Introduction
The treatment outcome for patients with esophageal
squamous cell carcinoma (ESCC) is poor, despite improvements in
care (1). There have been several
treatment strategies for ESCC; among them, surgery is considered as
the most effective. However, patients with unresectable or
inoperable disease are usually treated with chemoradiotherapy (CRT)
in the clinic; this is considered to be an effective therapeutic
strategy (2–4). At present, the standard regimen for
CRT regarding ESCC is the combined use of cisplatin and
5-fluorouracil with radiotherapy. CRT sometimes induces severe
adverse events, together with a favorable therapeutic effect
(5,6). Therefore, a CRT regimen involving
reduced side effects is desirable. Taxanes including docetaxel
(DTX) and paclitaxel (PTX) are anticancer agents that exhibit
cytotoxicity by inhibiting microtubule polymerization; they are
attracting attention as one of the key drugs in the treatment of a
variety of carcinomas (7). Among
them, DTX is useful for many types of cancer including
nasopharyngeal (8), gastric
(9,10), breast (11), prostate (12), lung (13), thyroid (14) and esophageal cancer (15). It has been widely recognized that
DTX enhances radiosensitivity in various cancers in vitro
and in vivo (16–18). To date, DTX has been shown to be
clinically useful as an active agent in CRT treatment regimens
involving laryngeal/hypopharyngeal cancers (19). DTX has been used in combination
with cisplatin as an agent for CRT in non-small cell lung cancer
(20). A phase I/II study
involving DTX and cisplatin with concurrent thoracic radiotherapy
for locally advanced non-small cell lung cancer has shown that the
dose limiting toxicity is radiation esophagitis (21). One of the approaches regarding the
reduction of adverse events in CRT is the administration of low
dose DTX. Recently, there have been several reports concerning the
favorable effects of weekly administration of low dose DTX as a
radiosensitizer in head and neck cancer (22–26),
non-small cell lung cancer (27,28)
and ovarian cancer (29). These
studies have shown that CRT with low dose DTX has an antitumor
effect comparable to high dose DTX, and induce less adverse events.
In recent years, CRT in combination with weekly DTX has been
performed safely in patients with advanced inoperable ESCC
(30,31). Preoperative CRT for locally
advanced ESCC using DTX results in similar or better long-term
outcomes as compared with cisplatin and 5-fluorouracil based CRT,
despite the patients demonstrating a better pathological response
to cisplatin and 5-fluorouracil based CRT when compared with DTX
based CRT (32).
DTX induces microtubule modification and causes
cells to accumulate in the radiosensitive G2/M phase of the cell
cycle (33). Recently, taxanes
have been reported to have a different mechanism of action
depending on dose intensity (34–36).
Most studies that have indicated a dual mechanism of action for
taxanes have been reported using PTX (34,36).
The mechanism of the radio-enhancing effect of DTX in relation to
the dose intensity is not clearly understood.
In the present study, we experimentally investigated
the radio-enhancing effects of various concentrations of DTX
against ESCC cells to elucidate an effective administration
schedule for DTX regarding CRT for ESCC. We also examined the
concentration-dependent radio-enhancing mechanism in respect to
cell cycle arrest in ESCC cells.
Materials and methods
Cell line and treatment
The esophageal squamous cell carcinoma cell line KES
has been established in our laboratory from endoscopic biopsy
specimens obtained from a patient carrying well differentiated
esophageal squamous cell carcinoma. KES was cultured in RPMI-1640
(Invitrogen, Osaka, Japan) supplemented with 10% heat-inactivated
fetal bovine serum (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan),
100 IU/ml penicillin, 100 μg/ml streptomycin (Invitrogen), 2 mM
glutamine (Nissui Pharmaceutical Co. Ltd.) and 0.5 mM sodium
pyruvate at 37°C in a humidified atmosphere of CO2 in
air. DTX was dissolved in phosphate-buffered saline (PBS) to a
stock concentration of 200 nM and stored at −20°C. Cultures were
irradiated using MBR-1520R-3 X-ray radiation apparatus (Hitachi
Medicotechnology, Hitachi, Japan) at a dose rate of 1 Gy/min. Power
output of the X-rays used for irradiation was 125 kV and the beam
current was 20 mA. Forward-scattered radiation, and 0.5 mm Al and
0.2 mm Cu filters were used.
Cell growth assay
The viability of cells treated with DTX was
determined using a standard
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. KES cells were treated with DTX at various concentrations
(0.1–50 nM) for 48 h. The percentage inhibition was determined by
comparing the cell density of the drug-treated cells with that of
untreated controls. All experiments were repeated at least three
times.
Cell cycle analysis
KES cells were treated with DTX at various
concentrations (0.1–50 nM), following treatment periods of 0, 3, 6,
12, 24 and 48 h. Cells were stained with propidium iodide and
analyzed using flow cytometry. The percentages of the cells in the
sub-G0, G0/G1, S and G2/M phases were calculated.
Nuclear form
Alteration of the nuclear form of the KES cells
treated with DTX was analyzed. KES cells were treated with DTX at
various concentrations (0, 1 and 10 nM), and were fixed with 4%
formaldehyde and stained with Hoechst 33342 (Thermo Fisher
Scientific K.K, Yokohama, Japan). Nuclear form was examined using
an Olympus immunofluorescence microscope (BX50/BX-FLA, Olympus,
Tokyo, Japan).
Clonogenic assay
The cells were plated into dishes and allowed to
attach for 4 h. The medium was then replaced by a medium with or
without DTX. Following incubation for 12 h, the cells were
irradiated at various doses (2–6 Gy). The cells were harvested
using trypsinization, counted, and known concentrations of cells
were re-plated into 100-mm culture dishes and returned to the
incubator. After incubation for 7–10 days, the cell colonies were
fixed and stained with 0.1% crystal violet. Colonies of >50
cells were manually counted to determine survival.
Assessment of apoptosis
The Annexin V binding assay was used to assess
phosphatidylserine externalization as a marker of apoptosis using
the Pacific Blue™ Annexin V/SYTOX® AADvanced™ Apoptosis
kit (Invitrogen) according to the manufacturer's instructions. The
extent of apoptosis was quantified using flow cytometry.
Immunofluorescent cytochemistry
Cells were cultured on Lab-Tec chamber slides (Nalge
Nunc International, Rochester, NY, USA). The cells were then
treated with various concentrations of DTX (0, 1 and 10 nM) for 12
h and subsequently irradiated. They were fixed in a mixture of
methanol and acetone (1:1) for 15 min. The slides were immersed in
methanol containing 0.3% H2O2 for 30 min,
blocked with 3.3% normal goat serum in PBS and incubated with
anti-phosphohistone H2AX (Ser 139) (Cell Signaling Technology, MA,
USA) at 4°C. After sections were washed in PBS, immunoreactivity
was visualized by incubating them in anti-rabbit IgG antibody
conjugated with Alexa Flour 488 (Molecular Probes, Eugene, OR,
Danvers, MA, USA) for 1 h at room temperature. The nucleus was
counterstained with Hoechst 33342 (Thermo Fisher Scientific K.K).
