Introduction
Cancer vaccines aim to stimulate a host immune
response that leads to tumor repression. Traditional cancer
vaccines target tumor-associated antigens. However, this is very
challenging since tumor cells of various tissue origins are not
only different but also genetically instable (1). Thus, researchers have been focusing
on anti-angiogenic vaccines because tumor vascular endothelium is
genetically stable and shows similar properties in different cancer
types (2). Moreover,
anti-angiogenic therapy is useful in combination therapies for
increasing tumor sensitivity to chemotherapy and radiotherapy
(3). Anti-angiogenic therapies can
be divided into two types. One is the application of monoclonal
antibodies or synthetic molecules against angiogenesis-associated
antigens (4). The other is the
whole endothelial vaccine. Recent studies have proven that
vaccination with HUVECs could prevent tumors by attacking on tumor
vasculature with both cellular and humoral immunity (5,6). One
pilot study treated patients who had recurrence of their brain
tumors or metastatic colorectal cancer with glutaraldehyde-fixed
HUVECs and found specific cellular immune responses against HUVECs.
Partial or complete tumor responses for at least 9 months were
shown in three patients with malignant brain tumors (7).
However, primary HUVECs, the most widely used cells
in anti-angiogenic immunity, have a very limited ability to
proliferate in vitro. They enter a growth arrest known as
replicative senescence after a certain number of cell divisions
(8). Senescent cells experience
both morphological changes and functional losses (9). This makes it difficult to apply
HUVECs on a large scale. Attempts to extend the life span of HUVECs
include spontaneous transformation, ectopic expression of viral
oncogenes, the provision of supportive matrix components and
ectopic expression of the human telomerase reverse transcriptase
(hTERT) gene (10–12). Several studies have proven that
hTERT-immortalized endothelial cells exhibited functional and
morphogenetic characteristics of parental cells while displaying a
survival advantage beyond the hurdle of replicative senescence
(13,14). However, whether hTERT-immortalized
HUVECs could preserve antitumor immunity has not yet been
confirmed. In the present study, we immortalized primary HUVECs by
virally introducing hTERT genes, which could renew replicative
capacity by means of telomere maintenance via the de novo
synthesis of telomeric DNA (15).
We also explored whether the antitumor immunity could be maintained
through vaccination with hTERT-immortalized HUVECs.
Materials and methods
Cell culture
Primary HUVECs were isolated from human umbilical
cord veins with 0.1% collagenase treatment and were cultured in
endothelial cell growth media (EBM-2) including 0.1% hEGF, 0.04%
hydrocortisone, 0.1% CA-1000, 2% FBS, 0.4% hFGF-B, 0.1% VEGF, 0.1%
R3-IGF-1, 0.1% heparin and 0.1% ascorbic acid (Lonza, Basel,
Switzerland). The purity of extracted HUVECs was identified by flow
cytometric analysis to compare the expression of specific
endothelial marker CD31 with that of HUV-EC-Cs, an established
endothelial cell line (American Type Culture Collection, Manassas,
VA, USA). HUVECs were passaged twice a week at a split ratio of
1:3–5 by TrypLE™ Select (Gibco, Waltham, MA, USA) digestion.
Recombinant vector creation and
lentiviral infection
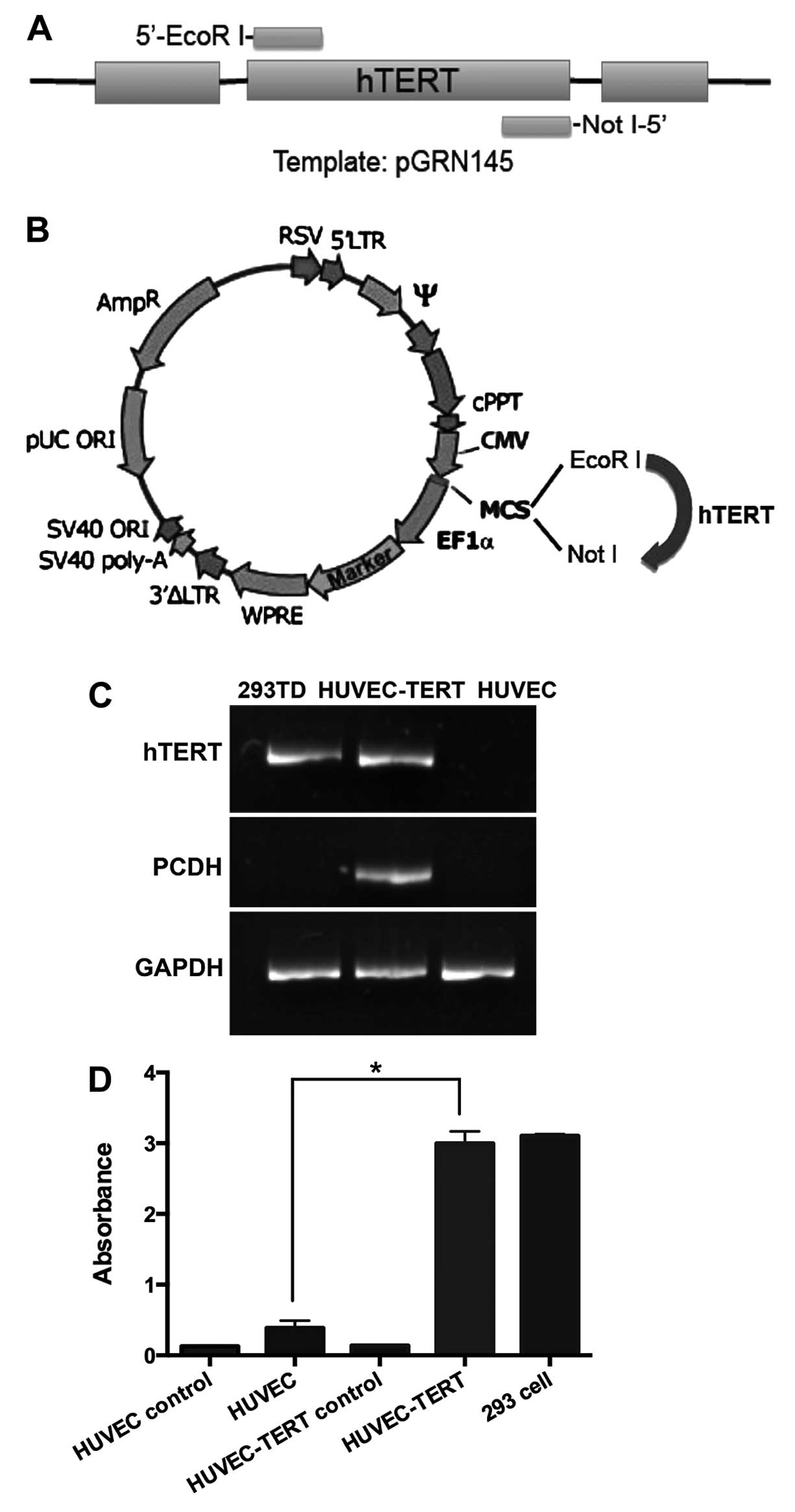
The cDNA sequence of human telomerase was amplified
by PCR with pGRN145 as the template (kindly provided by Dr Wei Wang
of our laboratory). The sense primer was 5′-agaaga
attcgccaccatgccgcgcgctccccgctgccgagcc-3′ with an EcoRI
linker at the 5′ end; the antisense primer was 5′-aga
agcggccgctcagtccaggatg gtcttgaagtc-3′ with a NotI linker at
the 5′ end. Amplified cDNA was then inserted into pCDH lenti-vector
plasmid at EcoRI/NotI site (System Biosciences,
Mountain View, CA, USA). HEK293 cells were transfected by the
plasmids of psPAX2, pMD2.G and pCDH-hTERT using
TransIT®-2020 transfection reagents (Mirus Bio
LLC, Madison, WI, USA). Forty-eight hours later, viral stocks were
harvested from cell supernatants and stored at −80°C in aliquots
(Fig. 1A and B).
