Introduction
Ovarian cancer is a significant cause of morbidity
and mortality in women, and the leading cause of death of
gynecologic malignancies (1).
Despite the use of operation-pathology stage, cytoreductive
surgery, and new adjuvant chemotherapy, it is still associated with
high mortality largely due to limited early detection methods.
Serial combined testing for tumor markers including
carcinoembryonic antigen (CEA), CA125 and CA153 are utilized.
However, the lack of specificity and sensitivity of these cancer
markers have hampered their general application in cancer detection
and diagnosis (2–5). Therefore, it would be beneficial if
an ovarian-specific specific biomarker was found.
In the late 1980s, Qian et al prepared a
series of monoclonal antibodies specifically reacting with ovarian
cancer tissues (6). COC166-9 is
one of these antibodies and has relatively high specificity against
ovarian cancer. Its reaction rate with ovarian cancer tissues was
75.6% in immunohistochemistry (7).
Targeted therapy using COC166-9 conjugated to adriamycin-entrapped
liposome resulted in longer survival for nude mice bearing ovarian
cancer cell xenografts and ascites (8). Additionally, F(ab′)2 fragment of
COC166-9 was obtained by ficin digestion, which had better
immunoactivity and radioimmunoimaging than the intact monoclonal
antibody (9). The anti-idiotypic
antibody 6B11 against COC166-9 was also generated and humanized
(10). The sera tested by 6B11
presented 67.3% positivity of ovarian epithelial carcinoma, much
higher than that of the benign ovarian tumor or uterine tumors
(11). Besides, the recombinant
single-chain variable fragment (ScFv) of 6B11 has demonstrated
killing effects against ovarian cancer cells in vitro and
in vivo (12–14). Moreover, a study on identification
of antigen-specific T cell eptitopes in 6B11 found that the light
chain CDR3 peptide and heavy chain CDR3 peptide are the MHC class I
and class II epitopes of 6B11, respectively. The combination of MHC
class I and class II epitopes is more effective than 6B11 in
inducing specific cellular immune response against ovarian cancer
(15). However, the clinical
significance of the antigen recognized by COC166-9 (COC166-9-Ag,
here we name this antigen as CA166-9) was not evaluated and the
CA166-9 remained to be characterized.
In this study, we analyzed the expression profile of
CA166-9 in tumor tissues from 111 ovarian cancer patients with
>5-year follow-up. The prognostic value of CA166-9 for ovarian
cancer was also assessed. We further identified CA166-9 as human
immunoglobulin γ-1 heavy chain (IGHG1) constant region. By in
vitro assays, CA166-9 was shown to promote proliferation,
migration, and invasion of a subset of ovarian cancer cells.
Materials and methods
Patients and clinical samples
The collection of tissue samples was approved and
supervised by the Research Ethics Committee of Peking University
People's Hospital. Written informed consents were obtained from all
the patients prior to operation. A total of 111 patients who
underwent surgical resection of primary ovarian cancer between
January 2001 and December 2005 in Peking University People's
Hospital were investigated. Formalin-fixed and paraffin-embedded
specimens from these patients were collected. No patients received
any type of pre-surgical therapy. Histological classification and
clinic pathological staging were performed according to the TNM
classification of UICC.
Ovarian cancer cell lines and cell
culture
The ovarian carcinoma cell line CaOV3 and SKOV3
(ATCC, Manassas, VA, USA) were cultured in DMEM and RPMI-1640
medium plus 10% fetal calf serum (FCS), respectively. The ovarian
carcinoma cell line HOC1A and 3AO, derived from poorly
differentiated ovarian cancer surgical specimens, were cultured in
RPMI-1640 medium with 10% FCS. These cells were routinely cultured
at 37°C in a humidified atmosphere of 5% CO2. All
culture media and FCS were from Invitrogen (Carlsbad, CA, USA).
Immunohistochemistry and
immunocytochemistry
Archived, formalin-fixed, paraffin-embedded tissue
sections were obtained from Gynecologic Oncology Center, Peking
University People's Hospital. Four-μm thick tissue slides were
deparaffinized with xylene and rehydrated through a graded alcohol
series. Endogenous peroxidase activity was then blocked by
incubation in 3% hydrogen peroxide-methanol for 10 min. After
washing with phosphate-buffered saline, the slides were blocked
with 5% skim milk for 60 min and then incubated with monoclonal
antibody COC166-9 (2 μg/ml) overnight at 4°C. Antibody binding was
visualized by a standard streptavidin immunoperoxidase reaction,
followed by chromogen detection with diaminobenzidine for 10 min
and haematoxylin counterstaining. The results were judged by two
pathologists independently and any specimen with >10% positive
staining cells was classified as positive. Normal mouse IgG was
used as negative control. For immunocytochemistry, the human
ovarian cancer CAOV3, SKOV3, HOC1A and 3AO cells were plated onto
glass coverslips. After 24 h, the cells were fixed in 4%
paraformaldehyde for 10 min, and the cells were stained for
immunocytochemistry by following the procedures used for
immunohistochemistry.
Preparation of immunoaffinity column
using COC166-9 antibody
Purified COC166-9 (6 mg) was dialyzed against the
coupling buffer (0.1 M NaHCO3 pH 8.3, 0.5 M NaCl) for
two times. 0.7 g cyanogen bromide (CNBr) activated Sepharose 4B
(Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) was added to
the 1 mM HCl and incubated at room temperature for 15 min. After
the reaction, the supernatant was disposed quickly with a funnel
with a vacuum filtration. COC166-9, dissolved in the coupling
buffer (5 ml), was added to the slurry immediately when the
supernatant was removed, and incubated for 2 h at room temperature
with gentle rotating. After centrifugation at 1,000 rpm for 2 min
at 4°C, the immunoaffinity resin was washed again with coupling
buffer. Ethanolamine (10 ml) (1 M pH 8.0) was added to the resin
and incubated for 2 h at room temperature. The immunoaffinity resin
was packed into a 5-ml plastic mini-column. The column was washed
three times alternately with acetate washing buffer (0.1 M sodium
acetate pH 4.0, 0.5 M NaCl) and Tris washing buffer (0.1 M Tris-HCl
pH 8.0, 0.5 M NaCl). The wash-out was recovered to measure unbound
protein by detecting their absorbance at 280 nm for determination
of coupling efficiency. The immunoaffinity resin was washed with
PBS until the absorbance at 280 nm was <0.02. The column was
stored at 4°C in PBS containing 0.02% sodium azide.
Purification of CA166-9 from the ascites
of ovarian cancer patient by immunoaffinity column
chromatography
The ascites of ovarian cancer patient was obtained
from Gynecologic Oncology Center, Peking University People's
Hospital. The ascites was diluted with PBS and then filtered using
a MILLEX-HV filter (0.45 μm, Millipore, MA, USA) to remove
insoluble portions. The filtrate was loaded on the immunoaffinity
column and allowed to stand overnight at 4°C. It was washed with
PBS until the unbinding proteins disappeared by analyzing the
absorbance at 280 nm. The column was then eluted with glycine
buffer (0.1 M, pH 2.7), and protein concentration in individual
fractions (1 ml each) was determined by absorbance at 280 nm.
