Introduction
Retinoblastoma is the most common malignant
intraocular tumor in children, typically occurring before 5 years
of age (1,2). Retinoblastoma affects ~1 in 18,000
children and can become fatal if left untreated (1,3,4). The
tumor is often initiated by the biallelic loss of the
retinoblastoma tumor suppressor gene (RB1) and progresses
quickly by accumulating additional genetic lesions and/or
epigenetic changes in key cancer pathways (3). Surgery is typically the initial
treatment followed by non-specific radiotherapy and/or
chemotherapy. There are no current therapies that specifically
target a unique molecular defect intrinsic to retinoblastoma. The
non-specific radio- and chemotherapy treatments can have side
effects including cognitive decline and secondary malignancy, which
is especially worrisome in such a young susceptible patient
population (5–7). Approximately 1% of patients with
retinoblastoma in both eyes develop second non-ocular tumors each
year and the cumulative probability of death from these secondary
malignancies is 26% at 40 years after diagnosis (8,9). In
order to identify prognostic factors and develop more efficacious
and safer treatments, a better understanding of the complex genomic
and proteomic regulatory pathways that control the development of
retinoblastoma is needed. Targeted chemotherapeutical agents
personalized to specific tumor biology have the tremendous
potential to be more clinically effective with less toxicity.
We previously identified the oncogene
orthodenticle homeobox 2 (OTX2) in the malignant
childhood brain tumor medulloblastoma (10,11).
OTX2 is a member of the highly conserved family containing
the bicoid-like homeodomain transcription factors that
control the developmental programs underlying brain morphogenesis
and plays a role in tumorigenesis if aberrantly expressed later in
life (12). This gene is expressed
in the metencephalon (cerebellum precursor), diencephalon (thalamus
and pineal gland precursor) and developing eye. OTX2 is also
expressed in the developing retinal pigment epithelium (RPE) of the
orbit (13). OTX2 is clearly
important to the development, maturation and function of retinal
cells. It is expressed in retinal photoreceptor and bipolar
precursor cells (14,15). The cell of origin for
retinoblastoma is speculated by some to reside in the RPE. Serial
Analysis of Gene Expression (SAGE) shows that the retina is one of
the very few normal adult tissues that continue to express the
gene, albeit at very low levels (http://cgap.nci.nih.gov/SAGE).
Our recent evidence has convincingly shown that
OTX2's aberrant overexpression results in tumorigenesis, notably as
medulloblastoma located in the cerebellum (10,11).
With genome-wide expression analysis, we identified genomic
amplification and overexpression of OTX2 in medulloblastoma
(10). A positive correlation of
OTX2 overexpression with aggressive tumor behavior (e.g.,
anaplasia) and worse patient survival was also evident (11). To demonstrate its oncogenic
ability, we showed that overexpression of OTX2 in non-tumorigenic
cells produced tumors (11).
Additionally, OTX2 regulates the medulloblastoma oncogene
MYC (11). These results
suggest that OTX2 plays a role in initiation and maintenance of a
large subset of tumors and thus represents a promising specific
therapeutic target.
Interestingly, three different malignant tumors
(pineoblastoma, retinoblastoma and medulloblastoma) can occur in
the same individual (16–18). The well-known syndrome of
trilateral retinoblastoma includes inherited unilateral or
bilateral retinoblastoma along with pineoblastoma, but reports of
concurrent medulloblastoma have also been seen (16–18).
Interestingly, OTX2 controls the cell fate of photoreceptor and
pineal gland cells (14),
suggesting a potential common cause of tumors in these tissues.
Because of the ontogenetic relationship of the common expression of
OTX2 in the developing precursor tissues to these adult structures,
the strikingly common histological appearance of these tumors and
common genetic aberrations (16,19–21),
we hypothesize that OTX2 may serve a similar oncogenic role in
retinoblastoma.
Materials and methods
Tissue samples
Retinoblastoma cell lines Y79 and WERI were
purchased from American Type Culture Collection (ATCC) (Manassas,
VA, USA). Medulloblastoma cells lines D425MED, D581MED and D324MED
were obtained from the Duke Preston Robert Tisch Brain Tumor Center
Biorepository. Acquisition of tissue specimens was approved by the
Duke University IRB (#Pro00008208) and done in accordance with
HIPPA regulations.
Cell culture
D425MED was selected as an OTX2-positive
control and D581MED/D324MED were selected as OTX2-negative
controls based on our prior data (10). Retinoblastoma cell lines were
cultured using RPMI-1640 medium with 10% FBS (Life Technologies;
Grand Island, NY, USA), while medulloblastoma cell lines were
cultured using a stock solution of 1X zinc option, HEPES and sodium
bicarbonate (Life Technologies). Cells were maintained at 5%
CO2 and 37°C.
Serial analysis of gene expression data
analysis
The serial analysis of gene expression (SAGE) data
were obtained from the National Center for Biotechnology
Information Cancer Genome Anatomy Project repository (http://cgap.nci.nih.gov/SAGE). The presence of OTX2
SAGE tags in a total of 157 tumors and 54 normal human tissues was
identified by using the SAGE Anatomic Viewer.
Quantitative real-time PCR
Q-PCR (Bio-Rad, Hercules, CA, USA) was used to
determine OTX2 copy number changes between normal human DNA
and retinoblastoma primary tumors or cell lines. Genomic DNA was
isolated by DNeasy Blood & Tissue kit (#69504; Qiagen,
Valencia, CA, USA) from 6 primary tumors (Rb1-6), cell lines Y79
and WERI, D425MED (positive control) and D581MED (negative
control). Line 1 was used to normalize DNA content.
Immunohistochemistry
Immunohistochemistry (IHC) was used to evaluate
protein expression and relationship with cellular morphology on
primary and mouse xenograft tumors. For IHC, 5-μm paraffin-embedded
tumors were obtained, deparaffinized by xylene and hydrated with a
series of graded alcohols. The sections were then washed with
distilled water and incubated in citrate buffer. Hydrogen peroxide
(0.3%) was used to block endogenous peroxidase activity. Sections
were blocked with 1% bovine serum albumin (BSA) and 20% mouse serum
(#M5905, Sigma-Aldrich, St. Louis, MO, USA) in PBS. The sections
were then screened with anti-OTX2 antibody at 1:200 (#MAB1979,
R&D Systems, Minneapolis, MN, USA) overnight at 4°C, followed
by HRP-conjugated anti-goat secondary antibody at 1:200 (#A8919,
Sigma-Aldrich) for 1 h at room temperature.
