Introduction
Globally, breast cancer in women is the leading
cause of cancer death with 1,383,500 estimated new cases each year
(1). This fact has generated an
interest to obtain insight into breast tumorigenesis and also to
develop drugs that effectively combat the disease. Currently, women
with advanced breast cancer develop metastases which account
significantly for morbidity and mortality. Ras is a proto-oncogene
that is activated transiently as a response to extracellular
signals such as growth factors, cytokines, and hormones which
stimulate cell surface receptors (2). Approximately 90% of the activating
mutations have been found in codons 12 (wild-type GGT) and 13
(wild-type GGC) of exon 1 identifying these codons as hot-spot
mutation points. The most frequently observed types of mutations
are G→A transitions and G→T transversions (3,4).
As a member of the Ras GTPase superfamily (5), Rho-A is an oncogenic and a critical
component of signaling pathways leading to downstream gene
regulation (6–9). Rho family proteins are prominent
members of the well-known Ras superfamily of small GTPases that can
cycle between inactive GDP-bound state and active GTP-bound state
and that exhibit intrinsic GTPase activities (10–12).
Rho-A is frequently over-expressed in human cancer (13). In terms of function, several Rho
GTPases have been shown to regulate diverse signal transduction
pathways and are involved in a variety of biological processes,
including cell morphology (14,15),
motility (16), proliferation
(17) and apoptosis (18,19).
Recently, a number of studies has shown that Rho-A expression was
upregulated in a group of malignancies, including breast cancer,
colon cancer, lung cancer, and ovarian cancer (20–24)
and that the expression level of Rho-A seemed to be positively
correlated with the progress of these carcinomas, suggesting that
Rho-A may play an important role in tumorigenesis and tumor
progression.
Rac1, belonging to the Rho family, is a Ras-related
small GTPase. Its activity is responsible for the regulation of
diverse cellular behaviors including, formation of cortical
actin-containing membrane ruffles, and induction of gene expression
programs (25). Rac1 activity is
implicated in various steps of oncogenesis including initiation,
progression, invasion and metastasis (26,27).
p53, considered as the ‘guardian of the genome’, is
the most frequently mutated gene in human malignancies such as
cancer, it is found inactivated in ~50% of tumors of any location
and histological type (generally, point mutations of one allele and
deletion of the other allele). Present in an inactive form in
normal cells, p53 becomes fully functional when activated in
response to cell stress (either oncogenic or genotoxic stress). p53
activation leads to the upregulation of various target genes
responsible for cell cycle arrest or apoptotic cell death,
depending on the cellular environment. Due to its crucial tumor
suppressor activity, TP53 thus appears to be an appealing target
for gene therapy or pharmacological intervention in cancer
treatment (28).
The signal transducers and activators of
transcription (Stats) belong to a family of seven cytoplasmic
proteins that function as signal messengers and transcription
factors participating in cellular responses to cytokines and growth
factors. Stat1 is deficient or inactive in many types of human
tumors whereas some tumors have activated Stat1. Whether Stat1
affects tumor growth and metastasis is unclear (29–31).
Apoptosis (programmed cell death), is a process of
cellular destruction that is required for the development and
homeostasis of multicellular organisms (32). Apoptosis is characterized by cell
shrinkage, condensation of nuclei and internucleosomal degradation
of DNA. Cells defective in apoptosis tend to survive with excess
DNA damage and thus lead to carcinogenesis by accumulating
mutations (33). In chemotherapy,
apoptosis is the predominant mechanism by which cancer cells die.
However, even when the apoptotic machinery remains intact, survival
signaling may antagonize the cell death by signals, such as growth
factor, steroid hormone, neuropeptide and the activation of
phosphatidylinositol 3-kinase and Akt (34,35).
In view of recent findings, specific patterns of resistance to
chemotherapy can occur depending on the genetic or epigenetic
abnormalities of the cancer cells (36,37).
The Bax gene, a member of the Bcl-2 family and an
apoptosis promoter, regulates the release of cytochrome c
from mitochondria (38), and its
forced expression is known to lead to the activation of caspases
and to programmed cell death (39,40).
However, it is controversial whether caspases are required for
Bax-induced apoptosis. Both caspase-dependent cell death (41,42)
and caspase-independent cell death (43) mediated by Bax have been reported.
Several caspase-3-like proteases exist and it is even uncertain
whether caspase-3 is absolutely required in Bax-mediated cell death
(44). Bcl-xL, one of several
additional proteins with sequence homology to Bcl-2, is 233 amino
acid protein with 43% sequence identity with Bcl-2 that suppresses
cell death (45).
Caspase-3 is a member of the cysteine protease
family, which plays a crucial role in apoptotic pathways by
cleaving a variety of key cellular proteins. Caspase-3 is the most
widely studied of the effector caspases, it can be activated by
diverse death-inducing signals, including the chemotherapeutic
agents. It plays a key role in both the death receptor pathway,
initiated by caspase-8, and the mitochondrial pathway, involving
caspase-9. In addition, several studies have shown that caspase-3
activation is required for apoptosis induction in response to
chemotherapeutic drugs e.g., taxanes, 5-fluorouracil (5-FU) and
doxorubicin (46–48).
NF-κB has been implicated in many inflammatory and
malignant diseases, such as breast cancer. NF-κB transcription
factors play a crucial role in oncogenesis (49). NF-κB is aberrantly activated in a
wide range of human cancers, in which it promotes survival and
malignancy by upregulating anti-apoptotic genes (50).
5-FU is a pyrimidine analog and is the most widely
used chemotherapeutic agent for the treatment of a variety of solid
cancers. Its mechanism of action has been attributed to the
production of cytotoxic metabolites incorporated into RNA and DNA
and inhibiting thymidylate synthase, finally leading to cell cycle
arrest and apoptosis in cancer cells (51). The aim of this study was to
evaluate 5-FU in cells transformed by low doses of ionizing
radiation α-particles in breast cancer cell lines (52) on apoptotic activity.
Materials and methods
Breast cancer cell lines
The immortalized breast cell line, MCF-10F (ATCC,
Manassas, VA, USA) retains all the characteristics of normal
epithelium in vitro, including anchorage-dependence,
non-invasiveness and non-tumorigenicity in nude mice. This cell
line was grown in DMEM/F-12 (1:1) medium supplemented with
antibiotics 100 U/ml penicillin, 2.5 μg/ml amphotericin B, 100
μg/ml streptomycin (all from Life Technologies, Grand Island, NY,
USA) and 0.5 μg/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO,
USA), 10 μg/ml and 5% equine serum (Biofluids, Rockville, MD, USA),
and 0.02 μg/ml epidermal growth factor (Collaborative Research,
Bedford, MA, USA). We used two cell lines from an in vitro
experimental breast cancer model, the MCF-10F and Tumor2 cells.