Western blotting
Equal amount of purified antigens were loaded in 12%
SDS-PAGE and electroblotted to a nitrocellulose membrane.
Non-specific binding was blocked with 5% non-fat milk in PBS for 1
h at room temperature. Then, the membrane was incubated with
COC166-9 (2 μg/ml) overnight at 4°C, rinsed with PBST five times,
followed by incubation with HRP-labeled goat anti-mouse IgG for 45
min. After five washes with PBST, the bands were developed with the
enhanced chemiluminescence (ECL) system (Amersham, Uppsala,
Sweden).
ELISA
The microplates were coated with 1:5,000 dilution of
ascites from ovarian cancer patients or 5 μg/ml human IgG in 0.1 M
of bicarbonate buffer (pH 9.5) then blocked with 5% skim milk in
PBS. COC166-9 (2 μg/ml) was added to each well. In ELISA inhibition
assay, 0.2, 0.5, 1, 2, and 5 μg/ml human IgG or purified antigen
protein were incubated with 2 μg/ml COC166-9 for 2 h at 4°C before
adding to the wells. Bound antibodies were detected using
HRP-conjugated anti-mouse IgG antibody (Zhong Shan Co., Beijing,
China) with a peroxidase substrate.
Peptide mass fingerprint by MALDI-TOF
MS
Protein bands were excised from coomassie blue
stained gel and transferred to microcentrifuge tubes. In gel
trypsin digestion was conducted. For MALDI-TOF MS analysis, 1 ml of
the peptide was mixed with 1 ml matrix solution [CHCA, saturated
solution in ACN: 0.1% TFA (1:1)] on the target plate, and analyzed
with a MALDI-TOF mass spectrometer (Autoflex; Bruker, Karlsruhe,
Germany) in reflector mode over a mass range of 1,000–3,000 Da. For
each sample, spectra at several different positions were combined
to generate a peptide mass finger-print (PMF) for database searches
for protein identification. The peptide mass fingerprints obtained
were searched against the MASCOT database.
Immunoprecipitation
To confirm that the antigen protein was human IgG,
immunoprecipitation with streptococcal protein G-conjugated beads
was performed. Ovarian cancer ascites were diluted 1,000-fold in
PBS, and incubated with 40 μl 25% protein G agarose slurry for 2 h
at 4°C. The protein G-conjugated beads were recovered by
centrifugation at 5,000 rpm at room temperature. The supernatant
was transferred to another tube. Proteins that bind to the beads
were then eluted with glycine buffer (0.1 M pH 2.7). Both the
supernatant and binding proteins were subjected to ELISA for
further evaluation.
Cloning and expression of IGHG1 constant
region
The total RNA was extracted from 5×106
CAOV3 cells with TRIzol reagent (Invitrogen). A CAOV3 cDNA library
was prepared by RT-PCR with Reverse Transcription kit (Promega,
Madison, WI, USA) according to the manufacturer's instructions. The
full length IGHG1 constant region cDNA was then obtained with the
CAOV3 cDNA library as template. Sense primer: 5′-CGC GGA TCC ATG
GGA CCG TCA GTC TTC CTC TT-3′; anti-sense primer: 5′-CCG CTC GAG
TCA TTT ACC CGG AGA CAG GGA G-3. The cDNA was inserted into the
pcDNA3 vector (Invitrogen) and pcDNA3-IGHG1 constant region
(pcDNA3-IGHG1cr) was transfected into CA166-9 negative ovarian
cancer 3AO cells by Lipofectimine 2000 (Invitrogen) according to
the manufacturer's instructions.
Generation of mouse polyclonal antibody
against CA166-9
Six- to 8-week-old BALB/c mice (Animal Center of the
Chinese Medical Academy, Beijing, China) were firstly immunized
subcutaneously with 50 μg purified antigen for each that was
emulsified in complete Freund's adjuvant (Sigma, St. Louis, MO,
USA). Mice were boosted four times with the antigen emulsified in
incomplete Freund's adjuvant every 3 weeks. Sera were taken on day
0 (preimmune) and 7 days after the third and fifth
immunization.
Cell proliferation assay
The MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay was used to assess proliferation of ovarian cancer cells.
Ovarian cancer cells were seeded in a 96-well culture plate at a
density of 2,000 cells per well in a volume of 100 μl. The cells
were allowed to adhere for 3 h at 37°C at 5% CO2, and 50
μl culture medium with or without purified IGHG1 or COC166-9 were
subsequently added. The concentrations of IGHG1 and COC166-9 were
20 and 10 μg/μl, respectively. The cells were cultured for 24, 48,
72 and 96 h. During the last 4 h of the assay, the culture was
pulsed with 0.5 mM MTT per well resulting in an insoluble purple
formazan product. The medium was aspirated and the precipitates
dissolved in 150 μl of dimethylsulfoxide (DMSO) buffered at pH
10.5. The absorbance was read at 492 nm using an enzyme-linked
immunosorbent assay (ELISA) plate reader. The results presented are
the mean of 3 separate experiments.
Cell migration and invasion assay
Cell migration assay was performed by using culture
medium-treated 6.5-mm Transwell chamber with 8.0-μm polycarbonate
membranes. The bottom chamber was filled with 800 μl medium
containing 10% FCS. Cells were harvested from tissue culture plates
by serum-free medium, and then were seeded onto the top chamber of
each Transwell at a density of 2×105–1×106
cells/ml per well (100 μl/chamber) in serum-free medium, and
subsequently, the antibody COC166-9 was added with a final
concentration of 10 μg/μl. Alternatively, purified IGHG1 was used
at 10 μg/μl. After incubation in a humidified incubator with 5%
CO2 at 37°C for 24–48 h, non-migratory cells were
scraped off from the top of the Transwell with a cotton swab. The
cells attached to the bottom side of the membrane were fixed by
methanol, stained with 5% crystal violet, and counted under a light
microscope. The results presented are the mean of 3–4 separate
experiments.
The invasion assay was similar to the migration
assay described above, except that the upper side of the membranes
was coated with a uniform thickness (2 mm) of 100 μg Matrigel for
60 min at room temperature before the cells were added.
Statistical analysis
A standard χ2 test was performed to
examine the association between CA166-9 expression and the
clinicopathologic parameters. Survival curves were estimated by the
Kaplan-Meier method and compared with the log-rank test. A
multivariate analysis was performed by using the Cox regression
model (a backward selection) to analysis whether a factor was an
independent predictor of DFS. Hazard ratios (HRs) with 95%
confidence intervals were estimated. A two-tailed P-value of
<0.05 was considered statistically significant. All statistical
analyses were performed with SPSS v13.0 software.