Lentiviral infection of passage-2 HUVECs was carried
out in the presence of 4 μg/ml polybrene (Sigma-Aldrich, St. Louis,
MO, USA). Stable hTERT-expressing HUVECs were selected with 0.5
μg/ml puromycin for the first 7 days and afterwards with 0.02 μg/ml
puromycin.
Reverse transcription-PCR
Transcriptional expression of telomerase was tested
using reverse transcription-PCR. Briefly, total RNA of HUVEC-TERTs
and primary HUVECs was extracted with RNeasy® Mini kit
(Qiagen Benelux B.V., Venlo, The Netherlands) and reverse
transcripted to cDNA with PrimeScript™ RT-PCR kit (Takara, Dalian,
China) following the protocols. PCR was performed with the sense
primer of hTERT as 5′-cacctcacccacgcgaaaa-3′ and the antisense
primer as 5′-ccaaagagtttgcgacgcatgtt-3′ (16). The products of RT-PCR were
visualized by Southern blot analysis in 1% soft agarose.
Telomeric repeat amplification protocol
assay
Telomerase activity was quantified by Telo
TAGGG telomerase PCR ELISA (Roche Diagnostics, Basel,
Switzerland). According to the manufacturer's recommendation,
2×105 cells for each sample were prepared for cell
extracts, 3 μl of which were transferred to PCR process for the
amplification of telomerase-specific 6 nucleotide increments
(TTAGGG). The PCR products were denatured and hybridized to
microplate modules and detected by ELISA assay.
Tube formation assay
The tube formation assay was conducted to examine
whether HUVEC-TERTs with extended passages could form vascular
tubes. Namely, Matrigel basement membrane matrix (Becton-Dickinson
and Company, Franklin Lakes, NJ, USA) was thawed at 4°C and coated
at pre-cooled 6-well plates (17).
Suspensions of 1×105 cells were plated on each well and
incubated in 5% CO2 at 37°C overnight. Tube formation
was observed under a microscope.
Senescence-associated β-galactosidase
staining
The staining was performed with senescence
β-galactosidase staining kit (Beyotime Institute of Biotechnology,
Shanghai, China) according to the protocol. Briefly, cells were
washed and fixed, followed by the staining with working mixture and
incubation at 37°C without CO2 overnight. Cells were
observed with the camera-equipped microscope.
Cell proliferation studies
Long-term cell growth in vitro was observed
by cell number count and population doubling level (PDL)
measurement. Cumulative population doubling to each passage was
calculated using the formula: PD (n) = log2
(Nn/N0), (n, passage; Nn, cell
number at passage n; N0, cell number at passage 0).
Western blot analysis
VEGFR-II and integrin α5, the two identified
proteins responsible for cross reaction in the anti-angiogenic
immunity, were detected by western blot analysis (5). Namely, proteins from HUVEC-TERTs and
HUVECs (30 μg/lane) were fractionated on an 8% SDS-PAGE gel and
electroblotted onto a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Hercules, CA, USA). The membrane blots were blocked
in 5% non-fat dry milk, and incubated with anti-human VEGFR-II
(1:500; Abcam, Cambridge, UK) or anti-human integrin α5 (1:500;
R&D Systems, Minneapolis, MN, USA) at 4°C overnight. Protein
bands were then probed with HRP conjugated secondary antibody
(Sigma-Aldrich) at 37°C for 1 h and were visualized on the
chemiluninescence imager (Beijing Sage Creation Science Co., Ltd.,
Beijing, China).
Vaccine preparation and animal tumor
challenge
To prepare the vaccine, HUVEC-TERTs (passage 30–35)
and primary HUVECs (passage 3–5) were harvested and washed three
times by M199 medium without fetal bovine or antibiotics. The cells
were then irradiated by 100 Gy of X-ray, when no proliferation of
cells was observed.
Six- to eight-week-old female C57BL/6 mice and
BALB/C mice were used for lung cancer model (LL2) and colorectal
cancer model (CT26), respectively. Mice were housed in a
specific-pathogen-free facility and were randomly divided into 3
groups (10 for each group). In preventive immunization, each group
was s.c. injected with 1×106 irradiated HUVEC-TERTs,
HUVECs or NS alone (100 μl/dose) at day 0, day 14 and day 21 in the
left flank. At day 28, mice were s.c. injected with
5×105 tumor cells in the right flank. In therapeutic
immunization, mice were firstly challenged with 5×105
tumor cells, and were then immunized with HUVEC-TERTs, HUVECs
(1×106 cells, 100 μl/dose) or NS alone once a week for
consecutive 4 weeks (18,19). The tumor volume was estimated every
3 days by the following formula: Tumor volume =
0.52xaxb2, where a represented the longer diameter and b
represented the shorter diameter (20). All animal experiments were approved
by the Institutional Animal Care and Treatment Committee of Sichuan
University in China.
ELISA
To evaluate the activation of IgG and other
regulatory factors after immunization, ELISA assays were performed
in mouse sera and spleen lymphocyte supernatants. As for
HUVEC-neutralizing IgG titer, mouse sera were obtained from the
orbital veins of mice 7 days after the third immunization of the
preventive model. Mouse sera diluted serially by PBS were then
added to HUVEC-seeded 96-well plates (1×104 cells, 100
μl/well) and labeled with horseradish peroxidase (HRP) anti-mouse
IgG. Enzyme activity was visualized by TMB substrate and the
absorbance was measured at 450 nm with an ELISA reader (Bio-Rad
Laboratories).
Using sandwich precoated ELISA kits (Dakewe Biotech
Co., Ltd., Beijing, China), TGF-β and VEGF levels in the sera were
tested 20 days after tumor inoculation of preventive model while
IL-4 and INF-γ levels in mouse lymphocyte supernatants were
evaluated at day 7 after tumor inoculation of preventive model. For
lymphocyte supernatant preparation, mice were sacrificed and spleen
lymphocytes were extracted by lymphocyte separation medium (Dakewe
Biotech) according to the manufacturer's recommendations. Cells
were cultured in a 6-well plate (1×107 cells/well with 2
ml serum-free Dulbecco's modified Eagle's medium) and supernatants
were harvested after 48 h of culture. Mouse sera or lymphocyte
supernatants were then added into the precoated microplates and
incubated with biotin-labeled antibodies as well as
streptavidin-labeled HRP. The reagents were visualized with TMB
substrate for 30 min at 37°C without light and were measured at 450
nm with an ELISA reader.
