Introduction
Hepatocellular carcinoma (HCC) is the most common
disease that causes primary liver tumors and the fifth most common
malignant disease in the world (1). HCC develops frequently in patients
with liver cirrhosis (LC) (2). HCC
has a poor prognosis and a high rate of recurrence, even after
treatment. Currently, sorafenib is the only available
chemotherapeutic agent for advanced HCC, but it cannot be used to
treat patients with LC and thrombocytopenia due to the risk of
bleeding (3–5). Therefore, there is an urgent need for
the development of effective therapeutic agents for HCC.
Thrombopoietin (TPO) is the most important growth
factor in the regulation of megakaryocyte development and platelet
production (6). TPO is the
endogenous ligand of the TPO receptor [(TPO-R, also known as
myeloproliferative leukemia virus oncogene (MPL)], which is
expressed on the surface of megakaryocytes, megakaryocyte
precursors and platelets (7–10).
Eltrombopag (EP) is an oral, nonpeptide, small
molecule TPO-R agonist that has proven efficacy in treating chronic
immune thrombocytopenic purpura (ITP) and hepatitis C-related
thrombocytopenia (11,12).
Despite concerns that some leukemia blast cells
express TPO-R, EP has been reported to have no proliferative
effects in patients with myelodysplastic syndromes or AML (13); instead, EP inhibits the
proliferation of leukemia cell lines (14).
Additionally, recent studies have demonstrated that
the antileukemic effect of EP is not related to the TPO-R pathway
(15) and that EP inhibits the
growth of breast, lung, and ovarian tumor cells (16). EP has been reported to increase
platelet counts in a short-term clinical study that included
patients with HCC (17). However,
it remains unclear whether EP has an antitumor effect in HCC.
The purpose of this study was to clarify whether EP
affects the growth of human HCC cells in vitro.
Materials and methods
Reagents
EP (SB-497115; Selleck Chemicals, Houston, TX, USA)
was dissolved as a 1 mg/ml stock solution in distilled water and
was stored in the dark at room temperature for up to 2 weeks.
Deferoxamine mesylate (DFO) and ferrous ammonium citrate (FAC) were
purchased from Sigma-Aldrich (St. Louis, MO, USA), and sorafenib
was purchased from Invitrogen (Grand Island, NY, USA).
Cell culture
Human hepatoblastoma cell lines (HepG2 and Hep3B)
and a well-differentiated human HCC cell line (Huh7) were used in
these experiments. HepG2, Hep3B and Huh7 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) supplemented with 10% fetal bovine
serum (FBS; HyClone, Logan, UT, USA) and 1% penicillin/streptomycin
(Invitrogen).
Cell proliferation
Following cell incubation at 37°C for 24 h, the
medium was changed to 100 μl of DMEM supplemented with 10%
FBS, and different concentrations (0.1, 0.4, 1, 4, 10, 40 and 100
μg/ml) of EP were added to each well. A recent double-blind,
placebo-controlled, randomized dose-escalation study showed that
maximum EP serum concentrations of >20 μg/ml are
clinically achievable with minimal toxicity (18). The reported IC50 values
of EP in other cancer cell lines range from 4.8 to 49.7
μg/ml (16). Additionally,
0.4 μg/ml EP is equivalent to 1 μM of EP. Therefore,
we selected the concentrations indicated above. After a 72-h
incubation at 37°C, the cells were counted based on DNA content
using a bromode-oxyuridine (BrdU) assay kit (Roche Diagnostics,
Penzberg, Germany) and Cell Counting Kit-8 (CCK-8) (Dojindo
Laboratories, Kumamoto, Japan) according to the instructions of the
manufacturers.
The mechanism by which EP inhibits HCC cell growth
has not been well characterized. EP reduces intracellular iron
concentrations, and iron chelators exhibit anti-proliferative
effects in myeloid leukemia cells (15). We investigated whether the
antitumor effect of EP is secondary to its ability to deplete
intracellular iron. Iron was pre-loaded into cells by treatment
with 500 μg/ml FAC for 24 h prior to EP addition.
Cell cycle analysis
Changes in the cell cycle after EP treatment were
assessed using a Muse™ cell cycle kit (EMD Millipore, Billerica,
MA, USA). The cells were cultured in 10-cm dishes with various
concentrations of EP (0–100 μg/ml) for 72 h. After
treatment, the cells were collected in DMEM supplemented with 1%
FBS, and the Muse™ cell cycle test reagent was added. The cells
were mixed by vortexing, and the reaction was allowed to proceed
for 30 min at room temperature in the dark. Then, the cells were
stained to determine the proportions of cells in the G0/G1, S and
G2/M phases using a Muse™ cell analyzer (EMD Millipore).
Western blot analysis of transcription
factors
Huh7 cells and hepatocytes were pre-cultured
separately in 6-well plates for 24 h; subsequently, the medium was
changed to DMEM supplemented with 10% FBS, and 0–100 μg/ml
EP was added to each well. Cells were harvested 72 h after the
addition of EP. For the western blot analysis, the cell lysates
were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a nitrocellulose
membrane (EMD Millipore). The membranes were exposed to primary
antibodies against cyclin-dependent kinase inhibitor 1A
(p21/CDKN1A) and the G1/S-specific protein cyclin D1 (CCND1).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling
Technology, Inc., Beverly, MA, USA) was used as an endogenous
control. An anti-rabbit immunoglobulin G horseradish
peroxidase-linked secondary antibody was used (Cell Signaling
Technology, Inc.).
The most common iron chelator: DFO
To compare the effects of DFO, with those of EP,
Huh7 cells were treated with DFO (0.1–100 μg/ml) for 72 h,
or not treated. Cell viability was measured at 72 h using a CCK-8
assay as described above.
Evaluation of the combined effects of EP
and sorafenib
Huh7 cells were exposed to combinations of EP (0–40
μg/ml) and sorafenib (4 μM) for 72 h. The effect on
cell proliferation was assessed using the CCK-8 assay, and the rate
of growth inhibition was calculated using the combination index
(CI) according to the method reported by Chou (19). The CI was obtained using Biosoft
CalcuSyn software (Biosoft, Cambridge, UK): CI=1, cumulative
effect; CI<1, synergistic effect; and CI>1, antagonistic
effect.
Statistical analysis
The data are presented as the mean and standard
deviation. Statistical analyses were performed using the
Mann-Whitney U test or one-way ANOVA, and significant results were
analyzed using the Bonferroni-Dunn multiple comparisons post hoc
test. In all cases, P<0.05 was considered significant.
