tumour grade, ER and PgR negativity, and basal phenotype. In
univariate survival analysis, higher total number of infiltrating
CD20+ cells was associated with significantly better
survival, therefore high B-cell numbers correlated with a
favourable prognosis independent of the size of the tumour, grade,
and lymph node invasion (69).
This investigation is supported by the study carried out by Schmidt
et al in node negative breast cancer (70). These contradictory studies
indicates more work need to be done on the contribution of the
humoral response, as Mahmoud et al point out ‘there is a
distinct lack of data regarding B-cell infiltrate in breast
carcinomas using large patient cohorts'. Interestingly, B-cell
depletion in mouse models (MMTV-PyMT) has been shown to have no
effect on early or late stage mammary carcinogenesis (71), which highlight potential
deficiencies in mouse models when studying the complexities of the
human immune response to cancer.
Due to immune-surveillance for defective cells solid
tumours are often weakly immunogenic and develop strategies to
avoid detection by immune cells, such as downregulation of
antigenic surface markers (e.g. integrins) and recruitment of
immune suppressive cells such as T-Regs and MDSCs (as discussed
previously). This has led to the idea that restoration of the
immunogenicity or depletion of the immune suppressive cells may
enhance the immune system's ability to detect and target cancers
(48).
There is increasing evidence that commonly used
cytotoxic drugs (e.g. anthracyclins) already increase antitumour
immunity and therefore increase their therapeutic effect due to the
apoptotic cells triggering antitumour immune signals (72,73).
Doxorubicin has been shown to increase tumour antigen specific
proliferation of CD8+ T-cells and biopsies of breast
cancer prior to neo-adjuvant therapy showed a correlation between
CD8/IFN-γ gene expression (indicative of Th1 recruitment) and
clinical response to doxorubicin (74). A more comprehensive overview of
this area can be read in Criscitiello and Curigliano (75). Subsequently targeted
immunotherapies are increasingly being developed, e.g. monoclonal
antibodies (mAbs) and tyrosine kinase inhibitors (TKIs).
Trastuzumab (mAb targeted to HER2) is the most well-known mAb in
breast cancer treatment, and antibody dependent cell-mediated
cytotoxicity (ADCC) has been implicated as a mechanism of action
(76).
Screening of H&E sections for lymphocytic
infiltration have been shown to have predictive and prognostic
value in triple-negative and HER2+ breast cancer, in
this context the TIL Working Group suggests that a strong
antitumour immunity directed to multiple targets may result in
improved control of a heterogeneous malignant cell population
(16). There are some examples of
adjuvant and neoadjuvant studies that have examined TILs, full
details can be found in the article by Salgado et al
(16). Two studies examined TILs
in triple-negative breast cancer (TNBC) and hormone receptor
positive breast cancer at diagnosis and were found to be a positive
prognostic marker in a subset of TNBCs (77,78).
Studies of HER2+ breast cancer and TILs have
demonstrated higher TILs in baseline samples or immune enriched
resulted in increased response to trastuzumab (79,80).
The T-cell inhibitory molecule B7-H1 (a.k.a. PD-L1)
has been shown to induce T-cell anergy when bound to the receptor
PD-1. Expression of PD-1+/FOXP3+ T-regs in
the breast cancer microenvironment has been demonstrated, raising
the possibility of using PD-L1 therapy in breast cancer (81,82).
PD-1 is an inhibitory receptor found on activated T- and B-cells,
which helps to suppress antitumour immunity (83). The efficacy of PD-1 blockade in the
treatment of bladder cancer was recently demonstrated (84), also it has previously been shown
that blockade of PD-1 exhibits positive responses in other advanced
carcinomas e.g. non-small cell lung carcinoma, melanoma, renal cell
carcinoma and ovarian carcinoma (85). There are several active clinical
trials currently running investigating the role of anti-PD1
(pembrolizumab) in various cancers. One phase 1b study by Merck
(Keynote-012) was recently presented at the San Antonio Breast
Cancer Conference 2014 (abstract S1-09) outlining an 18.5% response
to pembrolizumab in PD-L1-positive triple-negative breast
cancers.
