Introduction
Neuroendocrine (NE) carcinomas are rare neoplasms
that can develop into highly malignant and life-threatening tumors
(1,2). While they share a number of genetic
and phenotypic traits, NE carcinomas comprise a very heterogeneous
population of tumor types that can arise in various organs
throughout the body. The most common of these cancers include
neuroblastomas, retinoblastomas, medulloblastomas, pituitary
carcinomas, small cell lung carcinomas, and carcinoid tumors,
encompassing a broad spectrum of tumors that have so far required
multiple detection and treatment methods (3–7).
Despite their differences, many of these tumors express common
tumor-specific markers that can identify them as NE cancers
(8,9). Consequently, early detection of these
tumor markers can lead to better treatment response and outcomes.
The INSM1 gene encodes a NE tumor-specific marker that was
discovered using an insulinoma subtractive hybridization screen
(10,11). The INSM1-promoter regulates the
expression of INSM1, a transcription factor with a zinc-finger DNA
binding domain that is highly specific for NE tumors (12). Through an Insm1 knockout
mouse model, Insm1 transcription factor was found to be important
in the formation of endocrine pancreas and sympatho-adrenal lineage
during development (13,14). Most interestingly, INSM1 expression
was discovered to be restricted to the embryonic peripheral and
central nervous system, specifically in the cells of neuroendocrine
origin (15). The expression
pattern was detected in the embryonic tissues of pituitary,
pancreas, stomach, duodenum, thymus, adrenal glands, brain, and
spinal cord, which were all found to be Insm1-positive at E15.5 in
mice (16,17). However, INSM1 is silenced in normal
adult tissues, but reactivated in most of the human NE tumors,
including neuroblastoma, medulloblastoma, pheochromocytoma, small
cell lung carcinoma, insulinomas, pituitary tumors, carcinoid
tumors, medullary thyroid carcinoma, and retinoblastoma (18). Therefore, INSM1 is a NE-specific
tumor marker.
In order to assist with the detection of NE tumors
despite their heterogeneous population, we have taken advantage of
the INSM1-promoter's specificity in NE tumors to drive the
expression of a downstream Gaussia luciferase gene. Secreted
luciferases like Metridia or Gaussia luciferase have
been shown to be highly luminescent, exhibiting 2–4-fold higher
signal than Renilla or firefly luciferases (19,20).
We have constructed INSM1p-Met and INSM1p-Gau
reporter vectors to measure the INSM1 promoter activity in NE
tumors. In vitro cell lines and xenograft human tumor
cultured cells revealed positive luciferase secreted from NE
tumors. In addition, combining the INSM1p-Δ24E1A and
INSM1p-Gau luciferase vectors increased the sensitivity of
secreted Gaussia in vivo. The Δ24E1A gene, a mutant
form of the adenovirus E1A gene with a 24-bp deletion, is
inactive in retinoblastoma (Rb) protein expressing cells and active
in Rb-negative cancer cells (21).
The cancer specificity from the modified INSM1 promoter and the
Δ24E1A gene create a dual layer of safety against
non-specific expression.
Materials and methods
Construction of adenoviral vectors
The Ad-INSM1p-Met construct was cloned using
an original pGL3-INSM1p vector that contained the modified
INSM1-promoter with HS4 insulator upstream and 2X NRSE downstream
(22). The Metridia
luciferase gene was excised from the pMet-Reporter vector
(Clontech, Mountain View, CA, USA) and ligated downstream of the
modified INSM1-promoter in pGL3. The pGL3 vector was cut to release
the INSM1p-Met fragment, which was then ligated into the
pShuttle plasmid (Agilent Technologies, Santa Clara, CA, USA) for
adenoviral vector. The Ad-INSM1p-Gau and
Ad-INSM1p-Δ24E1A constructs were cloned using the modified
INSM1-promoter on the pGL3-INSM1p vector, created by shortening the
full insulator sequence into two copies of the core HS4 insulator.
The Gaussia luciferase gene was obtained from the
pMCS-Gaussia-Dura Luc vector (Thermo Fisher Scientific,
Waltham, MA, USA) and ligated downstream of the INSM1-promoter to
create pGL3-INSM1p-Gau. To clone the Δ24E1A gene,
site directed mutagenesis was performed on an existing E1A
gene in the pJet plasmid (Thermo Fisher Scientific) to delete 24 bp
from the original sequence. This Δ24E1A gene was then cloned
into the pGL3 vector to form pGL3-INSM1p-Δ24E1A. Both the
INSM1p-Gau and the INSM1p-Δ24E1A fragments were
excised from their vectors and placed into the pShuttle plasmid.
The Ad-SV40-Luc2 construct was generated by excising the
SV40 promoter from the pSEAP2-Control vector (Clontech) and ligated
upstream of the Luc2 reporter gene in the pGL4.10 vector
(Promega, Madison, WI, USA). The SV40-Luc2 fragment was
cloned into the pShuttle vector. The pShuttle plasmid was
linearized and electroporated into BJ5183-AD-1 cells (Agilent
Technologies) to undergo recombination. After selection for the
recombinants, linear adenoviral DNA was transfected into AD293
cells (Agilent Technologies) using FuGENE 6 reagent (Promega). The
virus was amplified onto forty 150-mm tissue culture dishes and
purified by CsCl gradient. This purified virus was then titered
using the Adeno-X-Rapid Titer kit (Clontech, Mountain View) and
stored at −80°C. All sequences in the cloning process were verified
through DNA sequencing.
In vivo luciferase imaging
Nu/Nu mice (National Cancer Institute, Bethesda, MD,
USA), aged 8–10 weeks, received intravenous tail vein injection of
either the modified first generation Ad-INSM1p-Luc2, the
second generation Ad-INSM1p-Luc2, or unmodified
Ad-INSM1p-Luc2. The viruses were prepared in
phosphate-buffered saline at a concentration of 1010
ifu/ml and 100 μl of the viral solution was delivered slowly into
the tail vein via a 27-gauge needle. To perform the imaging
analysis for luciferase activity, D-luciferin substrate (Biosynth,
Itasca, IL, USA) was prepared at a concentration of 15 mg/ml and
injected intraperitoneally into mice at a dose of 150 mg/kg. Once
injection was completed, the mice were anesthetized in an
isofluorane chamber (2–4% by inhalation) before being transferred
to a Kodak In-Vivo Multispectral FX imager (Carestream Health,
Rochester, NY, USA). Using the imager's software, luminescence was
acquired with a 10-min exposure and an X-ray image of the mice in
the same position was acquired with a 2-min exposure. Imaging was
performed 48 h after virus injection and periodically for ≤28 days.
