Introduction
Oral squamous cell carcinoma (OSCC), accounting for
over 90% of head and neck cancers, is the most common malignancy of
oral cavity cancers, with more than half a million new patients
diagnosed annually, and metastasis to lymph node frequently takes
place (1–3). Since most patients are diagnosed with
OSCC after the cancer has progressed to an advanced stage, the
5-year survival rate remains low (4,5). In
spite of great advances of early diagnosis and cancer treatment,
the 5-year survival rate for patients with OSCC remains <50% and
has not improved over the last few decades (3). Consequently, there is a need for
development of effective therapeutic agents that could prevent and
treat OSCC.
Herbal medicine has been regarded as an alternative
approach to modern medicine and there have been various efforts to
find active components with better anticancer potency and less side
effects (6). Licorice, the root
and rhizome of several Glycyrrhiza species
(Leguminosase) (7), has
been used as a flavouring agent, and traditional medicine for
gastric ulcer, bronchial asthma and inflammation (8). There have been many studies on the
biological effects of active ingredients of licorice, such as
anti-inflammatory, anti-microbial, anti-oxidative, antiulcer,
cytoprotective and cytotoxic activities (9). Licochalcone B (Lico B), a chalconoid
presents in the root of Glycyrrhiza species, not only
inhibit cell proliferation, but also to induce cell cycle arrest
and apoptosis (10). Possibly,
Lico B could be a promising alternative compound to traditional
anti-cancer agents.
Apoptosis (programmed cell death), triggers an
extrinsic (death receptor-mediated signaling) or an intrinsic
(mitochondria-mediated signaling) pathway to activate caspase-3,
leading to morphological alterations (e.g., DNA fragmentation, cell
shrinkage, membrane blebbing and chromatin condensation) (11,12).
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a
member of the TNF cytokine superfamily, interacts with five
different receptors to coordinate cancer cell apoptosis. Among
these receptors, only death receptor (DR)4 and DR5 have cytoplasmic
death domains and are able to transduce the apoptotic signal of
TRAIL by association with the Fas-associated death domain (FADD)
protein, transmitting the apoptotic death signals into the cancer
cells (13). Activation of
caspase-8 causes cleavage of Bid and Bcl-2 family proteins and
induces releasing of cytochrome c from mitochondria to cytosol. The
mitochondria-mediated pathway is regulated by Bcl-2 family proteins
such as Bax and Bak. Apoptotic induction is accompanied by the
release of cytochrome c from mitochondria, which mediate apoptosis
in both the extrinsic and intrinsic pathways. The resulting
cytochrome c forms the apoptosome with apoptotic protease
activating factor-1 (Apaf-1), ATP and procaspase-9 (14). Thus, many studies have shown that
the induced expression of DR4 and DR5 contributes to apoptosis
caused by cancer drugs (13).
Moreover, reactive oxygen species (ROS) induce endoplasmic
reticulum (ER) stress-induced apoptosis via modulation of
CCAAT/enhancer-binding protein homologous protein (CHOP) (14,15).
That is, overexpression of CHOP promotes apoptosis through
down-regulation of cell survival proteins and upregulation of DR4
and DR5 (16,17).
However, mechanism of Lico B-induced apoptosis in
OSCC have not been well defined. This study suggests that Lico B
inhibits cell growth and induces apoptosis in OSCC HN22 and HSC4
cells.
Materials and methods
Reagents and antibodies
We prepared Lico B with 95% purity according to the
method previously reported (18).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), trypsin, penicillin and streptomycin (P/S) and
phosphate-buffered saline (PBS) were purchased from Thermo
Scientific (Logan, UT, USA). Antibodies against cyclin D1, p21,
p27, actin, CHOP, DR4, DR5, cytochrome c, α-tubulin, MTCO1, Apaf-1,
survivin, Bid, Bax, Bcl-xl, myeloid cell leukemia-1
(Mcl-1) and poly (ADP-ribose) polymerase (PARP) were from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). A
4′,6-diamidino-2-phenylindole (DAPI) was obtained from
Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Cell culture
HN22 and HSC4 cells are human oral squamous cancer
cell lines. HN22 cells were provided by Dankook University
(Cheonan, Korea) and HSC4 cells were provided by Hokkaido
University (Hokkaido, Japan) (19). Both cell types were maintained in
DMEM supplemented with 10% FBS and 100 U/ml each of P/S at 37ºC in
a 5% CO2 incubator.
Trypan blue staining
HN22 (6×104/6-well plate) and HSC4
(7.5×104/6-well plate) cells were grown for 24 h, and
then treated with various concentrations of Lico B (0, 10, 20 and
30 μM). After treatment with Lico B for 24 and 48 h, cells were
trypsinized and resuspended in complete medium. Each sample was
mixed with trypan blue solution. Colored (non-viable) and
dye-excluding (viable) cells were counted on a haemocytometer.
Annexin V staining
HN22 (6×104/6-well plate) and HSC4
(7.5×104/6-well plate) cells were seeded and allowed to
incubate overnight in 10% FBS-containing DMEM. At 48 h after
treatment with different concentration of Lico B (0, 10, 20 and 30
μM), cells were harvested by trypsinization and analyzed for the
detection of early/late apoptosis and cell death using Annexin V
and Dead Cell kit (EMD Millipore Corp., Billerica, MA, USA)
according to the manufacturer's instructions. The percentage of
apoptotic cells was calculated from triplicate samples by
statistical analysis of dot plot using Muse Cell Analyzer (Merck
Millipore).
DAPI staining
After treatment with Lico B, the HN22 and HSC4 cells
were harvested by trypsin digestion. The cells were washed in
ice-cold PBS and fixed with 100% methanol at room temperature (RT)
for 1 h. Fixed cells were stained with DAPI solution (2 μg/ml) and
deposited on slides at RT in the dark. DAPI-stained cells were
observed through a FluoView confocal laser microscope (Flouview
FV10i, Olympus Corp., Tokyo, Japan).
Cell cycle
HN22 (6×104/6-well plate) and HSC4
(7.5×104/6-well plate) cells were seeded and exposed to
Lico B (0, 10, 20 and 30 μM) for 48 h. The harvested cells were
washed in PBS and 200 μl of Muse cell cycle reagent (EMD Millipore
Corp.) was added. The cells were incubated for 30 min at RT in the
dark. Cell cycle distribution was analyzed by Muse Cell Analyzer
(Merck Millipore).