Results
CorrelationbetweenCA166-9expressionandclinicopathologic
parameters
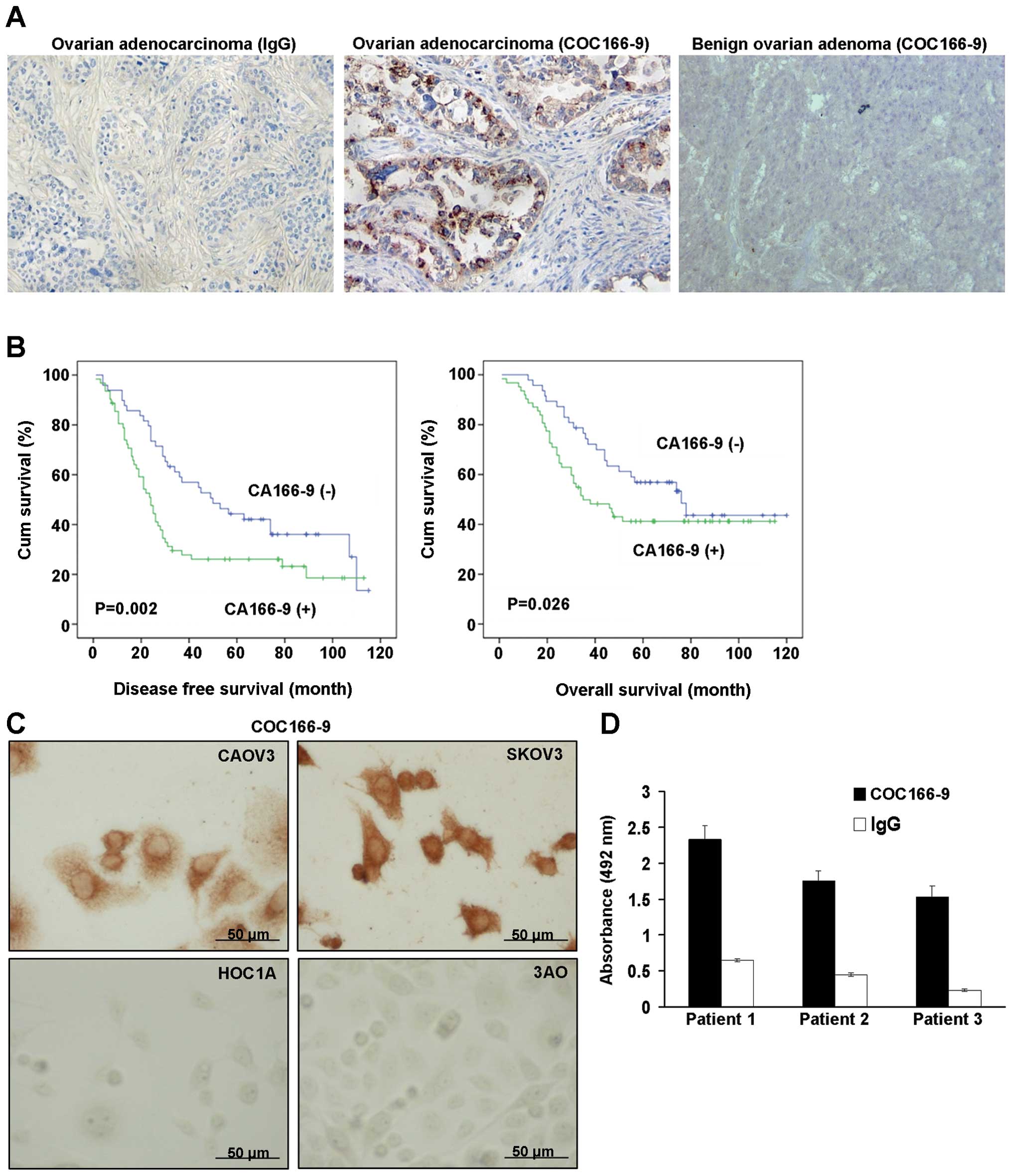
CA166-9 protein expression in tumor specimens of
ovarian cancer was determined by immunohistochemical staining
(IHC). CA166-9 protein localization was revealed as granulated loci
in ovarian adenocarcinoma (Fig.
1A). The staining was mainly localized in the cytoplasm and
loosely in the nucleus. There was no CA166-9 expression in benign
adenoma (Fig. 1A). High levels of
CA166-9 expression were found in 59 out of 111 neoplasms (53.1%).
Regarding the clinicopathologic parameters, we observed that
positive CA166-9 expression was closely associated with relapse
(χ2=5.316, P=0.021, Table
I). No significant correlation between CA166-9 status and other
clinicopathologic characteristics (e.g., age, clinical stage, tumor
size, or CA125) was found in this cohort (Table I).
 | Table ICorrelation of CA166-9 expression with
clinicopathologic factors. |
Table I
Correlation of CA166-9 expression with
clinicopathologic factors.
| | CA166-9 | | |
|---|
| |
| | |
|---|
| Classification | Cases (n) | Negative (%) | Positive (%) | χ2 | P-value |
|---|
| Age |
| <50 | 37 | 19 (51.4) | 18 (48.6) | 0.452 | 0.501 |
| ≥50 | 74 | 33 (44.6) | 41 (55.4) | | |
| Tumor size |
| <6 cm | 73 | 38 (52.1) | 35 (47.9) | 3.627 | 0.057 |
| ≥6 cm | 34 | 11 (32.4) | 23 (67.6) | | |
| FIGO stage |
| I/II | 38 | 17 (44.7) | 21 (55.3) | 0.103 | 0.748 |
| III/IV | 73 | 35 (47.9) | 38 (52.1) | | |
| CA125 |
| ≤35 | 7 | 3 (42.8) | 4 (57.2) | 1.169 | 0.280 |
| >35 | 104 | 46 (44.2) | 58 (55.8) | | |
| Relapse |
| Negative | 33 | 21 (63.6) | 12 (36.4) | 5.316 | 0.021 |
| Positive | 78 | 31 (39.7) | 47 (60.3) | | |
Relationship of CA166-9 expression with
the survival of ovarian cancer patients
As expected, stage and relapse were significantly
associated with clinical outcome (P<0.001, Table II). Patients with a high level of
CA166-9 expression also exhibited a trend towards shorter overall
survival (OS) and disease-free survival (DFS) compared to patients
with a low level of CA166-9 (OS, P=0.026; DFS, P=0.002, Fig. 1B and Table II). A multivariate analysis showed
that CA166-9 expression was an independent prognostic marker for OS
or DFS (OS: HR 2.454, P=0.016; DFS: HR 2.331, P=0.021, Table III).
 | Table IIUnivariate analysis of the prognostic
factors in ovarian cancer patients. |
Table II
Univariate analysis of the prognostic
factors in ovarian cancer patients.
