Introduction
Colorectal cancer (CRC) is one of the most common
cancers worldwide (1,2). In the United States, it is expected
that there will be 132,700 newly diagnosed CRC cases and 49,700
CRC-related deaths in 2015 (2),
indicating the inadequacies of currently available measures
(1,3). Herbal medication is an approach that
has recently gained more attention for colorectal cancer (CRC)
management (4,5). It is known that botanicals have been
a significant resource to several of the currently used efficacious
chemotherapeutic agents (6,7). The
identification of non-toxic natural compounds from herbal medicines
remains an essential step in advancing CRC therapeutics (8,9).
The root of Scutellaria baicalensis is a
widely used herbal medicine in the traditional medical systems of
China and Japan for a variety of inflammation related ailments
(10–13). The major constituents of this
botanical are a group of flavonoid glycosides, including baicalin,
and wogonoside, of which baicalin is the major constituent in the
herb (14,15).
S. baicalensis is most often orally
administered. After oral ingestion, the constituents in the herb
inevitably come into contact with intestinal microbiota. Many of
these constituents could be transformed by the intestinal bacteria
before being absorbed (16). As
reported before, for natural glycosides such as ginsenosides, the
most common metabolic pathway is the deglycosylation reaction
induced by intestinal bacteria via the stepwise cleavage of the
sugar moieties (17–19). After deglycosylation, compared to
their parent compounds, the intestinal microbiome metabolites may
have more potent biological activity (20–22).
Anticancer activities of S. baicalensis and
its constituents were reported, but previous studies focused more
on its natural sourced flavonoid glycosides (23,24).
We recently observed that the major constituent of S.
baicalensis, baicalin, can be converted to baicalein by
glycoside hydrolases or hydrolyzing during the herb's processing or
storage (20,25), but whether the enteric microbiome
will metabolize baicalin is still unclear. In addition, although
attempts have been made to evaluate the anticancer activities of
the two compounds (26,27), a chemopreventive effect comparison
between baicalin and baicalein on CRC has not been performed.
In this study, using the human microbiome, we
determined biotransformation from baicalin to baicalein. We
compared the antiproliferative effects of baicalin and baicalein
using a panel of human cancer cell lines, including three human CRC
cell lines. The in vitro antiproliferative effects on CRC
cells were verified using an in vivo xenograft nude mouse
model. Then, we selected HCT-116 colon cancer cells, which are most
sensitive to baicalein treatment, for further mechanistic
observations, including cell cycle arrest and apoptosis induction.
Due to the fact that caspases are highly conserved in multicellular
organisms and function as central regulators of apoptosis, levels
of caspase expression were subsequently determined. Finally, the
possible binding modes of baicalein at the catalytic domains of
caspase 3 and 9 were simulated using the receptor-ligand docking
analysis.
Materials and methods
Chemicals and materials
All cell culture plasticware were obtained from
Falcon Labware (Franklin Lakes, NJ, USA) and Techno Plastic
Products (Trasadingen, Switzerland). Trypsin, McCoy's 5A,
Leibovitz's L-15, RPMI-1640 and DMEM media, and phosphate-buffered
saline were obtained from Mediatech, Inc. (Herndon, VA, USA).
Penicillin and streptomycin were obtained from Sigma-Aldrich (St.
Louis, MO, USA). The MTS assay kit, CellTiter 96 Aqueous Solution
Cell Proliferation Assay, was obtained from Promega (Madison, WI,
USA). The Annexin V-FITC apoptosis detection kit was obtained from
BD Biosciences (Rockville, MD, USA). PI/RNase staining buffer was
obtained from BD Biosciences Pharmingen (San Diego, CA, USA).
Caspase 3 and 9 ELISA kits were obtained from BioVison (Mountain
View, CA, USA). Baicalin and wogonoside were obtained from Indofine
Chemical Co. Inc. (Hillsborough Township, NJ, USA). Baicalein and
wogonin were obtained from Sigma-Aldrich.
Plant materials and extraction
The roots of Scutellaria baicalensis were
obtained from Chengde (Hebei, China). The voucher samples were
deposited at the Tang Center for Herbal Medicine Research at The
University of Chicago. Dried S. baicalensis roots were
ground to powder, and the powdered roots were extracted with 70%
ethanol for 2 h. The extraction method was boiling under reflux.
The filtrate was collected and the extraction procedure was
repeated one more time on the residue. The combined filtrate was
condensed under vacuum and lyophilized to yield dried S.
baicalensis extract (SbE).
Biotransformation of SbE by human fecal
microflora
Fecal samples were obtained from five adult
volunteers, who were non-smokers and had not consumed antibiotics
for ≥3 months before the study. The samples were collected by the
donors in plastic cups, and were processed within 30 min of
passage. All five fecal samples were mixed and an aliquot of 5 g of
the mixed feces was homogenized with 20 ml of phosphate buffer (pH
7.0) to obtain a fecal slurry. The slurry was filtered through
muslin to remove particulate material. One microliter of the fecal
slurry was mixed with 4 ml anaerobic medium containing 2.5 mg of
SbE. They were anaerobically incubated at 37°C for 0, 2 or 8 h.
Then, 1 ml of reaction mixture was extracted three times with 400
μl n-butanol/each time. The combined n-butanol solution was dried
under nitrogen steam spray in a water bath (60°C). Then the residue
was dissolved in methanol. The methanol solution was centrifuged at
17,000 × g for 10 min before HPLC analysis.
High performance liquid chromatography
(HPLC) analysis
The HPLC system was a Waters 2960 instrument
(Milford, MA, USA) with a quaternary pump, an automatic injector, a
photodiode array detector (Model 996), and Waters Empower software
for peak identification and integration. The separations were
carried out on a Phenomenex Prodigy ODS(2) column (150×2.0 mm, 5 μm). A binary
gradient solvent system of acetonitrile (eluent A) −0.03% (v/v)
phosphoric acid in water (eluent B) was used as follows: 13% A and
87% B (0 min), 28% A and 72% B (17 min), 35% A and 65% B (27 min),
90% A and 10% B (30–31 min), 13% A and 87% B (34–39 min). The
flow-rate of 0.8 ml/min was used and absorbance was detected at 280
nm. All tested solutions were filtered through Millex 0.2-μm nylon
membrane syringe filters before use. The contents of the
constituents were calculated using standard curves of
flavonoids.
Cell lines and cultures
The human colorectal cancer cell lines HCT-116
(McCoy's 5A), SW-480 (Leibovitz's L-15), HT-29 (McCoy's 5A), NSCLC
non-small cell lung cancer cells (DMEM), and human breast cancer
cell lines MCF-7 (RPMI-1640), MDA-MB-231 (RPMI-1640) were obtained
from American Type Culture Collection (Manassas, VA, USA). The
cells were grown in the indicated medium supplemented with 10% FBS
and 50 IU penicillin/streptomycin in a humidified atmosphere with
5% CO2 at 37°C.
Cell proliferation analysis
Baicalin and baicalein were dissolved in DMSO and
were stored at −20°C before use. Cells were seeded in 96-well
plates (1×104 cells/well). After 24 h, indicated
concentrations of drugs were added to the wells. The final
concentration of DMSO was 1%. Controls were exposed to culture
medium containing 1% DMSO without drugs. All experiments were
performed in triplicate and repeated 3 times. Following the
indicated incubation period, cell proliferation was evaluated using
an MTS assay according to the manufacturer's instructions. Briefly,
the medium was replaced with 100 μl of fresh medium and 20 μl of
MTS reagent (CellTiter 96 Aqueous Solution) in each well, and the
plate was returned to the incubator for 1–2 h. A 60-μl aliquot of
medium from each well was transferred to an ELISA 96-well plate and
its absorbance at 490 nm was recorded. Since 1% DMSO did not
influence the proliferation of the two cell lines, results were
expressed as percent of control (DMSO vehicle set at 100%).