Cytotoxic T-lymphocyte assay
Lymphocytes extracted from mouse spleens at day 7
after the third immunization were used as effector cells to
incubate with HUVECs or LL2s in a 96-well plate for 4 h at 37°C.
Effector: target ratios were 12.5:1, 25:1, 50:1 and 100:1. Lactate
dehydrogenase released by lysed cells in supernatants was measured
by LDH cytotoxicity assay (GenMed Scientifics, DX Zoetermeer, The
Netherlands). The cytotoxicity of lymphocytes were calculated with
the following formula: % cytotoxicity = (experimental O.D. −
effector spontaneous O.D. − target spontaneous O.D.)/(maximal O.D.
− target spontaneous O.D.) × 100%.
Flow cytometric analysis
To test the activation of spleen cells, spleen
lymphocytes at day 7 after the third immunization were labeled with
anti-CD4-APC, anti-CD8-FITC and anti-CD69-PE or anti-CD4-APC,
anti-CD8-FITC and anti-IFN-γ-PE at 4°C for 30 min. Stained cells
were washed twice with 2 ml PBS and resuspended in 500 μl PBS. The
percentages of activated cells were calculated on FACSCalibur using
CellQuest software (Becton-Dickinson and Company).
To explore possible tumor microenvironment changes,
mice were sacrificed 20 days after tumor inoculation of the
preventive model. Tumor tissues were digested by 0.1% colla-genase
at 37°C for 3 h. Tumor cells were then labeled with anti-CD4-APC,
anti-CD8-FITC and anti-CD69-PE (activated CD4+ or
CD8+ T cells) or anti-CD25-PE and anti-Foxp3-FITC
(regulatory T cells) or anti-CD11b-PE and anti-Gr-1-FITC
(myeloid-derived suppressor cells) at 4°C for 30 min (21–23).
Stained cells were analyzed as described above.
Immunohistochemistry
To explore whether HUVEC-TERTs could inhibit
angiogenesis in vivo, paraffin sections of tumor tissues
were incubated overnight with the vascular endothelial antibody
against CD34 (1:500; Abcam), followed by incubation with
biotinylated secondary antibody at 37°C for 40 min and
streptavidin-biotin complex at 37°C for 40 min (ZSGB-Bio, Beijing,
China). The numbers of CD34-positive microvessels and infiltrating
lymphocytes were microscopically examined in the high-power field
of view at a magnification of ×200.
Statistical analysis
Statistical comparisons were made by 2-tailed
Student's t-test or log-rank test. Differences were considered
statistically significant at P<0.05.
Results
hTERT-transfected HUVECs show an extended
life span and maintain endothelial characteristics
The ectopic expression of hTERT in HUVECs was tested
at mRNA and protein levels. RT-PCR showed that both hTERT mRNA and
vector pCDH mRNA were expressed after transfection (Fig. 1C). In TRAP-ELISAs, while primary
HUVECs presented no obvious telomerase activity, the telomerase
level of HUVEC-TERTs was significantly higher (P<0.05) and was
96% of that of the 293 cells, the embryonic kidney tumor cell line
(Fig. 1D). These data suggested
that HUVECs were successfully transduced with human telomerase cDNA
and HUVEC-TERTs presented high telomerase activity.
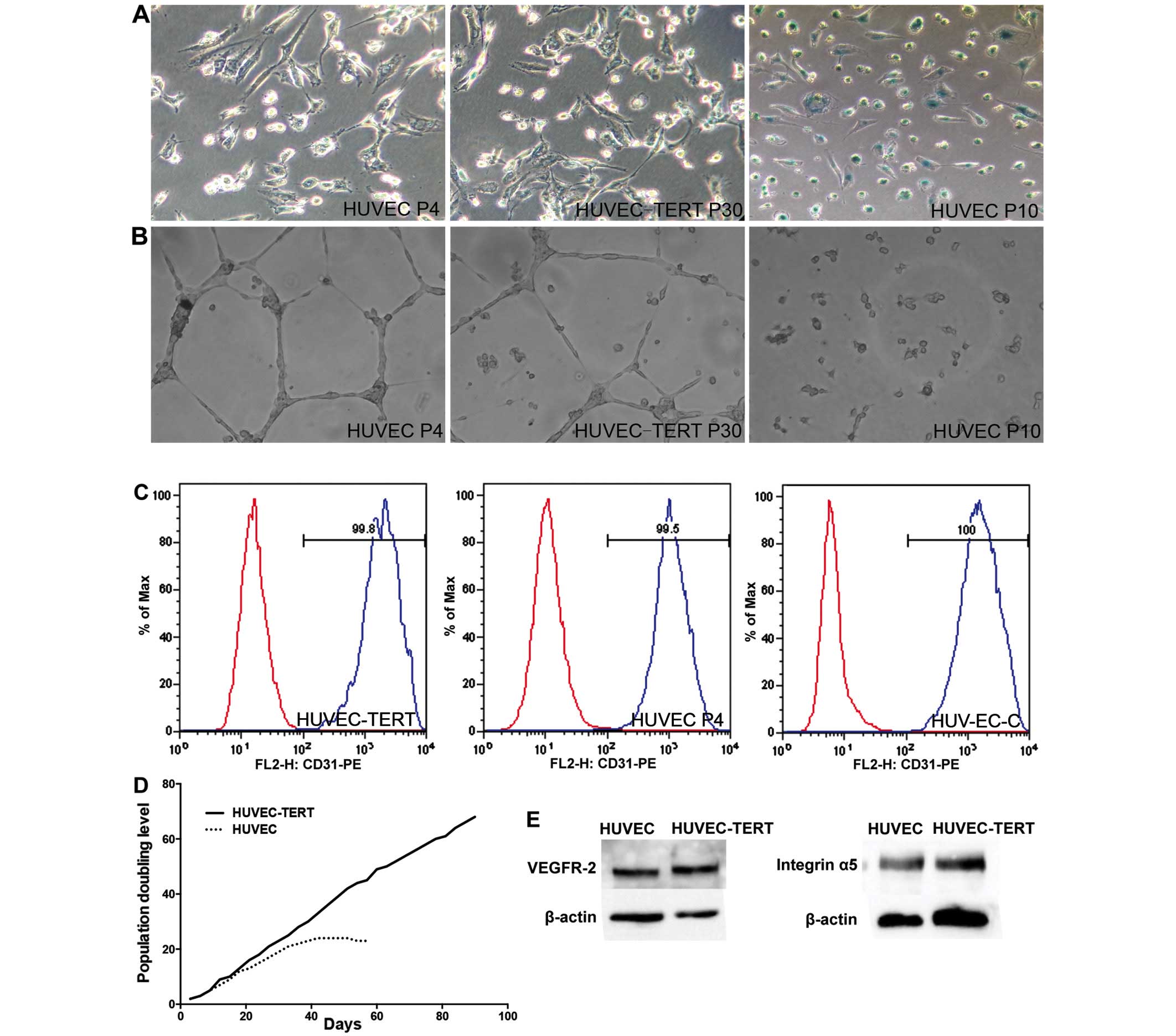
In contrast to primary HUVECs, which showed marked
β-galactosidase production and loss of endothelial tube formation
ability at passage 10, passage-30 HUVEC-TERTs presented no obvious
β-galactosidase staining and were able to form vascular tubes on
Matrigel (Fig. 2A and B). In
addition, HUVECs went into growth plateau after 24 PDs while
HUVEC-TERTs were still proliferative after 70 PDs (Fig. 2D). Moreover, HUVEC-TERTs expressed
CD31, a specific endothelial marker that was evaluated by flow
cytometric analysis (Fig. 2C)
(24). Passage-30 HUVEC-TERTs also
resembled young primary HUVECs in the expression of VEGFR-II and
integrin α5, the two identified proteins responsible for cross
reaction in the anti-angiogenic immunity (Fig. 2E). Thus, hTERT transfection
prolonged the life span of HUVECs while remained their endothelial
characteristics.
