Introduction
Colorectal cancer (CRC) was the third most common
malignant tumor in 2013 (1). Every
year, there are more than 1.2 million new cases and nearly 0.7
million people die of this disease, principally due to the tumor
relapse and metastasis (2,3). However, carcinogenesis is a
complicated biological process, in which most of the underlying
molecular mechanisms are unclear (4). Although limited evidence of molecular
biology has been used to explain why several genes are involved in
carcinogenesis and development of CRC, many efforts are being made
to develop some new interventions that target tumor-specific genes
by constructing tumor-selective replicating viruses.
Cluster of differentiation 147 (CD147) or
extracellular matrix metalloproteinase inducer (EMMPRIN) is a
glycosylated cell surface transmembrane protein of the
immunoglobulin superfamily (IgSF), also known as basigin (BSG)
(5–7). CD147 has a broad tissue distribution,
involved in many physiological processes. Previous studies have
indicated that aberrant CD147 expression is observed in several
cancers, including CRC (8–14), and that increased CD147 expression
is seen in CRC and is associated with poor prognosis (15). In addition, CD147 is also involved
in multidrug resistance (MDR) of cancer cells (16), and thus changes in its expression
levels can be used to predict tumor relapse and patient outcome
(11,14). Despite the importance of CD147 in
CRC, whether it is feasible to target CD147 for gene therapy in CRC
is still unknown.
Genomic imprinting is an epigenetic modification of
a gene, which is mono-allelic expression (17). Some genes are expressed in only one
allele that is known as maintenance of imprinting (MOI). In
contrast, the reactivation of the silenced allele of an imprinted
gene (which leads to expression of both paternal and maternal
alleles) is loss of imprinting (LOI). Insulin-like growth factor 2
(IGF2) is identified as the first endogenous imprinted gene,
which is expressed only in paternal alleles, and regulated by the
enhancer, DNA differentially methylated domain (DMD) and promoter
(18).
Upregulated expression of IGF2 has been
detected in CRC, indicating that IGF2 LOI may serve as a
potential biomarker for diagnosis of CRC (19,20).
In our previous studies, we found that IGF2 LOI is present
in both colorectal tissues and cancer cell lines (HCT-8 and HT-29),
but that such a phenomenon is not observed in normal cells
(21–23). Importantly, we successfully
constructed a recombinant adenovirus that carries the IGF2
imprinting system and it is specially expressed in the IGF2
LOI tumor cells, with the greater potential for targeted gene
therapy for CRC (21–23). In this study, we constructed the
adenovirus-mediated siRNA that targets CD147 and carries the
IGF2 imprinting system, and investigated its effects on the
efficacy of gene therapy for CRC as a novel therapeutic
strategy.
Materials and methods
Cell culture
HT-29, HCT-8 and HCT116 (human colon cancer cell
lines) were obtained from the Chinese Academy of Sciences Cell Bank
(Shanghai, China). The HEK293 cell line (human embryonic kidney
cells containing the E1A region of the adenovirus) was obtained
from Microbix Biosystems, Inc. (Ontario, Canada). HEK293, HT-29,
HCT-8 and HCT116 cell lines were, respectively, maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (all from HyClone, Logan, UT, USA). Cells
were incubated in a humidified incubator at 37°C with 5%
CO2. Both HT-29 and HCT-8 cell lines were characterized
by IGF2 LOI, whereas HCT116 cell line was known to possess
IGF2 MOI (21–23).
Plasmid construction and incorporation
into adenoviral vectors
The original adenoviral shuttle plasmid used in this
study was pYr-mir30-shRNA which included the enhanced green
fluorescent protein (EGFP) gene sequence, provided from the
Changsha Yingrun Biotechnology Co., Ltd., Hunan, China. The 808–828
fragment of CD147gene was selected as the RNAi target site,
and the scrambled control sequence was also synthesized by the
Changsha Yingrun Biotechnology Co., Ltd. These oligonucleotides
were annealed and subcloned into the BsaI sites of the
pYr-mir30-shRNA vector. These recombinant vectors were designated
as pYr-mir30-CMV-control-shRNA and pYr-mir30-CMV-CD147mirsh,
respectively. The IGF2 imprinting system, enhancer-DMD-H19 sequence
(1798 bp), were amplified by PCR with MluI and NheI
restriction enzyme digestion from pDC315-enhancer-DMD-H19-E1A
preserved in our laboratory (22).
Subsequently, T4 DNA ligase was added to reconnect the
enhancer-DMD-H19 sequence and pYr-mir30-CD147mirsh (at 16°C
overnight). The following day, the connected reaction solution was
transformed into competent E. coli DH5α cells (Center
Laboratory, the First Affiliated Hospital, Liaoning Medical
University, Jinzhou, China). The
pYr-mir30-enhancer-DMD-H19-CD147mirsh plasmid was obtained. All the
cloned genes were confirmed by DNA sequencing.
Construction of the adenovirus plasmid
and packaging of the adenovirus
pYr-mir30-CMV-control-shRNA/pYr-mir30-CMV-CD147mirsh/pYr-mir30-enhancer-DMD-H19-CD147mirsh
and adenovirus backbone vector pAD/PL-DEST were used to produce
recombinant vector pAd-control, pAd-CMV-CD147m irsh and
pAd-H19-CD147m irsh, respectively. A PacI restriction enzyme
reaction system was established. The recombinant vectors
pAd-control, pAd-CMV-CD147mirsh, and pAd-H19-CD147mirsh were
re-dissolved in ddH2O, and then transfected into HEK293
cells using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). The culture solution was changed after 6 h, and
the cytopathic effect (CPE) was observed continuously. When the
majority of the pathologically abnormal cells had detached from the
bottom of the culture flask, these abnormal cells and supernatant
were collected, frozen and thawed at −80/37°C three times and
centrifuged, then the supernatant was collected, and finally three
sets of adenoviruses, the rAd-control, rAd-CMV-CD147mirsh, and
rAd-H19-CD147mirsh, were obtained. Adenoviruses were plaque
purified, propagated in HEK293 cells, and purified again by a CsCl
gradient according to standard techniques. Functional particle
titers of all adenoviruses were determined by a plaque assay using
HEK293 cells.
Analysis of EGFP expression in the
constructed adenovirus
HT-29, HCT-8 and HCT116 cells were, respectively,
infected with rAd-control, rAd-CMV-CD147mirsh, and
rAd-H19-CD-147mirsh with multiplicity of infection (MOI) of 10
plaque forming units (PFU)/cell. EGFP expression was examined at 48
h after infection using an Olympus microscope with a fluorescent
filter set (excitation 450–490 nm).
