Like several other cancers, early detection of
pancreatic cancer increases the probability of a better prognosis.
However, pancreatic cancer is difficult to detect and diagnose
because it does not exhibit any specific, detectable symptoms and
is hidden behind other large abdominal organs (5). Moreover, pancreatic cancer causes
symptoms that are characteristic of other common illnesses, such as
jaundice, back/abdominal pain, loss of appetite, weight loss and
fatigue (6). In addition to the
lack of unique symptoms, pancreatic cancer is highly invasive and
metastatic and highly resistant to chemotherapy (7,8). The
standard treatment options available for patients with pancreatic
cancer are surgery, radiation, chemotherapy [5-FU, gemcitabine and
cetuximab) (9)], chemoradiation,
and some marginally effective targeted therapies [inhibitors for
VEGF, EGFR (9,10) mTOR (11)]. Despite remarkable global research
efforts by clinicians and cancer scientists, pancreatic cancer
remains fatal. Even though the incidence of pancreatic cancer is
lower relative to other cancers, it is the fourth leading cause of
cancer-related death in the U.S. According to the Pancreatic Cancer
Action Network pancreatic cancer is expected to become the 2nd
leading cause of cancer-related death by the year 2020. Because
there is not an effective treatment for this deadly cancer
(12), there is an immediate need
to improve our understanding of pancreatic cancer. The better
understanding of mechanism of disease pathogenesis will facilitate
early detection and development of effective treatments. The focus
of the present review is to summarize current knowledge regarding
the roles of miRNAs in pancreatic cancer diagnosis, treatment and
prognosis.
The importance of small non-coding microRNA (miRNA)
molecules in the regulation of gene and protein expression was
discovered and documented in early 90s. During the last decade,
remarkable amount of research efforts have been dedicated towards
understanding the role of miRNA regulatory networks in mammalian
genetics and human diseases (12,13).
miRNAs are a class of post-transcriptional
regulators, which mainly repress expression of genes and proteins.
miRNAs form a part of the cellular regulatory network that governs
various important biological functions, such as cell growth,
proliferation, differentiation, development and apoptosis (14). In the recent past, miRNAs have
become a central focus in cancer research because many miRNAs
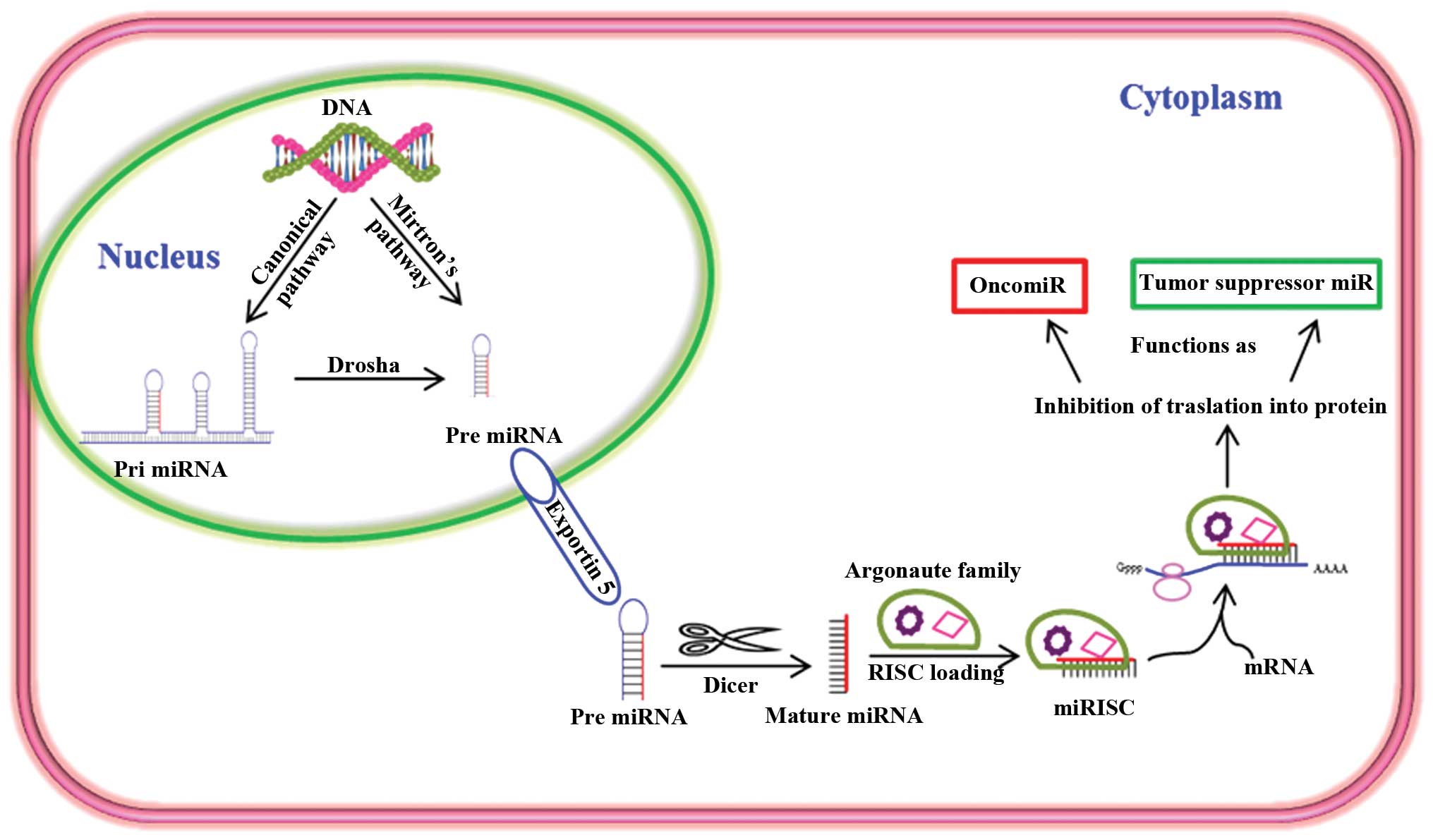
function as either tumor suppressors or oncogenes (Fig. 1). In addition, miRNAs are very
promising diagnostic and prognostic biomarkers for human diseases,
including pancreatic cancer. They are found in most biological
fluids [e.g., blood, amniotic fluid, breast milk, bronchial lavage,
cerebrospinal fluid (CSF), colostrum, peritoneal fluid, pleural
fluid, saliva, seminal fluid, tears and urine] (15) and are more stable than protein and
mRNA (16). Identification of
biomarkers in biological fluids is particularly attractive because
this represents a quick, non-invasive, and relatively inexpensive
method for detecting and diagnosing disease (17). Identification of a specific miRNA
profile in body fluids would be invaluable for early diagnosis,