CTLA4 has homology to PD-1, but acts differently to
downregulate immune signals and is found on T-cells where it
induces T-cell anergy when activated. Phase III trials have
demonstrated responses in advanced melanoma either alone (86) or in combination with other drugs
(87). There are currently several
active clinical trials using anti-CTLA-4 (tremelimumab) in
different cancers. There has been limited research into the
efficacy in breast cancer but one study by Vonderheide et al
examined the combination of tremelimumab and exemestane in advanced
breast cancer. While the trial was halted due to strong side
effects, 42% of the patients exhibited stable disease for ≥12 weeks
and treatment was associated (in most patients) with increased
peripheral CD4+ and CD8+ T-cells, and a
marked increase in the ratio of inducible costimulator
(ICOS)+ T-cells to FOXP3+ regulatory T-cells
indicating the treatment was working (88). New targets such as anti-PD-1 and
anti-CTLA-4 mAbs may improve outcome in breast cancer, perhaps in
combination with standard therapies, e.g. trastuzumab (89).
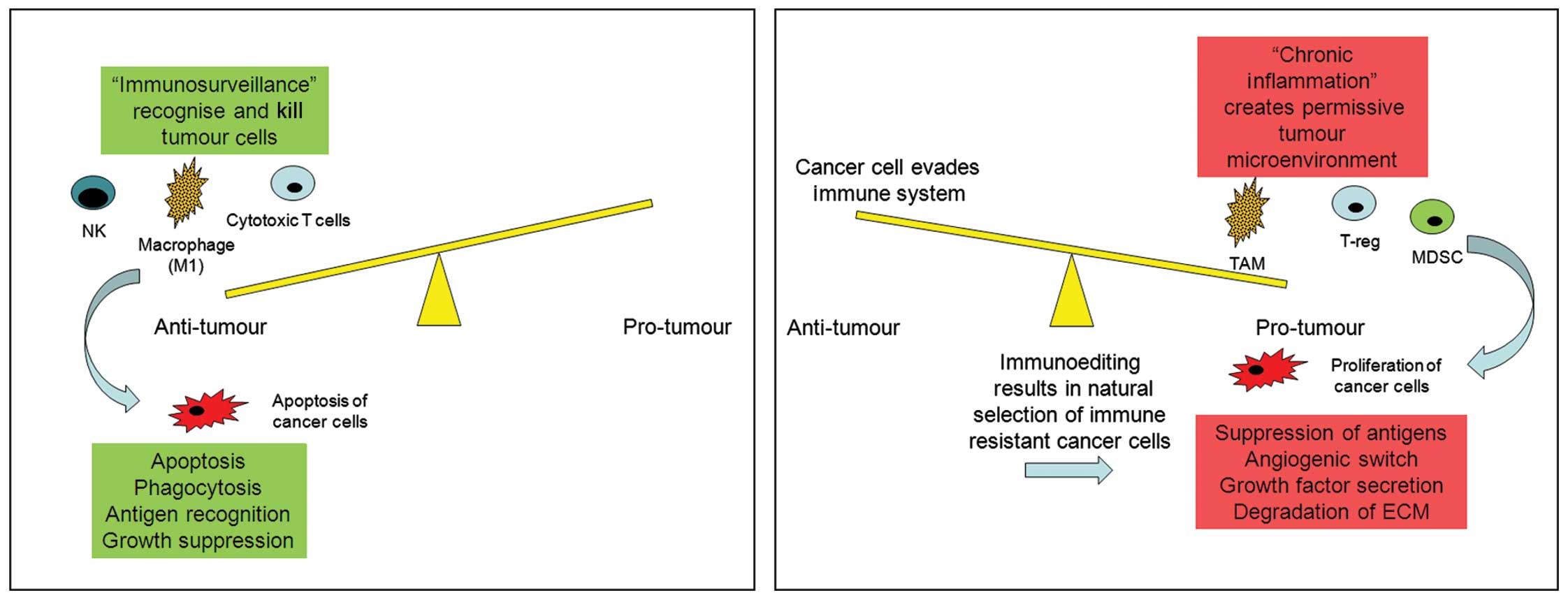
Over the past 20 years much has been learnt about
the mechanisms underlying the complex interactions between immune
cells and tumour progression. It is generally understood that the
way in which the immune system responds to a tumour depends on the
type of immune response generated by the microenvironment of that
tumour. If a tumour drives CD8+/Th1 T-cells, NK and M1
TAM recruitment, then the tumour progression is likely to be halted
and the tumour destroyed or at least severely compromised in its
ability to progress. However, if the tumour drives a
CD4+/Th2/T-reg, MDSC and M2 TAM response, then the
tumour is likely to not only escape immune destruction but the
immune cells may actively aid tumour progression to metastasis
(Fig. 1). We must bear in mind the
caveat that this is an extremely simplified understanding of how
the immune system responds to cancer cells and that it is much more
complex than this.
