Introduction
Colon cancer, also known as colorectal cancer, is
one of the leading causes of cancer deaths in men and women
worldwide (1). In colon cancer,
adjuvant chemotherapy after the primary surgical intervention is
suggested to be beneficial in eradicating the visually undetectable
disseminated small tumors and improving the prognosis (2). The nucleic acid antimetabolite
5-fluorouracil (5-FU) has mainly been used for adjuvant
chemotherapy in colon cancer treatment. However, adjuvant
chemotherapeutic clinical studies using 5-FU alone or in
combination with leucovorin or other drugs have shown limited
effectiveness (3,4). For example, a reduced risk of
recurrence was reported, but not of death in patients with stage II
cancer, and it was not effective in patients with stage III cancers
(3,4). Therefore, new efficacious drugs are
required to improve the outcome of adjuvant chemotherapy in colon
cancer treatment.
Recently, we developed a novel drug screening system
using a nanoimprinting 3-dimensional (3D) culture that creates
multicellular spheroids mimicking in vivo conditions
(5,6) (Fig.
1A). This 3D culture uses a microplate that possesses
well-regulated inorganic nanoscale indented patterns printed on the
base using nanoimprinting technology (nanoculture plate, NCP). The
nanoimprinted pattern acts as a scaffold to which cultured cells
adhered, albeit with less physical contact with the culture plates
than with traditional 2D plates. NCPs were shown to facilitate
spontaneous tumor cell migration, intercellular adhesion, and
formation of 3D multicellular spheroids similar to in vivo
tumors while maintaining cellular proliferation and viability
(5). In addition, we revealed that
the high-throughput drug screening using the nanoimprinting 3D
culture efficiently identified effective drugs for the treatment of
xenograft tumors subcutaneously transplanted in vivo,
compared to 2D culture (6). Here,
we noted that the nanoimprinting 3D culture forms adherent and
active spheroids, which have a considerable resemblance to the
disease condition of disseminated small tumors in vivo and
subcutaneous xeno-grafts. Therefore, in this study, we performed
drug selection using the nanoimprinting 3D culture to identify an
effective drug against disseminated small tumors, using a human
colon cancer model of HCT116-red fluorescent protein (RFP)
cells.
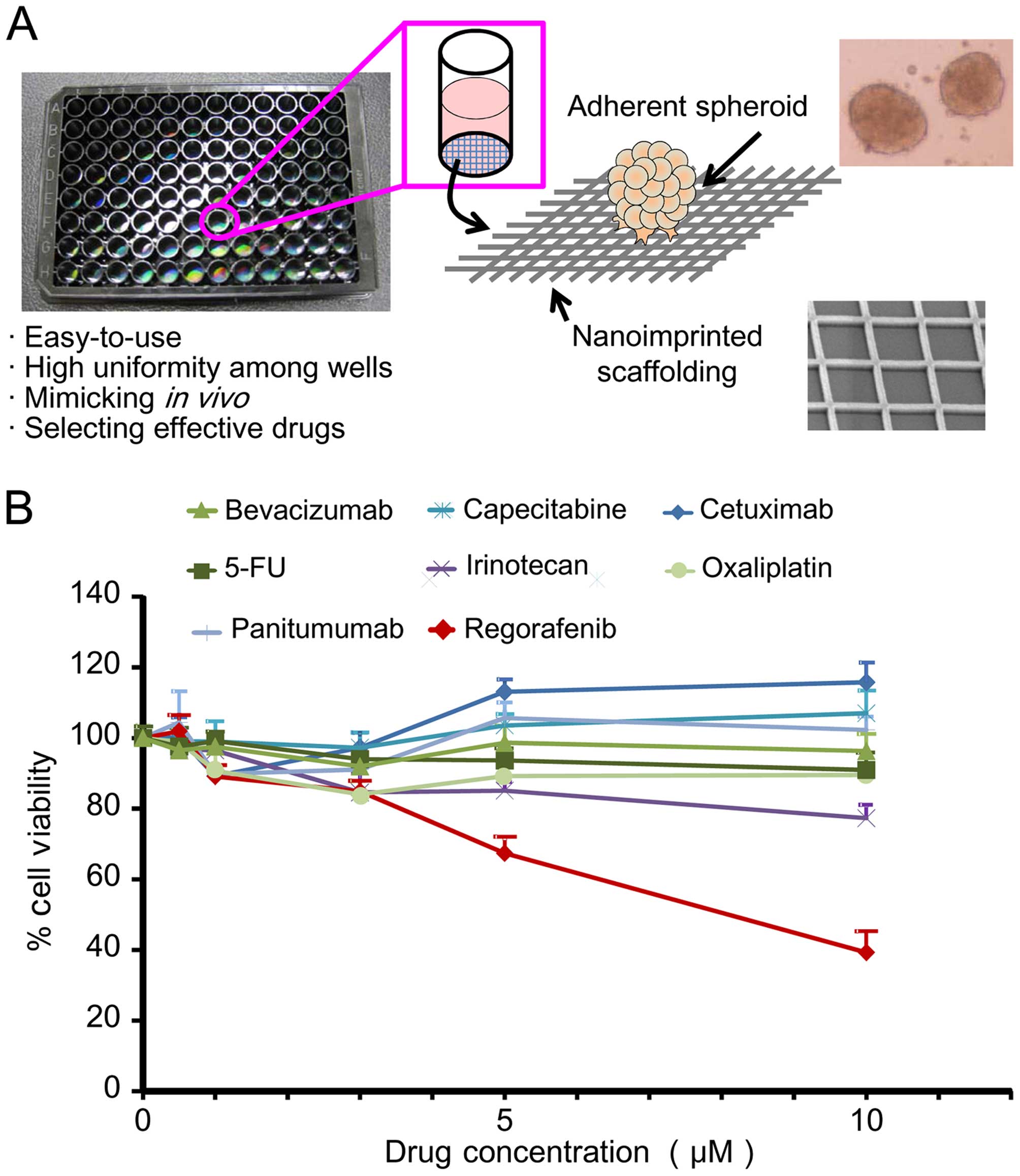 | Figure 1Drug selection using a nanoimprinting
3D culture screening system. (A) Schematic representation of
screening system with nanoimprinting 3D culture (6). NCP 3D culture system consists of
inorganic nanoscale scaffolding printed on transparent synthetic
resinous bases using a nanoimprinting technique, which reproduces
adherent cancer spheroids with characteristics resembling in
vivo tumors. (B) Effect of drugs on HCT116-RFP spheroids in
nanoimprinting 3D culture. Cells were treated with 8 clinically
used antitumor agents for colon cancer including bevacizumab,
capecitabine, cetuximab, 5-FU, irinotecan, oxaliplatin, panitumumab
and regorafenib was performed. Data represent the percentage (%)
cell viability after administration of each drug at 0, 0.5, 1, 3, 5
and 10 μM relative to control (0 μM). Data are mean ± SD; n=6,
P<0.05. |
Materials and methods
Cell cultivation
Human colon carcinoma cell line HCT116 (CCL-247,
American Type Cell Collection, ATCC) were used in this study.