therapy and prognosis of pancreatic cancer. Several miRNAs have
been shown to play a vital part in regulating pancreatic cancer by
influencing pancreatic cancer growth, progression, invasion,
metastasis and resistance to therapy (Table I) (18–28).
The miRNAs that are specifically involved in pancreatic cancer and
their target genes are shown in Table
II (29–39).
The overall sensitivity for the detection of
pancreatic cancer by established gastrointestinal tumor markers,
such as carcinoembryonic antigen (CEA) and CA 19-9 varies depending
on tumor grade. However, the sensitivity and specificity of CA 19-9
for accurate diagnosis of pancreatic cancer using serum is
debatable. Therefore, conventional serum antigen markers are
inappropriate tools for diagnosing pancreatic cancer (40,41).
There is a great need to develop better diagnostic markers for
pancreatic cancer. Some progress has been made towards delineating
the roles of miRNAs in the pathogenesis of pancreatic cancer. Blood
is one of the easiest and most preferable types of biofluid for
diagnosis and detection of most diseases. Plasma levels of miRNA-16
and miRNA196a in combination with CA 19-9 has been shown to work
very efficiently for screening early pancreatic cancer (85.2%)
(42). In addition, Wang et
al (43) studied the
expression levels of miR-21, miR-210, miR-155 and miR-196a in
plasma from PDAC patients in comparison to healthy individuals,
which showed that miR-155 overexpression was an early biomarker for
pancreatic neoplasia, while miR-196a expression correlated with the
progression of PDAC (43). In
another study, overexpression of miR-155 was found in 80% of early
pancreatic lesions (stage II) in microdissected panIN tissues
(44). In addition, blood samples
collected from pancreatic cancer patients had higher expression
levels of miR-200a, 200b and 210 (15). Furthermore, the combination of
miR-196a and miR-217 expression patterns differentiated PDAC from
healthy controls and chronic pancreatitis cases (45). Using a similar approach, another
group of researchers also observed much higher levels of
circulating miR-18a in the plasma of 36 pancreatic cancer patients
with compared to 30 healthy volunteers (46). These reports indicate the
importance of miRNAs as potential biomarkers for the diagnosis of
pancreatic cancer.
The widely used chemotherapeutic treatment for
pancreatic cancer is gemcitabine, which shows a moderate tumor
suppression response rate of ~12% (12). Therefore, the development of new
and improved therapies for the treatment of pancreatic cancer is
crucial. Clinical studies have demonstrated the efficacy of miRNA
as a therapeutic tool in the management of PDAC (12,47).
Tremendous efforts have been made in vitro and in
vivo using preclinical models of cancer, to inhibit oncogenic
miRNAs with antagomiRs (48,49).
AntagomiRs exhibit great potential as miRNA-based therapeutics for
cancer treatment. However, optimization and execution of
miRNA-based therapeutics lag behind other current therapies
(48,49). Additional research is needed in
order for miRNA-based therapy to become a standard anticancer
therapy. Based on current data, intense research efforts are
required to improve outcome for successful pancreatic cancer
treatment.