Analysis of the expression of CD147 mRNA
in virus-infected cells by real-time quantitative PCR
(RT-qPCR)
The CD147 mRNA expression was determined by
RT-qPCR. The HT-29, HCT-8 and HCT116 cells were infected with
rAd-control, rAd-CMV-CD147mirsh and rAd-H19-CD147mirsh (10
PFU/cell), respectively. After 24 h, total RNA was extracted from
the three cell lines with TRIzol (Invitrogen Life Technologies)
according to the manufacturer's instructions. Following treatment
with DNase I (Takara Bio, Inc., Otsu, Japan) at 37°C for 30 min,
RNA quantification was performed using spectrophotometry. The RNA
(1 μg) was subsequently incubated with 1 μl of
Oligo(dT) primer (50 μM), 1 μl of Random 6 mers (100
μM), 1 μl of PrimeScript™ RT Enzyme Mix I, 4
μl of 5X PrimeScript™ Buffer and RNase-free dH2O,
and first-strand cDNA synthesis was performed in a total volume of
20 μl. The primer sequences used for CD147 and β-actin are
listed in Table I. The PCR
reactions were performed in a LightCycler apparatus using real-time
PCR Master mix SYBR-Green I (Yotobo Biotech Co., Ltd., Osaka,
Japan). Thermocycling was done in a final volume of 25 μl
containing 1 μl of cDNA sample, 0.5 μl of the
up-primer, 0.5 μl down-primer, 12.5 μl of SYBR-Green
Real-Time PCR Master mix, and 10.5 μl of dH2O.
After 15 sec at 95°C to denature the cDNA and to activate Taq DNA
polymerase, the cycling conditions were as follows: 40 cycles
consisting of denaturation at 95°C for 5 sec, annealing at 60°C for
5 sec, and extension at 72°C for 30 sec. The Ct used in the
real-time quantification PCR was defined as the PCR cycle number
that crossed an arbitrarily chosen signal threshold in the log
phase of the amplification curve. To verify the fold change of
CD147 gene expression, calculated Ct values were normalized to Ct
values of β-actin amplified from the same sample
(ΔCt=CtCD147 − Ctβ-actin), and the
2−ΔΔCt method was used to calculate changes in gene
expression. Each sample had triplicates and all reactions were
triplicated independently to ensure the reproducibility of the
results.
 | Table IPrimers of CD147 and β-actin for
real-time PCR. |
Table I
Primers of CD147 and β-actin for
real-time PCR.
| Target | | Primers |
|---|
| CD147 | Sense: |
5′-CCATGCTGGTCTGCAAGTCAG-3′ |
| Antisense: |
5′-CCGTTCATGAGGGCCTTGTC-3′ |
| β-actin | Sense: |
5′-CTGGAACGGTGAAGGTGACA-3′ |
| Antisense: |
5′-AAGGGACTTCCTGTAACAACGCA-3′ |
Analysis of CD147 protein expression by
western blot analysis
The CD147 protein expression was evaluated by
western blot analysis. Cells were harvested and lysed by three
cycles of freeze/thaw at −80°C. Total protein was separated by 10%
SDS-PADE gels, and transferred to a polyvinylidene difluoride
(PVDF) membrane. After blocking with 5% skimmed milk powder
(soluble in TBST buffer solution) was used at room temperature
under sealed conditions for 2 h, the membrane was incubated with
mouse anti-CD147 primary antibodies (1:500) and rabbit anti-human
β-actin primary antibodies (1:500) at room temperature for 2 h,
followed by secondary antibodies conjugated to horseradish
peroxidase in a 1:2,000 dilution (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA) for 1 h at room temperature. The proteins were
visualized by ECL detection system (Boster Inc., Wuhan, China).
Analysis of the cytotoxic effect of the
adenoviruses by CCK-8
Cytotoxicity was assessed by Cell Counting Kit-8
(CCK-8) (Beyotime, China). Cells were seeded in 96-well plates at a
density of 1×105 cells/100 μl/well, and then
infected with adenoviruses at 10 PFU/cell for 24, 48 and 72 h,
respectively. After addition of 10 μl of CCK-8 into the
medium, each plate was measured at 450 nm with a microplate reader
(Bio-Rad Laboratories, Richmond, CA, USA).
In vitro invasion assay
Transwell plates (Corning Inc., Acton, MA, USA) were
coated with basement membrane Matrigel (20 mg/ml; Becton-Dickinson,
Franklin Lakes, NJ, USA) for 4 h at 37°C. After the Matrigel was
solidified, 1×105 cells were seeded onto the Matrigel
and infected with adenoviruses at 10 PFU/cell for 24 h. Cells that
migrated through the permeable membrane were fixed with 100%
methanol for 10 min. The membrane with cells was soaked in 0.1%
crystal violet for 10 min and then washed with distilled water. The
number of cells attached to the lower surface of the polycarbonate
filter was counted at ×400 magnification under a light microscope.
Each assay was carried out in triplicate and repeated three
times.
Drug sensitivity assay
To assess the sensitivity to the cancer drug, HT-29,
HCT-8 and HCT116 cells (1×104 cells/well) were seeded in
triplicates on 96-well plates and then infected with rAd-control,
rAd-CMV-CD147mirsh, and rAd-H19-CD147mirsh, respectively. After 24
h, the cells were treated by cisplatin or oxaliplatin (Sigma, St.
Louis, MO, USA) with varying concentrations at 0.1, 1 and 10
μM for 48 h, respectively. The cytotoxicity was assessed
using CCK-8 assay as described above. The absorbance was measured
with a microplate reader at 450 nm (Bio-Rad Laboratories).
Treatment of tumor-bearing nude mice with
the recombinant adenovirus
HT-29 cells were trypsinized to a single cell
suspension and resuspended in 109 cells/100 μl
PBS, then subcutaneously injected into the flank area of adult
(8-week-old) athymic male nude mice. The protocol was approved by
the Experimental Animal Center of University of Yangzhou, Yangzhou,
China. Two weeks after injection of HT-29 cells, the developed
tumors were measured in two dimensions, then rAd-H19-CD147mirsh
were injected into the growing tumor, and rAd-CMV-CD147mirsh, and
rAd-control served as viral vector controls. Each adenoviral vector
(a total dosage of 109 PFU/mouse) was injected into a
growing tumor from three directions for 3 consecutive days, and the
tumor volume was observed for 28 days. Tumor dimensions were
measured, and the tumor volume was calculated according to the
formula (width)2 × length × 0.5. Mice were euthanized 30
days post-inoculation. Harvested tissues were fixed in 10% buffered
formalin, embedded in paraffin, sectioned at 4 μm, and
stained with hematoxylin and eosin (H&E). Mouse survival was
recorded in a separate experiment. Animal experiments were
performed in accordance with the Institutional Guidelines for
Animal Care by the Nanjing Medical University, China.
Statistical analysis
Statistical analysis of data was conducted by SPSS
software. Experimental data are presented as the mean ± standard
deviation (SD) and assessed by Student's t-tests and one-way ANOVA
at a significance level of P<0.05.
