Introduction
Gastric cancer remains highly prevalent and accounts
for a notable proportion of global cancer mortality, with poor
survival rates (1). According to
GLOBOCAN estimation for 2015, 1034,124 new cases of gastric cancer
are predicted to be diagnosed, accounting for 785,558 new deaths
annually (2). It is the third
leading cause of cancer-related death (>8% of the total) and
fifth most common malignancy in both sexes worldwide. The
case-fatality ratio is higher than the common malignancies such as
lung, colon, breast, and prostate cancers (3) with 70% cases in developing countries
where, 50% accounts for only in Eastern Asia (4). Despite advancement in the current
diagnosis and major therapies including surgery and chemotherapy,
it carries a poor prognosis due to non-specific symptoms in early
stages with 5-year relative survival <20% in most countries
(5). Due to its multidrug
resistance to classical chemotherapies, potent bio-therapeutic
targets are now required as alternative preventive methods.
It is well known that sustaining proliferative
signals and resisting cell death are crucial hallmarks of cancer
cells (6). In addition it
possesses the capability to regulate cancer cell development and
progression by downregulating the growth-stimulatory signals, upon
activation of tumor suppressor genes. PI3K/Akt (protein kinase
B)/mTOR (mammalian target of rapamycin) cascade is
probably the most frequently altered signaling pathway in cancer
(7). These serine/theorine
proteins are regarded as important key regulators of many essential
cellular processes including cell survival, proliferation, growth,
and differentiation (8). The
activation of PI3K/Akt stimulates mTOR, which allows cells to
inhibit autophagic progression followed by cell death (9).
Autophagy is a ubiquitous physiological process in
all eukaryotic cells. The most prevalent form of autophagy known as
‘macro-autophagy', has been defined as type II programmed cell
death (PCD) (9). Autophagy begins
with the formation of double-membrane vesicles known as
autophagosomes that engulf cytoplasmic constituents including
organelles followed by maturation process upon fusion with
lysosomes and finally become autolysosomes, which undergoes a
cellular degradation process lead by lysosomal enzyme in response
to starvation and stress (10).
Several studies have reported that autophagy promotes cancer cell
death in response to various anticancer agents on apoptosis
defective cell (11–14). Accordingly, over-activation of
autophagy in cancer cell has been proposed to play an important
death mechanism during tumor progression, where apoptosis is
limited (15).
MAPK signaling has been implicated in numerous
cellular responses including inflammation, cell cycle, cell death,
development, differentiation, tumorigenesis and senescence
(16). Numerous studies have shed
light on activation of MAPKs including extracellular
signal-regulated kinase (ERK1/2), c-Jun N-terminal kinase (JNK) and
p38 MAPKs induced autophagy in cancer cells (17,18),
subsequently accompanied by an increase of autophagy regulatory
protein and tumor suppressor genes (19). Furthermore, induction of autophagic
cell death in cancer cell could be triggered by
p21WAF1/CIP1, popularly known as a potent master
effector of multiple tumor suppressor pathway promoting
anti-proliferative activities (20–22).
The use of herbal medicine and supplements increased
tremendously over the past three decades with people worldwide
gaining health benefits (23).
Over last few years, several investigations established dietary
substances from fruits, vegetables, tea and wine with health
promoting activities. Citrus fruits have been widely studied
for their therapeutic role in human cancer (24,25)
as they contain a great variety of phytochemicals such as
flavonoids, limonoids, phenolic acid and ascorbic acid. Flavonoids
are a large group of heterogeneous polyphenols carrying potential
anti-carcinogenic and antitumor activities. Naringin, a major
flavonoid mostly available in grape and citrus fruits, exerting a
variety of pharmacological effects such as antitumor (26), antioxidant (27), cholesterol-lowering,
anti-atherogenic (28),
anti-inflammatory (29), antiviral
and inhibitory activities followed by induction of apoptosis in
different cancer cells have been reported (30,31).
However, Naringin exhibiting growth regulatory mechanism relevant
to non-apoptotic cell death signaling pathways in cancer cells is
still unidentified.
Therefore, the present study evaluated the
inhibitory mechanism of flavonoid Naringin in AGS human cancer
cell, presenting a detailed observation on induction of autophagy
by downregulating PI3K/Akt/mTOR signaling cascade via activation of
MAPK families. This report unveils that Naringin induced autophagic
growth inhibition in human AGS gastric cancer cells.
Materials and methods
Chemical and reagents
Roswell Park Memorial Institute (RPMI)-1640 medium
was purchased from Hyclone (Logan, UT, USA). Fetal bovine serum
(FBS) and antibiotics (streptomycin/penicillin) were obtained from
Gibco (BRL Life Technologies, Grand Island, NY, USA).
3-(4,5-dimethyl-thiazol-2- yl)-2,5-diphenyltetrazolium bromide
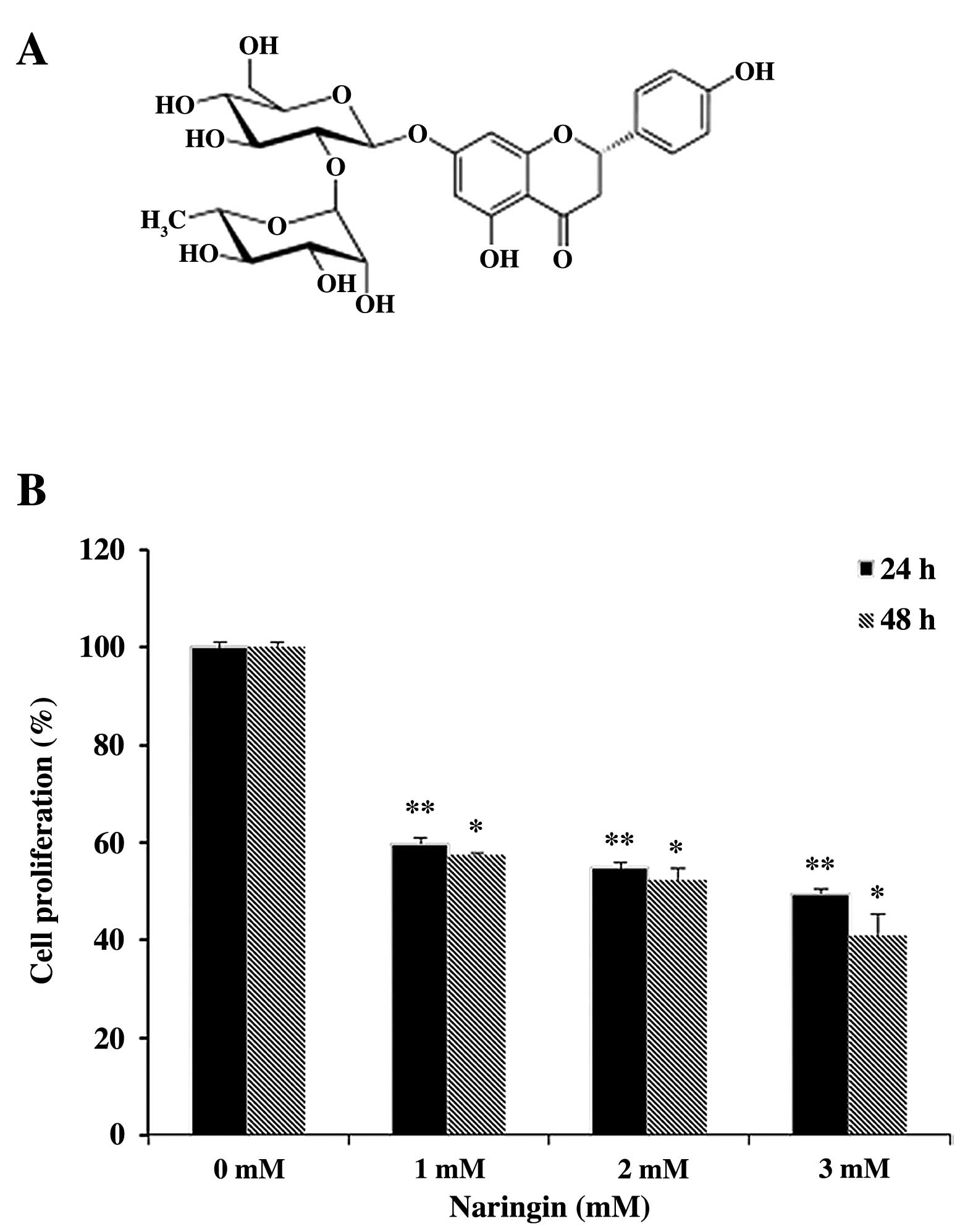
(MTT) and Naringin (Fig. 1A) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Materials and
chemicals used for electrophoresis were obtained from Bio-Rad
(Hercules, CA, USA). Primary antibodies ERK1/2, p-ERK1/2
(Thr202/Tyr204), JNK, p-JNK
(Thr183/tyr185), p38, p-p38
(Thr180/Try182), p-PI3K
(Tyr458/Tyr199), p-Akt (Ser473),
mTOR, p-mTOR (Ser2448), LC3B, Beclin 1, Bcl-xL and PI3K
inhibitor LY294002 were purchased from Cell Signaling (Beverly, MA,
USA). Akt (H-136) and Bax (P-19) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). PI3K p110δ antibody was
obtained from Enzo Life Sciences. Anti-p21WAF1/CIP1 and
β-actin were purchased from Millipore (Billerica, MA, USA).