To generate the complete image, the luminescence acquisition was
converted into a rainbow intensity scale and superimposed onto the
X-ray acquisition using ImageJ software (National Institutes of
Health, Bethesda MD, USA). For the NE tumor imaging, H1155 NE lung
tumor cells (1×107) were pre-infected with the second
generation modified Ad-INSM1p-Luc2 virus (50 MOI) for 24 h
and injected subcutaneously into the right hind flank of nude mice
(n=3). After one week, the tumor growth was evidenced and imaged to
show the modified INSM1 promoter specificity.
In vitro Metridia and Gaussia luciferase
secretion assay
Cells were seeded in a 96-well plate at a density of
10,000 cells per well. After incubation at 37°C and 5%
CO2 for 1 h, cells were infected with either no virus
(negative control), Ad-INSM1p-Met (0–50 MOI),
Ad-INSM1p-Gau (0–50 MOI), or Ad-SV40-Luc2 (5 MOI).
Infected cells were then incubated at 37°C, 5% CO2 for
24 h. After incubation, each well was washed gently with 1X PBS and
replaced with fresh media for another 24 h. Fifty microliters of
media per well were transferred to a 96-well white microplate.
Luminescence was detected using the Pierce Gaussia
Luciferase Glow Assay kit (Thermo Fisher Scientific), and read on a
TopCount NXT Microplate Scintillation and Luminescence Counter. The
adenoviral infection efficiency was determined by normalization
(ratio) with intracellular luciferase (Ad-SV40-Luc2) using
the Dual-Glo luciferase assay system (Promega) and read on a
TopCount NXT Microplate Scintillation and Luminescence counter. To
test the effects of Ad-INSM1p-Gau in combination with the
Ad-INSM1p-Δ24E1A conditionally replicating adenovirus, cells
were infected with a combination of 10 MOI Ad-INSM1p-Gau and
Ad-INSM1p-Δ24E1A for a total of 20 MOI (2×105
ifu). Media was collected 2, 4, and 6 days (20 μl each day) after
infection to determine secreted luciferase activity. Luminescence
was detected using the Pierce Gaussia Luciferase Glow Assay
kit (Thermo Fisher Scientific), then read on a TopCount NXT
Microplate Scintillation and Luminescence Counter. The Student's
t-test with a threshold of p<0.05 was used to determine
statistical significance. This process was repeated with the
Ad-INSM1p-Gau infected cells to determine the
Ad-INSM1p-Gau/Ad-SV40-Luc2 ratio.
Xenograft human tumor culture assay
Xenograft tumors were prepared by injecting human
tumor cells (1×107), such as HeLa, U87, D283, UMC-11,
SK-NBe(2), H1155, and H69 subcutaneously into the right hind flank
of nude mice. Tumor tissues were harvested and frozen (−80°C) in
RPMI-1640 culture medium with 10% DMSO. The cultured tumor cells
were prepared by rapidly thawing in a 37°C water bath and
subsequent mincing into small sections ~1 mm3 in size.
The minced tissues were then centrifuged at 250 x g for 1 min and
incubated in 2.5% trypsin for a total of 30 min at 37°C and 2 ml
growth media was added to neutralize the trypsin. The trypsinized
tissues were then filtered through a 70-μm sieve and centrifuged
again at 250 x g for 5 min. The 96-well clear-bottom plates were
coated with 75 μl per well of a 1:6 dilution of Matrigel in RPMI
growth media and then incubated for 30 min at 37°C. The tumor cells
(10,000 cells) were re-suspended in RPMI media and added to each
Matrigel coated well. Cells from each tumor were infected with
Metridia-luciferase and Ad-SV40-Luc2 at 37°C for 24
h. The ratio of Metridia/Luc2 luciferase was
calculated and averaged using RFU from 50 μl media per well.
Detection of serum Gaussia luciferase in
vivo
Eight-week-old Nu/Nu mice (National Cancer
Institute) were injected with H1155 NE lung tumor cells
(1×107) subcutaneously into the right hind flank. Tumors
were allowed to establish until tumor size grew to ≥0.1
cm3 in volume. The mice were injected intra-tumorally
with 1×109 ifu of Ad-INSM1p-Luc2 virus, or a
combination of 5×108 ifu of Ad-INSM1p-Gau and
Ad-INSM1p-Δ24E1A (for a total of 1×109 ifu). To
detect the Gaussia expression in the bloodstream, 100 μl of
blood was drawn at 3, 6, 9, and 12 days after virus injection. All
animal experiments were performed in accordance with the approved
protocol from the Institutional Animal Care and Use Committee,
Louisiana State University Health Sciences Center New Orleans. The
collected blood was allowed to clot for 30 min at room temperature
and centrifuged at 2,000 g for 10 min. Serum was collected from the
supernatant and diluted with PBS at a 1:10 ratio. To detect
Gaussia luciferase in the serum, 50 μl of the diluted serum
from each sample was added to a flat bottom 96-well plate for the
Gaussia luciferase assay.
Statistical analysis
Values were corrected and expressed relative to a
control group. All experiments were repeated three times. Results
are presented as mean ± SEM. Statistical analysis was performed
using wither the Student's t-test when only two groups were in the
experiment or by an one-way ANOVA comparison of multiple groups
using the Tukey-Kramer test with differences at p-value of <0.05
being considered significant.