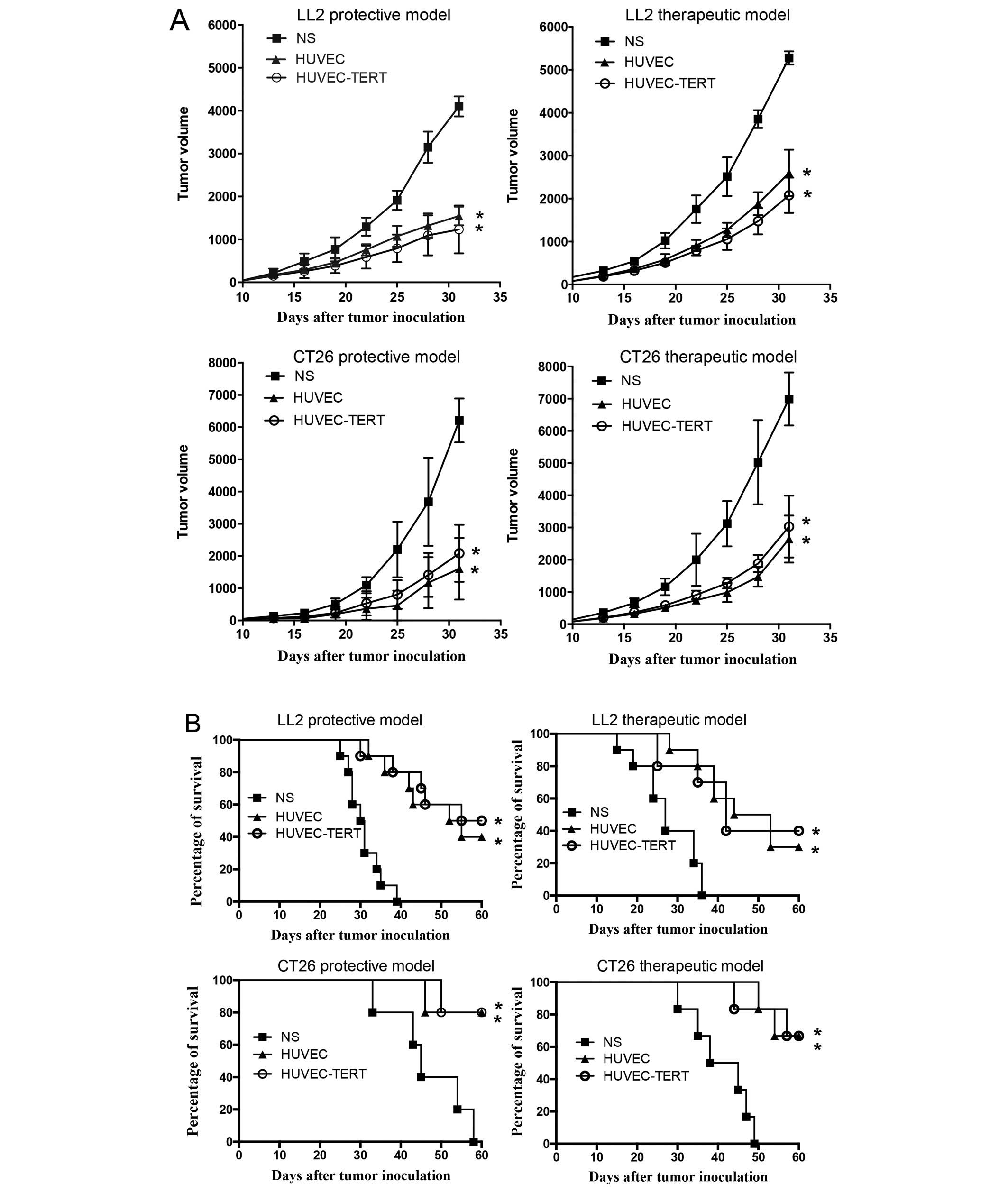
HUVEC-TERTs induced protective and
therapeutic anti-tumor immunity
To confirm whether hTERT-immortalized HUVECs
retained antitumor immunity similarly to primary HUVECs, both
protective and therapeutic mouse models were established in
HUVEC-TERT, HUVEC and NS groups. As shown in Fig. 3A, tumors in HUVEC-TERTs and
HUVEC-immunized mice grew significantly slower compared with NS
group. Thirty-one days after tumor inoculation, tumor inhibition
rates in HUVEC-TERT group and HUVEC group were 69. and 62.3% in the
protective LL2 model and were 66.0 and 74.0% in the protective CT26
model. In therapeutic models, treatment with HUVEC-TERTs and HUVECs
also significantly repressed tumor growth. In addition, survival
rates of HUVEC-TERTs and HUVECs treated mice were significantly
higher than controls in both tumor models (Fig. 3B).