The slides were examined with an Olympus immunofluorescence
microscope (BX50/BX-FLA, Olympus).
Results
Antitumor efficacy of DTX
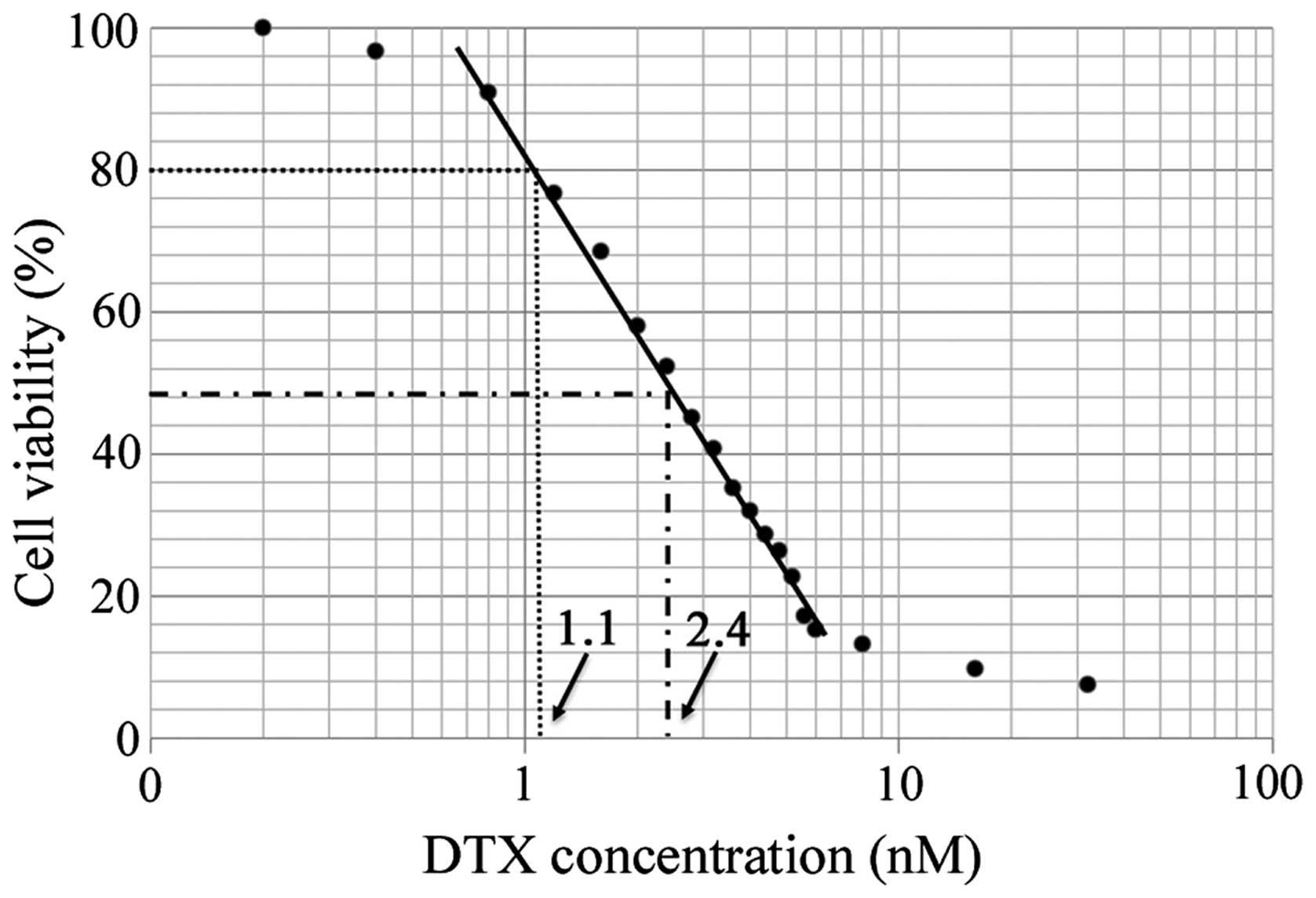
The inhibitory effect of DTX on KES cell growth was
assessed using the MTT assay. Cells were treated with various
concentrations of DTX for 48 h. DTX inhibited KES cell growth in a
dose-dependent manner (Fig. 1).
The 50% inhibitory concentration (IC50) value of DTX was
2.4 nM and the 20% inhibitory concentration (IC20) value
was 1.1 nM.
Cell cycle
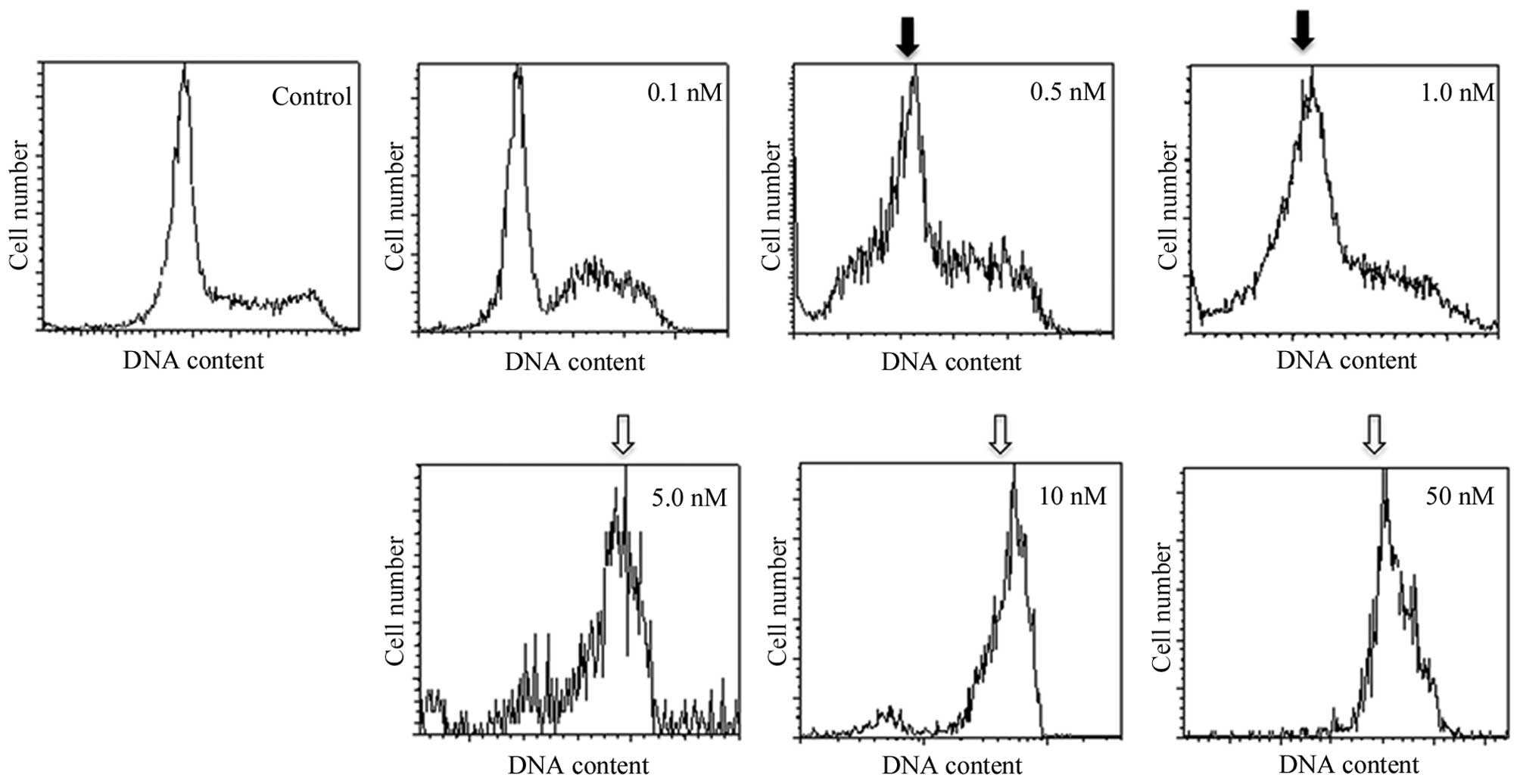
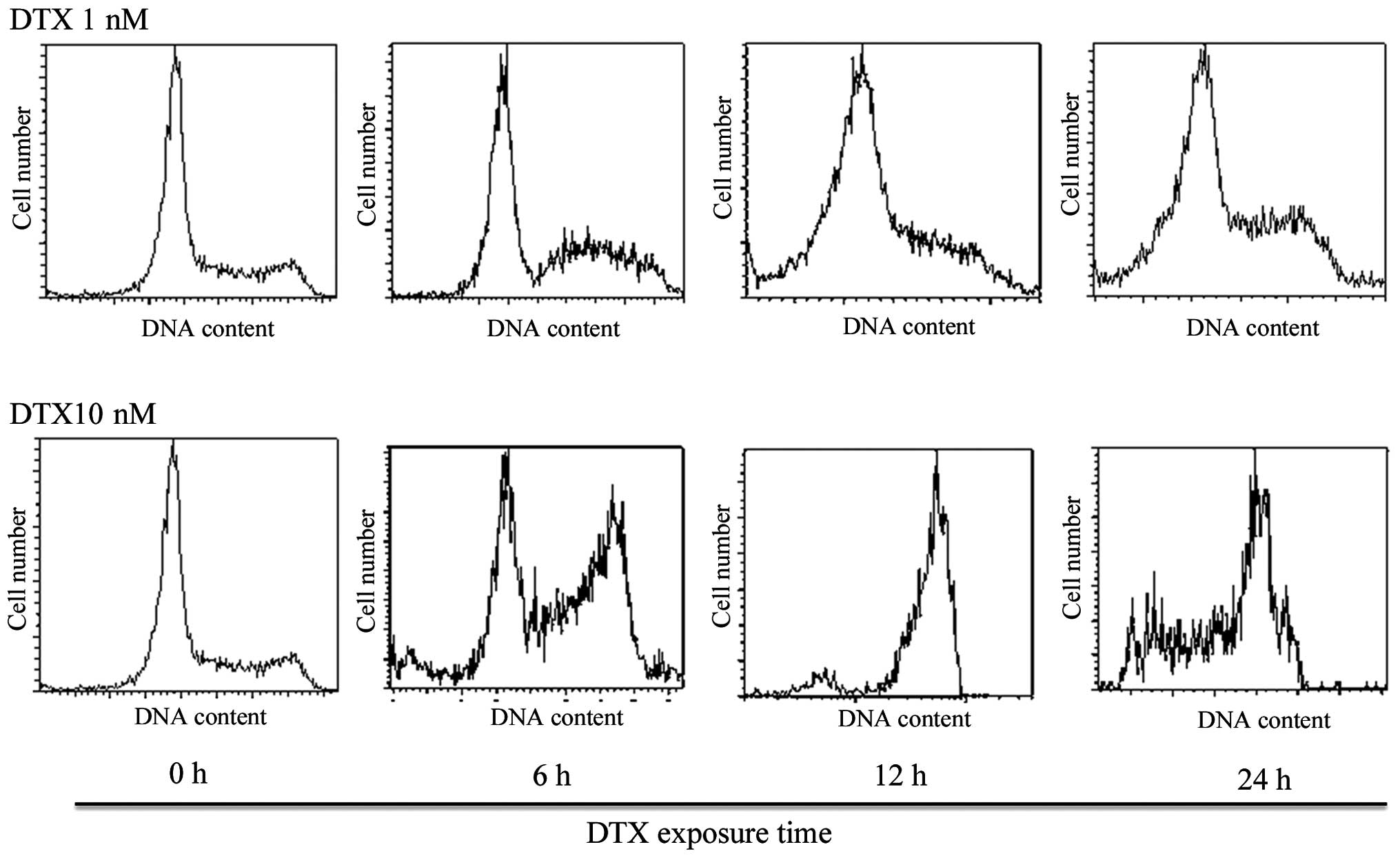
First, no obvious cell cycle change was observed
below 6-h exposure to DTX at any concentration. After 12-h
incubation of KES cells with the medium and high concentrations of
DTX (5–50 nM and more), ~65% of the cells were arrested in the G2-M
phase with a 4-n content of DNA (Figs.
2 and 3 and Table I). The accumulation of cells in the
sub-G0 and G0/G1 phases was observed after treatment using low
concentrations of DTX (0.5–1 nM). Low concentrations of DTX led to
the development of hypodiploid cells.
 | Table ICell cycle distribution of KES cells
following docetaxel (DTX) treatment. |
Table I
Cell cycle distribution of KES cells
following docetaxel (DTX) treatment.