Results
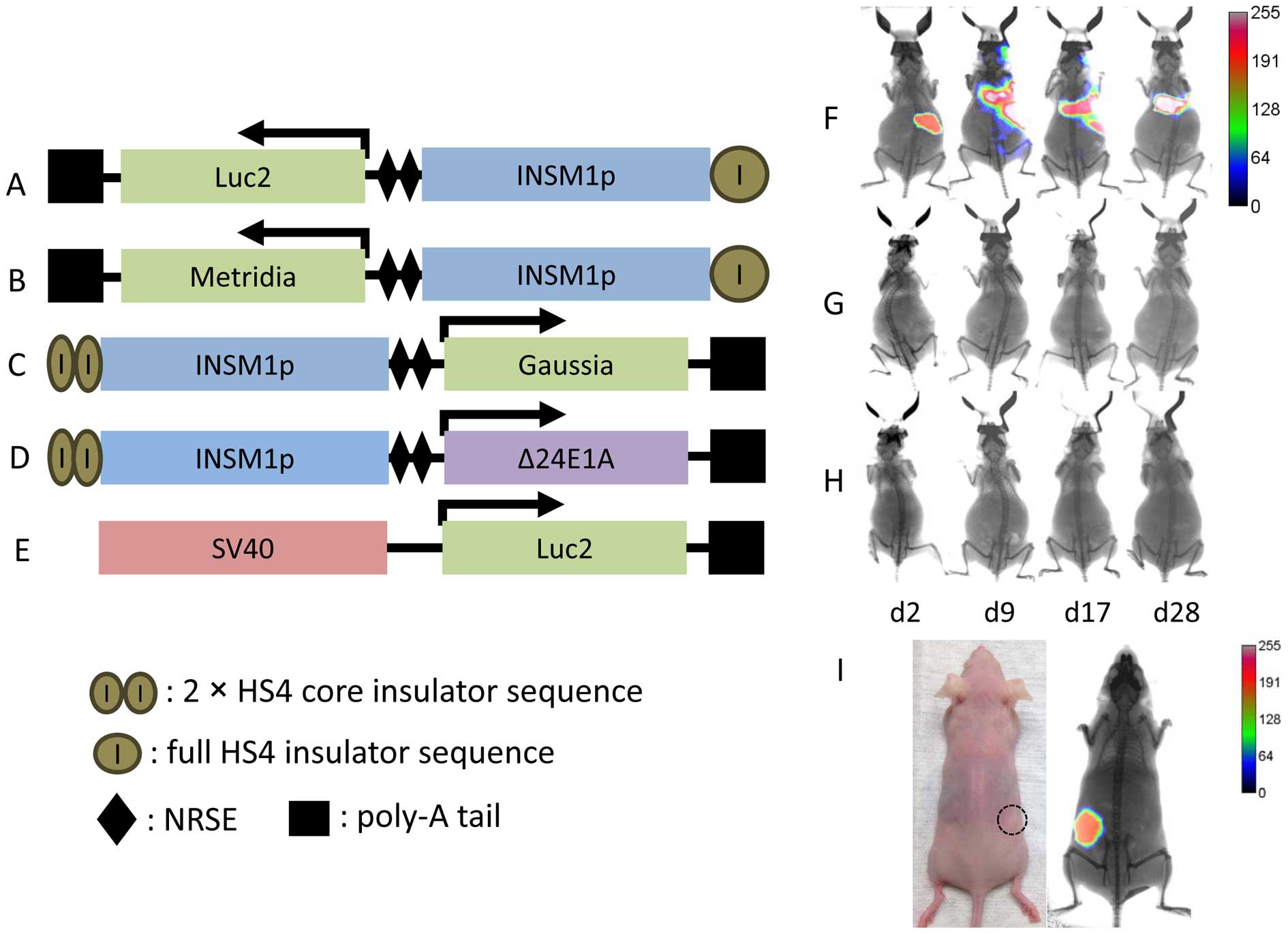
Cloning the INSM1-promoter driven
adenoviral constructs
To generate an adenoviral vector that is useful for
the diagnosis of INSM1-positive NE tumors, we constructed the first
generation modified INSM1-promoter by inserting a full HS4
insulator sequence upstream of the INSM1-promoter (~1.7 kb) along
with two NRSE enhancer sequences in tandem repeats downstream and
Luc2 gene (Fig. 1A)
(22). The modified INSM1-promoter
drives a downstream Metridia luciferase gene, resulting in
the construct Ad-HS4ins-INSM1p-2xNRSE-Metridia
(Ad-INSM1p-Met) (Fig. 1B).
A second generation of the modified INSM1-promoter was constructed
to drive the expression of Gaussia luciferase and
Δ24E1A. This promoter was created using two copies of the
HS4 core insulator in place of the full insulator sequence. The
final constructs Ad-2xHS4Core-INSM1p-2xNRSE-Gaussia
(Ad-INSM1p-Gau) and Ad-2xHS4Core-INSM1p-2xNRSE-Δ24E1A
(Ad-INSM1p-Δ24E1A), have a modified INSM1-promoter that is
~700 bp shorter than that of the promoter in Ad-INSM1p-Met
(Fig. 1C and D).
Ad-SV40-Luc2 vector was constructed as a control vector
(Fig. 1E).
We tested whether an adenoviral vector driven by the
modified INSM1-promoter would result in non-specific expression
in vivo. Tail vein injection was performed using three viral
vectors, the unmodified Ad-INSM1p-Luc2 (Fig. 1F), the first generation of the
modified Ad-INSM1p-Luc2 (Fig.
1G), and the second generation modified Ad-INSMp-Luc2
(Fig. 1H) injected into non-tumor
bearing Nu/Nu mice separately. Therefore, we examined the INSM1
promoter specificity with or without HS4 insulator and NRSE
enhancer sequence. After a period of 2–28 days, luciferase activity
was determined via in vivo imaging system after
intraperitoneal (i.p.) injection of luciferin substrate using a
Kodak In-Vivo Multispectral FX imager. The intravenous injected
adenovirus usually harbored in the liver (>90%). In the
non-tumor bearing mice that were injected with the original
unmodified Ad-INSM1p-Luc2, it was observed that non-specific
luciferase expression occurred and was focused primarily in the
liver area. In contrast, both the first and second generation
modified Ad-INSM1p-Luc2 did not exhibit non-specific
luciferase expression after luciferin administration. To further
demonstrate that the modified Ad-INSM1p-Luc2 virus maintains
NE tumor specificity, an Ad-INSM1p-Luc2 pre-infected H1155
NE tumor was established in nude mice and showed readily tumor
imaging by luciferase (Fig. 1I).
These results determined that the modified INSM1-promoter (both
first and second generation) is essential in blocking the effects
of adenoviral regulatory elements to retain tumor specificity in
vivo.
Ad-INSM1p-Met displays INSM1 specificity
in vitro
We constructed an adenoviral vector to express
secreted Metridia luciferase specifically driven by the
modified INSM1-promoter for the detection of NE tumors. Secreted
Metridia was measured in vitro by co-infecting tumor
cells with Ad-INSM1p-Met vector
(Ad-HS4ins-INSM1p-2xNRSE-Metridia) and Ad-SV40-Luc2.
The addition of Ad-SV40-Luc2 virus was used to normalize the
infection efficiency using the ratio between extracellular and
intracellular luciferase activity. Both INSM1-negative and
INSM1-positive tumor cell lines including lung carcinoma,
neuroblastoma, medulloblastoma, pheochromocytoma, and insulinoma
were infected with Ad-INSM1p-Met/Ad-SV40-Luc2 for 48
h (Fig. 2). The secreted
luciferase activity in the media was readily detected in all of the
INSM1-positive cell lines. In particular, the INSM1-positive cell
lines H82, βTC-1, and WERI-Rb-1 exhibited the highest
Ad-INSM1-Met/Ad-SV40-Luc2 luminescence ratios. At the
highest MOI (50:1), secreted luciferase activity reached >2-fold
that of the intracellular luciferase activity (Fig. 2). In contrast, the INSM1-negative
tumor cell lines showed no secreted Metridia luciferase
relative to intracellular luciferase.