Western blotting
The cell lysates were prepared using PRO-PREP™
Protein Extraction Solution (iNtRON Biotechnology, Korea) and then
supernatants were collected by centrifugation. Samples containing
equal amounts of protein were separated by SDS-PAGE and transferred
to polyvinylidene difluoride membrane. The membranes were blocked
with 5% skim milk in PBS with 0.1% Tween-20 at RT. Membranes were
probed with primary antibodies at 4ºC overnight with mild shaking
and then washed with PBST for a total of 30 min. Finally, membranes
were incubated with the secondary antibodies for 2 h at RT.
Antibody-bound protein bands were visualized using ECL Plus Western
Blotting Detection system (Santa Cruz Biotechnology, Inc.).
Measurement of intracellular ROS
Muse Oxidative Stress kit (EMD Millipore Corp.) was
used to examine the effect of Lico B on the generation of ROS.
Briefly, cells from each treatment were harvested, washed with PBS,
and then resuspended in Muse Oxidative Stress Reagent working
solution for 30 min at 37ºC in the dark. Intracellular reactive
oxygen species was measured by flow cytometry using Muse Cell
Analyzer (Merck Millipore).
Mitochondrial membrane potential (MMP)
detection assay
MMP was assessed using Muse MitoPotential kit (EMD
Millipore Corp.) according to the manufacturer's protocol. Briefly,
HN22 and HSC4 cells treated with Lico B were harvested to observe
quantitatively MMP. The cells were incubated with Muse
MitoPotential working solution at 37ºC for 20 min. Then, 5 μl of
7-aminoactinomycin D (7-AAD) was added and samples were further
incubated at RT for 5 min. Muse Cell Analyzer (Merk Millipore) was
employed to detect MMP.
Multi-caspase assay
The activity of caspase-1, -3, -4, -5, -6, -7, -8
and -9 was measured using a Muse Multi-caspase kit (MCH100109,
Merck Millipore) according to the manufacturer's instructions. HN22
and HSC4 cells were incubated with various concentrations of Lico B
(0, 10, 20 and 30 μM) for 48 h. Cells were harvested and incubated
with 5 μl of Muse Multi-Caspase reagent working solution at 37ºC
for 30 min. Then, 150 μl of Muse Caspase 7-AAD working solution was
added to each sample. Multi-caspase assay was performed with Muse
Cell Analyzer (Merck Millipore).
Statistical analysis
Using Student's t-test, the statistical significance
was assessed. The result with p-value <0.05, indicates
statistical significance of data.
Results
Inhibition of cell proliferation by Lico
B
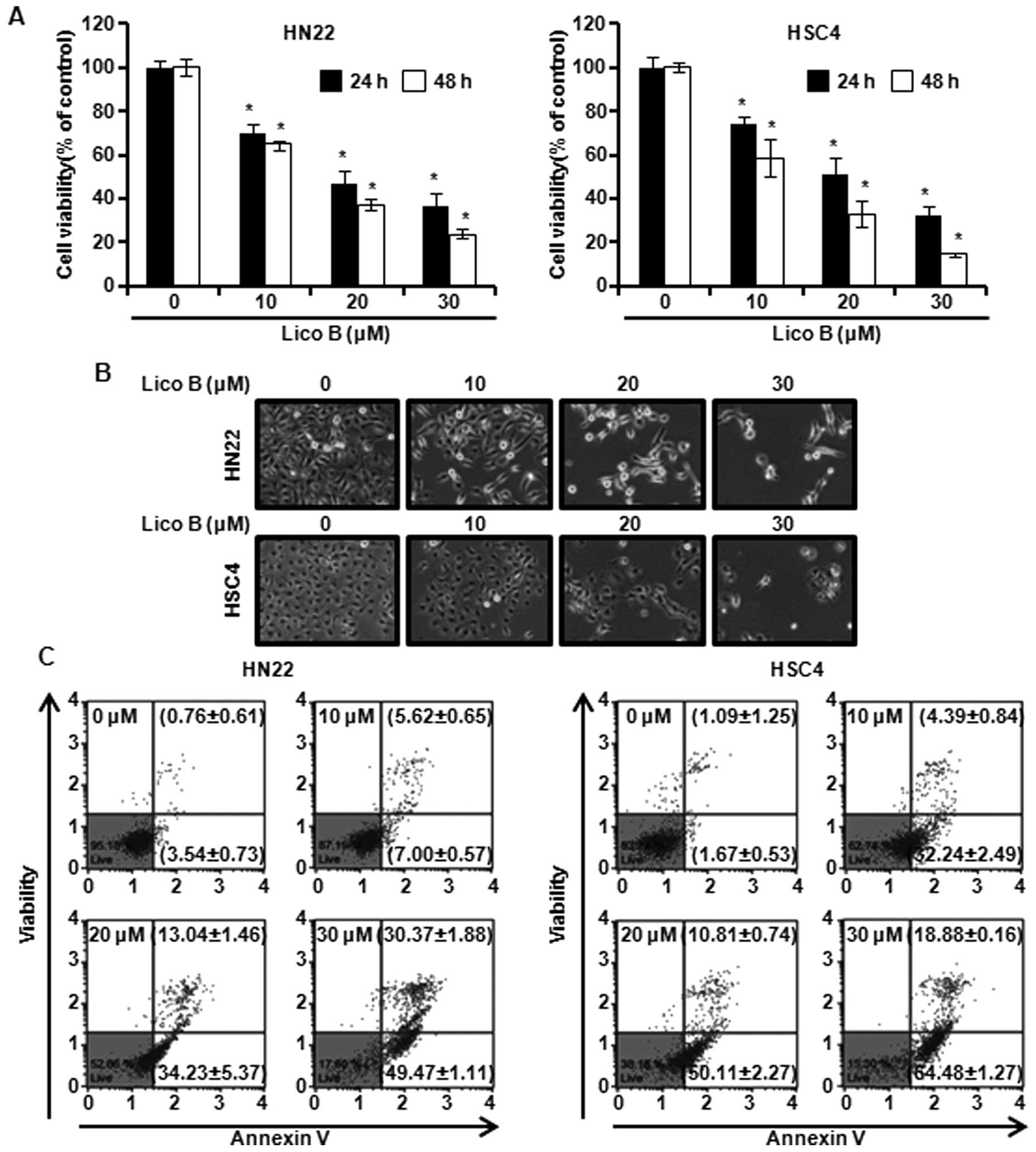
Trypan blue staining was performed to determine the
viability of HN22 and HSC4 cells treated with Lico B (0, 10, 20 and
30 μM) for 24 and 48 h, and then live cells were counted using a
haemocytometer. As shown in Fig.
