Introduction
The incidence and prevalence of pancreatic
neuroendocrine tumors (pNETs) are being found increasingly; pNETs
represent ~1.3 and 10% of pancreatic malignancy cases in incidence
and in prevalence, respectively (1–3).
Approximately 65% of pNET patients present with unresectable or
metastatic disease (4), and in
>40% patients, recurrence or metastasis occurs even after
radical surgical resection, resulting in a poor prognosis (5,6). It
is urgent to explore the molecular mechanisms of pNET development,
progression and metastasis in order to identify new molecular
targets for therapy. Indeed, there are currently only two drugs for
molecular target therapy, everolimus and sunitinib, which have been
approved for clinical use for pNETs.
Emerging evidence suggests that tumor metastasis and
recurrence might be caused by a small subpopulation of stemness
cells, so-called cancer stem cells (CSCs). CSCs promote tumor
invasion and metastasis (7,8),
therefore, CSCs are considered to be a promising therapeutic target
for cancer (9). There have been
numerous investigations for identification of the CSC population
based on their characteristics, including the cell surface markers,
ability to exclude a fluorescent Hoechst dye, the so-called ‘side
population’, aldehyde dehydrogenase (ALDH) activity and dormancy
(10). High ALDH activity has been
shown to be one of the reliable CSC markers in several types of
solid tumors, including breast, skin, bladder and prostate cancer
(11–13). Gaur et al reported that CSCs
were identified in gastrointestinal NETs, and that they were
isolated using ALDH activity and that the CSC property was verified
in 2011 (14). However, there has
been no study on the identification and isolation of pNET CSCs,
therefore, the clinical significance and a therapeutic target
remain unknown.
In this study, we isolated pNET CSCs by sorting with
ALDH activity and demonstrated that these cells have the property
of stemness. Additionally, in order to acquire a CSC gene profile,
genome-wide gene expression analysis was performed using DNA
microarray, which revealed CD73 was overexpressed in
ALDHhigh cells. We identified CD73 as not only a unique
biomarker for pNET CSCs but also as a novel molecular target for
pNET therapy.
Materials and methods
Patients and tissue samples
In all, 44 patients with histologically proven pNETs
underwent pancreatic resection at the Tokyo Medical and Dental
University Hospital between 2001 and 2013. Of them, 15 patients who
underwent surgery from 2012 to 2013 were recruited for Aldeflour
assay, 31 cases were randomly selected for immunohistochemical
analysis. Written informed consent was obtained from the patients,
and our institutional review board approved this study (no.
1080).
Cell culture and transient
transfection
Human pNET cell line QGP1 was obtained from the
Health Science Research Resources Bank (Osaka, Japan). A murine
insulinoma cell line MIN6 was provided by Professor J. Miyazaki
(Osaka University, Japan) (15).
Both cell lines were used in the present experiments within 10
passages after reception. All cell cultures were routinely tested
to rule out mycoplasma infection using PlasmoTest kit (InvivoGen,
San Diego, CA, USA). QGP1 cells were cultured in RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal
bovine serum (Biological Industries, Beit Haemek, Israel) and 1%
Pen/Strep (Gibco, Grand Island, NE, USA) as antibiotics. MIN6 cells
were cultured in Dulbecco's modified Eagle's medium containing 25
mmol/l glucose (DMEM; Sigma-Aldrich), supplemented with 10% fetal
bovine serum, 1% Pen/Strep and β-mercaptoethanol (2ME) (5 ml/l,
Wako, Osaka, Japan). All cell lines were cultivated in a humidified
incubator at 37°C in 5% CO2, and were collected with
0.25% trypsin-ethylene diamine tetraacetic acid (EDTA) (Gibco).
Luciferase expression plasmid pGL4.50
[luc2/CMV/Hygro] (Promega, Madison, WI, USA) was transfected into
QGP1 cells according to the manufacturer's instructions, and
luciferase-expressing QGP1 cells (QGP1-Luc) were generated as
described previously (16).
Aldeflour assay
A portion of a resected tumor was harvested under
sterile conditions in a pathology suite and placed on ice in
DMEM/F12 (Gibco) containing 10% fetal bovine serum, care being
taken to avoid contaminating adjacent normal tissue. Each specimen
was mechanically dissociated with sterile scalpels, digested for 1
h with 10 mg/ml type IV collagenase (Sigma-Aldrich) in fresh
DMEM/F12 medium containing 10% fetal bovine serum, and then
filtered through sterile 100-μm membranes to obtain single-cell
suspensions. Red blood cells in the surgical samples were lysed
using a red blood cell lysis buffer (Miltenyi Biotec, Bergisch
Gladbach, Germany). Cultured cells were collected with Accutase (BD
Bioscience, San Jose, CA, USA) as single cells. An Aldefluor kit
(Stemcell Technologies, Vancouver, Canada) was used to isolate the
population of cells with high ALDH enzymatic activity. Cells were
suspended in Aldefluor assay buffer containing ALDH substrate
(BAAA, 1 μmol/l) and incubated at 37°C for 30 min. As a negative
control, an aliquot of each sample was treated with 50 mmol/l
diethyl-aminobenzaldehyde (DEAB), a specific ALDH inhibitor. The
fluorescently labeled product, BODIPY-aminoacetate, was produced by
cells expressing the ALDH enzyme, and ALDHhigh cells
were quantified and purified using FACS Aria II (BD
Biosciences).
Spheroid assay
The spheroid assay was performed as described
previously (16–18). After FACS sorting,
ALDHhigh or bulk cells were plated separately at a
density of 300 cells on low attachment plates (96-well Ultra Low
Cluster Plate; Costar, Corning, New York, NY, USA), and then
incubated in serum-free DMEM/F12 medium (n=20 in each). To
investigate sphere formation with the CD73 inhibitor, after FACS
sorting, 5×104 ALDHhigh cells were seeded
into two 6-cm dishes. After 24 h, PBS or 12 μM α,β-methylene
adenosine 5′-diphosphate (APCP) (Sigma-Aldrich) was added to each
dish. After 48 h, the medium was changed to drug-free medium,
followed by incubation for 24 h. Using trypan blue exclusion, the
remaining viable cells were collected and plated separately at 300
cells per low attachment plates, and then incubated in serum-free
medium (n=20 in each). Sphere formation was observed using an
AxioObserver (Carl Zeiss, Oberkochen, Germany), and images were
acquired digitally using AxioVision software (Carl Zeiss).
Experiments were independently performed in triplicate.
Hypoxic treatment
Hypoxic treatment was performed as described
previously (18). PNET cell lines
were seeded into 96-well plates at 3×103 cells per well.
After 24 h, the cells were exposed to hypoxic conditions (1%
O2, 5% CO2, and 94% N2) in an
anaerobic workstation (Hirasawa Works, Tokyo, Japan). The oxygen
concentration inside the workstation was constantly monitored with
an oxygen sensor (MC-8G-S, Iijima Electrics, Gamagori, Japan) and
maintained at 1% during the experiment. After 2, 4 and 6 days, the
number of living cells was measured by the MTS assay using a Cell
Titer 96 AQueous One Solution Cell Proliferation Assay kit
(Promega), according to the manufacturer's instructions. The
absorbance was read at 490 nm, with 630 nm as the reference
wavelength, using a multiwall plate reader (Model 550, Bio-Rad,
Hercules, CA, USA), with wells containing medium but no cells
serving as blank controls (16,19).
The experiments were independently performed in triplicate.