Immunoblotting
Immunoblotting was used to detect the expression
levels of OTX2, MYC, CRX and phosphorylated RB (pRB). Per standard
protocol, 50 μg cell protein isolates were prepared, denatured by
heating at 97°C for 7 min, loaded on to polyacrylamide gels,
electrophoresed at 180 V 45 min and transferred to a nitrocellulose
membrane at 15 V for 30 min (Bio-Rad). Membranes were blocked with
5% non-fat milk and probed using primary antibodies (anti-OTX2,
1:5,000, MAB1979, R&D Systems; anti-GAPDH, 1:3,000, #sc-365062,
Santa Cruz Biotechnology, Santa Cruz, CA, USA; anti-MYC, 1:1,000,
#9402, Cell Signaling Technology, Danvers, MA, USA; anti-CRX,
1:1,000, #C7498, Sigma-Aldrich; anti-pRB, 1:1,000, #9308, Cell
Signaling Technology) overnight at 4°C with gentle shaking.
Subsequently, membranes were washed and probed with the appropriate
HRP-conjugated secondary antibodies (OTX2 needed anti-goat,
1:5,000, Invitrogen, Carlsbad, CA, USA; GAPDH/MYC/CRX/pRB needed
anti-rabbit, 1:3,000, Santa Cruz Biotechnology) for 1 h at room
temperature with gentle shaking. Proteins were visualized with
SuperSignal West Femto Chemiluminescent Substrate (#34095, Thermo
Scientific, Waltham, MA, USA) by autoradiography.
siRNA inhibition of OTX2
Y79 and WERI cell lines were either transfected with
one of the two OTX2-specific siRNAs or with a non-specific
scrambled siRNA control (siRNA#1, GGAGGU GGCACUGAAAAUCtt; siRNA#2,
GGACACUA AUUCA UCUGUAtt; Ambion, Austin, TX, USA; MISSION siRNA
Universal Negative Control, Invitrogen). Transfection was conducted
with 100 nM of each siRNA and Lipofectamine 2000 (siRNA to
Lipofectamine 2000 ratio of 1:2; #11668-019, Invitrogen). The cells
were incubated with the siRNA at 37°C and harvested at 24, 48, 72
and 96 h to ascertain maximum knockdown by immunoblotting.
Seventy-two hours was chosen as the best time-point by
immunoblotting, so cells were harvested at that time for assays.
For each siRNA, studies were done in triplicate.
Cell proliferation, apoptosis and colony
formation assays
After confirming OTX2 inhibition by immunoblotting,
functional in vitro assays were performed. Cell viability
and proliferation was measured using the MTT Cell Proliferation
assay (#30-1010k, ATCC). After OTX2 inhibition, cells were allowed
to grow for 7 days and MTT assay was performed (measured at OD540).
Cell density was normalized to untreated cells. Cell apoptosis in
response to OTX2 inhibition was measured using the FITC Annexin V
Apoptosis Detection kit (#556547, BD Pharmingen, San Diego, CA,
USA), normalized to untreated cells and analyzed by flow cytometry.
For colony formation, treated cells were plated at 5×103
in triplicates in 0.5% agarose-coated 24-well plates and the number
of colonies was counted at 2 weeks.
Pharmacologic inhibition of OTX2
All-trans-retinoic acid (#302-79-4, ATRA,
Sigma-Aldrich) in DMSO (#D8418, Sigma-Aldrich) at various
concentrations (0, 0.5, 2, 5 and 10 μM) was added to the cells.
Time experiments were done to ascertain maximum reduction in OTX2
expression by immunoblotting. Maximum reduction in OTX2 expression
was obtained after 72 h. Similar functional assays were done in
triplicate.
Conditional knockdown of OTX2 for in vivo
studies
Using the open reading frame of OTX2 from
GenBank (http://www.ncbi.nlm.nih.gov/genbank/), we designed 2
OTX2-specific and 1 non-specific scrambled shRNA with the
Invitrogen BLOCK-iT RNAi Designer (http://rnaidesigner.invitrogen.com/rnaiexpress/). The
sequence for shRNA#1 was
CGCGTCCCCGCTTGGATTATAAAGATCATTCAAGAGATGATCTTTATAATCCAAGCTTTTTGGAAAT;
shRNA#2 was
CGCGTCCCCGAGCTGCACTGAAACTTTATTCAAGAGATAAAGTTTCAGTGCAGCTCTTTTTGGAAAT;
scrambled shRNA was
CGCGTCCCCGTATCGGATAATATCAGTATTCAAGAGATACTGATATTATCCGATACTTTTTGGAAAT
(IDT, Coralville, IA, USA). The restriction enzymes MluI and
ClaI (#R0198L and #R0197L, New England Biolabs, Ipswich, MA,
USA) were used to ligate the gene into pLVTHM (#12247, Addgene,
Cambridge, MA, USA) cloning vector. MscI and FspI
(#R0534M and #R0135L, New England Biolabs) were used to ligate it
into the lentiviral construct pLVCT-tTR-KRAB (#11643, Addgene),
which contained a green fluorescent protein (GFP) tag and a Tet-on
system so that inserts are transcribed in the presence of
doxycycline. HEK293 cells (#CRL-1573, ATCC) were transfected with
the lentiviral vector, the packing plasmid psPAX2 (#12260) and the
envelope plasmid pMD2.G (#12259, Addgene) to make viral particles.
Lentiviral particles were collected and concentrated (Amicon Ultra
centrifuge filter, Millipore, Billerica, MA, USA). Y79 and WERI
were infected with lentiviral particles of each shRNA (2 μl of the
virus per 2×105 cells). After 24 h of incubation, the
cell media was changed and cells were allowed to grow for 5 days.
Fluorescent microscopy confirmed GFP expression.
OTX2 promoter assay
HEK293 cells were plated in 24-well plates with
4×105 cells per well the day before transfection. These
cells were confirmed not to have OTX2 expression by immunoblotting.
The next day, the medium was changed by adding different doses of
ATRA from 0, 0.5, 2, 5 and 10 μM in triplicates. Plasmids
pGL3-enhance-OTX2 and pRL-CMV were co-transfected into HEK293 cells
by Lipofectamine 2,000 per protocol. Forty-eight hours later, cells
were harvested and analyzed by dual luciferase reporter assay
system (#E1910, Promega, Madison, WI, USA).
In vivo tumor growth
Once a conditional knockdown system with shRNA was
successfully established and confirmed via immunoblotting, the
infected cells were harvested, washed by phosphate buffer saline
and injected into mice to create a xenograft model for in
vivo tumor growth studies. Immunodeficient athymic nu/nu
mice (Duke Cancer Center Isolation Facility; n=5 per group) were
injected with 1×107 cells on their flank subcutaneously
with a 27-gauge needle per approved animal protocol (#A054-10-03).
Doxycycline (#D9891, Sigma-Aldrich) was given orally to half the
mice in their water (2 mg doxycycline per 1 ml water). Control
groups included cells not infected with viral vectors, cells not
infected with viral vector and given doxycycline, cells infected
with viral vector containing scrambled shRNA and cells infected
with viral vector containing scrambled shRNA and given doxycycline.
Experimental groups included cells infected with viral vectors
containing one of the two shRNAs and cells infected with viral
vectors containing one of the two shRNAs and given doxycycline. The
doxycycline water was changed twice per week. Tumor growth was
measured in two dimensions and the study ended per approved animal
protocol.
Statistical analysis
Experiments were done in triplicate. The
significance level for all tests was set a priori at 0.05.