Results
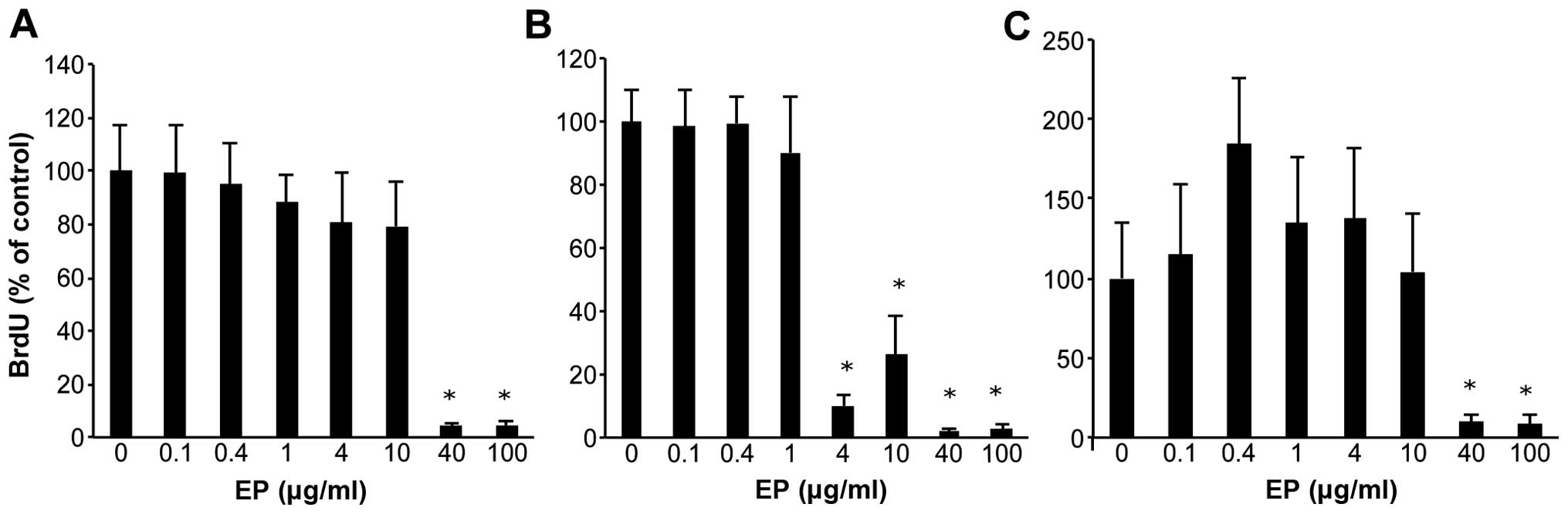
Effect of EP on human HCC cell lines
HCC cells were treated with various doses of EP for
72 h, and cell viability was assessed using the BrdU assay
(Fig. 1A–C). As the dose of EP
increased from 0.1 to 100 μg/ml, cell growth was inhibited
in a dose-dependent manner in Huh7, HepG2 and Hep3B cells. Compared
with the non-treated cells, EP significantly inhibited cell
proliferation at concentrations of 40–100 μg/ml based on the
BrdU assay results.
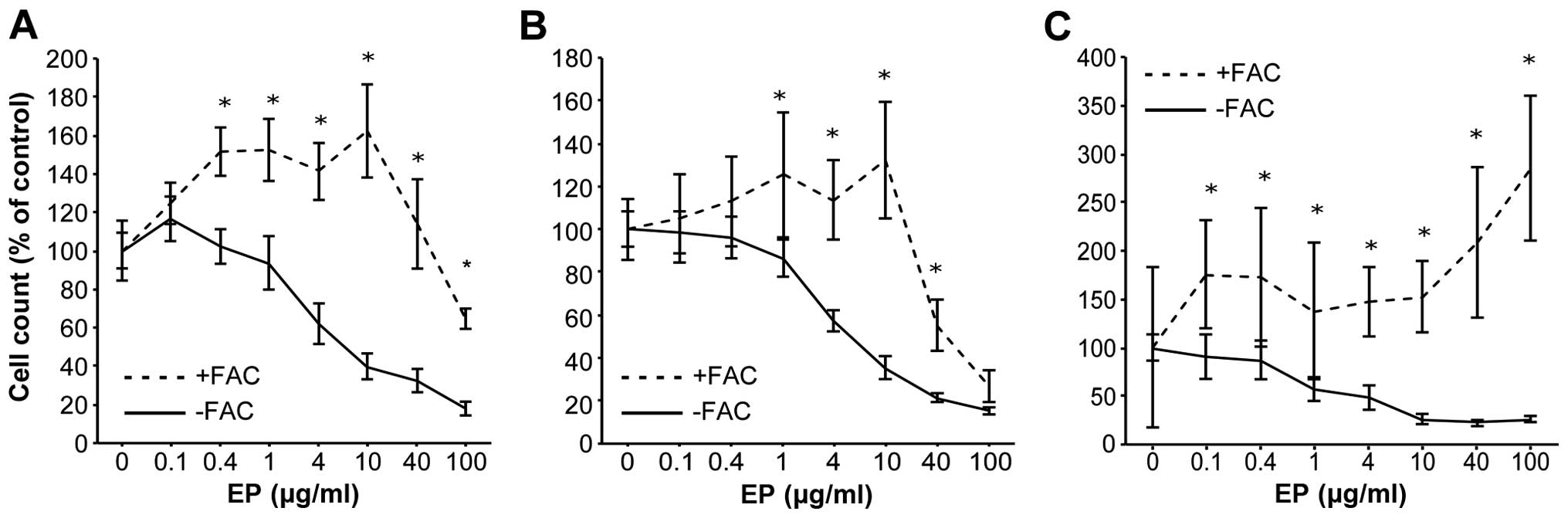
Preloading HCC cell lines with iron
diminishes the anti-proliferative effects of EP
Huh7, HepG2 and Hep3B cells were preloaded with iron
(500 μg/ml FAC) for 24 h before EP addition to clarify the
antitumor mechanism of action of EP. CCK-8 cell proliferation
assays were performed after 72 h. The IC50 values of EP
in the HCC cell lines were 5.7 μg/ml for Huh7, 5.4
μg/ml for HepG2, and 4.7 μg/ml for Hep3B. Iron-loaded
Huh7 cells were non-treated (control), or treated with EP.
Preloading Huh7 cells with iron nearly abolished the
anti-proliferative effects of EP (Fig.
2A–C).
