Introduction
Gastric cancer is the second leading cause of
cancer-related deaths worldwide (1,2),
with a particularly high rate in China (3). Despite recent advances in screening
and treatment, the 5-year survival rate of advanced gastric
adenocarcinoma patients is still low (4). Therefore, improved therapies are
essential. To date, many gastric cancer patients are treated with
chemotherapy, such as 5-fluorouracil (5-FU). Uncontrolled
proliferation, invasion and metastasis are the major poor
prognostic factors associated with advanced gastric cancer
(5). Additionally, there is a
close relationship between inflammation and cancer. This
relationship is not new. It was originally described in 1863 by
Virchow, who hypothesized that cancer originates at sites of
chronic inflammation (6).
Non-resolving inflammation has been linked to the development and
progression of gastric cancer (7,8). In
many cases, invading inflammatory cells can secrete growth factors
or cytokines that act directly on tumor cells or on the stroma to
promote cellular proliferation. Moreover, chemokine receptors and
their ligands have been linked to inflammation and have been
associated with cancer progression (9–12).
Some cancer cells express high levels of chemokine receptors, such
as CXCR1/2 (13–15). The interaction between chemokine
receptors and their ligands has been shown to regulate multiple
aspects of cancer cell biology, including interaction with the
tumor microenvironment (16,17),
cell survival, growth (18),
angiogenesis (16), invasion
(19) and metastasis (11,12,20).
CXCR1/2 are G protein-coupled receptors that bind
interleukin-8 (IL-8) and transduce its signal via a
G-protein-activating second messenger system. CXCR1 and CXCR2 share
76% amino acid identity, with the highest homology occurring within
the transmembrane domains (21).
CXCR1/2 are mainly expressed on neutrophils and were originally
characterized by their ability to induce chemotaxis of leukocytes,
but recent study has demonstrated that these receptors may play a
critical role in certain cancers. CXCR1/2 are overexpressed in
numerous solid tumors and in several cancer cell lines, including
the gastric cancer cell line MKN45 (22,23).
Several studies have correlated expression of CXCR1/2 with
increased proliferation, invasion, metastasis and resistance to
therapy (24–28). Many of these cancer phenotypes are
mediated by the binding of IL-8 to these receptors. In fact, most
primary and metastatic tumors constitutively express IL-8. Thus,
chemokine signaling can promote tumorigenesis and may represent a
useful therapeutic target in cancer.
Despite recent advances (22,29),
the exact role of CXCR1/2 in gastric cancer remains unclear.
However, several studies provide evidence that inhibition of
CXCR1/2 signaling may be a viable strategy for cancer treatment
showing that inhibition of these receptors can slow tumor
progression (30–32). Repertaxin is a non-competitive
allosteric inhibitor of CXCR1/2. Upon binding, repertaxin locks
these receptors in an inactive conformation, which prevents
receptor signaling (33). Although
repertaxin was originally developed to inhibit CXCR1/2 function to
reduce reperfusion injury (34,35),
the importance of chemokine signaling in cancer raises the
possibility that repertaxin may also be useful as an anticancer
agent. In fact, clinical phase I studies using this compound to
treat reperfusion injury have demonstrated a lack of toxicity
(26). This finding significantly
increases the potential utility of repertaxin as an anticancer
therapy (26). While some reports
have begun to explore this (36,37),
the potential efficacy of repertaxin in gastric cancer has not been
examined.
In the present study, we used both gastric cancer
cell lines and mouse xenograft models to investigate the effects of
the CXCR1/2 inhibitor repertaxin, either alone or in combination
with the commonly administered gastric cancer chemotherapy 5-FU. We
assessed the effect of repertaxin and 5-FU on several tumorigenic
phenotypes, including proliferation, cell cycle, apoptosis and
migration and invasion. We found that combined administration of
repertaxin and 5-FU significantly attenuates many of these
phenotypes when compared to treatment with either compound alone,
suggesting that combined inhibition may be clinically useful for
the treatment of gastric cancer.
Materials and methods
Cell culture
The poorly differentiated gastric carcinoma cell
line MKN45 was obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China) and maintained in
Dulbecco's modified Eagle's medium (DMEM; (Gibco-BRL, Life
Technologies, Gaithersburg, MD, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco-BRL) and 1% penicillin/streptomycin
(Invitrogen, Carlsbad, CA, USA) in a humidified cell culture
incubator at 37°C and in 5% CO2.