| Overall survival
(OS) | Disease-free
survival (DFS) |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
| ≥50 vs <50 | 1.693 | 0.953–3.005 | 0.073 | 1.239 | 0.775–1.983 | 0.371 |
| Tumor size |
| ≥6 vs <6
cm | 1.748 | 1.184–2.579 | 0. 592 | 0.698 | 0.432–1.127 | 0.141 |
| Relapse |
| Positive vs
negative | 5.150 | 1.107–21.264 | <0.001 | 8.615 | 5.806–12.781 | <0.001 |
| CA125 |
| >35 vs ≤35 | 4.875 | 0.675–35.210 | 0.116 | 3.268 | 0.802–13.320 | 0.098 |
| FIGO stage |
| III/IV vs
I/II | 3.994 | 1.960–18.141 | <0.001 | 3.539 | 1.945–17.505 | <0.001 |
| COC166-9-Ag |
| Positive vs
negative | 1.504 | 0.890–2.542 | 0.026 | 1.778 | 1.129–2.799 | 0.002 |
 | Table IIIMultivariate analysis of the
prognostic factors in ovarian cancer patients. |
Table III
Multivariate analysis of the
prognostic factors in ovarian cancer patients.
| Overall survival
(OS) | Disease-free
survival (DFS) |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
| ≥50 vs <50 | 0.964 | 0.658–1.410 | 0.848 | 1.485 | 0.893–2.467 | 0.127 |
| Tumor size |
| ≥6 vs <6
cm | 1.284 | 0.706–2.336 | 0.043 | 1.740 | 0.437–1.253 | 0.023 |
| Relapse |
| Positive vs
negative | 7.808 | 2.326–26.210 | 0.001 | 7.137 | 10.105–84.151 | <0.001 |
| CA125 |
| >35 vs ≤35 | 0.345 | 0.046–2.567 | 0.299 | 0.872 | 0.199–3.824 | 0.856 |
| FIGO stage |
| III/IV vs
I/II | 2.026 | 0.874–4.695 | 0.010 | 2.886 | 0.934–3.810 | 0.007 |
| COC166-9-Ag |
| Positive vs
negative | 2.454 | 0.825–2.563 | 0.016 | 2.331 | 1.383–3.929 | 0.021 |
CA166-9 expression in ovarian cancer cell
and ovarian cancer ascites
Immunocytochemistry analysis with COC166-9
demonstrated that CA166-9 was detectable in the cytoplasm and
nuclei of ovarian cancer cells CAOV3 and SKOV3, whereas the HOC1A
and 3AO did not show any CA166-9 expression (Fig. 1C), indicating the expression of
CA166-9 is cell type-specific. CA166-9 was also found in the
ascites from three ovarian cancer patients by ELISA assay (Fig. 1D).
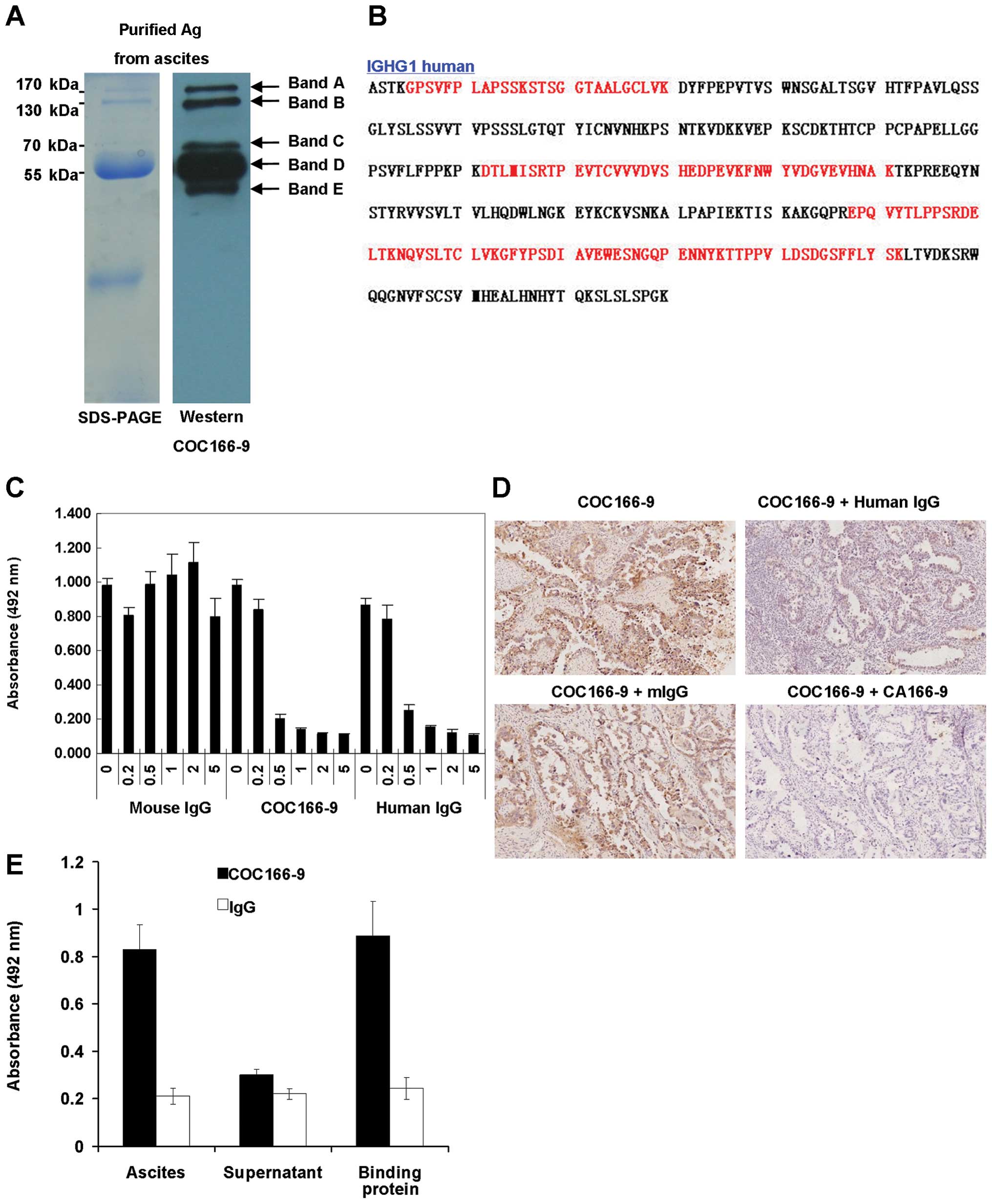
Identification of CA166-9 as IGHG1
constant region
To identify the CA166-9, ovarian cancer ascites were
subjected to the immunoaffinity column chromatography. The purified
antigens were resolved by SDS-PAGE (Fig. 2A, left) and probed with the
COC166-9 by western blotting (Fig.
2A, right). Five protein bands at 170, 130, 70 55 and 45 kDa
were successfully purified from the ovarian cancer ascites and were
named as CA166-9 band A, B, C, D, and E, respectively. By mass
spectrometry analysis, we obtained 13 peptide sequences from band
A, 23 peptide sequences from band B, 16 peptide sequences from band
C, 18 peptide sequences from band D, and 14 peptide sequences from
band E. The peptides were all matched to human immunoglobulin γ-1
heavy chain (IGHG1) constant region by basic local alignment search
tool (MASCOT) homology search (Fig.