The role of potential target, hsa-miR-155, which is
upregulated in PDAC was reported to be a regulator of the putative
tumor suppressor SEL1L. According to this report, inhibition of
this aberrantly upregulated miRNA in human pancreatic ductal
adenocarcinoma would serve as a potential therapeutic strategy for
PDAC by increasing expression of SEL1L (50). In another report, the therapeutic
efficacy of miR- 34b was demonstrated using pancreatic tissues from
64 pancreatic cancer patients. miR-34b was shown to act as a tumor
metastasis suppressor through negative modulation of oncogenic
SMAD3 (51). Another miRNA with
possible therapeutic potential in pancreatic cancer is miR-142-3p.
Triptolide, a diterpene triepoxide isolated from the Chinese herb
Tripterygium wilfordii inhibits the proliferation of
pancreatic cancer cells by upregulating miR-142-3p which negatively
regulates HSP70 expression in PDAC cell lines (52). Similarly, Qazi et al
(9) showed that enforced
expression of miR-101, enhanced the expression of E-cadherin levels
and reduced the pancreatic tumor growth rate in SCID mouse
xenograft model. Thus, miR-101 has demonstrated a potential
therapeutic target of PDAC (9).
However, despite these promising studies, it is
important to consider that not all patients respond the same way to
a given anticancer therapy. Therefore, the best treatment approach
is the one in which the therapy can be tailored to each individual
patient. miRNAs provide the foundation for developing tailored and
targeted treatment strategies against pancreatic cancer, because
both specific miRNAs and antagomiRs can be identified easily and
quickly in blood or other bodily fluids to determine the best
treatment strategy. Thus, instead of concentrating on one miRNA or
antagomiR, a more effective approach would be a combination of
therapies, in which a panel of miRNAs/antagomiRs in conjunction
with chemotherapy is tailored to meet the needs of each
patient.
Global miRNA microarray profiling may discriminate
miRNA expression in normal vs. pancreatic cancer tissues and serve
as a potential prognostic predictor of disease. High expression of
miR-452, miR-105, miR-127, miR-518a-2, miR-187, and miR-30a-3p
correlated with increased survival rates of more than two years
(53). Notably, deregulated levels
of miRNAs, miR-21, miR-155, and miR-196a in plasma, and miR-141 in
the sera were observed in pancreatic cancer patients who had a poor
overall survival rate (54).
Furthermore, another study also reported that the levels of
miR-196a were shown to be elevated in sera of the PDAC patients
(55) in correlation with poor
survival and advanced disease stage (55,56).
In addition, it has been suggested that miR-196a expression is a
more specific indicator of PDAC progression (45). Overexpression of miR-196a-2 and
miR-219 are also associated with reduced survival. The median
survival for patients with miR-196a-2 overexpression was 14.3
months compared with 26.5 months for those with low expression
(57). The median survival for
those with miR-219 overexpression was 13.6 months compared with
23.8 for those with low expression (58).
Comparative analysis of 98 normal and 88 PDAC
patients revealed miR-21 and miR-155 as good biomarkers for
predicting tumor stage and prognosis (58). Low expression of miR-21 was
associated with increased survival. On the contrary, high
expression of miR-34a and miR-30d was correlated with increased
survival in patients who did not receive surgical tumor resection.
Patients who underwent surgery for PDAC are expected to have
reduced overall survival if they have high expression of miR-212
and miR-675 and low expression of miR-148a, miR-187 and let-7g
(59). Compared with ductal
epithelia from normal pancreatic tissues of organ donors,
expression of miR-21 was upregulated by >1000-fold in
microdissected pancreatic cancer tumors (60). Overexpression of miR-21 was
associated with worse pancreatic cancer progression in
gemcitabine-treated PDAC patients (60). miR-10b is another important miRNA
marker that could predict successful treatment response and
suitability for surgery in PDAC patients (56). In a study, 106 patient samples
examined for the levels of expression of miR-10b, which revealed
low levels of miR-10b in epithelial cells from benign lesions
compared to those in pancreatic cancer lesions. The authors
proposed that decreased miR-10b could improve the response to
multimodality neoadjuvant therapy (61,62).
Two independent sets of samples obtained from
patients with resected PDAC (with subsets of 19 and 60 patients)
were subjected to miRNA microarray analysis. The analysis confirmed
that miR-211 expression was the most predictive biomarker for
treatment outcome in PDAC patients with significant associations
with both overall survival and disease-free survival (63). Schultz and others (59) studied the expression levels of
miRNAs in tissue samples from 225 patients. The results strongly
suggested that miRNAs, miR-212 and miR-675 were upregulated and
miR-148a, miR-187 and let-7g were downregulated and these miRNAs
could function as independent predictors for reduced overall
survival in PDAC patients (59).
Thus, altered expression of miRNAs are strongly associated with
poor survival, decreased response to treatment, and increased
metastatic disease in PDAC (Table
III) (16,64–68).
