Introduction
Human hepatocellular carcinoma (HCC) is one of the
most common malignant tumors and a significant cause of mortality
in several regions of Africa and Asia (1,2).
Triggering cancer cell death to reduce cancer cell number and
inhibiting cancer cell proliferation by phytochemicals or
chemotherapeutic agents represent some of the most effective
current anticancer strategies (3).
Cell death can occur through one of three pathways: necrosis,
apoptosis or autophagy (4).
Autophagy is induced by various physiological
conditions, such as mitochondrial damage, protein aggregation,
pathogen infection and nutrient starvation (5,6).
Autophagy is a multi-step cellular pathophysiological program,
including initiation (pre-autophagosome formation), autophagosome
formation, maturation and degradation (7). In these steps, phosphoinositide
3-kinases (PI3Ks) play key regulatory roles in many cellular
processes, including cell survival, proliferation and
differentiation (8–10). Both class I PI3K (PI3K-I)/Akt/
mammalian target of rapamycin (mTOR) and Beclin-1/class III PI3K
(PI3K-III)/LC3-II signaling pathways are involved in
pre-autophagosome formation (11–14).
Cytosolic and nuclear p53, damage-regulated autophagy modulator
(DRAM) and the p53-induced glycolysis and apoptosis regulator
(TIGAR) are involved in autophagosome formation (15). In addition, p53 initiates a cascade
of starvation signals and triggers a starvation-like response by
inhibiting mTOR (16).
Furthermore, p53 activates both the DRAM and PI3K-III pathways upon
autophagosome formation (15).
Phytochemicals or chemical agents that regulate p53- and
PI3K-mediated autophagy induction could serve as a potential
starting point for novel methods of cancer prevention and
treatment.
The transcription factor nuclear factor-kappa B
(NF-κB) plays an important role in autophagy-induced signaling
pathways (17,18). Several genes involved in autophagy,
such as the genes encoding Beclin-1, LC3-II and DRAM, are regulated
by NF-κB (15,19,20).
Regulation of NF-κB activation affects autophagy-relevant gene
expression, leading to the induction of autophagy.
Sedanolide (SN;
3-butyl-3a,4,5,6-tetrahydro-1(3H)-isobenzofuranone), is a
phthalide-like compound from celery (Apium graveolens L.)
seed oil (21,22). Celery seed contains 2% volatile
oil. The typical aroma of celery seed essential oils arises due to
SN, which is present at very low levels (1–3%) in the essential oil
(23). Previous studies have
demonstrated that SN protects against hydrogen peroxide- and
tert-butyl hydroperoxide-induced toxicity in HepG2 and CaCo-2 cells
(22). SN exhibits prostaglandin H
endoperoxide synthase-I (COX-I) and prostaglandin H endoperoxide
synthase-II (COX-II) inhibitory activities and topoisomerase-I and
-II enzyme inhibitory activities (24). In benzo[α]pyrene-(BP) induced
forestomach cancer in mice, SN reduced tumor incidence by 57% and
tumor multiplicity by 83% by increasing glutathione S-transferase
(GST) activity of mice (21).
However, the role that SN plays in cancer prevention remains
unclear, and research regarding its molecular mechanism through a
role in cell death induction is limited. If SN is cytotoxic or
induces cell death, its chemopreventative properties would be more
favorable.
The aim of this study was to determine whether SN
exhibits cytotoxic effects and whether these effects involve
autophagy induction in human liver cancer cells. To investigate the
effects of SN on cell viability and autophagy, an MTT assay and
monodansylcadaverine (MDC) staining of J5 cells were performed. To
understand the mechanism by which SN regulates pre-autophagosome
and autophagosome formation in autophagy induction, Beclin-1,
PI3K-III, LC3-II, PI3K-I, Akt and mTOR expression were investigated
using an immunoblot assay. In addition, the roles of p53 and NF-κB
on SN-induced autophagy were also investigated. These experimental
results will be helpful to determine the mechanism by which SN
induces human liver tumor cell death and its potential effects on
chemoprevention and/or cancer therapy.
Materials and methods
Chemicals and reagents
SN and MDC stain were purchased from Sigma Chemical
Co. (St Louis, MO, USA). Antiserum against p53, DRAM,
phosphorylated (p)-IκB, IκB or NF-κB (P65) were obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Antiserum against TIGAR
was obtained from Abcam, Inc. (Cambridge, MA, USA). Antiserum
against PI3K-I, PI3K-III, LC3-II, Beclin-1 and mTOR were purchased
from Cell Signaling Technology (Beverly, MA, USA).
Cell culture and SN treatment
The human hepatocellular carcinoma HepJ5 (J5) cell
line was obtained from the Food Industry Research and Development
Institute (Hsinchu, Taiwan). J5 cells (2.5×106) were
plated on 60-mm plastic tissue culture dishes (Becton-Dickinson
Labware, Franklin Lakes, NJ, USA) and incubated at 37°C (in
humidified 5% CO2 and 95% air at 1 atm) in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum, 1% penicillin and streptomycin (100 U/ml), and 1%
L-glutamine (0.33%, w/v).
After primary plating for 24 h, cells were treated
with 100, 250 or 500 μM of SN for 24 h. All SN was diluted in
dimethyl sulfoxide (DMSO). Cells treated with DMSO alone were
regarded as the control group.
Cell viability and morphology
examination
Cell viability was measured using an MTT (3-(4,
5-dimethyl-2-yl)-2, 5-diphenyl tetrazolium bromide) assay and
morphological examination. The MTT assay was performed as described
by Debizot and Lang (1986) (25).
Briefly, J5 cells were incubated in 3-cm plates (1×106
cells) for 24 h and treated with 100, 250 or 500 μM of SN for 24 h.
Then, MTT reagent (5 mg/ml) was added, and cell viability was
determined by measuring the optical density (OD) at 570 nm. A
phase-contrast microscope was used to examine morphological changes
(Olympus IX 51, Olympus, Tokyo, Japan).
Monodansylcadaverine staining
To determine whether J5 cell death induced by SN was
attributed to autophagy, the autophagic vacuole staining dye MDC
was used as an autophagy indicator (26). Cells were grown on cover slips and
treated with 100, 250 or 500 μM of SN for 24 h. After SN treatment,
cells were incubated with 0.1 mM MDC for 15 min. Micrographs were
acquired at ×400 magnification on an inverted fluorescence
microscope (Olympus IX 51, Olympus). MDC stained cells were
measured fluorometrically at an excitation wavelength of 360 nm and
an emission wavelength of 530 nm.