HCT116 cells were stably transfected with RFP lentivirus
(Lenti-Red, LG502, Biogenova, Rockville, MD, USA) following the
manufacturer's protocol. Then, a clone that strongly expressed the
RFP was established by limiting dilution and denoted as HCT116-RFP
cells. The cells were incubated in a humidified atmosphere of 5%
CO2 at 37°C in Dulbecco's modified Eagle's medium (DMEM,
11995-065, Invitrogen, Carlsbad, CA, USA), supplemented with 10%
fetal bovine serum (FBS), and antibiotics as culture medium.
Exponentially growing cells were used in the study, and were
detached from the plates with trypsin. The number of viable cells
was determined using the trypan blue dye exclusion method.
In vitro drug selection with NCPs
We used NCPs (96-wells, SCIVAX Life Sciences,
Kawasaki, Japan) with a nanoscale square grid pattern consisting of
500-nm line width as well as 1 and 2 μm line depth and spacing,
respectively, printed on the transparent synthetic resinous bases,
using the previously reported nanoimprinting technique(5). HCT116-RFP cells were seeded in the
96-well NCPs at a density of 1×104 cells/100 μl of
medium and incubated in a humidified atmosphere of 5%
CO2 at 37°C for 7 days to construct the spheroids. For
in vitro drug selection, we used 8 antitumor agents that are
clinically used for the treatment of patients with colon cancer
including bevacizumab, capecitabine, cetuximab, 5-FU, irinotecan,
oxaliplatin, panitumumab, and regorafenib (details shown in
Table I). In the experiments,
drugs were added to each well of the NCPs at a final concentration
of 10, 5, 3, 1, 0.5 and 0 μM in the medium and the doses were
chosen based on previous studies (6–16).
The spheroids were incubated for 3 days (n=6) in a CO2
incubator. During the incubation, the formation of the spheroid
morphology was monitored using time-lapse analysis by acquiring
images every 2 h at ×10 magnification using an IncuCyte imaging
system (Essen Bioscience, Ann Arbor, MI, USA). After a 3-day
incubation, the cell viability (percentage of control cells treated
with 0 μM drug) was evaluated by quantifying the levels of cellular
adenosine-5′-triphosphate (ATP) using the CellTiter-Glo Luminescent
Cell Viability assay (Promega, Madison, WI, USA) (5). The luminescence signals were detected
using a microplate reader (SpectraMax M5, Molecular Devices,
Sunnyvale, CA, USA).
 | Table IAnticancer drugs used in this
study. |
Table I
Anticancer drugs used in this
study.
| Drug | Target | Source | Location |
|---|
| Capecitabine | Nucleic acid | Sigma-Aldrich | St. Louis, MO,
USA |
| 5-FU | Nucleic acid | Wako | Osaka, Japan |
| Irinotecan | Topo I | Sigma-Aldrich | St. Louis, MO,
USA |
| Oxaliplatin | DNA | Tocris
Bioscience | Bristol, UK |
| Cetuximab
(Erbitux) | EGFR | Merck Serono | Darmstadt,
Germany |
| Panitumumab
(Vectibix) | EGFR | Takeda | Osaka, Japan |
| Bevacizumab
(Avastin) | VEGF | Chugai | Tokyo, Japan |
| Regorafenib
(Stivarga) | Multikinase | Bayer
HealthCare | Leverkusen,
Germany |
Ethics statement
Animal experiments in this study were carried out in
strict accordance with the recommendations of the Guide for the
Care and Use of Laboratory Animals of the National Institute of
Radiological Sciences, Japan. The protocol was approved by the
Animal Ethics Committee of the National Institute of Radiological
Sciences, Japan (permit no. M40-01). All efforts were made to
minimize suffering of the animals in the experiments.
In vivo antitumor study
We examined the in vivo effects of
regorafenib, which was selected in the nanoimprinting 3D culture,
using a mouse model of HCT116-RFP disseminated small tumors. The
model was established in BALB/c female nude mice (6-week-old with
uniform body weight) obtained from the Japan SLC (Shidzuoka,
Japan). A purified diet (AIN-93M, TestDiet, St. Louis, MO, USA) was
used during the experiments to avoid the effect of diet on
abdominal autofluorescence detected by in vivo fluorescence
microscopic imaging. Before the experiments, the mice were
acclimatized for >1 week, and then intraperitoneally (i.p.)
injected with 3×106 HCT116-RFP cells suspended in 500 μl
phosphate-buffered saline (PBS). On day 5 after inoculation (day 0
of treatment), mice were randomly divided into 3 groups of 5
animals each including i) control, ii) regorafenib, and iii) 5-FU
(as a reference drug). Mice were administered regorafenib (orally,
p.o.) or 5-FU (i.p.) at doses of 30 and 8 mg/kg/day, respectively,
on days 1, 2, 3, 4, 5, 15, 16, 17, 18 and 19, with interruption of
drug administration on days 6–14 and 20–28. The treatment dose and
administration route for each drug were determined based on
previous studies (17–20). Each drug was dissolved in 30 μl of
dimethyl sulfoxide (DMSO), and then further diluted with 70 μl of
saline just before injecting. As a control, an equivalent volume of
the vehicle was administered p.o. similar to regorafenib treatment.