1A, cell viability was significantly decreased in a
concentration- and time-dependent manner by Lico B (Fig. 1A). The IC50 value was 15
μM for HN22 cells and 13 μM for HSC4 cells at 48 h. Cell morphology
was observed under an inverted phase contrast microscope after the
cells were treated with Lico B for 48 h, and images were obtained
(Fig. 1B). Significant cell
shrinkage, formation of plasma membrane blebs and a decreased
cellular attachment rate were observed in the Lico B-treated
groups. These results suggest that Lico B has an inhibitory effect
on the cell growth of HN22 and HSC4 cells.
Lico B induces apoptosis in HN22 and HSC4
cells
Phosphatidylserine (PS) of normal cells are mainly
distributed on the inner layer of the membrane. However, early
apoptotic cells transpose PS on the outer layer of the membrane
(20). Annexin V can detect PS on
the outer membrane, designating early apoptotic cells while 7-AAD
can permeate into plasma membrane and bind to DNA when the membrane
of cells lose integrity. Annexin V and 7-AAD absorb laser with
different wavelengths so that both fluorophores can be analyzed at
the same time (20). To determine
whether the Lico B could induce apoptosis in HN22 and HSC4 cells,
we stained cells with Annexin V/7-AAD for analysis by flow
cytometry. As shown in Fig. 1C,
Lico B caused apoptosis of OSCC cells in a concentration-dependent
manner. The apoptotic cells of HN22 were 12.62±1.22, 47.27±6.83,
79.84±2.99% at 10, 20 and 30 μM of Lico B, respectively (Fig. 1C, left). In HSC4, population of
apoptotic cells was 36.63±3.33, 60.92±3.01, 83.36±1.43% at 10, 20
and 30 μM of Lico B, respectively (Fig. 1C, right). Additionally, the
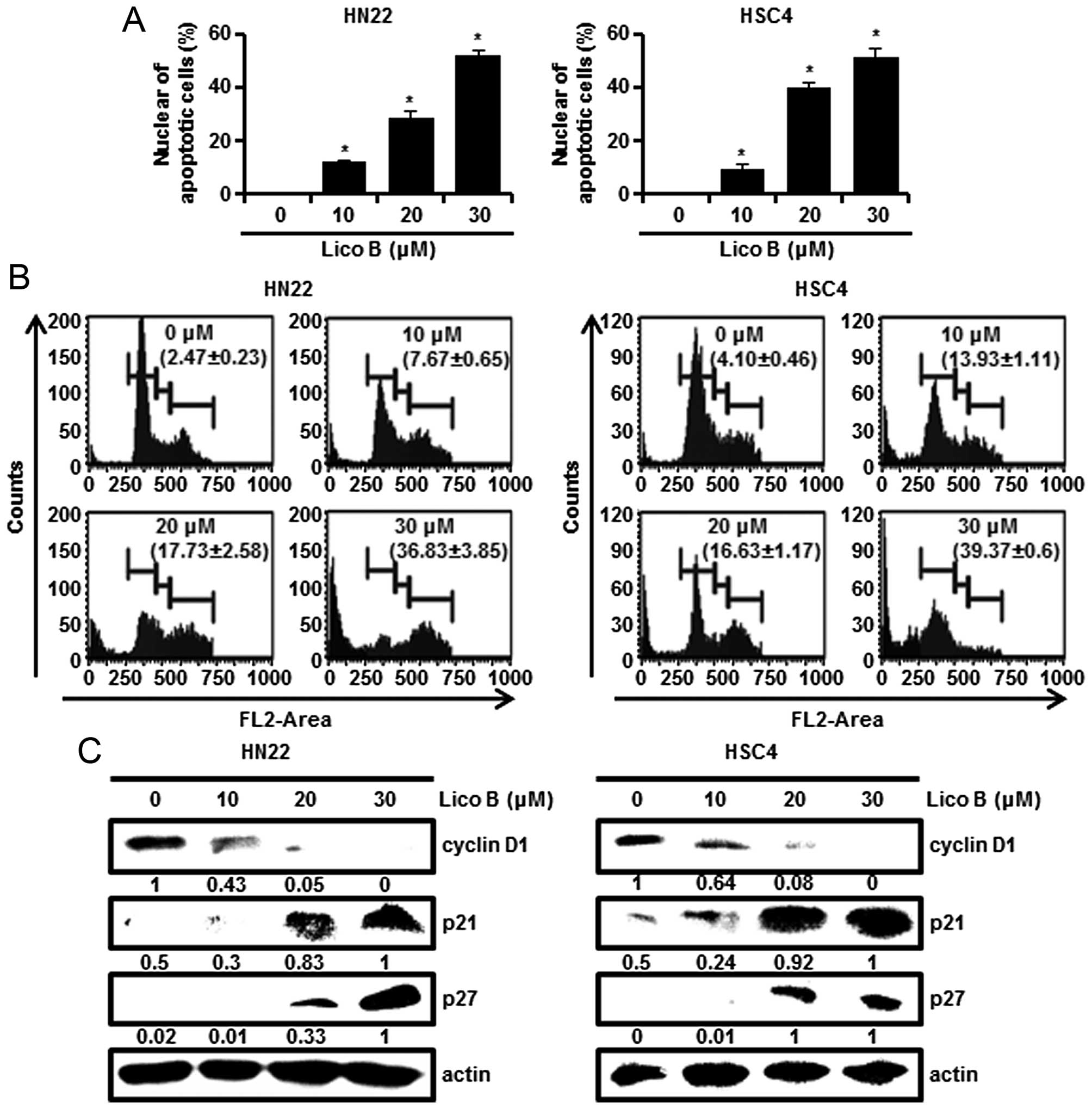
characteristic appearance of apoptosis was analyzed by DAPI
staining. HN22 and HSC4 cells treated with Lico B presented nuclear
shrinkage, chromatin condensation and fragmentation of nuclear
bodies compared to control (Fig.
2A). The cell cycle distribution was assessed after PI staining
by Muse Cell Analyzer. As shown in Fig. 2B, the treatment of cells with Lico
B at 30 μM caused 36.83±3.85 or 39.37±0.6% induction of
sub-G1 phase in HN22 (Fig.