Immunoblot analysis of
autophagy-associated protein expression
To elucidate the signal transduction pathways by
which autophagy was induced by SN in J5 cells, we analyzed the
protein expression of autophagy regulators of pre-autophagosome and
autophagosome formation. J5 cells were treated with 100, 250 or 500
μM of SN for 24 h. Total protein was collected and prepared from
cells. Nuclear extracts were obtained from cell pellets using an
NE-PER extraction kit (Thermo Scientific, Rockford, IL, USA) as per
the manufacturer's instructions.
Cellular protein concentration of cells was assayed
by the method of Lowry et al (27). Cytoplasmic PI3K-I, PI3K-III, Akt,
mTOR, Beclin-1, LC3-II, p53, DRAM and TIGAR as well as nuclear p53
expression were determined using sodium dodecylsulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot assay
(28,29). Bands were visualized using hydrogen
peroxide/tetrahydrochloride diaminobenzidine or an enhanced
chemiluminescent detection kit (Amersham Life Science,
Buckinghamshire, UK) and were quantitated with an AlphaImager 2000
(Alpha Innotech, San Leandro, CA, USA).
Analysis of NF-κB activation and
NF-κB-DNA binding activation
To determine whether autophagy-related molecules are
regulated by the NF-κB pathway after SN treatment, J5 cells were
treated with 100, 250 or 500 μM of SN for 24 h. Total protein was
collected and prepared from cells. Cytosolic p-IκB, IκB, and NF-κB
as well as nuclear NF-κB (p65) expression were determined using
SDS-PAGE and immunoblot assay (28,29).
Bands were visualized using hydrogen peroxide/ tetrahydrochloride
diaminobenzidine or an enhanced chemiluminescent detection kit
(Amersham Life Science) and were quantitated with an AlphaImager
2000 (Alpha Innotech).
For the NF-κB-DNA binding activity assay, nuclear
extracts were obtained from cell pellets using an NE-PER extraction
kit (Thermo Scientific) as per the manufacturer's instructions.
NF-κB-DNA binding activity within the nuclear fraction was
determined using an NF-κB (p65) transcription factor activity assay
kit (Cayman Chemical Co., Ann Arbor, MI, USA) as per the
manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using SAS
software (SAS Institute). Analysis of variance (ANOVA) and Duncan's
multiple-range test were used to identify significant differences
among the means (p<0.05).
Results
SN induces cytotoxicity in human liver
tumor cells
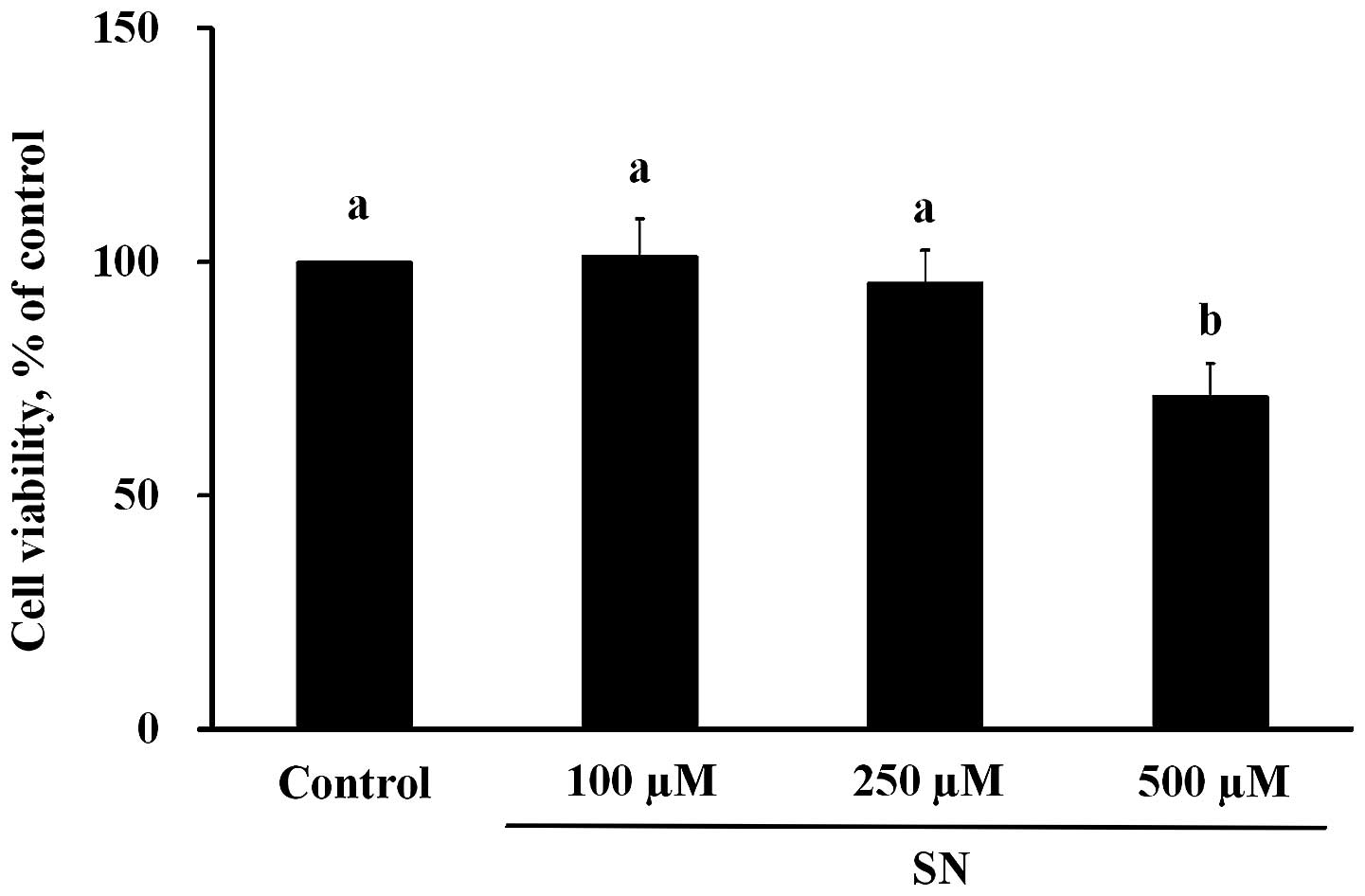
According to the results of the MTT assay (Fig. 1), when J5 cells were treated with
100, 250 or 500 μM SN for 24 h, the cell viability measurements
were 101±8, 96±7 and 71±7%, respectively. The cell viability of the
500 μM SN group was significantly reduced compared with the control
group (100%) (p<0.05). From the microscopic observation results,
no significant changes in the morphology of J5 cells treated with
100 or 250 μM SN were noted compared with the controls (data not
shown). However, significant changes in J5 cell morphology were
observed upon treatment with 500 μM SN after 24-h incubation
compared with controls.