Chemotherapy is the most common treatment option for
many cancers, including pancreatic cancer. However, it offers
little therapeutic value in many cases due to rapid development of
chemoresistance. Pancreatic cancer is a notoriously chemoresistant
cancer, which renders chemotherapy highly ineffective in a majority
of patients. Various miRNAs, notably oncomiR-21, miR-34a, miR-196a,
miR-221 and miR-214, have been implicated in the induction of
chemoresistance in PDAC (56,69).
miR-10b, miR-34a and members of the miR-200 family were reported to
have roles in gemcitabine chemoresistance. Upregulation of miR-21
was implicated in the induction of chemoresistance in PANC-1 cells
(70). In contrast, downregulation
of miR-21 increased the sensitivity of PANC-1 cells to gemcitabine
and indole-3-carbinol combination therapy. Similarly, miR-10b has
been shown to be responsible for chemoresistance in many tumor
types. Cancer stem cells (CSCs) are highly resistance to
chemotherapeutic drugs; miRNAs are shown to be involved in
developing resistance to chemotherapy by CSCs (71). In this regard, the roles of miRNAs
and stem cells in the development of PDAC chemoresistance need to
be examined. Pancreatic cancer stem cells sorted from the BxPC3
cell line exhibited much higher chemoresistance upon holoclone
formation, which coincided with high levels of miR-214, miR-21,
miR-221, miR-222 and miR-155 and lower levels of Let-7a, miR-30c,
miR-30b and miR-30a. Holoclone-forming stem cells may contribute to
pancreatic cancer chemoresistance. Therefore, the stem cell
holoclones represent a useful model for studying pancreatic cancer
chemoresistance and for designing treatment strategies to
effectively combat the development of chemoresistance (72).
During the past several decades, a great deal of
effort and resources have been utilized but only a few promising
advances have been made towards more effective prevention and
treatment strategies against pancreatic cancer. A major limitation
has been a lack of animal models that mimic the human disease.
Several chemotherapeutic drugs for pancreatic cancer may be highly
effective in two-dimensional (2D) in vitro cell culture
models, while many of these drugs fail to show the same effect in
clinical trials. A recent study by Longati et al (8) reported the use of three-dimensional
(3D) culture models, in which significant increases in chemo- and
radiation therapy resistance were observed in comparison with 2D
cell culture models. These 3D models were associated with increased
expression of matrix proteins, stromal markers, and various miRNAs,
which are known to be involved in the multidrug-resistant
phenotypes of many pancreatic cancers. Consequently, most drugs
tested in 3D cultures showed limited effects in contrast to 2D
cultures (8). Thus, the 3D culture
system is a more suitable model for predicting responsiveness to a
potential drug. Traditional cell culture-based assays and xenograft
models consume huge amounts of financial resources but do not
necessarily improve the clinical outcome of patients with PDAC.
Genetically engineered mouse models (GEMM) are suitable for
identifying early genetic alterations in pancreatic precursor
lesions and for studying early diagnosis and treatment. A major
advantage of using GEMM is the conditional strategy to activate or
inactivate expression of therapeutic or pathogenic genes,
respectively, temporarily in specific tissues. GEMM also reflect
intratumoral genetic heterogeneity, which is one of the hallmarks
of PDAC. A lack of intratumoral genetic heterogeneity in xenografts
and the cell lines may produce results that are not a true
representation of human pancreatic cancer (73). Another approach is a
patient-derived xenograft (PDX) model, a key model to translate the
effective miRNAs into clinics as potential biomarkers or
therapeutic targets for PDAC. PDX is increasingly used to
characterize and validate several diagnostic and therapeutic
strategies. The resemblance of intra and intertumoral genetic
heterogeneity along with complexity of the microenvironment of PDX
mimics more accurately the human PDAC. Direct representation of
parental tumor with realistic genetic integrity is an advantage of
PDX over the cell line-derived xenograft model. Another advantage
of clinically relevant PDX is the similar stromal compartments of
the parental tumor at the early passages. The key features of
orthotopic and subcutaneous PDX are accurate predictability of drug
response, identification of tumor specific treatment regimens, and
high correlation between pre-clinical and clinical outcome in terms
of relative sensitivity, resistance and therapeutic efficacy of the
drug with molecular insights (74,75).
Overall, PDX seems to be an appropriate and sufficient model to
recapitulate both genetic and morphological alterations that leads
to the development of PDAC. Together, these findings suggest that
advanced models are required to test and validate miRNAs that would
be effective in early diagnosis and development of suitable
treatment for pancreatic cancer.
Future research on the roles of miRNAs in the
pathogenesis of pancreatic cancer will unfold the mechanisms of
this deadly disease. Studies on the biogenesis of miRNAs will help
in understanding the regulatory machinery of the overall metabolic
state of the tumor. The identification of the key metabolic
switches that favor expression of a particular set of miRNAs should
help improve therapeutic odds of success in pancreatic cancer.
According to the current scenario, many key
oncogenic and tumor suppressor miRNAs and downstream molecular
targets have been identified in PDAC. Identification and targeting
of pancreatic cancer associated miRNA will have a tremendous impact
on non/minimally invasive diagnostics, that may open new doors to
improve the rate of curative outcome. The discovery of pancreatic
cancer related miRNA as a therapeutic agent is warranted to bring a
major breakthrough in many aspects of pancreatic cancer therapy.