Scar migration assay
After FACS sorting, pNET cells, ALDHhigh
or bulk, were seeded into 96-well plates at 3×103 cells
per well. The cell layer was scratched, a 200-μl pipette tip being
run the full length of each well. The well was washed once with
growth medium to remove cell debris. Images were taken at the time
of initial wounding as well as 24, 48 and 72 h post-wounding using
an INCell Analyzer 2000 (GE Healthcare, Waukesha, WI, USA). The
wound area was determined using INCell Developer Toolbox software
(GE Healthcare). We compared the ratio of the area at 72 h after
scratching to that at the time of initial wounding. Experiments
were independently performed in triplicate.
Tumor xenotransplantation and
tumorigenicity and drug sensitivity assaying in vivo
Female NOD.CB17-PRkdcScid/J mice, 16–19 g
and aged 4–6 weeks, were purchased from Charles River Laboratory
Inc. (Kanagawa, Japan). The care and use of animals were performed
in accordance with institutional guidelines. Various amounts of
cells (ALDHhigh and bulk, from 101 to
104 cells each) were mixed with Matrigel (BD
Biosciences), and then aliquots were injected subcutaneously into
both flanks of mice under isoflurane anesthesia. Tumor formation
was then monitored once a week. Cancer initiation frequency was
calculated using L-Calc software (Stem Cell Technologies) (20) and significance was determined by
Chi-square analysis using ELDA (The Walter and Eliza Hall Institute
of Medical Research) (20).
The peritoneal metastatic potential of cancer cells
was assessed as reported (21).
Briefly, 105 ALDHhigh pNET cells or unsorted
control cells were injected intraperitoneally into mice (n=4 mice
per group). The mice were monitored using a
luciferase-luciferin-based imaging IVIS system (Xenogen, Alameda,
CA, USA) under isoflurane anesthesia. Images of the mice were taken
every week for up to 14 weeks, and then the mice were euthanized by
cervical dislocation. Any mice with ascites formation or a loss of
body weight of >25% were euthanized. Animal survival data were
entered to the Kaplan-Meier Life Table format and presented as a
cumulative survival plot. Statistical differences were analyzed by
means of log-rank test. For the drug sensitivity assay,
104 freshly sorted ALDHhigh cells were used.
The mice were randomly divided into 2 groups for the experiments.
APCP (400 μg/tumor) or PBS was injected into the tumor twice a week
after tumor cell injection. Tumor formation was monitored once a
week. The tumor volume was estimated using the following equation:
volume = (length) × (width)2/2. All in vivo
procedures were approved by the Animal Care Committee of Tokyo
Medical and Dental University.
RNA extraction and gene expression
analysis
Total RNA was extracted from QGP1 freshly sorted
ALDHhigh cells and unsorted control cells using a RNeasy
kit (Qiagen, Hilden, Germany), and the integrity of the obtained
RNA was assessed using an Agilent 2100 Bioanalyzer (Agilent
Technologies, Palo Alto, CA, USA). All samples had an RNA Integrity
Number of >7.0. Anti-sense RNA was prepared from 100 ng of total
RNA using a 3′ IVT Express kit (Affymetrix, Santa Clara, CA, USA).
Hybridization and signal detection for HG-U133 Plus 2.0 arrays
(Affymetrix) were performed according to the manufacturer's
instructions. The microarray datasets for ALDHhigh and
unsorted control QGP1 cells were normalized simultaneously using
the robust multiarray average method found in R statistical
software (v. 2.12.1) together with the Bioconductor package. The
estimated gene-expression levels were obtained as log2-transformed
values. To reveal functional relationships among genes
differentially expressed in ALDHhigh QGP1 cells, a
protein interaction network was analyzed. Genes upregulated
>1.3-fold between ALDHhigh and unsorted control QGP1
cells were included in the network. Protein interaction data
obtained from BIND (http://bond.unleashedinformatics.com), Bio-GRID
(http://thebiogrid.org), and HPRD (http://www.hprd.org) were downloaded from the ftp site
of the National Center for Biotechnology Information (NCBI;
ftp://ftp.ncbi.nih.gov/gene/GeneRIF/interactions.gz).
The protein interaction network was analyzed using Cytoscape
software.
Immunocytochemistry
The cells were sorted using the FACSAria II and were
incubated for 24 h on glass slides. After fixation with 10%
trichloroacetic acid for 15 min at 4°C, the cells were incubated in
permeabilization buffer (0.2% Triton-PBS) for 5 min at room
temperature. After incubation in a blocking buffer, 3% bovine serum
albumin (BSA)-PBS, for 1 h, the slides were incubated with the
primary antibodies, CD73 (IE9; 1:50; Santa Cruz, Dallas, TX, USA)
for QGP1 or CD73 (C-20; 1:50; Santa Cruz) for MIN6 for another
hour. The secondary antibody, Alexa Fluor 647 tetramethyl rhodamine
isothiocyanate-conjugated goat anti-rabbit IgG (1:200;
Sigma-Aldrich), was diluted in 3% BSA-PBS, and then incubated with
the cells for 30 min. The Hoechst 33342 solution was added for
nuclear staining. After mounting, the slides were observed under a
fluorescent microscope, AxioObserver (Carl Zeiss).
Immunohistochemical analysis
Immunohistochemical analysis was performed on pNET
tissue samples. For the tissue analysis, 31 samples were available,
which were stained using the anti-CD73 primary antibody (IE9; Santa
Cruz) at 1:50 dilution with PBS followed by reactions in an
automated immunostainer (Ventana XT System; Ventana, Roche, Basel,
Switzerland) using a standard DAB detection kit (Ventana, Roche).
The immunostaining was evaluated quantitatively by counting ≥500
cells in three different random fields under a light microscope.
Tissue samples with >5% of strong staining in the pNET tumor
cells were diagnosed as positive, and the others were diagnosed as
negative immunohistochemically. The immunostaining was evaluated
under a light microscope by two independent investigators.
Statistical analysis
Statistical comparisons of clinicopathological
characteristics for significance were performed using a Chi-square
test or Fisher's exact test with a single degree of freedom, and
Student's t-test was used to determine the differences between
continuous values. p-values of <0.05 were considered to be
statistically significant. All statistical analyses were performed
using SPSS version 17.0 (SPSS, Chicago, IL, USA).