This model consisted of human breast epithelial cells in different
stages of transformation (52). In
brief, MCF-10F was exposed to low doses of high linear energy
transfer (LET) α-particle radiation (150 keV/μm) and subsequent
growth in the presence or absence of 17β-estradiol at
10−8 M (E) (Sigma-Aldrich) was evaluated. Tumor2, is a
malignant and tumorigenic cell line obtained from Alpha5
(60cGy+E/60cGy+E) injected into the nude mice given rise to this
cell line (52). The cells were
incubated at 37°C with 5% CO2 up to 70% of confluence.
The other cell line used was MDA-MB-231, a metastatic human breast
cancer cell line obtained from ATCC® HTB-26™ and grown
in RPMI supplemented with 10% fetal bovine serum.
Cellviabilityassay
The cytotoxic effect of 5-FU on cell viability was
examined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay in breast cancer cell lines. Briefly, cells were seeded in
24-well culture plates at a density of 5×104 cells/well.
After cells were attached, the cells were treated with 5-FU at
different concentrations ranging from 0–5 μM. The concentration of
DMSO was 0.01% (v/v). The plates were incubated at 37°C with 5%
CO2 for 48 h. The control cells received the vehicle
only. After 48-h incubation, the medium was removed, and 0.5 μmol/l
MTT was added into the wells. After another 4 h, 150 μl DMSO was
added into each well to dissolve the crystal. The absorbance was
read at 570 nm on a microplate reader (Autobio Labtec Instruments,
Zhengzhou, China). The drug concentration yielding 50% cell
inhibition (LD50) was determined. The treatment groups
were compared with the control group and the results were expressed
as percentage of viable cells. All experiments were performed in
triplicate.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted with TRIzol (Invitrogen,
Carlsbad, CA, USA), and the concentration and purity of RNA were
determined using a UV spectrophotometer. Total RNA was reverse
transcribed into cDNA using High capacity cDNA Reverse
Transcription kit and 10 units of RNase inhibitor (both from
Applied Biosystems, Carlsbad, CA, USA) according to the
manufacturer's protocol. A CFX 96 Touch Real-Time PCR Detection
Systems (Bio-Rad Laboratories, Hercules, CA, USA) was used with an
aliquot of cDNA (2 μl) in 20 μl qPCR reaction containing SYBR-Green
PCR Master Mix (Agilent, La Jolla, CA, USA) for measurement of
target genes such as c-Ha-ras, Rho-A, NF-κB,
Bcl-xL, Bax, p53 and β-actin was used
as reference to obtain the relative fold-change for target genes
using the comparative Ct method and using Bio-Rad CFX Manager 2.1
software. Relative expression was always normalized to the average
in normal breast cells. Table I
shows the primers for the genes selected to develop cDNA
probes.
 | Table ISelected primers for target genes to
develop cDNA probes. |
Table I
Selected primers for target genes to
develop cDNA probes.
| Gene name | Product length
(bp)a | Primer
sequenceb |
|---|
| H-ras | 112 |
1-CCAGTACAGGGAGCAGAT
1′-GAGCCTGCCGAGATTCCACA |
| Rho-A | 140 |
1-CCATCATCCTGGTTGGGAAT
1′-CATGTACCCAAAAGCGCCA |
| p53 | 128 |
1-CCTCAGCATCTTATCCGAGTGG
1′-TGGATGGTGGTACAGTCAGAGC |
| Bcl-xL | 211 |
1-CTGAATCGGAGATGGAGACC
1′-TGGGATGTCAGGTCACTGAA |
| Bax | 143 |
1-GCGAGTGTCTCAAGCGCATC
1′-CCAGTTGAAGTTGCCGTCAGAA |
| NF-κB
(RelA) | 114 |
1-ATCTGCCGAGTGAACCGAAACT
1′-CCAGCCTGGTCCCGTGAAA |
| β-actin | 569 |
1-ACTACCTCATGAAGATCCTC
1′-TAGAAGCATTTGCGGTGGACGATGG |
Western blot analysis
Cells were lysed with 1 ml lysis buffer (pH 7.2)
(Tris Base (50 mM), NaCl (100 mM), EDTA (1 mM), orthovanadate (1
mM), PMSF (1 mM), Triton X-100 (0,1%) and centrifuged (13,200 rpm ×
15 min). The supernatant with cellular proteins were dissolved in
SDS-PAGE sample solution (60 mM) Tris, pH 6.5, 10% (w/v) glycerol,
5% (w/v) β-mercaptoethanol, 20% (w/v) SDS, and 0.025% (w/v)
bromophenol blue and denatured by boiling (2×5 min), and vortex
mixing (2×30 seg). The total amount of protein was 30 μg in each
lane with standard protein markers (Bio-Rad Laboratories). After
fractionation by SDS-PAGE on gels (7×14 cm), proteins were
electro-blotted onto PVDF membrane (Amersham Biosciences,
Buckinghamshire, UK) using a blotting apparatus (Bio-Rad
Laboratories). Prestained SDS-PAGE (Standards) blots were blocked
for 2 h in 10% defatted dry milk-TBS-0.1% Tween-20 and then
incubated for 2 h at room temperature with corresponding primary
antibodies (1:200) Rac1 (sc-217), Rho-A (sc-418), Stat1 (sc-417),
caspase-3 (sc-7148), Bax (sc-7480), NF-κB (sc-53744) and β-actin
(sc-47778) followed by incubation with secondary
peroxidase-conjugated mouse IgG (1:5,000) (Cell Signaling
Technology, Danvers, MA, USA) in 5% defatted dry milk-TBS-0.1%
Tween-20. All steps were performed at room temperature, and blots
were rinsed between incubation steps with TBS-0.1% Tween-20. Cell
blots were probed with mouse anti β-actin antibody as control.
Immunoreactive bands were visualized by using the ECLTM
Western Blotting Detection Reagent detection method (Amersham,
Dübendorf, Switzerland) and exposure of the membrane to X-ray film.
Protein determination was performed using the Bicinchoninic Acid
Method (Bio-Rad Laboratories) and BSA as the standards. Experiments
were performed in triplicate.
Apoptosis assay
Annexin V, a Ca2+-dependent phospholipid
binding protein, has a strong binding affinity for
phosphatidylserine (PS) which is inside of cell membrane in normal
cells and is transferred to the surface during the early stage of
cell apoptosis. Thus, apoptotic cells were quantified using the
Annexin V-FITC apoptosis detection kit (Beckman Coulter, Fullerton,
CA, USA) after cells were treated with 5-FU at 2 μM for 48 h.
MCF-10F and Tumor2 cell lines were cultured until 70% confluent,
then 5-FU with indicated concentrations was added. After 48 h,
cells were trypsinized and washed twice with cold PBS, and then
resuspended in 1X binding buffer with 10 μl of Annexin V-FITC and
20 μl of 7-amino-actinomycin D (7-AAD, a nucleic acid dye) at
1×106 cells/ml in a total volume of 100 μl. Cells were
gently mixed and incubated in the dark for 15 min at room
temperature. A quantity of 1X binding buffer (400 μl) was then
added to a clean test tube and the number of apoptotic cells was
quantified using a flow cytometer (Beckman Coulter FC500 Flow
Cytometry System; Beckman Coulter) within 1 h. Cells that stain
positive for Annexin V-FITC and negative for 7-AAD are undergoing
apoptosis; cells that stain positive for both Annexin V-FITC and
7-AAD are either in the endstage of apoptosis, are undergoing
necrosis, or are already dead; cells that stain negative for both
Annexin V:FITC and 7-AAD are alive and not undergoing apoptosis.