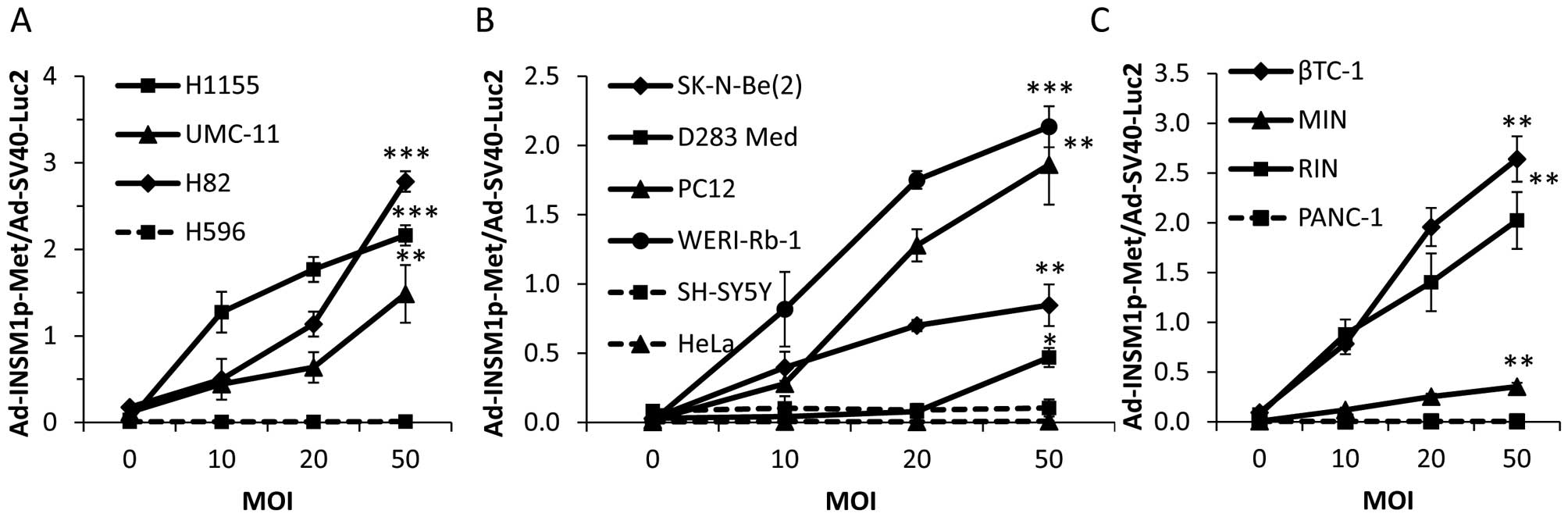 | Figure 2Ad-INSM1p-Met vector expressed
Metridia luciferase specifically in INSM1-positive cell
lines. An increasing Ad-INSM1p-Met concentration (0–50 MOI)
and a constant Ad-SV40-Luc2 concentration (5 MOI) was used
to infect INSM1-positive (solid lines) and -negative (dot lines)
cell lines in culture. (A) NE lung cancer H1155, UMC-11, H82, and
lung adenosquamous carcinoma H596 cells; (B) Neuroblastoma
SK-N-Be(2) and SH-SY5Y, retinoblastoma WERI-Rb-1, pheochromocytoma
PC-12, medulloblastoma D283 Med, and cervical adenocarcinoma HeLa
cells; (C) Insulinoma βTC-1, MIN, RIN, and pancreatic epithelioid
carcinoma PANC-1 cells were used. Values are expressed as ratios
between extracellular and intracellular luciferase activity.
*p<0.05, **p<0.01,
***p<0.001 (n=3). |
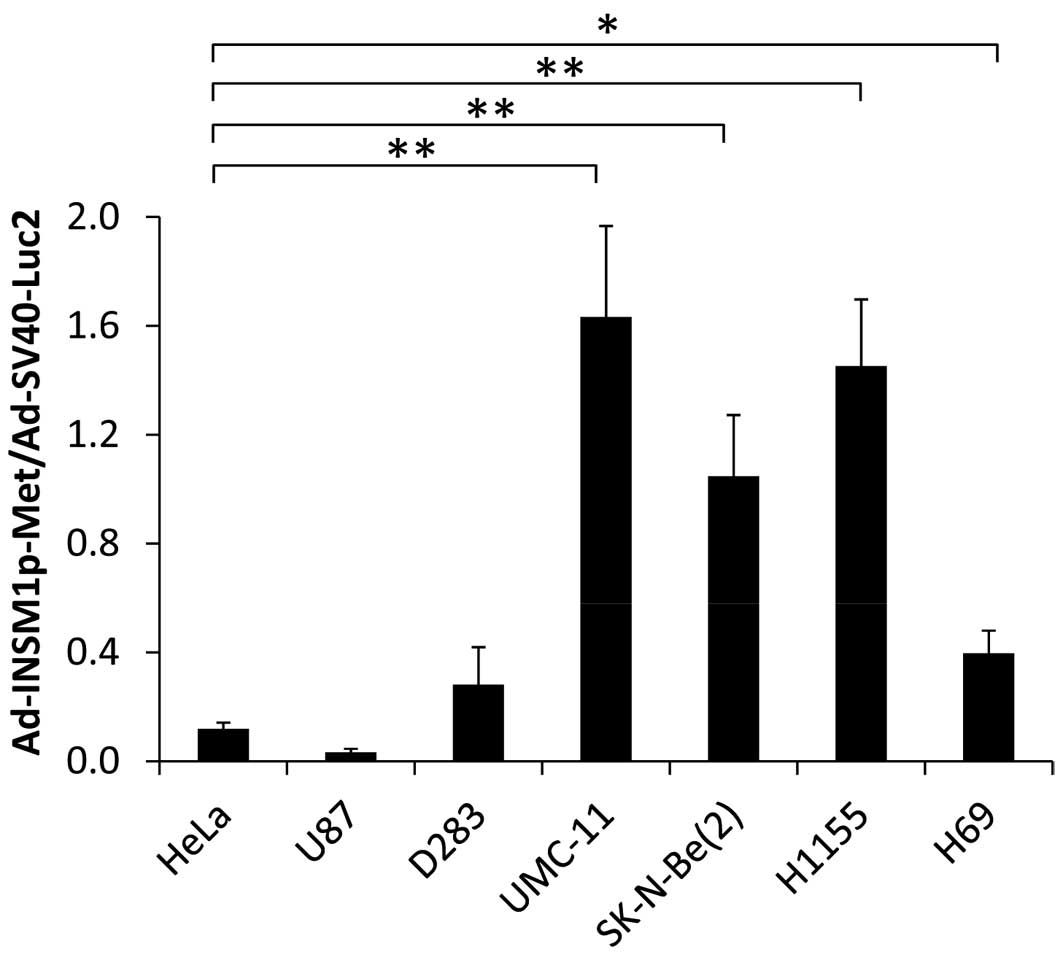
In order to assess the efficacy of INSM1-promoter
driven Metridia luciferase adenoviral vector in xenograft
human tumors, human tumor cultured cells derived from previously
established xenograft tumor were collected and grown in culture.