The mice were weighed, and their general conditions were monitored
weekly. Therapeutic effects were evaluated on day 28 using
3′-[18F]fluoro-3′-deoxythymidine (FLT)-positron emission
tomography (PET), which is a non-invasive method that is reportedly
useful for evaluating the effects of chemotherapy and detecting
peritoneal seeding nodules of colon cancer in clinical studies
(21,22). After the FLT-PET scan, the
abdominal cavity was opened and then fluorescence microscopy images
were acquired to confirm tumor development and detect small
tumors.
FLT-PET and fluorescence microscopy
imaging
FLT was synthesized as reported previously (23) and the radiochemical purity was >
99%. Each mouse was intravenously injected with 3.7 MBq of FLT
dissolved in 100 μl of saline via the tail vein. Ten minute
emission scans were performed 1 h after the FLT injection using a
small animal PET system (Inveon, Siemens Medical Solutions,
Malvern, PA, USA), while the mice were under 1.5–2% isoflurane
anesthesia. Body temperature was maintained with a heat pump during
the scans. The images were reconstructed using a 3D maximum a
posteriori with the Inveon Acquisition Workplace software (Siemens
Medical Solutions). Tracer uptake was quantified as the standard
uptake value (SUV) of each voxel within the mouse abdominal area
except for the urinary bladder using the ASIPro software (CTI
Molecular Imaging/Siemens) as described previously (24,25).
In this study, the tumor volume was calculated from the FLT-PET
images as the sum of the volume of any voxel with counts greater
than the fixed threshold value (SUV = 1), which was the peak
activity for the abdominal area of normal mice in the FLT-PET
images.
The fluorescent images of the open mouse abdominal
cavities were acquired using a fluorescent microscope (MZ16F,
Leica, Wetzlar, Germany) equipped with a camera system (DFC310FX,
Leica, ×0.71). Tiling images were constructed using Leica
Application Suite software (Leica).
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Cell viability curves in in vitro drug selection were
analyzed using two-way analysis of variance (ANOVA). Statistical