Anti-rabbit IgG horseradish conjugate secondary antibody was
purchased from Enzo Life Sciences. All the chemicals used were of
the highest grade commercially available.
Cell culture and treatment
AGS cancer cell line, which is a gastric
adenocarcinoma, was obtained from the Korean Cell Line Bank (Seoul,
Korea). AGS cells were maintained in RPMI-1640 supplemented with
10% heat inactivated FBS and 1% penicillin/streptomycin at 37°C in
a 5% CO2 incubator. Cells were treated with vehicle
alone (DMSO) or a series of concentrations of Naringin dissolved in
1% DMSO.
Cell proliferation activity
Cell proliferation of AGS cancer cells was assessed
using MTT. Cells were seeded at density of 2.5×104 cells
per well in a 24-well plate, incubated overnight at 37°C in a 5%
CO2 incubator and treated with various concentrations of
Naringin (1, 2 and 3 mM) or vehicle alone (DMSO) for 24 and 48 h.
After treatment, MTT solution (5 mg/ml in 1X PBS) was
added followed by incubation for 3 h at 37°C in the dark. The
formazan crystals formed were solubilized by incubating cells with
500 μl of DMSO. Cell absorbance was read by enzyme-linked
immunosorbent assay (ELISA) plate reader (BioTek Instruments Co.,
Korea) at 540 nm. Cell proliferation was quantified as a percentage
compared to the control group (untreated cells), which was set at
100%.
DNA fragmentation assay
DNA was isolated with little modification following
DNA extraction protocol (32).
Briefly, untreated and Naringin-treated cells incubated for 24 h
were harvested and were lysed with cell lysis buffer for 30 sec at
room temperature (RT). The supernatant was collected after
centrifugation at 3,000 rpm for 5 min followed by incubation at
56°C for 2 h after adding 10% SDS solution and RNase A. Proteinase
K 25 mg/ml was added and incubated overnight till complete lysis at
37°C. After adding saturated NaCl and absolute ethanol to the
samples, the mixture was incubated at −80°C for precipitation.
Centrifuging for 20 min at 12,000 rpm followed by washing the white
pellet with 80% ice cold ethanol and air-dried at RT. The obtained
pellets were dissolved in 1X TE buffer. The total DNA solutions
were then subjected to 1.5% agarose gel electrophoresis at 100 V
for 45 min at room temperature. Tris acetate EDTA was used as the
electrophoresis running buffer and DNA bands were visualized by UV
light and documented by photography.
Electron microscopy analysis
For the transmission electron microscopy analysis
(TEM) the cells were seeded in a 100-mm dish and incubated with
vehicle or 2 mM Naringin for 24 h. The cells were harvested and
fixed in 4% formaldehyde and 1% glutaraldehyde phosphate buffer
(1:1) for 48 h at 4°C. The fixative was pipetted and replaced with
8% sucrose in 1X PBS, followed by post-fixation with 1% osmium
tetraoxide for 1 h at 4°C. The cells were then washed with 1X PBS
three times for 10 min. After dehydration in 50–100% ethanol, the
cells were embedded in Poly/Bed 812 resin (Pelco, Redding, CA,
USA). The cells were polymerized overnight at 60°C. Ultrathin
sections were stained with lead citrate and examined on Tecnai 12,
FEI transmission electron microscope.
Western blot analysis
Briefly, AGS cells treated with 1 mM and 2 mM
Naringin or vehicle (as control) for 24 h were lysed overnight with
lysis buffer (RIPA) containing phosphatase inhibitor cocktail along
with protease inhibitor and EDTA (Thermo Scientific, MA, USA). The
extracted proteins were then centrifuged at 14000 rpm for 30 min at
4°C to remove debris. The proteins were resolved using 8–15%
SDS-PAGE and subsequently transferred to polyvinylidene difluoride
(PVDF) membrane (Immunobilon-P, 0.45 mm; Millipore) using the TE 77
Semi-Dry Transfer Unit (GE Healthcare Life Sciences,
Buckinghamshire, UK). The membranes were blocked with 5% non-fat
milk in Tris-buffered saline containing 1% Tween-20 (TBS-T, pH 7.4)
or 1X phospho-blocking solution (TransLab, Biosciences in Korea) at
RT for 1 h. Blots were probed with a 1:500 or 1:1,000 dilutions of
the respective primary antibodies at 4°C overnight. After washing
five times with TBS-T, the membranes were incubated with a 1:1,000
diluted enzyme-linked secondary antibodies at RT for 3 h. The
immune blots were visualized using an enhanced chemiluminescence
(ECL) kit and Western Blotting Detection Reagents (GE Healthcare
Life Sciences). Each protein band was quantified using ImageJ
software (http://rsb.info.nih.gov) followed by
densitometry reading, undertaken after normalization by β-actin
expression.
Inhibitor assay
To explore the effect of PI3K as upstream targets of
PI3K/Akt/mTOR signaling pathway on Naringin induced autophagy in
AGS cell growth inhibition, 10 μmol/ LY294002 (a PI3K specific
inhibitor) were pre-treated for 2 h prior to the addition of 2 mM
Naringin followed by 24-h incubation. The protein expression was
analyzed by immunoblotting as described above against the p-PI3K
and LC3B antibodies.
Statistical analysis
The obtained results were expressed as the mean ±
standard deviation (SD) of a minimum three replicates in
independent experiments. The data were analyzed by unpaired, two
tailed Student's t-test using SPSS version 10.0 for Windows (SPSS,
Chicago, IL, USA). The p-value of <0.05 and <0.01 was
considered statistically significant.