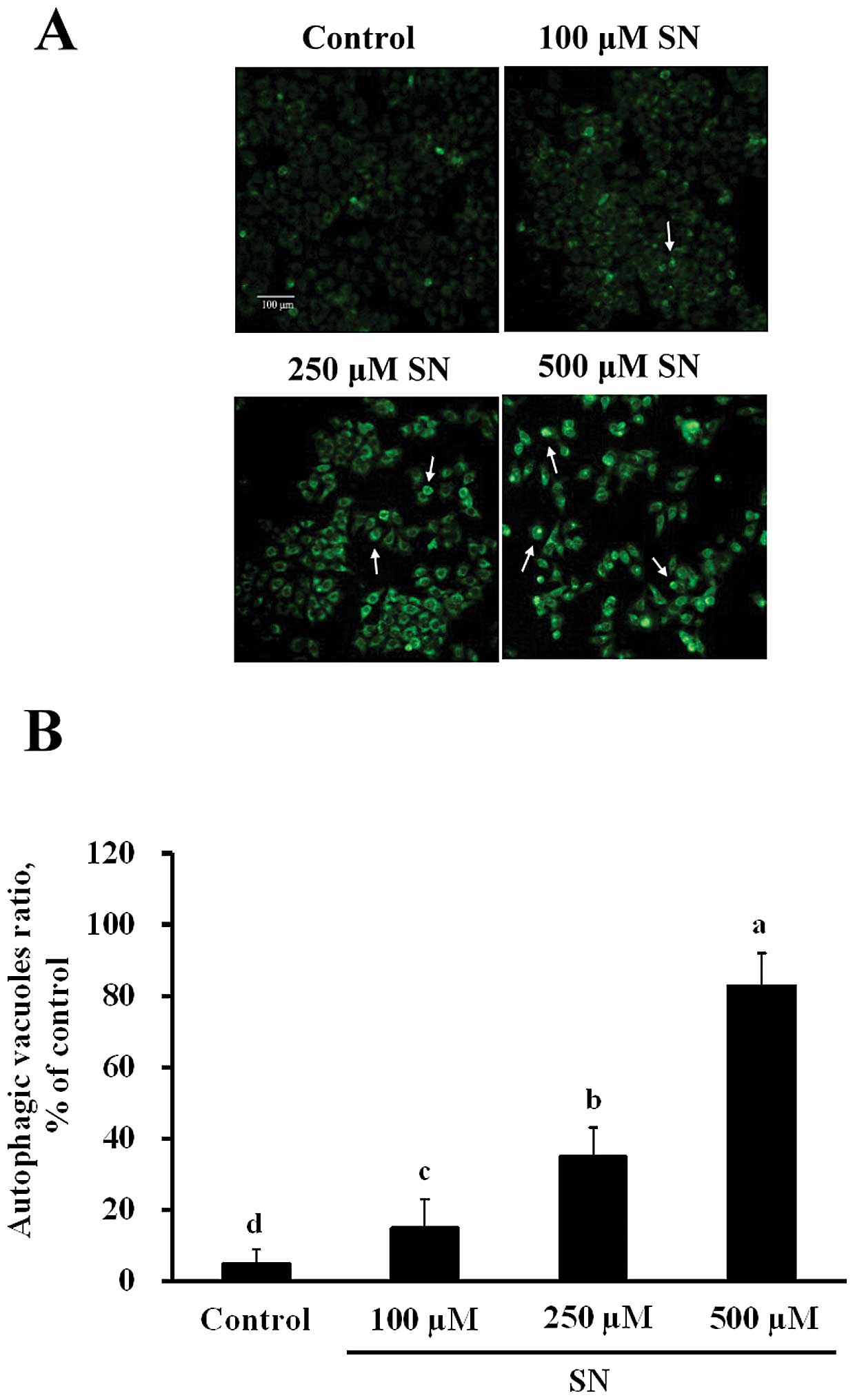
SN induces autophagy in human liver tumor
cells
As shown in Fig.
2A, J5 cells were stained by MDC after various concentrations
of SN treatment for 24 h, MDC-stained cell levels significantly
increased (p<0.05), as measured fluorometrically. After J5 cells
were treated with 100, 250 or 500 μM SN for 24 h, MDC fluorescence
(35±5, 46±8 and 83±9%, respectively) also significantly increased
in a dose-dependent manner compared with the control group (6±3%)
(Fig. 2B). These results
demonstrate that SN induces autophagy in J5 cells.