Detection of IL-8 by ELISA
The level of soluble IL-8 secreted into the culture
medium of MKN45 cells was performed using enzyme
linked-immunosorbent assay (ELISA; Sigma-Aldrich, St. Louis, CA,
USA) according to the manufacturer's instructions. In brief,
related reagents and samples were prepared, and the samples
containing IL-8 were added to each well at 100 μl. After incubation
for 90 min, plates were incubated with a biotinylated antibody.
Immunoreactivity was determined using the avidin-HRP-TMB detection
system. The reactions were stopped by addition of TMB stop buffer,
and absorbance was measured at 450 nm using a microplate reader
(Bio-Rad 680). A curve of the absorbance vs. concentrations of
standard wells was plotted. The concentration of human IL-8 in the
tested samples was determined by comparing the absorbance of the
samples to the standard curve.
Cell viability assay
Cells were seeded in a 96-well plate at a density of
5,000 cells/well for MTT assays. Treatments with different
concentrations of repertaxin (dissolved in 0.9% saline) alone
(0.025, 0.25, 2.5, 25 and 50 μg/ml) (Sigma-Aldrich), 5-FU alone
(0.01, 0.1, 1, 10 and 20 μg/ml) (Shanghai Xudong Haipu
Pharmaceutical, Co., Ltd., Shanghai, China), and repertaxin (0.025,
0.25, 2.5, 25 and 50 μg/ml) in combination with 5-FU (0.01, 0.1, 1,
10 and 20 μg/ml) were administered 12 h after seeding. Cell
viability was determined on days 1, 2, 3, 4 and 5 after treatment
by addition of 20 μl MTT solution (5 mg/ml in PBS) (Sigma-Aldrich).
Cells were incubated in MTT solution for 4 h at 37°C. Next, the
culture medium was removed and replaced with 150 μl DMSO to
dissolve the crystals. Samples were incubated at room temperature
for 10 min, and then absorbance was measured at 568 nm using a
microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Colony forming assay
Cells were seeded in a 6-well plate at a density of
200 cells/well and cultured for two weeks. After the medium was
removed, cells were washed three times with PBS and fixed for 15
min in pure methanol, and then stained for 25 min with crystal
violet (0.5% w/v) (Sigma). Colonies of more than 50 cells were
identified using an inverted microscope (Olympus IX70; Olympus
Corp., Tokyo, Japan). Colony efficiency (CE) and the rate of colony
inhibition (CI) were calculated as follow: CE = (colonies
formed/cells seeded) × 100% and CI = (CE in experimental group/CE
in control group) × 100%, respectively.
Cell cycle analysis
MKN45 cells were plated in 60-mm dishes and grown to
50% confluence. Complete medium was then removed and replaced with
medium containing repertaxin and/or 5-FU. After 48 h, treated cells
were harvested and fixed overnight with cold 70% ethanol at −20°C.
After washing with PBS, the samples were incubated with 50 μg/ml
propidium iodide (PI; Sigma-Aldrich), 100 μg/ml RNase A and 0.2%
Triton X-100 in the dark for 30 min. Flow cytometric analysis was
performed using flow cytometry system and ModFit software (B&D
Biosciences, San Diego, CA, USA) (38). Cell cycle distribution, including
the percentage of cells in G0/G1, S and G2/M was obtained.
Apoptosisassay
Cells were treated with repertaxin and/or 5-FU and
then incubated in buffer containing Annexin V-fluorescein
isothiocyanate (0.5 μg/ml; Annexin V-FITC apoptosis detection kit;
Sigma-Aldrich) and PI (0.6 μg/ml) in the dark, at room temperature
for 15 min. Samples were then measured by flow cytometry (B&D
Biosciences), and the percentage of apoptotic cells was calculated
using CellQuest software.
Cell migration and invasion assay
Cell migration and invasion were performed using
Transwell inserts (Corning Costar) with polycarbonate membranes (8
μm pore size) in 24-well plates. For invasion assays, the filter
was coated with 20 μg/ml Matrigel (Becton-Dickinson) overnight at
4°C. A total of 100 μl cells at 1.0×105/ml in serum-free
medium containing the indicated compounds, were seeded in the upper
chamber. The lower chamber was also treated with compounds in 800
μl culture medium containing 10% FBS. After 24-h incubation at 37°C
in 5% CO2, non-invading cells were removed from the
upper surface of the membrane by gently scrubbing with a cotton
swab. Cells that had successfully invaded the lower surface of the
membrane were fixed with methanol, stained with crystal violet,
rinsed with water and then air dried. The invading cells were
photographed and counted using an inverted microscope in ten random
fields (magnification, ×200). Experimental conditions for migration
assays were similar to those used for invasion assays with the
exception of Matrigel-coated chambers.