The development of state-of-the art microRNA delivery methods may
increase the potential of miRNAs as therapeutic agents of choice in
pancreatic cancer. Future analysis of miRNAs in a larger patient
cohort is warranted to verify if this is a useful biomarker for
early detection, prognosis, and therapy in a majority of pancreatic
cancer patients.
Recent studies demonstrate the diverse and
significant roles of non-protein-coding genomic regions (miRNAs) in
pancreatic cancer progression and metastasis. Therefore, future
research efforts that focus on miRNAs must be just as vigorous as
the efforts made towards understanding the contributions of the
coding genome (individual genes). A great deal of research in this
field is required before we can effectively translate this into the
clinicals to treat pancreatic cancer. Understanding the roles of
miRNAs and tapping into the excellent potential of miRNAs will have
a great beneficial impact on the early detection, diagnosis,
treatment and prognosis of pancreatic cancer.
We thank Dr Rebecca Lopez-Valdez for her
professional editing of the manuscript.
|
1
|
Moniri MR, Dai LJ and Warnock GL: The
challenge of pancreatic cancer therapy and novel treatment strategy
using engineered mesenchymal stem cells. Cancer Gene Ther.
21:12–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michaud DS: Epidemiology of pancreatic
cancer. Minerva Chir. 59:99–111. 2004.PubMed/NCBI
|
|
4
|
Tamburrino A, Piro G, Carbone C, Tortora G
and Melisi D: Mechanisms of resistance to chemotherapeutic and
anti-angiogenic drugs as novel targets for pancreatic cancer
therapy. Front Pharmacol. 4:562013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hingorani SR, Petricoin EF, Maitra A,
Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD,
Hitt BA, et al: Preinvasive and invasive ductal pancreatic cancer
and its early detection in the mouse. Cancer Cell. 4:437–450. 2003.
View Article : Google Scholar
|
|
6
|
Burris HA 3rd, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997.PubMed/NCBI
|
|
7
|
Cheng H1, Shi S, Cai X, Long J, Xu J, Liu
C and Yu X: microRNA signature for human pancreatic cancer invasion
and metastasis. Exp Ther Med. 4:181–187. 2012.PubMed/NCBI
|
|
8
|
Longati P, Jia X, Eimer J, Wagman A, Witt
MR, Rehnmark S, Verbeke C, Toftgård R, Löhr M and Heuchel RL: 3D
pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant
phenotype offering a better model for drug testing. BMC Cancer.
13:952013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qazi AM1, Gruzdyn O, Semaan A, Seward S,
Chamala S, Dhulipala V, Sethi S, Ali-Fehmi R, Philip PA, Bouwman
DL, et al: Restoration of E-cadherin expression in pancreatic
ductal adenocarcinoma treated with microRNA-101. Surgery.
152:704–711; discussion 711–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borja-Cacho D, Jensen EH, Saluja AK,
Buchsbaum DJ and Vickers SM: Molecular targeted therapies for
pancreatic cancer. Am J Surg. 196:430–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai ZJ, Gao J, Kang HF, Ma YG, Ma XB, Lu
WF, Lin S, Ma HB, Wang XJ and Wu WY: Targeted inhibition of
mammalian target of rapamycin (mTOR) enhances radiosensitivity in
pancreatic carcinoma cells. Drug Des Devel Ther. 7:149–159. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Papaconstantinou IG, Lykoudis PM, Gazouli
M, Manta A, Polymeneas G and Voros D: A review on the role of
microRNA in biology, diagnosis, and treatment of pancreatic
adenocarcinoma. Pancreas. 41:671–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu C, Ping Y and Li X, Zhao H, Wang L, Fan
H, Xiao Y and Li X: Prioritizing candidate disease miRNAs by
integrating phenotype associations of multiple diseases with
matched miRNA and mRNA expression profiles. Mol Biosyst.
10:2800–2809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu C, Chen G and Cui Q: Towards the
understanding of microRNA and environmental factor interactions and
their relationships to human diseases. Sci Rep. 2:3182012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weber JA, Baxter DH, Zhang S, Huang DY,
Huang KH, Lee MJ, Galas DJ and Wang K: The microRNA spectrum in 12
body fluids. Clin Chem. 56:1733–1741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Humeau M, Torrisani J and Cordelier P:
miRNA in clinical practice: Pancreatic cancer. Clin Biochem.
46:933–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vlastos G and Verkooijen HM: Minimally
invasive approaches for diagnosis and treatment of early-stage
breast cancer. Oncologist. 12:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sicard F, Gayral M, Lulka H, Buscail L and
Cordelier P: Targeting miR-21 for the therapy of pancreatic cancer.
Mol Ther. 21:986–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai B, An Y, Lv N, Chen J, Tu M, Sun J, Wu
P, Wei J, Jiang K and Miao Y: miRNA-181b increases the sensitivity
of pancreatic ductal adenocarcinoma cells to gemcitabine in vitro
and in nude mice by targeting BCL-2. Oncol Rep. 29:1769–1776.