Analysis was performed by Beckman Coulter FC500 Flow Cytometry
System with CXP Software (Beckman Coulter). All experiments were
performed at least three times.
Statistical analysis
Data are expressed as the average ± standard error
of the mean (SEM). Comparisons of multiple groups were performed
between treated groups and controls carried out by ANOVA and
Dunnet's test. P-values of p<0.05 and p<0.01 were considered
to be significant. Lethal dose at 50% (LD50) was
calculated by a non-linear regression curve using GraphPad Prism
5.0 for Windows (GraphPad Software, San Diego, CA, USA). Assays
were performed at least three times independently.
Results
MTT assay was carried out to evaluate the metabolic
activity of living cells as indicator of viability in MCF-10F,
Tumor2 and MDA-MB-231 cell lines and to determine the dose to be
used in the experiments. Concentration range of 0–5 μM was used of
5-FU for 48 h to calculate that LD50 values for all cell
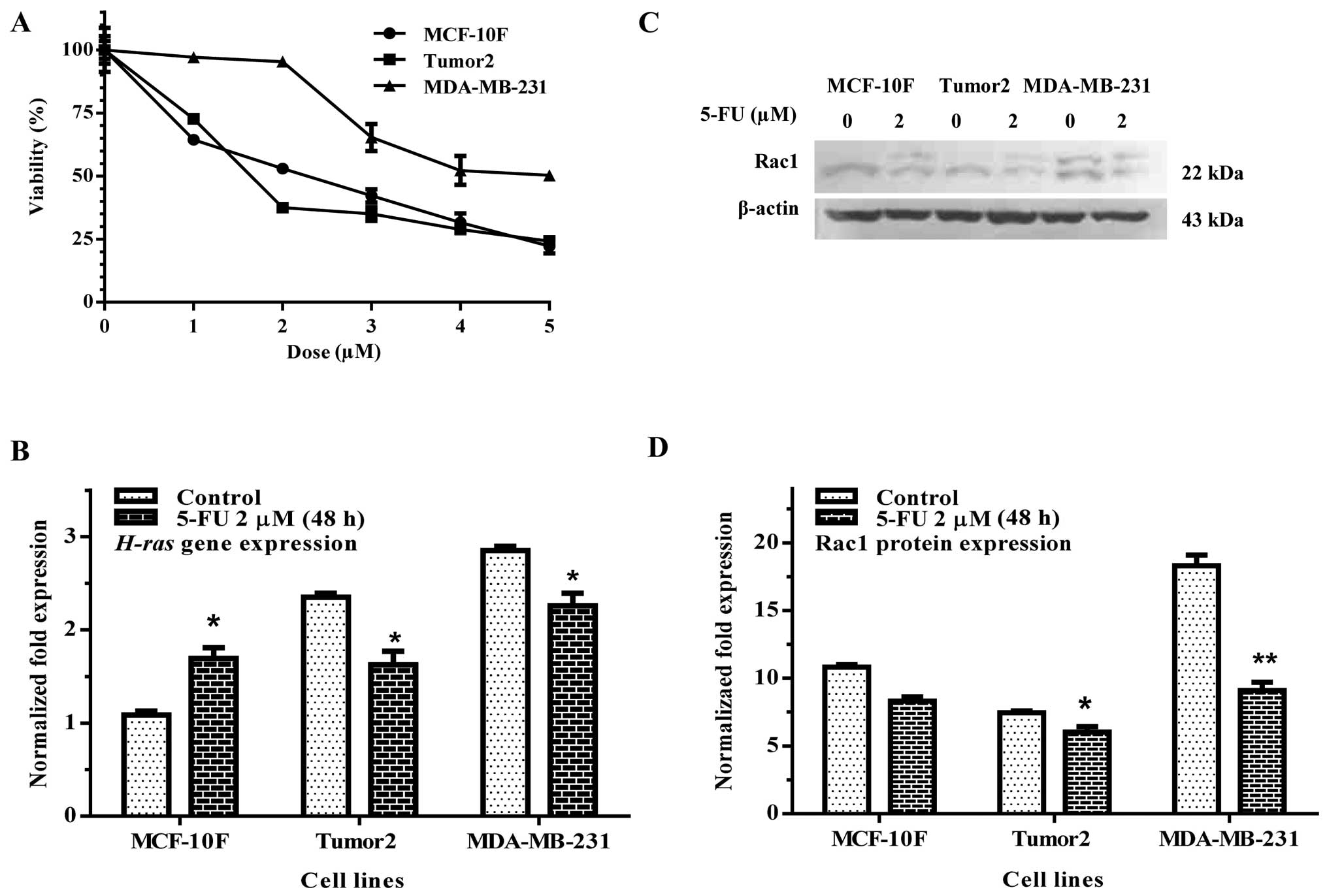
lines tested. Results in Fig. 1A
showed that the mean LD50 was at 2 μM after 48 h.
Therefore, all the following experiments were carried out with this
concentration of 5-FU.
Ras family is related to cell proliferation in
cancer cells. H-ras gene expression was studied by RT-qPCR.
Results of the experiments indicated that 5-FU significantly
decreased H-ras gene expression in Tumor2 and MDA-MB-231
cell lines (Fig. 1B). Rac1
(Fig. 1C and D) protein expression
was decreased in Tumor2 and MDA-MB-231 cells (p<0.05 and
p<0.01) in comparison with its counterpart.