These ex vivo tumor cells were co-infected with
Ad-INSM1p-Met and Ad-SV40-Luc2 to determine the ratio
between extracellular and intracellular luciferase activity. After
incubation for 3 days, it was determined that INSM1-positive cells
[UMC-11, SK-N-Be(2), H1155, H69, except D283] infected by
Ad-INSM1p-Met expressed extracellular Metridia
luciferase that produced signals as high as 1.6 times the
intracellular firefly luciferase (Fig. 3). In contrast, INSM1-negative cells
(HeLa and U87) produced low levels of extracellular Metridia
luciferase that did not exceed 0.11 times the activity of
intracellular firefly luciferase.
INSM1 promoter-driven Gaussia luciferase
retains specificity
In preparation for further in vivo assays, we
switched from Metridia luciferase to a Gaussia
luciferase expression vector due to the increased stability of
Gaussia luciferase in vivo. As recent studies have
shown, Gaussia luciferase signals were detectable after
tail-vein injection in mice, due to its increased temperature
stability compared to Metridia luciferase (19). To determine the specificity of our
newly constructed Gaussia luciferase construct
(Ad-INSM1p-Gau), we conducted an in vitro luciferase
assay to evaluate whether our Ad-INSM1p-Gau vector
(Ad-2xHS4Core-INSM1p-2xNRSE-Gaussia) could specifically
express Gaussia luciferase in INSM1-positive cell lines in a
similar manner as our Ad-INSMp-Met vector. After
co-infection with Ad-INSM1p-Gau/Ad-SV40-Luc2, we were
able to see significant secreted luminescent activity in the media
of all INSM1-positive cell lines as compared to INSM1-negative
control cell lines (Fig. 4). In
particular, the INSM1-positive cell lines βTC-1, RIN, H82, and
H1155 exhibited the highest Ad-INSM1-Gau/Ad-SV40-Luc2
ratios. Secreted Gaussia luciferase was not detected in any
INSM1-negative cell lines, indicating INSM1 promoter retains
specificity in vitro.
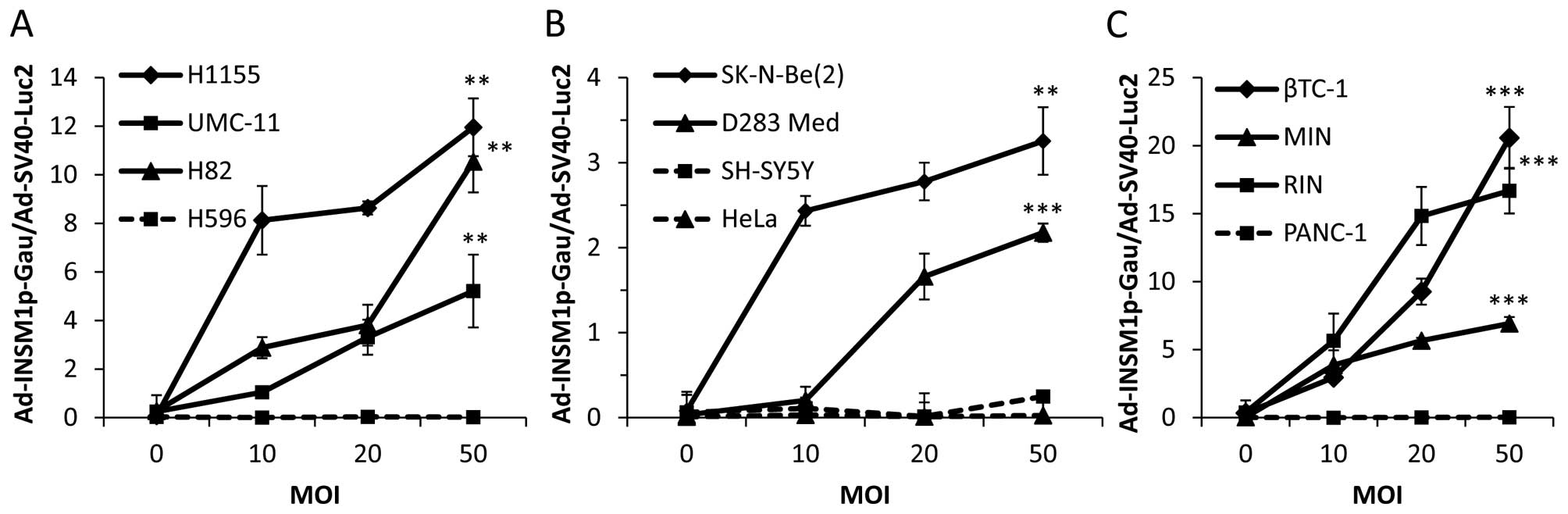 | Figure 4Ad-INSM1p-Gau vector expressed
Gaussia luciferase specifically in INSM1-positive cell
lines. An increasing Ad-INSM1p-Gau concentration (0–50 MOI)
and a constant AdSV40-Luc2 concentration (5 MOI) was used to
infect INSM1-positive (solid lines) and -negative (dot lines) cell
lines in culture. (A) NE lung cancer H1155, UMC-11, H82, and lung
adenosquamous carcinoma H596 cells; (B) Neuroblastoma SK-N-Be(2)
and SH-SY5Y, medulloblastoma D283Med, and cervical adenocarcinoma
HeLa cells; (C) Insulinoma βTC-1, MIN, RIN, and pancreatic
epithelioid carcinoma PANC-1 cells were used. Values are expressed
as ratios between extracellular and intracellular luciferase
activity. *p<0.05, **p<0.01,
***p<0.001 (n=3). |
Conditional replicating vector
(Ad-INSM1p-Δ24E1A) enhances Gaussia luciferase secretion and
sensitivity over time in an in vivo mouse xenograft tumor
model
After establishing the specificity of the
Ad-INSM1p-Gau vector, we attempted to determine whether
infecting cells with Ad-INSMp-Δ24E1A in combination with
Ad-INSM1p-Gau could increase the secreted luciferase signal.