2013.PubMed/NCBI
|
|
20
|
Kawaguchi T, Komatsu S, Ichikawa D,
Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T,
Hirajima S, et al: Clinical impact of circulating miR-221 in plasma
of patients with pancreatic cancer. Br J Cancer. 108:361–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang WS, Liu LX, Li GP, Chen Y, Li CY, Jin
DY and Wang XL: Combined serum CA19-9 and miR-27a-3p in peripheral
blood mononuclear cells to diagnose pancreatic cancer. Cancer Prev
Res (Phila). 6:331–338. 2013. View Article : Google Scholar
|
|
22
|
Huang J, Egger M, Grizzle W and McNally L:
MicroRNA-100 regulates IGF1-receptor expression in metastatic
pancreatic cancer cells. Biotech Histochem. 88:397–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallardo E, Navarro A, Viñolas N, Marrades
RM, Diaz T, Gel B, Quera A, Bandres E, Garcia-Foncillas J and
Ramirez J: miR-34a as a prognostic marker of relapse in surgically
resected non-small-cell lung cancer. Carcinogenesis. 30:1903–1909.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang P, Chen L, Zhang J, Chen H, Fan J,
Wang K, Luo J, Chen Z, Meng Z and Liu L: Methylation-mediated
silencing of the miR-124 genes facilitates pancreatic cancer
progression and metastasis by targeting Rac1. Oncogene. 33:514–524.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM and
Chen J: The miR-217 microRNA functions as a potential tumor
suppressor in pancreatic ductal adenocarcinoma by targeting KRAS.
Carcinogenesis. 31:1726–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delpu Y, Lulka H, Sicard F, Saint-Laurent
N, Lopez F, Hanoun N, Buscail L, Cordelier P and Torrisani J: The
rescue of miR-148a expression in pancreatic cancer: An
inappropriate therapeutic tool. PLoS One. 8:e555132013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Druz A, Chen YC, Guha R, Betenbaugh M,
Martin SE and Shiloach J: Large-scale screening identifies a novel
microRNA, miR-15a-3p, which induces apoptosis in human cancer cell
lines. RNA Biol. 10:287–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou B, Jian Z, Chen S, Ou Y, Li S and Ou
J: Expression of miR-216a in pancreatic cancer and its clinical
significance. Nan Fang Yi Ke Da Xue Xue Bao. 32:1628–1631.
2012.PubMed/NCBI
|
|
29
|
Jiao LR, Frampton AE, Jacob J, Pellegrino
L, Krell J, Giamas G, Tsim N, Vlavianos P, Cohen P, Ahmad R, et al:
MicroRNAs targeting oncogenes are down-regulated in pancreatic
malignant transformation from benign tumors. PLoS One.
7:e320682012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Drakaki A and Iliopoulos D: MicroRNA-gene
signaling pathways in pancreatic cancer. Biomed J. 36:200–208.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J and Sen S: MicroRNA functional
network in pancreatic cancer: From biology to biomarkers of
disease. J Biosci. 36:481–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner
MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ and Schmittgen
TD: Expression profiling identifies microRNA signature in
pancreatic cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar
|
|
33
|
Bloomston M, Frankel WL, Petrocca F,
Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce
CM, et al: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Li M, Wang H, Fisher WE, Lin PH,
Yao Q and Chen C: Profiling of 95 microRNAs in pancreatic cancer
cell lines and surgical specimens by real-time PCR analysis. World
J Surg. 33:698–709. 2009. View Article : Google Scholar
|
|
35
|
Mees ST, Mardin WA, Wendel C, Baeumer N,
Willscher E, Senninger N, Schleicher C, Colombo-Benkmann M and
Haier J: EP300 - a miRNA-regulated metastasis suppressor gene in
ductal adenocarcinomas of the pancreas. Int J Cancer. 126:114–124.
2010. View Article : Google Scholar
|
|
36
|
Szafranska AE1, Davison TS, John J, Cannon
T, Sipos B, Maghnouj A, Labourier E and Hahn SA: MicroRNA
expression alterations are linked to tumorigenesis and
non-neoplastic processes in pancreatic ductal adenocarcinoma.
Oncogene. 26:4442–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szafranska AE1, Doleshal M, Edmunds HS,
Gordon S, Luttges J, Munding JB, Barth RJ Jr, Gutmann EJ,
Suriawinata AA, Marc Pipas J, Tannapfel A, et al: Analysis of
microRNAs in pancreatic fine-needle aspirates can classify benign
and malignant tissues. Clin Chem. 54:1716–1724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Cai X, Huang F, Zhong W and Yu Z:
Effect of trichostatin a on viability and microRNA expression in
human pancreatic cancer cell line BxPC-3. Exp Oncol. 30:265–268.