To compare the treatment and control means, Student's paired
t-tests (2 tailed) were performed.
Results
OTX2 has high expression in
retinoblastoma, minimal expression in normal retina and no
expression in other normal tissues
In order to examine OTX2 expression, we evaluated
OTX2 transcript levels by SAGE data mining of 10 libraries
obtained from normal adult human tissues and tumors (http://cgap.nci.nih.gov/SAGE). The data revealed an
average tag density of 22 for retinoblastoma, compared to an
average tag density of 5.7 for normal retina. Consistent with SAGE
analysis, quantification of OTX2 mRNA by Q-PCR showed minimal
expression in adult retina, no detectable expression in other
normal adult tissues tested and no expression in 18 adult
glioblastoma multiforme tumors (data not shown) (10).
OTX2 is overexpressed in primary
retinoblastoma tumors and cell lines
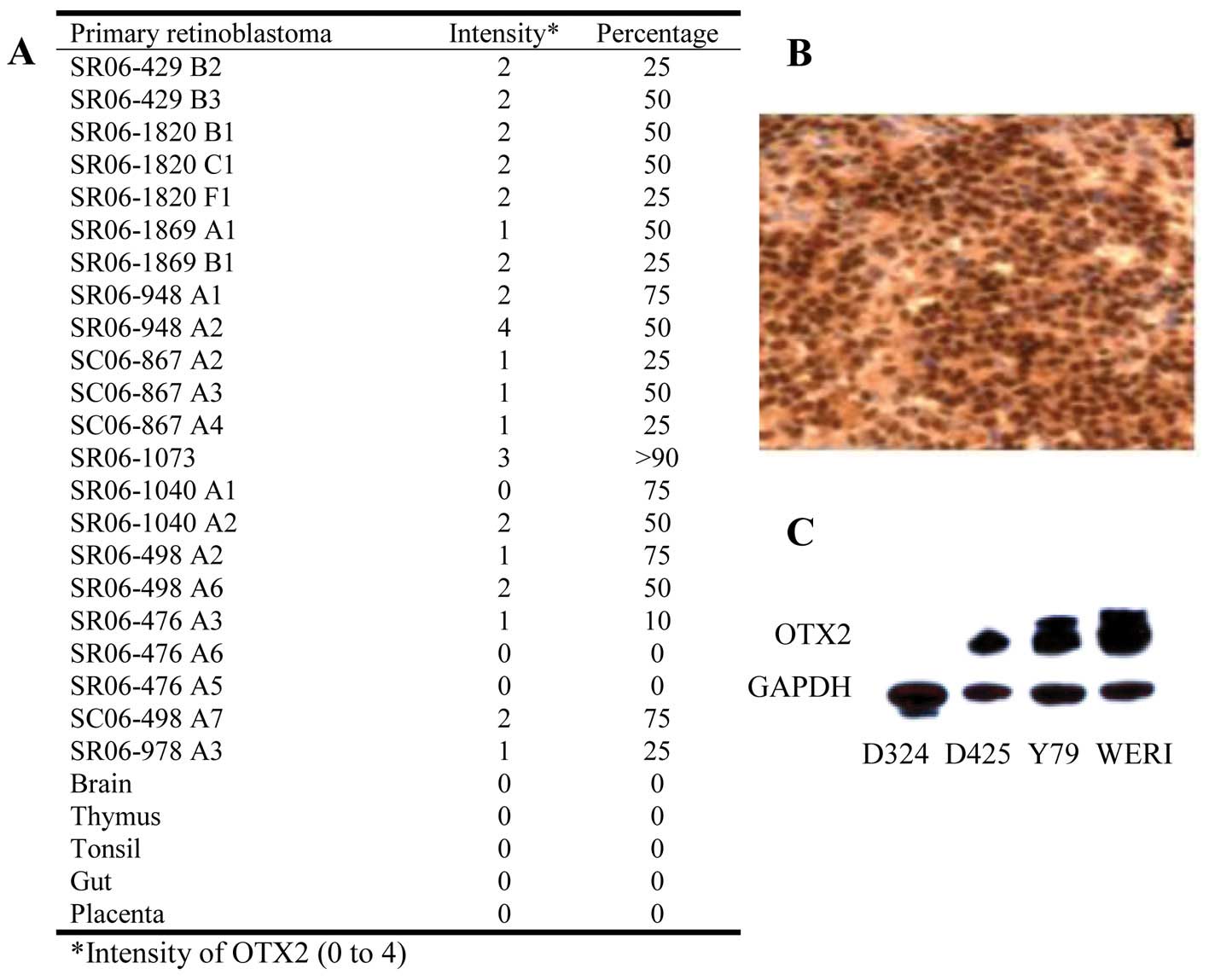
OTX2 overexpression was seen in 86% (19/22) of
primary adult retinoblastoma tumors tested (Fig. 1A). High expression (Fig. 1B, representative tumor with >2
intensity and >50% of cells immunopositive) was seen diffusely
throughout the entire tumors and did not correlate with any
specific histological features including rosettes, vascularity, or
necrosis. OTX2 was robustly overexpressed in Y79 and WERI (RB cell
lines) (Fig. 1C). D425 and D324
medulloblastoma cells served as previously reported positive and
negative controls, respectively. Using Q-PCR, OTX2
amplification was observed in 33% (2/6) of the primary tumors
tested (data not shown).