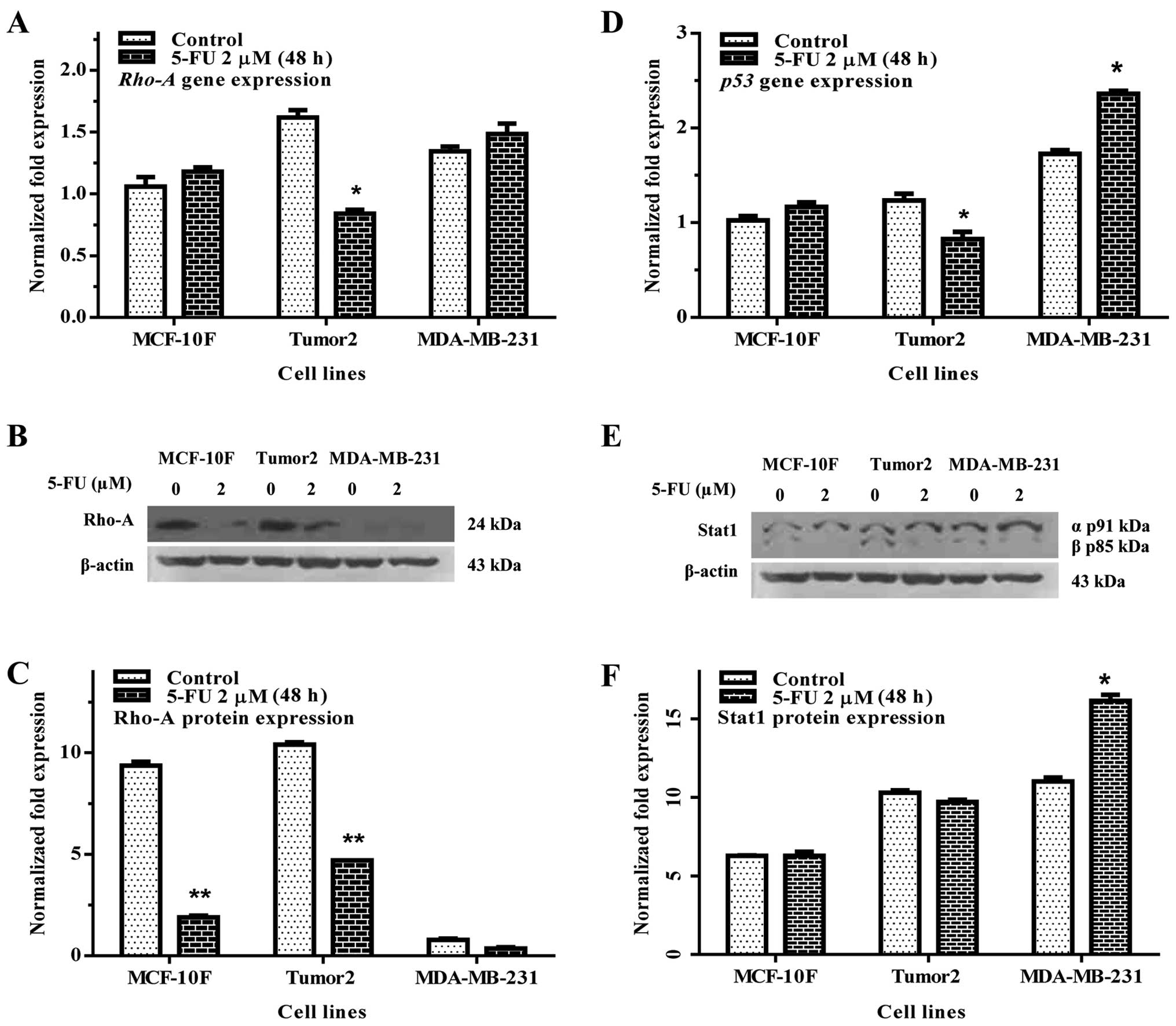
Rho-A is member of Ras family known to regulate the
actin cytoskeleton and it is distributed in the nuclei of cancer
cells. Rho-A gene and protein expression were studied by RT-qPCR
and western blot analysis, respectively. Results of the experiments
indicated that 5-FU significantly decreased Rho-A gene expression
and protein expression of the Tumor2 cells (p<0.01) in
comparison with its counterpart, however, the MDA-MB-231 cells were
not altered (Fig. 2A–C).
Analysis of gene expression indicated that 5-FU
decreased p53 in Tumor2 cells in comparison to its counterparts.
However, MDA-MB-231 cells showed an increase in gene expression in
comparison with their counterparts (Fig. 2D). Fig. 2E and F show Stat1 protein
expression. There was no effect on Stat1 either in Tumor2 or in