Wound-healing assay
Wound-healing assays were performed to assess cancer
cell migration. Briefly, MKN45 cells were seeded at a density of
105 cells/well in a 6-well plate and grown to near
confluent monolayers in DMEM containing 10% FBS. Wounds were made
by scratching the cell monolayer with a sterile 200 μl pipette tip.
The cells were then washed twice with PBS. The scratched areas were
photographed at ×10 magnification using computer-assisted
microscopy (Olympus IX70; Olympus Corp.). Images were captured at
0, 24, 48 and 72 h after the scratch was made. Images were analyzed
using CellProfiler 2.0 cell image analysis software (http://www.cellprofiler.org) (39).
Quantitative real-time reverse
transcriptase-polymerase chain reaction (RT-PCR)
Total RNA from cells was isolated using TRIzol
reagent (Invitrogen) and quantified spectrophotometrically.
Single-stranded cDNA was synthesized using SuperScript First-Strand
Synthesis System (Life Technologies, Carlsbad, CA, USA) according
to the manufacturer's instructions. Sequences of all primers used
are listed in Table I. All
real-time PCR reactions were performed with SYBR-Green Master Mix
kit (all-in-One™ qPCR SYBR-Green I Mix from GeneCopoeia, Rockville,
MD, USA) using an ABI PRISM 7500 sequence detection system. GADPH
was used as an internal loading control. The reactions were
performed as follows: denaturation for 10 min at 95°C, 40 cycles of
95°C for 10 sec, and 60°C for 20 sec. The 2−ΔΔCt method
was performed to calculate the relative mRNA expression of target
genes.
 | Table IPrimer sequences used for
quantitative real-time RT-PCR. |
Table I
Primer sequences used for
quantitative real-time RT-PCR.
| Gene | Forward primer | Reverse primer | Product length
(bp) |
|---|
| GAPDH |
5′-TGAACGGGAAGCTCACTGG-3′ |
5′-TCCACCACCCTGTTGCTGTA-3′ | 307 |
| Bax |
5′-CCCGAGAGGTCTTTTTCCGAG-3′ |
5′-CCAGCCCATGATGGTTCTGAT-3′ | 155 |
| Bcl-2 |
5′-CCTGGGCAATTCCGCATT-3′ |
5′-AACAGGCCACGTAAAGCAAC-3′ | 158 |
| Cyclin D1 |
5′-GCTGCGAAGTGGAAACCATC-3′ |
5′-CCTCCTTCTGCACACATTTGAA-3′ | 135 |
| EGFR |
5′-AGGCACGAGTAACAAGCTCAC-3′ |
5′-ATGAGGACATAACCAGCCACC-3′ | 177 |
| VEGF |
5′-ATTATGCGGATCAAACCTC-3′ |
5′-ATTTCTTGCGCTTTCGTT-3′ | 157 |
| MMP-9 |
5′-ACTACTGTGCCTTTGAGTCC-3′ |
5′-AGAATCGCCAGTACTTCCCA-3′ | 115 |
| MMP-2 |
5′-ACTCTGGACTTAGACCGCTTG-3′ |
5′-ACAGGTTGCAGCTCTCCTTG-3′ | 217 |
| TIMP-2 |
5′-ACCCCTGTTCGCTTCCTGT-3′ |
5′-GGGTCAAATGCTTCCACGAT-3′ | 196 |
| E-cadherin |
5′-GCTAACGTCGTAATCACCAC-3′ |
5′-AATGCCATCGTTGTTCACTG-3′ | 141 |
| Ki-67 |
5′-AGAAGACCTGCTACTCCAAAGA-3′ |
5′-AGTTTGCGTGGCCTGTACTAA-3′ | 70 |
Western blot analysis
Cells were lysed in buffer (20 mM Tris, pH 7.4, 150
mM NaCl, 2 mM EDTA and 1% Triton X-100) containing protease
inhibitor, and then samples were centrifuged at 12,000 rpm for 12
min at 4°C. Equal amounts of protein (50 μg), quantified by BCA
protein assay kit (Pierce Biotechnology, Rockford, IL, USA), were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE, 8–12%). After electrophoresis, separated
proteins were transferred from the gel to polyvinylidene fluoride
(PVDF) membranes (Millipore). Membranes were then incubated in
blocking solution containing 5% non-fat milk for 1.5 h. After
blocking, membranes were incubated with primary antibody.