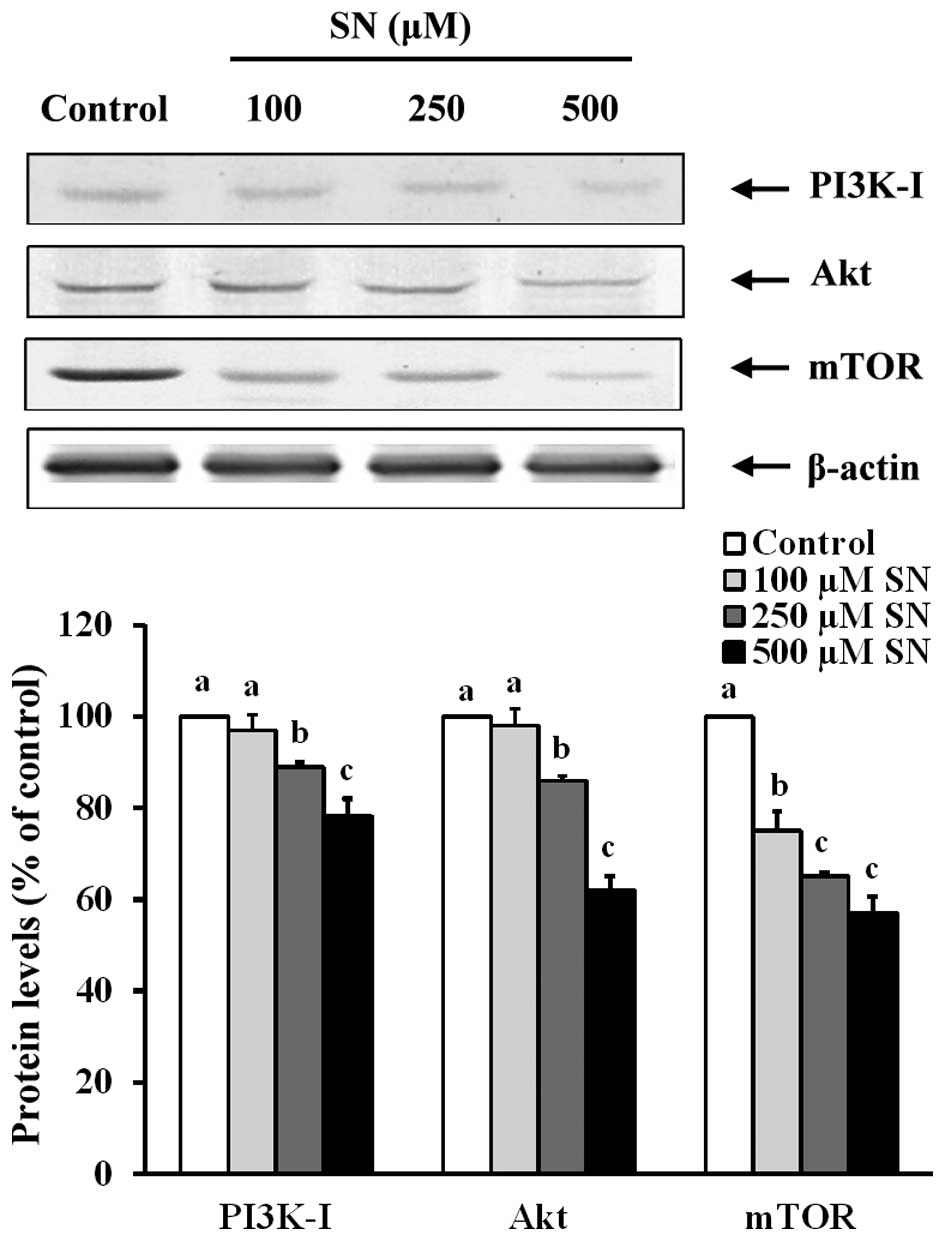
Effects of SN on protein levels of
autophagy regulators
Fig. 3 indicates
that in J5 cells treated with 250 or 500 μM SN for 24 h, PI3K-I
protein levels (89±7 and 78±4%, respectively) significantly
decreased following 24 h incubation compared with the control group
(100%) (p<0.05). After J5 cell treatment with 250 or 500 μM SN
for 24 h, the Akt protein levels were decreased (86±5 and 62±3%,
respectively) compared with the control group (100%) (p<0.05).
mTOR protein levels of the 100, 250 or 500 μM SN-incubated groups
were significantly decreased (75±4, 65±3 and 53±4%, respectively)
compared with the control group (100%) (p<0.05). These results
demonstrate that SN suppresses the PI3K/Akt/mTOR pathways. Thus,
this compound may play an important role in pre-autophagosome
formation.
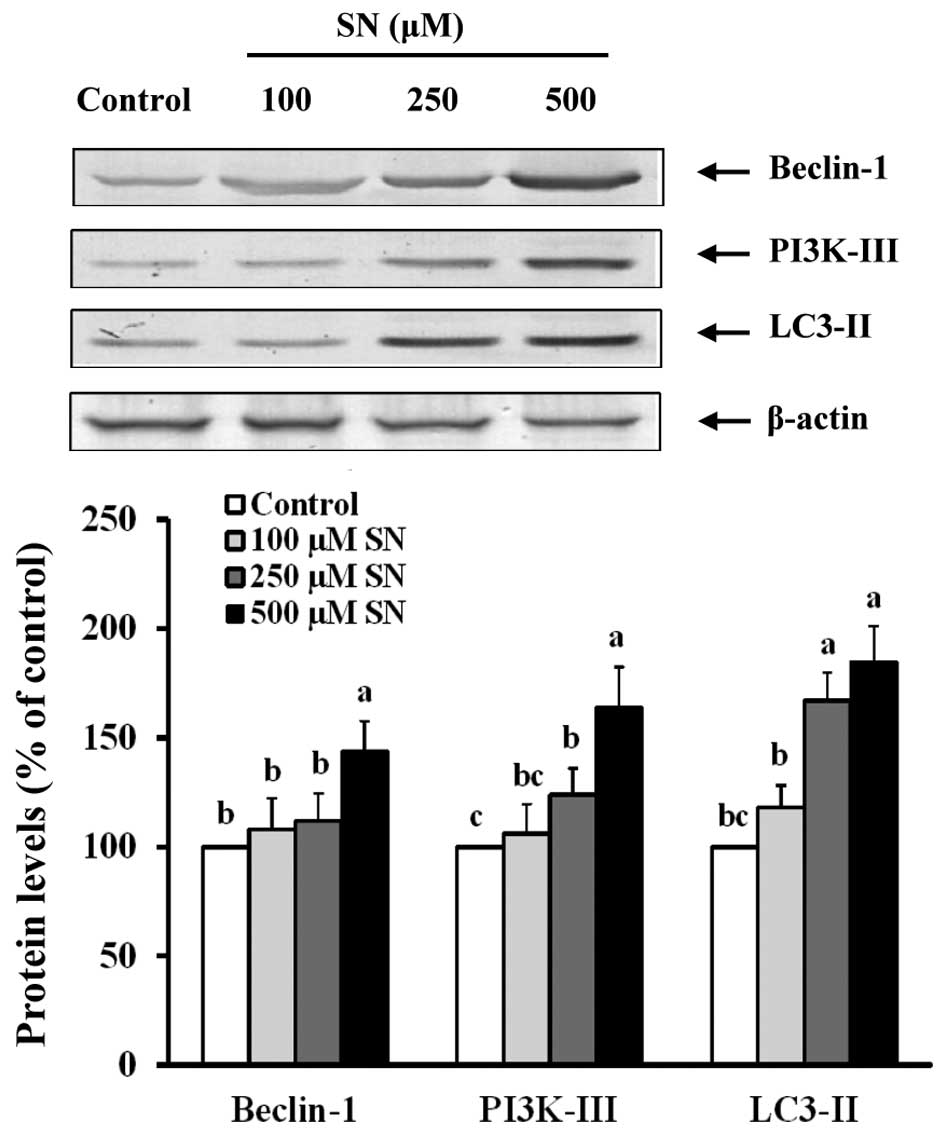
Conversely, Fig. 4
indicates that when J5 cells are treated with 500 μM SN for 24 h,
Beclin-1 protein levels were increased (144±14%) compared with the
control group (100%) (p<0.05). When J5 cells were treated with
250 or 500 μM SN, PI3K-III (124±12 and 164±18%, respectively) and
LC3-II (167±13 and 185±15%, respectively) protein levels were also
significantly increased compared with the control group (100%)
(p<0.05). These results suggest that SN regulates the
PI3K-I/Akt/mTOR and Beclin-1/PI3K-III/LC3-II pathways that induce
autophagy in J5 cells.