Results
Construction and characterization of the
recombinant adenovirus
The recombinant adenovirus rAd-H19-CD147mirsh was
successfully constructed in this study, and the control virus,
rAd-CMV-CD147mirsh and rAd-control, were also constructed. After
infection of cells with rAd-control, rAd-CMV-CD147mirsh, and
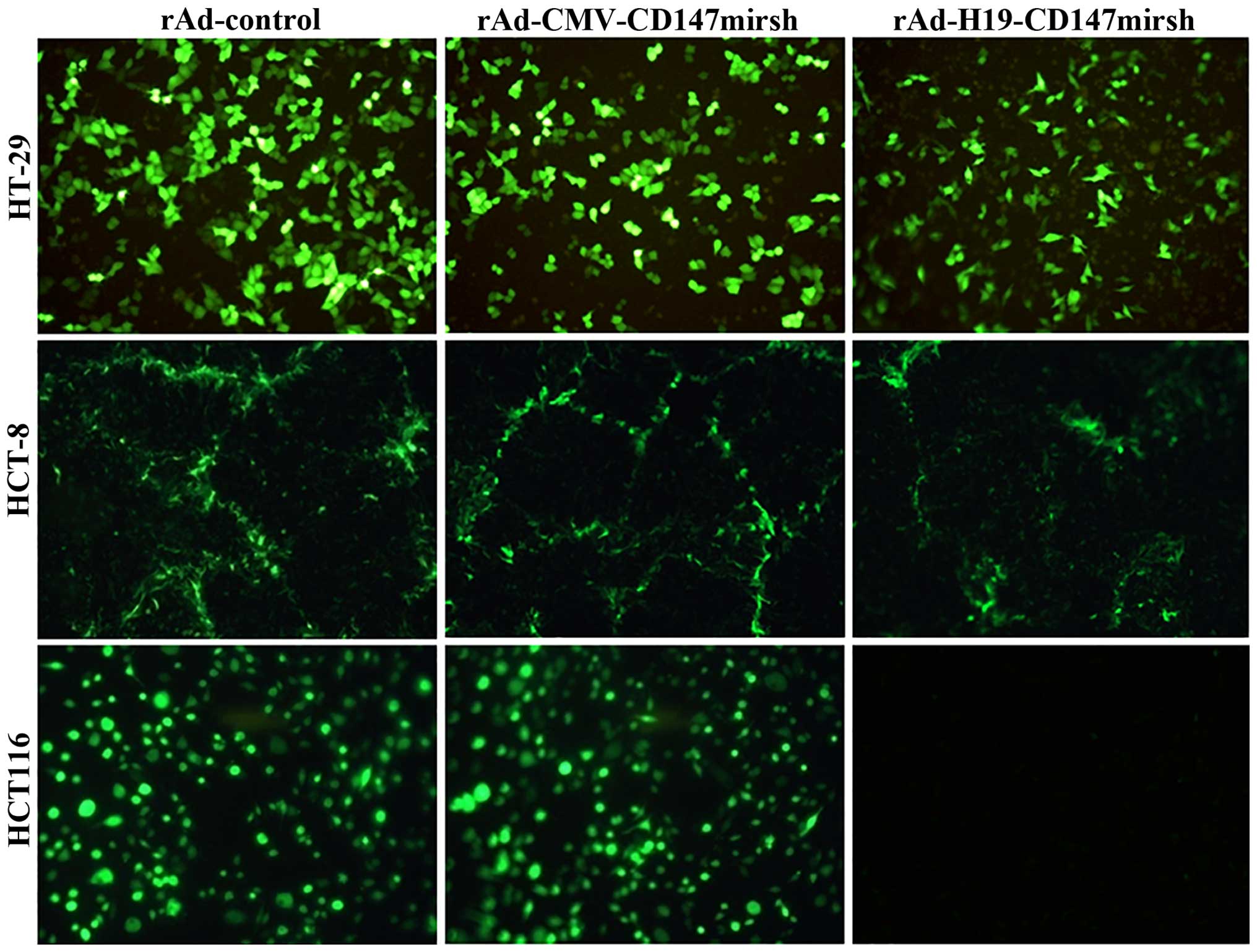
rAd-H19-CD147mirsh (10 PFU/cell) for 24 h, respectively, we tested
the applicability of the expression system through detection of the
expression of EGFP, and observed that the expression of EGFP
protein was positive in the three cell lines (HT-29, HCT-8 and
HCT116) as shown in Fig. 1.
The recombinant adenovirus-mediated gene
silencing inhibits CD147 expression in colon cancer cells
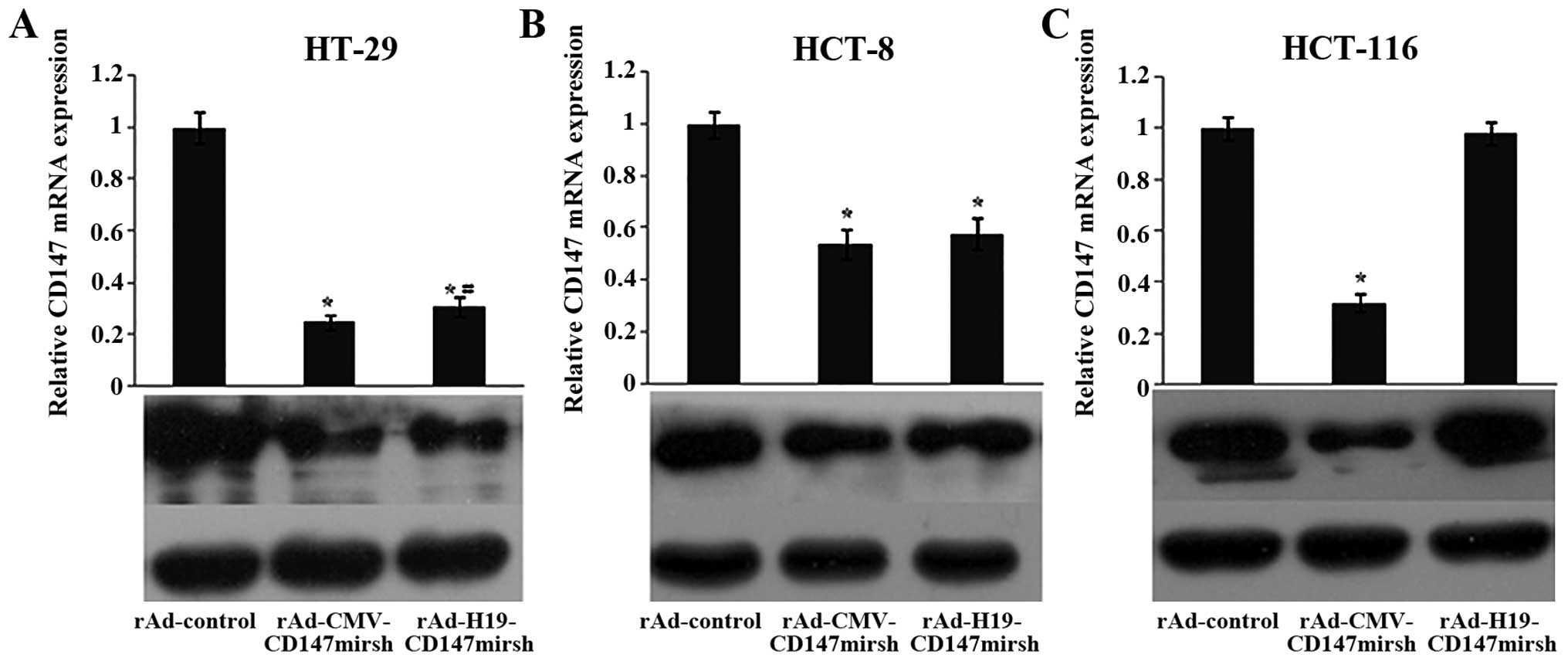
We also examined CD147 mRNA and protein
expression in these cell lines (HT-29, HCT-8 and HCT116) after
infection with rAd-control, rAd-CMV-CD147mirsh and
rAd-H19-CD147mirsh (10 PFU/cell), respectively. Cells were
harvested to determine CD147 mRNA expression by RT-PCR at 24
h after infection, and CD147 protein expression by western blot
analysis at 48 h after infection. As shown in Fig. 2, a significantly reduced
CD147 mRNA and protein expression was seen in HT-29, and
HCT-8 cells (LOI) treated with rAd-CMV-CD147mirsh, and
rAd-H19-CD-147mirsh compared with rAd-control, respectively
(P<0.01). The same results were also seen in HT-29 cells
(P<0.05), but not in HCT-8 cells (P>0.05), which were both
infected with rAd-CMV-CD147mirsh vs. rAd-H19-CD147mirsh.