Results
Effect of Naringin on AGS cell
proliferation
In order to assess the potential anti-proliferative
activity of Naringin on AGS cancer cells, MTT assay was conducted.
The anti-proliferative effect of Naringin on AGS cancer cells were
examined dose- and time-dependently. It has been observed that
treatment with different doses of Naringin (0–3 mM) or vehicle
alone at two time points (24 and 48 h) exhibited significantly
decreased cell proliferation (Fig.
1B). Besides, >50% cell growth inhibition was observed at 3
mM dose within 24-h, but the opted effective concentration (EC) of
Naringin was 2 mM based on the morphological observation of treated
AGS cells with visible increased vacuolization. Moreover, the cell
growth was efficiently attenuated dose- and time-dependently
followed by 59.8, 54.8 and 49.5% in 24 h and 57.4, 52.5 and 41.02%
in 48-h durations respectively at subsequent doses of Naringin.
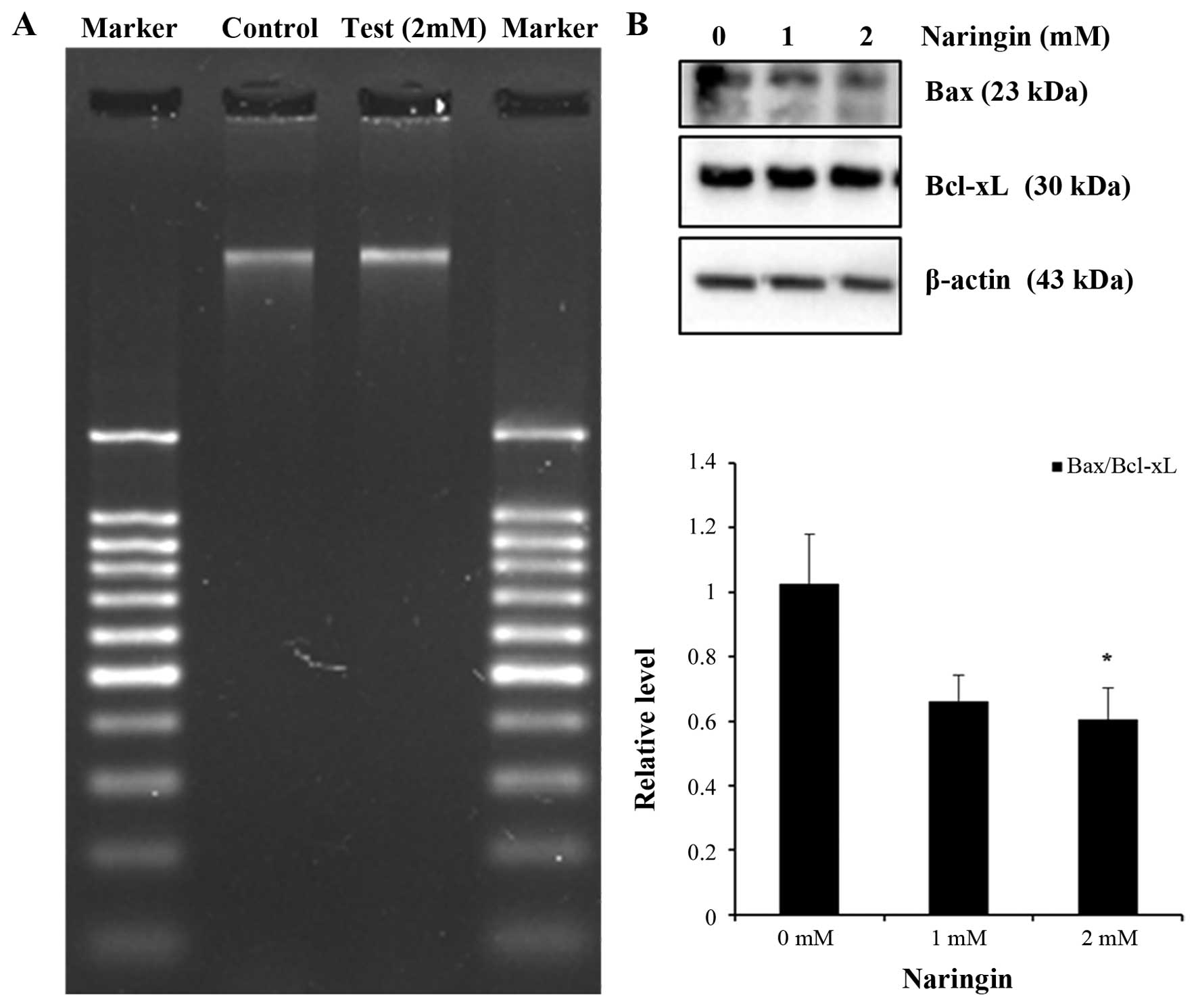
Naringin attenuates AGS cancer cell
growth: non apoptotic cell death
DNA fragmentation has been considered as hallmark
for apoptotic cell death which proceeds before the onset of
morphological changes during apoptosis. On the contrary, Naringin
attenuated AGS cell growth, investigation was conducted to confirm
the induction of apoptotic cell death by 1.5% agarose gel
electrophoresis analysis. DNA ladder assay presented no apparent
DNA inter-nucleosomal fragmentation in the 2 mM Naringin-treated
AGS cells for 24 h compared with control (Fig. 2A), suggesting the cell death
occurrence was not due to apoptosis. Further confirmation was done
to observe the potential role of apoptosis related proteins in
Naringin-treated cells. Western blot analysis for Bax and Bcl-xL
(Bax/Bcl-xL) relative ratio revealed a gradual decreasing trend in
Naringin-treated AGS cells (Fig.
2B). Thus, collectively these results supported non-apoptotic
cell death in the Naringin-treated AGS cell line.