2B, left) and HSC4 (Fig. 2B,
right), respectively. Overall, these findings suggest that Lico B
can induce apoptosis in HN22 and HSC4 cells.
Lico B induces G1 phase arrest
in HN22 and HSC4 cells
To determine the effect of Lico B on the cell cycle
progression, HN22 and HSC4 cells were treated with different
concentrations of Lico B (0, 10, 20 and 30 μM) for 48 h and the
cell cycle distribution was assessed by Muse Cell Analyzer.
Fig. 2B showed that HN22 and HSC4
cells accumulated in G1 phase after treatment of Lico B
(Fig. 2B). To confirm
G1 block in cell cycle progression, the level of
G1 cell cycle components was monitored by western blot
analysis. Fig. 2C showed that Lico
B increased the protein levels of p21 and p27, and decreased the
levels of cyclin D1 (Fig. 2C).
These results support that Lico B causes G1 phase arrest
in OSCC cells.
Lico B induces endoplasmic reticulum (ER)
stress and expression of death receptor (DR) 4, DR5 in HN22 and
HSC4 cells
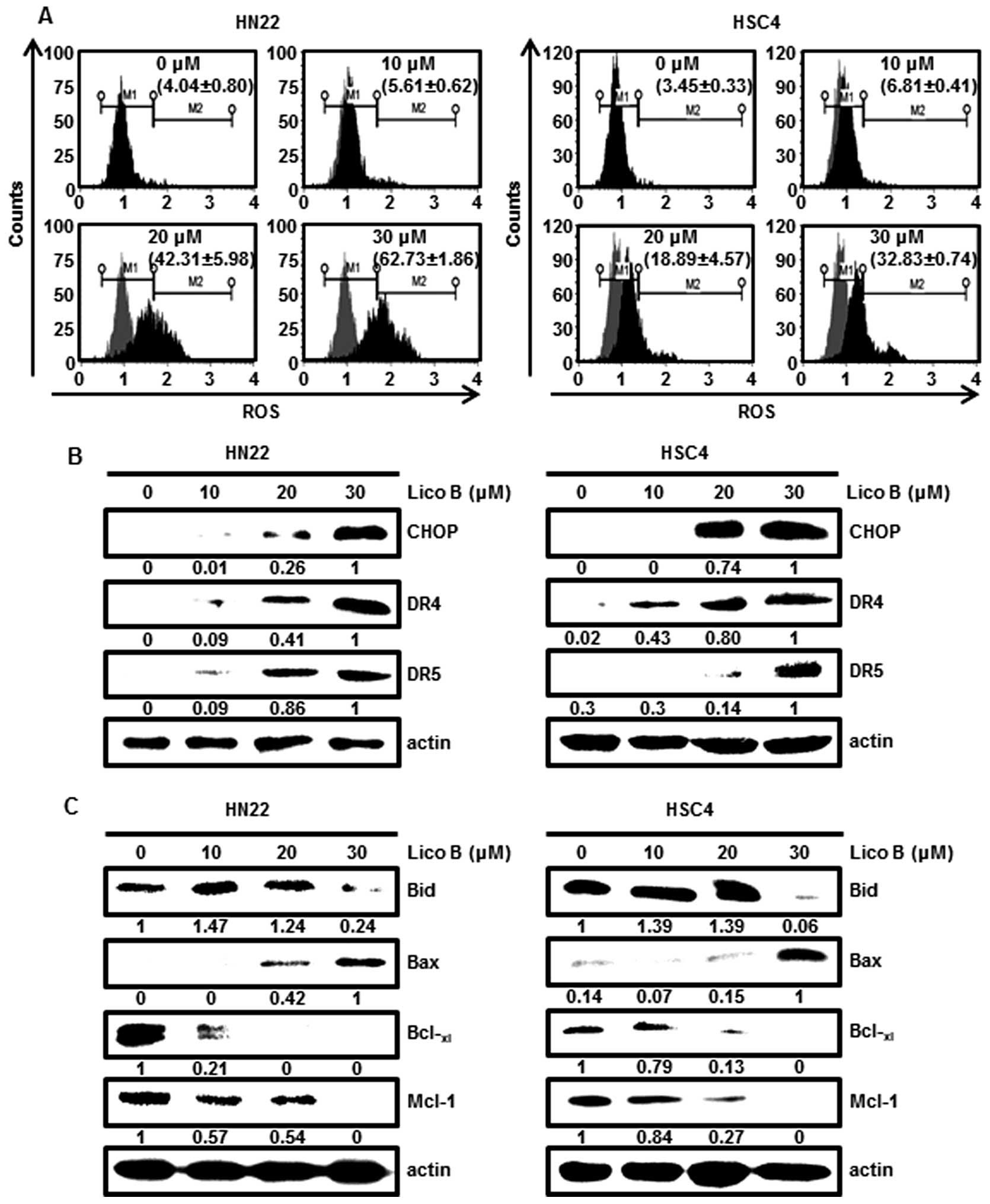
Overwhelming ER stress resulted from accumulation of
ROS has been known to trigger apoptotic signaling (15,21).
As shown in Fig. 3A, ROS
production from HN22 and HSC4 cells treated with Lico B was
significantly increased. CHOP is one of the components of ER
stress-mediated apoptosis pathway (16). To assess the effect of Lico B on
CHOP expression in HN22 and HSC4 cells, western blotting was
performed. Fig. 3B showed that the
protein levels of CHOP were increased in a dose-dependent manner by
Lico B. CHOP is suggested to induce apoptosis through upregulation
of DR4 and DR5 expression (16).
We also observed that Lico B treatment increased dose-dependently
DR4 and DR5 in HN22 and HSC4 cells (Fig. 3B). These results showed that
treatment of OSCC cells with Lico B induced ER stress, which, in
turn, upregulates CHOP, DR4 and DR5.
Lico B modulates the level of proteins
related to apoptosis in HN22 and HSC4 cells
Dynamic changes in Bcl-2 family proteins were
associated with death receptor-mediated apoptosis (22). Bcl-xl functions as an
anti-apoptotic protein, whereas Bax is pro-apoptotic protein
(23). Bid is then truncated to
induce apoptosis. Downregulation of Mcl-1 protein may be required
to initiate a cascade of apoptotic signals leading to release of
cytochrome c (5). To investigate
whether expression of apoptosis regulatory proteins may be
modulated by Lico B, the protein levels of Bid, Bax,
Bcl-xl and Mcl-1 in HN22 and HSC4 cells treated with
Lico B were analyzed by western blotting. Fig. 3C showed that the levels of
pro-apoptotic Bax were increased and the expression levels of Bid,
Bcl-xl and Mcl-1 were decreased in a
concentration-dependent manner.