We hypothesized that if an INSM1-positive cell is infected
concurrently with both viruses, Δ24E1A expression from
Ad-INSM1p-Δ24E1A can be utilized by both viruses to
facilitate replication. The replication of Ad-INSM1p-Gau
should lead to viral amplification and increased Gaussia
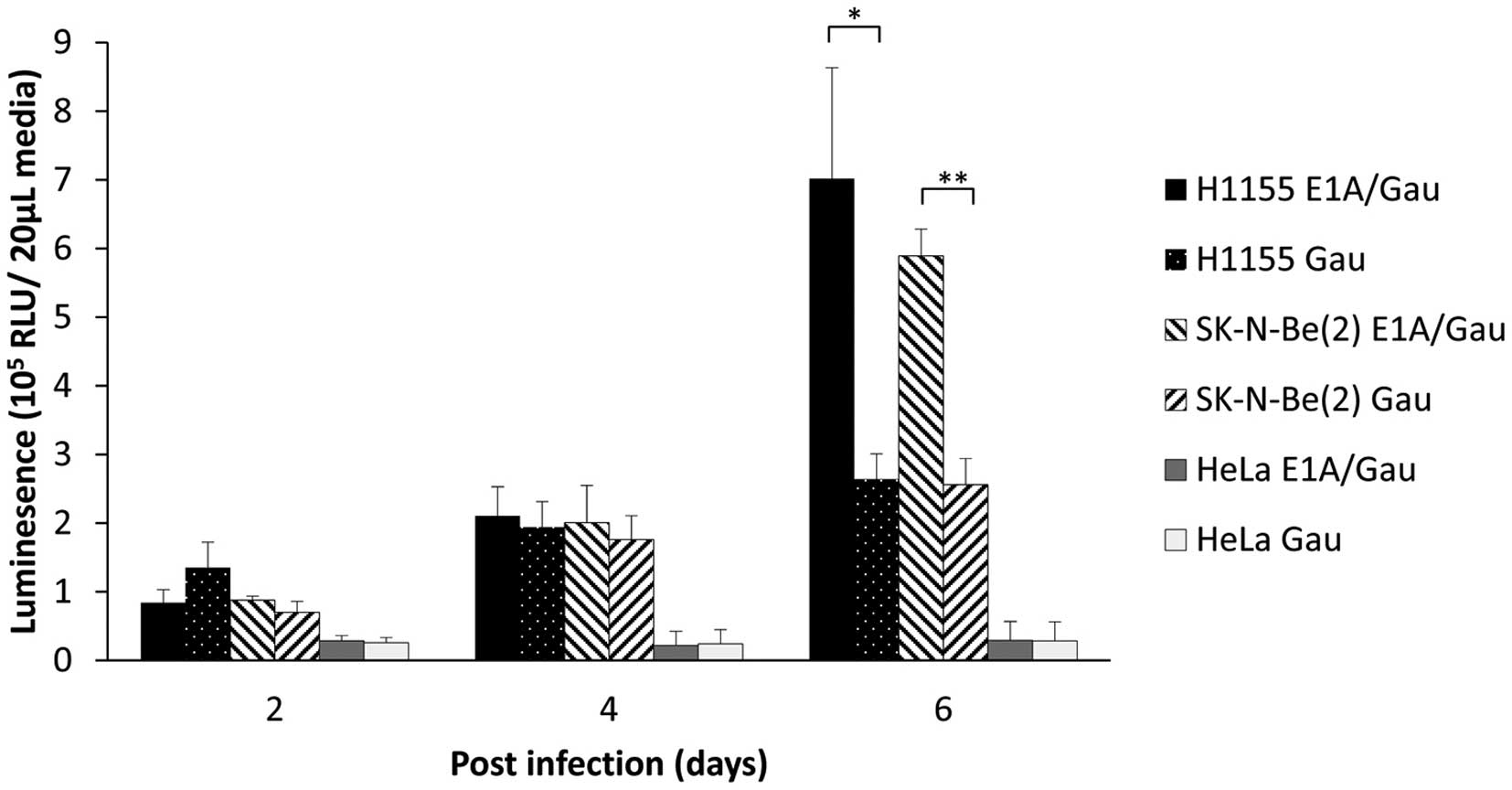
luciferase secretion over time. Therefore, we co-infected H1155 NE
lung carcinoma cells or SK-N-Be(2) neuroblastoma cells with
Ad-INSM1p-Gau alone (20 MOI) or in combination with
Ad-INSM1p-Δ24E1A at a concentration of 10 MOI each (Fig. 5). Six days after infection, the
combination viruses displayed a 2-fold increase in secreted
luminescent activity as compared to infection with
Ad-INSM1p-Gau alone. This result suggested that our
combination viruses could indeed amplify inside an INSM-positive
cell in vitro.
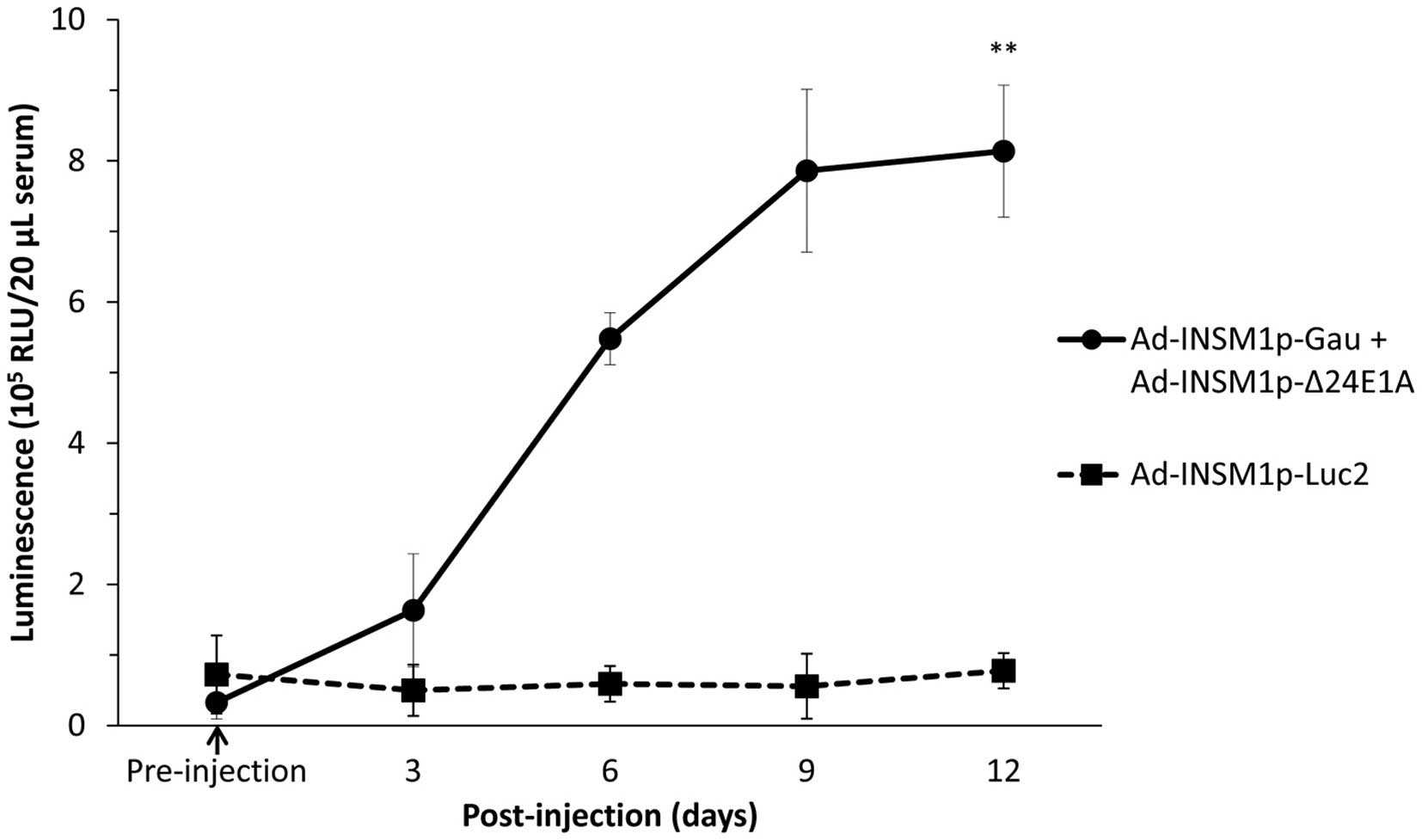
We further analyzed whether the combination of
Ad-INSM1p-Gau and Ad-INSM1p-Δ24E1A viruses could
secrete detectable amount of Gaussia luciferase into the
circulation from a tumor-bearing animal for an extended period of
time. In this experiment, subcutaneous H1155 tumors (~0.1
cm3) were first established on the right flank of Nu/Nu
mice. These tumor-bearing mice (n=3) were then injected
intra-tumorally with either Ad-INSM1p-Luc2 or the
Ad-INSM1p-Gau and Ad-INSM1p-Δ24E1A virus combination
at a total concentration of 1×109 ifu (Fig. 6). After infection with the virus
combination for 6 days, detectable luciferase signal was observed
in the serum (p<0.01). The signal increased in intensity by day
9 and lasted up to 12 days, the humane endpoint for the tumor
bearing animals. The Ad-INSM1p-Luc2 infected tumor released
no Gaussia luciferase into the circulation and was used as
the control.
Discussion
Although the original INSM1-promoter possesses
NE-tumor specificity, it was discovered that the promoter loses its
specificity when used in an adenoviral setting. In a recent study,
Akerstrom et al demonstrated that an INSM1-promoter driven
adenoviral reporter construct displayed non-specific expression
after tail vein injection in an in vivo mouse model
(22). It was hypothesized that
this loss of specificity was due to the presence of overpowering
viral enhancers that were otherwise not present in normal cells. To
override these adenoviral regulatory elements, an insulator
sequence derived from the HS4 chicken β-globin insulator was placed
upstream of the INSM1-promoter to block effects from any viral
enhancers. In addition, two copies of the neuron-restrictive
silencer element (NRSE), a regulatory element with dual functions
to silence the INSM1 promoter in non-neuronal cells while enhancing
it in neuronal cells, were placed downstream of the promoter. Once
these elements were added, the modified INSM1-promoter was able to
retain its high specificity in an adenoviral vector (22). To further improve upon this
original design, the present study replaced the 1.2-kb full
insulator sequence with two copies of the HS4 core insulator (250
bp x 2) to create the second generation modified INSM1 promoter.
Although the 1.2-kb full insulator sequence has been well
characterized functionally, the 250-bp core insulator was observed
to exhibit the same protective activity as the full sequence
(23). The main benefit of
switching from a full insulator sequence to the core sequence is