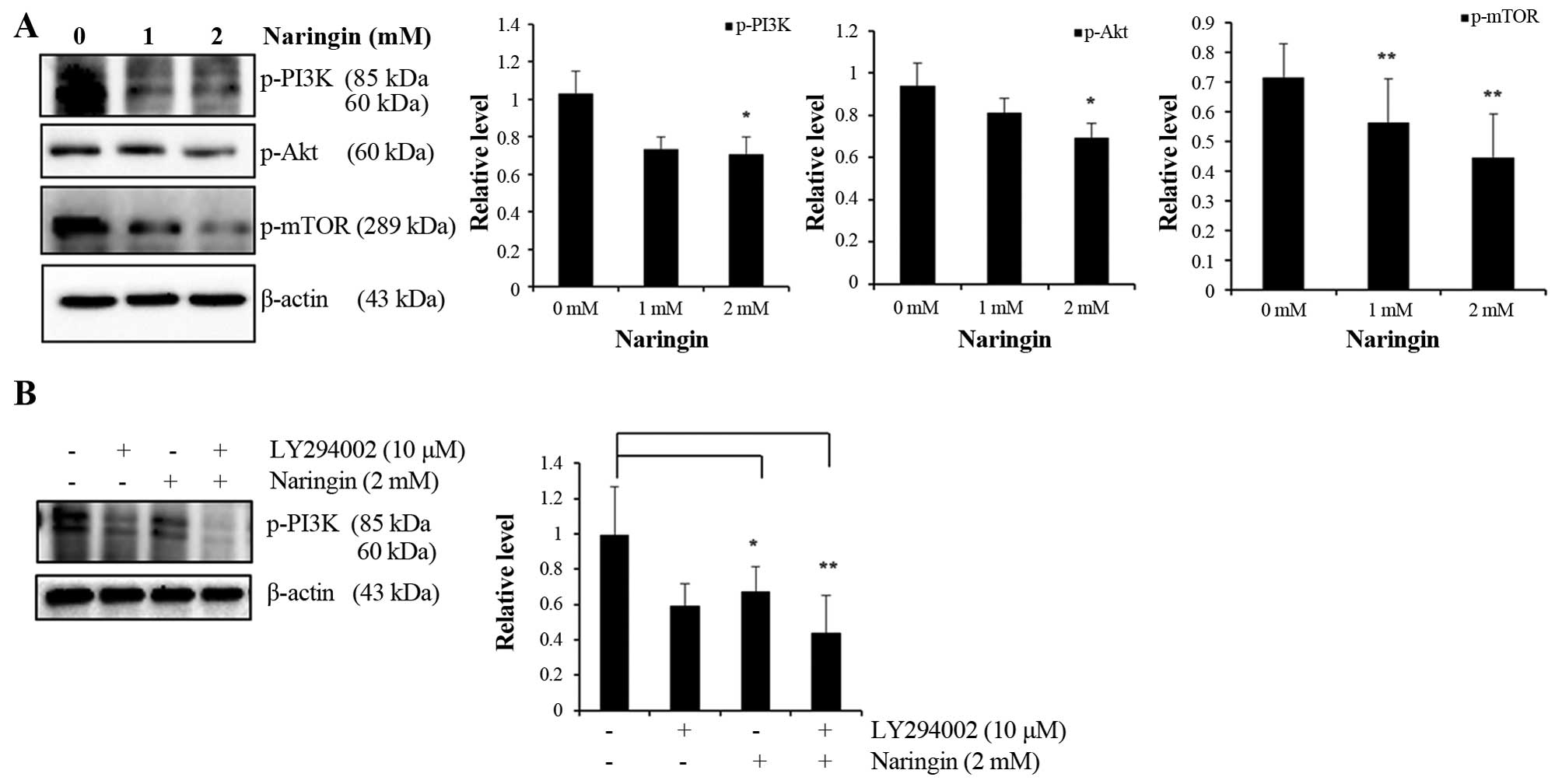
Naringin downregulates the expression of
PI3K/Akt/mTOR cascades
In human cancer cells, activated Akt and mTOR
stimulates cell growth through activation of PI3K. The present
experimental results determined that phosphorylation of PI3K and
its activated downstream targets p-Akt and p-mTOR are significantly
decreased at 2 mM in Naringin-treated AGS cells, observed by
immunoblot analysis (Fig. 3A). To
further validate the effect of Naringin on cell growth inhibition
via PI3K pathway, pre-treatment of AGS cells with 10 μM of LY294002
as described earlier was done by western blot analysis (Fig. 3B). It was observed that
pre-treatment with LY294002 inhibited the expression of PI3K in
Naringin-treated cells. The above data represent the
anti-proliferative role of Naringin in AGS cancer cells.
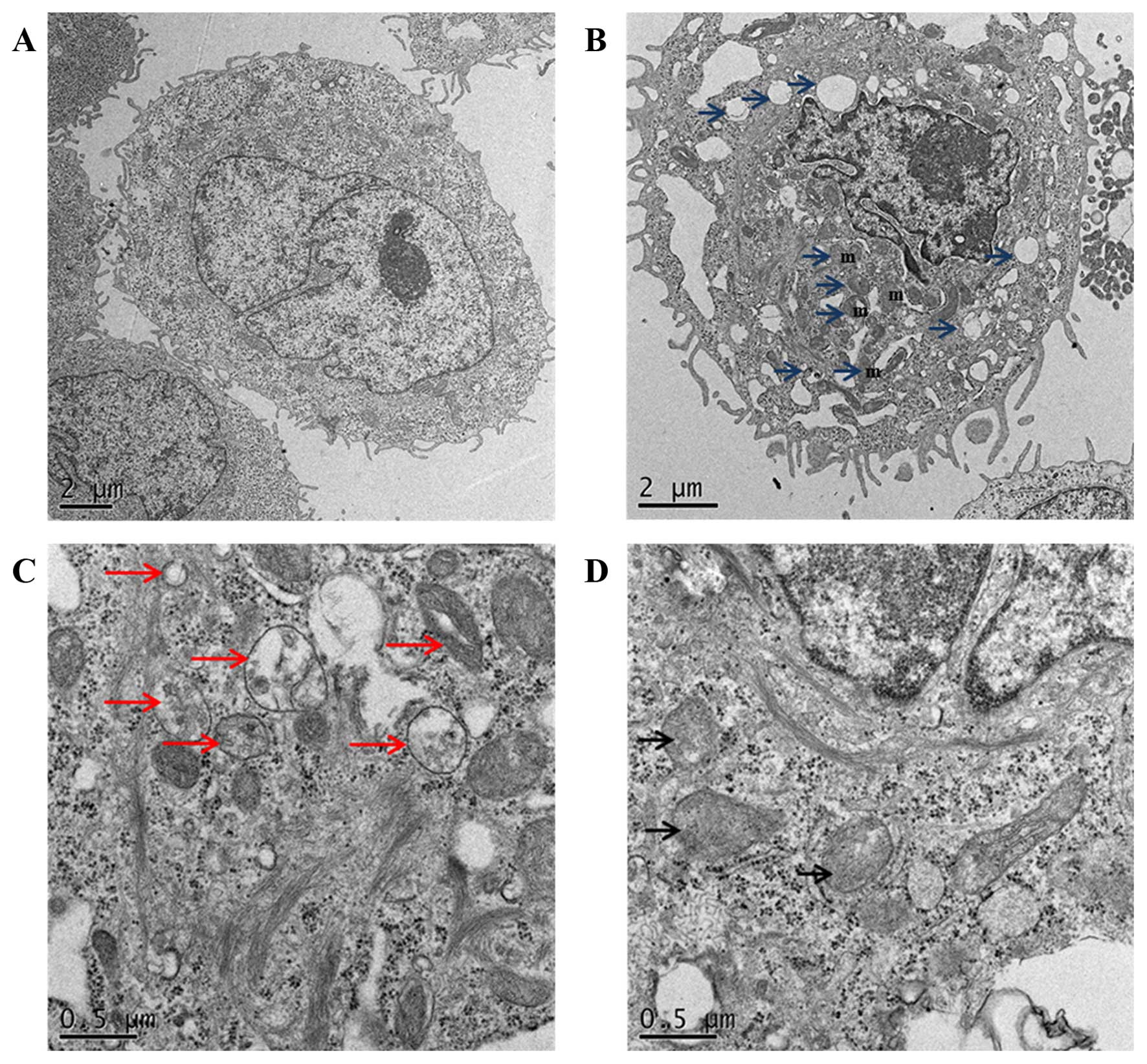
Naringin induces autophagosome
Electron microscopic investigation is still the most
reliable method for monitoring autophagic morphology (33). The TEM observation reports revealed
formation of double-membrane vesicles containing subcellular
materials, representing formation of phagophore in 2 mM
Naringin-treated cells when compared with the non-treated AGS cells
(Fig. 4) showing the vesicle
formation in 2 mM Naringin treated cells with damaged organelles,
such as swollen mitochondria/lysosomes surrounded by
double-membrane vacuoles, which further formed autophagosomes.