2B). ELISA assay revealed that both purified CA166-9 and human
IgG could abolish COC166-9's reaction with ovarian cancer ascites
in a dose-dependent manner, however purified mouse IgG had no
inhibitory effect (Fig. 2C).
Similarly, results of immunohistochemistry inhibition analysis
demonstrated that staining of successive sections of ovarian cancer
specimens by COC166-9 was interfered by purified human IgG and
CA166-9, but not by mouse IgG (Fig.
2D). Since the streptococcal protein G binds to the Fc fragment
of IgG (16), it is reasonable to
assume that the putative CA166-9, IGHG1 constant region protein
could also associate with protein G. To confirm this predication,
immunoprecipitation was performed with protein G-conjugated beads
plus ascites solution from ovarian cancer patient. The ascites,
protein eluted from protein G-conjugated beads after incubated with
the ascites, and ascites after absorbed with the protein G were,
respectively, coated onto the microtiter plates for ELISA assay
with COC166-9. The results showed that COC166-9 had strong positive
reactivity with the ascites or eluted protein, but a negative
reactivity with the ascites after being absorbed with the protein G
(Fig. 2E). These results indicated
the antigen recognized by COC166-9 was IgG-like protein.
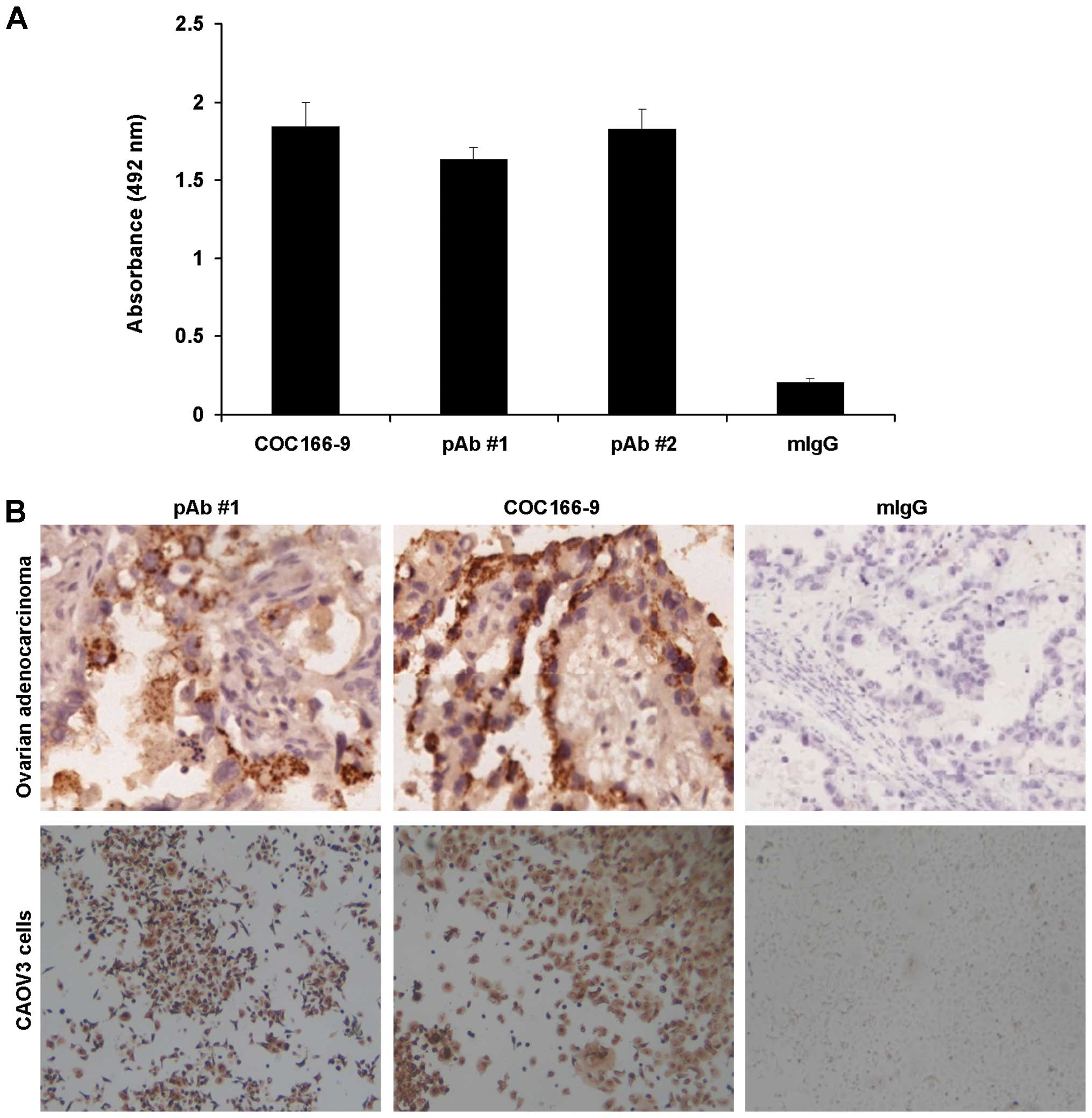
Mice immunized with purified antigen
produced polyclonal antibody against CA166-9
Two of 3 mice immunized with purified antigen
generated polyclonal antibody against CA166-9. The sera were
subjected to ELISA assay in which ovarian cancer ascites were
coated in microtiter plates. The results showed that the two
polyclonal antibodies, but not pre-immune mouse serum, could
recognize ovarian cancer ascites (Fig.
3A). To compare the immunoreactivity of prepared polyclonal
antibody against CA166-9 with CA166-9, immunocytochemistry and
immunohistochemistry analysis were performed. In all the five cases
of ovarian cancer tissues, similar expression patterns were
detected by the polyclonal anti-CA166-9 and monoclonal antibody
COC166-9 (Fig. 3B).
Immunocytochemical analysis of CA166-9 positive CAOV3 cells also
obtained similar expression pattern with these two antibodies, with
light or dark brown staining in the cytoplasm and the nuclei
(Fig. 3B). These results further
validated CA166-9 as IGHG1.