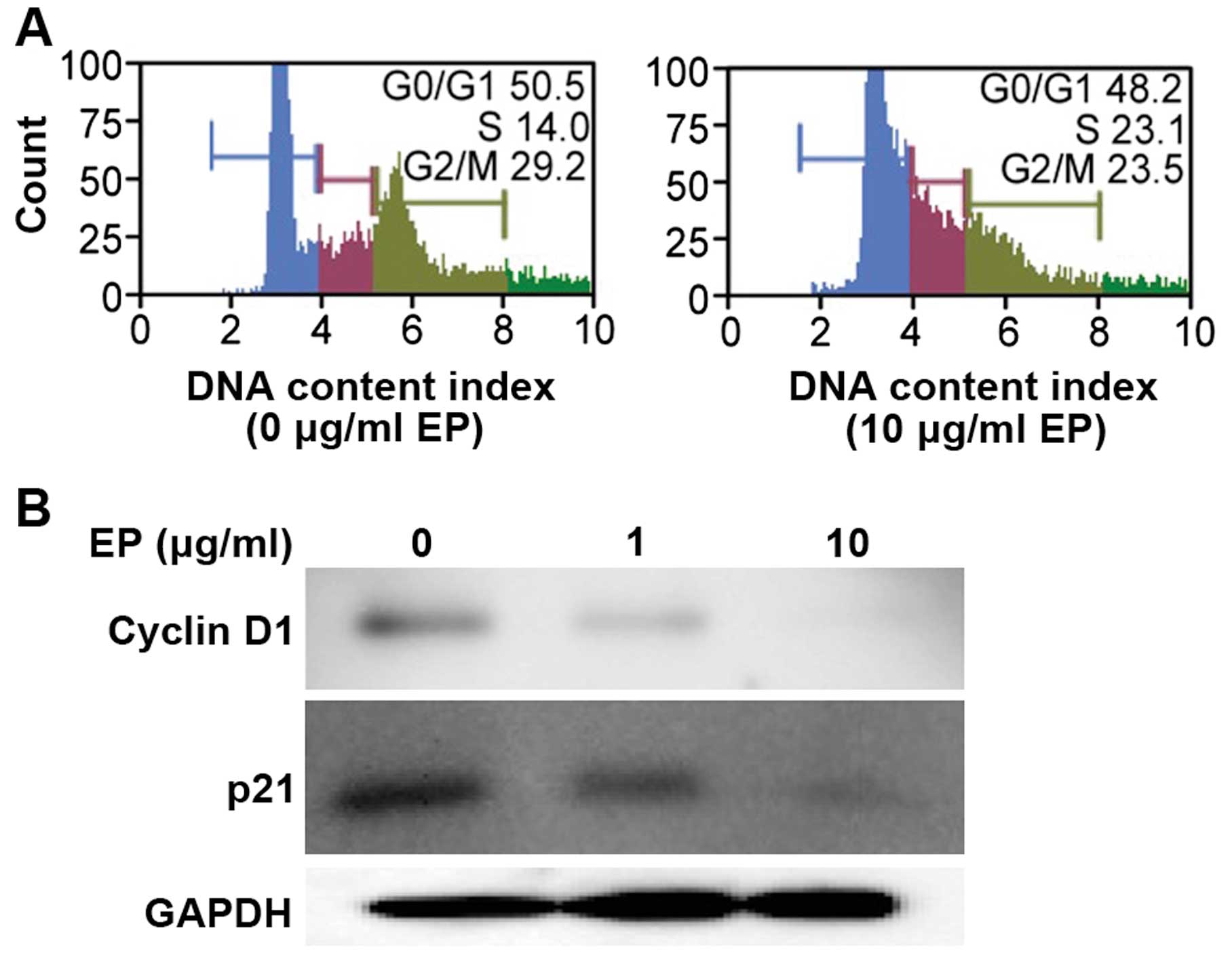
EP elicited G1 arrest in a human HCC cell
line
Huh7 cells were treated with EP (0 or 10
μg/ml) for 72 h, and the cells were monitored during the
transition from the G0/G1 phase to S phase. Treatment with 10
μg/ml EP significantly induced G0/G1 phase arrest compared
with control treatment (Fig.
3A).
Effects of EP on cell cycle-related
protein expression in an HCC cell line
Treatment with iron chelators significantly
decreases CCND1 levels in various cancer cell types (20). Therefore, we measured the protein
levels of CCND1 and p21/CDKN1A using western blot analysis.
Treatment with EP (0, 1, or 10 μg/ml) for 72 h downregulated
the protein levels of CCND1 and p21/CDKN1A in a dose-dependent
manner (Fig. 3B).
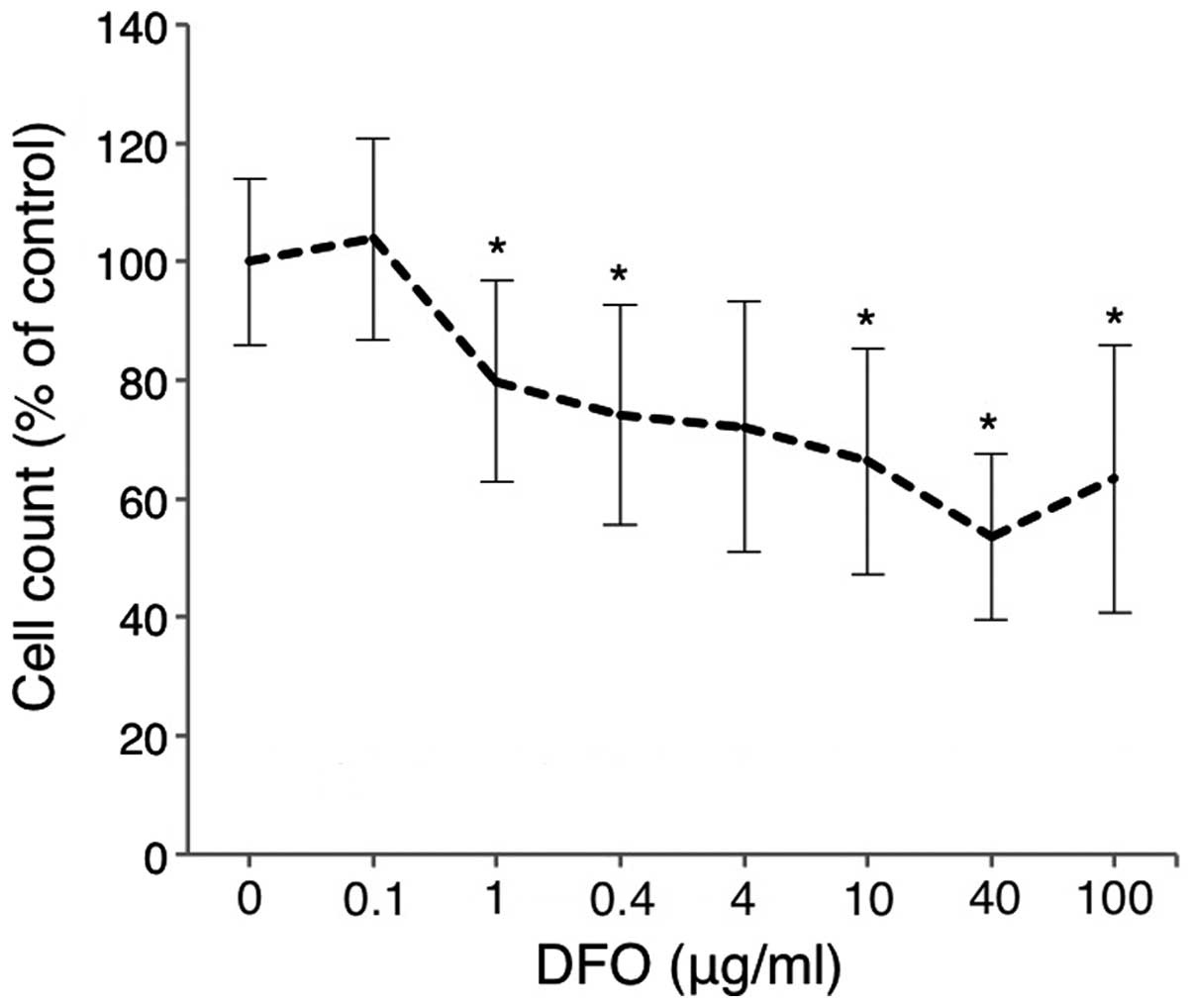
Comparison of the antitumor effects of
DFO and EP
Huh7 cells were treated with various doses of DFO
for 72 h, and cell viability was analyzed using the CCK-8 assay.