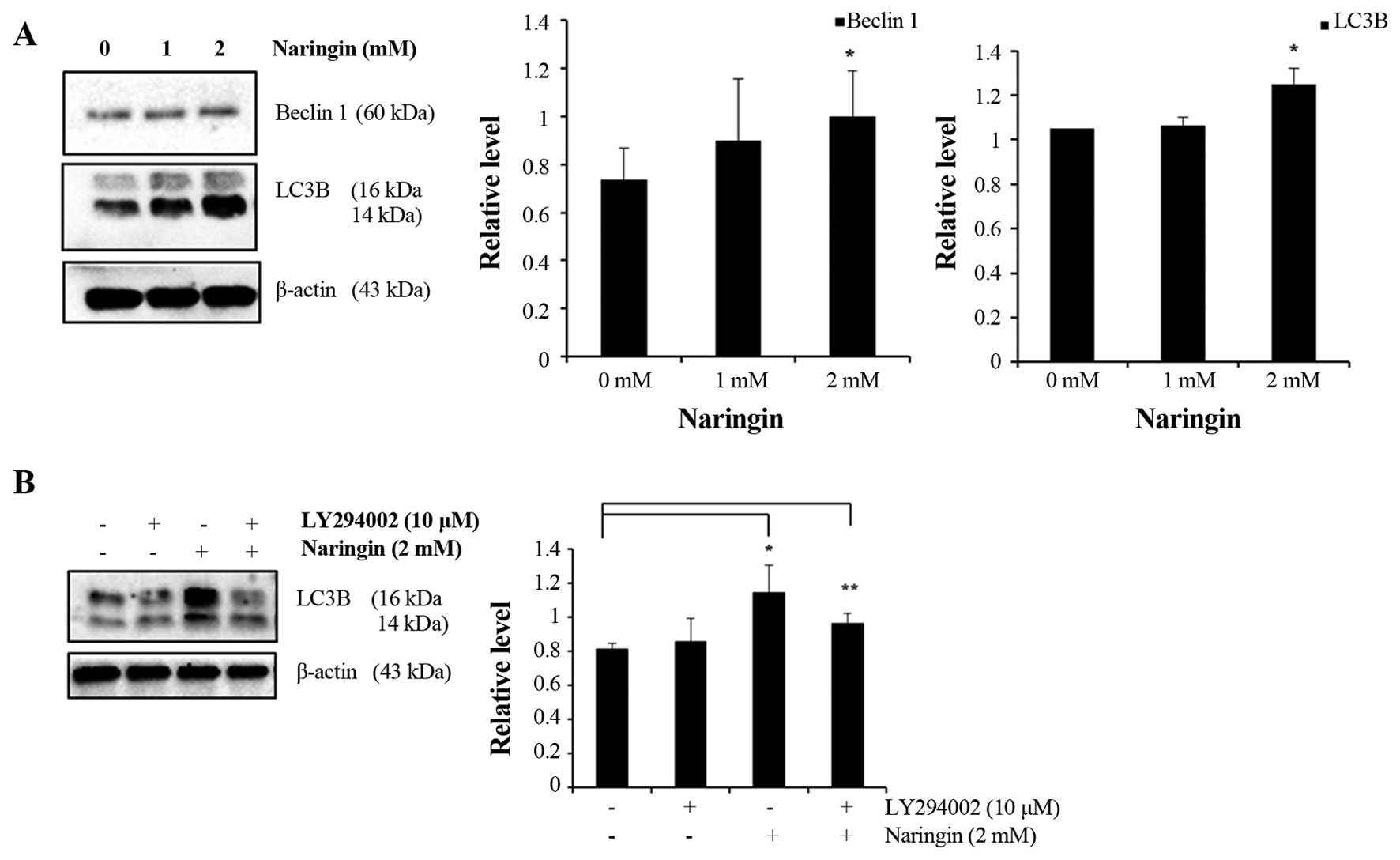
To further elucidate the molecular mechanisms that
underlie Naringin-induced autophagosome, examination was done to
assess the expression of vacuolar protein Beclin1 and
microtubule-associated protein light chain 3 (LC3) in AGS cancer
cells. The observed data represented a gradual increase of Beclin 1
protein, including an increased conversion of cytosolic LC3-I
protein to autophagic isoform LC3-II (LC3 II/LC3 I ratio) that was
significant at 2 mM Naringin-treated AGS cells compared with
control (Fig. 5A). In addition to
confirming the effect of Naringin on LC3B conversion, examination
was done with 10 μM of LY294002 pre-treatment. Western blot
analysis result (Fig. 5B) revealed
that the conversion of LC3B was inhibited in the presence of
LY294002. Taken together, these results indicated that Naringin
induces autophagy in AGS cancer cells.
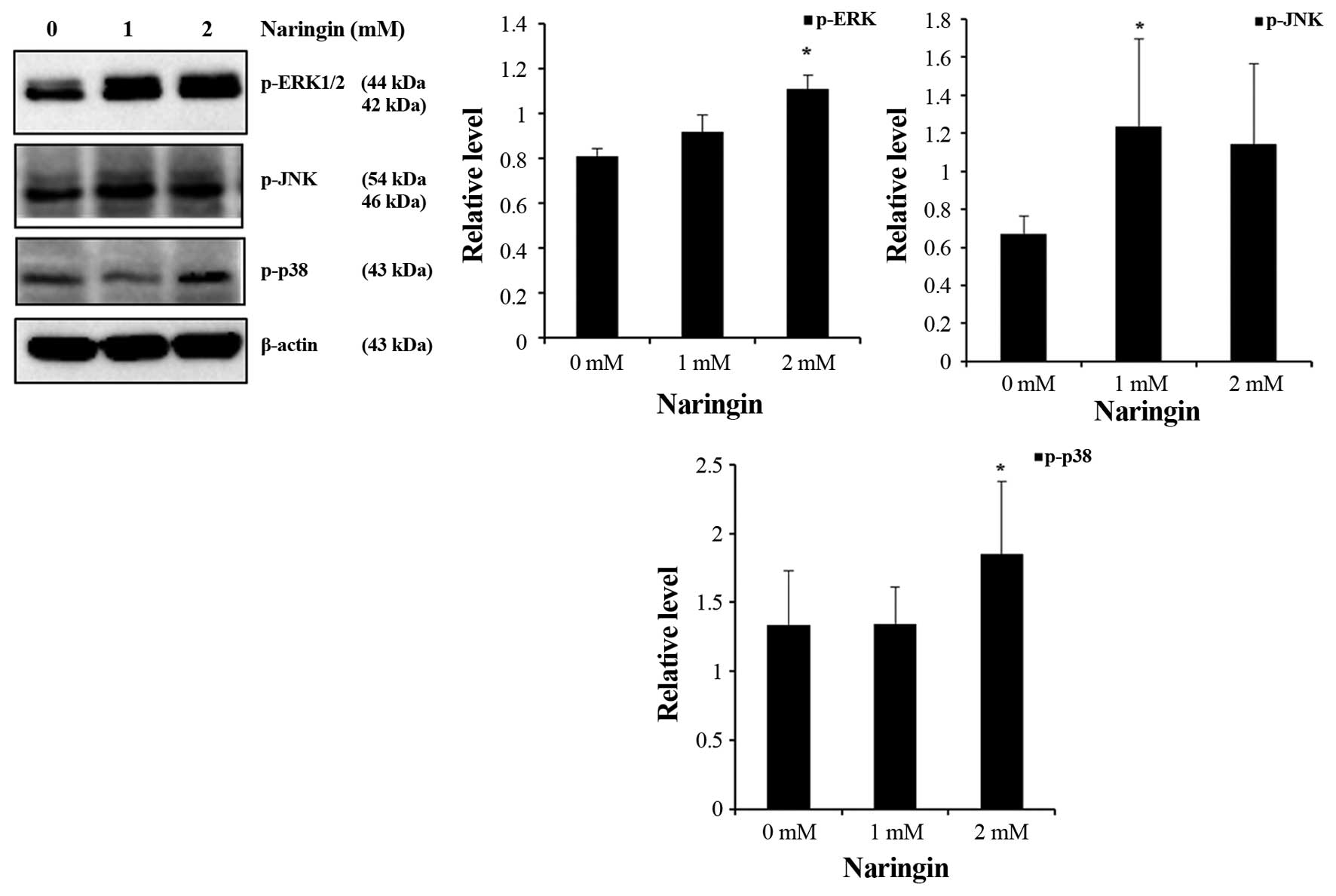
Activation of MAPK signaling pathways in
Naringin-treated AGS cells
MAPK signaling pathways play an important role in
cell growth inhibition and upregulation of autophagic protein
followed by autophagy-mediated cell death. To further investigate
the role of MAPK family proteins in Naringin-treated AGS cells
inducing autophagy, western blot analysis was done. As shown
(Fig. 6), Naringin induced
significant activation of p-ERK1/2, p-p38 at 2 mM and p-JNK at 1 mM
concentration in AGS cells during 24-h incubation. Taken together,
these results demonstrated that MAPK signaling pathways are
involved in Naringin-induced autophagic cell growth inhibition in
AGS cells.