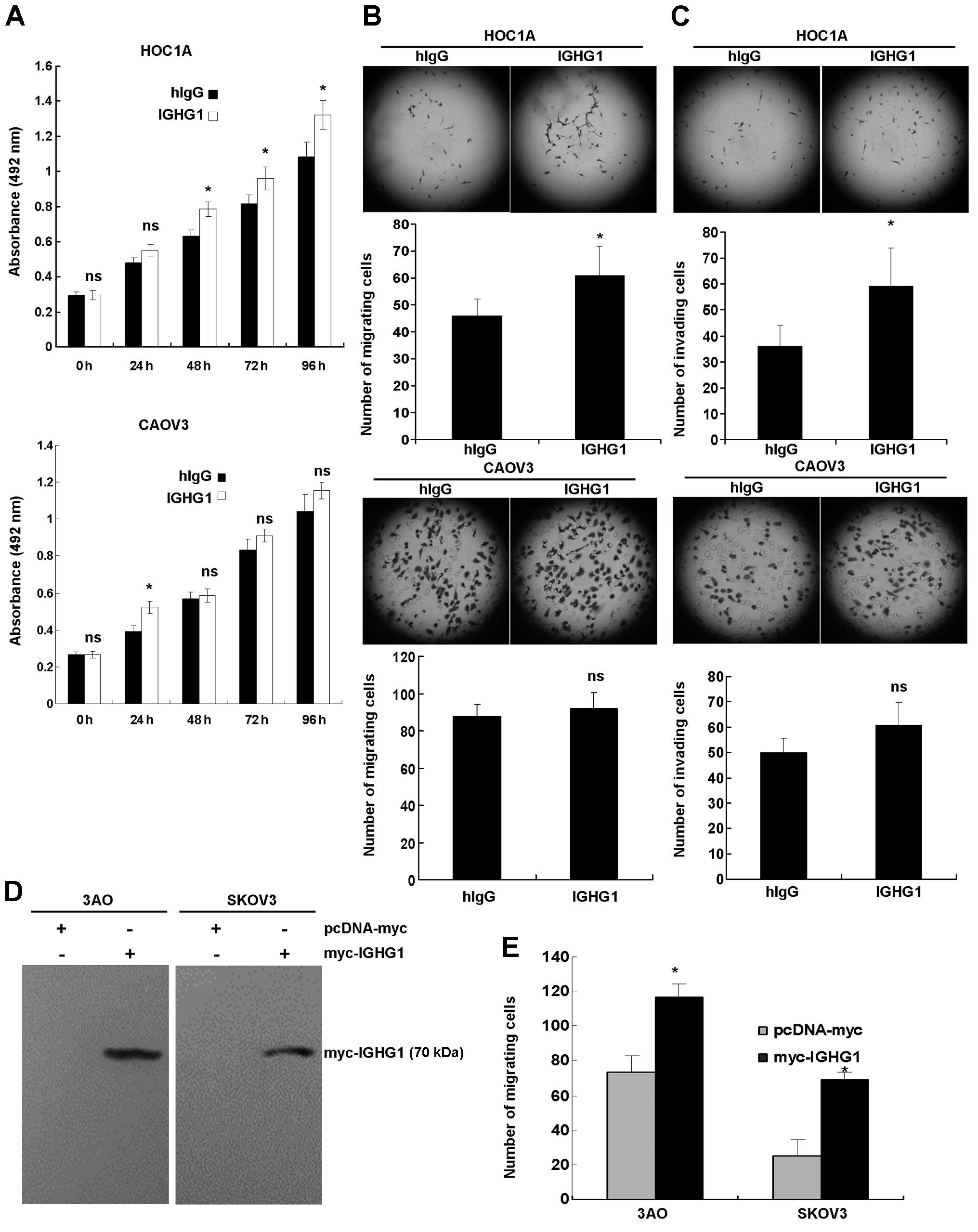
CA166-9 promotes ovarian cancer cell
proliferation, migration and invasion in vitro
To explore the biological function of CA166-9,
proliferation assay was conducted in the presence of purified
IGHG1. The results showed that purified IGHG1 significantly
promoted proliferation of CA166-9-negative HOC1A cells (P<0.05),
whereas it had minimal effects on the proliferation of CA166-9
positive CAOV3 cells (Fig. 4A). By
performing transwell chamber assays, we observed that IGHG1
elevated migration (Fig. 4B, upper
panel) and invasion (Fig. 4C,
upper panel) of HOC1A cells, but those of CAOV-3 were not affected
by IGHG1 (Fig. 4B and C, lower
panel). To better substantiate the above results, IGHG1 was cloned
and ectopically expressed in 3AO and SKOV3 cells (Fig. 4D). We found that exogenous IGHG1
markedly enhanced migration of these cells in the migration assay
(Fig. 4E). To examine the effects
of IGHG1 on non-cancerous cells, we treated human microvascular
endothelial cells (HMEC) with IGHG1, but no obvious changes in the
proliferation, migration, or invasion were found (data not
shown).
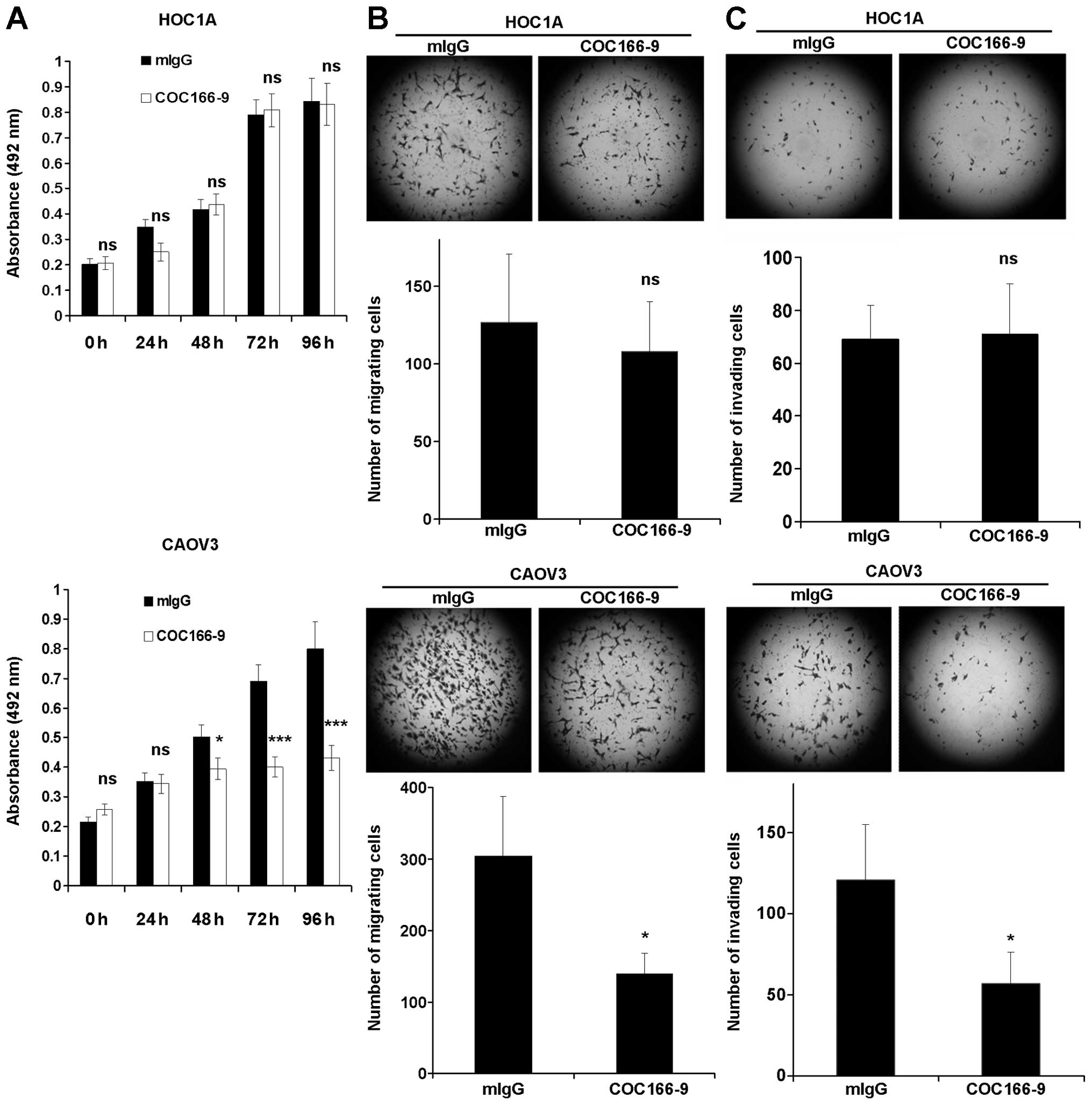
Antibody COC166-9 decreases ovarian
cancer cell proliferation, migration and invasion in vitro
Since IGHG1 promoted proliferation, migration and
invasion of a subset of ovarian cancer cells, it is likely that
IGHG1 has oncogenic functions in the tumor development. We thus
explored whether COC166-9 has any therapeutical effects. It was
shown that COC166-9 decreased the proliferation, migration, and
invasion of CAOV3 cells (Fig.