| Proportion of cell
cycle fraction (%) |
|---|
|
|
|---|
| DTX concentration
(nM) | Sub-G0/G1+
G0/G1 | S | G2/M |
|---|
| 0 | 59.0 | 11.6 | 29.2 |
| 1 | 71.9 | 9.6 | 18.3 |
| 10 | 21.1 | 11.8 | 66.8 |
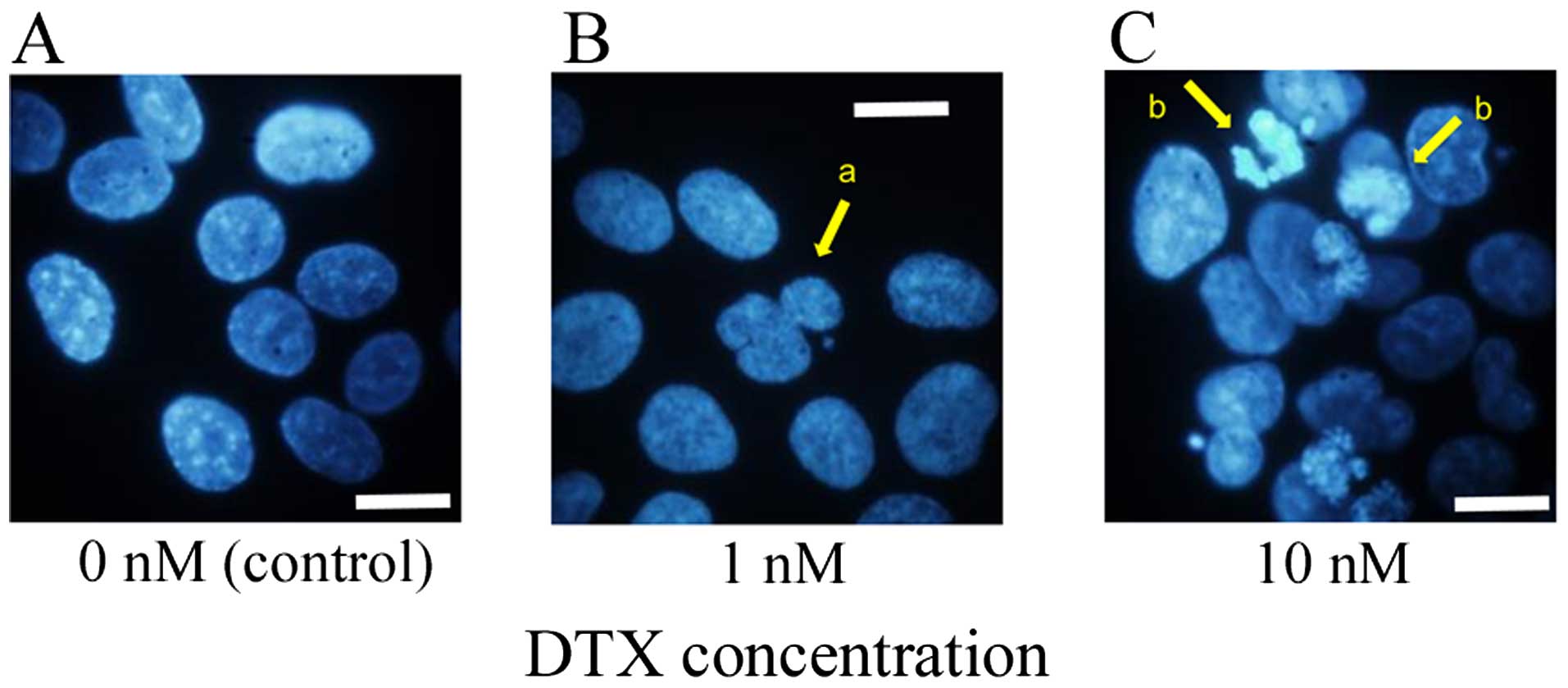
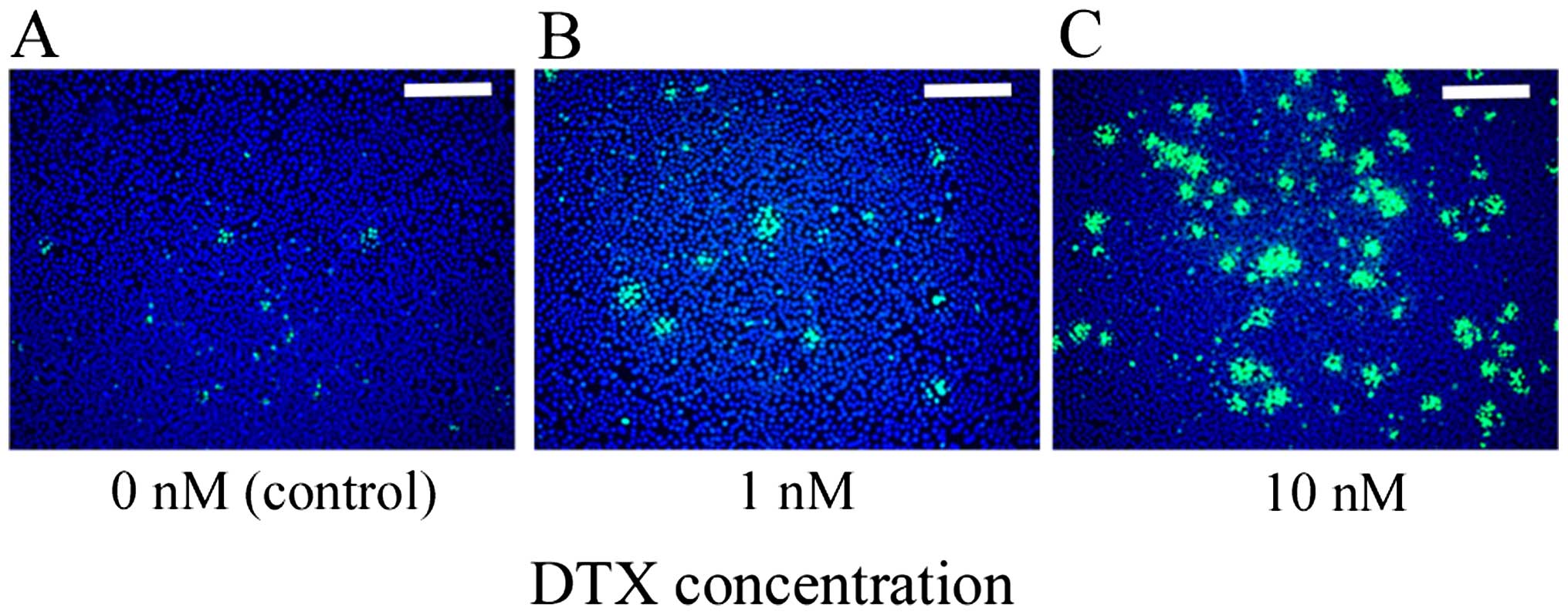
Nuclear form
KES cells were stained with Hoechst 33342 after
treatment with two concentrations of DTX (0.1 and 10 nM) for 12 h.
Nuclear form was investigated using an immunofluorescence
microscope. Cells treated with a high concentration of DTX
exhibited nuclear aggregation associated with apoptotic change in
some nuclei. In contrast, cells treated with a low concentration of
DTX exhibited multi-nucleation or unequal division in some nuclei
(Fig. 4).