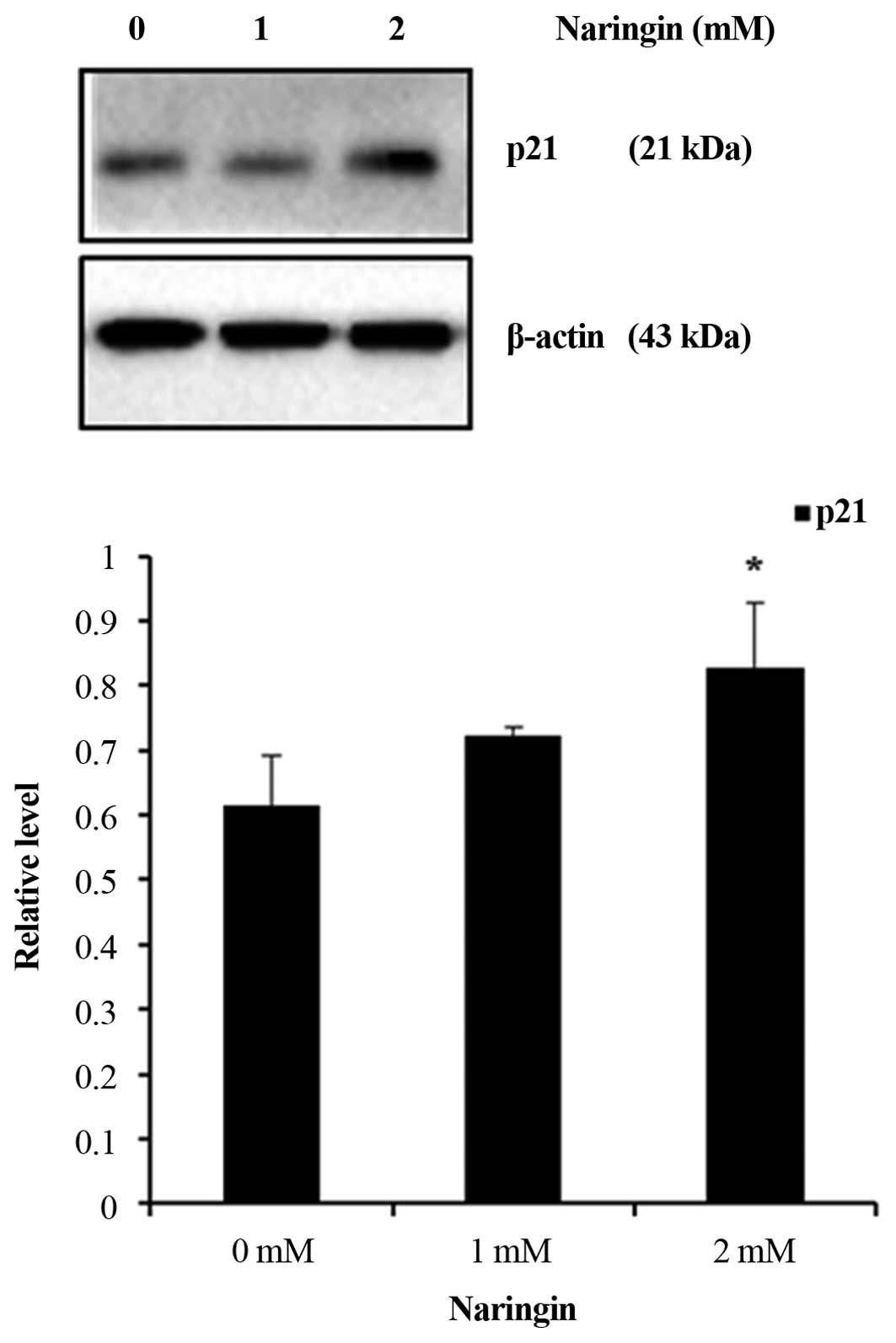
Upregulation of p21 plays a role in AGS
cell anti-proliferation
A marked overexpression of p21WAF1/CIP1
in Naringin-treated cancer cells inducing apoptotic cell death in
breast cancer and cell cycle arrest in bladder cell carcinoma have
been reported (34,35). To further assess the cell
anti-proliferative mechanism by p21WAF1/CIP1 protein,
expression study was determined by immunoblot analysis. The result
demonstrated a significant increase of p21WAF1/CIP1
expression at 2 mM in Naringin-treated AGS cells (Fig. 7). The observed data could be
correlated with the induction of autophagy depicting the role of
p21 in Naringin-inducing cell death in AGS cancer cells.
Discussion
The study of plant flavonoids as anticancer agents
has increased substantially, due in part to their profound effects
in cell death signaling pathways (36,37).
Flavonoids are a group of polyphenolic secondary metabolites with
diphenyl propane (C6C3C6) skeletons. Major classes of flavonoids
are anthocyanins, flavonols, flavanols and proanthocyanidins or
condensed tannins (38). Naringin,
as one of the most abundant flavonoids in citrus containing
anaglycone moiety named naringenin, linked to a dioside
neohesperidoside (39). It has
been implicated for its pharmacological values based on recent
reports on its ability to inhibit cell growth in breast cancer
cells through β-catenin pathway (34) or as anti-oxidant in mouse leukemia
P388 cells (40). In addition,
activation of Ras/Raf/ERK inducing G1-cell cycle arrest via
p21WAF1 has been observed in Naringin-treated bladder
carcinoma cells (35) or induction
of apoptosis through both death receptor and mitochondrial pathway
in cervical cancer (SiHa) cells (41). Herein, the present study revealed
that Naringin inhibited cell proliferation dose- and
time-dependently with an opted EC value of 2 mM at 24 h inducing
autophagy in human AGS cells, which suggested Naringin possesses a
potential anti-proliferative effect on AGS cancer cells.