Interestingly, the expression of CD147 was significantly
reduced in HCT116 cells (MOI) when treated with rAd-CMV-CD147mirsh
vs. rAd-control, but there was no marked difference when treated
with rAd-H19-CD147mirsh vs. rAd-control, suggesting that the
recombinant adenovirus carrying IGF2 impriting system could
be specially expressed in the LOI cells.
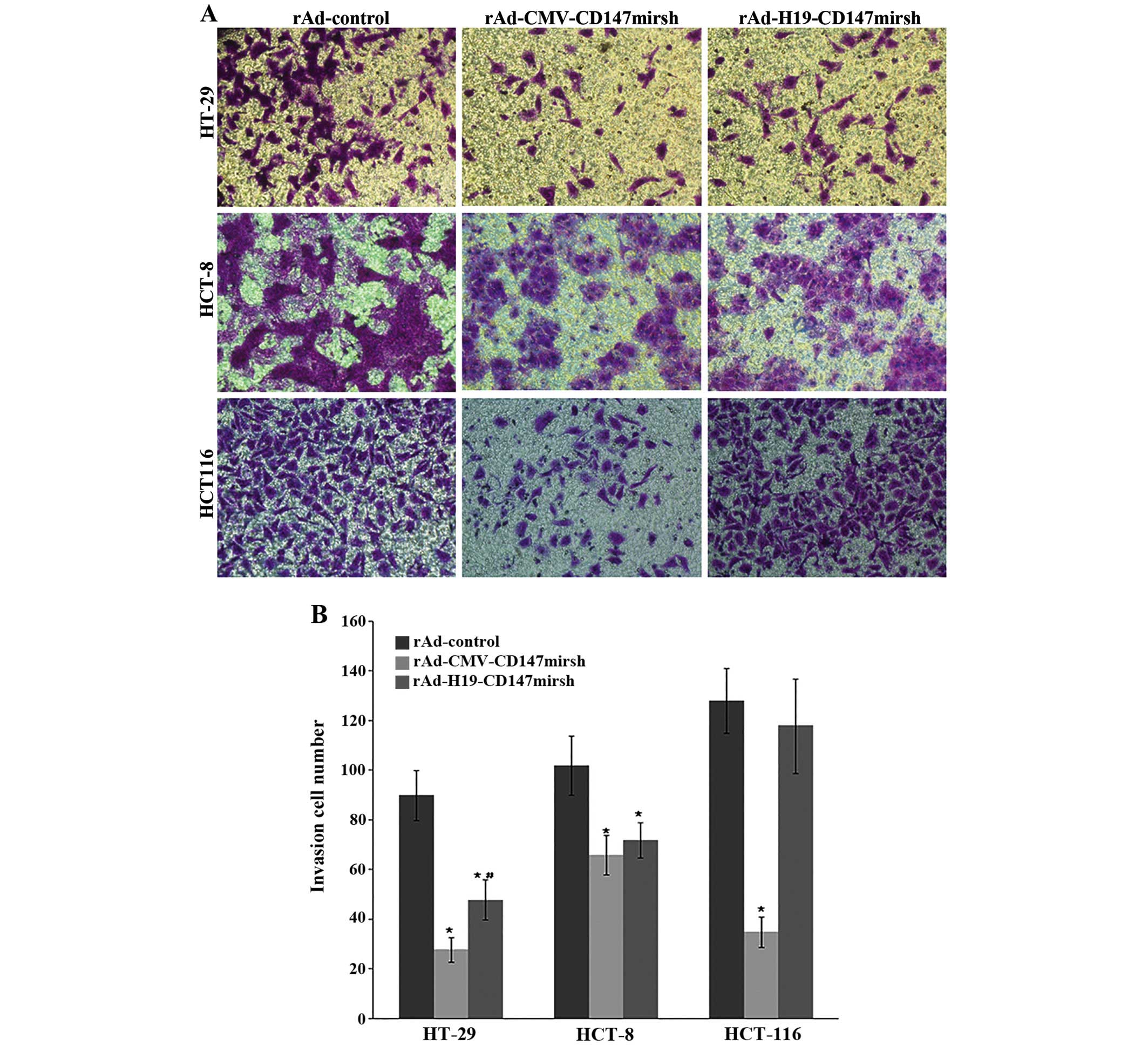
CD147 silencing reduces the invasive
ability of tumor cells in vitro
To examine whether the downregulation of CD147 in
tumor cells could affect their invasive ability, we performed a
Matrigel Transwell analysis in vitro. The results showed
that the number of HT-29 and HCT-8 cells that passed through the
Matrigel was markedly reduced when infected with rAd-CMV-CD147mirsh
or rAd-H19-CD147mirsh vs. rAd-control (P<0.05), the same results
were observed with the HT-29 cells passed through the Matrigel when
infected with rAd-CMV-CD147mirsh vs. rAd-H19-CD147mirsh
(P<0.05), but that there was no marked difference in the HCT-8
cells infected with rAd-H19-CD147mirsh vs. rAd-CMV-CD147mirsh as
measured with the number of the cells that passed through the
Matrigel-coated filter (P>0.05). Similarly, the number of the
filtered cells was significantly decreased in HCT116 when infected
with rAd-CMV-CD147mirsh vs. rAd-control group (P<0.05), but not
infected with rAd-H19-CD147mirsh vs. rAd-control group (P>0.05)
as shown in Fig. 3.