Results
Identification of ALDHhigh
cells in pNET specimens and cell lines
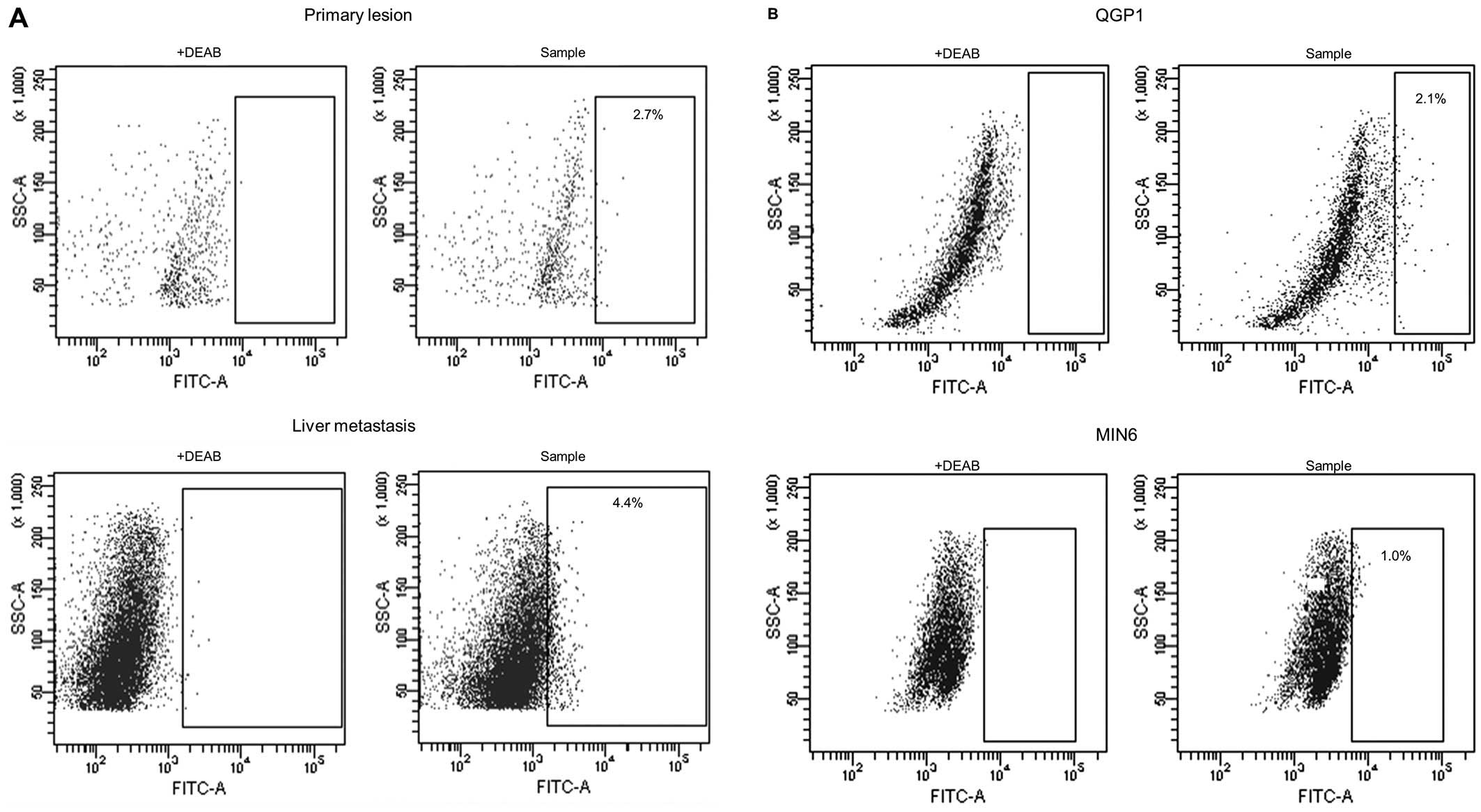
A total of 15 pNET specimens (7 primary lesions, 7
liver metastatic lesions and 1 lymph node metastatic lesion) were
analyzed by Aldefluor assay. Flow cytometry was used to isolate
viable single ALDHhigh cells from pNET specimens, with
ALDH expression greater than that of the top 0.1% of DEAB-treated
negative control cells (Fig. 1A).
The ALDHhigh cells were observed in pNET surgical
sections, the mean proportion of the subpopulation being 2.1±1.7%
(range, 0.2–4.6%).
We also found a small population of cells expressing
ALDHhigh in pNET cell lines, QGP1 (human
somatostatinoma) and MIN6 (murine insulinoma). Flow cytometric
analysis revealed that ALDHhigh cells represented from 1
to 4% of the population in both pNET cell lines, i.e., QGP1 and
MIN6 (Fig. 1B).
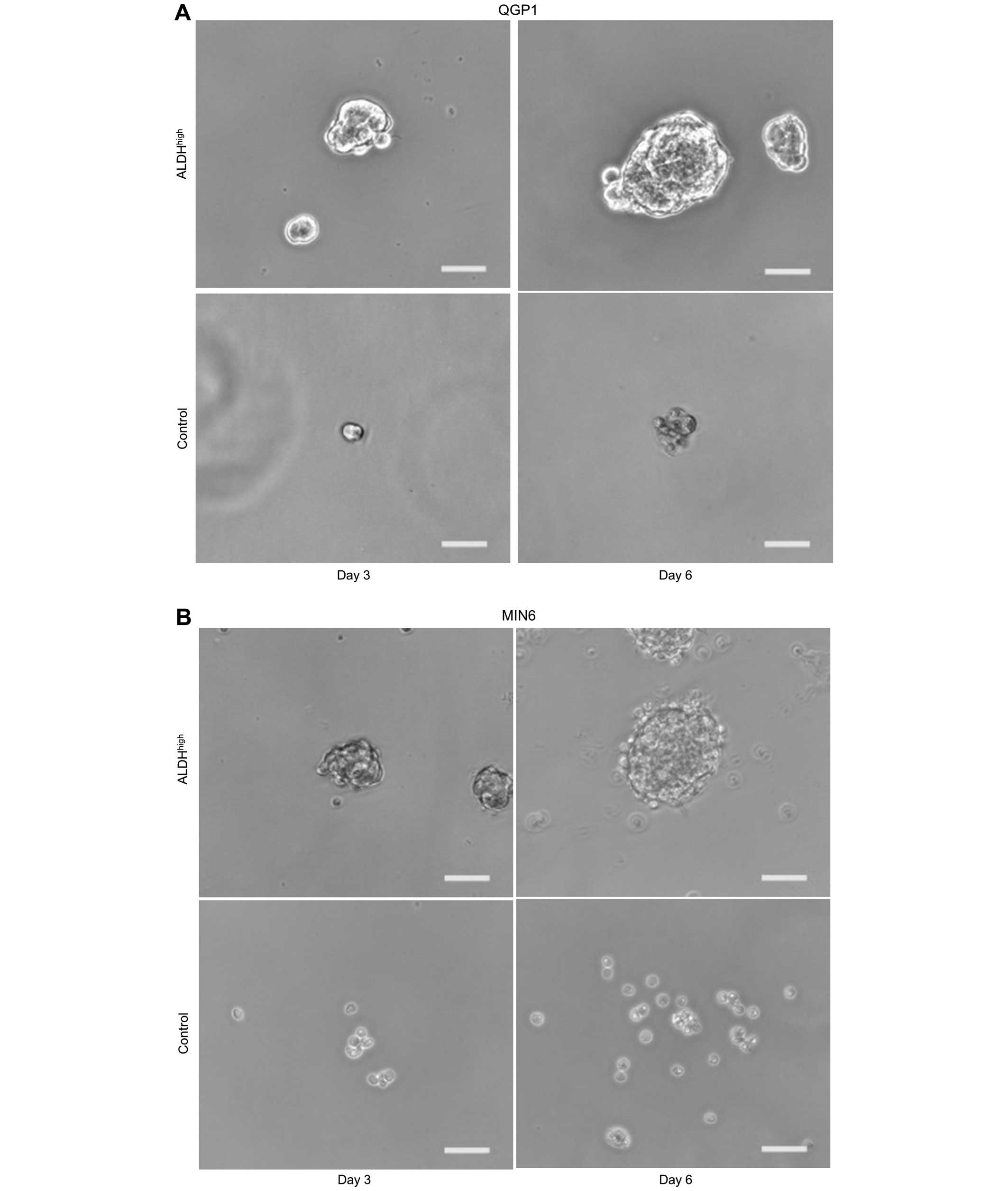
CSC property of the pNET subpopulation
among ALDHhigh cells in vitro
In order to investigate whether or not
ALDHhigh cells were characterized as CSCs,
sphere-forming ability was evaluated by sphere formation assay. The
results showed that ALDHhigh cells formed spheres in
contrast to unsorted cells for both QGP1 (Fig. 2A and C; p<0.001) and MIN6
(Fig. 2B and D; p<0.001).