significance was determined using ANOVA along with Bonferroni post
hoc test for comparison of estimated tumor volume in FLT-PET study.
P-values <0.05 were considered statistically significant.
Results
In vitro drug selection with a
nanoimprinting 3D culture
First, we performed in vitro drug selection
using a nanoimprinting 3D culture with HCT116-RFP cells to
determine the most effective candidate drug among 8 clinically used
antitumor agents. Fig. 1B shows
the dose-dependent effects of each drug in the HCT116-RFP spheroid
viability assay. From the results, 7 drugs including bevacizumab,
capecitabine, cetuximab, 5-FU, irinotecan, oxaliplatin, and
panitumumab did not show the apparent decrease of the cell
viability at any measured drug concentration. In contrast,
regorafenib showed decrease of the cell viability by ≤39% at 10 μM
with a half-maximal inhibitory concentration (IC50) of
8.1 μM. The statistical analysis on the cell viability curves
showed significant inhibition in a regorafenib treatment group
compared to the other treatment groups (P<0.05). Therefore, the
in vitro drug selection using the nanoimprinting 3D culture
of HCT116-RFP determined regorafenib as the most effective drug
among the typical clinical antitumor agents evaluated in this
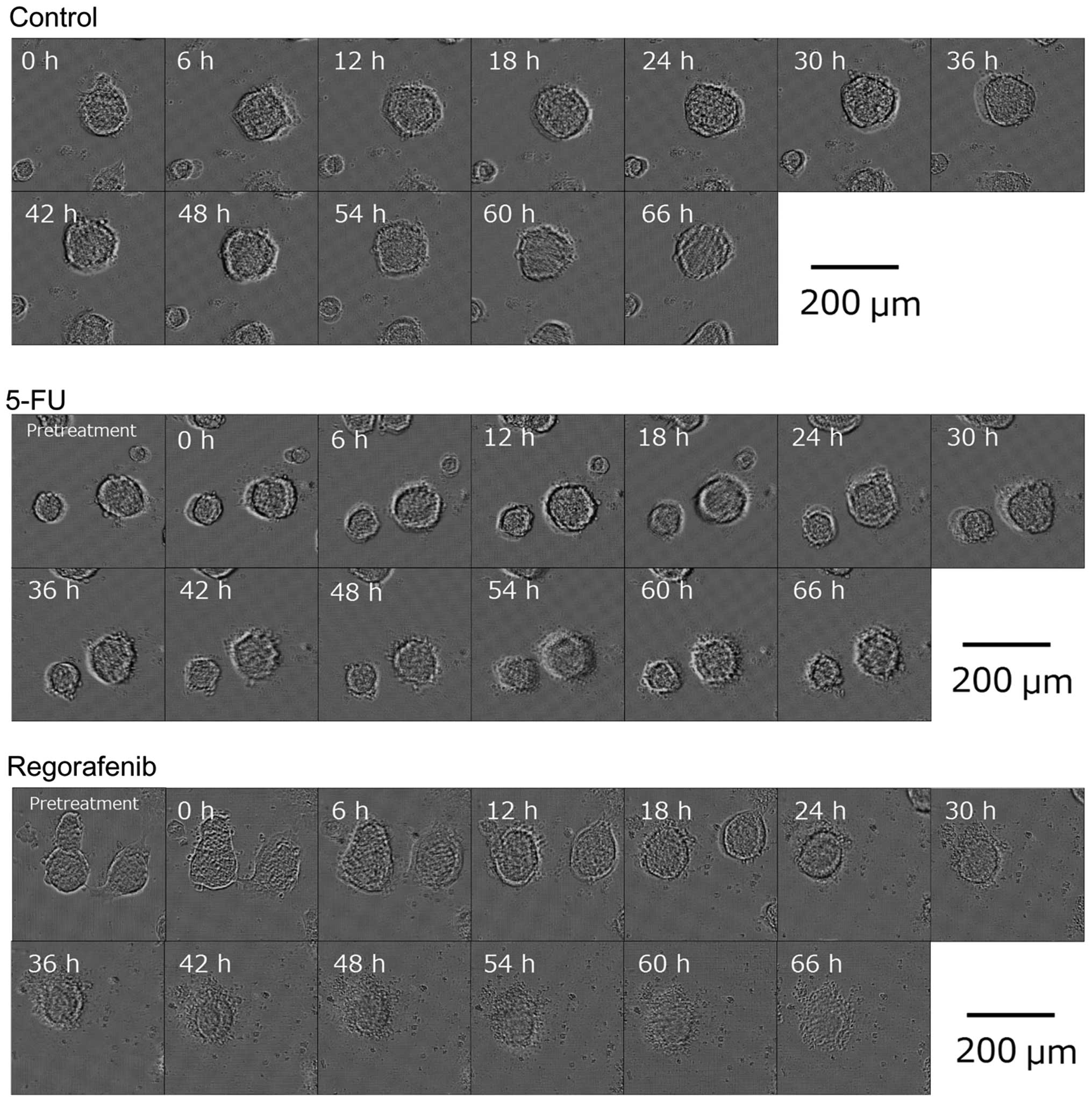
study. Fig. 2 shows representative
time-lapse microscopic images acquired during incubation with 10 μM
of regorafenib, compared to those with 0 μM control and 10 μM 5-FU
as the reference drug. The results demonstrate that spheroids
treated with regorafenib started to collapse around 24 h after drug
administration while the control and 10 μM 5-FU-treated spheroids
did not show clear morphological changes.
In vivo effects of regorafenib against
disseminated tumors
Next, we tested the antitumor effects of regorafenib
in a mouse model of HCT116-RFP-induced intraperitoneally
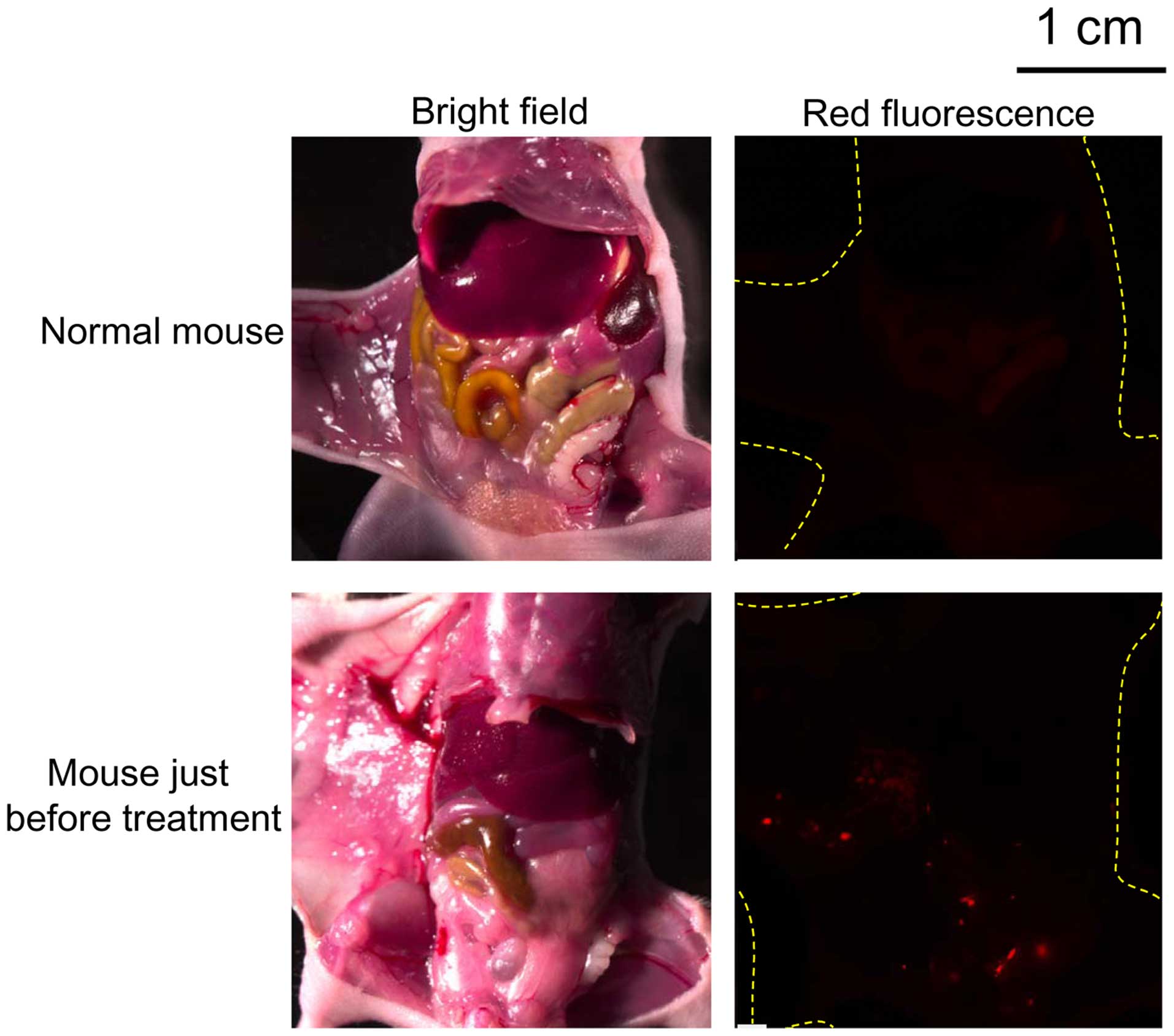
disseminated tumors. Prior to the study, we confirmed that the
model used in this study had developed intraperitoneally
disseminated small HCT116-RFP tumors (1 mm or less) before drug
administration (day 0 of the treatment), which were difficult to
identify by the naked eye (Fig.