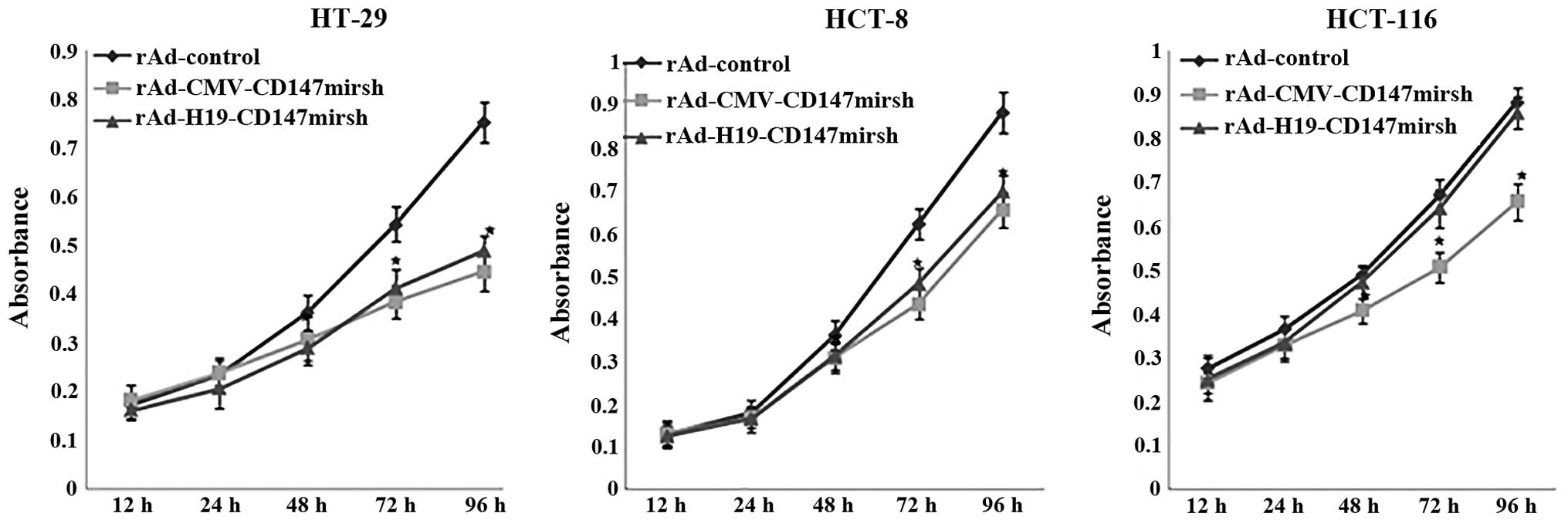
Inhibition of the growth of colon cancer
cells by CD147 silencing
We were interested in examining whether CD147
silencing would effectively suppress tumor cell proliferation. For
this purpose, the growth inhibition and cytotoxicity of tumor cells
by CD147 silencing were investigated in the cell lines by CCK-8
assay. As shown in Fig. 4, the
colon cancer cells (HT-29, HCT-8 and HCT116) infected with
rAd-CMV-CD147mirsh for 48 h displayed decreased viability
(P<0.05). The rAd-H19-CD147mirsh induced CPEs as efficiently as
did rAd-CMV-CD147mirsh only in the LOI cells (HT-29 and HCT-8), but
not in the MOI cells (HCT116) infected with rAd-CMV-CD147mirsh.
Increased sensitivity to the
chemotherapeutic drug in colon cancer cells by CD147 silencing
CD147 has been found to be over expressed in tumor
cells resistant to multiple drugs, and thus could confer resistance
to some (if not all) antitumor drugs. In order to test whether
CD147 silencing could affect the sensitivity of the tumor cells to
the cancer drugs in colon cancer cells, we investigated the
sensitivity of colon cancer cells to the antitumor drugs cisplatin
and oxaliplatin. As shown in Fig.
5A, CD147 silencing significantly increased the inhibition rate
of HT-29 cells treated with cisplatin at the concentration of 0.1,
1 and 10 μM for 48 h (P<0.05), and increased the
inhibition rate of HCT-8 cells treated with cisplatin (1 and 10
μM) for 48 h (P<0.05). Similar results were obtained in
the HCT116 cells (MOI) when treated with rAd-control plus cisplatin
(0.1, 1 and 10 μM) (P<0.05), but not infected with
rAd-H19-CD147mirsh vs. rAd-control group (P>0.05). However,
oxaliplatin did not show any effects on the sensitivity when
treated with rAd-CMV-CD147mirsh and rAd-H19-CD147mirsh,
respectively, compared with rAd-control in all infected groups
(P>0.05) (Fig. 5B).
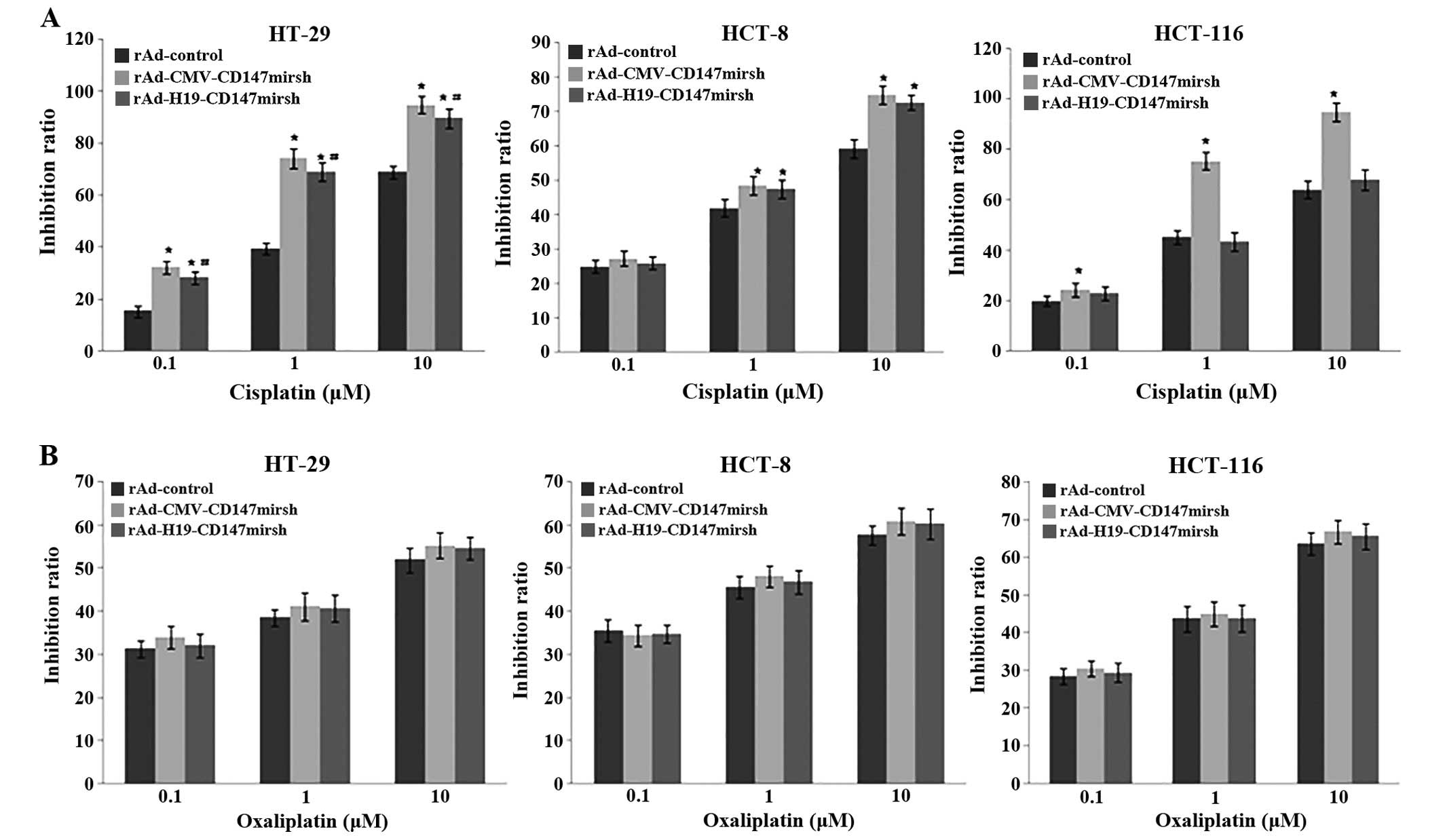 | Figure 5Multidrug chemosensitivity analysis
in colon cancer cells after CD147 silencing. (A) The inhibition
rate of HT-29 cells treated by cisplatin (0.1, 1 and 10 μM)
for 48 h, which infected with rAd-CMV-CD147mirsh and