Bcl-2 family has been reported as the best
characterized protein family, playing an important role in
regulation of apoptotic cell death (42). Previous reports of Naringin showed
induction of apoptosis in human cervical cancer cells, breast
cancer cells and mouse leukemia P388 cells (34,40,41).
In the present study, Naringin-treated AGS cells presented no DNA
fragmentation with a decreasing expression of pro-apoptotic protein
Bax and anti-apoptotic proteins Bcl-xL ratio. Similar reports
(11,13) on non-apoptotic cell death confirmed
that the effect of Naringin did not trigger apoptotic pathway in
AGS cells.
PI3K/Akt/mTOR cascades are the most frequently
deregulated and inappropriately activated cancer signaling pathway,
controlling cellular energy, cell growth, proliferation, senescence
and angiogenesis in cancer cells. Blocking different nodes of this
pathway is a relevant treatment strategy for human malignancies
(8). Several bioactive components
such as samsoeum (17),
polyphenols of Korean Lonicera japonica (43), araguspongine C (44), justicidin A (45), flavonoids including luteolin
(46), baicalein (47) possess anticancer activity by
suppressing PI3K/Akt/mTOR pathway in cancer cells. To date, mTOR is
the most well characterized negative regulator of autophagy in
cancer cells suggesting that decrease of autophagic activity is
related to tumorigenesis (8).
Similarly, the present study showed the potential involvement of
PI3K/Akt/mTOR signaling pathway in Naringin-treated AGS
cells, further confirmed by the PI3K specific inhibitor
LY294002.
Induction of autophagy in Naringin-treated AGS cells
evidencing the accumulation of biochemical hallmark proteins of
autophagy, Beclin 1 and LC3-II, known to play pivotal roles in the
formation of autophagosomes. The mammalian autophagy gene Beclin 1,
as part of the PI3K complexes, participates in the formation of
autophagic vesicles and localizing autophagic proteins (48). Elevation of Beclin 1 and the
conversion of LC3-I to LC3-II correlates with the extent of
autophagosome formation, indicating LC3-II as the most widely used
biomarker of autophagosomes formation in tumor suppressor mechanism
(10). Similarly, the present
finding supports Beclin1 and LC3-II activation in a dose-dependent
manner, as evidenced by immunoblotting. In addition, the conserved
positive role of Class III PI3K in the autophagic process, 3-MA as
a PI3K inhibitor, has been reported as a specific autophagy
inhibitor of the conversion of LC3 expression (49). Similarly, the present study also
suggested that PI3K inhibitor LY294002, which blocked the LC3B
conversion, confirms that Naringin induces autophagic cell death in
AGS cancer cells.
MAPK kinases play an integral role in the inception
and execution of autophagy. It leads to phosphorylation-dependent
activation of other kinases and transcription factors. The
best-studied MAP kinases are ERK, p38 and JNK. While ERK is
activated in response to proliferative signals, p38 and JNK are
activated in response to various stresses (19). Activation of ERK1/2 during growth
inhibition and apoptosis have been observed in cancer cells
(18,35), while activation of JNK is required
for the upregulation of Beclin 1 triggering autophagy- mediated
cell death (14,17). Subsequently, p38 MAPK activation
has been addressed during autophagic cell death (50). Therefore, the observed result
indicated that Naringin-induced autophagy in AGS cells is
associated with the activation of MAPK signaling pathways.
p21 gene has been widely studied as an antitumor
gene, regulated directly by p53 gene. In addition, p21 can bind to
proliferating cell nuclear antigen (PCNA) thereby blocking DNA
synthesis (51). It has been
reported (22) that increased
expression of p21CIPI/WAFI by Clozapine-treated lung
cancer cells in a time-dependent manner simultaneously increase the
number of autophagosomes, which correlate with the present study on
anti-proliferative effect of Naringin-inducing autophagosome in AGS
cancer cells.
The present findings clearly demonstrate that
anti-proliferative activity of Naringin-treated human AGS cancer
cells leads to induction of autophagy by suppressing PI3K/ Akt/mTOR
signaling pathway through activation of MAPKs. Further study will
be undertaken to explore the molecular mechanism of
autophagy-related growth inhibition and anticancer activities of
Naringin-treated AGS cancer cells. Therefore, induction of
autophagy or autophagic cell death by bioactive flavonoid Naringin
would play an important role as an anticancer therapeutic agent
enhancing the treatment responses for human gastric carcinoma.
Acknowledgements
This study was supported by a grant from the
National Research Foundation (NRF) of Korea funded by the Ministry
of Science (no. 2012M3A9B8019303), ICT & Future Planning (no.
2012R1A2A2A06045015) and National R&D Program for Cancer
Control, Ministry for Health, Welfare and Family Affairs, Republic
of Korea (no. 0820050).
References
|
1
|
McLean MH and El-Omar EM: Genetics of
gastric cancer. Nat Rev Gastroenterol Hepatol. 11:664–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization. GLOBOCAN 2012:
Estimated cancer incidence, mortality and prevalence worldwide in
2012. Available online: http://globocan.iarc.fr.
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
5
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie X, White EP and Mehnert JM: Coordinate
autophagy and mTOR pathway inhibition enhances cell death in
melanoma. PLoS One. 8:e550962013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meijer AJ and Codogno P: Regulation and
role of autophagy in mammalian cells. Int J Biochem Cell Biol.
36:2445–2462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De A, De A, Papasian C, Hentges S,
Banerjee S, Haque I and Banerjee SK: Emblica officinalis extract
induces autophagy and inhibits human ovarian cancer cell
proliferation, angiogenesis, growth of mouse xenograft tumors. PLoS
One. 8:e727482013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Law BY, Chan WK, Xu SW, Wang JR, Bai LP,
Liu L and Wong VK: Natural small-molecule enhancers of autophagy
induce autophagic cell death in apoptosis-defective cells. Sci Rep.
4:55102014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao W, Zhang X, Zhao W and Chen X:
Psoralidin induces autophagy through ROS generation which inhibits
the proliferation of human lung cancer A549 cells. Peer J.
2:e5552014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YJ, Chi CW, Su WC and Huang HL:
Lapatinib induces autophagic cell death and inhibits growth of
human hepatocellular carcinoma. Oncotarget. 5:4845–4854.
2014.PubMed/NCBI
|
|
16
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
17
|
Kim A, Yim NH and Ma JY: Samsoeum, a
traditional herbal medicine, elicits apoptotic and autophagic cell
death by inhibiting akt/mTOR and activating the JNK pathway in
cancer cells. BMC Complement Altern Med. 13:233,6882-13-233.
2013.
|
|
18
|
Lee JW, Kim KS, An HK, Kim CH, Moon HI and
Lee YC: Dendropanoxide induces autophagy through ERK1/2 activation
in MG-63 human osteosarcoma cells and autophagy inhibition enhances
dendropanoxide-induced apoptosis. PLoS One. 8:e836112013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sridharan S, Jain K and Basu A: Regulation
of autophagy by kinases. Cancers (Basel). 3:2630–2654. 2011.