The importance of this complexity cannot be
underestimated as the immunotherapeutic clinical trials in cancer
expand. While the success of the PD-1 inhibitors is an exciting
prospect and new investigations into mono and combinatorial
treatment for breast cancer are very important, lesson must be
learnt from past failures and decreased emphasis must be placed on
the reliability of mouse and animal models when making decision
about dose, from a toxicity and side effect stand point. It was
only 9 years ago, in 2006, that a phase I trial for the drug
TGN1412 (the CD28-SuperMaB) resulted in horrific side-effects for
the 6 volunteers. It took 6 years to understand how the lack of
toxicity in animal models failed to predict the ‘cytokine storm' in
humans. This failing was a breakdown in the fundamental
understanding that laboratory animals are not exposed to infection
on a daily basis as are their wild-type counterparts. This
difference results in an under-representation of effector T-cells
(T-em) vs. T-regs in the laboratory animals compared to the human
volunteers. The T-em cells are a reservoir of cytokines stored in
tissues waiting to be released which is what happened in the
response to treatment with TGN1412 in humans. Other failings were
also exposed about the methods of deciding on dose (90). What this study highlights is the
need to gain a more comprehensive and detailed understanding of the
complexities of the human immune system, which can only be achieved
with more research, better animal replacement models and carefully
designed clinical trials.
We need to develop a more detailed understanding of
how these different cell types interact and use immune cells and
cytokines as biomarkers to predict outcome and measure response to
therapy. New targets such as anti-PD-1 and anti-CTLA-4 mAbs may
improve outcome in breast cancer, perhaps in combination with
standard therapies, e.g. trastuzumab (89). Eventually we will be able to
harness our own immune system to target and destroy cancers and
hopefully immunise patients from relapse.
|
1
|
Kerlikowske K, Molinaro AM, Gauthier ML,
Berman HK, Waldman F, Bennington J, Sanchez H, Jimenez C, Stewart
K, Chew K, et al: Biomarker expression and risk of subsequent
tumors after initial ductal carcinoma in situ diagnosis. J Natl
Cancer Inst. 102:627–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Price P, Sinnett HD, Gusterson B, Walsh G,
A'Hern RP and McKinna JA: Duct carcinoma in situ: Predictors of
local recurrence and progression in patients treated by surgery
alone. Br J Cancer. 61:869–872. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Welch HG and Black WC: Overdiagnosis in
cancer. J Natl Cancer Inst. 102:605–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma XJ, Salunga R, Tuggle JT, Gaudet J,
Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, et
al: Gene expression profiles of human breast cancer progression.
Proc Natl Acad Sci USA. 100:5974–5979. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castro NP, Osório CA, Torres C, Bastos EP,
Mourão-Neto M, Soares FA, Brentani HP and Carraro DM: Evidence that
molecular changes in cells occur before morphological alterations
during the progression of breast ductal carcinoma. Breast Cancer
Res. 10:R872008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moelans CB, de Weger RA, Monsuur HN, Maes
AH and van Diest PJ: Molecular differences between ductal carcinoma
in situ and adjacent invasive breast carcinoma: A multiplex
ligation-dependent probe amplification study. Anal Cell Pathol
(Amst). 33:165–173. 2010. View Article : Google Scholar
|
|
7
|
Solin LJ, Gray R, Baehner FL, Butler SM,
Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW
Jr, et al: A multigene expression assay to predict local recurrence
risk for ductal carcinoma in situ of the breast. J Natl Cancer
Inst. 105:701–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allen M and Jones LJ: Jekyll and Hyde: The
role of the micro-environment on the progression of cancer. J
Pathol. 223:162–176. 2011. View Article : Google Scholar
|
|
9
|
Bissell MJ, Hall HG and Parry G: How does
the extracellular matrix direct gene expression? J Theor Biol.