rAd-H19-CD147mirsh, respectively, were significantly upregulated
compared with rAd-control group (*P<0.05). Moreover
the inhibition rate of HCT-8 cells treated by cisplatin (1 and 10
μM) for 48 h were significantly upregulated compared with
rAd-control group (*P<0.05). Otherwise, the
inhibition rate of HCT116 cells treated by cisplatin (0.1, 1 and 10
μM) for 48 h, which infected with rAd-CMV-CD147mirsh, were
significantly upregulated compared with rAd-control group
(*P<0.05), whereas there was no significant
difference between rAd-H19-CD147mirsh group and rAd-control group
(P>0.05). (B) The inhibition rate of colon cancer cells (HT-29,
HCT-8 and HCT116) had no significant difference treated by
oxaliplatinl infected with rAd-CMV-CD147mirsh and
rAd-H19-CD147mirsh respectively, compared with rAd-control group
(P>0.05). |
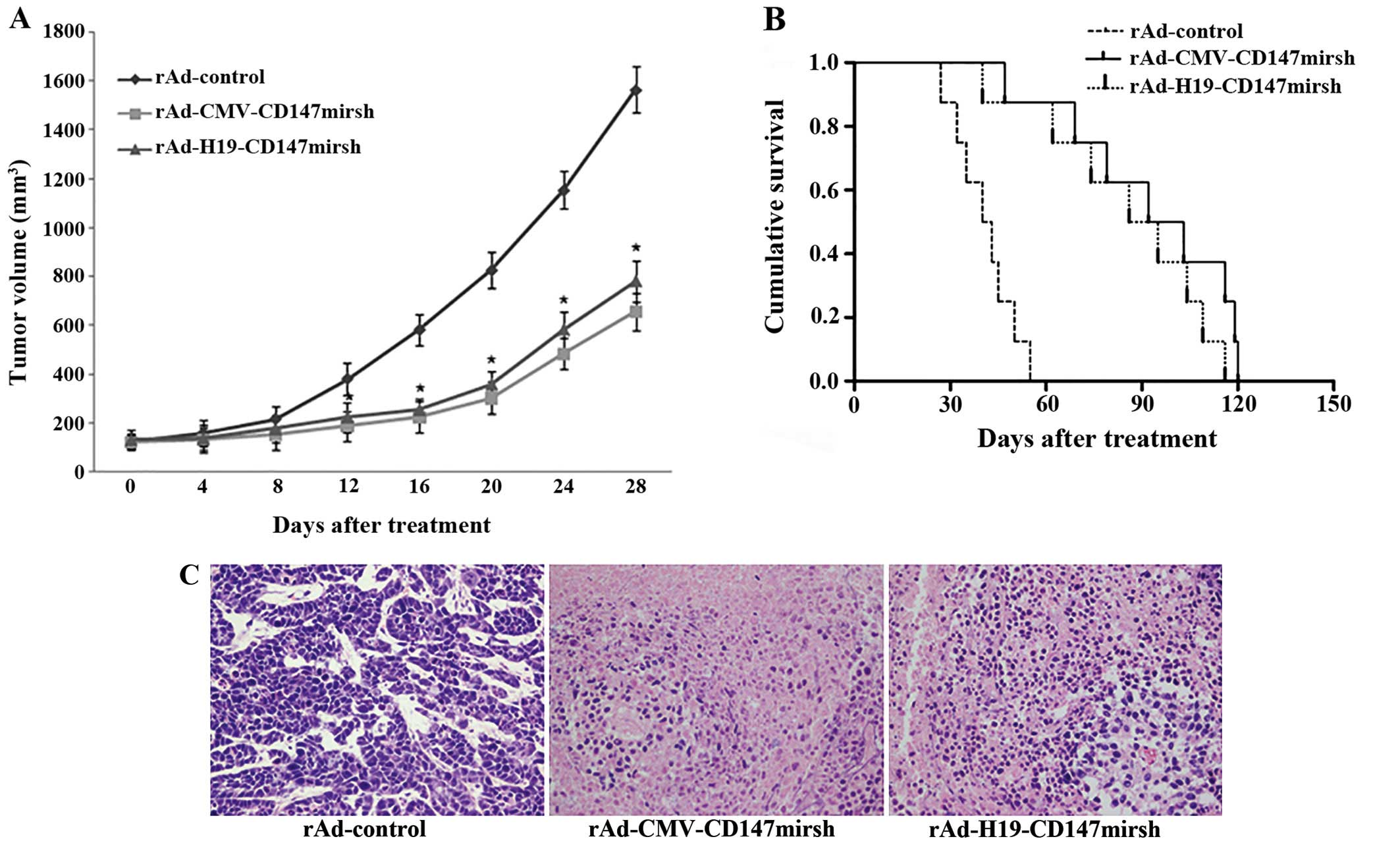
CD147 silencing inhibits the tumor
formation in nude mice
Because the use of RNAi can effectively reduce the
proliferative ability of colon cancer cells in vitro, we
investigated the antitumor efficacy of RNAi in vivo. Every 4
days after the date of vaccination, we measured the length and
width of the tumors and calculated their volume. As shown in
Fig. 6A, tumor growth of the HT-29
cells was slower when infected with rAd-H19-CD-147mirsh or
rAd-CMV-CD147mirsh than with rAd-control groups after 12 days,
respectively (P<0.05), but there was no significant difference
between Ad-H19-CD147mirsh and rAd-CMV-CD147mirsh groups
(P>0.05). Moreover, the average duration of survival for mice
treated with rAd-control, rAd-CMV-CD147mirsh and rAd-H19-CD147mirsh
were 41, 93 and 85 days, respectively, as shown in Fig. 6B. The results showed that the
survival time of the mice was obviously prolonged when injected
with rAd-CMV-CD147mirsh or rAd-H19-CD147mirsh vs. rAd-control
(P<0.05). H&E-stained examination did not reveal obvious
morphological changes among the tumors generated from the three
groups, with the exception of larger areas of necrosis in the group
injected with the rAd-CMV-CD147mirsh or rAd-H19-CD147mirsh vs.
rAd-control (Fig. 6C).