Inhibition of OTX2 causes increased
apoptosis and decreased proliferation in retinoblastoma cell
lines
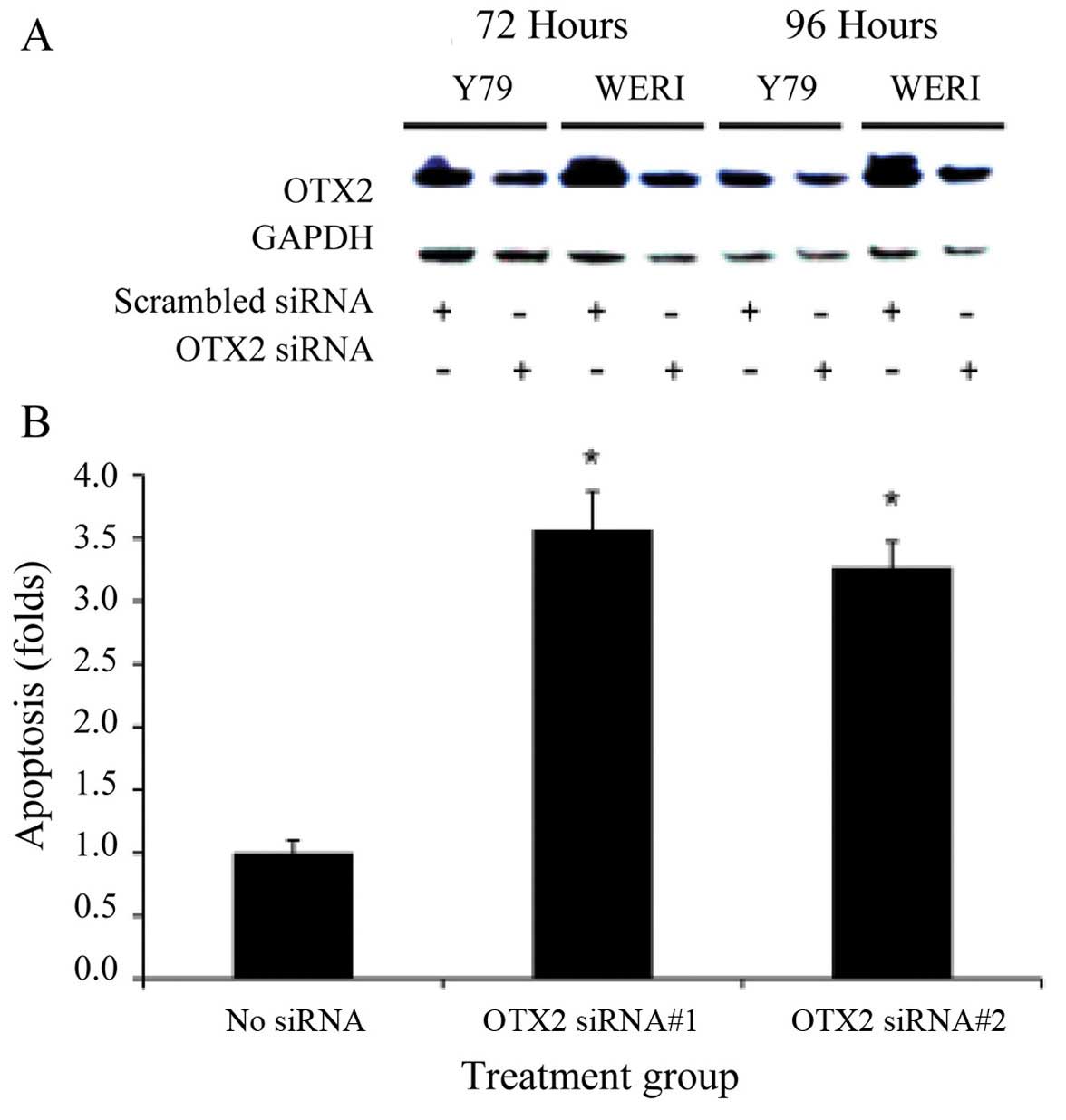
After 72 and 96 h of exposure to siRNA specific for
OTX2, there is a significant reduction of OTX2 expression in Y79
and WERI as normalized to scrambled siRNA treated cells (Fig. 2A, *p<0.05).
Densitometric analysis confirmed significant reduction in OTX2 with
both siRNAs tested (p<0.05). Inhibition of OTX2
expression significantly increased the level of apoptosis in Y79
and WERI as compared to no inhibition by scrambled siRNA (Fig. 2B, *p<0.05). When
measuring Annexin V levels, we routinely get 2–3% (or <0.1-fold
change) apoptosis in our baseline, untreated cells. We also
observed a significant reduction in cell proliferation after 7 days
in cells treated with OTX2-specific siRNA, whereas minimal
effect were seen using the scrambled-siRNA (Fig. 2C, *p<0.05),
normalized to untreated cells. Inhibition by two different siRNAs
strongly supports specificity on the OTX2 gene. Furthermore, a
dose-dependent response to siRNA knockdown was demonstrated while
selecting the optimal siRNA conditions.
Pharmacologic inhibition of OTX2 causes
increased apoptosis, decreased proliferation and colony formation
in retinoblastoma cell lines
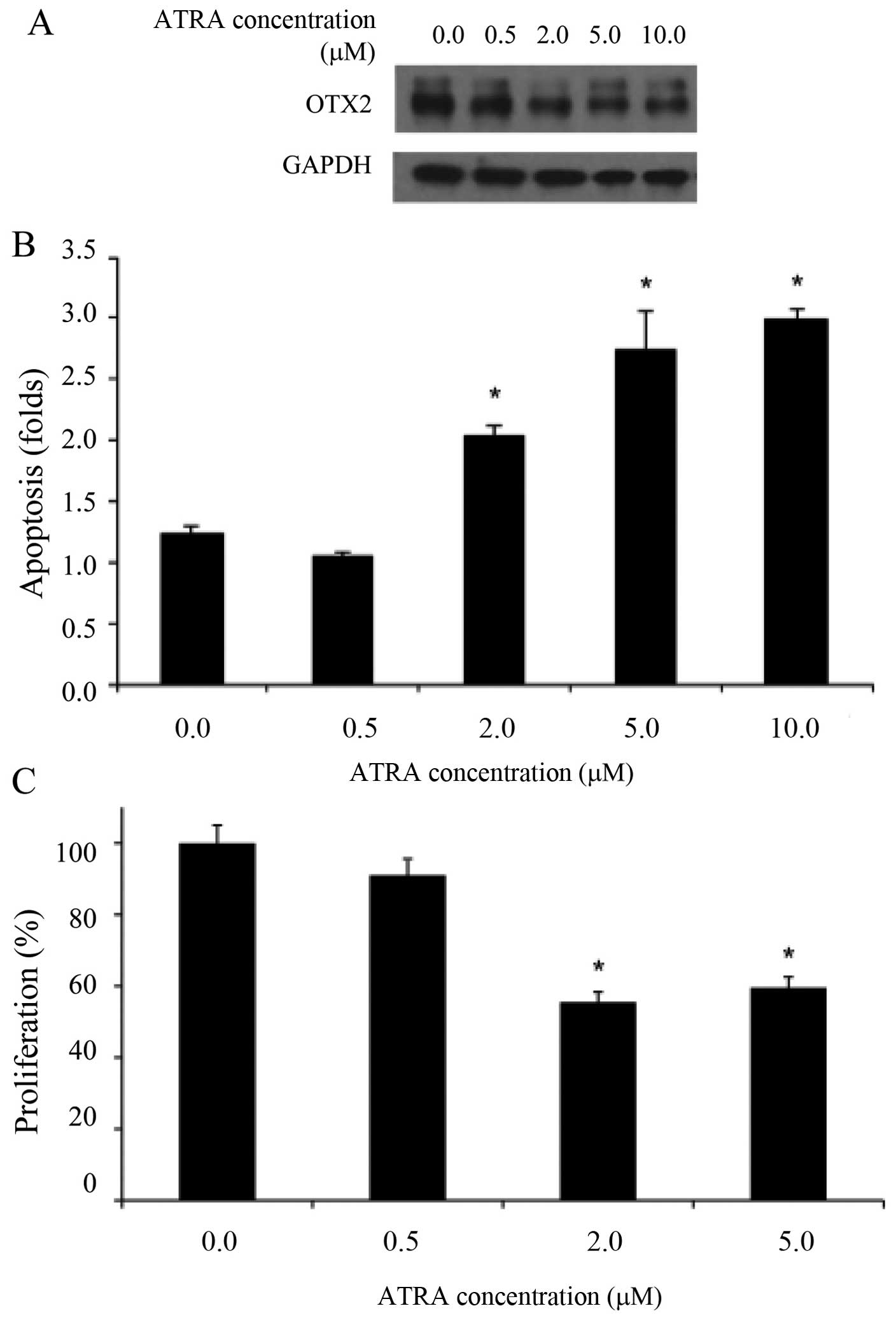
We previously demonstrated that ATRA can
pharmacologically inhibit OTX2 in medulloblastoma cells (10). We confirmed that OTX2 is repressed
after ATRA treatment in retinoblastoma cells in a dose-dependent
manner by immunoblotting (Fig.