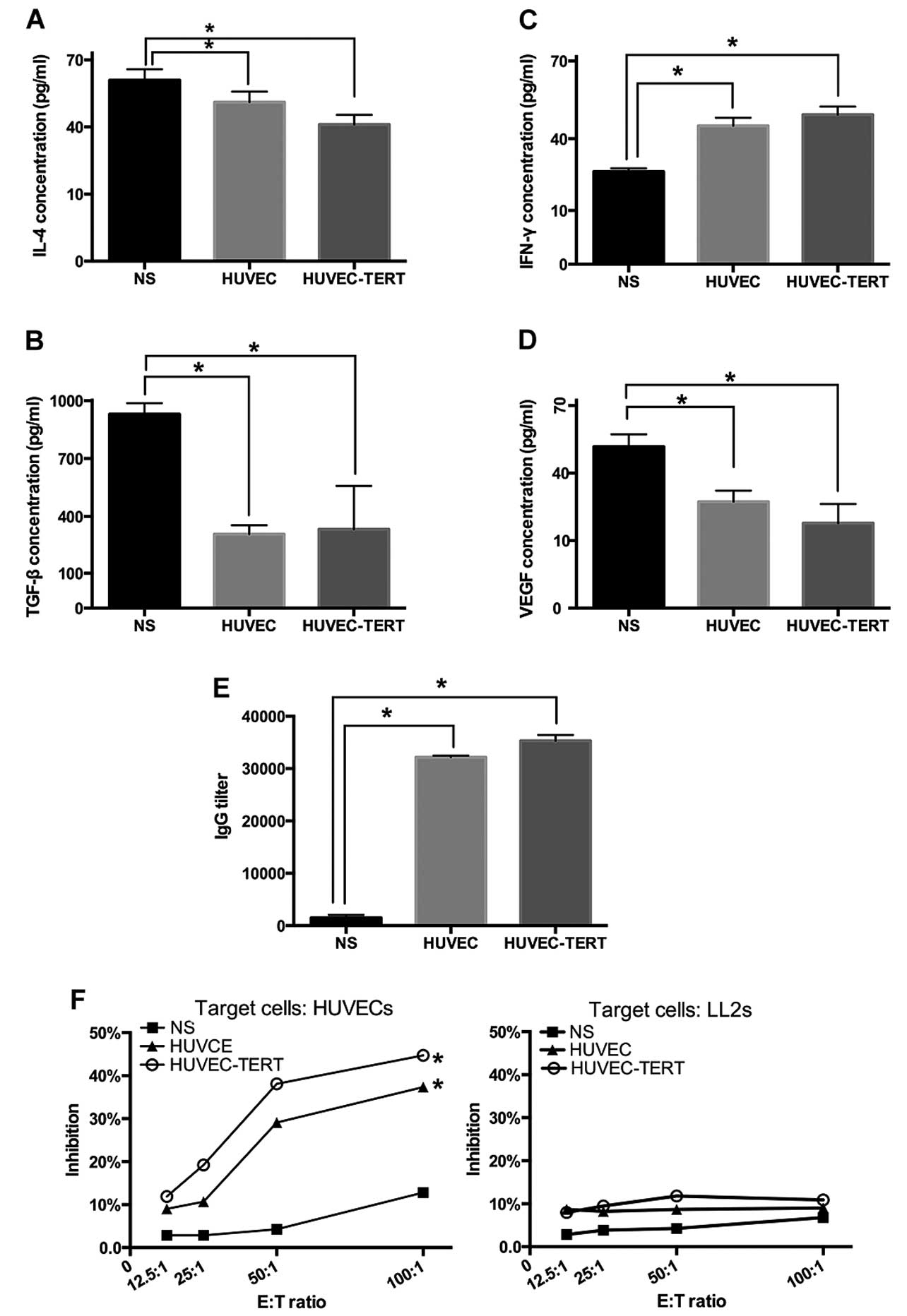
The production of immunoglobulin and
regulatory factors in mouse sera
The IgG titers tested by ELISAs in HUVET-TERT group
and HUVEC group were 1:35,600 and 1:32,200, respectively (Fig. 4E). Moreover, compared with NS
group, lymphatic secretion of IFN-γ (secreted mainly by Th1) was
increased while IL-4 (secreted mainly by Th2) was decreased
significantly in both groups (P<0.05) 7 days after tumor
inoculation (25,26). Whereas, VEGF and TGF-β, the two
important immunosuppressive mediators, were diminished (P<0.05)
in sera 20 days after tumor inoculation (Fig. 4A and D) (27,28).
These data suggested that immunization with irradiated HUVEC-TERTs
increased the production of immunoglobulin against endothelial
cells and changed secretion levels of immune mediators.
T lymphocytes from HUVEC-TERT-immunized
mice are cytotoxic to HUVECs, but not to tumor cells
In CTL assay based on LDH release of lysed target
cells, T lymphocytes isolated from spleens of HUVEC-TERTs and
HUVECs vaccinated mice were found to kill HUVECs in vitro
dose-dependently. At 100:1 E:T ratio, the cytotoxicity of
HUVEC-TERT group was 3.5 times that of the NS group. However, no
obvious cytotoxicity to LL2 tumor cells was observed in any of the
three groups (Fig. 4F), suggesting
that lymphocytes were specifically and dose-dependently cytotoxic
to the endothelium in the antitumor immunity.
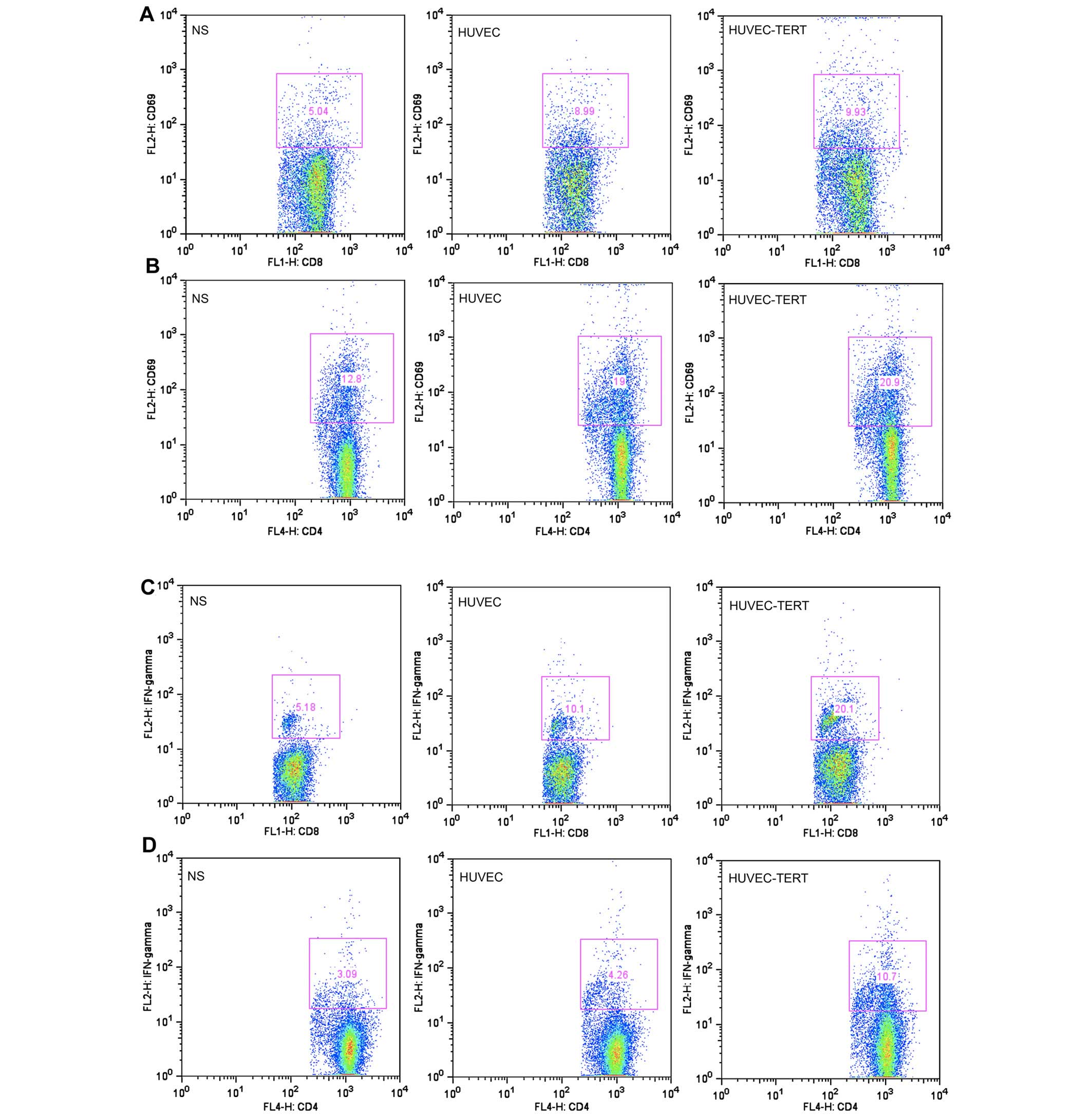
Spleen lymphocytes were activated by
HUVEC-TERT immunization
The flow cytometric analysis showed that HUVEC-TERT
group and HUVEC group presented higher ratios of activated
CD4+ T cells (CD4+CD69+) and
activated CD8+ T cells (CD8+CD69+)
at day 7 after the third immunization (Fig. 5A, B and F). In accordance with
ELISAs, the flow cytometric analysis also revealed a significant
increase in the secretion of IFN-γ in CD4+ T cells and
CD8+ T cells (Fig. 5C, D
and F). The above results suggested that subsets of both
CD4+ T cells and CD8+ T cells were activated
in the HUVEC-TERT-induced antitumor immunity.