3). Using this model, we examined the effects of treatment with
regorafenib and compared it to that of the untreated control and
5-FU-treated group. The evaluation was performed using the FLT-PET
and fluorescence microscopy imaging.
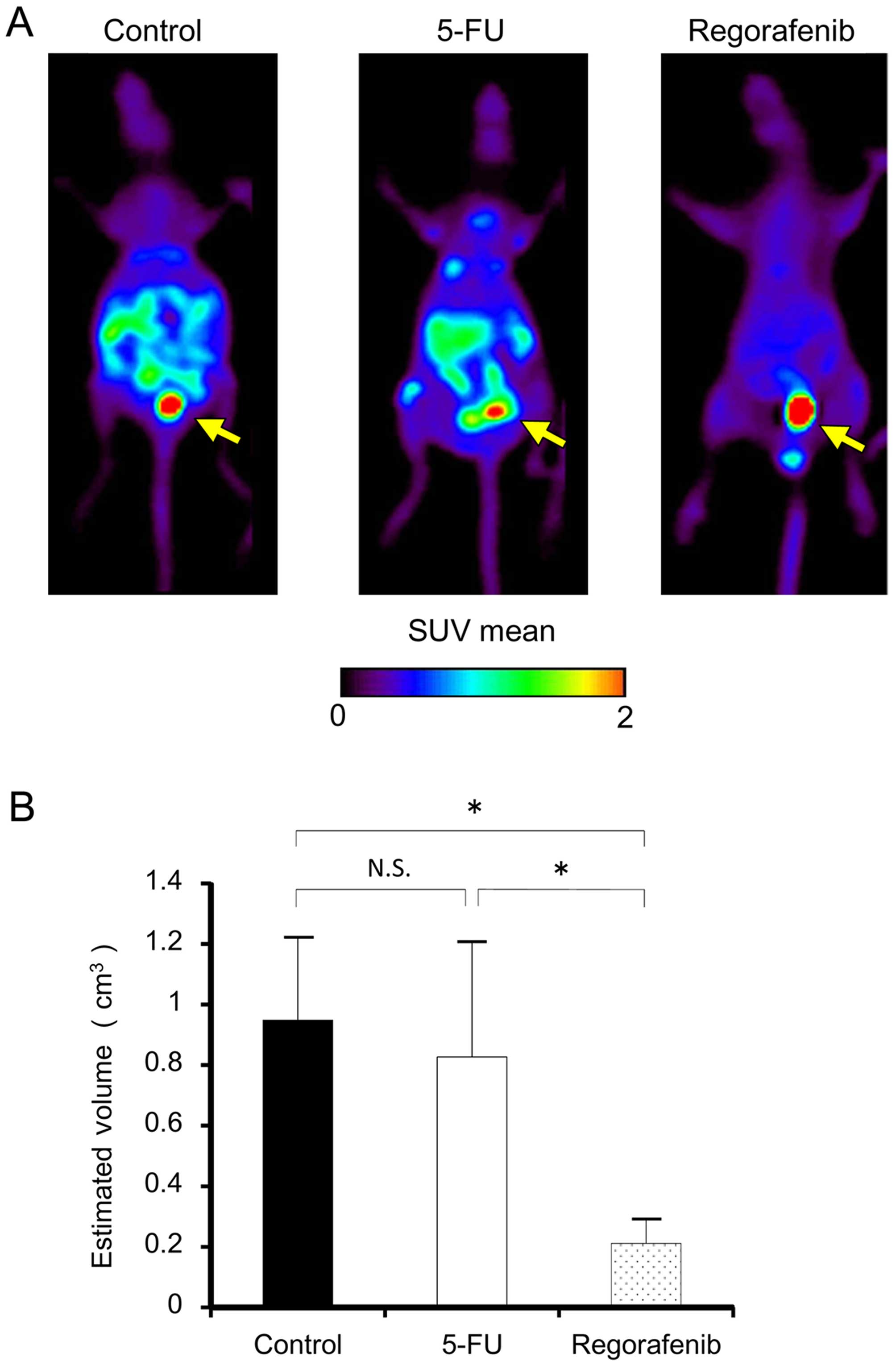
Fig. 4A shows
representative FLT-PET images of the control, 5-FU- and
regorafenib-treated mice on day 28. Control and 5-FU treatment
showed apparent FLT uptake in the mouse abdominal cavity while
regorafenib treatment showed low uptake. Fig. 4B shows the summary of the estimated
tumor volume calculated from the FLT-PET images. The regorafenib
treatment group showed significantly lower estimated tumor volume
than the control and 5-FU groups did (P<0.05). There was no
significant difference between the control and 5-FU groups in
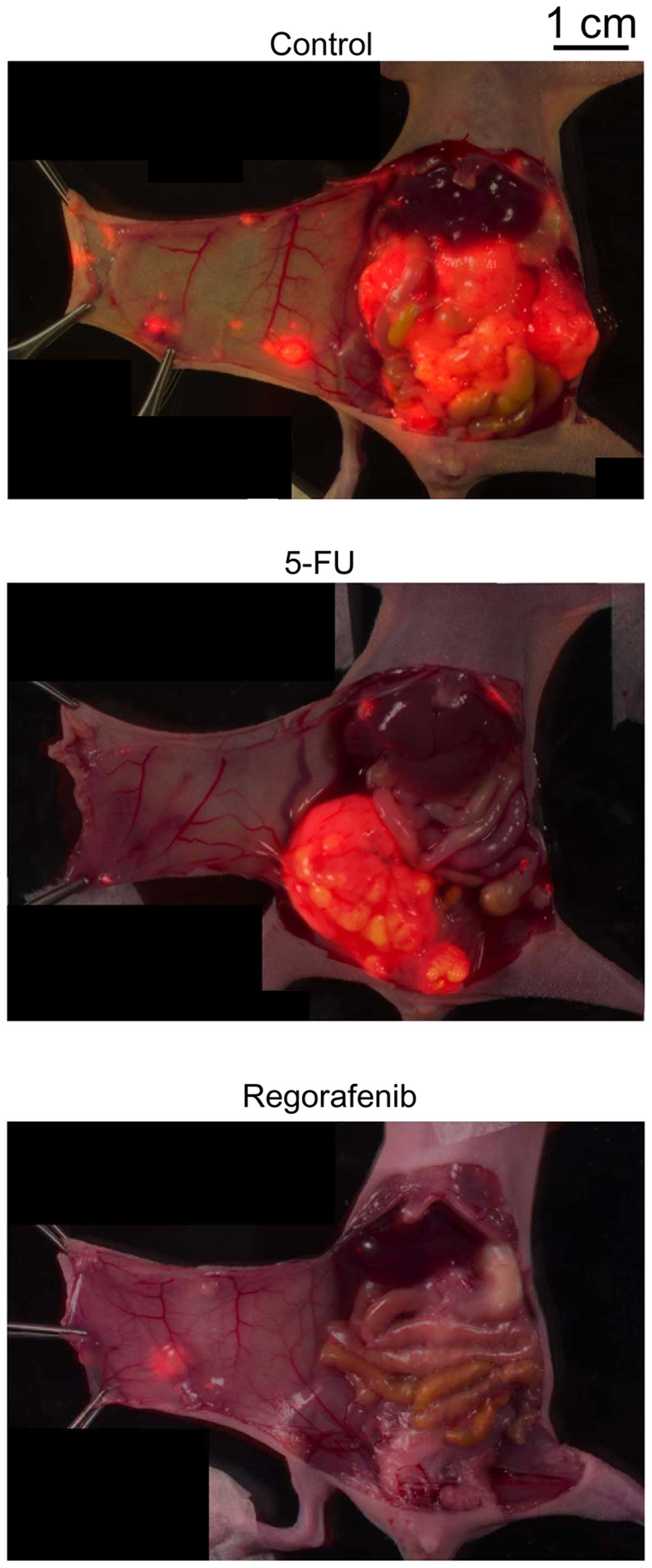
estimated tumor volume from the FLT-PET analysis. Fig. 5 shows representative fluorescence
microscopy images of the opened abdominal cavities of the mice
acquired after the FLT-PET scan. Highly grown and spreading small
tumors are evident in the abdominal cavity of the control and 5-FU
groups and less marked in the regorafenib group. Body weight loss
>10% of initial body weight and apparent changes in physical
condition were not observed in the mice during regorafenib
treatment.
Discussion
In this study, we found that regorafenib was the
most effective drug out of 8 clinically used antitumor agents for
colon cancer, using drug selection with a nanoimprinting 3D culture
of colon cancer HCT116-RFP cells and demonstrated that this drug
was efficacious against the in vivo disseminated small tumor
model. In colon cancer treatment, surgery is usually performed as
the primary treatment intervention (2). In most patients with colon cancer,
the diagnosis is made at a stage when the tumor tissue can be
surgically removed. However, tumor recurrence is likely to occur
due to undetectable residual cancer cells in the affected patients
(2). In clinical practice,
patients with stage II and III colon cancers who are considered at
high risk for recurrence are administered adjuvant chemotherapy
(2,4). 5-FU-based chemotherapy (alone or in
combination with leucovorin or other drugs) is used as a standard
adjuvant chemotherapy for colon cancer (3,4);
however, the efficacy of adjuvant chemotherapy with 5-FU is
reportedly limited and the benefit is still under contention
(3,4). In this study, we showed the efficacy
of regorafenib against disseminated small tumors in a HCT116-RFP
model. Though our findings were limited to the HCT116-RFP cell
line, this study demonstrated the therapeutic potential of