3A). Densitometric analysis confirmed significant reduction
(not shown). Inhibition of OTX2 expression with 2 μM ATRA
significantly increased cell apoptosis in Y79 (Fig. 3B, *p<0.05). ATRA (5
μM) increased cell death further at which dose this effect appeared
to plateau since additional ATRA had similar effect. ATRA (2 μM)
also significantly reduced cell proliferation in Y79 (Fig. 3C, *p<0.05).
Similarly, 2 μM ATRA significantly reduced colony formation in Y79
(Fig. 3D, *p<0.05).
Again, 5 μM had additional effect on colony formation at which dose
this effect plateaued. ATRA can target multiple molecular pathways,
so we tested the effect of ATRA in cells transfected with a
luciferase-driven OTX2 promoter. These cells did not have
endogenous OTX2 expression. Fig.
3E shows that ATRA can specifically inhibit the OTX2 promoter
in a dose-dependent manner, suggesting a direct effect of this
agent on OTX2 expression (*p<0.05).
In vivo inhibition of OTX2 in
retinoblastoma xenografts reduces tumor growth and tumor size
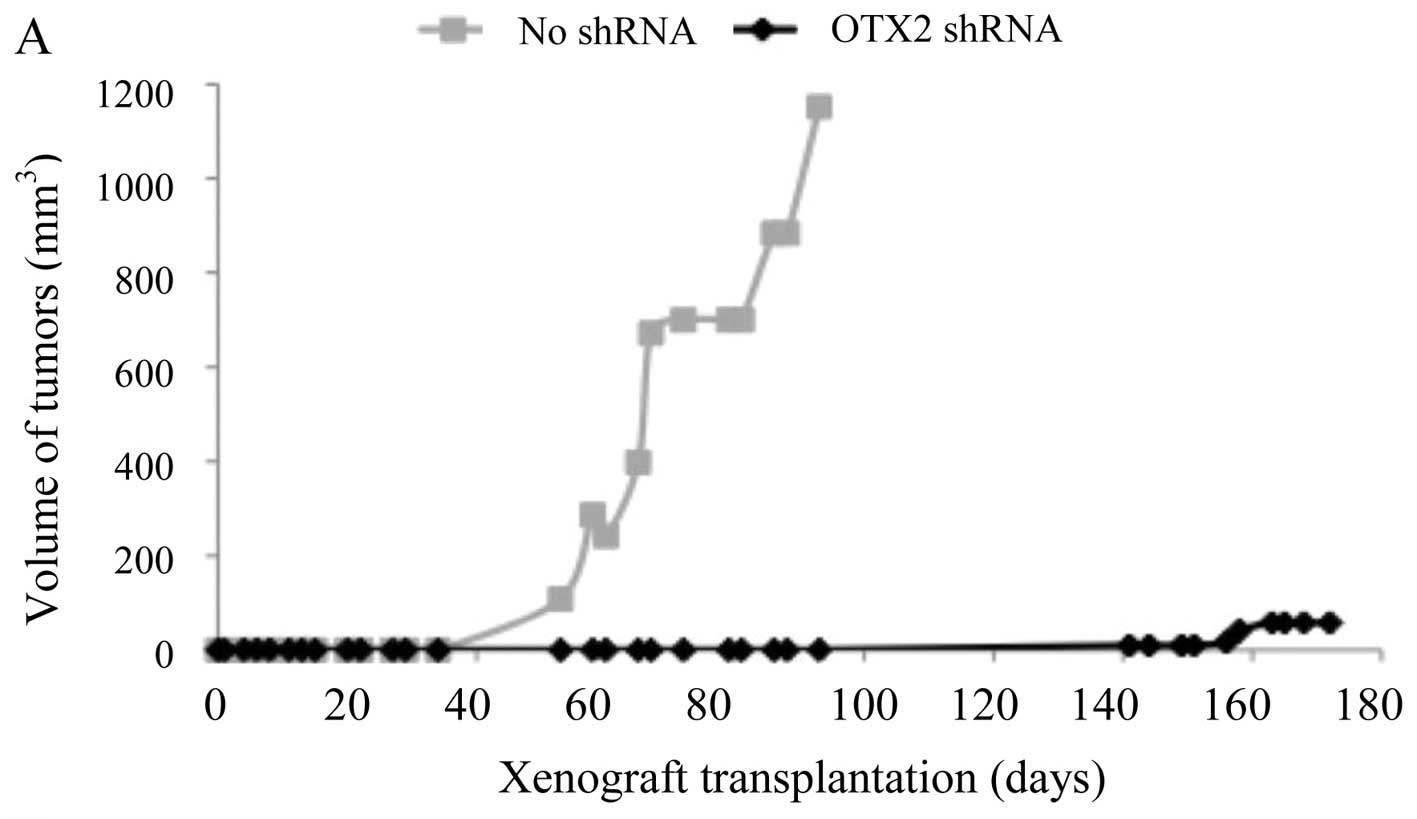
To examine the oncogenic effects of OTX2 in
vivo, we used a conditional knockdown strategy in xenograft
flank models. We observed significant decreases in tumor growth
when OTX2 was suppressed, when compared to controls (Fig. 4A, *p<0.05). Tumors in
the control group appeared significantly earlier (~64 days;
Fig. 4A, gray line) than the
tumors in the knockdown group (~152 days; Fig. 4A, black line;
*p<0.05). At the end of the study, tumors from the
control group (no shRNA, 611.60 mm3) were significantly
larger than the tumors from the knockdown group (OTX2 shRNA, 32.83
mm3; *p<0.05). On average, tumors in the
control group had a doubling time of 7 days, while tumors in the
knockdown group had a doubling time of 15 days. To illustrate that
the difference in tumor growth was mediated through inhibition of
OTX2, IHC on tumors showed robust OTX2 expression in controls (80%
of cells with an intensity of 2+), while knockdown tumors showed
less OTX2 expression (20% of the cells with an intensity of 1+)
(Fig. 4B).