Cell proliferation was inhibited in a dose-dependent manner in Huh7
cells treated with DFO (0–100 μg/ml) (Fig. 4). EP was nearly equivalent to the
most common iron chelator DFO, in terms of the inhibition of cell
proliferation.
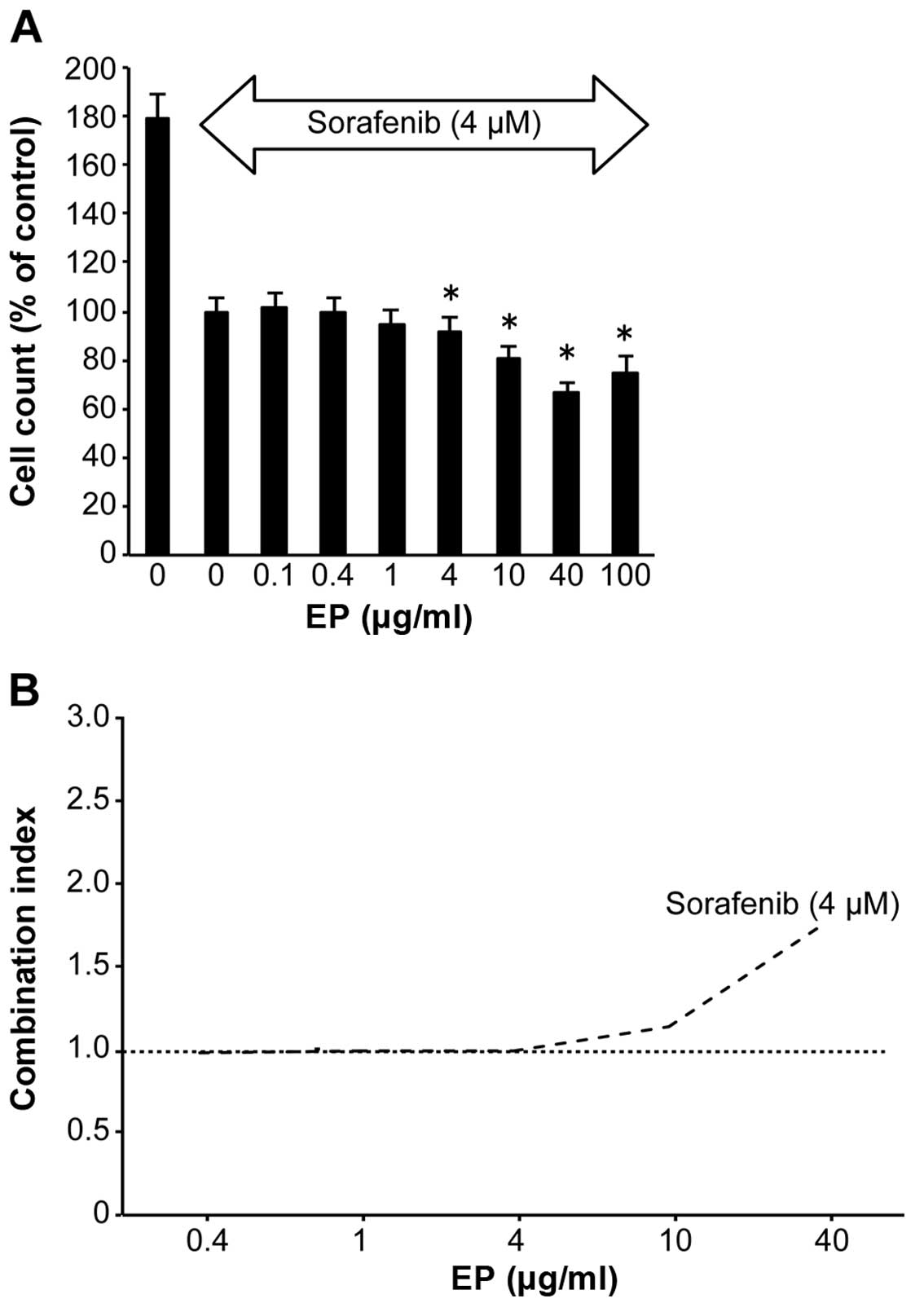
Combined effect of sorafenib and EP on
HCC cells
Compared with untreated cells, EP (4–100
μg/ml) significantly inhibited Huh7 cell proliferation in a
dose-dependent manner, as evidenced by the CCK-8 assay results, in
the presence of the antitumor agent 4 μM of sorafenib
(Fig. 5A). The CI values were ~1
at 0.1–10 μg/ml EP (Fig.
5B). EP clearly did not antagonize sorafenib.
Discussion
Patients with advanced HCC and LC often present with
thrombocytopenia. Therefore, it is difficult to use sorafenib,
which currently is the only recognized effective treatment for
advanced HCC (4).
EP, a second-generation TPO-R agonist that has the
ability to increase platelet count, has been reported to have
antitumor effects in several types of cancer (16).
The purpose of our study was to clarify the effects
of EP on the growth of human HCC cells.
In the present study, EP exhibited strong antitumor
activity in HCC by eliciting cell cycle arrest through iron
chelation rather than through TPO-R. We concluded that EP
represents a novel treatment for HCC.
TPO-R, also known as myeloproliferative leukemia
virus oncogene (MPL), has been reported to be expressed in liver
sinusoidal endothelial cells (LSEC) in mice and in liver progenitor
cells in rats, and TPO promotes the proliferation of both of these
cell types (21,22).
However, we have already reported that TPO has no
proliferative effect on HCC in vitro or in vivo
(23).