Discussion
CD147, a multifunctional glycoprotein, forms
homo-oligomers in a cis-dependent manner in the plasma membrane
(24), is commonly overexpressed
in many tumors, and is associated with tumor progression and
invasion (25,26). Previous studies have indicated that
CD147 can regulate colon cancer growth by mediating tumor-host
interactions (27), and that CD147
is associated with the lymph node metastasis in CRC patients. This
suggests that CD147 contributes to the progression of CRC and may
serve as a diagnosis marker of CRC (28).
In general, only paternal IGF2 alleles
expressed and maternal alleles closed, which is known as the MOI,
however, the maternal IGF2 allele abnormally expressed,
triggered by the abnormal binding of insulator CTCF to DMD, which
was caused by the impaired function of CTCF or the hypomethylation
status of DMD (29,30). IGF2 LOI as a hallmark of
various human neoplasms has been widely investigated (31,32).
In addition, upregulation of IGF2 has been detected in CRC,
indicating IGF2 LOI may serve as a potential biomarker in
diagnosis of CRC (19,20).
Based on the above findings, we have constructed the
recombinant adenovirus carrying IGF2 imprinting system,
which is specially expressed in IGF2 LOI tumor cells
(21–23). Also based on the association of
CD147 with the development of colon and its high expression in CRC,
we constructed the adenovirus-mediated siRNA targeting CD147 that
carries the IGF2 imprinting system Ad-H19-CD147mirsh,
recombinant adenovirus rAd-CMV-CD147mirsh as a positive control,
and rAd-control as a negative control, respectively. Initially the
utility of our expression system was tested using EGFP reporter
assays. The results showed that the EGFP was detected in both LOI
and MOI cell lines (HT-29, HCT-8 and HCT116) infected with
rAd-CMV-CD147mirsh or rAd-control, respectively. The EGFP was
detected only in LOI colon cancer cell lines (HT-29 and HCT-8),
with no marked changes due to increased time and multiplicity of
the infections, suggesting that the recombinant adenovirus carrying
IGF2 imprinting system could be specifically expressed in
the LOI colon cancer cells. We used RT-PCR and western blotting to
detect CD147 expression at mRNA and protein levels, respectively.
The results showed that the expression of CD147 was reduced
significantly in the LOI colon cancer cell lines (HT-29 and HCT-8)
infected with rAd-CMV-CD147mirsh or rAd-H19-CD147mirsh,
respectively, compared with rAd-control. Moreover, the
rAd-H19-CD147mirsh exerted a potent inhibitory effect on HT-29
cells, which was similar to rAd-CMV-CD147mirsh, but this inhibition
was impaired with HCT-8 cells, possibly due to the difference in
the ability of adenovirus infection across the different cells. All
of the above results indicate that the adenovirus-mediated siRNA
targeting CD147 that carries the IGF2 imprinting system is
practicable and effective.
To examine whether the downregulation of CD147 in
colon cancer cells could affect their proliferation and invasive
ability, we performed CCK-8 and Matrigel Transwell analysis in
vitro. The results revealed that CD147 silencing reduced the
proliferation and invasive ability in both MOI and LOI cell lines,
but that there was no significantly change of proliferation and
invasive ability in MOI cell line (HCT116) infected with
rAd-H19-CD147mirsh. This suggests that CD147 may play a potential
role in promoting proliferation and invasion of colon cancer
cells.
MDR occurred in tumor cells, which is the main cause
of treatment failure and mortality in colon cancer patients,
abnormal expression of CD147 was also observed in many MDR cancer
cells (33), and the CD147 could
decrease the sensitivity of the cancer cells to certain
chemotherapeutic drugs (34,35).
In the present study, we found a possible role of
CD147 in drug resistance in colon cancer cells. Our results
demonstrated that CD147 silence increased chemosensitivity to
cisplatin in both MOI and LOI colon cancer cells, but that there
was no significant difference in response to oxaliplatin in the
cells after CD147 silencing. In addition, there was no effect in
MOI cells (HCT116) infected with rAd-H19-CD147mirsh vs. control,
because the high expression of CD147 could not be inhibited,
indicating that the expression of CD147 is closely related to drug
resistance in colon cancer cells. Therefore, CD147 regulated the
sensitivity of colon cancer cells to the cancer drug, but the
underlying mechanism is still unclear. We also carried out animal
experiments to validate the results. After use of the colon cancer
cell line HT-29 as a model, we observed that the currently
developed therapeutic strategy of CD147 silence can significantly
inhibit the tumor formation and potentiate the death of the cancer
cell death in vivo.
In conclusion, our results show that the
adenovirus-mediated siRNA targeting CD147 carrying the IGF2
imprinting system, rAd-H19-CD147mirsh, confers a significant
anti-tumor effect by inhibiting CD147 expression, in particular in
the LOI colon cancer cells, but neither had effect in MOI colon
cancer cells nor toxic side effects on the normal cells (i.e.,
IGF2 MOI). In terms of aberrant expression of CD147 and
IGF2 LOI in the cancer cells, the use of recombinant viruses
in the context of the IGF2 LOI system and upregulated CD147
shows promise as a novel approach for targeted CRC gene
therapy.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China to S.K. W. (nos.
81172141 and 81200401) and Y.Q.P. (no. 81501820), the Medical
Science and Technology Development Foundation, Nanjing Department
of Health (no. YKK13107), the Nanjing Science and Technology
Committee Project (no. 201108025), the Nanjing Medical Technology
Development Project (no. ZKX11025), the Nanjing Health Young Talent
Project, Jiangsu Provincial Key Medical Talents awarded to S.-K.W.,
Nanjing Medical Science and Technique Development Foundation
awarded to Y.-Q.P. (no. QRX11255) and B.-S.H. (no. QRX11254).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS,
Matsuda T, Ng SS, Lau JY, Zheng S, Adler S, et al; Asia Pacific
Working Group. An updated Asia Pacific Consensus Recommendations on
colorectal cancer screening. Gut. 64:121–132. 2015. View Article : Google Scholar
|
|
3
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stein U and Schlag PM: Clinical,
biological, and molecular aspects of metastasis in colorectal
cancer. Recent Results Cancer Res. 176:61–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simon-Chazottes D, Matsubara S, Miyauchi
T, Muramatsu T and Guénet JL: Chromosomal localization of two cell
surface-associated molecules of potential importance in
development: Midkine (Mdk) and basigin (Bsg). Mamm Genome.