2008.PubMed/NCBI
|
|
39
|
Gironella M1, Seux M, Xie MJ, Cano C,
Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, et
al: Tumor protein 53-induced nuclear protein 1 expression is
repressed by miR-155, and its restoration inhibits pancreatic tumor
development. Proc Natl Acad Sci USA. 104:16170–16175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pawa N, Arulampalam T and Norton JD:
Screening for colorectal cancer: Established and emerging
modalities. Nat Rev Gastroenterol Hepatol. 8:711–722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee KJ, Yi SW, Chung MJ, Park SW, Song SY,
Chung JB and Park JY: Serum CA 19-9 and CEA levels as a prognostic
factor in pancreatic adenocarcinoma. Yonsei Med J. 54:643–649.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Gao J, Du Y, Li Z, Ren Y, Gu J,
Wang X, Gong Y, Wang W and Kong X: Combination of plasma microRNAs
with serum CA19-9 for early detection of pancreatic cancer. Int J
Cancer. 131:683–691. 2012. View Article : Google Scholar
|
|
43
|
Wang J, Chen J, Chang P, LeBlanc A, Li D,
Abbruzzesse JL, Frazier ML, Killary AM and Sen S: MicroRNAs in
plasma of pancreatic ductal adenocarcinoma patients as novel
blood-based biomarkers of disease. Cancer Prev Res (Phila).
2:807–813. 2009. View Article : Google Scholar
|
|
44
|
Ryu JK, Hong SM, Karikari CA, Hruban RH,
Goggins MG and Maitra A: Aberrant MicroRNA-155 expression is an
early event in the multistep progression of pancreatic
adenocarcinoma. Pancreatology. 10:66–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park JY, Helm J, Coppola D, Kim D, Malafa
M and Kim SJ: MicroRNAs in pancreatic ductal adenocarcinoma. World
J Gastroenterol. 17:817–827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Morimura R, Komatsu S, Ichikawa D,
Takeshita H, Tsujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H,
Okamoto K, et al: Novel diagnostic value of circulating miR-18a in
plasma of patients with pancreatic cancer. Br J Cancer.
105:1733–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Keklikoglou I, Hosaka K, Bender C, Bott A,
Koerner C, Mitra D, Will R, Woerner A, Muenstermann E, Wilhelm H,
et al: MicroRNA-206 functions as a pleiotropic modulator of cell
proliferation, invasion and lymphangiogenesis in pancreatic
adenocarcinoma by targeting ANXA2 and KRAS genes. Oncogene. Dec
15–2014, http://dx.doi.org/10.1038/onc.2014.408.
|
|
48
|
Song S and Ajani JA: The role of microRNAs
in cancers of the upper gastrointestinal tract. Nat Rev
Gastroenterol Hepatol. 10:109–118. 2013. View Article : Google Scholar
|
|
49
|
Pramanik D, Campbell NR, Karikari C,
Chivukula R, Kent OA, Mendell JT and Maitra A: Restitution of tumor
suppressor microRNAs using a systemic nanovector inhibits
pancreatic cancer growth in mice. Mol Cancer Ther. 10:1470–1480.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pramanik D, Campbell NR, Karikari C,
Chivukula R, Kent OA, Mendell JT and Maitra A: Putative tumor
suppressor gene SEL1L was downregulated by aberrantly upregulated
hsa-mir-155 in human pancreatic ductal adenocarcinoma. Mol
Carcinog. 53:711–712. 2013.
|
|
51
|
Liu C, Cheng H, Shi S, Cui X, Yang J, Chen
L, Cen P, Cai X, Lu Y, Wu C, et al: MicroRNA-34b inhibits
pancreatic cancer metastasis through repressing Smad3. Curr Mol
Med. 13:467–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
MacKenzie TN, Mujumdar N, Banerjee S,
Sangwan V, Sarver A, Vickers S, Subramanian S and Saluja AK:
Triptolide induces the expression of miR-142-3p: A negative
regulator of heat shock protein 70 and pancreatic cancer cell
proliferation. Mol Cancer Ther. 12:1266–1275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun T, Kong X, Du Y and Li Z: Aberrant
MicroRNAs in pancreatic cancer: Researches and clinical
implications. Gastroenterol Res Pract. 2014:3865612014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Di Leva G and Croce CM: miRNA profiling of
cancer. Curr Opin Genet Dev. 23:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kong X, Du Y, Wang G, Gao J, Gong Y, Li L,
Zhang Z, Zhu J, Jing Q, Qin Y and Li Z: Detection of differentially
expressed microRNAs in serum of pancreatic ductal adenocarcinoma
patients: miR-196a could be a potential marker for poor prognosis.
Dig Dis Sci. 56:602–609. 2011. View Article : Google Scholar
|
|
56
|
Frampton AE, Krell J, Jacob J, Stebbing J,
Jiao LR and Castellano L: microRNAs as markers of survival and
chemo-resistance in pancreatic ductal adenocarcinoma. Expert Rev
Anticancer Ther. 11:1837–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mardin WA and Mees ST: MicroRNAs: Novel
diagnostic and therapeutic tools for pancreatic ductal
adenocarcinoma? Ann Surg Oncol. 16:3183–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Papaconstantinou IG, Manta A, Gazouli M,
Lyberopoulou A, Lykoudis PM, Polymeneas G and Voros D: Expression
of microRNAs in patients with pancreatic cancer and its prognostic
significance. Pancreas. 42:67–71. 2013. View Article : Google Scholar
|
|
59
|
Schultz NA, Andersen KK, Roslind A,
Willenbrock H, Wojdemann M and Johansen JS: Prognostic microRNAs in
cancer tissue from patients operated for pancreatic cancer--five
microRNAs in a prognostic index. World J Surg. 36:2699–2707. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Giovannetti E, Funel N, Peters GJ, Del
Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A,
Falcone A, et al: MicroRNA-21 in pancreatic cancer: Correlation
with clinical outcome and pharmacologic aspects underlying its role
in the modulation of gemcitabine activity. Cancer Res.