Lico B induces mitochondrial membrane
depolarization and mitochondria-mediated apoptosis in OSCC
cells
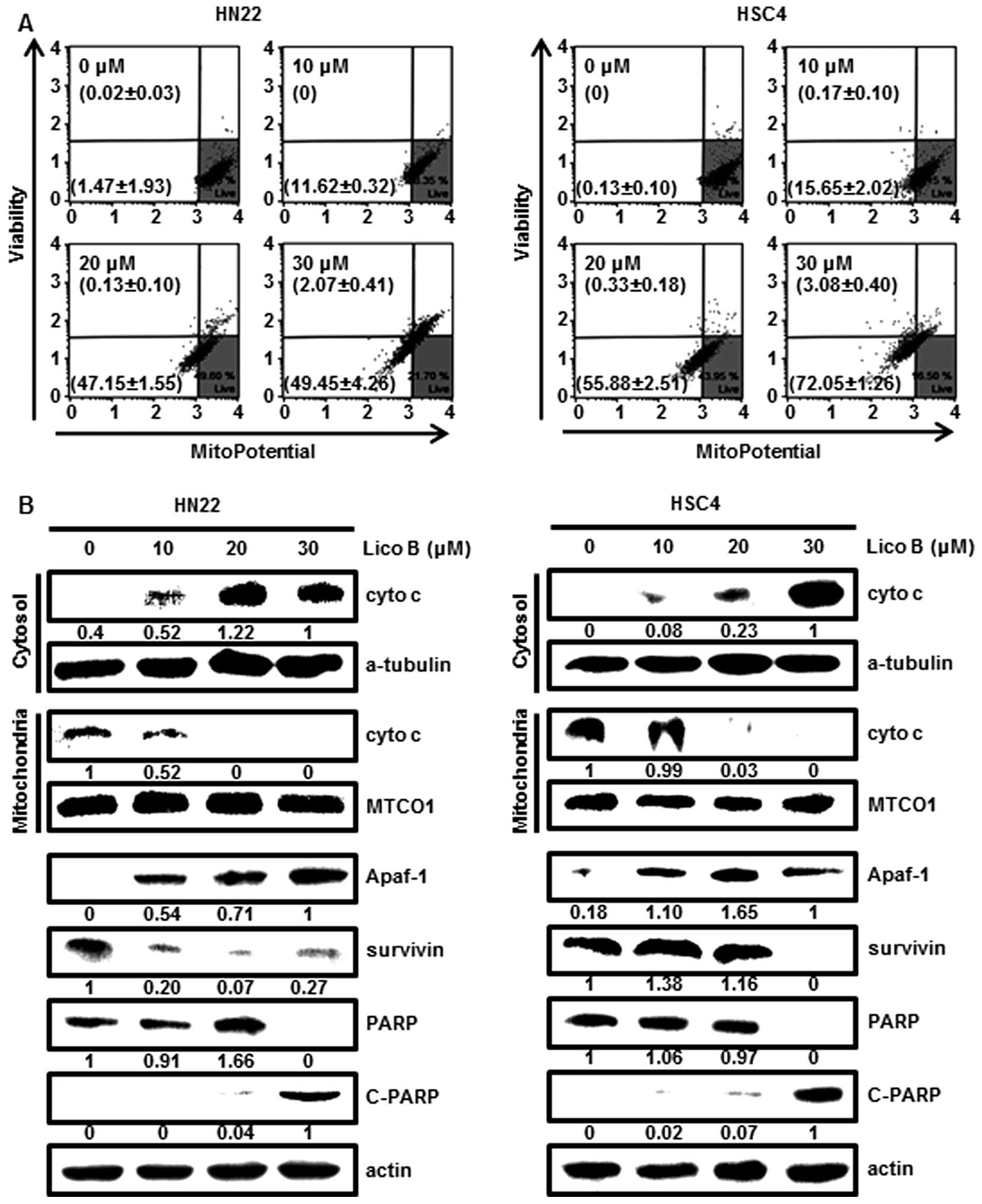
Death receptor induces apoptosis through the
disruption of the mitochondrial membrane potential (MMP) and
sequential release of cytochrome c from mitochondria into the
cytosol (24). The MMP was
measured by Muse Cell Analyzer with cationic, lipophilic dye. The
dye permeates mitochondrial membrane of intact cells and
accumulates within inner membrane. Thus, cells with depolarized
mitochondria show a decrease in fluorescence (25). The data indicated that treatment of
cells with Lico B induced a loss of MMP in HN22 and HSC4 cells
(Fig. 4A). The loss of MMP leads
to the release of cytochrome c into the cytosol then, triggering
the downstream processes in the apoptotic cascade (26). To examine whether Lico B could
cause the release of cytochrome c into the cytosol, subcellular
distribution of cytochrome c in cytosol and mitochondria fraction
were analyzed by western blotting. The results showed that
cytochrome c in cytosol fraction of HN22 and HSC4 cells treated
with Lico B (0, 10, 20 and 30 μM) was concentration-dependently
increased (Fig. 4B). These results
indicated that Lico B facilitated mitochondrial membrane
depolarization and release of cytochrome c into the cytosol.
Additionally, to investigate downstream target proteins including
Apaf-1, survivin and PARP in mitochondria-mediated apoptosis, their
expression was analyzed by western blotting with specific
antibodies. As shown in Fig. 4B,
the expression levels of survivin and PARP were diminished, but
Apaf-1 and C-PARP were increased in a concentration-dependent
manner by Lico B.