Cell cycle analysis
HCT-116 cells were seeded in 24-well tissue culture
plates. On the second day, the medium was changed and cells were
treated with test compounds. Cells were incubated for 48 h before
they were harvested. These cells were fixed gently with 80% ethanol
in a freezer for 2 h and were then treated with 0.25% Triton X-100
for 5 min on an ice bath. Cells were resuspended in 300 μl of PBS
containing 40 μg/ml propidium iodide (PI) and 0.1 mg/ml RNase
(28). Then the cells were
incubated in the dark for 20 min at room temperature, and cell
cycle analysis was performed using a FACScan flow cytometer
(Becton-Dickinson, Mountain View, CA, USA) and FlowJo 7.1.0
software (Tree Star, Ashland, OR, USA). For each measurement,
≥10,000 cells were counted.
Apoptotic analysis
The apoptosis assay was performed by flow cytometry
following a previously described procedure (29). Briefly, HCT-116 cells were seeded
in 24-well tissue culture plates. After culturing for 1 day, the
medium was changed and test compounds were added. After treatment
for 48 h, cells floating in the medium were collected. The adherent
cells were detached with trypsin. Then, culture medium containing
10% FBS (and floating cells) was added to inactivate trypsin. After
gentle pipetting, the cells were centrifuged for 5 min at 1,500 g.
The supernatant was removed and cells were stained with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
according to the manufacturer's instructions. Untreated cells
served as controls. The cells were analyzed immediately after
staining using a flow cytometer. For each measurement, ≥20,000
cells were counted.
Caspase 3 and 9 analyses
HCT-116 cells were seeded in 6-well tissue culture
plates. After 24 h, the medium was changed and baicalein was added.
After treatment for 24 h, cell lysates were collected. Expression
levels of caspase 3 and 9 were determined by the colorimetric
method according to the manufacturer's instructions. Briefly, cell
lysates were diluted with 50 μl of 2X reaction buffer (containing
10 mM DTT) to a protein concentration of 0.5 mg/ml in an ELISA
96-well plate. Then, 5 μl of colorimetric tetrapeptide substrate
(DEVD-pNA for caspase 3 and LEHDpNA for caspase 9) and cell lysate
were added, and plate was incubated at 37°C for 24 h. Then, the
absorbance was recorded at 405 nm. The change in caspase activity
was calculated as absorbance of baicalein treated cells/absorbance
of untreated controls.
Receptor docking analysis
The possible binding modes of baicalein at the
catalytic domains of human caspase 3 and caspase 9 were predicted
using the docking program Surflex-Dock (Tripos, St. Louis, MO,
USA). The structure of baicalein was generated (through Ligand
model in Sybyl), and protein crystal structures were obtained (PDB
code 3H0E for caspase 3 and 2AR9 for caspase 9). To prepare for
docking analysis, the protein structures were prepared by adding
hydrogen atoms and missing sidechain atoms and removing water
molecules. Intermolecular interaction between baicalein and
caspases were analyzed, and the key pharmacophore in the ligand was
identified (30,31).
Statistical analysis
Data are presented as mean ± standard error (SE)
(n=3). A one-way ANOVA was employed to determine the statistical
significance of the results. When necessary, a Student's t-test was
used to compare the two groups. The level of statistical
significance was set at P<0.05.
Results
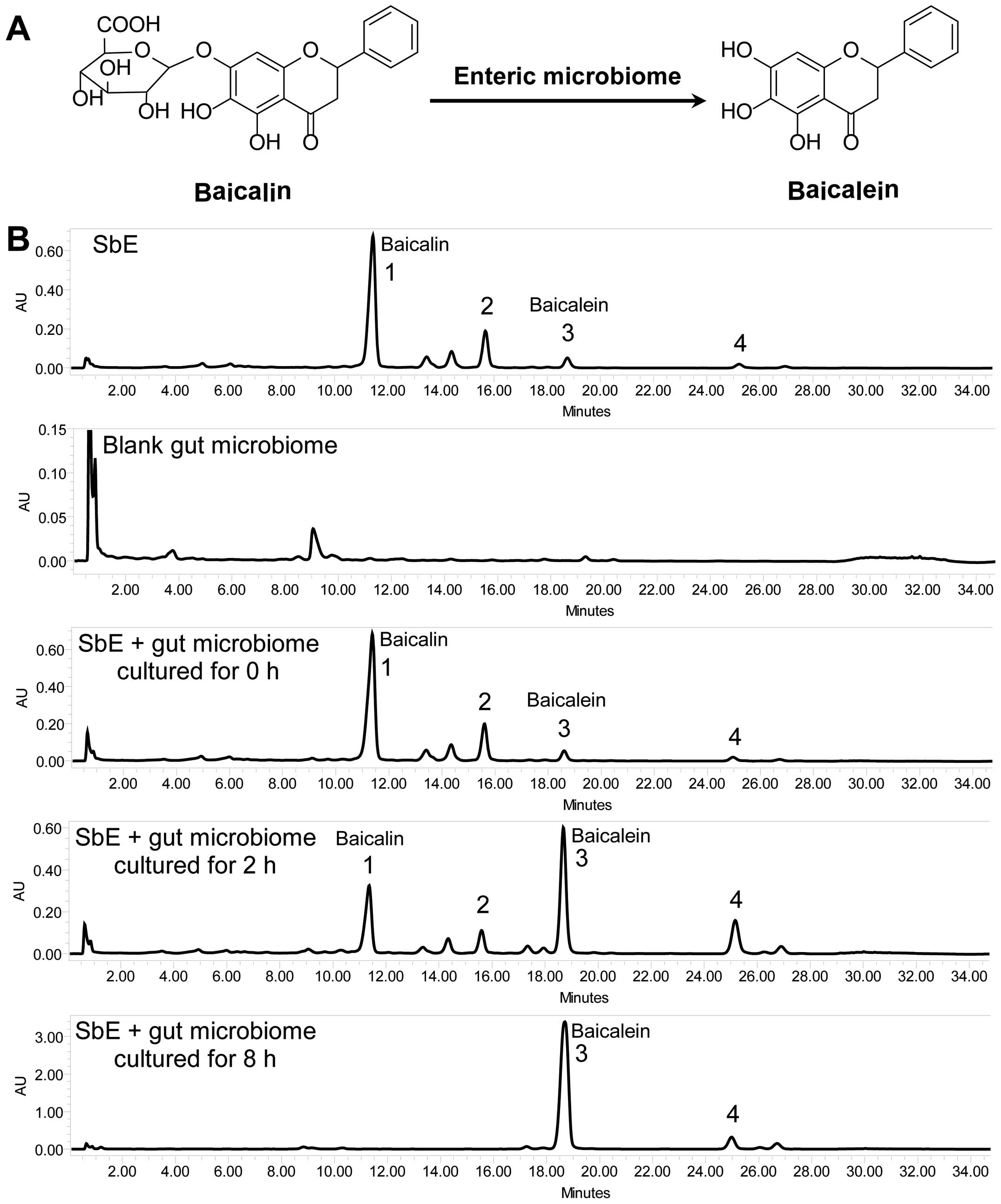
Baicalin metabolism by human enteric
microbiome to produce baicalein
HPLC analysis was used to monitor SbE flavonoid
changes during the biotransformation of human enteric microbiome.