HUVEC-TERTs increase lymphocyte
infiltration and impair regulatory T cells (Tregs) and
myeloid-derived suppressor cell (MDSCs) infiltration in tumor
tissues
To test possible tumor microenvironmental changes
despite anti-angiogenesis, flow cytometric analysis was conducted
on digested tumor tissues. As to tumor infiltrating lymphocytes,
the numbers of activated CD4+ T cells
(CD4+CD69+) and activated CD8+ T
cells (CD8+CD69+) per 10,000-cell storage
were significantly larger in HUVEC-TERT group and HUVEC group
compared with NS group. Besides, regulatory T cells
(CD25+Foxp3+), an important type of
immunosuppressive cells, were significantly decreased in both
groups (29). Furthermore,
myeloid-derived suppressor cells (MDSCs), which were related to
antigen-specific CD8+ T cell tolerance, were also
significantly suppressed in tumor tissues of HUVEC-TERTs and HUVECs
treated mice (Fig. 5E and F)
(30). These data suggested that
in addition to vascular damage, tumor microenvironment was also
regulated in the HUVEC-TERT-induced antitumor immunity.
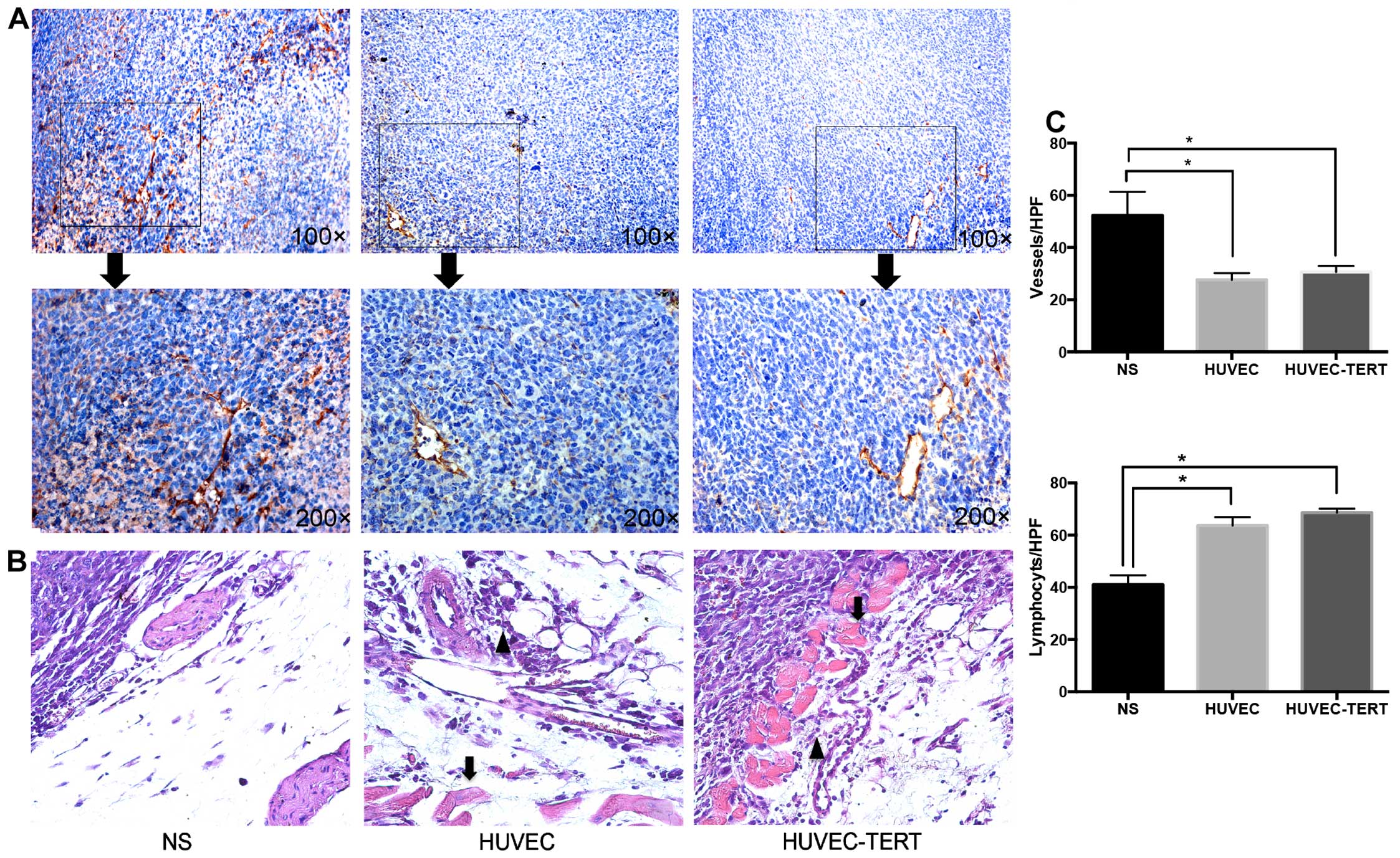
Immunization with HUVEC-TERTs inhibits
angiogenesis but shows no damage to major organs
Paraffin sections of tumor tissues showed that
HUVEC-TERT and HUVEC groups experienced a significant decrease in
CD34+ microvessels (Fig. 6A
and C) (31). H&E staining
of tumor sections presented symptoms of stromal hemorrhage and
inflammatory infiltrates in HUVEC-TERT and HUVEC groups while tumor
cells of NS group were highly proliferative, with no obvious
symptoms of hemorrhage or inflammatory infiltrates (Fig. 6B and C). Notaby, the results of
H&E staining also showed that mice vaccinated with HUVEC-TERTs
and HUVECs had no pathologic changes in heart, liver, spleen, lungs
and kidneys (data not shown). These data indicated that HUVEC-TERTs
inhibited tumor angiogenesis and had no toxicity to major
organs.