In contrast to TPO, the second-generation small
molecule TPO-R agonist EP does not induce the production of
neutralizing antibodies (24–28).
Recent studies have demonstrated that EP inhibits
leukemia cell growth by depleting intracellular iron (15) and inhibits the growth of breast,
lung and ovarian tumor cells (16).
These findings of tumor cell growth inhibition by EP
in vitro and in vivo demonstrate that these effects
of EP are TPO-R-independent.
The reported IC50 values of EP in various
cell lines are 9.6–19.0 μg/ml for breast, 3.7–10.3
μg/ml for lung, and 4.8–49.7 μg/ml for ovarian
cancer. These results in our study supported the previous studies
that indicated IC50 of EP in tumor cells.
The observed median Cmax for EP in patients with ITP
is 11.4 μg/ml at a 75-mg dose (29). The efficacy of EP in hepatitis C
virus-infected patients with thrombocytopenia before the initiation
of pegylated interferon and ribavirin therapy has been reported
(12). A recent double-blind,
placebo-controlled, randomized dose-escalation study showed that
maximum serum concentrations of EP of >20 μg/ml are
clinically achievable with minimal toxicity (18).
EP has three primary features: a lipophilic end, an
acidic end, and a chelator backbone (30,31).
These elements enable EP to reduce intracellular iron as well as
possibly other polyvalent cations (24,32).
Iron (Fe) is a metal that is vital for the
sustenance of life (33–35). It is an essential component of many
proteins and enzymes that are involved in cell growth and
replication (33,35). Cellular Fe depletion typically
results in G1/S arrest (36,37),
which indicates that this metal is essential for cell cycle
progression as well as cell growth and division (38,39).
Compared with normal cells, neoplastic cells require
a greater amount of Fe because they generally proliferate at a
higher rate than their normal counterparts (39,40).
This is reflected by the upregulated expression of the transferrin
receptor protein 1 (TfR1) (41)
and the higher rate of Fe uptake from transferrin (Tf) in cancer
cells (42,43). Furthermore, neoplastic cells
express high levels of ribonucleotide reductase (RR) (44,45),
rendering them more susceptible to Fe chelators than normal cells
(39,46). Early studies showed that the
clinically utilized Fe chelator, deferoxamine (DFO), exerts some
inhibitory effects on the growth of neuroblastoma and leukemia
cells in culture and in clinical trials (47–51).
One mechanism by which Fe chelators exert
anti-proliferative effects on tumors is through targeting molecules
that are critical for regulating cell cycle progression (52,53).
Importantly, the effects of Fe chelators on CCND1
expression were determined to be due to Fe depletion, as Fe
supplementation reversed these effects (20).
Furthermore, Fe depletion appears to have similar
effects on both CCND1 and p21CIP1/WAF1 protein expression: it
induces the ubiquitin-independent degradation of these proteins
(20,54).
The following important findings were also obtained
via our experiments.
In addition to DFO, the most common iron chelator
(55), which is used in the
clinic, recent studies have characterized new iron chelators, such
as deferasirox, Dp44mT (56) and
O-Trensox (57). These iron
chelators induce growth inhibition in breast cancer cells and
neuroblastoma (37,58). Although DFO, which exhibited a
similar antitumor effect as EP in our study, is commonly used in
patients, its poor membrane permeability and inability to permit
redox cycling of iron are disadvantageous in terms of eliciting an
anti-proliferative effect (55).
Additionally, DFO is poorly absorbed in the intestine, and it must
be administered via an intravenous route; furthermore, DFO has a
very short plasma half-life (55).
These disadvantages prompted the search for more effective
chelators that are easier to administer to patients. From the
results of our study, EP would be a candidate for drug as effective
chelator in a clinical study.
It is well known that patients with the congenital
iron overload disease hemochromatosis are more likely to develop
HCC compared with the general population (2). A review by Gangaidzo and Gordeuk
suggest that iron overload may contribute to the high incidence of
HCC in Africa (59).
Furthermore, iron chelators have been reported to be
useful for HCC treatment in vitro, in vivo and in the
clinic (57,60,61).
Iron reduction therapy, such as phlebotomy or a low
iron diet, is used to improve liver function and to prevent
carcinogenesis in patients with chronic hepatitis C (62,63).
Conversely, it has been reported that the iron
chelator DFO protects against the cytotoxic effects of sorafenib in
HCC cells (64). The findings
suggest that sorafenib can induce ferroptosis, which is a novel
form of programmed cell death.
Thus, a potential method to attenuate the effects of
sorafenib is to reduce intracellular iron levels. The cytotoxic
effect of sorafenib is markedly offset when the concentration of EP