Since the pluripotent potential in embryonic stem
cells is efficiently maintained under low oxygen levels (22), and hypoxia contributes to CSCs
maintenance (23), the effects of
hypoxic treatment on ALDHhigh and unsorted pNET cells
were evaluated utilizing QGP1. Cell proliferation was 39% reduced
under hypoxic conditions (1% O2) compared to normoxic
conditions for control cells, in contrast, no significant
difference in proliferation was observed between under hypoxic and
normoxic conditions for ALDHhigh cells (Fig. 2E). These results are consistent
with the reports showing that hypoxic conditions serve as a
stimulus for reprograming cells towards normal stem cells and CSCs
(22,23).
To determine whether or not ALDHhigh
cells promote cell motility, scar migration assay was performed for
QGP1. Cell motility was quantified by measuring the scratch wound
closure as a percentage. There was a 20% greater reduction in wound
size for ALDHhigh cells than for control unsorted cells
(Fig. 2F and G; p=0.006).
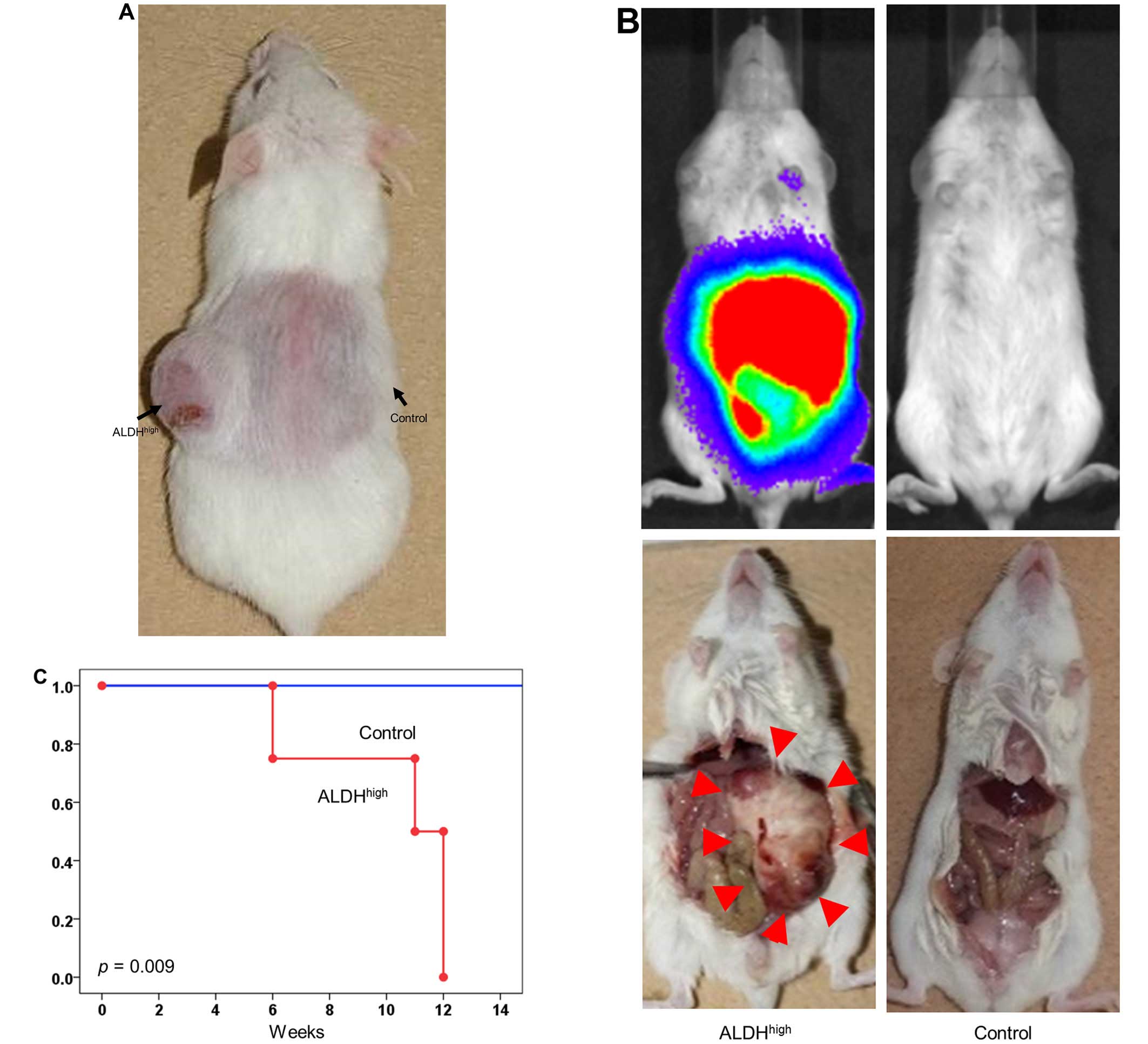
CSC property of the pNET subpopulation
among ALDHhigh cells in vivo
Different numbers of QGP1 ALDHhigh or
unsorted cells were injected subcutaneously into non-obese
diabetic/severe combined immunodeficient (NOD/SCID) mice in numbers
ranging from 101 to 104 cells per injection.
The results showed ALDHhigh pNET cells had higher
tumorigenicity than unsorted cells. Surprisingly, for a very small
number of ALDHhigh cells, as few as 101
cells, subcutaneous tumor formation was observed (Fig. 3A and Table I). The cancer initiation frequency
was 1 in 83 (95% CI, 33–211) for ALDHhigh pNET cells and
1 in 720 (95% CI, 292–1,777) for unsorted cells (p=0.002). These
results suggested that CSCs are enriched in the ALDHhigh
subpopulation.
 | Table IFraction (%) of injected mice that
developed tumors. |
Table I
Fraction (%) of injected mice that
developed tumors.
| No. of cells
injected | Injected with
ALDHhigh cells (%) | Injected with
control cells (%) |
|---|
| 101 | 1/6 (17) | 0/6 (0) |
| 102 | 4/6 (67) | 0/6 (0) |
| 103 | 6/6 (100) | 5/6 (83) |
| 104 | 6/6 (100) | 6/6 (100) |
Furthermore, to investigate whether or not the pNET
cells establish in vivo metastasis, ALDHhigh or
unsorted pNET cells were injected intraperitoneally into a NOD/SCID
mouse utilizing QGP1. Peritoneal metastasis was assessed with IVIS
imaging system. ALDHhigh cells (105) were
sufficient to establish metastasis in all transplanted mice, in
contrast, no established metastasis was observed in mice
transplanted with 105 unsorted cells (Fig. 3B and C).
Genome-wide gene expression correlates to
ALDH activity of pNET cells
Comprehensive gene expression analysis of
ALDHhigh and unsorted control QGP1 cells was performed
to acquire a CSC gene profile. The microarray data have been
deposited in the Gene Expression Omnibus (GEO) database under
accession number GSE62079. The gene expression changes in 185 probe
sets were detected by microarray analysis (fold-change value >2
between ALDHhigh pNET and control cells). Genes
associated with mesenchymal stem cells, including CD73
(5′-nucleotidase, ecto) and CD109, and
epithelial-mesenchymal transition (EMT), including
caveolin-1 (CAV1) and SLUG (SNAI2), were
overexpressed in ALDHhigh cells.