regorafenib as an adjuvant chemotherapy agent in colon cancer
treatment.
In this study, we used a mouse model with
intraperitoneally disseminated small tumors. In clinical settings,
peritoneal metastasis is one of the major types of colon cancer
metastasis observed at the time of primary resectioning along with
liver metastasis (26,27). Importantly, a higher incidence of
peritoneal metastasis is reported to be observed in cases of
recurrent cancer after surgery (60%), than of liver metastasis
(50%). In addition, this phenomenon is considered to occur because
of the dissemination of exfoliated primary malignancy into the free
peritoneal space (26).
Consequently, small undetectable tumors that are intraperitoneally
disseminated before as well as after surgery are important targets
in the radical cure of colon cancer. Therefore, the model used in
this study is appropriate for determining the efficacy and benefits
of an adjuvant chemotherapy agent for colon cancer treatment.
Regorafenib is an orally administered small
molecular multikinase inhibitor that targets tumor cells,
vasculature, and tumor microenvironment (8,28).
To date, preclinical studies with orthotopic colon cancer
xenografts have revealed that regorafenib reduced tumor
angiogenesis and inhibited tumor growth (20). In recent years, multinational phase
III trials (CORRECT and CONCUR) have been conducted and revealed
that regorafenib therapy showed survival benefits in metastatic
colorectal cancer, which had progressed after standard therapies
(29,30). These preclinical and clinical
studies showed that regorafenib is effective in primary and
metastatic solid colon cancer tumors even when they had developed
resistance to standard treatments. Furthermore, the present study
showed that regorafenib is effective against disseminated
undetectable small colon cancer. This suggests that regorafenib
could be useful not only for the treatment of drug-resistant solid
tumors but also for prophylactic use as an adjuvant chemotherapy
agent after surgery by killing undetectable disseminated small
tumors and inhibiting recurrence of colon cancer. From our in
vivo study, side effects were not observed in the drug-treated
mice. In clinical trials, adverse events such as hand-foot skin
reaction, fatigue, diarrhea, and hypertension were observed but
were manageable (31). Therefore,
the side effects of regorafenib are considered minor or manageable,
and this agent could be potentially beneficial for colon cancer
treatment.
In this study, a novel screening system involving
nanoimprinting 3D culture was used to select an effective drug
against disseminated small tumors of colon cancer HCT116-RFP cells.