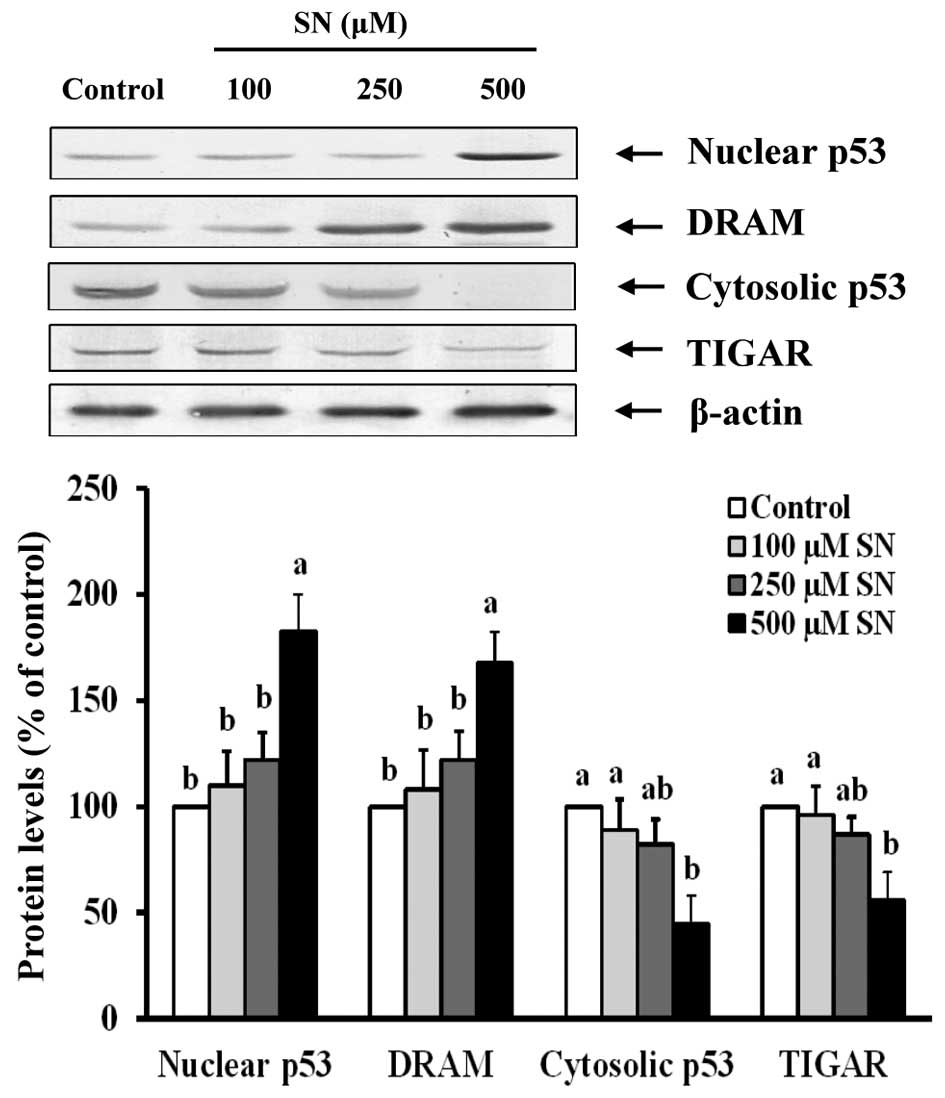
Immunoblot analysis also demonstrated that nuclear
p53 protein levels of the 500 μM SN group were significantly
increased (183±17%) compared with the control group (100%)
(p<0.05) (Fig. 5). DRAM protein
levels were significantly increased (168±14%) after 500 μM SN
treatment compared with the control group (100%) (p<0.05).
However, cytoplasmic p53 significantly decreased (45±13%) after J5
cells were treated with 500 μM SN for 24 h compared with the
control group (100%) (p<0.05). When J5 cells were treated with
500 μM SN, TIGAR protein levels (56±13%) were significantly reduced
compared with the control group (100%) (p<0.05). These results
indicate that SN affects autophagosome formation via p53
signaling.