increases, which is consistent with our results (Fig. 5).
Drug repositioning, in which existing drugs are used
for new purposes, is a cost-effective strategy for identifying new
treatments for existing conditions, and finding use for drugs for
which development has ceased; this strategy has been used to
identify new usage for several drugs, including thalidomide and
plerixafor (65,66). In recent years, significant
progress in drug discovery technology and bioinformatics has
facilitated drug repositioning, and new drug discovery tools are
eliminating the innovation gap (67). This study identified EP as a
candidate for drug repositioning efforts, which should lead to the
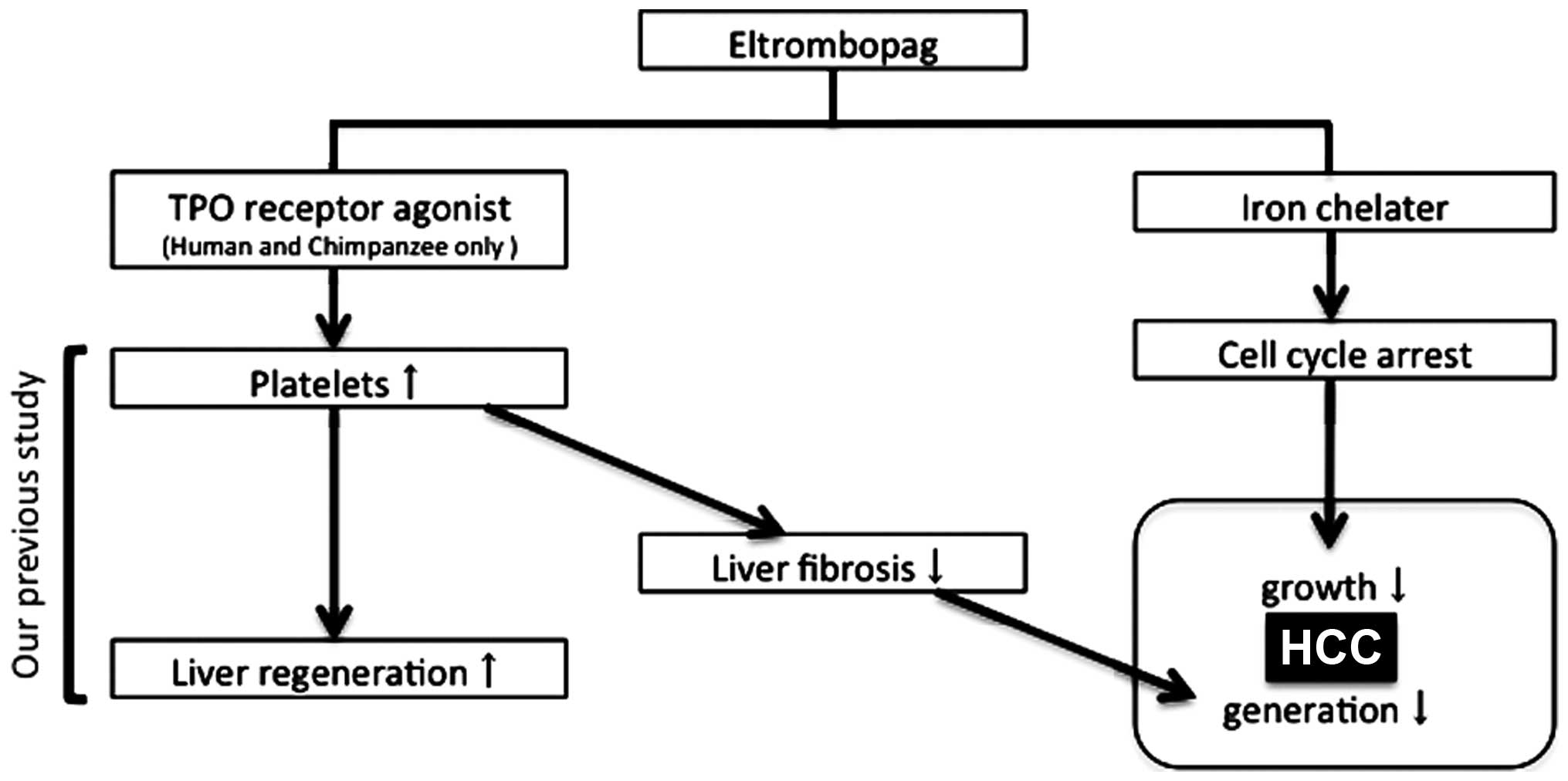
novel clinical use of EP in patients with HCC and LC (Fig. 6).
EP interacts with the transmembrane domain of TPO-R.
This interaction and subsequent TPO-R downstream signaling is
highly species-specific and occurs only in humans and primates, not
in murine cells (68).
Therefore, it is difficult to conduct an in
vivo study on EP, and the lack of in vivo data
represents a limitation of this study.
However, short-term EP treatment is useful and safe
in combination with radio-frequency ablation in HCC patients with
LC and severe thrombocytopenia, as the risk of bleeding is
mitigated by the increase in platelets (69); moreover, EP does not have a
proliferative effect on HCC (69).
This option should be considered in clinical trials.
In conclusion, the present study demonstrated that
EP is a promising and safe chemotherapeutic agent for the treatment
of HCC in patients with LC and thrombocytopenia.
Acknowledgements
The present study was supported in part by grants
from the Ministry of Education, Culture, Sports, and Science and
Technology of Japan (MEXT).
References
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montalto G, Cervello M, Giannitrapani L,
Dantona F, Terranova A and Castagnetta LA: Epidemiology, risk
factors, and natural history of hepatocellular carcinoma. Ann N Y
Acad Sci. 963:13–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abou-Alfa GK, Schwartz L, Ricci S, Amadori
D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz
B, et al: Phase II study of sorafenib in patients with advanced
hepatocellular carcinoma. J Clin Oncol. 24:4293–4300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al; SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterology.
140:1410–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolber E-M and Jelkmann W: Thrombopoietin:
The novel hepatic hormone. News Physiol Sci. 17:6–10.
2002.PubMed/NCBI
|
|
7
|
de Sauvage FJ, Carver-Moore K, Luoh SM,
Ryan A, Dowd M, Eaton DL and Moore MW: Physiological regulation of
early and late stages of megakaryocytopoiesis by thrombopoietin. J
Exp Med. 183:651–656. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaushansky K and Drachman JG: The
molecular and cellular biology of thrombopoietin: The primary
regulator of platelet production. Oncogene. 21:3359–3367. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deutsch VR and Tomer A: Megakaryocyte
development and platelet production. Br J Haematol. 134:453–466.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaushansky K, Broudy VC, Lin N, Jorgensen
MJ, McCarty J, Fox N, Zucker-Franklin D and Lofton-Day C:
Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte
development. Proc Natl Acad Sci USA. 92:3234–3238. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bussel JB, Cheng G, Saleh MN, Psaila B,
Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, et
al: Eltrombopag for the treatment of chronic idiopathic
thrombocytopenic purpura. N Engl J Med. 357:2237–2247. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McHutchison JG, Dusheiko G, Shiffman ML,
Rodriguez-Torres M, Sigal S, Bourliere M, Berg T, Gordon SC,
Campbell FM, Theodore D, et al; TPL102357 Study Group. Eltrombopag
for thrombocytopenia in patients with cirrhosis associated with
hepatitis C. N Engl J Med. 357:2227–2236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Will B, Kawahara M, Luciano JP, Bruns I,
Parekh S, Erickson-Miller CL, Aivado MA, Verma A and Steidl U:
Effect of the nonpeptide thrombopoietin receptor agonist
Eltrombopag on bone marrow cells from patients with acute myeloid
leukemia and myelodysplastic syndrome. Blood. 114:3899–3908. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erickson-Miller CL, Kirchner J, Aivado M,
May R, Payne P and Chadderton A: Reduced proliferation of
non-megakaryocytic acute myelogenous leukemia and other leukemia
and lymphoma cell lines in response to eltrombopag. Leuk Res.
34:1224–1231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roth M, Will B, Simkin G, Narayanagari S,
Barreyro L, Bartholdy B, Tamari R, Mitsiades CS, Verma A and Steidl
U: Eltrombopag inhibits the proliferation of leukemia cells via
reduction of intracellular iron and induction of differentiation.