99:31–68. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aaltomaa S, Lipponen P, Eskelinen M, Kosma
VM, Marin S, Alhava E and Syrjänen K: Lymphocyte infiltrates as a
prognostic variable in female breast cancer. Eur J Cancer.
28A:859–864. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stewart T, Tsai SC, Grayson H, Henderson R
and Opelz G: Incidence of de-novo breast cancer in women
chronically immunosuppressed after organ transplantation. Lancet.
346:796–798. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee AH, Happerfield LC, Bobrow LG and
Millis RR: Angiogenesis and inflammation in ductal carcinoma in
situ of the breast. J Pathol. 181:200–206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu
L, Grzesik DA, Qian H, Xue XN and Pollard JW: Macrophages regulate
the angiogenic switch in a mouse model of breast cancer. Cancer
Res. 66:11238–11246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee AH, Dublin EA and Bobrow LG:
Angiogenesis and expression of thymidine phosphorylase by
inflammatory and carcinoma cells in ductal carcinoma in situ of the
breast. J Pathol. 187:285–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leek RD, Lewis CE, Whitehouse R, Greenall
M, Clarke J and Harris AL: Association of macrophage infiltration
with angiogenesis and prognosis in invasive breast carcinoma.
Cancer Res. 56:4625–4629. 1996.PubMed/NCBI
|
|
16
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
International TILs Working Group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar
|
|
17
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang X: Tumor-associated macrophages as
potential diagnostic and prognostic biomarkers in breast cancer.
Cancer Lett. 332:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bögels M, Braster R, Nijland PG, Gül N,
van de Luijtgaarden W, Fijneman RJ, Meijer GA, Jimenez CR, Beelen
RH and van Egmond M: Carcinoma origin dictates differential skewing
of monocyte function. OncoImmunology. 1:798–809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hagemann T, Lawrence T, McNeish I, Charles
KA, Kulbe H, Thompson RG, Robinson SC and Balkwill FR:
‘Re-educating' tumor-associated macrophages by targeting NF-kappaB.
J Exp Med. 205:1261–1268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coussens LM and Pollard JW: Leukocytes in
mammary development and cancer. Cold Spring Harb Perspect Biol.
3:32011. View Article : Google Scholar
|
|
22
|
Guy CT, Cardiff RD and Muller WJ:
Induction of mammary tumors by expression of polyomavirus middle T
oncogene: A transgenic mouse model for metastatic disease. Mol Cell
Biol. 12:954–961. 1992.PubMed/NCBI
|
|
23
|
Scholl SM, Pallud C, Beuvon F, Hacene K,
Stanley ER, Rohrschneider L, Tang R, Pouillart P and Lidereau R:
Anticolony-stimulating factor-1 antibody staining in primary breast
adenocarcinomas correlates with marked inflammatory cell
infiltrates and prognosis. J Natl Cancer Inst. 86:120–126. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony-stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DeNardo DG, Brennan DJ, Rexhepaj E,
Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD,
Junaid SA, et al: Leukocyte complexity predicts breast cancer
survival and functionally regulates response to chemotherapy.
Cancer Discov. 1:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soria G and Ben-Baruch A: The inflammatory
chemokines CCL2 and CCL5 in breast cancer. Cancer Lett.
267:271–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murdoch C, Giannoudis A and Lewis CE:
Mechanisms regulating the recruitment of macrophages into hypoxic
areas of tumors and other ischemic tissues. Blood. 104:2224–2234.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chavey C, Bibeau F, Gourgou-Bourgade S,
Burlinchon S, Boissière F, Laune D, Roques S and Lazennec G:
Oestrogen receptor negative breast cancers exhibit high cytokine
content. Breast Cancer Res. 9:R152007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ueno T, Toi M, Saji H, Muta M, Bando H,
Kuroi K, Koike M, Inadera H and Matsushima K: Significance of
macrophage chemoattractant protein-1 in macrophage recruitment,
angiogenesis, and survival in human breast cancer. Clin Cancer Res.
6:3282–3289. 2000.PubMed/NCBI
|
|
30
|
Goede V, Brogelli L, Ziche M and Augustin
HG: Induction of inflammatory angiogenesis by monocyte
chemoattractant protein-1. Int J Cancer. 82:765–770. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valković T, Lucin K, Krstulja M,
Dobi-Babić R and Jonjić N: Expression of monocyte chemotactic
protein-1 in human invasive ductal breast cancer. Pathol Res Pract.