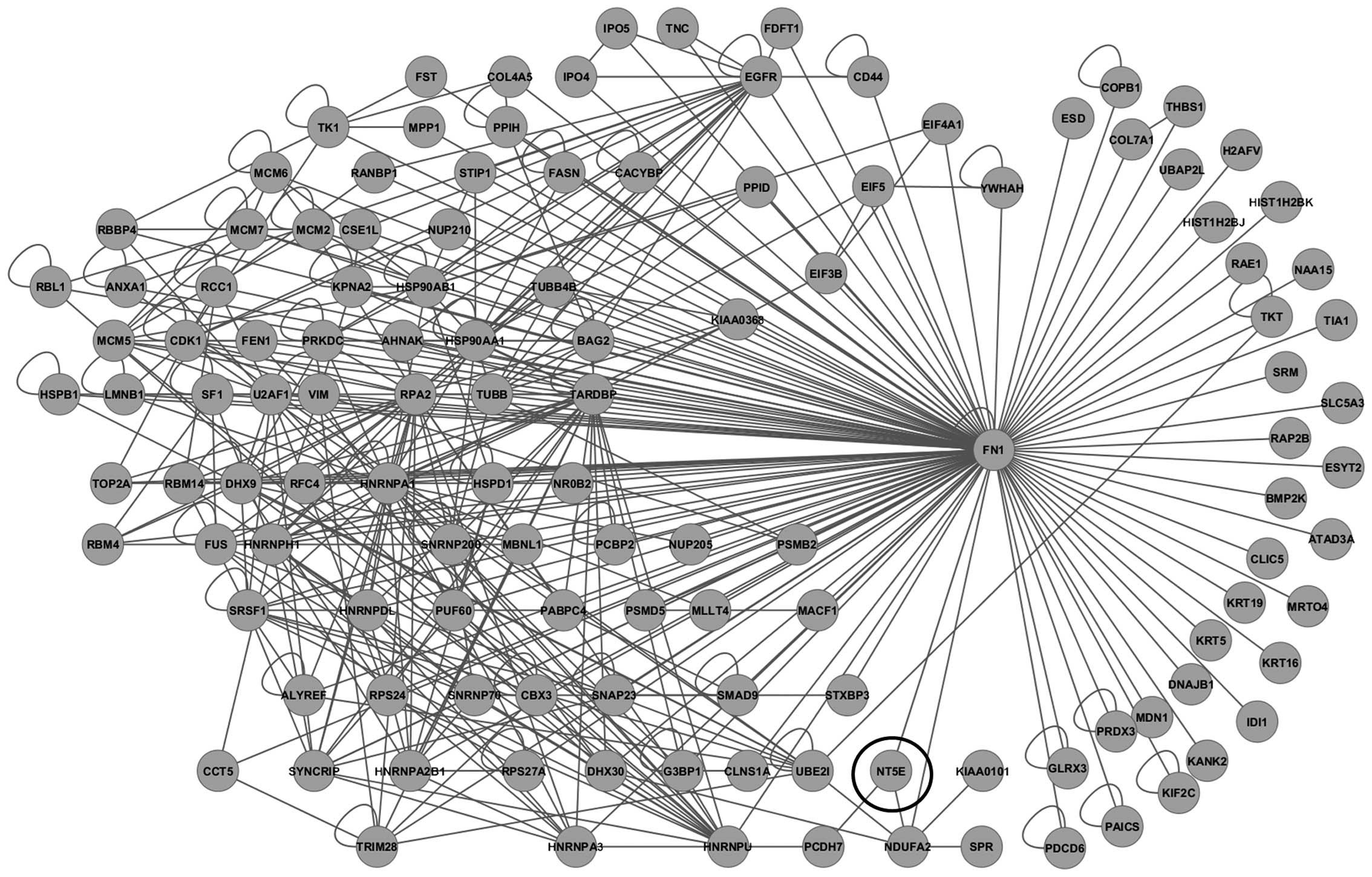
A protein interaction network was then constructed
using 4,438 probe sets with ≥1.3-fold overexpression in
ALDHhigh pNET cells. To more closely investigate
molecular networks associated with CSCs, a sub-network of 3-hop
neighbors from the cell adhesion molecule fibronectin 1
(FN1) genes was generated, including CD73 (Fig. 4), which is 12.332-fold
overexperessed in ALDHhigh cells compared to the
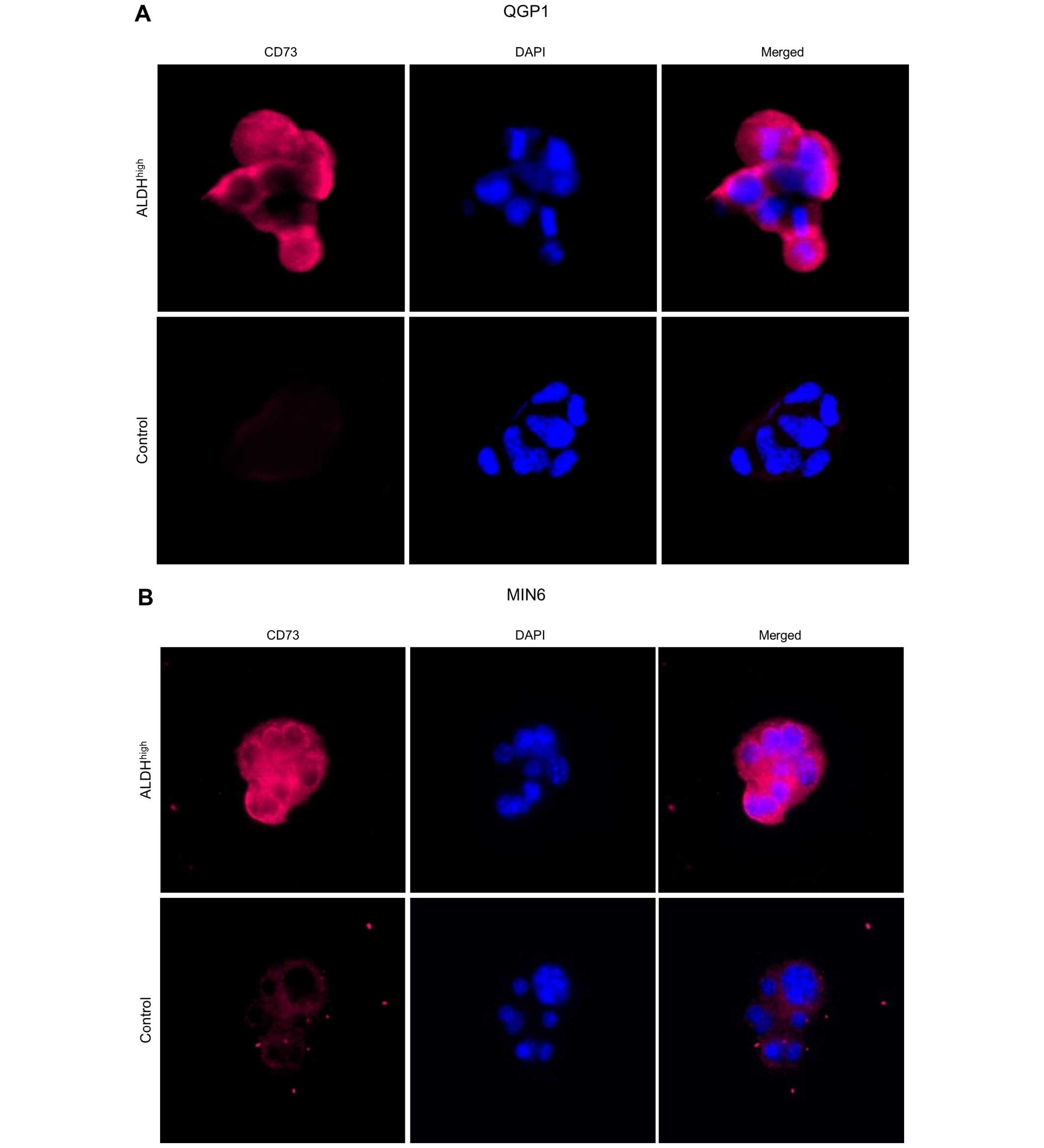
unsorted control cells. On immunocytochemical analysis of QGP1 and
MIN6, CD73 overexpression was observed in ALDHhigh cells
(Fig. 5).