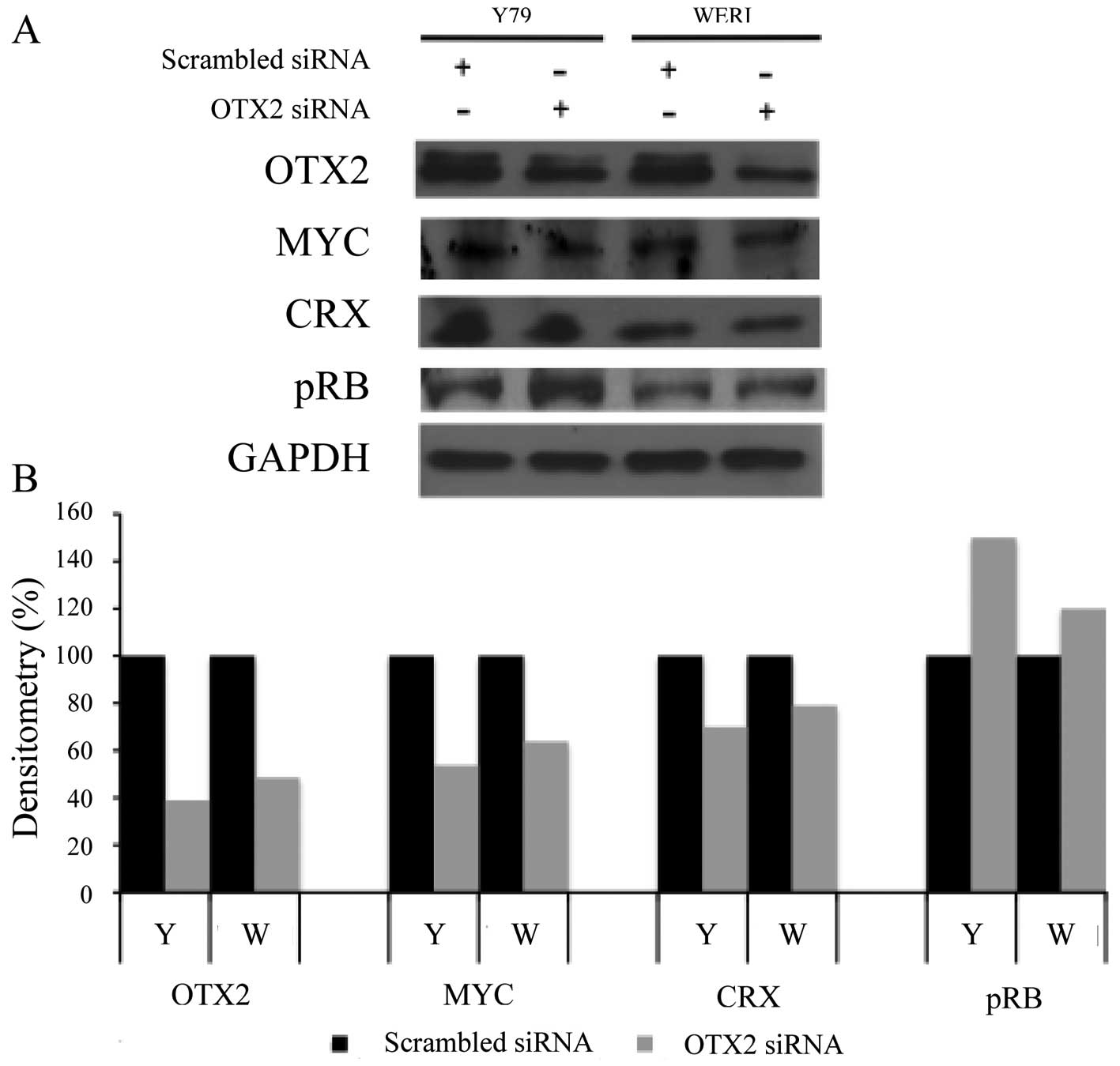
OTX2 inhibition decreases MYC and CRX
expression, but increases pRB expression
To explore possible downstream effects of
OTX2 in retinoblastoma, we examined the effects of
OTX2 inhibition on the expression of genes previously
suggested as OTX2 targets [MYC (11), CRX (22)], or implicated in majority of these
tumors [phosphorylated RB (23)].
OTX2-specific siRNA knockdown in retinoblastoma resulted in
the decreased expression of MYC and CRX but the increased
expression of pRB by immunoblotting (Fig. 5A). Densitometry of the
immunoblotting revealed that after OTX2 inhibition, the
expression of MYC and CRX were decreased by 30% while pRB
expression was increased by 40% (Fig.
5B).
Discussion
Current therapy for retinoblastoma, a malignant
intraocular tumor in children, is non-specific and has many
consequences. Because enucleation causes irreversible vision loss
and because radiation therapy can result in the development of
secondary malignancies in this young patient population, there is
great interest in developing alternative chemotherapeutic agents as
the primary form of treatment (24–27).
A better understanding of specific genetic alterations in
retinoblastoma could offer insight to potential targets for the
treatment of this cancer. We recently identified OTX2 as an
oncogene for medulloblastoma, another common childhood malignant
tumor (10,11). OTX2 is a transcription factor that
is crucial to the patterning of brain development and early brain
morphogenesis, facilitates cell fate in the maturing retina
(14,15) and plays a role in tumorigenesis if
aberrantly expressed later in life (12).