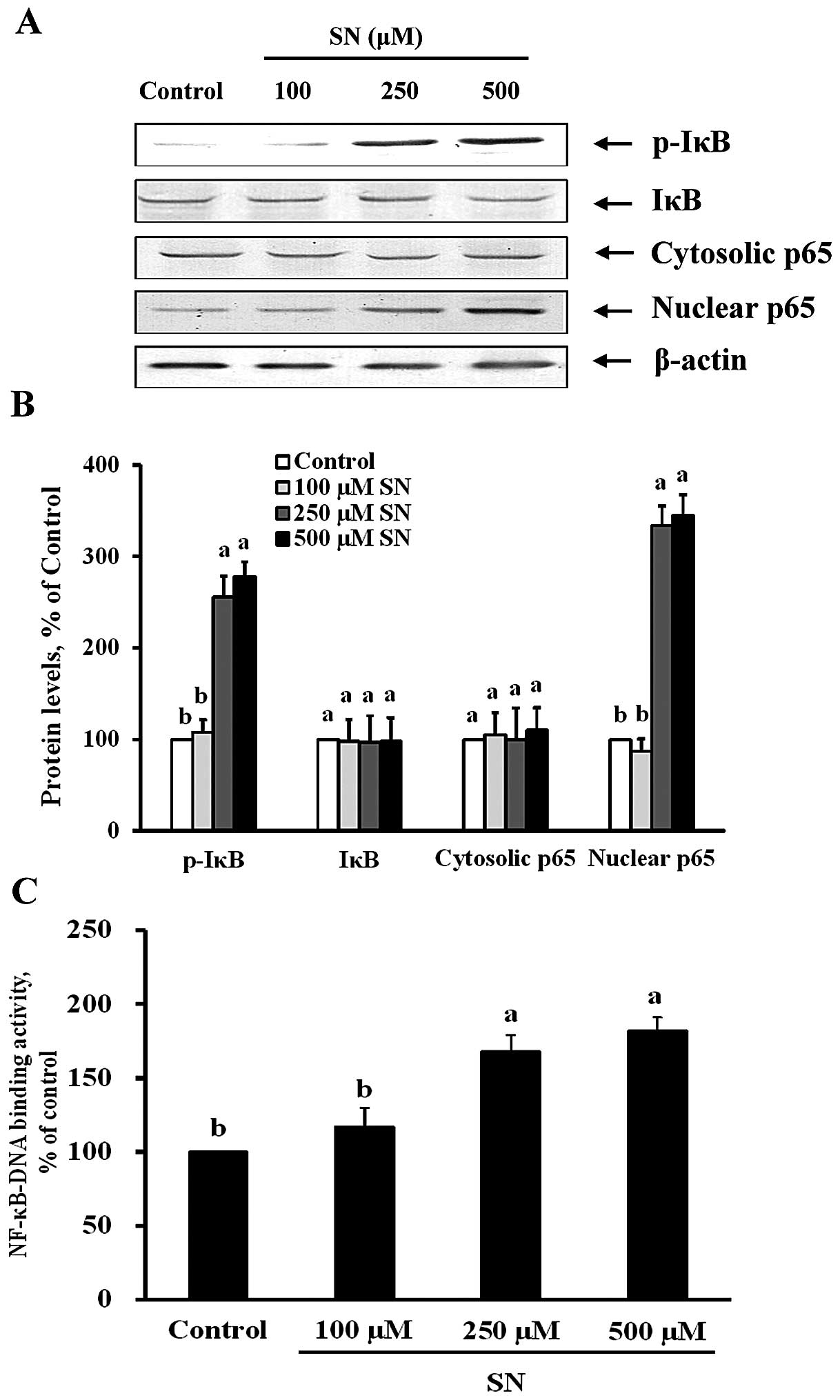
SN induces NF-κB activation
To examine whether the suppression of Beclin-1,
LC3-II and DRAM expression by SN is dependent on the inhibition of
NF-κB activation, both immunoblot and NF-κB-DNA binding activity
assays were performed. Fig. 6A and
B demonstrate that p-IκB protein was significantly increased by
256 and 278% after 250 and 500 μM SN treatment, respectively
(p<0.05). Nuclear p65 protein levels were also significantly
decreased by 334 and 345% after 250 and 500 μM SN treatment,
respectively (p<0.05). The changes in p65 protein levels in the
nuclear fraction indicate that SN could significantly increase
translocation of NF-κB from the cytoplasm to the nucleus in J5
cells. Furthermore, Fig. 6C
demonstrates that NF-κB nuclear protein DNA-binding activity was
significantly increased after 250 and 500 μM SN treatment
(p<0.05). These results suggest that SN regulates NF-κB
activation in J5 cells.
Discussion
SN is one of the major flavor compounds from celery
seed oil (30). Our study
demonstrates that SN suppresses human tumor cell viability through
the induction of autophagy. This finding is novel in the anticancer
research of SN. Autophagy plays an important role in tumor
inhibition because autophagy defects are associated with metabolic
stress, genomic damage, and tumorigenesis (3). By genetically or pharmacologically
triggering autophagy, these autophagy inducers could act as
potential therapeutic candidates to kill tumor cells (5,31).
Various clinical anticancer agents, such as polyoxomolybdates,
platonin, phenethyl isothiocyanate, tamoxifen, rapamycin,
temozolomide, histone deacetylase inhibitors and camptothecin,
involve the induction of autophagy to enhance chemotherapeutic
efficacy (32–36). In this study, SN significantly
reduced human liver cell viability and induced autophagy.
Therefore, SN may act as a potential cancer preventative or
therapeutic.
SN is a phthalide-like compound. Phthalide
structures contain a lactone core with a variety of complex
chemical compound substituents typically found in herbs or
vegetables, such as celery, Dong quai (Angelica sinensis)
and Chuanxiong (Rhizoma Chuanxiong) (37,38).
SN has a similar chemical structure to butylphthalide
(3-n-butylphthalide) in celery seed oil, and both compounds are
primarily responsible for the aroma and taste of celery (30). Previous studies demonstrate that
Angelica sinensis phthalides, including
n-butylidene-phthalide, senkyunolide A and z-ligustilide,
significantly inhibit cell proliferation of human colon cancer
HT-29 cells (39). Noscapine, a
phthalide isoquinoline alkaloid derived from opium, inhibits cell
proliferation by inducing apoptosis in colon cancer cells (40). Furthermore,
4-(3′,3′-dimethyl-allyloxy)-5-methyl-6-methoxyphthalide, which is
isolated from Podocarpus macrophyllus, inhibits
proliferation of HeLa tumor cell lines by inducing G1 cell cycle
arrest and apoptosis (41). No
study has investigated the effects of SN, a phthalide-like
structure, on cancer cell death. Our results demonstrate that SN
induces autophagy in human liver tumor cells, and this specific
physiological effect may be attributed to its phthalide-like
structure.
The PI3K pathway, a critical signal transduction
system linking oncogenes and multiple receptor classes to many
essential cellular functions, is perhaps the most commonly
activated signaling pathway in human cancer (42). PI3K-I is highly correlated with Akt
activation. Constitutive activation of the PI3K/ AKT signaling
pathway often causes cells to proliferate in an uncontrolled manner
(10). PI3K-III is an important
regulator of autophagy, a cellular response to nutrient starvation
(43). Among the signaling
pathways that promote autophagy induction, the PI3K-I/Akt/mTOR and
Beclin-1/PI3K-III/LC3-II signaling pathways are the major
transduction pathways that regulate autophagy (44). These pathways negatively and
positively regulate autophagy, respectively (45,46).
In this study, SN significantly suppresses PI3K-I, Akt and mTOR
protein levels in J5 cells, indicating that SN is a critical
suppressor of PI3K-I/Akt/mTOR signaling that leads to autophagy. In
contrast, when J5 cells were treated with SN, Beclin-1 and PI3K-III
expression increased, thus triggering LC-3 II activation and
inducing autophagy. Clinical anticancer drugs, such as
polyoxomolybdates, platonin, phenethyl isothiocyanate and
rapamycin, regulate PI3K, LC-3 or mTOR expression and lead to
decreased cancer cell viability (32–34,47).