As shown in Fig. 1, four
flavonoids were detected in SbE, i.e., baicalin, wogonoside,
baicalein and wogonin. Baicalin is the major constituent in SbE. To
determine whether fecal compounds influence SbE flavonoid analysis,
we assayed the vehicle fecal sample. A major peak at retention time
(Rt) of 9.061 min was observed in the chromatogram of the fecal
sample. For the SbE, the closest flavonoid peak to this fecal peak
is baicalin, with an Rt of 11.366 min. This fecal peak was
separated from the baicalin peak at baseline. Thus, compounds from
fecal sample did not influence SbE flavonoid determination.
When SbE was cultured with human enteric microbiome
for 2 h, compared to un-transformed SbE, the baicalin peak was
significantly reduced. Data showed that 75.3% of baicalin in SbE
was converted to baicalein after being cultured for 2 h.
Furthermore, after being cultured for 8 h, baicalin was not
detected, while baicalein was the major constituent in the reaction
mixture, indicating that all baicalin in SbE was converted to
baicalein. Similar results were also observed in wogonoside. Our
data showed that the gut microbiome can quickly transform baicalin
to baicalein.
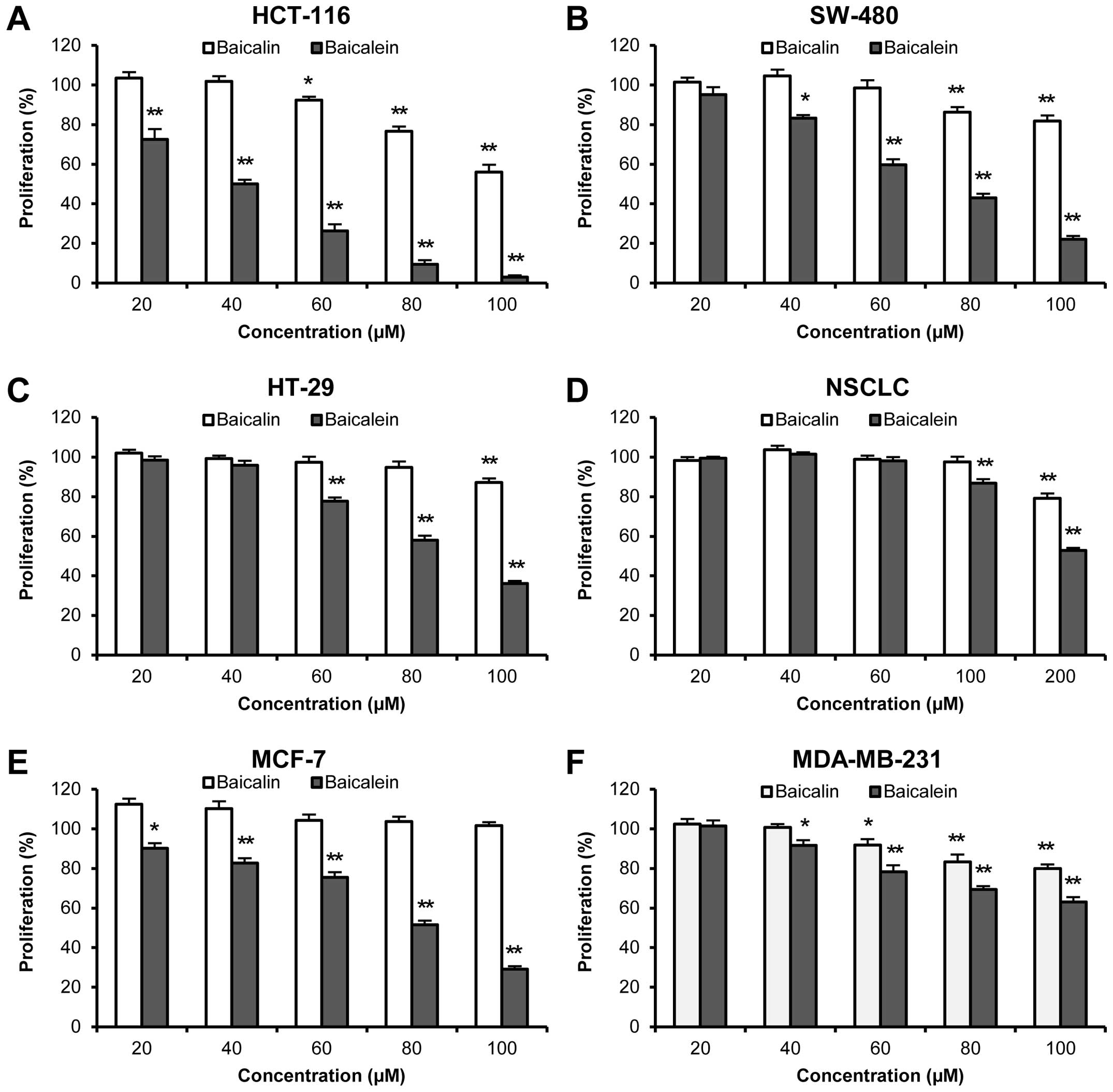
Antiproliferative effects of baicalin and
baicalein
In this study, six human cancer cell lines from
three of the most common human cancers were used, including
colorectal cancer (HCT-116, SW-480 and HT-29), non-small cell lung
cancer (NSCLC), and breast cancer (MCF-7 and MDA-MB-231).
As shown in Fig.
2A–C, while 48-h treatment with baicalin inhibited cancer cell
growth in relatively high concentrations, baicalein caused much
stronger growth suppression in all three colorectal cancer cell
lines. At 60 μM, only baicalin showed 7.6±1.7% of an
antiproliferative effect on HCT-116 cells (P<0.05 vs. control),
while no significant effects were observed on the other two cancer
cell lines. In the same concentration (60 μM), baicalein inhibited
cancer cell growth by 73.7±3.4% in HCT-116, 40.3±2.9% in SW-480,
and 22.3±1.8% in HT-29 cells, respectively (all P<0.01 vs.
control). Among the three cell lines, baicalein showed the most
potent antiproliferative effects in HCT-116 cells with an
IC50 value of 40.1 μM.
Baicalein also showed significant antiproliferative
effects on the other three cancer cell lines, but NSCLC cells were
not sensitive to baicalein treatment. Compared to baicalein,
baicalin showed weaker antiproliferative effects on NSCLC and
MDA-MB-231 cells (Fig. 2D and F).
MCF-7 cells were the only exception. Although baicalein showed
significant effects on MCF-7 cells at concentrations even as low as
20 μM, when treatment concentration was increased to 100 μM,
baicalin still did not inhibit cancer cell growth (Fig. 2E).
Our data suggested that baicalin showed less
antiproliferative effects in the tested concentrations. Its enteric
microbiome metabolite, baicalein, showed very significant
antiproliferative effects in different human cancer cell lines,
especially HCT-116 cells (Fig.
2).