2:269–271. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasinrerk W, Fiebiger E, Stefanová I,
Baumruker T, Knapp W and Stockinger H: Human leukocyte activation
antigen M6, a member of the Ig superfamily, is the species
homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J
Immunol. 149:847–854. 1992.PubMed/NCBI
|
|
7
|
Saxena DK, Oh-Oka T, Kadomatsu K,
Muramatsu T and Toshimori K: Behaviour of a sperm surface
transmembrane glycoprotein basigin during epididymal maturation and
its role in fertilization in mice. Reproduction. 123:435–444. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenthal EL, Shreenivas S, Peters GE,
Grizzle WE, Desmond R and Gladson CL: Expression of extracellular
matrix metalloprotease inducer in laryngeal squamous cell
carcinoma. Laryngoscope. 113:1406–1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gallagher SM, Castorino JJ, Wang D and
Philp NJ: Monocarboxylate transporter 4 regulates maturation and
trafficking of CD147 to the plasma membrane in the metastatic
breast cancer cell line MDA-MB-231. Cancer Res. 67:4182–4189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan Y, He B, Song G, Bao Q, Tang Z, Tian F
and Wang S: CD147 silencing via RNA interference reduces tumor cell
invasion, metastasis and increases chemosensitivity in pancreatic
cancer cells. Oncol Rep. 27:2003–2009. 2012.PubMed/NCBI
|
|
11
|
Sato M, Nakai Y, Nakata W, Yoshida T,
Hatano K, Kawashima A, Fujita K, Uemura M, Takayama H and Nonomura
N: EMMPRIN promotes angiogenesis, proliferation, invasion and
resistance to sunitinib in renal cell carcinoma, and its level
predicts patient outcome. PLoS One. 8:e743132013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Omi Y, Shibata N, Okamoto T, Obara T and
Kobayashi M: The role of CD147 in the invasiveness of follicular
thyroid carcinoma cells. Thyroid. 22:383–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stenzinger A, Wittschieber D, von
Winterfeld M, Goeppert B, Kamphues C, Weichert W, Dietel M, Rabien
A and Klauschen F: High extracellular matrix metalloproteinase
inducer/CD147 expression is strongly and independently associated
with poor prognosis in colorectal cancer. Hum Pathol. 43:1471–1481.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu S, Chu D, Zhang Y, Wang X, Gong L, Han
X, Yao L, Lan M, Li Y and Zhang W: EMMPRIN/CD147 expression is
associated with disease-free survival of patients with colorectal
cancer. Med Oncol. 30:3692013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng HC, Wang W, Xu XY, Xia P, Yu M,
Sugiyama T and Takano Y: Up-regulated EMMPRIN/CD147 protein
expression might play a role in colorectal carcinogenesis and its
subsequent progression without an alteration of its glycosylation
and mRNA level. J Cancer Res Clin Oncol. 137:585–596. 2011.
View Article : Google Scholar
|
|
16
|
Toole BP and Slomiany MG: Hyaluronan, CD44
and Emmprin: Partners in cancer cell chemoresistance. Drug Resist
Updat. 11:110–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Falls JG, Pulford DJ, Wylie AA and Jirtle
RL: Genomic imprinting: Implications for human disease. Am J
Pathol. 154:635–647. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engel N, Thorvaldsen JL and Bartolomei MS:
CTCF binding sites promote transcription initiation and prevent DNA
methylation on the maternal allele at the imprinted H19/Igf2 locus.
Hum Mol Genet. 15:2945–2954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lambert S, Vivario J, Boniver J and
Gol-Winkler R: Abnormal expression and structural modification of
the insulin-like growth-factor-II gene in human colorectal tumors.
Int J Cancer. 46:405–410. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui H, Cruz-Correa M, Giardiello FM,
Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR and
Feinberg AP: Loss of IGF2 imprinting: A potential marker of
colorectal cancer risk. Science. 299:1753–1755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan Y, He B, Li T, Zhu C, Zhang L, Wang B,
Xu Y, Qu L, Hoffman AR, Wang S, et al: Targeted tumor gene therapy
based on loss of IGF2 imprinting. Cancer Biol Ther. 10:290–298.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nie ZL, Pan YQ, He BS, Gu L, Chen LP, Li
R, Xu YQ, Gao TY, Song GQ, Hoffman AR, et al: Gene therapy for
colorectal cancer by an oncolytic adenovirus that targets loss of
the insulin-like growth factor 2 imprinting system. Mol Cancer.
11:862012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun H, Pan Y, He B, Deng Q, Li R, Xu Y,
Chen J, Gao T, Ying H, Wang F, et al: Gene therapy for human
colorectal cancer cell lines with recombinant adenovirus 5 based on
loss of the insulin-like growth factor 2 imprinting. Int J Oncol.
46:1759–1767. 2015.PubMed/NCBI
|
|
24
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
25
|
Lynch CC and Matrisian LM: Matrix
metalloproteinases in tumor-host cell communication.
Differentiation. 70:561–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abraham D, Zins K, Sioud M, Lucas T and
Aharinejad S: Host CD147 blockade by small interfering RNAs
suppresses growth of human colon cancer xenografts. Front Biosci.
13:5571–5579. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu T, Zhou M, Peng L, Kong S, Miao R, Shi
Y, Sheng H and Li L: Upregulation of CD147 promotes cell invasion,
epithelial-to-mesenchymal transition and activates MAPK/ERK
signaling pathway in colorectal cancer. Int J Clin Exp Pathol.
7:7432–7441. 2014.
|
|
29
|
Yang Y, Hu JF, Ulaner GA, Li T, Yao X, Vu
TH and Hoffman AR: Epigenetic regulation of Igf2/H19 imprinting at
CTCF insulator binding sites. J Cell Biochem. 90:1038–1055. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paradowska A, Fenic I, Konrad L, Sturm K,
Wagenlehner F, Weidner W and Steger K: Aberrant epigenetic
modifications in the CTCF binding domain of the IGF2/H19 gene in
prostate cancer compared with benign prostate hyperplasia. Int J
Oncol. 35:87–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ito Y, Koessler T, Ibrahim AE, Rai S,
Vowler SL, Abu-Amero S, Silva AL, Maia AT, Huddleston JE,
Uribe-Lewis S, et al: Somatically acquired hypomethylation of IGF2
in breast and colorectal cancer. Hum Mol Genet. 17:2633–2643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaneda A and Feinberg AP: Loss of
imprinting of IGF2: A common epigenetic modifier of intestinal
tumor risk. Cancer Res. 65:11236–11240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang JM, Xu Z, Wu H, Zhu H, Wu X and Hait
WN: Overexpression of extracellular matrix metalloproteinase
inducer in multidrug resistant cancer cells. Mol Cancer Res.
1:420–427. 2003.PubMed/NCBI
|
|
34
|
Zou W, Yang H, Hou X, Zhang W, Chen B and
Xin X: Inhibition of CD147 gene expression via RNA interference
reduces tumor cell invasion, tumorigenicity and increases
chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett.
248:211–218. 2007. View Article : Google Scholar
|
|
35
|
Jia L, Wang H, Qu S, Miao X and Zhang J:
CD147 regulates vascular endothelial growth factor-A expression,
tumorigenicity, and chemosensitivity to curcumin in hepatocellular
carcinoma. IUBMB Life. 60:57–63. 2008. View
Article : Google Scholar : PubMed/NCBI
|