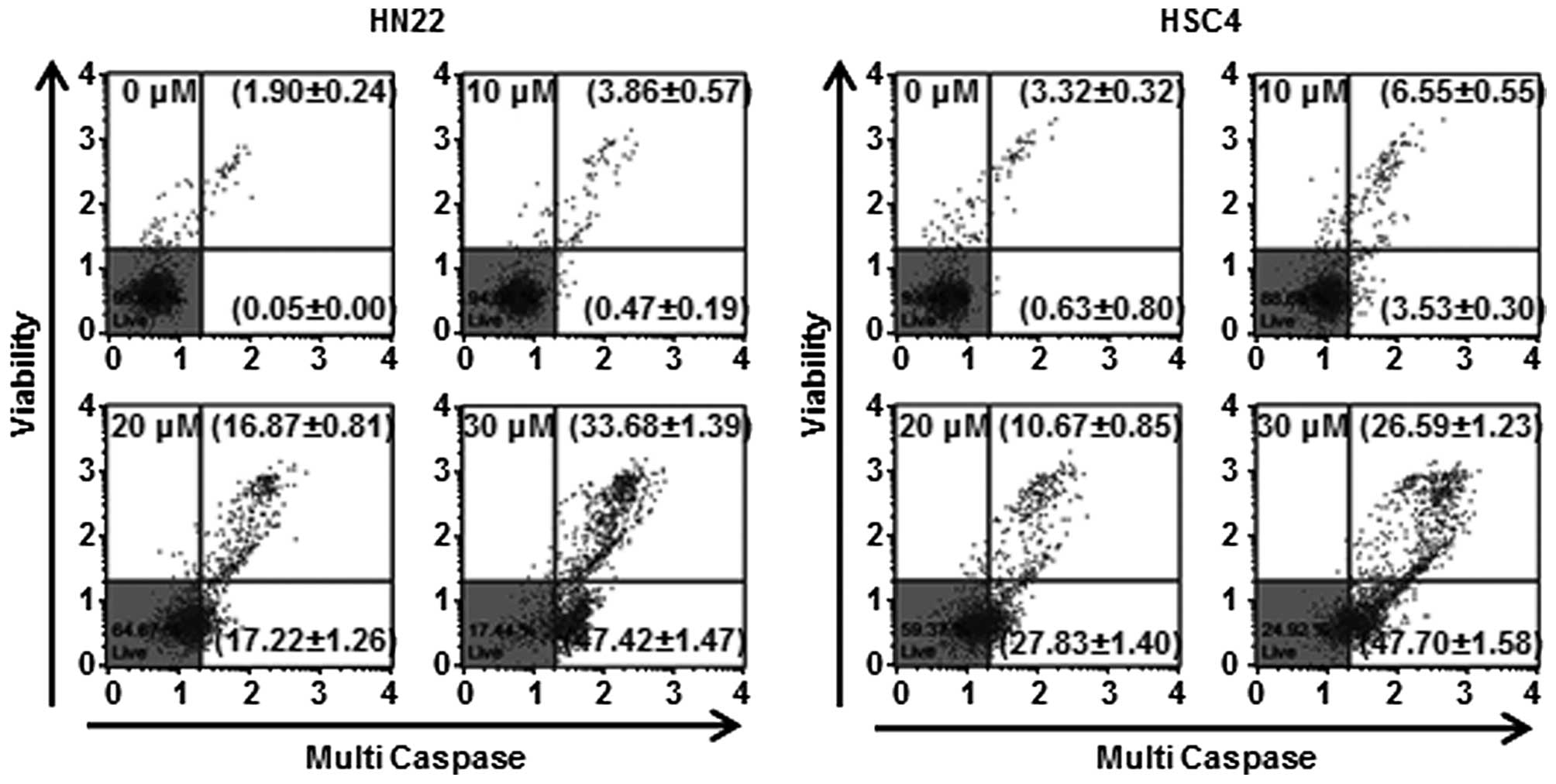
Lico B induces caspase-mediated apoptosis
in HN22 and HSC4 cells
To examine whether Lico B-induced apoptosis would be
associated with the activation of caspases, the expression levels
and activity of caspases such as caspase-1, -3, -4, -5, -6, -7, -8
and -9 in the Lico B-treated OSCC cells were assessed using Muse
Cell Analyzer. As shown in Fig. 5,
HN22 and HSC4 cells treated with Lico B exhibited enhanced
multi-caspase activity in a concentration-dependent manner. The
multi-caspase activity of HN22 cells (Fig. 5, left) was 4.33±0.76, 34.09±2.07
and 81.1±2.86%, respectively, at 10, 20 and 30 μM of Lico B
compared with control. In HSC4 cells (Fig. 5, right), multi-caspase activity was
10.08±0.85, 38.5±2.25 and 74.29±2.81% at 10, 20 and 30 μM of Lico
B, respectively.
Discussion
Natural extracts derived from edible plants have
been investigated as alternative products of cancer drugs based on
safety and efficacy (27).
Chalcones are ubiquitous natural products with a wide variety of
reported biological activities such as antiviral, anti-microbial,
anti-inflammatory and anti-cancer effect (6,28).
Recent studies have reported that Lico B, a chalcone extracted from
licorice, inhibits cell proliferation and induces cell cycle arrest
and apoptosis in tumor cells (6).
Lico B has been reported to attenuate bladder cancer cell
migration, adhesion and invasion by downregulating the protein
expression and suppressing the activity of matrix metalloproteinase
9 (10).
Oral squamous cell carcinoma (OSCC) is the most
common neoplasm of oral cavity cancers (4). Despite great advances in cancer
diagnosis and therapy, there has been little improvement in the
5-year survival rate of oral cancer patients (27). Anticancer effects of the Lico B
have been reported in a variety of cancers, but has not been
examined previously in OSCC. To better understand the action
mechanisms of Lico B on OSCC cell lines, we further checked the
intracellular signaling pathway. We found that Lico B induced
apoptosis by intrinsic and extrinsic apoptotic pathways in HN22 and
HSC4 cells. We found that Lico B significantly not only inhibited
cell growth in a concentration- and time-dependent manner, but also
induced apoptosis in OSCC cells (HN22 and HSC4).
The morphological alterations such as cell
shrinkage, chromatin condensation and DNA fragmentation supported
Lico B-induced apoptotic induction. p21 and p27 proteins are
members of the Cip/Kip family, and regulate cell cycle progression
(29). Cyclin D1 is a key protein
at G1 phase in the cell cycle progression. This protein
dimerizes with CDK4/6 to regulate the G1/S phase
transition and enter the S phase. Thus, cyclin D1 is involved in
the development and progression of various tumors (30). Our results showed that percentage
of cells was increased in the sub-G1 phase during Lico B
treatment, arresting in G1 phase. In OSCC cells, Lico B
inhibits cell cycle progression as a result of reduced cyclin D1,
and increased p21 and p27 expression. Deregulation of the cell
cycle is frequent during cancer development (6). Therefore, inhibition of cell cycle
progression is considered as useful approach to control of cancer
cell growth.