194:335–340. 1998. View Article : Google Scholar
|
|
32
|
Dwyer RM, Potter-Beirne SM, Harrington KA,
Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T and Kerin MJ:
Monocyte chemotactic protein-1 secreted by primary breast tumors
stimulates migration of mesenchymal stem cells. Clin Cancer Res.
13:5020–5027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lebrecht A, Grimm C, Lantzsch T, Ludwig E,
Hefler L, Ulbrich E and Koelbl H: Monocyte chemoattractant
protein-1 serum levels in patients with breast cancer. Tumour Biol.
25:14–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lyon DE, McCain NL, Walter J and Schubert
C: Cytokine comparisons between women with breast cancer and women
with a negative breast biopsy. Nurs Res. 57:51–58. 2008. View Article : Google Scholar :
|
|
35
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luboshits G, Shina S, Kaplan O, Engelberg
S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I and Ben-Baruch
A: Elevated expression of the CC chemokine regulated on activation,
normal T cell expressed and secreted (RANTES) in advanced breast
carcinoma. Cancer Res. 59:4681–4687. 1999.PubMed/NCBI
|
|
37
|
Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki
Y and Abe A: Correlation of tissue and plasma RANTES levels with
disease course in patients with breast or cervical cancer. Clin
Cancer Res. 7:285–289. 2001.PubMed/NCBI
|
|
38
|
Yaal-Hahoshen N, Shina S, Leider-Trejo L,
Barnea I, Shabtai EL, Azenshtein E, Greenberg I, Keydar I and
Ben-Baruch A: The chemokine CCL5 as a potential prognostic factor
predicting disease progression in stage II breast cancer patients.
Clin Cancer Res. 12:4474–4480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stormes KA, Lemken CA, Lepre JV, Marinucci
MN and Kurt RA: Inhibition of metastasis by inhibition of
tumor-derived CCL5. Breast Cancer Res Treat. 89:209–212. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robinson SC, Scott KA, Wilson JL, Thompson
RG, Proudfoot AE and Balkwill FR: A chemokine receptor antagonist
inhibits experimental breast tumor growth. Cancer Res.
63:8360–8365. 2003.PubMed/NCBI
|
|
41
|
Velasco-Velázquez M, Jiao X, De La Fuente
M, Pestell TG, Ertel A, Lisanti MP and Pestell RG: CCR5 antagonist
blocks metastasis of basal breast cancer cells. Cancer Res.
72:3839–3850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Biswas SK, Gangi L, Paul S, Schioppa T,
Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F,
et al: A distinct and unique transcriptional program expressed by
tumor-associated macrophages (defective NF-kappaB and enhanced
IRF-3/STAT1 activation). Blood. 107:2112–2122. 2006. View Article : Google Scholar
|
|
44
|
Jubb AM, Soilleux EJ, Turley H, Steers G,
Parker A, Low I, Blades J, Li JL, Allen P, Leek R, et al:
Expression of vascular notch ligand delta-like 4 and inflammatory
markers in breast cancer. Am J Pathol. 176:2019–2028. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsutsui S, Yasuda K, Suzuki K, Tahara K,
Higashi H and Era S: Macrophage infiltration and its prognostic
implications in breast cancer: The relationship with VEGF
expression and microvessel density. Oncol Rep. 14:425–431.
2005.PubMed/NCBI
|
|
46
|
Mahmoud SM, Lee AH, Paish EC, Macmillan
RD, Ellis IO and Green AR: Tumour-infiltrating macrophages and
clinical outcome in breast cancer. J Clin Pathol. 65:159–163. 2012.
View Article : Google Scholar
|
|
47
|
Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A,
Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al:
Expression of interleukin-1beta in human breast carcinoma. Cancer.