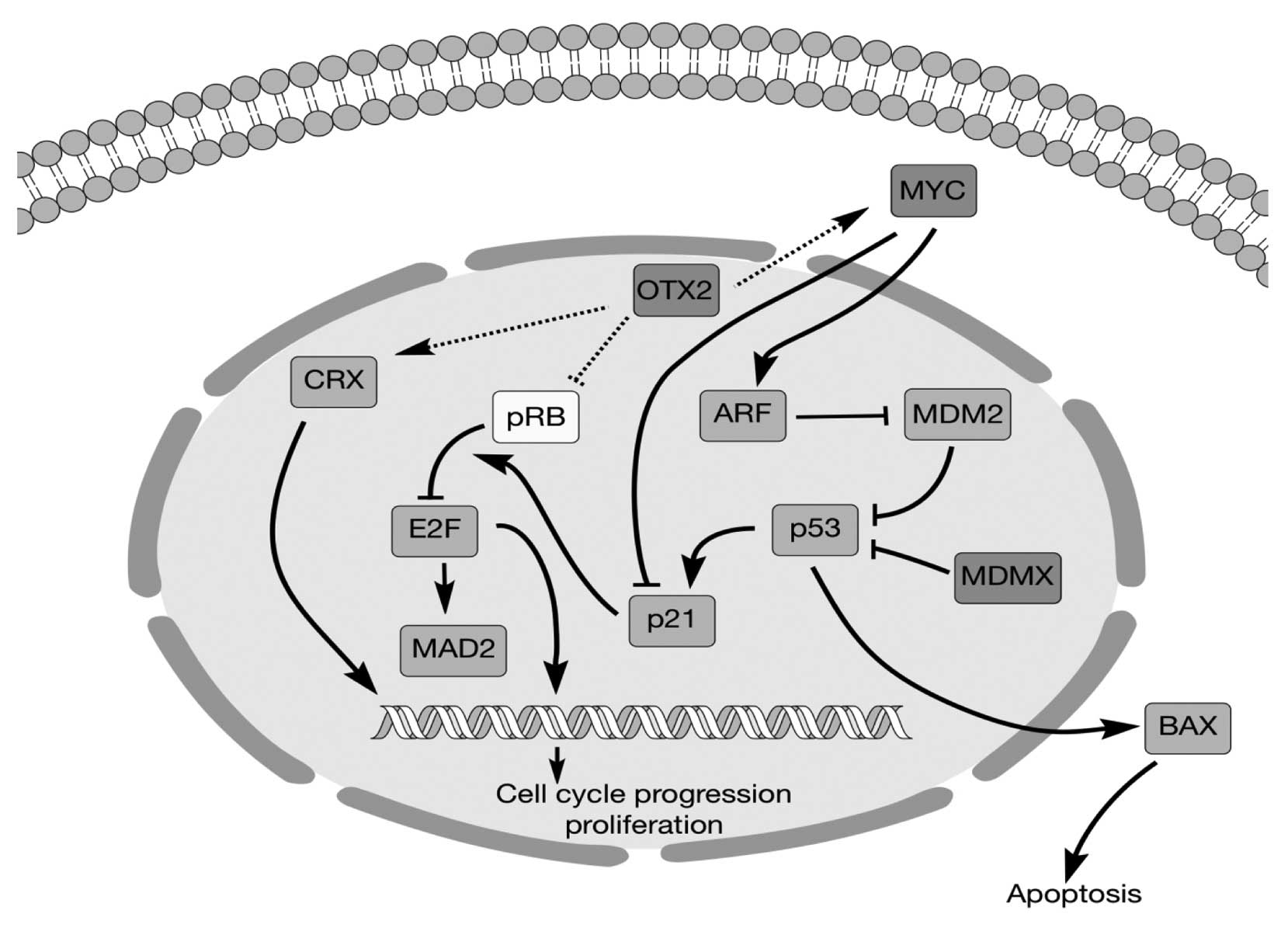
This study shows that the oncogene OTX2,
previously demonstrated in medulloblastoma, may also play an
important oncogenic role in retinoblastoma and can be targeted.
These tumors have distinct characteristics and are typically not
grouped together; yet, they do share some histological and genetic
features. This study and others have now confirmed OTX2 as being
frequently overexpressed in retinoblastoma primary tumors and cell
lines, as well as alteration of related signaling networks
(Fig. 6). In a study of 21
hereditary and non-hereditary retinoblastoma patients, Mol et
al (28) found OTX2 gain and
MYCN gain in non-hereditary tumors. Glubrecht et al
(29) saw widespread
overexpression of OTX2 and CRX in retinoblastoma tumors and cell
lines and suggested a link between these and the retinoblastoma
cell of origin. We further these observations by showing that OTX2
can be specifically targeted to reduce tumorigenesis in
vitro and in vivo. Further experiments are necessary to
clarify how abnormal OTX2 expression may result in the
dysregulation of the developmental programs in retinal progenitor
cells and cause neoplastic transformation.
It is of great interest that aberrant OTX2
expression affects other pathways implicated in retinoblastoma
tumorigenesis (Fig. 6), suggesting
possible mechanisms for initiating or maintaining tumorigenesis and
a possible common therapeutic target. CRX encodes for a
transcription factor that is crucial to the differentiation of
photoreceptor cells (30). A
recent study by Omori et al (22) showed that OTX2 conditional
knockout mice exhibited strong downregulation of CRX in the retina
and here we show that CRX expression is decreased by specific OTX2
inhibition in retinoblastoma cells. OTX2 can actually directly
regulate CRX expression by binding to the cis-regulatory
elements in its promoter region (20). MYC is an oncogene that
promotes cell proliferation in many cancers and is a known
downstream target of oncogenic OTX2 (11,31).
Our previous study showed that OTX2 also directly upregulates the
expression of MYC in medulloblastoma via cis-regulatory
elements in the MYC promoter region (11). RB is a well-known tumor
suppressor gene for retinoblastoma and other cancers that inhibits
cell cycle progression and suppresses cell growth (32). Bunt et al (23) showed that OTX2 expression in
medulloblastoma cells decreased the levels of phosphorylated RB
(pRB). Consistent with this result, our results showed that OTX2
inhibition increased pRB levels in retinoblastoma. Previous studies
by others support the possibility that OTX2 regulates the
expression of all three genes (11,22,23)
and it will be interesting to further clarify the role of OTX2 in
these signaling networks. As seen here, directly targeting OTX2 or
these related factors may be a viable option with siRNA-like
strategies. Alternatively, pharmacologic agents that can drive
terminal differentiation of tumor cells may have a beneficial
impact on curtailing uncontrolled growth of tumors. For example,
retinoic acid can drive photoreceptor cell differentiation
(33). It is conceivable that
retinoid agents may combat tumorigenesis via multiple mechanisms,
e.g., by specifically inhibiting oncogenic factors like OTX2 and at
the same time by promoting the expression of differentiating
factors in retinoblastoma. In our study, 2 μM ATRA consistently
decreased proliferation and colony formation, while increasing cell
death. Furthermore, ATRA specifically repressed OTX2 expression at
its promoter. Although ATRA and other retinoids may affect multiple
molecular pathways, the connection between OTX2 repression and
growth inhibition effect of ATRA suggests that OTX2 expressing
tumors may be amenable to therapy with minimal doses of retinoids
that are less toxic. ATRA is approved clinically for the treatment
of acute promyelocytic leukemia, a disease in which another nuclear
receptor is a predominant determinant of pathogenesis.
Our studies of OTX2, in conjunction with the studies
of others, lay the conceptual framework for clinical trials of
retinoids and OTX2-specific agents in the treatment of this
challenging pediatric tumor. The opportunity to develop new
targeted therapeutic agents against retinoblastoma is of great
interest. Current treatments of enucleation followed by
non-specific adjuvant therapies may cure the tumor, but at grave
costs. Loss of vision is universal and the long-term toxicities of
these adjuvant therapies on this young patient population can be
devastating. More specific agents may be less toxic.
References
|
1
|
Wilson WG: Retinoblastoma. Pediatr Rev.
28:37–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eldebawy E, Parker W, Abdel Rahman W and
Freeman CR: Dosimetric study of current treatment options for
radiotherapy in retinoblastoma. Int J Radiat Oncol Biol Phys.
82:e501–e505. 2012. View Article : Google Scholar
|
|
3
|
Zhang J, Benavente CA, McEvoy J,
Flores-Otero J, Ding L, Chen X, Ulyanov A, Wu G, Wilson M, Wang J,
et al: A novel retinoblastoma therapy from genomic and epigenetic
analyses. Nature. 481:329–334. 2012.PubMed/NCBI
|
|
4
|
Devesa SS: The incidence of
retinoblastoma. Am J Ophthalmol. 80:263–265. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhagia P, Colanta AB, Abramson DH, Carlson
DL, Kleinerman RA, Kraus D and Dunkel IJ: Sinonasal adenocarcinoma:
a rare second malignancy in long term retinoblastoma survivors.