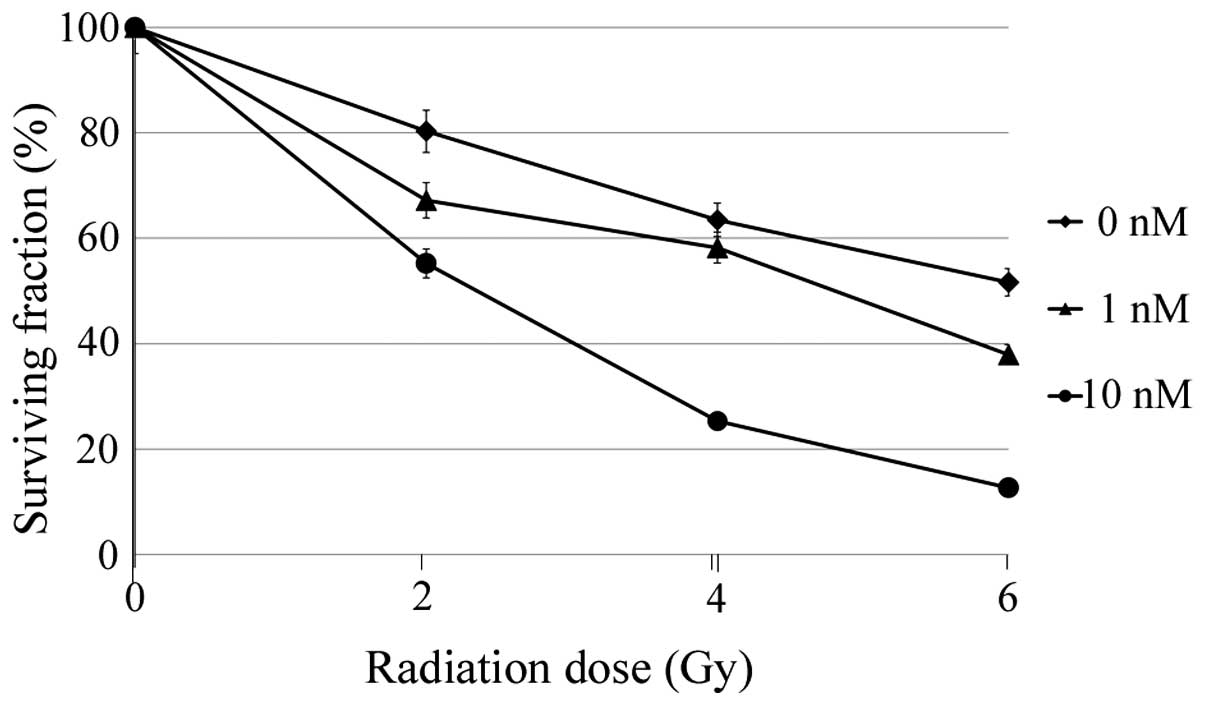
Radiosensitizing effect of DTX
The radio-sensitizing effect of DTX was assessed
using a clonogenic assay. As expected, cells treated with 10 nM DTX
(high concentration) had enhanced radio-sensitivity (Fig. 5). In addition, cells treated with 1
nM DTX (low concentration) exhibited enhanced radio-sensitivity
although it was slightly lower than after treatment with the high
concentration of DTX.
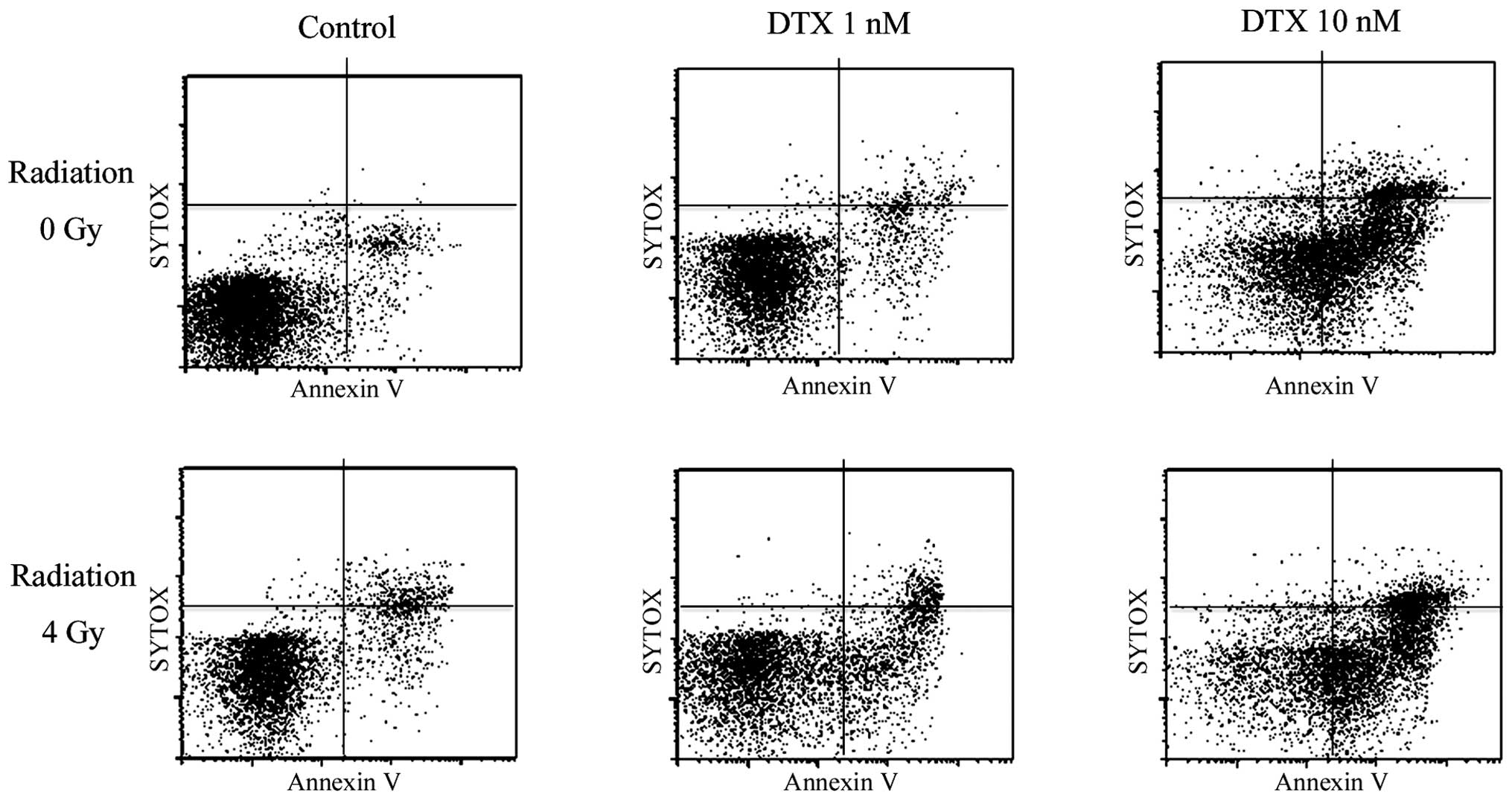
Enhancement of radiation-induced
apoptosis using DTX
The apoptotic response to irradiation alone or the
combination of irradiation and DTX treatment was assessed. After
treatment with various concentrations of DTX (0, 1 and 10 nM) for
12 h, KES cells were irradiated (4 Gy) and returned to culture for
6 h. The cells were then examined for the detection of apoptosis.