Discussion
HUVECs have been proved to be effective in the
anti-angiogenic immunity against cancer but their restricted
ability to proliferate and expensive culture condition limit their
application on a large scale (32). In the present study, we
immortalized primary HUVECs by virally introducing hTERT genes and
explored whether the antitumor immunity could be maintained through
vaccination with hTERT-expressing HUVECs. The present study found
that hTERT-expressing HUVECs displayed high telomerase activity at
both transcription and protein levels. Passage-30 HUVEC-TERTs
presented no obvious β-galactosidase staining and were able to form
vascular tubes on matrigel. They also expressed endothelial
specific marker CD31 and the two identified proteins for cross
reaction (VEGFR-II and integrin α5). This proved that virally
introducing hTERT cDNA was an effective method to elongate the life
span of primary HUVECs while maintaining their endothelial
characteristics.
HUVEC-TERTs were found to elicit both protective and
therapeutic antitumor immunity in tumor-bearing mice and we
believed that the previously reported anti-angiogenesis was still
responsible due to the following reasons: firstly,
immunohistochemistry revealed significantly less CD34 positive
microvessels in HUVEC-TERT group; secondly, ELISAs tested higher
production of neutralizing IgG against HUVECs from HUVEC-TERTs
treated mouse sera; thirdly, CTL assay showed that spleen
lymphocytes extracted from HUVEC-TERT-treated mice were
dose-dependently and specifically cytotoxic to HUVECs; fourthly,
immune suppressive mediators that mainly prompt angiogenesis were
downregulated. TGF-β, which was secreted by endothelial cells,
tumor cells and tumor-associated macrophages was decreased while
VEGF, which was mainly produced by endothelial cells, was also
diminished significantly (27).
The subsets of activated T lymphocytes in the
antitumor immunity were further elucidated. The flow cytometric
analysis of spleens demonstrated that both CD4+ and
CD8+ T lymphocytes were almost doubled in HUVEC-TERT
group compared with NS group. This was different from the previous
report that mice were not protected from tumor challenge only when
they were depleted of CD4+ T lymphocytes and vaccinated
with xenogeneic endothelial cells (5). We suspected that this contradiction
was caused by the different vaccine-preparing methods. In previous
studies, methods to prepare HUVEC vaccines differed from 3%
paraformaldehyde fixation, 0.025% glutaraldehyde fixation to no
special treatment by using viable HUVECs (5,26,32).
In the present study, we treated HUVEC-TERTs and HUVECs with X-ray,
when no proliferation was observed. As is known to us, irradiation
was able to restrict cell proliferation, increase antigen
presentation and enhance cytotoxic T lymphocyte recognition
(18). This might be the reason
that the present study revealing a stronger cellular immunity.
Despite CD4+ and CD8+ T
lymphocytes, Th1 and Th2 also played a role since the Th1 cytokine
IFN-γ was increased while the Th2 cytokine IL-4 was decreased,
which was consistent with the previous observation that IFN-γ
production was reduced and IL-4 production was enhanced in a
variety of human malignancies, including melanoma, gastric cancer,
lung cancer, glioblastoma, nasopharyngeal carcinoma, colorectal
cancer and head and neck cancer (33,34).
In recent years, the tumor microenvironment is being
increasingly recognized as a key factor in multiple stages of tumor
progression. Inappropriate activation of the stroma, which involves
migration of stromal cells, remodeling of matrix, and expansion of
vasculature, is able to convert the physiological microenvironment
into a pathological entity (35).
For instance, it was proven that tumors employed various strategies
to recruit MDSCs, a major component of tumor-adjacent stroma, to
positively regulate tumor development (36). It was also reported that MDSCs
could stimulate Tregs, inhibit T lymphocytes proliferation and
promote angiogenesis by secreting VEGF, TGF-β and FGF (37). In the present study, the flow
cytometric analysis of tumor tissues revealed that both MDSCs and
Tregs were decreased while the H&E staining showed intense
CD4+ and CD8+ T cells migration in the stroma
of HUVEC-TERT group, indicating that the antitumor immunity of
HUVEC-TERTs involve both anti-angiogenesis and the regulation of
other factors in the tumor microenvironment.
To the best of our knowledge, the present study is
the first to confirm the antitumor immunity of irradiated
hTERT-immortalized HUVECs. Both anti-angiogenesis and tumor
microenvironmental regulation were responsible for the antitumor
activity. Transducing hTERT genes might be a new strategy to allow
HUVECs to be applied on a large scale in tumor immunotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81372246 and 81123003)
and the National High Technology Research and Development Program
of China (no. 2014AA020708).
References
|
1
|
Okaji Y, Tsuno NH, Saito S, Yoneyama S,
Tanaka M, Nagawa H and Takahashi K: Vaccines targeting tumour
angiogenesis - a novel strategy for cancer immunotherapy. Eur J
Surg Oncol. 32:363–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boehm T, Folkman J, Browder T and O'Reilly
MS: Antiangiogenic therapy of experimental cancer does not induce
acquired drug resistance. Nature. 390:404–407. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dranoff G, Jaffee E, Lazenby A, Golumbek
P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D and Mulligan
RC: Vaccination with irradiated tumor cells engineered to secrete
murine granulocyte-macrophage colony-stimulating factor stimulates
potent, specific, and long-lasting anti-tumor immunity. Proc Natl
Acad Sci USA. 90:3539–3543. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu JY, Wei YQ, Yang L, Zhao X, Tian L,
Hou JM, Niu T, Liu F, Jiang Y, Hu B, et al: Immunotherapy of tumors
with vaccine based on quail homologous vascular endothelial growth
factor receptor-2. Blood. 102:1815–1823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei YQ, Wang QR, Zhao X, Yang L, Tian L,
Lu Y, Kang B, Lu CJ, Huang MJ, Lou YY, et al: Immunotherapy of
tumors with xenogeneic endothelial cells as a vaccine. Nat Med.
6:1160–1166. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen XY, Zhang W, Zhang W, Wu S, Bi F, Su
YJ, Tan XY, Liu JN and Zhang J: Vaccination with viable human
umbilical vein endothelial cells prevents metastatic tumors by
attack on tumor vasculature with both cellular and humoral
immunity. Clin Cancer Res. 12:5834–5840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okaji Y, Tsuno NH, Tanaka M, Yoneyama S,
Matsuhashi M, Kitayama J, Saito S, Nagura Y, Tsuchiya T, Yamada J,
et al: Pilot study of anti-angiogenic vaccine using fixed whole
endothelium in patients with progressive malignancy after failure
of conventional therapy. Eur J Cancer. 44:383–390. 2008. View Article : Google Scholar
|
|
8
|
Chang MW, Grillari J, Mayrhofer C,
Fortschegger K, Allmaier G, Marzban G, Katinger H and Voglauer R:
Comparison of early passage, senescent and hTERT immortalized
endothelial cells. Exp Cell Res. 309:121–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Böcker W, Yin Z, Drosse I, Haasters F,
Rossmann O, Wierer M, Popov C, Locher M, Mutschler W, Docheva D, et
al: Introducing a single-cell-derived human mesenchymal stem cell
line expressing hTERT after lentiviral gene transfer. J Cell Mol
Med. 12:1347–1359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freedman DA and Folkman J: Maintenance of
G1 checkpoint controls in telomerase-immortalized endothelial
cells. Cell Cycle. 3:811–816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bodnar AG, Ouellette M, Frolkis M, Holt
SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S and
Wright WE: Extension of life-span by introduction of telomerase
into normal human cells. Science. 279:349–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu J, Wang H, Bishop JM and Blackburn EH:
Telomerase extends the lifespan of virus-transformed human cells
without net telomere lengthening. Proc Natl Acad Sci USA.