View Article : Google Scholar
|
|
20
|
Capparelli C, Chiavarina B,
Whitaker-Menezes D, Pestell TG, Pestell RG, Hulit J, Andò S, Howell
A, Martinez-Outschoorn UE, Sotgia F, et al: CDK inhibitors
(p16/p19/p21) induce senescence and autophagy in cancer-associated
fibroblasts, ‘fueling' tumor growth via paracrine interactions,
without an increase in neo-angiogenesis. Cell Cycle. 11:3599–3610.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin YC, Lin CC, Chen TT, Chen JY, Tsai HJ,
Wang CY and Chen SY: Clozapine induces autophagic cell death in
non-small cell lung cancer cells. Cell Physiol Biochem. 35:945–956.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ekor M: The growing use of herbal
medicines: Issues relating to adverse reactions and challenges in
monitoring safety. Front Pharmacol. 4:1772014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park KI, Park HS, Nagappan A, Hong GE, Lee
H, Kang SR, Kim JA, Zhang J, Kim EH, Lee WS, et al: Induction of
the cell cycle arrest and apoptosis by flavonoids isolated from
Korean Citrus aurantium L. in non-small-cell lung cancer cells.
Food Chem. 135:2728–2735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delle Monache S, Sanità P, Trapasso E,
Ursino MR, Dugo P, Russo M, Ferlazzo N, Calapai G, Angelucci A and
Navarra M: Mechanisms underlying the anti-tumoral effects of Citrus
Bergamia juice. PLoS One. 8:e614842013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Camargo CA, Gomes-Marcondes MC, Wutzki NC
and Aoyama H: Naringin inhibits tumor growth and reduces
interleukin-6 and tumor necrosis factor α levels in rats with
Walker 256 carcinosarcoma. Anticancer Res. 32:129–133.
2012.PubMed/NCBI
|
|
27
|
Kumar A, Prakash A and Dogra S: Naringin
alleviates cognitive impairment, mitochondrial dysfunction and
oxidative stress induced by D-galactose in mice. Food Chem Toxicol.
48:626–632. 2010. View Article : Google Scholar
|
|
28
|
Lee EJ, Kim DI, Kim WJ and Moon SK:
Naringin inhibits matrix metalloproteinase-9 expression and AKT
phosphorylation in tumor necrosis factor-alpha-induced vascular
smooth muscle cells. Mol Nutr Food Res. 53:1582–1591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nie YC, Wu H, Li PB, Luo YL, Long K, Xie
LM, Shen JG and Su WW: Anti-inflammatory effects of naringin in
chronic pulmonary neutrophilic inflammation in cigarette
smoke-exposed rats. J Med Food. 15:894–900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanno S, Tomizawa A, Hiura T, Osanai Y,
Shouji A, Ujibe M, Ohtake T, Kimura K and Ishikawa M: Inhibitory
effects of naringenin on tumor growth in human cancer cell lines
and sarcoma S-180-implanted mice. Biol Pharm Bull. 28:527–530.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng L, Zhen Y, Chen Y, Zou L, Zhang Y, Hu
F, Feng J, Shen J and Wei B: Naringin inhibits growth and induces
apoptosis by a mechanism dependent on reduced activation of
NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int J
Oncol. 45:1929–1936. 2014.PubMed/NCBI
|
|
32
|
Rogakou EP, Nieves-Neira W, Boon C,
Pommier Y and Bonner WM: Initiation of DNA fragmentation during
apoptosis induces phosphorylation of H2AX histone at serine 139. J
Biol Chem. 275:9390–9395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H, Yang B, Huang J, Xiang T, Yin X, Wan
J, Luo F, Zhang L, Li H and Ren G: Naringin inhibits growth
potential of human triple-negative breast cancer cells by targeting
β-catenin signaling pathway. Toxicol Lett. 220:219–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim DI, Lee SJ, Lee SB, Park K, Kim WJ and
Moon SK: Requirement for Ras/Raf/ERK pathway in naringin-induced
G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis.
29:1701–1709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi MD, Liao YC, Shih YW and Tsai LY:
Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways
in HGF-treated liver cancer HepG2 cells. Phytomedicine. 20:743–752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yumnam S, Park HS, Kim MK, Nagappan A,
Hong GE, Lee HJ, Lee WS, Kim EH, Cho JH, Shin SC, et al: Hesperidin
induces paraptosis like cell death in hepatoblatoma, HepG2 cells:
Involvement of ERK1/2 MAPK. PLoS One. 9:e1013212014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chahar MK, Sharma N, Dobhal MP and Joshi
YC: Flavonoids: A versatile source of anticancer drugs. Pharmacogn
Rev. 5:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fuhr U and Kummert AL: The fate of
naringin in humans: A key to grapefruit juice-drug interactions?
Clin Pharmacol Ther. 58:365–373. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanno S, Shouji A, Asou K and Ishikawa M:
Effects of naringin on hydrogen peroxide-induced cytotoxicity and
apoptosis in P388 cells. J Pharmacol Sci. 92:166–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ramesh E and Alshatwi AA: Naringin induces
death receptor and mitochondria-mediated apoptosis in human
cervical cancer (SiHa) cells. Food Chem Toxicol. 51:97–105. 2013.
View Article : Google Scholar
|
|
42
|
Asnaghi L, Calastretti A, Bevilacqua A,
D'Agnano I, Gatti G, Canti G, Delia D, Capaccioli S and Nicolin A:
Bcl-2 phosphorylation and apoptosis activated by damaged
microtubules require mTOR and are regulated by Akt. Oncogene.
23:5781–5791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park HS, Park KI, Lee DH, Kang SR,
Nagappan A, Kim JA, Kim EH, Lee WS, Shin SC, Hah YS, et al:
Polyphenolic extract isolated from Korean Lonicera japonica Thunb.
induce G2/M cell cycle arrest and apoptosis in HepG2 cells:
Involvements of PI3K/Akt and MAPKs. Food Chem Toxicol.
50:2407–2416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akl MR, Ayoub NM, Ebrahim HY, Mohyeldin
MM, Orabi KY, Foudah AI and El Sayed KA: Araguspongine C induces
autophagic death in breast cancer cells through suppression of
c-Met and HER2 receptor tyrosine kinase signaling. Mar Drugs.
13:288–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Won SJ, Yen CH, Liu HS, Wu SY, Lan SH,
Jiang-Shieh YF, Lin CN and Su CL: Justicidin A-induced autophagy
flux enhances apoptosis of human colorectal cancer cells via class
III PI3K and Atg5 pathway. J Cell Physiol. 230:930–946. 2015.
View Article : Google Scholar
|
|
46
|
Lee WJ, Wu LF, Chen WK, Wang CJ and Tseng
TH: Inhibitory effect of luteolin on hepatocyte growth
factor/scatter factor-induced HepG2 cell invasion involving both
MAPK/ERKs and PI3K-Akt pathways. Chem Biol Interact. 160:123–133.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aryal P, Kim K, Park PH, Ham S, Cho J and
Song K: Baicalein induces autophagic cell death through AMPK/ULK1
activation and downregulation of mTORC1 complex components in human
cancer cells. FEBS J. 281:4644–4658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li X, Li X, Wang J, Ye Z and Li JC:
Oridonin up-regulates expression of P21 and induces autophagy and
apoptosis in human prostate cancer cells. Int J Biol Sci.
8:901–912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Oridonin induced autophagy in human cervical carcinoma
HeLa cells through Ras, JNK, and P38 regulation. J Pharmacol Sci.
105:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mohapatra P, Preet R, Das D, Satapathy SR,
Choudhuri T, Wyatt MD and Kundu CN: Quinacrine-mediated autophagy
and apoptosis in colon cancer cells is through a p53- and
p21-dependent mechanism. Oncol Res. 20:81–91. 2012. View Article : Google Scholar : PubMed/NCBI
|