80:421–434. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Slaney CY, Rautela J and Parker BS: The
emerging role of immunosurveillance in dictating metastatic spread
in breast cancer. Cancer Res. 73:5852–5857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bidwell BN, Slaney CY, Withana NP, Forster
S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, et
al: Silencing of Irf7 pathways in breast cancer cells promotes bone
metastasis through immune escape. Nat Med. 18:1224–1231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang L, Huang J, Ren X, Gorska AE, Chytil
A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, et al:
Abrogation of TGF beta signaling in mammary carcinomas recruits
Gr-1+CD11b+ myeloid cells that promote
metastasis. Cancer Cell. 13:23–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Melani C, Sangaletti S, Barazzetta FM,
Werb Z and Colombo MP: Amino-biphosphonate-mediated MMP-9
inhibition breaks the tumor-bone marrow axis responsible for
myeloid-derived suppressor cell expansion and macrophage
infiltration in tumor stroma. Cancer Res. 67:11438–11446. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Lv D, Kim HJ, Kurt RA, Bu W, Li Y
and Ma X: A novel role of hematopoietic CCL5 in promoting
triple-negative mammary tumor progression by regulating generation
of myeloid-derived suppressor cells. Cell Res. 23:394–408. 2013.
View Article : Google Scholar :
|
|
53
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garrett-Mayer E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden, and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar
|
|
54
|
Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu
W, Shen C, Liu J and Ren X: Myeloid-derived suppressor cells
suppress antitumor immune responses through IDO expression and
correlate with lymph node metastasis in patients with breast
cancer. J Immunol. 190:3783–3797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chin Y, Janseens J, Vandepitte J,
Vandenbrande J, Opdebeek L and Raus J: Phenotypic analysis of
tumor-infiltrating lymphocytes from human breast cancer. Anticancer
Res. 12:1463–1466. 1992.PubMed/NCBI
|
|
56
|
Watanabe MA, Oda JM, Amarante MK and Cesar
Voltarelli J: Regulatory T cells and breast cancer: Implications
for immuno-pathogenesis. Cancer Metastasis Rev. 29:569–579. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kohrt HE, Nouri N, Nowels K, Johnson D,
Holmes S and Lee PP: Profile of immune cells in axillary lymph
nodes predicts disease-free survival in breast cancer. PLoS Med.
2:e2842005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of
CD4+CD25+ regulatory T cells. Nat Immunol.
4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bacchetta R, Passerini L, Gambineri E, Dai
M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C,
Matthes-Martin S, Lawitschka A, et al: Defective regulatory and
effector T cell functions in patients with FOXP3 mutations. J Clin
Invest. 116:1713–1722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gavin MA, Torgerson TR, Houston E, DeRoos
P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD and
Rudensky AY: Single-cell analysis of normal and FOXP3-mutant human
T cells: FOXP3 expression without regulatory T cell development.
Proc Natl Acad Sci USA. 103:6659–6664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li B, Saouaf SJ, Samanta A, Shen Y,
Hancock WW and Greene MI: Biochemistry and therapeutic implications
of mechanisms involved in FOXP3 activity in immune suppression.
Curr Opin Immunol. 19:583–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gupta S, Joshi K, Wig JD and Arora SK:
Intratumoral FOXP3 expression in infiltrating breast carcinoma: Its
association with clinicopathologic parameters and angiogenesis.
Acta Oncol. 46:792–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bates GJ, Fox SB, Han C, Leek RD, Garcia
JF, Harris AL and Banham AH: Quantification of regulatory T cells
enables the identification of high-risk breast cancer patients and
those at risk of late relapse. J Clin Oncol. 24:5373–5380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu Y and Zheng P: FOXP3 and breast
cancer: Implications for therapy and diagnosis. Pharmacogenomics.
8:1485–1487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mahmoud SM, Paish EC, Powe DG, Macmillan
RD, Grainge MJ, Lee AH, Ellis IO and Green AR: Tumor-infiltrating
CD8+ lymphocytes predict clinical outcome in breast
cancer. J Clin Oncol. 29:1949–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wernicke M: Quantitative morphologic
assessment of immuno-reactivity in regional lymph nodes of patients
with carcinoma of the breast. Surg Gynecol Obstet. 140:919–924.
1975.PubMed/NCBI
|
|
67
|
Urdiales-Viedma M, Nogales-Fernandez F,
Martos-Padilla S and Sanchez-Cantalejo E: Breast tumors:
Immunoglobulins in axillary lymph nodes. Tumori. 72:575–579.