Even without DTX, apoptosis was induced in a few cells by
irradiation. A high concentration of DTX (10 nM) remarkably
enhanced radiation-induced apoptosis. In addition, a low
concentration of DTX (1 nM) also enhanced radiation and induced a
certain degree of apoptosis in KES cells (Fig. 6).
Enhancement of radiation induced DNA
double strand breaks (DSBs) using DTX
After irradiation, H2AX phosphorylation (γH2AX) was
measured as an indicator of DNA DSBs using immunofluorescent
cytochemistry. In the absence of DTX, few cells showed γH2AX foci
after irradiation. A high concentration of DTX (10 nM) induced
multiple γH2AX foci after irradiation in many cells. A low
concentration of DTX (1 nM) induced a certain degree of γH2AX foci
after irradiation in several KES cells (Fig. 7).
Discussion
In the present study, we found that DTX had a
concentration-dependent radio-enhancing effect in ESCC cells. A
high cytotoxic concentration (>10 nM) of DTX strongly enhanced
radiosensitivity. A low concentration (<1 nM) of DTX, that did
not elicit a cytotoxic effect, also achieved a radio-enhancing
effect by inducing DNA DSBs and apoptosis after irradiation. Low
and high concentrations of DTX induced radiosensitive G0/G1 and
G2/M phase arrest, respectively, in ESCC cells.
Taxanes have demonstrated activity as a
radiosensitizer in a number of preclinical and clinical studies by
inhibiting the depolymerization of microtubules, and blocking the
cell cycle in the most radiosensitive phase of the cell cycle
(G2/M). In various tumor cells, the G2/M phase is the most
sensitive regarding radiation in comparison with normal cells
because of deficient DNA repair during the G2-prophase period of
the cell cycle (37,38). Conversely, cells are known to be
most radio-resistant in the late S phase. We found that a high
concentration of DTX (10 nM) induced the accumulation of cells in
the G2/M phase and strongly enhanced the radiosensitivity of ESCC
cells. Recently, taxanes have been reported to have a different
mechanism of action depending on DTX dose intensity (34–36).
It is well known that a high concentration of DTX (100 nM) arrests
cells in the G2/M phase. A low concentration of DTX can induce
aberrant mitosis (39,40). There has been limited evidence
published concerning the effect of low concentrations of DTX
concerning cell cycle arrest. The present study showed that a low
concentration of DTX (1 nM) induced the accumulation of ESCC cells
in the sub-G0 and G0/G1 phases, indicating G0/G1 arrest.
Morphologically, a low concentration of DTX induces
multi-nucleation or unequal division. The cells are known to be
most radioresistant in the S phase and most radiosensitive in the
G2/M phase. A low concentration of DTX may contribute to the
enhancement of radiosensitivity in ESCC cells by means of cell
cycle arrest in the radiosensitive G0/G1 phase and the induction of
multi-nucleation or unequal division.
In the present study, although a low concentration
of DTX induced very little apoptosis, irradiated cells after
pretreatment with a low concentration of DTX exhibited a higher
population of apoptotic cells relative to those irradiated without
DTX pretreatment. In addition, after irradiation apoptotic cells
are associated with an increased level of γH2AX. When DNA DSBs are
induced by radiation, the histone H2AX is rapidly phosphorylated
and forms γH2AX at the sites of the DSBs. Thus, γH2AX is considered
as an indicator of DSBs. In the present study, we found that a low
concentration of DTX, that did not cause a cytotoxic effect, could
enhance radiosensitivity by enhancing radiation-induced DSBs and
apoptosis.
Pradier et al (41) speculated that DTX seemed to be a
better radiosensitizer for squamous cell carcinoma cells than PTX,
because DTX enhances the radiosensitivity at lower concentrations
than PTX. Cancer cells tested in vitro had IC50
values for DTX ranging from 5 to 50 nM (42). However, the radiosensitizing
activity of DTX in vitro was achieved even at subnanomolar
concentrations. DTX concentrations as low as 0.07 nM have been
shown to potentiate the effects of irradiation in cell lines
(41). Plasma levels of DTX reach
their peak just after injection, and are metabolized relatively
quickly. The pharmacokinetics and metabolism of DTX in vivo
has been shown to have a three phase disposition profile with a
terminal half-life of 12 h (43).
DTX may be present for as long as 1 week, thus supporting the use
of weekly DTX (44). From the
elimination curve for patients receiving 20 mg/m2 of
DTX, it can be speculated that the plasma concentration of DTX is
maintained at a relatively high level (>5 nM) for ~48 h after
administration, and at a relatively low level (~1 nM) after 48 h
(44). It is speculated that DTX
at a high plasma concentration has a cytotoxic effect and a strong
radio-enhancing effect as a result of cell cycle arrest in the G2/M
phase within 48 h after administration; however, thereafter the
cytotoxic effect may disappear because of drug metabolization,
maintaining its radio-enhancing activity as a result of cell cycle
arrest in the G0/G1 phase. These results support the clinical use
of weekly low dose DTX in combination with radiotherapy as reported
in patients with oropharyngeal and hypopharyngeal carcinoma,
non-small cell lung cancer and ESCC (26,28,31).
Hennequin et al (16) reported that the radio-enhancing
effect of taxoids varies from one cell line to another. DTX and PTX
can reduce or enhance radiation cell killing, depending on the drug
concentration. The full dosage of taxoids may provide suitable
conditions for supra-additive interaction with radiation during
concomitant exposure; conversely, induced radiation resistance by
taxoids may occur in the low-drug dose range. An in vivo
study indicated that the high level of radiation enhancement
achieved by PTX occurs not at the time of the highest mitotic
arrest but at 1 day after PTX treatment (45); this indicates that PTX potentiates
tumor radio-responsiveness by mechanisms in addition to the
blocking of the cell cycle in mitosis, by tumor reoxygenation of
hypoxic tumor cells (46). In
addition, wild-type p53 function is required to confer tumor cell
sensitivity to DNA-damaging agents, such as ionizing radiation; the
loss of p53 function in certain human tumor cells can lead to
resistance to ionizing radiation (47). In the present study, we examined
the radio-enhancing effect of DTX in ESCC using the KES cell line
that was newly established in our department, because KES cells are
transplantable into the nude mouse. The p53 status in KES cells has
not yet been investigated. To confirm the radiosensitizing effect
of DTX according to its concentration in ESCC cells, further study
to investigate its effects in other ESCC cell lines showing
different p53 function in vitro is required. Furthermore,
the radio-enhancing effect of DTX should be confirmed in
vivo using a KES cell xenograft nude mouse model.