MDA-MB-231 cells.
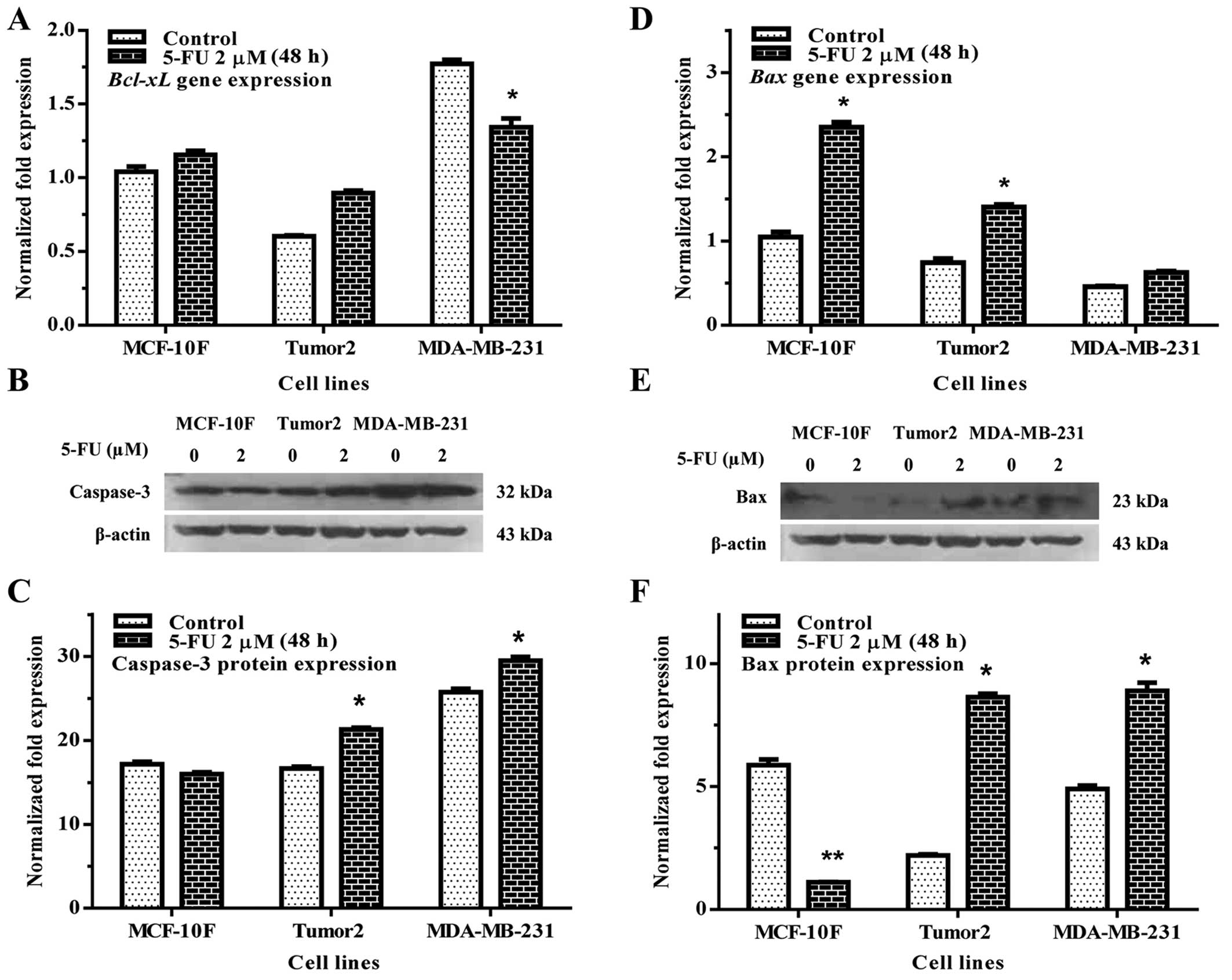
The apoptotic activity of 5-FU on MCF-10F, Tumor2
and MDA-MB-231 cell lines were analyzed. Results indicated that
Bcl-xL (Fig. 3A) gene
expression significantly decreased in MDA-MB-231 with regard to its
counterpart (p<0.01). However, there was no effect in Tumor2
cells. 5-FU significantly increased caspase-3 protein expression in
Tumor2 and MDA-MB-231 cells in comparison to its counterparts
(Fig. 3B and C). It also increased
Bax gene (Fig. 3D) and protein
(Fig. 3E and F) expression in
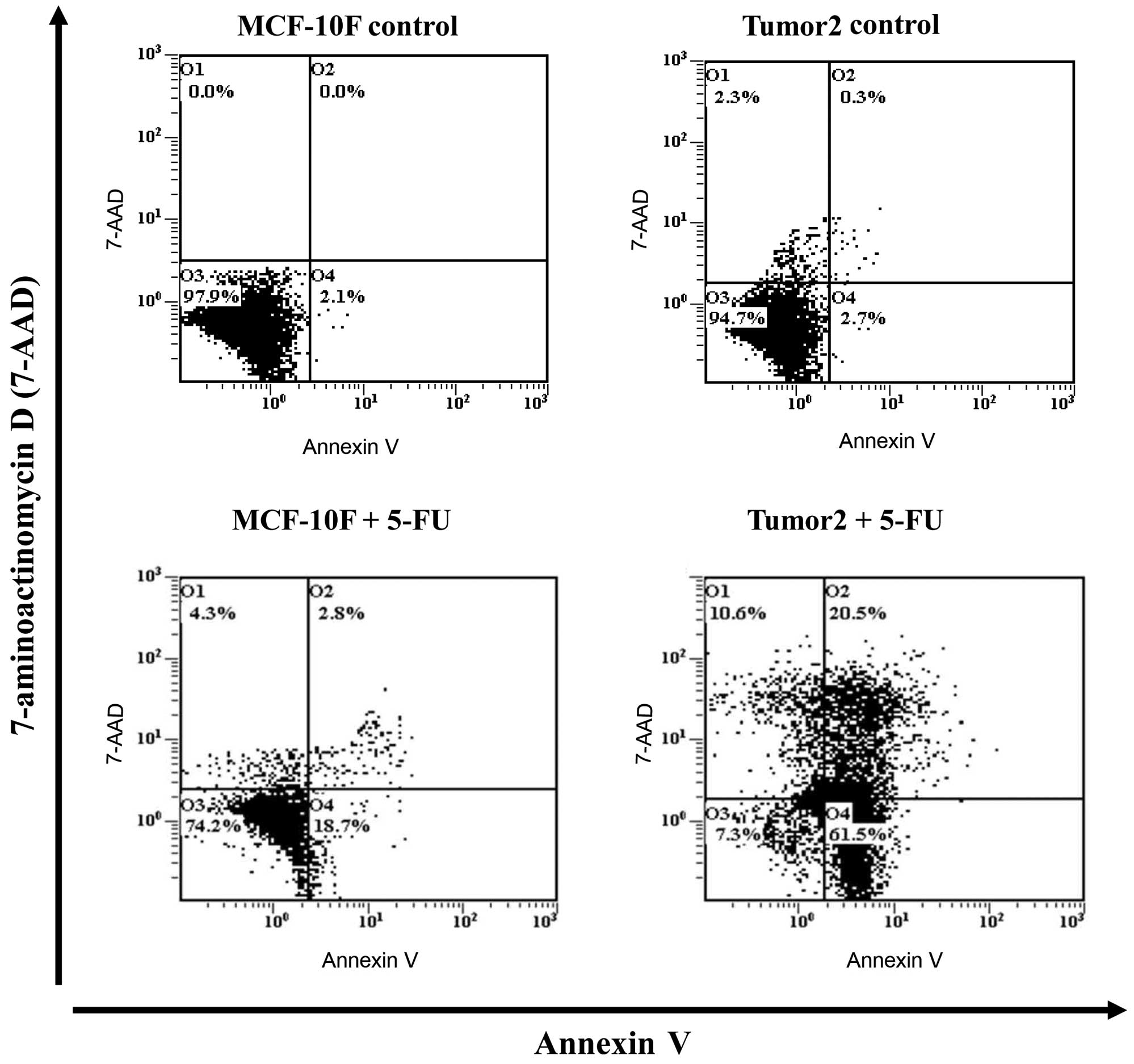
Tumor2 and MDA-MB-231 cell lines. Apoptotic cells were also
measured by flow cytometry, the results indicated 21.5% of cell
death in the control MCF-10F and 80% in Tumor2 cells (Fig. 4).