Previous studies demonstrate that caffeine suppresses HeLa cell
growth and enhances autophagy by inhibiting the PI3K/Akt/mTOR
signaling pathway (48). Wang
et al also report that quercetin activates Akt-mTOR
signaling in human gastric cancer cells, leading to increased
LC3-II and Beclin-1 expression and subsequent induction of
autophagy (49). Phytochemicals or
chemicals that affect autophagy induction by suppressing
PI3K-I/Akt/mTOR signaling and/or enhancing Beclin-1/
PI3K-III/LC3-II signaling show chemopreventative or chemotherapy
potential.
p53 is a tumor suppressor that regulates numerous
responses, such as cell cycle arrest, apoptosis, and senescence,
each of which may lead to tumor inhibition (50). Recent studies indicate a new role
for p53 in autophagy induction. Two p53-associated signaling
pathways are involved in autophagy induction and based on p53
cellular localization. p53 promotes autophagy as a transcription
factor in the nucleus by activating genes involved in apoptosis,
cell cycle arrest and autophagy. However, autophagy is suppressed
when cytosolic p53 expression is inhibited (15,51,52).
In addition, p53 may induce autophagy by binding to the promoter
region of multiple genes that code for pro-autophagic modulators,
such as DRAM and TIGAR (53,54).
Cytosolic p53 inhibits autophagy by inducing TIGAR, which
indirectly affects reactive oxygen species (ROS) through modulation
of the glycolytic pathway (53,55).
To understand the effect of SN on p53 localization and its
downstream regulation of autophagy, our study demonstrated that SN
induces J5 cell autophagy by upregulating nuclear p53 and DARM and
downregulating cytoplasmic p53 and TIGAR in J5 cells. Furthermore,
SN not only regulates cytosolic and nuclear p53 levels but also
modifies the expression of mTOR and DRAM in our study. Clearly, p53
plays an important role in SN-induced J5 cell autophagy. Previous
studies demonstrate a similar biochemical regulation phenomenon
wherein T-47D breast cancer cells treated with sulphathiazole, a
common antibacterial drug, enhanced expression of p53/DRAM and
downregulated the Akt/mTOR pathway, resulting in autophagy
(56). Plumbagin
(5-hydroxy-2-methyl-1, 4-naphthoquinone; PLB), a naturally
occurring naphthoquinone isolated from the roots of
Plumbaginaceae plants, induces autophagy in tongue squamous
cell carcinoma (TSCC) through regulation of p53 and
PI3K/Akt/mTOR-mediated pathways (57).
In this study, SN was significantly affected by
transcript-specific autophagy genes, such as Beclin-1, LC3-II and
DRAM, through upregulation of NF-κB activation, which results in
increased Beclin-1, LC3-II and DRAM protein levels. Previous
studies have demonstrated that the NF-κB pathway is activated in
response to cellular starvation. When wild-type mouse embryonic
fibroblasts are starved in medium, IκB phosphorylation and
degradation is triggered. Then, NF-κB activation and increased LC3
expression is noted following starvation. These results also
demonstrate that NF-κB-DNA binding activity and target gene
expression were significantly increased under starvation conditions
(19).
In conclusion, 250 and 500 μM SN induce autophagy in
J5 cells, leading to human liver cancer cell death. The molecular
mechanism involves regulation of PI3K, p53 and NF-κB expression
during autophagy induction.
Acknowledgements
This study was supported by a grant
(NSC101-2815-C-309-010-B) from the National Science Council,
Taiwan, R.O.C..
Abbreviations:
|
SN
|
sedanolide
|
|
MDC
|
monodansylcadaverine
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
DRAM
|
damage-regulated autophagy
modulator
|
|
TIGAR
|
Tp53-induced glycolysis and apoptosis
regulator
|
|
IκB
|
inhibitor of kappa B
|
|
NF-κB
|
nuclear factor-kappa B
|
|
HCC
|
human hepatocellular carcinoma
|
References
|
1
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al; World Gastroenterology Organisation Guidelines and
Publications Committee. World Gastroenterology Organisation
Guideline. Hepatocellular carcinoma (HCC): A global perspective. J
Gastrointestin Liver Dis. 19:311–317. 2010.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding WX, Ni HM, Gao W, Chen X, Kang JH,
Stolz DB, Liu J and Yin XM: Oncogenic transformation confers a
selective susceptibility to the combined suppression of the
proteasome and autophagy. Mol Cancer Ther. 8:2036–2045. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizushima N: The pleiotropic role of
autophagy: From protein metabolism to bactericide. Cell Death
Differ. 12(Suppl 2): 1535–1541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcription and translation. Nat Rev
Cancer. 5:921–929. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arico S, Petiot A, Bauvy C, Dubbelhuis PF,
Meijer AJ, Codogno P and Ogier-Denis E: The tumor suppressor PTEN
positively regulates macroautophagy by inhibiting the
phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol
Chem. 276:35243–35246. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: A hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kondapaka SB, Singh SS, Dasmahapatra GP,
Sausville EA and Roy KK: Perifosine, a novel alkylphospholipid,
inhibits protein kinase B activation. Mol Cancer Ther. 2:1093–1103.
2003.PubMed/NCBI
|
|
15
|
Sui X, Jin L, Huang X, Geng S, He C and Hu
X: p53 signaling and autophagy in cancer: A revolutionary strategy
could be developed for cancer treatment. Autophagy. 7:565–571.
2011. View Article : Google Scholar
|
|
16
|
Feng Z, Zhang H, Levine AJ and Jin S: The
coordinate regulation of the p53 and mTOR pathways in cells. Proc
Natl Acad Sci USA. 102:8204–8209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trocoli A and Djavaheri-Mergny M: The
complex interplay between autophagy and NF-κB signaling pathways in
cancer cells. Am J Cancer Res. 1:629–649. 2011.
|
|
18
|
Criollo A, Chereau F, Malik SA,
Niso-Santano M, Mariño G, Galluzzi L, Maiuri MC, Baud V and Kroemer
G: Autophagy is required for the activation of NFκB. Cell Cycle.
11:194–199. 2012. View Article : Google Scholar
|
|
19
|
Comb WC, Cogswell P, Sitcheran R and
Baldwin AS: IKK-dependent, NF-κB-independent control of autophagic
gene expression. Oncogene. 30:1727–1732. 2011. View Article : Google Scholar
|
|
20
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng GQ, Kenney PM, Zhang J and Lam LK:
Chemoprevention of benzo[a]pyrene-induced forestomach cancer in
mice by natural phthalides from celery seed oil. Nutr Cancer.