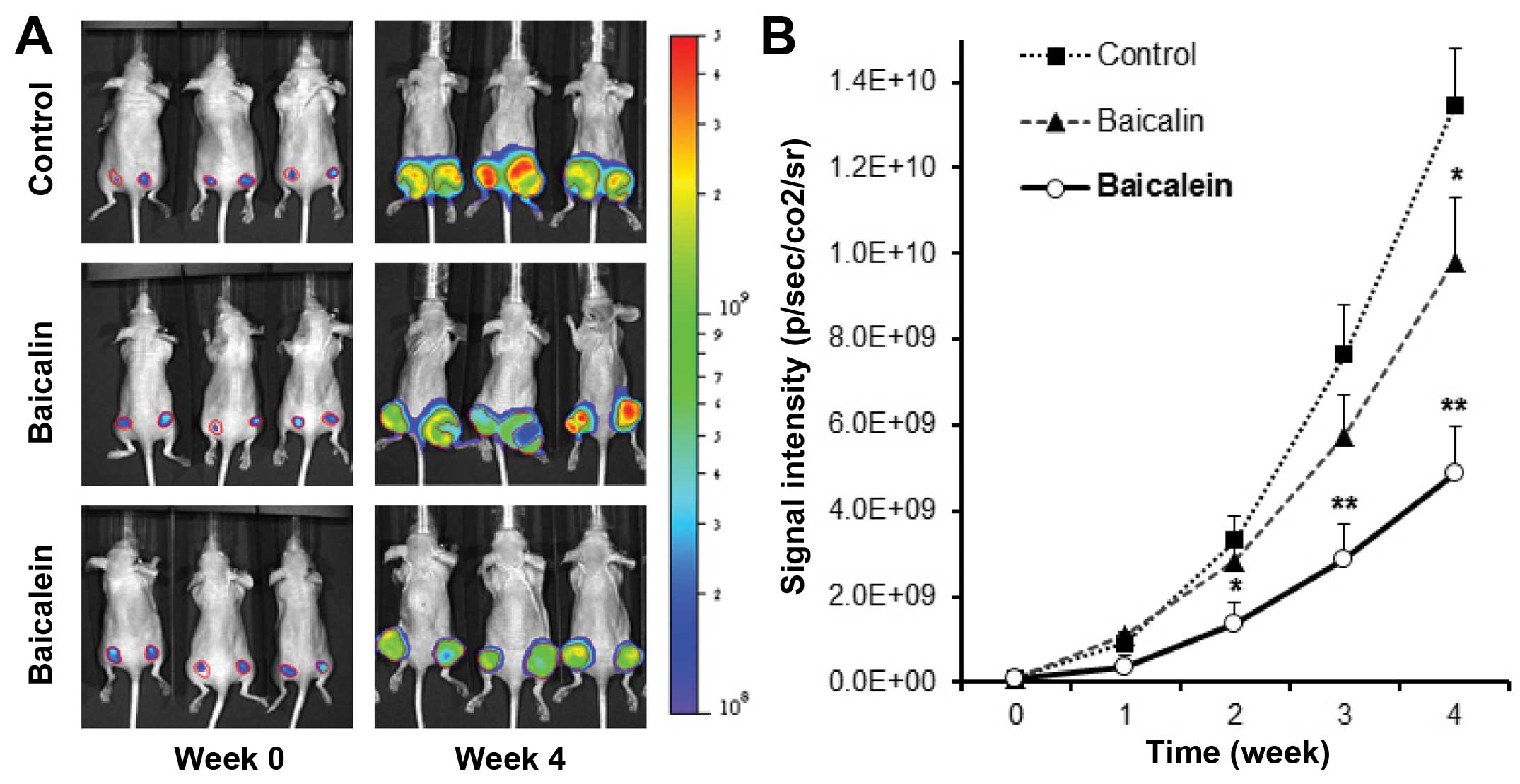
Antitumor effects of baicalin and
baicalein in xenograft tumor model
To confirm the in vitro antiproliferative
effect of baicalein on HCT-116 colorectal cancer cells, the in
vivo antitumor activities of baicalin and baicalein were
evaluated. Firefly luciferase-tagged HCT-116 cells were inoculated
into the flanks of athymic nude mice. Beginning on day 1, animals
were also administered with baicalin or baicalein at 30 mg/kg or
vehicle intraperitoneally every other day. Tumor growth was
measured by xenogeny bioluminescence imaging on a weekly basis.
Representative xenogen imaging results at weeks 0–4 are shown in
Fig. 3A. Tumor size at indicated
time-points as assessed by imaging signal intensities is summarized
in Fig. 3B. The data showed that
at weeks 2 and 3, baicalin suppressed tumor growth, but there is no
significant differences compared to control. At week 4, baicalin
significantly inhibited tumor growth (P<0.05). For the
baicalein, at week 2, baicalein exhibited significantly decreased
xenogeny imaging signal intensities compared with those of the
control (P<0.05). Weeks 3 and 4 exhibited more significant
antitumor effects than week 2 (both P<0.01). Our data suggested
the metabolite baicalein showed more significant antitumor effects
than those of its parent compound baicalin. The enteric microbiome
metabolism plays an important role in enhancing the anti-cancer
activity of SbE.
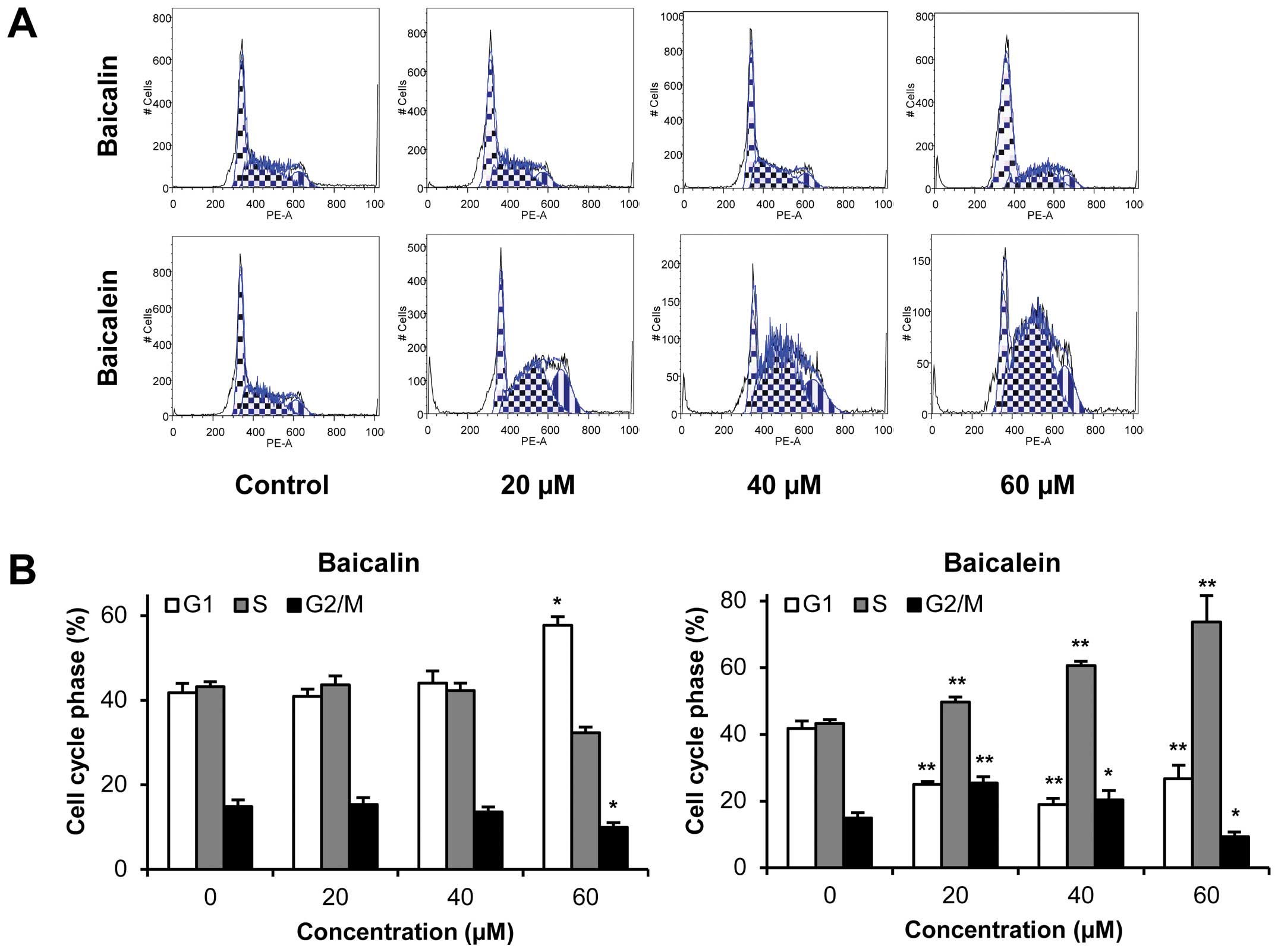
Effects of baicalin and baicalein on cell
cycle
Since HCT-116 colorectal cancer cells were sensitive
to baicalein treatment both in vitro and in vivo, we
selected this cell line for further mechanistic evaluations. As
shown in Fig. 4, compared to the
control, the effects of baicalein on the cell cycle profile were
observed at concentrations as low as 20 μM. Treatment of HCT-116