Effects of a CD73 inhibitor on
ALDHhigh cells
We evaluated the efficacy of CD73 inhibition in
ALDHhigh cells of pNETs using APCP, which is a known
small molecule inhibitor of CD73 in vitro and in vivo
(2425). ALDHhigh cells treated with or without APCP
showed no significant difference in proliferation (data was not
shown). To determine whether or not APCP inhibits the CSC
properties of ALDHhigh cells, sphere-forming and scar
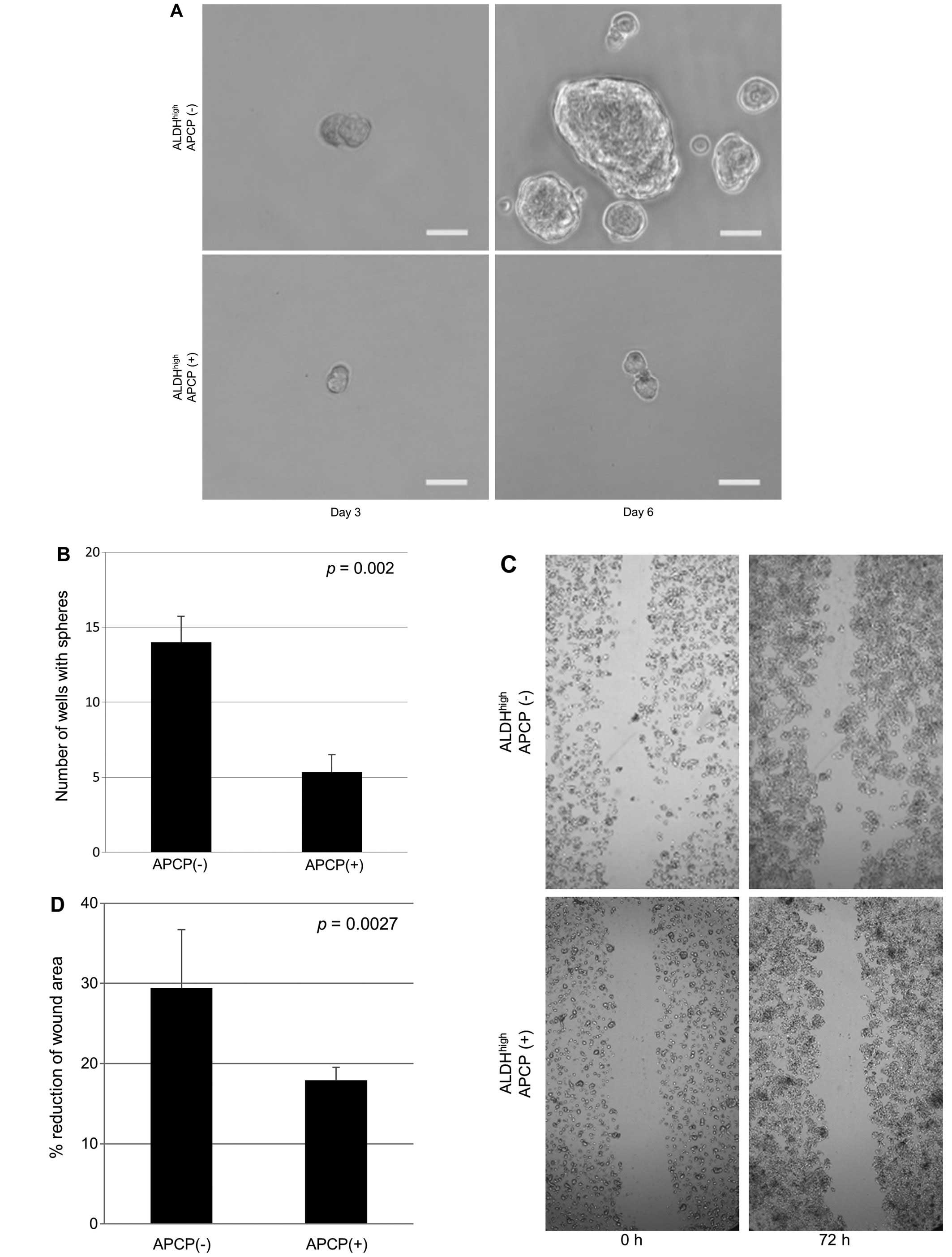
migration assay were performed for QGP1 cells. The results showed
that the number of spheres was 62.2% decreased (Fig. 6A and B; p=0.002) and cell motility
was 39% reduced (Fig. 6C and D;
p=0.027) on exposure to APCP in ALDHhigh cells.
In order to evaluate the in vivo efficacy of
APCP, peritumoral injection of APCP was performed twice a week into
the QGP1 ALDHhigh subcutaneous murine xenograft model.
As shown in Fig. 6E and F, ≤43%
tumor growth inhibition was observed in the group of mice treated
with APCP compared with in the control group on day 49 (p=0.003).
None of the APCP-treated mice showed signs of wasting or other
toxicity compared to control mice. APCP was tolerated at the dose
at which antitumor efficacy was observed.