In several studies, phytochemicals exhibited
anticancer activities by the activation of death receptor and loss
of MMP in tumor cells (31), and
anticancer agents can enhance ROS production associated with
apoptosis in cells (32). ER
stress-induced response mediates the expression of the
apoptosis-relevant gene such as CHOP, DR4 and DR5 (15,33).
CHOP plays a decisive role in the expression of death receptor
(17). Our results indicated that
Lico B induced DR4 and DR5 expression through regulation of
ROS-CHOP mediated pathway. The results suggest that Lico B induces
apoptosis through the extrinsic pathway. The expression of Bcl-2
family proteins is directly linked to cell damage and there are
important factors that make up the apoptosome (34). The mitochondrial function has been
reported to be regulated by Bcl-2 family proteins such as
anti-apoptotic (Bcl-2, Bcl-xl and Mcl-1) and
pro-apoptotic (Bax, Bad and Bak) proteins (35). Thus, changes in the levels of Bcl-2
family proteins can affect the mitochondrial physiology positively
or negatively (36). The results
showed that Lico B led to downregulation of anti-apoptotic Bid,
Bcl-xl and Mcl-1, and upregulation of pro-apoptotic Bax.
Subsequently, Lico B resulted in a significant decrease in the
level of MMP and release of cytochrome c from mitochondria to
cytosol, finally executing cell death by activation of caspases
(37).
The release of cytochrome c into the cytosol is
regulated by the equilibrium between anti-apoptotic and
pro-apoptotic proteins and plays an important role in the execution
of apoptosis (26,34). Additionally, Lico B treatment
increased the expression of Apaf-1 and cleavage of PARP, while it
decreased inhibitor of apoptosis (IAP) family proteins (survivin)
which are direct inhibitors of activated caspases (38). The activation of Bax and caspase is
critical for initiation and amplification of apoptosis, and their
activation is normally blocked by Bcl-xl or IAP proteins
(6). All these studies support
that Lico B also induces apoptosis through the intrinsic
pathway.
In summary, this study demonstrated that Lico B
induced apoptosis in OSCC cells. Apoptosis was triggered through
the extrinsic pathway by upregulating death receptor and also the
intrinsic pathway by modulating Bcl-2 and IAP family members. These
findings provide the underlying molecular mechanisms for the
anticancer effects of Lico B that can be useful in the treatment of
human oral cancer.
Acknowledgements
This study was supported by Basic Science Research
program through the National Research Foundation Korea (NRF).
Funded by the Ministry of Education, Science and Technology
(2014R1A1A2053500) and the Next-Generation BioGreen 21 Program
(PJ01116401) from Rural Development Administration, Republic of
Korea.
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
Lico
|
licochalcone
|
|
ROS
|
reactive oxygen species
|
|
DR
|
death receptor
|
|
MMP
|
mitochondrial membrane potential
|
|
Apaf-1
|
apoptotic protease activating
factor-1
|
|
C-PARP
|
cleaved poly (ADP-ribose)
polymerase
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
FADD
|
Fas-associated death domain
|
|
ER
|
endoplasmic reticulum
|
|
CHOP
|
CCAAT/enhancer-binding protein
homologous protein
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
P/S
|
penicillin and streptomycin
|
|
PBS
|
phosphate buffered saline
|
|
Mcl-1
|
myeloid cell leukemia-1
|
|
DAPI
|
4′-6-diamidino-2-phenylindole
|
|
RT
|
room temperature
|
|
7-AAD
|
7-aminoactinomycin D
|
|
PS
|
phosphatidylserine
|
|
MMP-9
|
matrix metalloproteinases-9
|
|
IAP
|
inhibitor of apoptosis
|
|
PI
|
propidium iodide
|
References
|
1
|
Zeng G, Shen H, Yang Y, Cai X and Xun W:
Licochalcone A as a potent antitumor agent suppresses growth of
human oral cancer SCC-25 cells in vitro via caspase-3 dependent
pathways. Tumour Biol. 35:6549–6555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ni ZY, Lin FO, Liu DF and Xiao J:
Decreased microRNA-143 expression and its tumor suppressive
function in human oral squamous cell carcinoma. Genet Mol Res.
14:6943–6952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin JA, Jung JY, Ryu MH, Safe S and Cho
SD: Mithramycin A inhibits myeloid cell leukemia-1 to induce
apoptosis in oral squamous cell carcinomas and tumor xenograft
through activation of Bax and oligomerization. Mol Pharmacol.
83:33–41. 2013. View Article : Google Scholar
|
|
5
|
Shin JA, Kim JS, Kwon KH, Nam JS, Jung JY,
Cho NP and Cho SD: Apoptotic effect of hot water extract of
Sanguisorba officinalis L. in human oral cancer cells. Oncol Lett.
4:489–494. 2012.
|
|
6
|
Yuan X, Li T, Xiao E, Zhao H, Li Y, Fu S,
Gan L and Wang Z, Zheng Q and Wang Z: Licochalcone B inhibits
growth of bladder cancer cells by arresting cell cycle progression
and inducing apoptosis. Food Chem Toxicol. 65:242–251. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu Y, Chen J, Li YJ, Zheng YF and Li P:
Antioxidant and anti-inflammatory activities of six flavonoids
separated from licorice. Food Chem. 141:1063–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furusawa J, Funakoshi-Tago M, Mashino T,
Tago K, Inoue H, Sonoda Y and Kasahara T: Glycyrrhiza
inflata-derived chalcones, Licochalcone A, Licochalcone B and
Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS
signaling pathway. Int Immunopharmacol. 9:499–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park SY, Kim EJ, Choi HJ, Seon MR, Lim SS,
Kang YH, Choi MS, Lee KW and Yoon Park JH: Anti-carcinogenic
effects of non-polar components containing licochalcone A in
roasted licorice root. Nutr Res Pract. 8:257–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao H, Yuan X, Jiang J, Wang P, Sun X,
Wang D and Zheng Q: Antimetastatic effects of licochalcone B on
human bladder carcinoma T24 by inhibition of matrix
metalloproteinases-9 and NF-κB activity. Basic Clin Pharmacol
Toxicol. 115:527–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee MS, Cherla RP, Lentz EK, Leyva-Illades
D and Tesh VL: Signaling through C/EBP homologous protein and death
receptor 5 and calpain activation differentially regulate THP-1
cell maturation-dependent apoptosis induced by Shiga toxin type 1.