cells with 20, 40 and 60 μM baicalein for 48 h decreased G1 phase
to 25.0, 19.0 and 26.7%, respectively, compared to 41.8% in vehicle
treated cells, while increasing S phase to 49.7, 60.7 and 73.6%,
respectively, compared to 43.3% in vehicle treated cells (all
P<0.01). Thus, baicalein significantly decreased the number of
cancer cells in G1 phase and increased the number of cancer cells
in S phase. On the other hand, baicalin treatment did not influence
the cell cycle at 20 and 40 μM. Treatment with 60 μM of baicalin
for 48 h changed cell proportions by increasing G1 phase and
decreasing G2/M phase (P<0.05 vs control). These results
suggested that the metabolite baicalein, not parent compound
baicalin, significantly induced S phase cell cycle arrest in
HCT-116 cells.
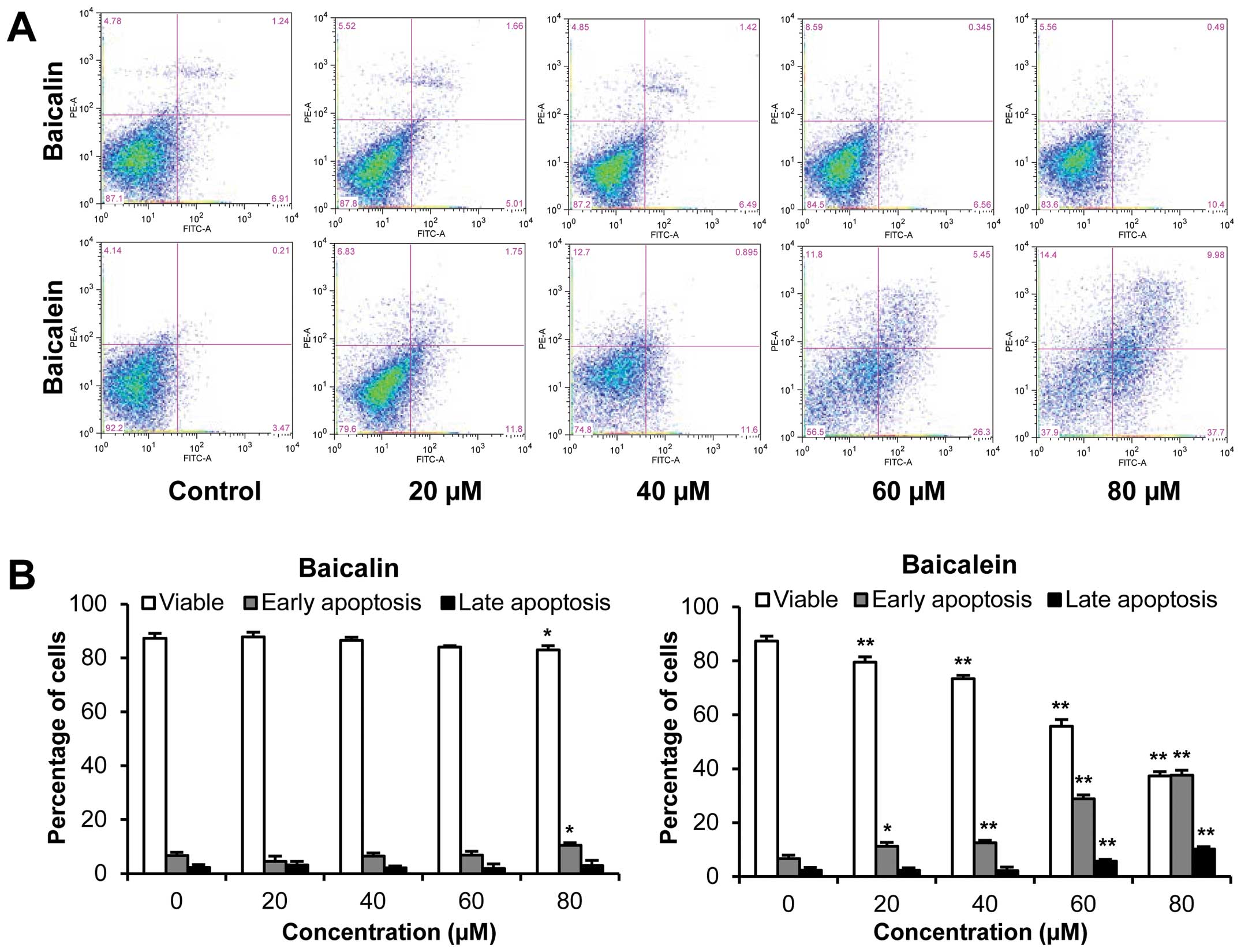
Effects of baicalin and baicalein on
apoptosis
The apoptotic effects of baicalin and baicalein were
evaluated by flow cytometry after staining with Annexin V and PI.
Annexin V can be detected in both early and late stages of
apoptosis, whereas PI stained cells only in late apoptosis or
necrosis. Early apoptotic cells were positive for Annexin V and
negative for PI (lower right quadrant); late apoptotic cells
stained for both Annexin V and PI (upper right quadrant). As shown
in Fig. 5, following treatment
with 20, 40, 60 and 80 μM of baicalein for 48 h, compared to the
control (6.7%), the percentage of early apoptotic SW-480 cells was
increased to 11.2, 12.5, 28.8 and 37.5%, respectively (P<0.05,
P<0.01, P<0.01 and P<0.01). However, baicalin did not
induce apoptosis at the concentrations of 20–60 μM. Only 80 μM of
baicalin showed an antiproliferative effect, which induced 10.5% of
early apoptotic cells (P<0.05). These data demonstrate that
baicalein significantly induces cell apoptosis.