5A–C, lower panels), but had no effect on those of HOC1A cells
(Fig. 5A–C, upper panels) and HMEC
(data not shown). These results also suggest that the impact of
COC166-9 on ovarian cancer cell is CA166-9-specific.
Discussion
COC166-9, a monoclonal antibody raised against human
epithelial ovarian adenocarcinoma, was potential for
immunodiagnosis and immunotherapy (7). However, the general application of
this antibody had been precluded in the last two decades because
its antigen, CA166-9, remained to be identified. In this study,
firstly we evaluated prognostic value of CA166-9 expression in
ovarian cancer. Our study revealed CA166-9 as an independent
prognostic marker for patients with ovarian cancer. Although
CA166-9 status had no correlation with some clinicopathologic
characteristics, we did find that CA166-9 was closely associated
with relapse. This could be explained by the results of functional
analysis showing CA166-9's stimulatory effect on the proliferation,
migration, and invasion of a subset of ovarian cancer cells.
Therefore, CA166-9 may play some oncogenic roles in the progression
of ovarian cancer cells. Importantly, antibody COC166-9 has a
capacity of decreasing the proliferation, migration, and invasion
of ovarian cancer cells in a CA166-9-dependent manner, further
highlighting the therapeutic potential of this antibody.
Our results identified CA166-9 as IGHG1 constant
region. Experiments including immunohistochemistry inhibition
analysis and immunoprecipitation with streptococcal protein G
proved that the CA166-9 was IgG-like protein. It is the first time
that IGHG1 constant region, part of immunoglobulin, is demonstrated
to be the tumor antigen of a monoclonal antibody. However, since
the mass spectra we obtained after trypsin digestion did not show
any information of the immunoglobulin heavy chain variable region,
we could not determine the intact human immunoglobulin heavy chain
sequence. Moreover, as the epitope recognized by COC166-9 appears
to be confined within the constant region of IGHG1, it is deducible
that different kinds of immunoglobulin heavy chains sharing the
same IGHG1 constant region could be recognized by COC166-9, which
explains why the antigen purified from ovarian cancer ascites
migrated as five distinct bands.
Traditionally, it was believed that the only source
of immunoglobulins (Igs) is mature B lymphocytes and plasma cells.
However, some groups reported that Ig could also be detected in
non-lymphoid cells, including epithelial cancer cells and
proliferating epithelial cells. In 1998, Kimoto detected gene
transcripts of the Ig heavy chain constant regions of IgG1, IgG3,
IgA, IgD, IgE and IgM in five carcinoma cell lines by using nested
RT-PCR (17). By employing cDNA
microarray, a human immunoglobulin heavy chain constant region was
found in hepatocellular carcinoma total RNA (18). Ig heavy chain protein could also be
detected by two-dimensional electrophoresis in human nasopharyngeal
carcinoma cell line (19). In
2003, IgG expression was reported in ovarian cancer CaOV3 cells by
FACS analysis (20). In the same
study, mRNA of IgG heavy chain was also identified in breast,
colon, liver, and lung cancer cells and corresponding human cancers
of epithelial origin (20).
Immunohistochemistry analysis showed this IgG was localized in the
cytoplasm or on the plasma membrane of these cells (20), in accordance with COC166-9 staining
pattern in ovarian cancer cells and tissues observed in this study.
In 2006, immunoglobulin heavy chain (IgH) gene transcripts was
detected by nested RT-PCR in four well-defined breast cancer cell
lines (21). Additionally, IgG
protein expression was found in breast cancer, colorectal cancer,
prostate cancer, and soft tissue tumors (22–25).
Regardless of the reports of Ig expression in
epithelial cancer cells, the literature on its biological function
remains limited. By using either antisense oligodeoxynucleotide or
anti-human IgG antibody, it was found that blockade of
tumor-derived IgG significantly increased programmed cell death and
inhibited cancer cells growth in vitro (20). In addition, IgG produced by cancer
cells had some unidentified capacity to promote the growth and
survival of tumor cells (20).
Treatment of human colorectal cancer cell line LoVo with anti-human
IgG resulted in decreased proliferation (23). Similarly, small interfering
RNA-mediated silencing of cancerous Ig inhibited cell proliferation
in vitro and suppressed xenograft growth in nude mice
(26). These results support the
concept that cancer-derived Ig may function as a growth factor for
cancer cells, in line with our study using purified CA166-9.
Although the exact mechanism of Igs-induced tumor growth is
unclear, it has been hypothesized that Igs may block target
epitopes on the cancer cells (27). Hellstrom et al suggested
that the ability of lymphocytes to eliminate their targets may be
compromised in vivo by serum factors that protect the
neoplastic cells specifically (akin to enhancing antibodies) or
non-specifically (28). However,
these hypotheses could not explain the inhibitory effect of cancer
cell-derived Igs observed in the in vitro studies, thus it
will be interesting to find the receptors of Igs on cancer cells
and delineate the signaling events downstream of the engagement of
Igs with cancer cells.