that utilization of two copies of the 250-bp core would free ~700
bp of space for the assembly of larger transgenic sequences in the
viral vector. Essentially, this more compact form of the modified
INSM1-promoter displays the same NE tumor specificity with the
additional advantage of allowing more flexible cloning
strategies.
Retaining the specificity of the INSM1-promoter in
an adenoviral vector has allowed us to construct a Gaussia
luciferase reporter vector that can detect the presence of NE tumor
in vivo. When paired with a conditionally replicating
oncolytic virus, the virus combination allowed for continuous
expression of Gaussia luciferase for the duration of the
tumor's progression. These results could have a significant impact
on monitoring tumor progression during the treatment of patients.
Given that the viruses can selectively replicate in NE tumor cells,
Gaussia luciferase expression should persist and intensify
as the tumor increases in size. Conversely, if treatment of the
tumor is successful, luciferase expression in the patient's blood
should decrease as tumor size is reduced. Our study is a proof in
principle that the Gaussia vector can be used in combination
with a treatment protocol to monitor a patient's treatment outcome.
An alternative use for this virus during the treatment of a NE
tumor would be to discern whether a tumor is removed completely
after surgical resection. By injecting the virus combination into
the resection site during the surgical procedure, clinicians would
be able to monitor the presence of INSM1-positive NE tumor cells
based on a Gaussia luciferase readings from the patient's
blood. Continuous monitoring of expression levels would allow for a
better prognosis in these patients post-procedure by alerting
clinicians to an incomplete resection.
Using the Ad-INSM1p-Gau vector in combination
with the Ad-INSM1p-Δ24E1A was discovered to be more
advantageous as compared to using Ad-INSM1p-Gau alone. In NE
tumor cells infected by the virus combination, Gaussia
luciferase expression was significantly higher than that of the
Ad-INSM1p-Gau virus (20 MOI) alone after 6 days
post-infection, even though the number of infectious units of
Ad-INSM1p-Gau (10 MOI) was lower at the start for the
combination. This indicates that the addition of Δ24E1A
expression in cells infected by our Gaussia virus allowed
for conditional replication of the reporter vector. This
replication has the potential to significantly increase the copy
number of the virus over several days, leading to an increase in
sensitivity of Gaussia luciferase detection. Therefore, the
most efficient method of increasing the sensitivity of infection
seems to involve utilization of conditionally replicating viruses,
as opposed to simply increasing the infectious units during
administration of the virus.
Taken together, the Ad-INSM1p-Gau virus has
the potential to be an easy-to-use and highly sensitive tool for
the detection of NE tumors in the clinical setting. While a viral
construct cannot be used as a diagnostic tool for the general
population, it can be an alternative approach to track the tumor
progression in patients with existing NE cancers. Additionally, it
could also be used diagnostically in populations where a NE tumor
is suspected. In these cases, the virus combination could act as
both a diagnostic tool and as a way to monitor tumor
progression.