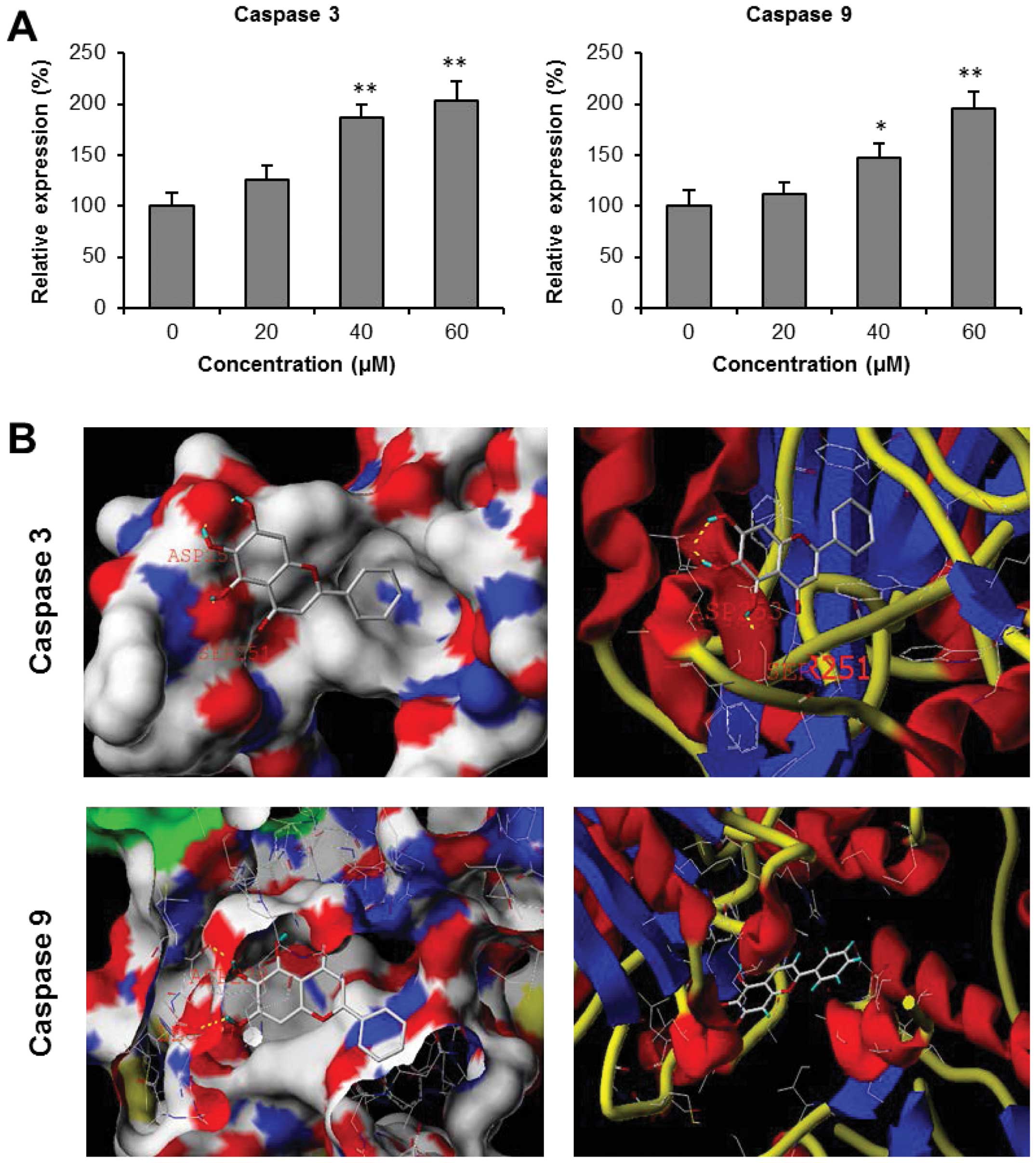
Effects baicalein on activities of
caspase 3 and 9
Caspase 3 and caspase 9 are two key proteins of the
caspase family of proteases, which are highly conserved in
multicellular organisms and function as central regulators of
apoptosis. They have been identified as playing a key role in the
progression of apoptosis (32). To
characterize the potential mechanism of baicalein's anticancer
activity, we assayed the activities of two caspases since baicalein
increased cancer cell apoptosis. As shown in Fig. 6A, treatment of HCT-116 cells with
40 μM baicalein for 24 h upregulated caspase 3 and 9 activities
significantly. These activities were further enhanced with 60 μM of
baicalein, increasing caspase 3 and 9 activities to 103.7±18.5 and
95.4±16.4% above those of vehicle treated cells, respectively (both
P<0.01). Our results suggested that baicalein significantly
induced the expression of caspase 3 and 9.
Molecular modeling of caspase 3 and 9 and
the binding mode of baicalein
To further explore the potential effects of
baicalein on caspase 3 and 9, we performed docking analysis to
characterize the physical interactions of baicalein with these
caspases. We examined baicalein docking for human caspase 3 (PDB
code: 3H0E) and human caspase 9 (PDB code: 2AR9). The Surflex-Dock
program was used to predict the binding sites of baicalein to
caspase 3 and 9. The energetically most favorable positions for
baicalein interaction with these caspases are shown in Fig. 6B. The in silico modeling
suggested that baicalein forms hydrogen bonds with residues Ser251
and Asp253 at the active site of caspase 3, while baicalein forms
hydrogen bonds with residues Leu227 and Asp228 in caspase 9 through
its hydroxyl groups. In addition, baicalein is predicted to show
significant binding affinity for caspase 3 (CScore 3.76) and
caspase 9 (CScore 3.18), suggesting that baicalein may directly
interact with these caspases.
Discussion
Colorectal cancer is the second leading cause of
cancer related death in the United States, and the second most
prevalent cancer worldwide (1,2). The
clinical CRC management involves diverse conventional modalities,
including surgery, radiation, and chemotherapy (2,33).
The complex characteristics of human cancer also require some
complementary approaches, including herbal medication, to improve
the therapeutic efficacy of conventional therapies (34,35).
In the past 30 years, nearly 80% of approved anticancer drugs were
derived from natural compounds (6). Herbal medicine has contributed
significantly to CRC therapies and many of the novel compounds with
significant anticancer properties are likely to be found in plant
sources.
S. baicalensis is one of the most commonly
used herbs in traditional medicine for the treatment of various
inflammatory diseases in Asia. The representative constituents of
S. baicalensis are a group of flavonoids that include
glycosides (baicalin, wogonoside) and their aglycon metabolites
(baicalein and wogonin), while baicalin occupies the major content
of the total flavonoids (14,15).
In recent years, the anticancer activities of S. baicalensis
extract and its constituents were reported (36,37).
However, most studies focused on its glycosides, such as baicalin,
which possess only limited anticancer activities.
S. baicalensis is most often orally
administered. In natural products research, many previous studies
employed primarily reductionist approaches in screening compounds
for bioactivity, and often only parent compounds were investigated.
In fact, in the gut, parent flavonoid glycosides in S.
baicalensis could be metabolized by the gut microbiome.
However, whether baicalin can be metabolized by the enteric
microbiome and its microbial metabolite leads to modified
anti-colorectal cancer activities are largely unclear.