Acknowledgements
This study was supported by the Capital Medical
Developing Foundation (2007–2052), the National Natural Science
Foundation of China (30872750).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Memarzadeh S, Lee SB, Berek JS and
Farias-Eisner R: CA125 levels are a weak predictor of optimal
cytoreductive surgery in patients with advanced epithelial ovarian
cancer. Int J Gynecol Cancer. 13:120–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bast RC Jr, Xu FJ, Yu YH, Barnhill S,
Zhang Z and Mills GB: CA 125: The past and the future. Int J Biol
Markers. 13:179–187. 1998.
|
|
4
|
Bast RC Jr, Skates S, Lokshin A and Moore
RG: Differential diagnosis of a pelvic mass: Improved algorithms
and novel biomarkers. Int J Gynecol Cancer. 22(Suppl 1): S5–S8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salani R, Backes FJ, Fung MF, Holschneider
CH, Parker LP, Bristow RE and Goff BA: Posttreatment surveillance
and diagnosis of recurrence in women with gynecologic malignancies:
Society of Gynecologic Oncologists recommendations. Am J Obstet
Gynecol. 204:466–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian HN, Feng J, Cui H, Fu TY, Wei P and
Fu ZY: Generation and characterization of three monoclonal
antibodies to human ovarian epithelial adenocarcinomas. Chin Med J
(Engl). 102:839–843. 1989.
|
|
7
|
Qian HN: Immunohistological analysis of
monoclonal antibody COC166-9 against primary ovarian epithelial
cancer. Zhonghua Bing Li Xue Za Zhi. 17:207–209. 1988.(In Chinese).
PubMed/NCBI
|
|
8
|
Qian HN and Li WJ: Target therapy of
ovarian carcinoma by monoclonal antibodies bearing chemical drugs
entrapped in liposomes. Chin Med J (Engl). 106:343–347. 1993.
|
|
9
|
Li X, Qian H and Feng J: Preparation of
F(ab′)2 fragment of monoclonal antibody COC166-9 and its
experimental study of radioimmunoimaging for ovarian carcinoma.
Zhonghua Fu Chan Ke Za Zhi. 32:152–155. 1997.(In Chinese).
PubMed/NCBI
|
|
10
|
Chang X, Cui H, Feng J, Li Y, Liu B, Cao
S, Cheng Y and Qian H: Preparation of humanized ovarian carcinoma
anti-idiotypic minibody. Hybrid Hybridomics. 22:109–115. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng J, Lü J and Qian H: Serological
analysis of antibodies by anti-idiotypic monoclonal antibody 6B11
against ovarian carcinoma. Zhonghua Fu Chan Ke Za Zhi. 31:493–495.
1996.(In Chinese). PubMed/NCBI
|
|
12
|
Cui H, Chang XH, Liu B, Feng J, Li Y, Ye
X, Cao SJ, Fu TY, Yao Y, Li HQ, et al: The anti-tumor immune
responses induced by a fusion protein of ovarian carcinoma
anti-idiotypic antibody 6B11ScFv and murine GM-CSF in BALB/c mice.
Int J Gynecol Cancer. 14:234–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi J, Chang X, Feng J, Cheng Y, Cheng H,
Guo H, Ye X and Cui H: Expression of an ovarian cancer
anti-idiotype antibody (6B11VLVHCH3) in Chinese hamster ovary (CHO)
cells with improved immunoactivity and stability over proteins
expressed in prokaryotic cells. Hybridoma (Larchmt). 26:289–295.
2007. View Article : Google Scholar
|
|
14
|
Yang W, Feng J, Chang X, Fu T, Ye X, Zhang
H, Li X, Wen H, Feng L, Tong C, et al: Cytotoxic effects of T cells
induced by fusion protein 6B11-pulsed dendritic cells on ovarian
carcinoma cells. Gynecol Oncol. 105:238–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Cui H, Meng FQ, Chang XH, Zhang G,
Liu B and Li ZH: New T cell epitopes identified from an
anti-idiotypic antibody mimicking ovarian cancer associated
antigen. Cancer Immunol Immunother. 57:143–154. 2008. View Article : Google Scholar
|
|
16
|
Stone GC, Sjöbring U, Björck L, Sjöquist
J, Barber CV and Nardella FA: The Fc binding site for streptococcal
protein G is in the C gamma 2-C gamma 3 interface region of IgG and
is related to the sites that bind staphylococcal protein A and
human rheumatoid factors. J Immunol. 143:565–570. 1989.PubMed/NCBI
|
|
17
|
Kimoto Y: Expression of heavy-chain
constant region of immunoglobulin and T-cell receptor gene
transcripts in human non-hematopoietic tumor cell lines. Genes
Chromosomes Cancer. 22:83–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okabe H, Satoh S, Kato T, Kitahara O,
Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y and Nakamura Y:
Genome-wide analysis of gene expression in human hepatocellular
carcinomas using cDNA microarray: Identification of genes involved
in viral carcinogenesis and tumor progression. Cancer Res.
61:2129–2137. 2001.PubMed/NCBI
|
|
19
|
Li J, Tan C, Xiang Q, Zhang X, Ma J, Wang
JR, Yang J, Li W, Shen SR, Liang S, et al: Proteomic detection of
changes in protein synthesis induced by NGX6 transfected in human
nasopharyngeal carcinoma cells. J Protein Chem. 20:265–271. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao
P, Li G, Lv P, Li Z, Sun X, et al: Human epithelial cancers secrete
immunoglobulin G with unidentified specificity to promote growth
and survival of tumor cells. Cancer Res. 63:6488–6495.
2003.PubMed/NCBI
|
|
21
|
Babbage G, Ottensmeier CH, Blaydes J,
Stevenson FK and Sahota SS: Immunoglobulin heavy chain locus events
and expression of activation-induced cytidine deaminase in
epithelial breast cancer cell lines. Cancer Res. 66:3996–4000.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma C, Wang Y, Zhang G, Chen Z, Qiu Y, Li
J, Luo J, Huang B, Jiang C, Huang G, et al: Immunoglobulin G
expression and its potential role in primary and metastatic breast
cancers. Curr Mol Med. 13:429–437. 2013.PubMed/NCBI
|
|
23
|
Niu N, Zhang J, Huang T, Sun Y, Chen Z, Yi
W, Korteweg C, Wang J and Gu J: IgG expression in human colorectal
cancer and its relationship to cancer cell behaviors. PLoS One.
7:e473622012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Chen Z, Niu N, Chang Q, Deng R,
Korteweg C and Gu J: IgG gene expression and its possible
significance in prostate cancers. Prostate. 72:690–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Z, Huang X, Ye J, Pan P, Cao Q, Yang
B, Li Z, Su M, Huang C and Gu J: Immunoglobulin G is present in a
wide variety of soft tissue tumors and correlates well with
proliferation markers and tumor grades. Cancer. 116:1953–1963.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li M, Zheng H, Duan Z, Liu H, Hu D, Bode
A, Dong Z and Cao Y: Promotion of cell proliferation and inhibition
of ADCC by cancerous immunoglobulin expressed in cancer cell lines.
Cell Mol Immunol. 9:54–61. 2012. View Article : Google Scholar
|
|
27
|
Manson LA: Anti-tumor immune responses of
the tumor-bearing host: The case for antibody-mediated immunologic
enhancement. Clin Immunol Immunopathol. 72:1–8. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hellström I, Hellström KE, Evans CA,
Heppner GH, Pierce GE and Yang JP: Serum-mediated protection of
neoplastic cells from inhibition by lymphocytes immune to their
tumor-specific antigens. Proc Natl Acad Sci USA. 62:362–368. 1969.
View Article : Google Scholar : PubMed/NCBI
|