Expression and clinicopathological
analysis of CD73 in pNET tissues
Immunohistochemical analysis of tissue samples from
31 patients with pNETs was performed to explore the clinical
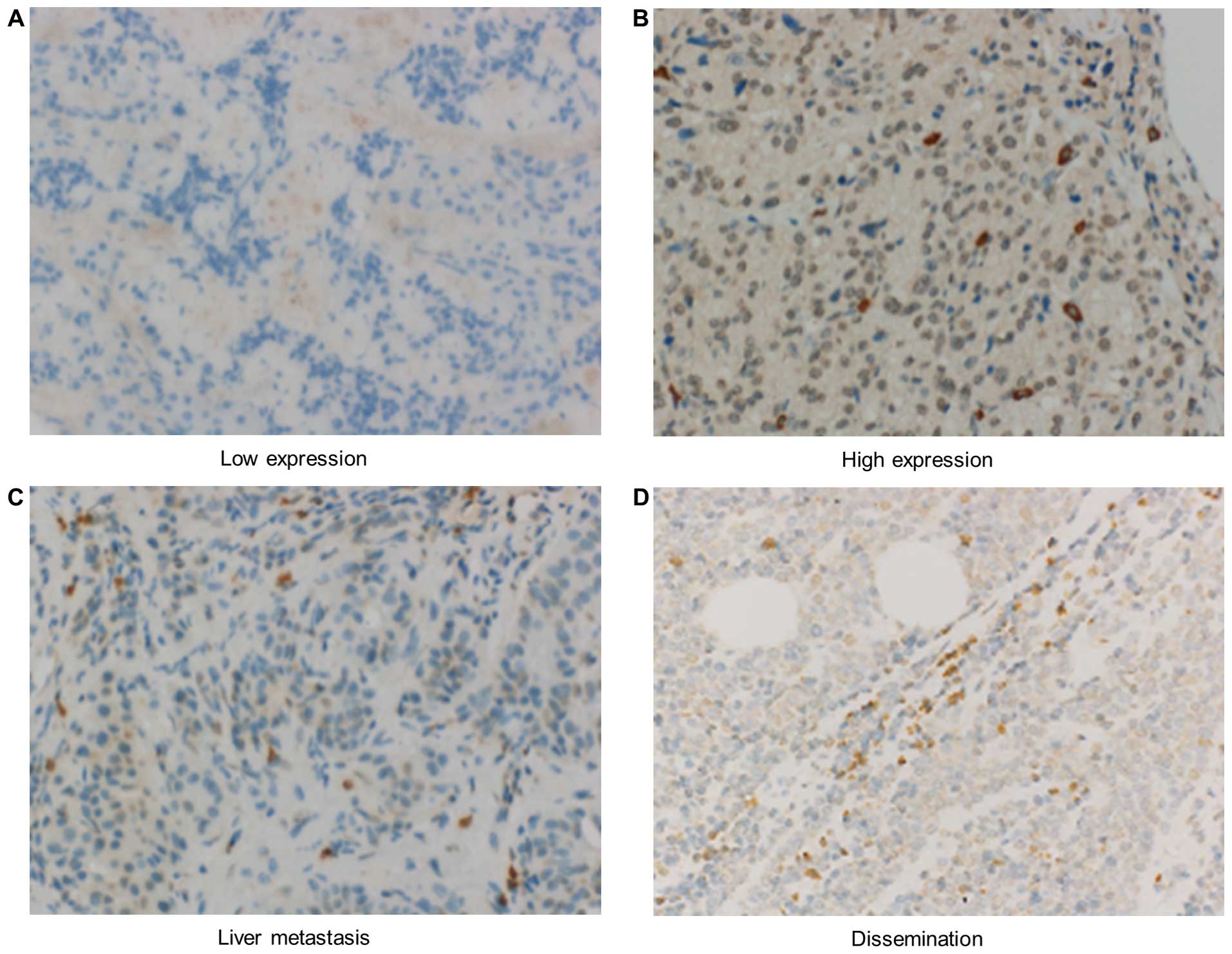
significance of CD73 expression. As shown in Fig. 7, the CD73 protein was mainly
distributed on the cytoplasm of pNET cells. In cases of liver
metastasis and peritoneal dissemination, CD73 overexpression was
observed (Fig. 7C and D).
Consequently, specific overexpression of CD73 was observed in 17
out of 31 cases of primary lesions (54.8%) (Fig. 7A and B). The clinicopathological
significance of CD73 expression was evaluated in the CD73-high
expression group (n=17), and compared to the low expression group
(n=14) of patients with pNETs (Table
II). CD73 expression was significantly correlated with invasion
into adjacent organs (35% vs 0%; p=0.024).
 | Table IIAssociation of different patient and
tumor specific characteristics with CD73 expression in tumor
tissues. |
Table II
Association of different patient and
tumor specific characteristics with CD73 expression in tumor
tissues.
| CD73 low expression
(n=14) | CD73 high
expression (n=17) | p-value |
|---|
| Age | 53.1±14.4 y.o. | 56.4±9.9 y.o. | 0.483 |
| Male | 4 (29%) | 9 (53%) | 0.158 |
| Functionality | 4 (29%) | 6 (35%) | 0.497 |
| Size of primary
lesion | 32.9±33.4 mm | 41.6±49.2 mm | 0.563 |
| Ki-67 index | 6.4±13.2 | 3.3±4.2 | 0.417 |
| Invasion into
adjacent organs | 0 (0%) | 6 (35%) | 0.024a |
| Synchronous LNs
metastasis | 2 (14%) | 4 (24%) | 0.429 |
| Synchronous liver
metastasis | 0 (0%) | 2 (12%) | 0.292 |
| Synchronous
peritoneal metastasis | 0 (0%) | 1 (6%) | 0.356 |
Discussion
Cancer cells include a small subpopulation of CSCs,
which selectively possess tumor initiation, a self-renewal
capability and the ability to give rise to bulk populations of
non-tumorigenic progeny through differentiation (9). In the present study, a small
population of ALDHhigh was observed in both clinical
specimens and cell lines of pNETs, and the ALDHhigh
population showed the stemness feature in vitro. On the
other hand, stem cell markers, such as CD44 and CD133 were not
increased in the ALDHhigh cells, indicating that they
might not overlap completely with these populations (data not
shown). Cellular spheres have been claimed to be enriched with stem
cells in normal neural and mammary tissues, as well as in cancers
(16,17); hence, the sphere formation assay is
a valuable tool for identifying cells with the characteristics of
CSCs (26). On sphere-forming
assay, the number of spheres observed for unsorted bulk cells is
significantly lower than that for ALDHhigh cells
(Fig. 2A–D). As an important
feature, CSC displayed high tumorigenicity (17,18,27),
ALDHhigh cells showed an ~9-fold higher rate of
tumorigenicity than unsorted bulk cells (Fig. 3A and Table I), which suggests that CSCs were
enriched in the ALDHhigh population of pNETs. According
to several studies, CSCs are highly resistant to hypoxia (8,18), a
hypoxic microenvironment maintains CSCs in a stem-like state, which
leads to invasion and metastasis (28,29).
Metastasis and dissemination-initiating cells were found within the
subpopulation of CSCs that expressed the EMT markers (10). In this study, ALDHhigh
cells exhibited higher resistance to hypoxia and higher motility
(Fig. 2E, F and G). Moreover,
these cells exhibited a higher rate of dissemination occurrence in
the murine peritoneal metastasis model (Fig. 3B and C). These findings strongly
suggest that ALDHhigh cells of pNET have CSC features
and a high malignant potential, which might be promising for
therapeutic targeting for pNET treatment.
In order to explore the molecular and biological
characteristics associated with ALDHhigh cells, gene
expression profiling was further evaluated in the pNET cell line,
which revealed mesenchymal stem cell markers and EMT associated
genes were overexpressed in ALDHhigh pNET cell lines. We
focused on CD73, also known as ecto-5′-nucleotidase (ecto-5′-NT),
which is a 12.332-fold overexpressed gene in ALDHhigh
cells. CD73 is a glycosyl-phosphatidylinositol (GPI)-linked
nucleotidase present in cell membrane lipid rafts (24). CD73 is also a well-known surface
marker of mesenchymal stem cells (30,31),
defined as a multipotent population of slow-growing, self-renewing
cells (3). Bussolati et al
reported that the population of CD73-overexpressed cells showed
several stem cell properties: clonogenic ability, capacity of
non-adhesive spheroid formation, bipotent differentiation potential
into epithelial and endothelial cell types, and in vivo
generation of serially transplantable carcinomas in renal cancer
cells (32). Moreover, the
expression of CD73, CD39 and adenosine receptors is induced by
hypoxia, that is one of the most essential stemness features
(33).
Overexpression of CD73 was observed in various human
carcinomas, associated with tumor neovascularization, invasiveness,
metastasis, and with shorter patient survival time (24). In cancer tissues, CD73 functions as
a rate-limiting enzyme, together with CD39, in the generation of
extracellular adenosine (34).
CD39 hydrolyzes adenosine triphosphate (ATP) and adenosine
diphosphate (ADP) to adenosine monophosphate (AMP), which is
further hydrolyzed to adenosine by CD73 (34). Chronically increased adenosine
leads to immune tolerance by accumulating in the tumor environment
and stroma, and contributes to the generation of an angiogenic and
matrix remodeling environment that is suitable for cancer growth.