In this study, we investigated the biotransformation
of baicalin by the human enteric microbiome. In our pilot study, we
selected 2, 8 and 24-h incubation periods, based on our previous
ginseng research (25), and
observed 8 h was enough to allow for the full metabolism of the
parent compounds. In this study, we observed that after 2 h of
incubation time, ¾ of baicalin was converted to baicalein. After
8-h incubation, all baicalin had been metabolized to baicalein.
Similar results were also observed on another pair of compounds:
>50% of wogonoside was converted to wogonin at 2 h. After 8-h
incubation, all wogonoside had been converted to wogonin. Our data
suggested that the human gut microbiome can effectively metabolize
baicalin to baicalein. Due to the possibility that water soaking
may structurally modify baicalin, as a control, we tested if water
only (without microbiome) can convert baicalin to baicalein. Our
results showed that after 8 h of culture, water soaking (without
enzymes) did not induce such transformation. The experimental
condition in this water control was different from those of our
previously published report, in which botanical enzymes
significantly converted baicalin to baicalein (15). Thus, our results suggested that the
enteric microbiome played a critical role in metabolizing baicalin
to baicalein.
To compare the antiproliferative effects between
baicalin and baicalein, in addition to three human colorectal
cancer cell lines (HCT-116, SW-480 and HT-29), we also used three
other cancer cell lines from two common solid tumors, small cell
lung cancer (NSCLC) and breast cancer (MCF-7 and MDA-MB-231). We
observed that, compared to baicalin, baicalein showed much stronger
antiproliferative effects on all the cancer cell lines. Basically,
baicalin was not effective on NSCLC and MCF-7 cells. At 100 μM,
baicalin did not inhibit cancer cell growth in these two cell
lines. Although baicalin showed antiproliferative effects in other
cancer cell lines, however, the active concentration was >60 μM.
On the other hand, baicalein showed very significant
antiproliferative effects on all the tested cancer cell lines.
For the three human colorectal cancer cell lines,
HT-29 was relatively resistant to baicalein treatment, with an
IC50 of 87.3 μM. Baicalein showed the most potent
effects on HCT-116 cells, with an IC50 of 40.1 μM. Thus,
we select this cell line to further validate its effects and
explore anticancer metabolisms of action.
A subcutaneous HCT-116 tumor model using xenograft
nude mice was established to confirm the in vitro
antiproliferative effect of baicalein on colorectal cancer cells.
Our data indicated that the daily administration of 30 mg/kg of
baicalin inhibited the HCT-116 tumor growth at week 4. Compare to
limited effects of baicalin, baicalein showed much stronger
antitumor effects. After mice received 30 mg/kg of baicalein,
HCT-116 tumor growth was significantly inhibited in weeks 2–4
(Fig. 3). The in vivo
antitumor evaluation supported the in vitro
antiproliferative effects that the enteric microbiome metabolite
baicalein is an active anti-colorectal cancer compound.
Because the inhibition of cell cycle progression and
induction of apoptosis are important mechanisms mediating the
effects of many anticancer agents, in this study, we compared the
activities of baicalin and baicalein on the cell cycle and
apoptosis. Cell cycle effects of baicalin were observed only at
high concentrations. At 60 μM, baicalin increased cell proportions
in G1 phase and decreased G2/M phase. On the other hand, at as low
as 20 μM, baicalein showed potent effects on the cell cycle.
Compared to the control, baicalein significantly dose-dependently
induced HCT-116 cell cycle arrest in S phase. We previously
isolated the glycoside fraction (contains baicalin and wogonoside)
and aglycon fraction (contains baicalein and wogonin) from SbE
(36). Since the aglycon fraction
showed potent antiproliferative effects, we assayed cell cycle
effects of aglycon fractions on HCT-116 cells. Aglycon fraction
induced cell cycle arrest in both S and G2/M phases (38). Data from this study supported our
previous observations, that baicalein contributed to the S phase
arrest that was also observed by the aglycon fraction
treatment.
Effects of baicalin and baicalein on HCT-116 cell
apoptosis were evaluated. Baicalein markedly induced colon cancer
cell apoptosis in concentrations of 20–80 μM, while baicalin
appeared to have positive effect at high concentration (80 μM).
Because caspase 3 and 9 are situated at critical points in
apoptotic pathways (39), to
further explore the mechanism mediating baicalein-induced
apoptosis, we assayed the activities of caspase 3 and 9. At
concentrations of 40 and 60 μM, baicalein significantly upregulated
the activities of these caspases. Our docking analysis further
suggested interaction sites between baicalein and caspases.
Baicalein induced apoptosis may be mediated by direct physical
interactions with these targets. Our results suggest that the
cancer cell growth inhibitory effect of baicalein was predominantly
mediated by induction of apoptosis.
In conclusion, using the human enteric microbiome,
the biotransformation and metabolic profile of S.
baicalensis flavonoids was determined. Baicalin can be easily
metabolized to baicalein. Using a panel of human cancer cell lines,
we tested the antiproliferative effects of baicalin and baicalein.
As a major parent compound from S. baicalensis, baicalin
showed limited antiproliferative effects on some of these cell
lines. Baicalein, however, showed significant antiproliferative
effects in all the tested cancer cell lines, especially on HCT-116
human colorectal cancer cells. In vivo antitumor results
supported our in vitro data. We demonstrated that baicalein
exerts potent S phase cell cycle arrest and pro-apoptotic effects
in HCT-116 cells. Baicalein upregulated the expression of caspase 3
and 9. Baicalein-induced apoptosis could be through direct physical
interactions with these apoptotic regulators. Data from this study
suggested that baicalein is a potent anticancer metabolite derived
from S. baicalensis. Enteric microbiota play a key role in
the colon cancer chemoprevention of S. baicalensis.
Acknowledgements
This study was supported in part by the NIH/NCCAM
grants K01 AT005362 and P01 AT004418.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gray R, Barnwell J, McConkey C, Hills RK,
Williams NS and Kerr DJ; Quasar Collaborative Group. Adjuvant
chemotherapy versus observation in patients with colorectal cancer:
A randomised study. Lancet. 370:2020–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ernst E: The role of complementary and
alternative medicine in cancer. Lancet Oncol. 1:176–180. 2000.
View Article : Google Scholar
|
|
5
|
Sampson W: Natural products and cancer. A
Textbook of Complementary and Alternative Medicine. 2nd edition.
Yuan CS, Bieber EJ and Bauer BA: Parthenon/CRC; London: pp.
645–654. 2006
|
|
6
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Butler MS, Robertson AA and Cooper MA:
Natural product and natural product derived drugs in clinical
trials. Nat Prod Rep. 31:1612–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen R, Zhang J, Hu Y, Wang S, Chen M and
Wang Y: Potential antineoplastic effects of Aloe-emodin: A
comprehensive review. Am J Chin Med. 42:275–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim EH, Shim B, Kang S, Jeong G, Lee JS,
Yu YB and Chun M: Anti-inflammatory effects of Scutellaria
baicalensis extract via suppression of immune modulators and MAP
kinase signaling molecules. J Ethnopharmacol. 126:320–331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arweiler NB, Pergola G, Kuenz J, Hellwig
E, Sculean A and Auschill TM: Clinical and antibacterial effect of
an anti-inflammatory toothpaste formulation with Scutellaria
baicalensis extract on experimental gingivitis. Clin Oral Investig.