This system consisted of nanoscale rectangular grid patterns
printed on transparent synthetic resinous bases using a
nanoimprinting technique and provided the optimal scaffold for
cultured cancer cells to adhere to, actively migrate, and aggregate
to form spheroids (5,6). These characteristics of the
nanoimprinting 3D culture are unique in comparison with both
conventional 2D and other types of 3D cultures. In this study, we
revealed that this system is useful in determining the efficacy of
drugs against disseminated small tumors. This indicates that the
nanoimprinting 3D culture might have a potential to facilitate the
selection of effective drugs against disseminated small tumors by
mimicking the characteristics of these tumors such as adhesion,
migration, and proliferation as multicellular aggregates. Previous
studies with HCT116 cells in 2D culture reported the
IC50 as 4.3 μM for 5-FU (32) and 2.5–5 μM for regorafenib
(33). On the other hand, in this
study with the 3D culture treatment with 5-FU did not show apparent
decrease in cell viability at ≤10 μM while regorafenib showed and
IC50 of 8.1 μM. Our in vivo study in the
intraperitoneally disseminated tumor model showed that regorafenib,
but not 5-FU was effective. These data indicate the nanoimprinting
3D culture could be a better tool for selecting effective drugs
against disseminated small tumors than the 2D culture.
In conclusion, this study demonstrated the efficacy
of regorafenib against disseminated small colon cancer, via drug
selection using a novel screening system with a nanoimprinting 3D
culture in HCT116-RFP cells. Furthermore, this drug could be a
potentially useful adjuvant chemotherapy agent for colon cancer
treatment and, therefore, warrants continued investigation for
future clinical application.
Acknowledgements
We would like to thank Mr. Hisashi Suzuki (National
Institute of Radiological Sciences, Japan) for providing the
radiophar-maceuticals. This study was supported by Grants-in-Aid
for Scientific Research C (grant no. 25461863) from the Japanese
Society for the Promotion of Science, Japan to Y.Y.
Abbreviations:
|
ANOVA
|
analysis of variance
|
|
FLT
|
3′-[18F]fluoro-3′-deoxythymidine
|
|
IC50
|
half-maximal inhibitory
concentration
|
|
NCP
|
nanoculture plate
|
|
PET
|
positron emission tomography
|
|
RFP
|
red fluorescent protein
|
|
SD
|
standard deviation
|
|
SUV
|
standard uptake value
|
|
3D
|
3-dimensional
|
|
5-FU
|
5-fluorouracil
|
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA,
Tormey DC, Glick JH, et al: Levamisole and fluorouracil for
adjuvant therapy of resected colon carcinoma. N Engl J Med.
322:352–358. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Vos tot Nederveen Cappel WH, Meulenbeld
HJ, Kleibeuker JH, Nagengast FM, Menko FH, Griffioen G, Cats A,
Morreau H, Gelderblom H, Vasen HF, et al: Survival after adjuvant
5-FU treatment for stage III colon cancer in hereditary
nonpolyposis colorectal cancer. Int J Cancer. 109:468–471. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaillant JC, Nordlinger B, Deuffic S,
Arnaud JP, Pelissier E, Favre JP, Jaeck D, Fourtanier G, Grandjean
JP, Marre P, et al: Adjuvant intraperitoneal 5-fluorouracil in
high-risk colon cancer: A multicenter phase III trial. Ann Surg.
231:449–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshii Y, Waki A, Yoshida K, Kakezuka A,
Kobayashi M, Namiki H, Kuroda Y, Kiyono Y, Yoshii H, Furukawa T, et
al: The use of nanoimprinted scaffolds as 3D culture models to
facilitate spontaneous tumor cell migration and well-regulated
spheroid formation. Biomaterials. 32:6052–6058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshii Y, Furukawa T, Waki A, Okuyama H,
Inoue M, Itoh M, Zhang MR, Wakizaka H, Sogawa C, Kiyono Y, et al:
High-throughput screening with nanoimprinting 3D culture for
efficient drug development by mimicking the tumor environment.
Biomaterials. 51:278–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawada M, Inoue H, Masuda T and Ikeda D:
Insulin-like growth factor I secreted from prostate stromal cells
mediates tumor-stromal cell interactions of prostate cancer. Cancer
Res. 66:4419–4425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73-4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar
|
|
9
|
Schmieder R, Hoffmann J, Becker M,
Bhargava A, Müller T, Kahmann N, Ellinghaus P, Adams R, Rosenthal
A, Thierauch KH, et al: Regorafenib (BAY 73-4506): Antitumor and
antimetastatic activities in preclinical models of colorectal
cancer. Int J Cancer. 135:1487–1496. 2014. View Article : Google Scholar :
|
|
10
|
Kehoe SM, Ma C, Rosales N, Rao T, Dupont J
and Spriggs DR: Effect of combination inhibition of vascular
endothelial growth factor (VEGF) and epidermal growth factor
receptor (EGF-R) on ovarian cancer cell lines. J Clin Oncol.
24(Suppl 13112)2006.
|
|
11
|
Kunnumakkara AB, Diagaradjane P, Anand P,
Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S and
Aggarwal BB: Curcumin sensitizes human colorectal cancer to
capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and
CXCR4 expression in an orthotopic mouse model. Int J Cancer.
125:2187–2197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto Y, Fukuda K, Fuchimoto Y,
Matsuzaki Y, Saikawa Y, Kitagawa Y, Morikawa Y and Kuroda T:
Cetuximab promotes anticancer drug toxicity in rhabdomyosarcomas
with EGFR amplification in vitro. Oncol Rep. 30:1081–1086.
2013.PubMed/NCBI
|
|
13
|
Jiang P, Mukthavaram R, Chao Y, Bharati
IS, Fogal V, Pastorino S, Cong X, Nomura N, Gallagher M, Abbasi T,
et al: Novel anti-glioblastoma agents and therapeutic combinations
identified from a collection of FDA approved drugs. J Transl Med.