Pediatr Blood Cancer. 57:693–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Draf C, Schaberg MR, Anand VK, Nyquist G
and Hoda S: Radiation induced malignancy in retinoblastoma: new
pathology in a case report. Laryngoscope. 120(Suppl 4): S2382010.
View Article : Google Scholar
|
|
7
|
Lohmann D: Retinoblastoma. Adv Exp Med
Biol. 685:220–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abramson DH, Melson MR, Dunkel IJ and
Frank CM: Third (fourth and fifth) nonocular tumors in survivors of
retinoblastoma. Ophthalmology. 108:1868–1876. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eng C, Li FP, Abramson DH, Ellsworth RM,
Wong FL, Goldman MB, Seddon J, Tarbell N and Boice JD Jr: Mortality
from second tumors among long-term survivors of retinoblastoma. J
Natl Cancer Inst. 85:1121–1128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di C, Liao S, Adamson DC, Parrett TJ,
Broderick DK, Shi Q, Lengauer C, Cummins JM, Velculescu VE, Fults
DW, et al: Identification of OTX2 as a medulloblastoma oncogene
whose product can be targeted by all-trans retinoic acid. Cancer
Res. 65:919–924. 2005.PubMed/NCBI
|
|
11
|
Adamson DC, Shi Q, Wortham M, Northcott
PA, Di C, Duncan CG, Li J, McLendon RE, Bigner DD, Taylor MD, et
al: OTX2 is critical for the maintenance and progression of
Shh-independent medulloblastomas. Cancer Res. 70:181–191. 2010.
View Article : Google Scholar :
|
|
12
|
Mattox A, Li J, Di C and Adamson DC:
Medulloblastoma: role of OTX2 transcription factors. Tumors of the
Central Nervous System. 8. Hayat MA: Springer Science; 2012
|
|
13
|
Martinez-Morales JR, Dolez V, Rodrigo I,
Zaccarini R, Leconte L, Bovolenta P and Saule S: OTX2 activates the
molecular network underlying retina pigment epithelium
differentiation. J Biol Chem. 278:21721–21731. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishida A, Furukawa A, Koike C, Tano Y,
Aizawa S, Matsuo I and Furukawa T: Otx2 homeobox gene controls
retinal photoreceptor cell fate and pineal gland development. Nat
Neurosci. 6:1255–1263. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato S, Inoue T, Terada K, Matsuo I,
Aizawa S, Tano Y, Fujikado T and Furukawa T: Dkk3-Cre BAC
transgenic mouse line: a tool for highly efficient gene deletion in
retinal progenitor cells. Genesis. 45:502–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elias WJ, Lopes MBS, Golden WL, Jane JA
and Gonzalez-Fernandez F: Trilateral retinoblastoma variant
indicative of the relevance of the retinoblastoma tumor-suppressor
pathway to medulloblastomas in humans. J Neurosurg. 95:871–878.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jurkiewicz E, Pakuła-Kościesza I,
Rutynowska O and Nowak K: Trilateral retinoblastoma: an
institutional experience and review of the literature. Child's
Nervous System. 26:129–132. 2010. View Article : Google Scholar
|
|
18
|
Mouratova T: Trilateral retinoblastoma: a
literature review, 1971–2004. Bull Soc Belge Ophtalmol. 297:25–35.
2005.
|
|
19
|
Jaffey PB, To GT, Xu HJ, Hu SX, Benedict
WF, Donoso LA and Campbell GA: Retinoblastoma-like phenotype
expressed in medulloblastomas. J Neuropathol Exp Neurol.
54:664–672. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santagata S, Maire CL, Idbaih A, Geffers
L, Correll M, Holton K, Quackenbush J and Ligon KL: CRX is a
diagnostic marker of retinal and pineal lineage tumors. PLoS One.
4:e79322009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stephan H, Zakrzewski JL, Bölöni R,
Grasemann C, Lohmann DR and Eggert A: Neurotrophin receptor
expression in human primary retinoblastomas and retinoblastoma cell
lines. Pediatr Blood Cancer. 50:218–222. 2008. View Article : Google Scholar
|
|
22
|
Omori Y, Katoh K, Sato S, Muranishi Y,
Chaya T, Onishi A, Minami T, Fujikado T and Furukawa T: Analysis of
transcriptional regulatory pathways of photoreceptor genes by
expression profiling of the Otx2-deficient retina. PLoS One.
6:e196852011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bunt J, De Haas TG, Hasselt NE,
Zwijnenburg DA, Koster J, Versteeg R and Kool M: Regulation of cell
cycle genes and induction of senescence by overexpression of OTX2
in medulloblastoma cell lines. Mol Cancer Res. 8:1344–1357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong FL, Boice JD Jr, Abramson DH, Tarone
RE, Kleinerman RA, Stovall M, Goldman MB, Seddon JM, Tarbell N,
Fraumeni JF Jr, et al: Cancer incidence after retinoblastoma.
Radiation dose and sarcoma risk. JAMA. 278:1262–1267. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohney BG, Robertson DM, Schomberg PJ and
Hodge DO: Second nonocular tumors in survivors of heritable
retinoblastoma and prior radiation therapy. Am J Ophthalmol.
126:269–277. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith LM, Donaldson SS, Egbert PR, Link MP
and Bagshaw MA: Aggressive management of second primary tumors in
survivors of hereditary retinoblastoma. Int J Radiat Oncol Biol
Phys. 17:499–505. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shields CL and Shields JA: Retinoblastoma
management: advances in enucleation, intravenous chemoreduction,
and intra-arterial chemotherapy. Curr Opin Ophthalmol. 21:203–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mol BM, Massink MP, van der Hout AH,
Dommering CJ, Zaman JM, Bosscha MI, Kors WA, Meijers-Heijboer HE,
Kaspers GJ, Riele Ht, et al: High resolution SNP array profiling
identifies variability in retinoblastoma genome stability. Genes
Chromosomes Cancer. 53:1–14. 2014. View Article : Google Scholar
|
|
29
|
Glubrecht DD, Kim JH, Russell L, Bamforth
JS and Godbout R: Differential CRX and OTX2 expression in human
retina and retinoblastoma. J Neurochem. 111:250–263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Furukawa T, Morrow EM and Cepko CL: Crx, a
novel otx-like homeobox gene, shows photoreceptor-specific
expression and regulates photoreceptor differentiation. Cell.
91:531–541. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nilsson JA and Cleveland JL: Myc pathways
provoking cell suicide and cancer. Oncogene. 22:9007–9021. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wiman K: The retinoblastoma gene: role in
cell cycle control and cell differentiation. FASEB J. 7:841–845.
1993.PubMed/NCBI
|
|
33
|
Khanna H, Akimoto M, Siffroi-Fernandez S,
Friedman JS, Hicks D and Swaroop A: Retinoic acid regulates the
expression of photoreceptor transcription factor NRL. J Biol Chem.
281:27327–27334. 2006. View Article : Google Scholar : PubMed/NCBI
|