15:909–913. 2011. View Article : Google Scholar
|
|
12
|
Li-Weber M: New therapeutic aspects of
flavones: The anti-cancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
13
|
Chen HM, Liou SF, Hsu JH, Chen TJ, Cheng
TL, Chiu CC and Yeh JL: Baicalein inhibits HMGB1 release and
MMP-2/-9 expression in lipopolysaccharide-induced cardiac
hypertrophy. Am J Chin Med. 42:785–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Zhou L, Lin G and Zuo Z: Contents of
major bioactive flavones in proprietary traditional Chinese
medicine products and reference herb of radix Scutellariae. J Pharm
Biomed Anal. 50:298–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu C, Qu F, Mao Y, Li D, Zhen Z, Nass R,
Calway T, Wang Y, Yuan CS and Wang CZ: Different extraction
pretreatments significantly change the flavonoid contents of
Scutellaria baicalensis. Pharm Biol. 51:1228–1235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi R, Zhou H, Liu Z, Ma Y, Wang T, Liu Y
and Wang C: Influence of coptis Chinensis on pharmacokinetics of
flavonoids after oral administration of radix Scutellariae in rats.
Biopharm Drug Dispos. 30:398–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hasegawa H: Proof of the mysterious
efficacy of ginseng: Basic and clinical trials: metabolic
activation of ginsenoside: Deglycosylation by intestinal bacteria
and esterification with fatty acid. J Pharmacol Sci. 95:153–157.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Yang J, Du F, Gao X, Ma X, Huang Y,
Xu F, Niu W, Wang F, Mao Y, et al: Absorption and disposition of
ginsenosides after oral administration of Panax notoginseng extract
to rats. Drug Metab Dispos. 37:2290–2298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tawab MA, Bahr U, Karas M, Wurglics M and
Schubert-Zsilavecz M: Degradation of ginsenosides in humans after
oral administration. Drug Metab Dispos. 31:1065–1071. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu C, Zhang Z, Zhang H, Zhen Z, Calway T,
Wang Y, Yuan CS and Wang CZ: Pretreatment of baicalin and
wogonoside with glycoside hydrolase: A promising approach to
enhance anti-cancer potential. Oncol Rep. 30:2411–2418.
2013.PubMed/NCBI
|
|
21
|
Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T,
Zhen Z, Musch MW, Bissonnette M, Chang EB and Yuan CS: Ginsenoside
compound K, not Rb1, possesses potential chemopreventive activities
in human colorectal cancer. Int J Oncol. 40:1970–1976.
2012.PubMed/NCBI
|
|
22
|
Gao JL, Lv GY, He BC, Zhang BQ, Zhang H,
Wang N, Wang CZ, Du W, Yuan CS and He TC: Ginseng saponin
metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting
multiple cancer signaling pathways. Oncol Rep. 30:292–298.
2013.PubMed/NCBI
|
|
23
|
Gong WY, Wu JF, Liu BJ, Zhang HY, Cao YX,
Sun J, Lv YB, Wu X and Dong JC: Flavonoid components in Scutellaria
baicalensis inhibit nicotine-induced proliferation, metastasis and
lung cancer-associated inflammation in vitro. Int J Oncol.
44:1561–1570. 2014.PubMed/NCBI
|
|
24
|
Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC,
Lin MW, Hsu YM, Lee MR, Amagaya S, Huang WW, et al: AKT
serine/threonine protein kinase modulates baicalin-triggered
autophagy in human bladder cancer T24 cells. Int J Oncol.
42:993–1000. 2013.PubMed/NCBI
|
|
25
|
Wan JY, Liu P, Wang HY, Qi LW, Wang CZ, Li
P and Yuan CS: Biotransformation and metabolic profile of American
ginseng saponins with human intestinal microflora by liquid
chromatography quadrupole time-of-flight mass spectrometry. J
Chromatogr A. 1286:83–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen WC, Kuo TH, Tzeng YS and Tsai YC:
Baicalin induces apoptosis in SW620 human colorectal carcinoma
cells in vitro and suppresses tumor growth in vivo. Molecules.
17:3844–3857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD,
Choi CS, Nam JS and Jung JY: Antitumor actions of baicalein and
wogonin in HT-29 human colorectal cancer cells. Mol Med Rep.
6:1443–1449. 2012.PubMed/NCBI
|
|
28
|
Xu Z, Wu G, Wei X, Chen X, Wang Y and Chen
L: Celastrol induced DNA damage, cell cycle arrest, and apoptosis
in human rheumatoid fibroblast-like synovial cells. Am J Chin Med.
41:615–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sai K, Li WY, Chen YS, Wang J, Guan S,
Yang QY, Guo CC, Mou YG, Li WP and Chen ZP: Triptolide
synergistically enhances temozolomide-induced apoptosis and
potentiates inhibition of NF-κB signaling in glioma initiating
cells. Am J Chin Med. 42:485–503. 2014. View Article : Google Scholar
|
|
30
|
Jain AN: Morphological similarity: A 3D
molecular similarity method correlated with protein-ligand
recognition. J Comput Aided Mol Des. 14:199–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giganti D, Guillemain H, Spadoni JL,
Nilges M, Zagury JF and Montes M: Comparative evaluation of 3D
virtual ligand screening methods: Impact of the molecular alignment
on enrichment. J Chem Inf Model. 50:992–1004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim R, Tanabe K, Uchida Y, Emi M, Inoue H
and Toge T: Current status of the molecular mechanisms of
anticancer drug-induced apoptosis. The contribution of
molecular-level analysis to cancer chemotherapy. Cancer Chemother
Pharmacol. 50:343–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanna RK and Soper JT: The role of surgery
and radiation therapy in the management of gestational
trophoblastic disease. Oncologist. 15:593–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Russo R, Corasaniti MT, Bagetta G and
Morrone LA: Exploitation of cytotoxicity of some essential oils for
translation in cancer therapy. Evid Based Complement Alternat Med.
2015:3978212015.PubMed/NCBI
|
|
35
|
Wang CZ, Li XL, Wang QF, Mehendale SR,
Fishbein AB, Han AH, Sun S and Yuan CS: The mitochondrial pathway
is involved in American ginseng-induced apoptosis of SW-480 colon
cancer cells. Oncol Rep. 21:577–584. 2009.PubMed/NCBI
|
|
36
|
Wang CZ, Li XL, Wang QF, Mehendale SR and
Yuan CS: Selective fraction of Scutellaria baicalensis and its
chemopreventive effects on MCF-7 human breast cancer cells.
Phytomedicine. 17:63–68. 2010. View Article : Google Scholar
|
|
37
|
Zhang J, Park HS, Kim JA, Hong GE,
Nagappan A, Park KI and Kim GS: Flavonoids identified from Korean
Scutellaria baicalensis induce apoptosis by ROS generation and
caspase activation on human fibrosarcoma cells. Am J Chin Med.
42:465–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang CZ, Calway TD, Wen XD, Smith J, Yu C,
Wang Y, Mehendale SR and Yuan CS: Hydrophobic flavonoids from
Scutellaria baicalensis induce colorectal cancer cell apoptosis
through a mitochondrial-mediated pathway. Int J Oncol.
42:1018–1026. 2013.PubMed/NCBI
|
|
39
|
Ismail B, Ghezali L, Gueye R, Limami Y,
Pouget C, Leger DY, Martin F, Beneytout JL, Duroux JL, Diab-Assaf
M, et al: Novel methylsulfonyl chalcones as potential
antiproliferative drugs for human prostate cancer: Involvement of
the intrinsic pathway of apoptosis. Int J Oncol. 43:1160–1168.
2013.PubMed/NCBI
|