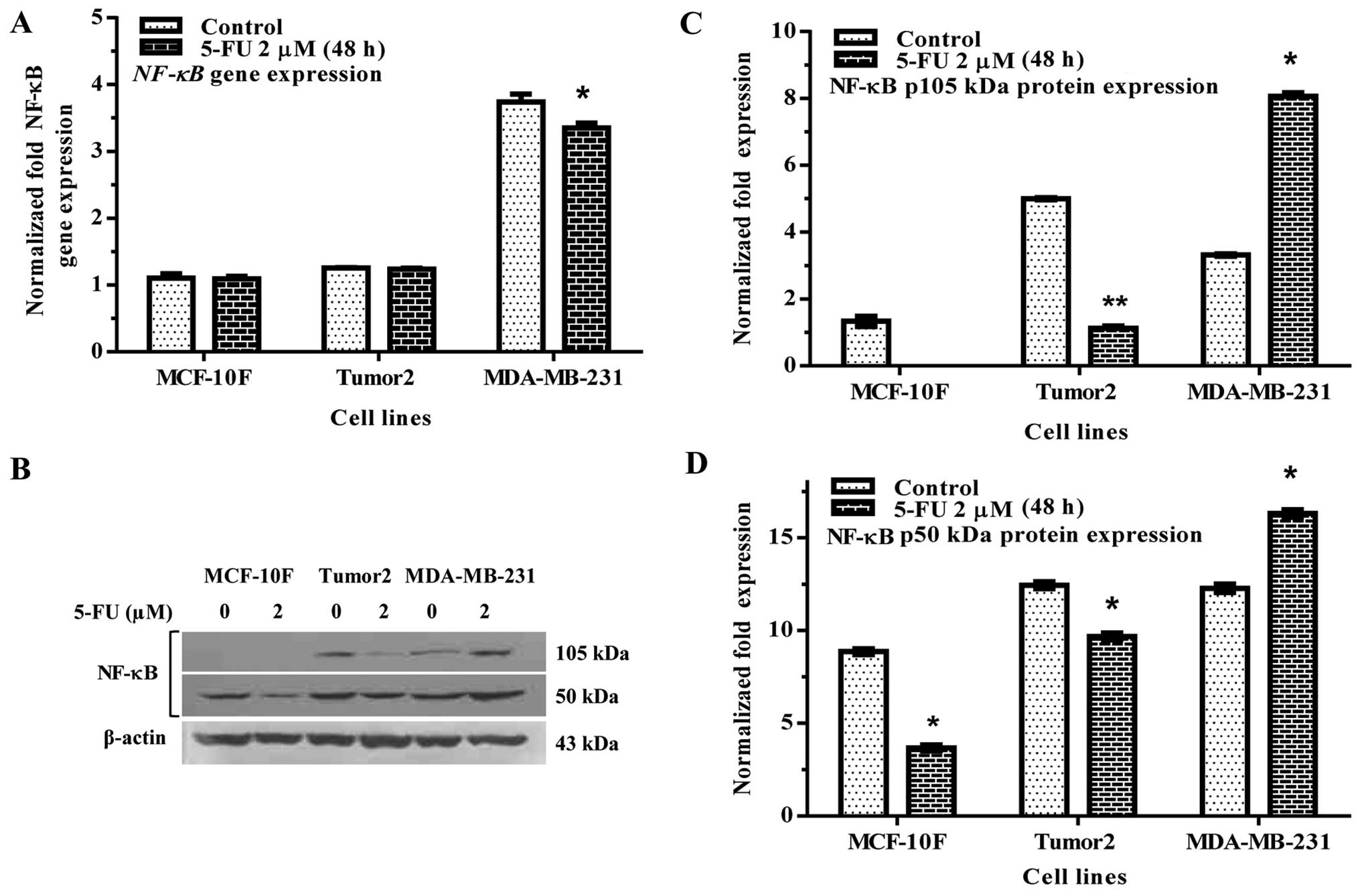
The activation of NF-κB is frequently observed in
breast cancer cells. 5-FU significantly decreased NF-κB gene
expression in MDA-MB-231 but not in Tumor2 in comparison to its
counterparts (Fig. 5A). 5-FU also
decreased protein expression in Tumor2 cell, but not in MDA-MB-231
cells (Fig. 5B–D), where we
observed and increase in the expression in both subunits p105 and
p50 kDa as shown in Fig. 5B.
Discussion
Breast cancer is one of the most common causes of
cancer-related death among women (1). 5-FU is frequently used to treat
breast cancer. This agent can inhibit breast cancer progression by
a variety of different mechanisms such as apoptosis by affecting
cell death pathways. Therefore, several clinical trials are
currently under investigation to overcome drug resistance due to
modulation of apoptosis (51). In
the present study, the in vitro effects of 5-FU in breast
cancer cell lines were evaluated by several parameters. 5-FU showed
a direct apoptotic activity in breast cancer cell lines, which is
in agreement with results from previous studies (51).
5-FU decreased H-ras gene and protein
expression in Tumor2 and MDA-MB-231 cell lines in comparison to its
counterparts and MCF-10F. Authors have demonstrated that resistance
to 5-FU may result from low levels of GTPase-activating proteins,
such as N-ras and H-ras in tumor cells (20). 5-FU has been shown to be a highly
effective inhibitor of human cell proliferation by inactivating the
Ras/ERK pathway (20,21). The effects of H-ras on cell
motility appeared to be through activation of a MAP kinase cascade,
presumably via the Ras effector Raf (24).
Rac1 is responsible for Ras-induced phenotype
changes by regulating motility mammary epithelial cells (53). Our results have shown that 5-FU
significantly decreased Rac1 protein expression in Tumor2 and
MDA-MB-231 cells. Rac is related to a profound change in cell
phenotype such as motility, invasiveness, and resistance to
apoptosis or the ability to adapt to environmental changes and
continue to invade successfully (54). Anti-apoptotic activity of Rac has
been indicated, although the molecular mechanism through which Rac
inactivation promotes apoptosis has yet to be elucidated (55).
Previous studies have highlighted the role of signal
transduction pathways controlled by the Rho family of small GTPases
(21). 5-FU decreased Rho-A gene
and protein expression in Tumor2 cell line in comparison to their
counterparts. It is of interest to note that MDA-MB-231 were not
altered by this chemotherapeutic drug which is highly resistant.
The inhibition of Rho proteins may provide a possibility to reduce
metastasis and apoptosis. Recent studies have indicated that 5-FU
induced apoptotic effects in myeloma cells in vitro
(19–27).
p53 acts as a transcription regulator and has been
shown to block the entry of DNA-damaged cells into the S-phase and
also to trigger an apoptotic pathway in many transformed cells by
inducing the expression of a set of genes related to the control of
cell proliferation (28). The
present results indicated that p53 gene expression decreased
by 5-FU in Tumor2 in comparison to its counterpart. Others, have
showed that 5-FU induces apoptosis of human gastric cancer cells
via wild-type p53 gene expression (56) which is consistent with our results.
In addition to the high levels of anti-apoptotic Bcl-2 and Bcl-xL
proteins combined with a low level of Bax were correlated to high
5-FU resistance of wild-type p53 cell lines (57). 5-FU did not affect p53 gene
expression in MDA-MB-231 cell line; however, this cell line as well
as T47D, or SKBR-3 with GnRH-p53 in combination with 5-FU
significantly enhanced p53-activated apoptotic signals including
BAX translocation to mitochondria, and activated caspase-3.
Intratumoral injection of the GnRH-p53 protein inhibited MDA-MB-231
xenograft growth and induced p53-mediated apoptosis in the tumors
(58).
Stat1 participates in regulation of tumor
angiogenesis, growth, and metastasis (29). Our results did not show any
significant difference in Stat1 protein expression with the
treatment of 5-FU in Tumor2 and MDA-MB-231. Stat1 has been shown to
be associated with cell growth modulation and cell death signaling
(59). This implied that Stat1 may
have a modulatory role in cell death signaling when tumor cell
growth is blocked by another Stat such as Stat3 inhibition
(59).
The caspases, a family of cysteine proteases, are
major mediators of the execution phase of apoptosis; possibly by
direct activation of the death receptor or following mitochondrial
changes (57,58). The cytotoxic effect of 5-FU induced
apoptosis in cancer cells. Our results showed that 5-FU
significantly increased caspase-3 expression in Tumor2 and
MDA-MB-231 cell lines suggesting activation of apoptosis. Other
authors have confirmed that 5-FU induced increased activity of
caspase-3 and -8 (57,58).