19:77–86. 1993. View Article : Google Scholar
|
|
22
|
Woods JA, Jewell C and O'Brien NM:
Sedanolide, a natural phthalide from celery seed oil: Effect on
hydrogen peroxide and tert-butyl hydroperoxide-induced toxicity in
HepG2 and CaCo-2 human cell lines. In Vitr Mol Toxicol. 14:233–240.
2001. View Article : Google Scholar
|
|
23
|
Sowbhagya HB: Chemistry, technology, and
nutraceutical functions of celery (Apium graveolens L.): An
overview. Crit Rev Food Sci Nutr. 54:389–398. 2014. View Article : Google Scholar
|
|
24
|
Momin RA and Nair MG: Antioxidant,
cyclooxygenase and topoisomerase inhibitory compounds from Apium
graveolens Linn. seeds. Phytomedicine. 9:312–318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Itoh T, Ohguchi K, Nozawa Y and Akao Y:
Intracellular glutathione regulates sesquiterpene lactone-induced
conversion of autophagy to apoptosis in human leukemia HL60 cells.
Anticancer Res. 29:1449–1457. 2009.PubMed/NCBI
|
|
27
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
28
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gold HJ and Wilson CW III: The volatile
flavor substances of celery. J Food Sci. 28:484–488. 1963.
View Article : Google Scholar
|
|
31
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ogata A, Yanagie H, Ishikawa E, Morishita
Y, Mitsui S, Yamashita A, Hasumi K, Takamoto S, Yamase T and
Eriguchi M: Antitumour effect of polyoxomolybdates: Induction of
apoptotic cell death and autophagy in in vitro and in vivo models.
Br J Cancer. 98:399–409. 2008. View Article : Google Scholar
|
|
33
|
Bommareddy A, Hahm ER, Xiao D, Powolny AA,
Fisher AL, Jiang Y and Singh SV: Atg5 regulates phenethyl
isothiocyanate-induced autophagic and apoptotic cell death in human
prostate cancer cells. Cancer Res. 69:3704–3712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen YJ, Huang WP, Yang YC, Lin CP, Chen
SH, Hsu ML, Tseng YJ, Shieh HR, Chen YY and Lee JJ: Platonin
induces autophagy-associated cell death in human leukemia cells.
Autophagy. 5:173–183. 2009. View Article : Google Scholar
|
|
35
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fleming A, Noda T, Yoshimori T and
Rubinsztein DC: Chemical modulators of autophagy as biological
probes and potential therapeutics. Nat Chem Biol. 7:9–17. 2011.
View Article : Google Scholar
|
|
37
|
Uhlig JW, Chang A and Jen JJ: Effect of
phthalides on celery flavor. J Food Sci. 52:658–660. 1987.
View Article : Google Scholar
|
|
38
|
Zhang X, Xiao H, Xu Q, Li X, Wang J and
Liang X: Characterization of phthalides in Ligusticum chuanxiong by
liquid chromatographic-atmospheric pressure chemical
ionization-mass spectrometry. J Chromatogr Sci. 41:428–433. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kan WL, Cho CH, Rudd JA and Lin G: Study
of the anti-proliferative effects and synergy of phthalides from
Angelica sinensis on colon cancer cells. J Ethnopharmacol.
120:36–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang ZR, Liu M, Peng XL, Lei XF, Zhang JX
and Dong WG: Noscapine induces mitochondria-mediated apoptosis in
human colon cancer cells in vivo and in vitro. Biochem Biophys Res
Commun. 421:627–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen C and Yang RL: A phthalide derivative
isolated from endophytic fungi Pestalotiopsis photiniae induces G1
cell cycle arrest and apoptosis in human HeLa cells. Braz J Med
Biol Res. 46:643–649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Backer JM: The regulation and function of
Class III PI3Ks: Novel roles for Vps34. Biochem J. 410:1–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Meijer AJ and Codogno P: Regulation and
role of autophagy in mammalian cells. Int J Biochem Cell Biol.
36:2445–2462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shinojima N, Yokoyama T, Kondo Y and Kondo
S: Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in
curcumin-induced autophagy. Autophagy. 3:635–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pyo JO, Nah J and Jung YK: Molecules and
their functions in autophagy. Exp Mol Med. 44:73–80. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Georgakis GV and Younes A: From Rapa Nui
to rapamycin: Targeting PI3K/Akt/mTOR for cancer therapy. Expert
Rev Anticancer Ther. 6:131–140. 2006. View Article : Google Scholar
|
|
48
|
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri
S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K,
et al: Caffeine induces apoptosis by enhancement of autophagy via
PI3K/Akt/ mTOR/p70S6K inhibition. Autophagy. 7:176–187. 2011.
View Article : Google Scholar :
|
|
49
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR- and
hypoxia-induced factor 1α-mediated signaling. Autophagy. 7:966–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Murray-Zmijewski F, Slee EA and Lu X: A
complex barcode underlies the heterogeneous response of p53 to
stress. Nat Rev Mol Cell Biol. 9:702–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zilfou JT and Lowe SW: Tumor suppressive
functions of p53. Cold Spring Harb Perspect Biol. 1:a0018832009.
View Article : Google Scholar :
|
|
52
|
Maiuri MC, Galluzzi L, Morselli E, Kepp O,
Malik SA and Kroemer G: Autophagy regulation by p53. Curr Opin Cell
Biol. 22:181–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bensaad K, Tsuruta A, Selak MA, Vidal MN,
Nakano K, Bartrons R, Gottlieb E and Vousden KH: TIGAR, a
p53-inducible regulator of glycolysis and apoptosis. Cell.
126:107–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bensaad K, Cheung EC and Vousden KH:
Modulation of intracellular ROS levels by TIGAR controls autophagy.
EMBO J. 28:3015–3026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mohammadpour R, Safarian S, Sheibani N,
Norouzi S and Razazan A: Death inducing and cytoprotective
autophagy in T-47D cells by two common antibacterial drugs:
Sulphathiazole and sulphacetamide. Cell Biol Int. 37:348–358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pan ST, Qin Y, Zhou ZW, He ZX, Zhang X,
Yang T, Yang YX, Wang D, Qiu JX and Zhou SF: Plumbagin induces G2/M
arrest, apoptosis, and autophagy via p38 MAPK- and
PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell
carcinoma cells. Drug Des Devel Ther. 9:1601–1626. 2015.PubMed/NCBI
|