In conclusion, enhancement of the radiosensitivity
of ESCC cells using DTX was demonstrated in vitro in the
present study, even at a nanomolar concentration that did not
induce cytotoxic effects. DTX has different radio-enhancing
mechanisms that are concentration-dependent. Weekly administration
of DTX might effectively enhance radiation cytotoxicity against
ESCC. It will be necessary to elucidate the radio-enhancing
mechanism of DTX in vivo. In addition, the benefit of weekly
administration of low dose DTX in CRT regimens will need to be
verified in the clinic.
Abbreviations:
|
CRT
|
chemoradiotherapy
|
|
DTX
|
docetaxel
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate-buffered saline
|
|
PTX
|
paclitaxel
|
|
γH2AX
|
H2AX phosphorylation
|
References
|
1
|
Castro C, Bosetti C, Malvezzi M, Bertuccio
P, Levi F, Negri E, La Vecchia C and Lunet N: Patterns and trends
in esophageal cancer mortality and incidence in Europe (1980–2011)
and predictions to 2015. Ann Oncol. 25:283–290. 2014. View Article : Google Scholar
|
|
2
|
Ohtsu A, Boku N, Muro K, Chin K, Muto M,
Yoshida S, Satake M, Ishikura S, Ogino T, Miyata Y, et al:
Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous
cell carcinoma of the esophagus. J Clin Oncol. 17:2915–2921.
1999.PubMed/NCBI
|
|
3
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar
|
|
4
|
Ishida K, Ando N, Yamamoto S, Ide H and
Shinoda M: Phase II study of cisplatin and 5-fluorouracil with
concurrent radiotherapy in advanced squamous cell carcinoma of the
esophagus: A Japan Esophageal Oncology Group (JEOG)/Japan Clinical
Oncology Group trial (JCOG9516). Jpn J Clin Oncol. 34:615–619.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herskovic A, Martz K, al-Sarraf M,
Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L
and Emami B: Combined chemotherapy and radiotherapy compared with
radiotherapy alone in patients with cancer of the esophagus. N Engl
J Med. 326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaneko K, Ito H, Konishi K, Kurahashi T,
Ito T, Katagiri A, Yamamoto T, Kitahara T, Mizutani Y, Ohtsu A, et
al: Definitive chemoradiotherapy for patients with malignant
stricture due to T3 or T4 squamous cell carcinoma of the
oesophagus. Br J Cancer. 88:18–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choy H: Taxanes in combined modality
therapy for solid tumors. Crit Rev Oncol Hematol. 37:237–247. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ngeow J, Lim WT, Leong SS, Ang MK, Toh CK,
Gao F, Chowbay B and Tan EH: Docetaxel is effective in heavily
pretreated patients with disseminated nasopharyngeal carcinoma. Ann
Oncol. 22:718–722. 2011. View Article : Google Scholar
|
|
9
|
Bang YJ, Kang WK, Kang YK, Kim HC, Jacques
C, Zuber E, Daglish B, Boudraa Y, Kim WS, Heo DS, et al: Docetaxel
75 mg/m(2) is active and well tolerated in patients with metastatic
or recurrent gastric cancer: A phase II trial. Jpn J Clin Oncol.
32:248–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbrederis K, Lorenzen S, von Weikersthal
LF, Vehling-Kaiser U, Schuster T, Rothling N, Peschel C and Lordick
F: Weekly docetaxel monotherapy for advanced gastric or
esophagogastric junction cancer. Results of a phase II study in
elderly patients or patients with impaired performance status. Crit
Rev Oncol Hematol. 66:84–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nuzzo F, Morabito A, Gravina A, Di Rella
F, Landi G, Pacilio C, Labonia V, Rossi E, De Maio E, Piccirillo
MC, et al: Effects on quality of life of weekly docetaxel-based
chemotherapy in patients with locally advanced or metastatic breast
cancer: Results of a single-centre randomized phase 3 trial. BMC
Cancer. 11:752011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gravis G, Bladou F, Salem N,
Macquart-Moulin G, Serment G, Camerlo J, Genre D, Bardou VJ,
Maraninchi D and Viens P: Weekly administration of docetaxel for
symptomatic metastatic hormone-refractory prostate carcinoma.
Cancer. 98:1627–1634. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perng RP, Shih JF, Chen YM, Chou KC, Lee
YC and Tsai CM: A phase II study of single-agent docetaxel
chemotherapy for non-small cell lung cancer. Jpn J Clin Oncol.
30:429–434. 2000. View Article : Google Scholar
|
|
14
|
Kawada K, Kitagawa K, Kamei S, Inada M,
Mitsuma A, Sawaki M, Kikumori T, Fujimoto Y, Arima H, Imai T, et
al: The feasibility study of docetaxel in patients with anaplastic
thyroid cancer. Jpn J Clin Oncol. 40:596–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin
K, Hyodo I, Fujita H, Takiyama W and Ohtsu T: A phase II study of
single-agent docetaxel in patients with metastatic esophageal
cancer. Ann Oncol. 15:955–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hennequin C, Giocanti N and Favaudon V:
Interaction of ionizing radiation with paclitaxel (Taxol) and
docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res.
56:1842–1850. 1996.PubMed/NCBI
|
|
17
|
Balcer-Kubiczek EK, Attarpour M, Wang JZ
and Regine WF: The effect of docetaxel (taxotere) on human gastric
cancer cells exhibiting low-dose radiation hypersensitivity. Clin
Med Oncol. 2:301–311. 2008.PubMed/NCBI
|
|
18
|
Mason KA, Hunter NR, Milas M, Abbruzzese
JL and Milas L: Docetaxel enhances tumor radioresponse in vivo.
Clin Cancer Res. 3:2431–2438. 1997.
|
|
19
|
Yoshida T, Tokashiki R, Itoh H, Nakamura
K, Hiramatsu H, Tsukahara K, Shimizu S, Takada D, Okamoto I, Abe K,
et al: A phase I–II study of bi-weekly docetaxel combined with
radiation therapy for patients with cancer of the
larynx/hypopharynx. Jpn J Clin Oncol. 37:641–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YQ, Shi AH, Li FH, Yu R and Zhu GY:
Phase I study to determine MTD of docetaxel and cisplatin with
concurrent radiation therapy for Stage III non-small cell lung
cancer. Chin J Cancer Res. 23:129–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiura K, Ueoka H, Segawa Y, Tabata M,
Kamei H, Takigawa N, Hiraki S, Watanabe Y, Bessho A, Eguchi K, et
al; Okayama Lung Cancer Study Group. Phase I/II study of docetaxel
and cisplatin with concurrent thoracic radiation therapy for
locally advanced non-small-cell lung cancer. Br J Cancer.
89:795–802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tishler RB, Norris CM Jr, Colevas AD, Lamb
CC, Karp D, Busse PM, Nixon A, Frankenthaler R, Lake-Willcutt B,
Costello R, et al: A Phase I/II trial of concurrent docetaxel and
radiation after induction chemotherapy in patients with poor
prognosis squamous cell carcinoma of the head and neck. Cancer.