NF-κB is an important signaling pathway involved in
chemoresistance induced by 5-FU. Constitutive activation of NF-κB
is observed in several cancer cells and such activation results in
the control of a signaling network, which includes the expression
of anti-apoptotic genes, cell cycle regulatory genes and genes
encoding cell surface receptors. The activation of NF-κB is
frequently observed in breast cancer cells. The present study shows
that 5-FU decreased NF-κB gene expression in MDA-MB-231. 5-FU also
decreased protein expression in Tumor2 cell line in comparison to
its counterparts. It has been indicated that inhibition of
inducible NF-κB activity reduces chemoresistance to 5-FU in human
stomach cancer cell line (60).
Other studies have shown that downregulation of NF-κB was able to
enhance therapeutic efficacy of 5-FU (60–63).
The regulation of the genes by NF-κB is related to
apoptosis (60) since it is a key
positive regulator of cancer cell proliferation and survival. It
has the ability to transcriptionally activate many pro-survival and
anti-apoptotic genes such as Bax and Bcl-xL (64). In the present study, 5-FU decreased
genes related to apoptosis such as Bcl-xL in Tumor2 cell line. It
can be concluded that 5-FU may exert apoptotic activity in breast
cancer cells transformed by low doses of ionizing α-particles in
vitro regulating Bax and Bcl-xL and NF-κB expression,
respectively.
Acknowledgements
The technical assistance of Guiliana Rojas, Georgina
Vargas and Leodán Crispin is greatly appreciated. This study was
supported by Grant support FONDECYT #1120006 (GMC) and MINEDUC-UTA
(GMC).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campbell SL, Khosravi-Far R, Rossman KL,
Clark GJ and Der CJ: Increasing complexity of Ras signaling.
Oncogene. 17:1395–1413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bos JL, Fearon ER, Hamilton SR, Verlaan-de
Vries M, van Boom JH, van der Eb AJ and Vogelstein B: Prevalence of
ras gene mutations in human colorectal cancers. Nature.
327:293–297. 1987. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finkelstein SD, Sayegh R, Christensen S
and Swalsky PA: Genotypic classification of colorectal
adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation
type. Cancer. 71:3827–3838. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bourne HR, Sanders DA and McCormick F: The
GTPase superfamily: Conserved structure and molecular mechanism.
Nature. 349:117–127. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khosravi-Far R, Solski PA, Clark GJ, Kinch
MS and Der CJ: Activation of Rac1, RhoA, and mitogen-activated
protein kinases is required for Ras transformation. Mol Cell Biol.
15:6443–6453. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moorman JP, Bobak DA and Hahn CS:
Inactivation of the small GTP binding protein Rho induces
multinucleate cell formation and apoptosis in murine T lymphoma
EL4. J Immunol. 156:4146–4153. 1996.PubMed/NCBI
|
|
8
|
Perona R, Esteve P, Jiménez B, Ballestero
RP, Ramón y Cajal S and Lacal JC: Tumorigenic activity of rho genes
from Aplysia californica. Oncogene. 8:1285–1292. 1993.PubMed/NCBI
|
|
9
|
Prendergast GC, Khosravi-Far R, Solski PA,
Kurzawa H, Lebowitz PF and Der CJ: Critical role of Rho in cell
transformation by oncogenic Ras. Oncogene. 10:2289–2296.
1995.PubMed/NCBI
|
|
10
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
12
|
Wherlock M and Mellor H: The Rho GTPase
family: A Racs to Wrchs story. J Cell Sci. 115:239–240.
2002.PubMed/NCBI
|
|
13
|
Yoshioka K, Nakamori S and Itoh K:
Overexpression of small GTP-binding protein RhoA promotes invasion
of tumor cells. Cancer Res. 59:2004–2010. 1999.PubMed/NCBI
|
|
14
|
Paterson HF, Self AJ, Garrett MD, Just I,
Aktories K and Hall A: Microinjection of recombinant p21rho induces
rapid changes in cell morphology. J Cell Biol. 111:1001–1007. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramakers GJ and Moolenaar WH: Regulation
of astrocyte morphology by RhoA and lysophosphatidic acid. Exp Cell
Res. 245:252–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soga N, Namba N, McAllister S, Cornelius
L, Teitelbaum SL, Dowdy SF, Kawamura J and Hruska KA: Rho family
GTPases regulate VEGF-stimulated endothelial cell motility. Exp
Cell Res. 269:73–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sahai E, Olson MF and Marshall CJ:
Cross-talk between Ras and Rho signalling pathways in
transformation favours proliferation and increased motility. EMBO
J. 20:755–766. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Senger DL, Tudan C, Guiot MC, Mazzoni IE,
Molenkamp G, LeBlanc R, Antel J, Olivier A, Snipes GJ and Kaplan
DR: Suppression of Rac activity induces apoptosis of human glioma
cells but not normal human astrocytes. Cancer Res. 62:2131–2140.
2002.PubMed/NCBI
|
|
19
|
Embade N, Valerón PF, Aznar S,
López-Collazo E and Lacal JC: Apoptosis induced by Rac GTPase
correlates with induction of FasL and ceramides production. Mol
Biol Cell. 11:4347–4358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abraham MT, Kuriakose MA, Sacks PG, Yee H,
Chiriboga L, Bearer EL and Delacure MD: Motility-related proteins
as markers for head and neck squamous cell cancer. Laryngoscope.
111:1285–1289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horiuchi A, Imai T, Wang C, Ohira S, Feng
Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA
and RhoC, is associated with tumor progression in ovarian
carcinoma. Lab Invest. 83:861–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamai T, Arai K, Tsujii T, Honda M and
Yoshida K: Overexpression of RhoA mRNA is associated with advanced
stage in testicular germ cell tumour. BJU Int. 87:227–231. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamai T, Kawakami S, Koga F, Arai G,
Takagi K, Arai K, Tsujii T and Yoshida KI: RhoA is associated with
invasion and lymph node metastasis in upper urinary tract cancer.
BJU Int. 91:234–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jaffe AB and Hall A: Rho GTPases:
Biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ellenbroek SI and Collard JG: Rho GTPases:
Functions and association with cancer. Clin Exp Metastasis.
24:657–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olivier M, Eeles R, Hollstein M, Khan MA,
Harris CC and Hainaut P: The IARC TP53 database: New online
mutation analysis and recommendations to users. Hum Mutat.
19:607–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang S, Bucana CD, Van Arsdall M and
Fidler IJ: Stat1 negatively regulates angiogenesis, tumorigenicity
and metastasis of tumor cells. Oncogene. 21:2504–2512. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schindler C, Levy DE and Decker T:
JAK-STAT signaling: From interferons to cytokines. J Biol Chem.