Blood. 120:386–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erickson-Miller CL, Pillarisetti K,
Kirchner J, Figueroa DJ, Ottesen L, Martin AM, Liu Y, Kamel YM and
Messam C: Low or undetectable TPO receptor expression in malignant
tissue and cell lines derived from breast, lung, and ovarian
tumors. BMC Cancer. 12:4052012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawaguchi T, Komori A, Seike M, Fujiyama
S, Watanabe H, Tanaka M, Sakisaka S, Nakamuta M, Sasaki Y, Oketani
M, et al: Efficacy and safety of eltrombopag in Japanese patients
with chronic liver disease and thrombocytopenia: A randomized,
open-label, phase II study. J Gastroenterol. 47:1342–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matthys G, Park JW, McGuire S, Wire MB,
Bowen C, Williams D, Jenkins J and Peng B: Clinical
pharmacokinetics, platelet response, and safety of eltrombopag at
supratherapeutic doses of up to 200 mg once daily in healthy
volunteers. J Clin Pharmacol. 51:301–308. 2011. View Article : Google Scholar
|
|
19
|
Chou T-C: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nurtjahja-Tjendraputra E, Fu D, Phang JM
and Richardson DR: Iron chelation regulates cyclin D1 expression
via the proteasome: A link to iron deficiency-mediated growth
suppression. Blood. 109:4045–4054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cardier JE and Dempsey J: Thrombopoietin
and its receptor, c-mpl, are constitutively expressed by mouse
liver endothelial cells: Evidence of thrombopoietin as a growth
factor for liver endothelial cells. Blood. 91:923–929.
1998.PubMed/NCBI
|
|
22
|
Schmelzer E, Deiwick A, Bruns H, Fiegel HC
and Bader A: Thrombopoietin is a growth factor for rat hepatic
progenitors. Eur J Gastroenterol Hepatol. 20:209–216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nozaki R, Murata S, Nowatari T, Maruyama
T, Ikeda N, Kawasaki T, Fukunaga K and Ohkohchi N: Effects of
thrombopoietin on growth of hepatocellular carcinoma: Is
thrombopoietin therapy for liver disease safe or not? Hepatol Res.
43:610–620. 2013. View Article : Google Scholar
|
|
24
|
Erickson-Miller CL, DeLorme E, Tian SS,
Hopson CB, Stark K, Giampa L, Valoret EI, Duffy KJ, Luengo JL,
Rosen J, et al: Discovery and characterization of a selective,
nonpeptidyl thrombopoietin receptor agonist. Exp Hematol. 33:85–93.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erickson-Miller C, Delorme E, Giampa L,
Hopson C, Valoret E, Tian SS, Miller SG, Keenan R, Rosen J, Dillon
S, et al: Biological activity and selectivity for Tpo receptor of
the orally bioavailable, small molecule Tpo receptor agonist,
SB-497115. Blood (ASH Annual Meeting Abstracts). 104:29122004.
|
|
26
|
Kalota A, Brennan K, Erickson-Miller CL,
Danet G, Carroll M and Gewirtz AM: Effects of SB559457, a novel
small molecule thrombopoietin receptor (TpoR) agonist, on human
hematopoietic cell growth and differentiation. Blood (ASH Annual
Meeting Abstracts). 104:29132004.
|
|
27
|
Safonov IG, Heerding DA, Keenan RM, Price
AT, Erickson-Miller CL, Hopson CB, Levin JL, Lord KA and Tapley PM:
New benzimidazoles as thrombopoietin receptor agonists. Bioorg Med
Chem Lett. 16:1212–1216. 2006. View Article : Google Scholar
|
|
28
|
Luengo JI, Duffy KJ, Shaw AN, Delorme E,
Wiggall KJ, Giampa L, Liu N, Smith H, Tian SS, Miller SG, et al:
Discovery of SB-497115, a small-molecule thrombopoietin (TPO)
receptor agonist for the treatment of thrombocytopenia. Blood (ASH
Annual Meeting Abstracts). 104:29102004.
|
|
29
|
Peeters K, Stassen J-M, Collen D, Van Geet
C and Freson K: Emerging treatments for thrombocytopenia:
Increasing platelet production. Drug Discov Today. 13:798–806.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duffy KJ, Shaw AN, Delorme E, Dillon SB,
Erickson-Miller C, Giampa L, Huang Y, Keenan RM, Lamb P, Liu N, et
al: Identification of a pharmacophore for thrombopoietic activity
of small, non-peptidyl molecules. 1. Discovery and optimization of
salicylaldehyde thiosemicarbazone thrombopoietin mimics. J Med
Chem. 45:3573–3575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duffy KJ, Price AT, Delorme E, Dillon SB,
Duquenne C, Erickson-Miller C, Giampa L, Huang Y, Keenan RM, Lamb
P, et al: Identification of a pharmacophore for thrombopoietic
activity of small, non-peptidyl molecules. 2. Rational design of
naphtho[1,2-d]imidazole thrombopoietin mimics. J Med Chem.
45:3576–3578. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Williams DD, Peng B, Bailey CK, Wire MB,
Deng Y, Park JW, Collins DA, Kapsi SG and Jenkins JM: Effects of
food and antacids on the pharmacokinetics of eltrombopag in healthy
adult subjects: Two single-dose, open-label, randomized-sequence,
crossover studies. Clin Ther. 31:764–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hershko C: Control of disease by selective
iron depletion: A novel therapeutic strategy utilizing iron
chelators. Baillieres Clin Haematol. 7:965–1000. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Buss JL, Greene BT, Turner J, Torti FM and
Torti SV: Iron chelators in cancer chemotherapy. Curr Top Med Chem.
4:1623–1635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andrews NC: Disorders of iron metabolism.
N Engl J Med. 341:1986–1995. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucas JJ, Szepesi A, Domenico J, Takase K,
Tordai A, Terada N and Gelfand EW: Effects of iron-depletion on
cell cycle progression in normal human T lymphocytes: Selective
inhibition of the appearance of the cyclin A-associated component
of the p33cdk2 kinase. Blood. 86:2268–2280. 1995.PubMed/NCBI
|
|
37
|
Brodie C, Siriwardana G, Lucas J,
Schleicher R, Terada N, Szepesi A, Gelfand E and Seligman P:
Neuroblastoma sensitivity to growth inhibition by deferrioxamine:
Evidence for a block in G1 phase of the cell cycle. Cancer Res.
53:3968–3975. 1993.PubMed/NCBI
|
|
38
|
Kwok JC and Richardson DR: The iron
metabolism of neoplastic cells: Alterations that facilitate
proliferation? Crit Rev Oncol Hematol. 42:65–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Le NTV and Richardson DR: The role of iron
in cell cycle progression and the proliferation of neoplastic
cells. Biochim Biophys Acta. 1603:31–46. 2002.PubMed/NCBI
|
|
40
|
Kalinowski DS and Richardson DR: The
evolution of iron chelators for the treatment of iron overload
disease and cancer. Pharmacol Rev. 57:547–583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Larrick JW and Cresswell P: Modulation of
cell surface iron transferrin receptors by cellular density and
state of activation. J Supramol Struct. 11:579–586. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Richardson DR and Baker E: The uptake of
iron and transferrin by the human malignant melanoma cell. Biochim
Biophys Acta. 1053:1–12. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Richardson DR and Baker E: The effect of
desferrioxamine and ferric ammonium citrate on the uptake of iron
by the membrane iron-binding component of human melanoma cells.