70:4528–4538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bauer AS, Keller A, Costello E, Greenhalf
W, Bier M, Borries A, Beier M, Neoptolemos J, Büchler M, Werner J,
et al: Diagnosis of pancreatic ductal adenocarcinoma and chronic
pancreatitis by measurement of microRNA abundance in blood and
tissue. PLoS One. 7:e341512012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Costello E, Greenhalf W and Neoptolemos
JP: New biomarkers and targets in pancreatic cancer and their
application to treatment. Nat Rev Gastroenterol Hepatol. 9:435–444.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Giovannetti E1, van der Velde A, Funel N,
Vasile E, Perrone V, Leon LG, De Lio N, Avan A, Caponi S, Pollina
LE, et al: High-throughput microRNA (miRNAs) arrays unravel the
prognostic role of MiR-211 in pancreatic cancer. PLoS One.
7:e491452012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Preis M, Gardner TB, Gordon SR, Pipas JM,
Mackenzie TA, Klein EE, Longnecker DS, Gutmann EJ, Sempere LF and
Korc M: MicroRNA-10b expression correlates with response to
neoadjuvant therapy and survival in pancreatic ductal
adenocarcinoma. Clin Cancer Res. 17:5812–5821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ali S, Ahmad A, Banerjee S, Padhye S,
Dominiak K, Schaffert JM, Wang Z, Philip PA and Sarkar FH:
Gemcitabine sensitivity can be induced in pancreatic cancer cells
through modulation of miR-200 and miR-21 expression by curcumin or
its analogue CDF. Cancer Res. 70:3606–3617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu J1, Ohuchida K, Mizumoto K, Sato N,
Kayashima T, Fujita H, Nakata K and Tanaka M: MicroRNA,
hsa-miR-200c, is an independent prognostic factor in pancreatic
cancer and its upregulation inhibits pancreatic cancer invasion but
increases cell proliferation. Mol Cancer. 9:1692010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ohuchida K, Mizumoto K, Kayashima T,
Fujita H, Moriyama T, Ohtsuka T, Ueda J, Nagai E, Hashizume M and
Tanaka M: MicroRNA expression as a predictive marker for
gemcitabine response after surgical resection of pancreatic cancer.
Ann Surg Oncol. 18:2381–2387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Greither T, Grochola LF, Udelnow A,
Lautenschlager C, Wurl P and Taubert H: Elevated expression of
microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated
with poorer survival. Int J Cancer. 126:73–80. 2010. View Article : Google Scholar
|
|
69
|
Hasegawa S, Eguchi H, Nagano H, Konno M,
Tomimaru Y, Wada H, Hama N, Kawamoto K, Kobayashi S, Nishida N, et
al: MicroRNA-1246 expression associated with CCNG2-mediated
chemoresistance and stemness in pancreatic cancer. Br J Cancer.
111:1572–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Paik WH, Kim HR, Park JK, Song BJ, Lee SH
and Hwang JH: Chemosensitivity induced by down-regulation of
microRNA-21 in gemcitabine-resistant pancreatic cancer cells by
indole-3-carbinol. Anticancer Res. 33:1473–1481. 2013.PubMed/NCBI
|
|
71
|
Bitarte N, Bandres E, Boni V, Zarate R,
Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM,
Fortes P and Garcia-Foncillas J: MicroRNA-451 is involved in the
self-renewal, tumorigenicity, and chemoresistance of colorectal
cancer stem cells. Stem Cells. 29:1661–1671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tan L, Sui X, Deng H and Ding M: Holoclone
forming cells from pancreatic cancer cells enrich tumor initiating
cells and represent a novel model for study of cancer stem cells.
PLoS One. 6:e233832011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mazur PK and Siveke JT: Genetically
engineered mouse models of pancreatic cancer: Unravelling tumour
biology and progressing translational oncology. Gut. 61:1488–1500.
2012. View Article : Google Scholar
|
|
74
|
Choi SY, Lin D, Gout PW, Collins CC, Xu Y
and Wang Y: Lessons from patient-derived xenografts for better in
vitro modeling of human cancer. Adv Drug Deliv Rev. 79–80. 222–237.
2014.
|
|
75
|
Mazur PK, Herner A, Neff F and Siveke JT:
Current methods in mouse models of pancreatic cancer. Methods Mol
Biol. 1267:185–215. 2015. View Article : Google Scholar : PubMed/NCBI
|