12:132014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dahan L, Sadok A, Formento JL, Seitz JF
and Kovacic H: Modulation of cellular redox state underlies
antagonism between oxaliplatin and cetuximab in human colorectal
cancer cell lines. Br J Pharmacol. 158:610–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freeman DJ, Bush T, Ogbagabriel S,
Belmontes B, Juan T, Plewa C, Van G, Johnson C and Radinsky R:
Activity of panitumumab alone or with chemotherapy in non-small
cell lung carcinoma cell lines expressing mutant epidermal growth
factor receptor. Mol Cancer Ther. 8:1536–1546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cordel S, Heymann MF, Boisteau O, Oliver
L, Le Pendu J, Grégoire M and Meflah K: 5-Fluorouracil-resistant
colonic tumors are highly responsive to sodium
butyrate/interleukin-2 bitherapy in rats. Int J Cancer. 73:924–928.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Looy T, Gebreyohannes YK, Wozniak A,
Cornillie J, Wellens J, Li H, Vanleeuw U, Floris G, Debiec-Rychter
M, Sciot R, et al: Characterization and assessment of the
sensitivity and resistance of a newly established human
gastrointestinal stromal tumour xenograft model to treatment with
tyrosine kinase inhibitors. Clin Sarcoma Res. 4:102014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gold GL, Hall TC, Shnider BJ, Selawry O,
Colsky J, Owens AH Jr, Dederick MM, Holland JF, Brindley CO and
Jones R: A clinical study of 5-fluorouracil. Cancer Res.
19:935–939. 1959.PubMed/NCBI
|
|
19
|
Miyake M, Anai S, Fujimoto K, Ohnishi S,
Kuwada M, Nakai Y, Inoue T, Tomioka A, Tanaka N and Hirao Y:
5-fluorouracil enhances the antitumor effect of sorafenib and
sunitinib in a xenograft model of human renal cell carcinoma. Oncol
Lett. 3:1195–1202. 2012.PubMed/NCBI
|
|
20
|
Abou-Elkacem L, Arns S, Brix G, Gremse F,
Zopf D, Kiessling F and Lederle W: Regorafenib inhibits growth,
angiogenesis, and metastasis in a highly aggressive, orthotopic
colon cancer model. Mol Cancer Ther. 12:1322–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wieder HA, Geinitz H, Rosenberg R, Lordick
F, Becker K, Stahl A, Rummeny E, Siewert JR, Schwaiger M and
Stollfuss J: PET imaging with
[18F]3′-deoxy-3′-fluorothymidine for prediction of
response to neoadjuvant treatment in patients with rectal cancer.
Eur J Nucl Med Mol Imaging. 34:878–883. 2007. View Article : Google Scholar
|
|
22
|
Hong YS, Kim HO, Kim KP, Lee JL, Kim HJ,
Lee SJ, Lee SJ, Oh SJ, Kim JS, Ryu JS, et al:
3′-Deoxy-3′-18F-fluorothymidine PET for the early
prediction of response to leucovorin, 5-fluo-rouracil, and
oxaliplatin therapy in patients with metastatic colorectal cancer.
J Nucl Med. 54:1209–1216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wodarski CEJ, Weber K, Henze M, Haberkorn
U and Eisenhut M: Synthesis of
3′-deoxy-3′-[18F]fluoro-thymidine with
2,3′-anhydro-5′-O-(4,4′-dimethoxytrityl)-thymidine. J Labelled Comp
Radiopharm. 43:1211–1218. 2000. View Article : Google Scholar
|
|
24
|
Tsuji AB, Kato K, Sugyo A, Okada M, Sudo
H, Yoshida C, Wakizaka H, Zhang MR and Saga T: Comparison of
2-amino-[3-11C]isobutyric acid and
2-deoxy-2-[18F]fluoro-D-glucose in nude mice with
xenografted tumors and acute inflammation. Nucl Med Commun.
33:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuji AB, Morita M, Li XK, Sogawa C, Sudo
H, Sugyo A, Fujino M, Sugioka A, Koizumi M and Saga T:
18F-FDG PET for semiquantitative evaluation of acute
allograft rejection and immunosuppressive therapy efficacy in rat
models of liver transplantation. J Nucl Med. 50:827–830. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugarbaker PH: Colorectal cancer:
Prevention and management of metastatic disease. BioMed Res Int.
2014:7828902014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomassen I, van Gestel YR, Lemmens VE and
de Hingh IH: Incidence, prognosis, and treatment options for
patients with synchronous peritoneal carcinomatosis and liver
metastases from colorectal origin. Dis Colon Rectum. 56:1373–1380.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brodowicz T, Liegl-Atzwager B, Tresch E,
Taieb S, Kramar A, Gruenwald V, Vanseymortier M, Clisant S, Blay
JY, Le Cesne A, et al: Study protocol of REGOSARC trial: activity
and safety of regorafenib in advanced soft tissue sarcoma: a
multinational, randomized, placebo-controlled, phase II trial. BMC
Cancer. 15:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar
|
|
30
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: CONCUR Investigators: Regorafenib
plus best supportive care versus placebo plus best supportive care
in Asian patients with previously treated metastatic colorectal
cancer (CONCUR): A randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jitawatanarat P and Wee W: Update on
antiangiogenic therapy in colorectal cancer: Aflibercept and
regorafenib. J Gastrointest Oncol. 4:231–238. 2013.PubMed/NCBI
|
|
32
|
Boyer J, McLean EG, Aroori S, Wilson P,
McCulla A, Carey PD, Longley DB and Johnston PG: Characterization
of p53 wild-type and null isogenic colorectal cancer cell lines
resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin
Cancer Res. 10:2158–2167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan LC, Teng HW, Shiau CW, Lin H, Hung MH,
Chen YL, Huang JW, Tai WT, Yu HC and Chen KF: SHP-1 is a target of
regorafenib in colorectal cancer. Oncotarget. 5:6243–6251. 2014.
View Article : Google Scholar : PubMed/NCBI
|