Biochim Biophys Acta. 1103:275–280. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Elford HL, Freese M, Passamani E and
Morris HP: Ribonucleotide reductase and cell proliferation. I.
Variations of ribonucleotide reductase activity with tumor growth
rate in a series of rat hepatomas. J Biol Chem. 245:5228–5233.
1970.PubMed/NCBI
|
|
45
|
Takeda E and Weber G: Role of
ribonucleotide reductase in expression in the neoplastic program.
Life Sci. 28:1007–1014. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Witt L, Yap T and Blakley RL: Regulation
of ribonucleotide reductase activity and its possible exploitation
in chemotherapy. Adv Enzyme Regul. 17:157–171. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Donfrancesco A, Deb G, Dominici C, Pileggi
D, Castello MA and Helson L: Effects of a single course of
deferoxamine in neuroblastoma patients. Cancer Res. 50:4929–4930.
1990.PubMed/NCBI
|
|
48
|
Estrov Z, Tawa A, Wang XH, Dubé ID, Sulh
H, Cohen A, Gelfand EW and Freedman MH: In vitro and in vivo
effects of deferoxamine in neonatal acute leukemia. Blood.
69:757–761. 1987.PubMed/NCBI
|
|
49
|
Kaplinsky C, Estrov Z, Freedman MH,
Gelfand EW and Cohen A: Effect of deferoxamine on DNA synthesis,
DNA repair, cell proliferation, and differentiation of HL-60 cells.
Leukemia. 1:437–441. 1987.PubMed/NCBI
|
|
50
|
Blatt J, Taylor SR and Stitely S:
Mechanism of antineuroblastoma activity of deferoxamine in vitro. J
Lab Clin Med. 112:433–436. 1988.PubMed/NCBI
|
|
51
|
Blatt J and Stitely S: Antineuroblastoma
activity of desferoxamine in human cell lines. Cancer Res.
47:1749–1750. 1987.PubMed/NCBI
|
|
52
|
Gao J and Richardson DR: The potential of
iron chelators of the pyridoxal isonicotinoyl hydrazone class as
effective antiproliferative agents, IV: The mechanisms involved in
inhibiting cell-cycle progression. Blood. 98:842–850. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Le NTV and Richardson DR: Iron chelators
with high antiproliferative activity up-regulate the expression of
a growth inhibitory and metastasis suppressor gene: A link between
iron metabolism and proliferation. Blood. 104:2967–2975. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fu D and Richardson DR: Iron chelation and
regulation of the cell cycle: 2 mechanisms of posttranscriptional
regulation of the universal cyclin-dependent kinase inhibitor
p21CIP1/WAF1 by iron depletion. Blood. 110:752–761. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Keberle H: The Biochemistry of
desferrioxamine and its relation to iron metabolism. Ann NY Acad
Sci. 119:758–768. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Whitnall M, Howard J, Ponka P and
Richardson DR: A class of iron chelators with a wide spectrum of
potent antitumor activity that overcomes resistance to
chemotherapeutics. Proc Natl Acad Sci USA. 103:14901–14906. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rakba N, Loyer P, Gilot D, Delcros JG,
Glaise D, Baret P, Pierre JL, Brissot P and Lescoat G:
Antiproliferative and apoptotic effects of O-Trensox, a new
synthetic iron chelator, on differentiated human hepatoma cell
lines. Carcinogenesis. 21:943–951. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rao VA, Klein SR, Agama KK, Toyoda E,
Adachi N, Pommier Y and Shacter EB: The iron chelator Dp44mT causes
DNA damage and selective inhibition of topoisomerase IIalpha in
breast cancer cells. Cancer Res. 69:948–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gangaidzo IT and Gordeuk VR:
Hepatocellular carcinoma and African iron overload. Gut.
37:727–730. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ba Q, Hao M, Huang H, Hou J, Ge S, Zhang
Z, Yin J, Chu R, Jiang H, Wang F, et al: Iron deprivation
suppresses hepatocellular carcinoma growth in experimental studies.
Clin Cancer Res. 17:7625–7633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yamasaki T, Terai S and Sakaida I:
Deferoxamine for advanced hepatocellular carcinoma. N Engl J Med.
365:576–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tanaka H, Fujita N, Sugimoto R, Urawa N,
Horiike S, Kobayashi Y, Iwasa M, Ma N, Kawanishi S, Watanabe S, et
al: Hepatic oxidative DNA damage is associated with increased risk
for hepatocellular carcinoma in chronic hepatitis C. Br J Cancer.
98:580–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kato J, Kobune M, Nakamura T, Kuroiwa G,
Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T,
et al: Normalization of elevated hepatic
8-hydroxy-2′-deoxyguanosine levels in chronic hepatitis C patients
by phlebotomy and low iron diet. Cancer Res. 61:8697–8702.
2001.PubMed/NCBI
|
|
64
|
Louandre C, Ezzoukhry Z, Godin C, Barbare
JC, Mazière JC, Chauffert B and Galmiche A: Iron-dependent cell
death of hepatocellular carcinoma cells exposed to sorafenib. Int J
Cancer. 133:1732–1742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Musto P, D'Auria F, Pietrantuono G,
Bringhen S, Morabito F, Di Raimondo F, Pozzi S, Sacchi S, Boccadoro
M and Palumbo A; Gruppo Italiano Malatte Ematologiche dell'Adulto
Multiple Myeloma Working Party; Italian Myeloma Network and Gruppo
Italiano Studio Linfomi. Role of thalidomide in previously
untreated patients with multiple myeloma. Expert Rev Anticancer
Ther. 8:1569–1580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mark TM, Reid W, Niesvizky R, Gergis U,
Pearse R, Mayer S, Greenberg J, Coleman M, Van Besien K and Shore
T: A phase 1 study of bendamustine and melphalan conditioning for
autologous stem cell transplantation in multiple myeloma. Biol
Blood Marrow Transplant. 19:831–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sleigh SH and Barton DCL: Repurposing
strategies for therapeutics. Pharmaceut Med. 24:151–159. 2010.
|
|
68
|
Erickson-Miller CL, Delorme E, Tian S-S,
Hopson CB, Landis AJ, Valoret EI, Sellers TS, Rosen J, Miller SG,
Luengo JI, et al: Preclinical activity of eltrombopag (SB-497115),
an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells.
27:424–430. 2009. View Article : Google Scholar :
|
|
69
|
Kawaguchi T, Nakano M, Satani M, Sumie S,
Yamada S, Amano K, Kuromatsu R and Sata M: Usefulness of short-term
eltrombopag treatment as a supportive treatment in hepatocellular
carcinoma patients with cirrhosis and severe thrombocytopenia: A
report of two cases. Oncol Lett. 7:2130–2134. 2014.PubMed/NCBI
|