Acknowledgements
This study was supported in part by the Research
Institute for Children, Children's Hospital at New Orleans
Louisiana.
Abbreviations:
|
NE
|
neuroendocrine
|
|
INSM1
|
insulinoma-associated-1
|
|
NRSE
|
neuronal restrictive silencing
element
|
|
Luc2
|
luciferase2
|
|
Gau
|
Gaussia
|
|
Met
|
Metridia
|
|
MOI
|
multiplicity of infection
|
|
RFU
|
reflective fluorescence unit
|
References
|
1
|
Rindi G and Wiedenmann B: Neuroendocrine
neoplasms of the gut and pancreas: New insights. Nat Rev
Endocrinol. 8:54–64. 2012. View Article : Google Scholar
|
|
2
|
Jann H, Roll S, Couvelard A, Hentic O,
Pavel M, Müller-Nordhorn J, Koch M, Röcken C, Rindi G, Ruszniewski
P, et al: Neuroendocrine tumors of midgut and hindgut origin:
Tumor-node-metastasis classification determines clinical outcome.
Cancer. 117:3332–3341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brodeur GM, Pritchard J, Berthold F,
Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE,
Favrot M, Hedborg F, et al: Revisions of the international criteria
for neuroblastoma diagnosis, staging, and response to treatment. J
Clin Oncol. 11:1466–1477. 1993.PubMed/NCBI
|
|
4
|
Hayes FA, Green A, Hustu HO and Kumar M:
Surgicopathologic staging of neuroblastoma: Prognostic significance
of regional lymph node metastases. J Pediatr. 102:59–62. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oyharcabal-Bourden V, Kalifa C, Gentet JC,
Frappaz D, Edan C, Chastagner P, Sariban E, Pagnier A, Babin A,
Pichon F, et al: Standard-risk medulloblastoma treated by adjuvant
chemotherapy followed by reduced-dose craniospinal radiation
therapy: A French Society of Pediatric Oncology Study. J Clin
Oncol. 23:4726–4734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Packer RJ, Goldwein J, Nicholson HS,
Vezina LG, Allen JC, Ris MD, Muraszko K, Rorke LB, Wara WM, Cohen
BH, et al: Treatment of children with medulloblastomas with
reduced-dose craniospinal radiation therapy and adjuvant
chemotherapy: A Children's Cancer Group Study. J Clin Oncol.
17:2127–2136. 1999.PubMed/NCBI
|
|
7
|
Richardson GE and Johnson BE: The biology
of lung cancer. Semin Oncol. 20:105–127. 1993.PubMed/NCBI
|
|
8
|
Mountain CF: Clinical biology of small
cell carcinoma: Relationship to surgical therapy. Semin Oncol.
5:272–279. 1978.PubMed/NCBI
|
|
9
|
Argiris A and Murren JR: Staging and
clinical prognostic factors for small-cell lung cancer. Cancer J.
7:437–447. 2001.PubMed/NCBI
|
|
10
|
Goto Y, De Silva MG, Toscani A, Prabhakar
BS, Notkins AL and Lan MS: A novel human insulinoma-associated
cDNA, IA-1, encodes a protein with ‘zinc-finger’ DNA-binding
motifs. J Biol Chem. 267:15252–15257. 1992.PubMed/NCBI
|
|
11
|
Lan MS, Russell EK, Lu J, Johnson BE and
Notkins AL: IA-1, a new marker for neuroendocrine differentiation
in human lung cancer cell lines. Cancer Res. 53:4169–4171.
1993.PubMed/NCBI
|
|
12
|
Breslin MB, Zhu M, Notkins AL and Lan MS:
Neuroendocrine differentiation factor, IA-1, is a transcriptional
repressor and contains a specific DNA-binding domain:
Identification of consensus IA-1 binding sequence. Nucleic Acids
Res. 30:1038–1045. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gierl MS, Karoulias N, Wende H, Strehle M
and Birchmeier C: The zinc-finger factor Insm1 (IA-1) is essential
for the development of pancreatic beta cells and intestinal
endocrine cells. Genes Dev. 20:2465–2478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wildner H, Gierl MS, Strehle M, Pla P and
Birchmeier C: Insm1 (IA-1) is a crucial component of the
transcriptional network that controls differentiation of the
sympatho-adrenal lineage. Development. 135:473–481. 2008.
View Article : Google Scholar
|
|
15
|
Farkas LM, Haffner C, Giger T, Khaitovich
P, Nowick K, Birchmeier C, Pääbo S and Huttner WB:
Insulinoma-associated 1 has a panneurogenic role and promotes the
generation and expansion of basal progenitors in the developing
mouse neocortex. Neuron. 60:40–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie J, Cai T, Zhang H, Lan MS and Notkins
AL: The zinc-finger transcription factor INSM1 is expressed during
embryo development and interacts with the Cbl-associated protein.
Genomics. 80:54–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mellitzer G, Bonné S, Luco RF, Van De
Casteele M, Lenne-Samuel N, Collombat P, Mansouri A, Lee J, Lan M,
Pipeleers D, et al: IA1 is NGN3-dependent and essential for
differentiation of the endocrine pancreas. EMBO J. 25:1344–1352.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lan MS and Breslin MB: Structure,
expression, and biological function of INSM1 transcription factor
in neuroendocrine differentiation. FASEB J. 23:2024–2033. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Amouri SS, Cao P, Miao C and Pan D:
Secreted luciferase for in vivo evaluation of systemic protein
delivery in mice. Mol Biotechnol. 53:63–73. 2013. View Article : Google Scholar
|
|
20
|
Koutsoudakis G, Pérez-del-Pulgar S,
González P, Crespo G, Navasa M and Forns X: A Gaussia luciferase
cell-based system to assess the infection of cell culture- and
serum-derived hepatitis C virus. PLoS One. 7:e532542012. View Article : Google Scholar
|
|
21
|
Whyte P, Buchkovich KJ, Horowitz JM,
Friend SH, Raybuck M, Weinberg RA and Harlow E: Association between
an oncogene and an anti-oncogene: The adenovirus E1A proteins bind
to the retinoblastoma gene product. Nature. 334:124–129. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akerstrom V, Chen C, Lan MS and Breslin
MB: Modifications to the INSM1 promoter to preserve specificity and
activity for use in adenoviral gene therapy of neuroendocrine
carcinomas. Cancer Gene Ther. 19:828–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aker M, Tubb J, Groth AC, Bukovsky AA,
Bell AC, Felsenfeld G, Kiem HP, Stamatoyannopoulos G and Emery DW:
Extended core sequences from the cHS4 insulator are necessary for
protecting retroviral vectors from silencing position effects. Hum
Gene Ther. 18:333–343. 2007. View Article : Google Scholar : PubMed/NCBI
|