95:1472–1481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujii M, Tsukuda M, Satake B, Kubota A,
Kida A, Kohno N, Okami K and Inuyama Y; Japan Cooperative Head and
Neck Oncology Group (JCHNOG). Phase I/II trial of weekly docetaxel
and concomitant radiotherapy for squamous cell carcinoma of the
head and neck. Int J Clin Oncol. 9:107–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tishler RB, Posner MR, Norris CM Jr,
Mahadevan A, Sullivan C, Goguen L, Wirth LJ, Costello R, Case M,
Stowell S, et al: Concurrent weekly docetaxel and concomitant boost
radiation therapy in the treatment of locally advanced squamous
cell cancer of the head and neck. Int J Radiat Oncol Biol Phys.
65:1036–1044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark JI, Eisner RM, Hofmeister C, Norton
J, Thomas S, Choudhury A, Petruzzelli G, Lathers D, Young MR, Lau
A, et al: Phase I adjuvant radiation with docetaxel in high-risk
head and neck cancer. Am J Clin Oncol. 32:396–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukada J, Shigematsu N, Takeda A, Ohashi
T, Tomita T, Shiotani A, Kunieda E, Kawaguchi O, Fujii M and Kubo
A: Weekly low-dose docetaxel-based chemoradiotherapy for locally
advanced oropharyngeal or hypopharyngeal carcinoma: A
retrospective, single-institution study. Int J Radiat Oncol Biol
Phys. 76:417–424. 2010. View Article : Google Scholar
|
|
27
|
Onishi H, Kuriyama K, Yamaguchi M,
Komiyama T, Tanaka S, Araki T, Nishikawa K and Ishihara H:
Concurrent two-dimensional radiotherapy and weekly docetaxel in the
treatment of stage III non-small cell lung cancer: A good local
response but no good survival due to radiation pneumonitis. Lung
Cancer. 40:79–84. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brunsvig PF, Hatlevoll R, Berg R, Lauvvang
G, Owre K, Wang M and Aamdal S: Weekly docetaxel with concurrent
radiotherapy in locally advanced non-small cell lung cancer: A
phase I/II study with 5 years' follow-up. Lung Cancer. 50:97–105.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kunos CA, Sill MW, Buekers TE, Walker JL,
Schilder JM, Yamada SD, Waggoner SE, Mohiuddin M and Fracasso PM:
Low-dose abdominal radiation as a docetaxel chemosensitizer for
recurrent epithelial ovarian cancer: A phase I study of the
Gynecologic Oncology Group. Gynecol Oncol. 120:224–228. 2011.
View Article : Google Scholar :
|
|
30
|
Font A, Arellano A, Fernández-Llamazares
J, Casas D, Boix J, Cardenal J, Margelí M, Manzano JL, Abad A and
Rosell R: Weekly docetaxel with concomitant radiotherapy in
patients with inoperable oesophageal cancer. Clin Transl Oncol.
9:177–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Makino I, Okamoto K, Kinoshita J, Hayashi
H, Nakamura K, Oyama K, Nakagawara H, Fujita H, Tajima H, Takamura
H, et al: A pilot study of chemoradiotherapy with weekly docetaxel
for thoracic esophageal carcinoma with T4 and/or M1 lymph node
metastasis. World J Oncol. 2:252–258. 2011.
|
|
32
|
Kushida T, Nohara S, Yoshino K, Fujiwara
D, Ouchi K, Amano T, Isayama F, Tomita N, Iwanuma Y, Sasai K, et
al: Utility of weekly docetaxel combined with preoperative
radiotherapy for locally advanced esophageal cancer from
pathological analysis. Dis Esophagus. 27:368–373. 2014. View Article : Google Scholar
|
|
33
|
Moos PJ and Fitzpatrick FA: Taxanes
propagate apoptosis via two cell populations with distinctive
cytological and molecular traits. Cell Growth Differ. 9:687–697.
1998.PubMed/NCBI
|
|
34
|
Torres K and Horwitz SB: Mechanisms of
Taxol-induced cell death are concentration dependent. Cancer Res.
58:3620–3626. 1998.PubMed/NCBI
|
|
35
|
Hernández-Vargas H, Palacios J and
Moreno-Bueno G: Telling cells how to die: Docetaxel therapy in
cancer cell lines. Cell Cycle. 6:780–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang D, Yang R, Wang S and Dong Z:
Paclitaxel: New uses for an old drug. Drug Des Devel Ther.
8:279–284. 2014.PubMed/NCBI
|
|
37
|
Chaffey JT and Hellman S: Differing
responses to radiation of murine bone marrow stem cells in relation
to the cell cycle. Cancer Res. 31:1613–1615. 1971.PubMed/NCBI
|
|
38
|
Parshad R, Gantt R, Sanford KK and Jones
GM: Chromosomal radiosensitivity of human tumor cells during the G2
cell cycle period. Cancer Res. 44:5577–5582. 1984.PubMed/NCBI
|
|
39
|
Paoletti A, Giocanti N, Favaudon V and
Bornens M: Pulse treatment of interphasic HeLa cells with nanomolar
doses of docetaxel affects centrosome organization and leads to
catastrophic exit of mitosis. J Cell Sci. 110:2403–2415.
1997.PubMed/NCBI
|
|
40
|
Morse DL, Gray H, Payne CM and Gillies RJ:
Docetaxel induces cell death through mitotic catastrophe in human
breast cancer cells. Mol Cancer Ther. 4:1495–1504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pradier O, Rave-Fränk M, Lehmann J, Lücke
E, Boghun O, Hess CF and Schmidberger H: Effects of docetaxel in
combination with radiation on human head and neck cancer cells
(ZMK-1) and cervical squamous cell carcinoma cells (CaSki). Int J
Cancer. 91:840–845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bruno R and Sanderink GJ: Pharmacokinetics
and metabolism of Taxotere (docetaxel). Cancer Surv. 17:305–313.
1993.PubMed/NCBI
|
|
44
|
Brunsvig PF, Andersen A, Aamdal S,
Kristensen V and Olsen H: Pharmacokinetic analysis of two different
docetaxel dose levels in patients with non-small cell lung cancer
treated with docetaxel as monotherapy or with concurrent
radiotherapy. BMC Cancer. 7:1972007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Milas L, Hunter NR, Mason KA, Kurdoglu B
and Peters LJ: Enhancement of tumor radioresponse of a murine
mammary carcinoma by paclitaxel. Cancer Res. 54:3506–3510.
1994.PubMed/NCBI
|
|
46
|
Milas L, Hunter NR, Mason KA, Milross CG,
Saito Y and Peters LJ: Role of reoxygenation in induction of
enhancement of tumor radioresponse by paclitaxel. Cancer Res.
55:3564–3568. 1995.PubMed/NCBI
|
|
47
|
McIlwrath AJ, Vasey PA, Ross GM and Brown
R: Cell cycle arrests and radiosensitivity of human tumor cell
lines: Dependence on wild-type p53 for radiosensitivity. Cancer
Res. 54:3718–3722. 1994.PubMed/NCBI
|