96:3723–3728. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anno K, Hayashi A, Takahashi T, Mitsui Y,
Ide T and Tahara H: Telomerase activation induces elongation of the
telomeric single-stranded overhang, but does not prevent chromosome
aberrations in human vascular endothelial cells. Biochem Biophys
Res Commun. 353:926–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young AT, Lakey JR, Murray AG, Mullen JC
and Moore RB: In vitro senescence occurring in normal human
endothelial cells can be rescued by ectopic telomerase activity.
Transplant Proc. 35:2483–2485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Zglinicki T: Oxidative stress shortens
telomeres. Trends Biochem Sci. 27:339–344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Chang E, Cherry AM, Bangs CD, Oei
Y, Bodnar A, Bronstein A, Chiu CP and Herron GS: Human endothelial
cell life extension by telomerase expression. J Biol Chem.
274:26141–26148. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Nagavarapu U, Relloma K, Sjaastad
MD, Moss WC, Passaniti A and Herron GS: Telomerized human
microvasculature is functional in vivo. Nat Biotechnol. 19:219–224.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Shen G, Nie W, Li Z, Sang Y, Zhang B
and Wei Y: Irradiated tumor cells of lipopolysaccharide stimulation
elicit an enhanced anti-tumor immunity. J Cancer Res Clin Oncol.
140:1815–1823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Z, Yao Y, Ding Z, Chen X, Xie K, Luo
Y, Zhang J, Chen X, Wu X, Xu J, et al: Antitumour immunity mediated
by mannan-modified adenovirus vectors expressing VE-cadherin.
Vaccine. 29:4218–4224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou X, Li J, Wang Z, Chen Z, Qiu J, Zhang
Y, Wang W, Ma Y, Huang N, Cui K, et al: Cellular immunotherapy for
carcinoma using genetically modified EGFR-specific T lymphocytes.
Neoplasia. 15:544–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupta S, Marcel N, Sarin A and
Shivashankar GV: Role of actin dependent nuclear deformation in
regulating early gene expression. PLoS One. 7:e530312012.
View Article : Google Scholar
|
|
22
|
Zhang C, Shan J, Lu J, Huang Y, Feng L,
Long D, Li S, Li Q and Li Y: Combination of rapamycin and IL-2 do
not affect antigen presentation ability of rat B cell and could
promote Tregs proliferation and inhibitory activity. Cell Immunol.
264:180–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Skabytska Y, Wölbing F, Günther C, Köberle
M, Kaesler S, Chen KM, Guenova E, Demircioglu D, Kempf WE, Volz T,
et al: Cutaneous innate immune sensing of Toll-like receptor 2–6
ligands suppresses T cell immunity by inducing myeloid-derived
suppressor cells. Immunity. 41:762–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Zhao YJ, Zou QY, Zhang K, Wu YM,
Zhou C, Wang K and Zheng J: Preeclampsia does not alter vascular
growth and expression of CD31 and vascular endothelial cadherin in
human placentas. J Histochem Cytochem. 63:22–31. 2015. View Article : Google Scholar
|
|
25
|
Bürgler S, Gimeno A, Parente-Ribes A, Wang
D, Os A, Devereux S, Jebsen P, Bogen B, Tjønnfjord GE and Munthe
LA: Chronic lymphocytic leukemia cells express CD38 in response to
Th1 cell-derived IFN-γ by a T-bet-dependent mechanism. J Immunol.
194:827–835. 2015. View Article : Google Scholar
|
|
26
|
Lohoff M, Giaisi M, Köhler R, Casper B,
Krammer PH and Li-Weber M: Early growth response protein-1 (Egr-1)
is preferentially expressed in T helper type 2 (Th2) cells and is
involved in acute transcription of the Th2 cytokine interleukin-4.
J Biol Chem. 285:1643–1652. 2010. View Article : Google Scholar :
|
|
27
|
Mulligan JK and Young MR: Tumors induce
the formation of suppressor endothelial cells in vivo. Cancer
Immunol Immunother. 59:267–277. 2010. View Article : Google Scholar
|
|
28
|
Blank S, Deck C, Dreikhausen L, Weichert
W, Giese N, Falk C, Schmidt T and Ott K: Angiogenic and growth
factors in gastric cancer. J Surg Res. 194:420–429. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shatry A, Chirinos J, Gorin MA, Jones M
and Levy RB: Targeting Treg cells in situ: Emerging expansion
strategies for (CD4+CD25+) regulatory T
cells. Biol Blood Marrow Transplant. 15:1239–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernández A, Oliver L, Alvarez R,
Fernández LE, Lee KP and Mesa C: Adjuvants and myeloid-derived
suppressor cells: Enemies or allies in therapeutic cancer
vaccination. Hum Vaccin Immunother. 10:3251–3260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing X, Gu X, Ma T and Ye H: Biglycan
up-regulated vascular endothelial growth factor (VEGF) expression
and promoted angiogenesis in colon cancer. Tumour Biol.
36:1773–1780. 2015. View Article : Google Scholar
|
|
32
|
Okaji Y, Tsuno NH, Kitayama J, Saito S,
Takahashi T, Kawai K, Yazawa K, Asakage M, Hori N, Watanabe T, et
al: Vaccination with autologous endothelium inhibits angiogenesis
and metastasis of colon cancer through autoimmunity. Cancer Sci.
95:85–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toomer KH and Chen Z: Autoimmunity as a
double agent in tumor killing and cancer promotion. Front Immunol.
5:1162014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vierboom MP, Nijman HW, Offringa R, van
der Voort EI, van Hall T, van den Broek L, Fleuren GJ, Kenemans P,
Kast WM and Melief CJ: Tumor eradication by wild-type p53-specific
cytotoxic T lymphocytes. J Exp Med. 186:695–704. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi
Y, Hu G and Sun Y: New horizons in tumor microenvironment biology:
Challenges and opportunities. BMC Med. 13:452015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koh BI and Kang Y: The pro-metastatic role
of bone marrow-derived cells: A focus on MSCs and regulatory T
cells. EMBO Rep. 13:412–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pacini S: Deterministic and stochastic
approaches in the clinical application of mesenchymal stromal cells
(MSCs). Front Cell Dev Biol. 2:502014. View Article : Google Scholar : PubMed/NCBI
|