Infect Immun. 78:3378–3391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu H, Liu XW, Ding WJ, Xu DQ, Zhao YC, Lu
W, He QJ and Yang B: Up-regulation of death receptor 4 and 5 by
celastrol enhances the anti-cancer activity of TRAIL/Apo-2L. Cancer
Lett. 297:155–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang TT, Liu FG, Wei CF, Lu CC, Chen CC,
Lin HC, Ojcius DM and Lai HC: Activation of multiple apoptotic
pathways in human nasopharyngeal carcinoma cells by the prenylated
isoflavone, osajin. PLoS One. 6:e183082011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farooqi AA, Li KT, Fayyaz S, Chang YT,
Ismail M, Liaw CC, Yuan SS, Tang JY and Chang HW: Anticancer drugs
for the modulation of endoplasmic reticulum stress and oxidative
stress. Tumour Biol. 36:5743–5752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L, Su L and Liu X: PKCδ regulates death
receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis
in human lung cancer cells. Mol Cancer Ther. 11:2174–2182. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prasad S, Yadav VR, Ravindran J and
Aggarwal BB: ROS and CHOP are critical for dibenzylideneacetone to
sensitize tumor cells to TRAIL through induction of death receptors
and down-regulation of cell survival proteins. Cancer Res.
71:538–549. 2011. View Article : Google Scholar :
|
|
18
|
Wang Z, Liu Z, Cao Y, Paudel S, Yoon G and
Cheon SH: Short and efficient synthesis of licochalcone B and D
through acid-mediated Claisen-Schmidt condensation. Bull Korean
Chem Soc. 34:3906–3908. 2013. View Article : Google Scholar
|
|
19
|
Cho JJ, Chae JI, Yoon G, Kim KH, Cho JH,
Cho SS, Cho YS and Shim JH: Licochalcone A, a natural chalconoid
isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1
and Sp1 regulatory proteins in oral squamous cell carcinoma. Int J
Oncol. 45:667–674. 2014.PubMed/NCBI
|
|
20
|
Janes SM, Ofstad TA, Campbell DH, Eddaoudi
A, Warnes G, Davies D and Watt FM: PI3-kinase-dependent activation
of apoptotic machinery occurs on commitment of epidermal
keratinocytes to terminal differentiation. Cell Res. 19:328–339.
2009. View Article : Google Scholar :
|
|
21
|
Fu HY, Okada K, Liao Y, Tsukamoto O,
Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, et al:
Ablation of C/EBP homologous protein attenuates endoplasmic
reticulum-mediated apoptosis and cardiac dysfunction induced by
pressure overload. Circulation. 122:361–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin DY, Park YS, Yang K, Kim GY, Kim WJ,
Han MH, Kang HS and Choi YH: Decitabine, a DNA methyltransferase
inhibitor, induces apoptosis in human leukemia cells through
intracellular reactive oxygen species generation. Int J Oncol.
41:910–918. 2012.PubMed/NCBI
|
|
23
|
Habibie YS, Yokoyama S, Abdelhamed S,
Awale S, Sakurai H, Hayakawa Y and Saiki I: Survivin suppression
through STAT3/β-catenin is essential for resveratrol-induced
melanoma apoptosis. Int J Oncol. 45:895–901. 2014.PubMed/NCBI
|
|
24
|
Kim YS, Kim EA, Park KG, Lee SJ, Kim MS,
Sohn HY and Lee TJ: Dioscin sensitizes cells to TRAIL-induced
apoptosis through downregulation of c-FLIP and Bcl-2. Oncol Rep.
28:1910–1916. 2012.PubMed/NCBI
|
|
25
|
Law CK, Kwok HH, Poon PY, Lau CC, Jiang
ZH, Tai WC, Hsiao WW, Mak NK, Yue PY and Wong RN: Ginsenoside
compound K induces apoptosis in nasopharyngeal carcinoma cells via
activation of apoptosis-inducing factor. Chin Med. 9:112014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hearps AC, Burrows J, Connor CE, Woods GM,
Lowenthal RM and Ragg SJ: Mitochondrial cytochrome c release
precedes transmembrane depolarisation and caspase-3 activation
during ceramide-induced apoptosis of Jurkat T cells. Apoptosis.
7:387–394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi ES, Cho SD, Jeon JG and Cho NP: The
apoptotic effect of the hexane extract of Rheum undulatum L. in
oral cancer cells through the down-regulation of specificity
protein 1 and survivin. Lab Anim Res. 27:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mahapatra DK, Bharti SK and Asati V:
Chalcone scaffolds as anti-infective agents: Structural and
molecular target perspectives. Eur J Med Chem. 101:496–524. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yalcin A, Clem BF, Imbert-Fernandez Y,
Ozcan SC, Peker S, O'Neal J, Klarer AC, Clem AL, Telang S and
Chesney J: 6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle
progression and suppresses apoptosis via Cdk1-mediated
phos-phorylation of p27. Cell Death Dis. 5:e13372014. View Article : Google Scholar
|
|
30
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han MH, Lee WS, Jung JH, Jeong JH, Park C,
Kim HJ, Kim G, Jung JM, Kwon TK, Kim GY, et al: Polyphenols
isolated from Allium cepa L. induces apoptosis by suppressing IAP-1
through inhibiting PI3K/Akt signaling pathways in human leukemic
cells. Food Chem Toxicol. 62:382–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Federico A, Cardaioli E, Da Pozzo P,
Formichi P, Gallus GN and Radi E: Mitochondria, oxidative stress
and neurodegeneration. J Neurol Sci. 322:254–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim IY, Kang YJ, Yoon MJ, Kim EH, Kim SU,
Kwon TK, Kim IA and Choi KS: Amiodarone sensitizes human glioma
cells but not astrocytes to TRAIL-induced apoptosis via
CHOP-mediated DR5 upregulation. Neuro Oncol. 13:267–279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim CD, Cha JD, Li S and Cha IH: The
mechanism of acacetin-induced apoptosis on oral squamous cell
carcinoma. Arch Oral Biol. 60:1283–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giam M, Huang DC and Bouillet P: BH3-only
proteins and their roles in programmed cell death. Oncogene.
27(Suppl 1): S128–S136. 2008. View Article : Google Scholar
|
|
36
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sha SH, Chen FQ and Schacht J: Activation
of cell death pathways in the inner ear of the aging CBA/J mouse.
Hear Res. 254:92–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|