Adenosine not only functions in the tumor micro-environment, but is
also involved in the regulation of proliferation, differentiation
and apoptosis of cancer cells (35). The adenosine receptor activated by
extracellular adenosine leads to reduction of cell adhesion and to
subsequent cell scattering, and these responses promote metastasis
and dissemination (35). In this
study, CD73 inhibition by a small molecule inhibitor impaired the
stemness property of ALDHhigh cells in pNETs including
sphere-formation ability and cell motility in vitro, and
significantly reduced tumor growth in the murine xenograft model of
pNET.
In conclusion, the ALDHhigh cell
population of pNET displayed CSC characteristics in vitro
and in vivo. Furthermore, CD73 might play a critical role in
the maintenance and progression of ALDHhigh cells, and
be promising as a novel therapeutical target for pNETs. More
recently, CD73 was identified as a potential biomarker of anti-PD-1
immune checkpoint therapy (36).
Urgent studies on CD73 are expected to confirm that it is a target
for the treatment of pNETs.
References
|
1
|
Yao JC, Eisner MP, Leary C, Dagohoy C,
Phan A, Rashid A, Hassan M and Evans DB: Population-based study of
islet cell carcinoma. Ann Surg Oncol. 14:3492–3500. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al:
One hundred years after ‘carcinoid’: Epidemiology of and prognostic
factors for neuroendocrine tumors in 35,825 cases in the United
States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song L, Webb NE, Song Y and Tuan RS:
Identification and functional analysis of candidate genes
regulating mesenchymal stem cell self-renewal and multipotency.
Stem Cells. 24:1707–1718. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: RAD001 in Advanced Neuroendocrine Tumors, Third Trial
(RADIANT-3) Study Group: Everolimus for advanced pancreatic
neuroendocrine tumors. N Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strosberg JR, Cheema A, Weber JM, Ghayouri
M, Han G, Hodul PJ and Kvols LK: Relapse-free survival in patients
with nonmetastatic, surgically resected pancreatic neuroendocrine
tumors: An analysis of the AJCC and ENETS staging classifications.
Ann Surg. 256:321–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Herder WW: Gastroenteropancreatic
neuroendocrine tumors (GEP-NETs). Best Pract Res Clin
Gastroenterol. 26:689–690. 2012. View Article : Google Scholar
|
|
7
|
Miletti-González KE, Chen S, Muthukumaran
N, Saglimbeni GN, Wu X, Yang J, Apolito K, Shih WJ, Hait WN and
Rodríguez-Rodríguez L: The CD44 receptor interacts with
P-glycoprotein to promote cell migration and invasion in cancer.
Cancer Res. 65:6660–6667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baccelli I and Trumpp A: The evolving
concept of cancer and metastasis stem cells. J Cell Biol.
198:281–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma I and Allan AL: The role of human
aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell
Rev. 7:292–306. 2011. View Article : Google Scholar
|
|
12
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
13
|
Luo Y, Dallaglio K, Chen Y, Robinson WA,
Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris
DA, et al: ALDH1A isozymes are markers of human melanoma stem cells
and potential therapeutic targets. Stem Cells. 30:2100–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gaur P, Sceusi EL, Samuel S, Xia L, Fan F,
Zhou Y, Lu J, Tozzi F, Lopez-Berestein G, Vivas-Mejia P, et al:
Identification of cancer stem cells in human gastrointestinal
carcinoid and neuroendocrine tumors. Gastroenterology.
141:1728–1737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyazaki J, Araki K, Yamato E, Ikegami H,
Asano T, Shibasaki Y, Oka Y and Yamamura K: Establishment of a
pancreatic beta cell line that retains glucose-inducible insulin
secretion: Special reference to expression of glucose transporter
isoforms. Endocrinology. 127:126–132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogawa K, Tanaka S, Matsumura S, Murakata
A, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, Tanabe M, et al:
EpCAM-targeted therapy for human hepatocellular carcinoma. Ann Surg
Oncol. 21:1314–1322. 2014. View Article : Google Scholar
|
|
17
|
Adikrisna R, Tanaka S, Muramatsu S, Aihara
A, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, Yamaoka S, et al:
Identification of pancreatic cancer stem cells and selective
toxicity of chemotherapeutic agents. Gastroenterology.
143:234–45.e7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muramatsu S, Tanaka S, Mogushi K,
Adikrisna R, Aihara A, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N,
et al: Visualization of stem cell features in human hepatocellular
carcinoma reveals in vivo significance of tumor-host interaction
and clinical course. Hepatology. 58:218–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshitake K, Tanaka S, Mogushi K, Aihara
A, Murakata A, Matsumura S, Mitsunori Y, Yasen M, Ban D, Noguchi N,
et al: Importin-α1 as a novel prognostic target for hepatocellular
carcinoma. Ann Surg Oncol. 18:2093–2103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishizawa K, Rasheed ZA, Karisch R, Wang Q,
Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N,
Rajeshkumar NV, et al: Tumor-initiating cells are rare in many
human tumors. Cell Stem Cell. 7:279–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka S, Pero SC, Taguchi K, Shimada M,
Mori M, Krag DN and Arii S: Specific peptide ligand for Grb7 signal
transduction protein and pancreatic cancer metastasis. J Natl
Cancer Inst. 98:491–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida Y, Takahashi K, Okita K, Ichisaka
T and Yamanaka S: Hypoxia enhances the generation of induced
pluripotent stem cells. Cell Stem Cell. 5:237–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Bao S, Wu Q, Wang H, Eyler C,
Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al:
Hypoxia-inducible factors regulate tumorigenic capacity of glioma
stem cells. Cancer Cell. 15:501–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang B: CD73: A novel target for cancer
immunotherapy. Cancer Res. 70:6407–6411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stagg J, Divisekera U, McLaughlin N,
Sharkey J, Pommey S, Denoyer D, Dwyer KM and Smyth MJ: Anti-CD73
antibody therapy inhibits breast tumor growth and metastasis. Proc
Natl Acad Sci USA. 107:1547–1552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chojnacki A and Weiss S: Production of
neurons, astrocytes and oligodendrocytes from mammalian CNS stem
cells. Nat Protoc. 3:935–940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of
CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahmood A, Harkness L, Abdallah BM,
Elsafadi M, Al-Nbaheen MS, Aldahmash A and Kassem M: Derivation of
stromal (skeletal and mesenchymal) stem-like cells from human
embryonic stem cells. Stem Cells Dev. 21:3114–3124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: mesenchymal stem cells: their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bussolati B, Bruno S, Grange C, Ferrando U
and Camussi G: Identification of a tumor-initiating stem cell
population in human renal carcinomas. FASEB J. 22:3696–3705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eltzschig HK, Köhler D, Eckle T, Kong T,
Robson SC and Colgan SP: Central role of Sp1-regulated CD39 in
hypoxia/ischemia protection. Blood. 113:224–232. 2009. View Article : Google Scholar :
|
|
34
|
Stagg J and Smyth MJ: Extracellular
adenosine triphosphate and adenosine in cancer. Oncogene.
29:5346–5358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Antonioli L, Blandizzi C, Pacher P and
Haskó G: Immunity, inflammation and cancer: A leading role for
adenosine. Nat Rev Cancer. 13:842–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beavis PA, Slaney CY, Milenkovski N,
Henderson MA, Loi S, Stagg J, Kershaw MH and Darcy PK: CD73: A
potential biomarker for anti-PD-1 therapy. OncoImmunology.
4:e10466752015. View Article : Google Scholar : PubMed/NCBI
|