282:20059–20063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stark GR and Darnell JE Jr: The JAK-STAT
pathway at twenty. Immunity. 36:503–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McKenna SL, McGowan AJ and Cotter TG:
Molecular mechanisms of programmed cell death. Adv Biochem Eng
Biotechnol. 62:1–31. 1998.PubMed/NCBI
|
|
33
|
Leist M and Jäättelä M: Four deaths and a
funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell
Biol. 2:589–598. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carson JP, Kulik G and Weber MJ:
Antiapoptotic signaling in LNCaP prostate cancer cells: A survival
signaling pathway independent of phosphatidylinositol 3′-kinase and
Akt/protein kinase B. Cancer Res. 59:1449–1453. 1999.PubMed/NCBI
|
|
35
|
Lin J, Adam RM, Santiestevan E and Freeman
MR: The phosphatidylinositol 3′-kinase pathway is a dominant growth
factor-activated cell survival pathway in LNCaP human prostate
carcinoma cells. Cancer Res. 59:2891–2897. 1999.PubMed/NCBI
|
|
36
|
Beale PJ, Rogers P, Boxall F, Sharp SY and
Kelland LR: BCL-2 family protein expression and platinum drug
resistance in ovarian carcinoma. Br J Cancer. 82:436–440.
2000.PubMed/NCBI
|
|
37
|
Moorehead RA and Singh G: Influence of the
proto-oncogene c-fos on cisplatin sensitivity. Biochem Pharmacol.
59:337–345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kagawa S, Pearson SA, Ji L, Xu K,
McDonnell TJ, Swisher SG, Roth JA and Fang B: A binary adenoviral
vector system for expressing high levels of the proapoptotic gene
bax. Gene Ther. 7:75–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rossé T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Finucane DM, Bossy-Wetzel E, Waterhouse
NJ, Cotter TG and Green DR: Bax-induced caspase activation and
apoptosis via cytochrome c release from mitochondria is inhibitable
by Bcl-xL. J Biol Chem. 274:2225–2233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kitanaka C, Namiki T, Noguchi K, Mochizuki
T, Kagaya S, Chi S, Hayashi A, Asai A, Tsujimoto Y and Kuchino Y:
Caspase-dependent apoptosis of COS-7 cells induced by Bax
overexpression: Differential effects of Bcl-2 and Bcl-xL on
Bax-induced caspase activation and apoptosis. Oncogene.
15:1763–1772. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiang J, Chao DT and Korsmeyer SJ:
BAX-induced cell death may not require interleukin 1
beta-converting enzyme-like proteases. Proc Natl Acad Sci USA.
93:14559–14563. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kagawa S, Gu J, Honda T, McDonnell TJ,
Swisher SG, Roth JA and Fang B: Deficiency of caspase-3 in MCF7
cells blocks Bax-mediated nuclear fragmentation but not cell death.
Clin Cancer Res. 7:1474–1480. 2001.PubMed/NCBI
|
|
45
|
Sethi G, Ahn KS and Aggarwal BB: Targeting
nuclear factor-kappa B activation pathway by thymoquinone: Role in
suppression of antiapoptotic gene products and enhancement of
apoptosis. Mol Cancer Res. 6:1059–1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bellarosa D, Ciucci A, Bullo A, Nardelli
F, Manzini S, Maggi CA and Goso C: Apoptotic events in a human
ovarian cancer cell line exposed to anthracyclines. J Pharmacol Exp
Ther. 296:276–283. 2001.PubMed/NCBI
|
|
47
|
Keane MM, Ettenberg SA, Nau MM, Russell EK
and Lipkowitz S: Chemotherapy augments TRAIL-induced apoptosis in
breast cell lines. Cancer Res. 59:734–741. 1999.PubMed/NCBI
|
|
48
|
Kottke TJ, Blajeski AL, Martins LM, Mesner
PW Jr, Davidson NE, Earnshaw WC, Armstrong DK and Kaufmann SH:
Comparison of paclitaxel-, 5-fluoro-2′-deoxyuridine-, and epidermal
growth factor (EGF)-induced apoptosis. Evidence for EGF-induced
anoikis. J Biol Chem. 274:15927–15936. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Staudt LM: Oncogenic activation of
NF-kappaB. Cold Spring Harb Perspect Biol. 2:a0001092010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Calaf GM and Hei TK: Establishment of a
radiation- and estrogen-induced breast cancer model.
Carcinogenesis. 21:769–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koh MS and Moon A: Activation of H-Ras and
Rac1 correlates with epidermal growth factor-induced invasion in
Hs578T and MDA-MB-231 breast carcinoma cells. Biochem Biophys Res
Commun. 406:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Parri M and Chiarugi P: Rac and Rho
GTPases in cancer cell motility control. Cell Commun Signal.
8:232010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang B, Zhang Y and Shacter E: Caspase
3-mediated inactivation of rac GTPases promotes drug-induced
apoptosis in human lymphoma cells. Mol Cell Biol. 23:5716–5725.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Osaki M, Tatebe S, Goto A, Hayashi H,
Oshimura M and Ito H: 5-Fluorouracil (5-FU) induced apoptosis in
gastric cancer cell lines: Role of the p53 gene. Apoptosis.
2:221–226. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Violette S, Poulain L, Dussaulx E, Pepin
D, Faussat AM, Chambaz J, Lacorte JM, Staedel C and Lesuffleur T:
Resistance of colon cancer cells to long-term 5-fluorouracil
exposure is correlated to the relative level of Bcl-2 and Bcl-X(L)
in addition to Bax and p53 status. Int J Cancer. 98:498–504. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lu Y, Zhang Z, Yan Z, Chen L, Deng W,
Lotze M, Wang Z, Lin X and Li LY: Recombinant GnRH-p53 protein
sensitizes breast cancer cells to 5-fluorouracil-induced apoptosis
in vitro and in vivo. Apoptosis. 18:1214–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shen Y, Devgan G, Darnell JE Jr and
Bromberg JF: Constitutively activated Stat3 protects fibroblasts
from serum withdrawal and UV-induced apoptosis and antagonizes the
proapoptotic effects of activated Stat1. Proc Natl Acad Sci USA.
98:1543–1548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Uetsuka H, Haisa M, Kimura M, Gunduz M,
Kaneda Y, Ohkawa T, Takaoka M, Murata T, Nobuhisa T, Yamatsuji T,
et al: Inhibition of inducible NF-kappaB activity reduces
chemoresistance to 5-fluorouracil in human stomach cancer cell
line. Exp Cell Res. 289:27–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kodach LL, Bos CL, Durán N, Peppelenbosch
MP, Ferreira CV and Hardwick JC: Violacein synergistically
increases 5-fluorouracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar
|
|
62
|
Wang W, McLeod HL and Cassidy J:
Disulfiram-mediated inhibition of NF-kappaB activity enhances
cytotoxicity of 5-fluorouracil in human colorectal cancer cell
lines. Int J Cancer. 104:504–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-kappaB activation through modulation of I
kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human
gastric carcinoma cells. Biomed Pharmacother. 62:659–666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vinod BS, Antony J, Nair HH,
Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A and Anto RJ:
Mechanistic evaluation of the signaling events regulating
curcumin-mediated chemosensitization of breast cancer cells to
5-fluorouracil. Cell Death Dis. 4:e5052013. View Article : Google Scholar : PubMed/NCBI
|