1986.PubMed/NCBI
|
|
68
|
Tan EM and Shi FD: Relative paradigms
between autoantibodies in lupus and autoantibodies in cancer. Clin
Exp Immunol. 134:169–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mahmoud SM, Lee AH, Paish EC, Macmillan
RD, Ellis IO and Green AR: The prognostic significance of B
lymphocytes in invasive carcinoma of the breast. Breast Cancer Res
Treat. 132:545–553. 2012. View Article : Google Scholar
|
|
70
|
Schmidt M, Böhm D, von Törne C, Steiner E,
Puhl A, Pilch H, Lehr HA, Hengstler JG, Kölbl H and Gehrmann M: The
humoral immune system has a key prognostic impact in node-negative
breast cancer. Cancer Res. 68:5405–5413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
DeNardo DG, Barreto JB, Andreu P, Vasquez
L, Tawfik D, Kolhatkar N and Coussens LM: CD4(+) T cells regulate
pulmonary metastasis of mammary carcinomas by enhancing protumor
properties of macrophages. Cancer Cell. 16:91–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
van der Most RG, Currie AJ, Robinson BW
and Lake RA: Decoding dangerous death: How cytotoxic chemotherapy
invokes inflammation, immunity or nothing at all. Cell Death
Differ. 15:13–20. 2008. View Article : Google Scholar
|
|
73
|
Zitvogel L, Apetoh L, Ghiringhelli F,
André F, Tesniere A and Kroemer G: The anticancer immune response:
Indispensable for therapeutic success? J Clin Invest.
118:1991–2001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mattarollo SR, Loi S, Duret H, Ma Y,
Zitvogel L and Smyth MJ: Pivotal role of innate and adaptive
immunity in anthracycline chemotherapy of established tumors.
Cancer Res. 71:4809–4820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Criscitiello C and Curigliano G:
Immunotherapeutics for breast cancer. Curr Opin Oncol. 25:602–608.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Musolino A, Naldi N, Bortesi B, Pezzuolo
D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R,
Bisagni G, et al: Immunoglobulin G fragment C receptor
polymorphisms and clinical efficacy of trastuzumab-based therapy in
patients with HER-2/neu-positive metastatic breast cancer. J Clin
Oncol. 26:1789–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Loi S, Sirtaine N, Piette F, Salgado R,
Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al:
Prognostic and predictive value of tumor-infiltrating lymphocytes
in a phase III randomized adjuvant breast cancer trial in
node-positive breast cancer comparing the addition of docetaxel to
doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin
Oncol. 31:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Loi S, Michiels S, Salgado R, Sirtaine N,
Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V,
Desmedt C, et al: Tumor infiltrating lymphocytes are prognostic in
triple negative breast cancer and predictive for trastuzumab
benefit in early breast cancer: Results from the FinHER trial. Ann
Oncol. 25:1544–1550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Perez EA, Thompson EA, Ballman KV,
Anderson SK, Asmann YW, Kalari KR, Eckel-Passow JE, Dueck AC,
Tenner KS, Jen J, et al: Genomic analysis reveals that immune
function genes are strongly linked to clinical outcome in the North
Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J
Clin Oncol. 33:701–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ghebeh H, Barhoush E, Tulbah A, Elkum N,
Al-Tweigeri T and Dermime S: FOXP3+ Tregs and
B7-H1+/PD-1+ T lymphocytes co-infiltrate the
tumor tissues of high-risk breast cancer patients: Implication for
immunotherapy. BMC Cancer. 8:572008. View Article : Google Scholar
|
|
82
|
Muenst S, Soysal SD, Gao F, Obermann EC,
Oertli D and Gillanders WE: The presence of programmed death 1
(PD-1)-positive tumor-infiltrating lymphocytes is associated with
poor prognosis in human breast cancer. Breast Cancer Res Treat.
139:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
meta-static melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Vonderheide RH, LoRusso PM, Khalil M,
Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Rüter J,
Mariani GL, et al: Tremelimumab in combination with exemestane in
patients with advanced breast cancer and treatment-associated
modulation of inducible costimulator expression on patient T cells.
Clin Cancer Res. 16:3485–3494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Stagg J, Loi S, Divisekera U, Ngiow SF,
Duret H, Yagita H, Teng MW and Smyth MJ: Anti-ErbB-2 mAb therapy
requires type I and II interferons and synergizes with anti-PD-1 or
anti-CD137 mAb therapy. Proc Natl Acad Sci USA. 108:7142–7147.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hünig T: The storm has cleared: Lessons
from the CD28 super-agonist TGN1412 trial. Nat Rev Immunol.
12:317–318. 2012. View Article : Google Scholar
|