Phosphorylated antibodies (p-AKT-Ser473, anti-AKT,
p-ERK-Thr202/Tyr204 and anti-ERK1/2) were
obtained from Anbo Biotechnology Co., Ltd. (San Francisco, CA,
USA). Additionally, primary antibodies detecting CXCR1, CXCR2,
Bcl-2, Bax, cyclin D1, EGFR, Ki-67, VEGF, MMP-9, MMP-2, TIMP-2 and
E-cadherin were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA); each of these antibodies were prepared at a 1:500
dilution in TBST overnight at 4°C. Following three washes, the
blots were incubated with the appropriate horseradish peroxidase
conjugated secondary antibody (1:2,000 dilution in TBST) for 1 h at
room temperature. GADPH was used as a loading control. Proteins
were visualized using enhanced chemiluminescence (Pierce
Biotechnology).
MKN45 gastric carcinoma xenograft mouse
model
Four to six-week old athymic female Balb/c-nu/nu
nude mice with a body weight of 15–20 g were obtained from the
Institute of Zoology, Chinese Academy of Sciences (Beijing, China).
Mice were housed in individually ventilated cages with isolated
ventilation at the Animal Care Center of the Third Xiang-ya
Hospital, Central South University under specific pathogen-free
conditions. Animals were allowed to adapt to their environment for
1 week prior to experimentation.
Twenty-four mice were randomly divided into four
groups, and 0.2 ml 1.0×107 MKN45 cells were implanted
subcutaneously (s.c.) into the right back side of each mouse. Once
the tumor reached ~5 mm in size (14 days after cell inoculation),
we initiated treatment with repertaxin alone (30 mg/kg, s.c. next
to the tumor, once every two days for 3 weeks), 5-FU alone (10
mg/kg, s.c. once every two days for 3 weeks), repertaxin plus 5-FU
in combination, or saline (s.c. once every two days for 3 weeks),
which was used as a control. Tumor volume was measured using
digital calipers every other day, and calculated according to the
following formula: [length × (width)2]/2. After three
weeks of therapy, all mice were euthanized with disoprofol
according to the institutional guidelines and local tumors were
resected and analyzed. A portion of the tumor tissue was fixed and
processed for H&E, immunohistochemistry for PCNA and CD34 and
another portion was used for TUNEL staining.
All experimental procedures were conducted in
conformity with the National Institutes of Health Guide for Care
and Use of Laboratory Animals and approved by the Animal Care and
Ethics Committee of Third Xiang-ya Hospital, Central South
University (no. 2012–10). All procedures were in line with the most
recent specifications of the National Research Council (US)
Committee for the Care and Use of Laboratory Animals (2011) Guide
for the Care and Use of Laboratory Animals (National Academies
Press, Washington, DC), 8th edition.
TUNEL
Terminal deoxyribonucleotidyl transferase-mediated
dUTP nick end labeling (TUNEL) assay was performed to assess
apoptosis. Tumor tissue sections were stained according to the
manufacturer's instructions (In situ Cell Death Detection kit,
fluorescein; Roche Diagnostics, Indianapolis, IN, USA; cat. no.
11684795910). Sections were detected with a fluorescent microscope
(Olympus Corp.) and apoptotic cell nuclei were stained in
green.
H&E staining and immunohistochemical
(IHC) analysis
At least 10 serial, thin (4 μm) sections were cut
from each paraffin block. Sections were deparaffinized and then
hydrated for hematoxylin and eosin staining and immunohistochemical
detection of PCNA and CD34. For immunohistochemistry, endogenous
peroxidase activity was inhibited, and then slides were treated
with antigen retrieval buffer (citrate buffer, pH 6.0, at 100°C, 2
min or EDTA buffer, pH 8.0, at 100°C, 2 min in a pressure cooker).
Samples were then blocked in normal goat serum and stained with
primary antibodies including PCNA (Santa Cruz Biotechnology) and
CD34 (Santa Cruz Biotechnology) at 4°C overnight. PBS, instead of
primary antibody, was applied as a negative control. Slides were
washed and then incubated with the appropriate horseradish
peroxidase conjugated secondary antibody for 1 h. DAB was used as a
chromogen and sections were counterstained with hematoxylin.
CD34-positive microvessels were counted under a light microscope
(Olympus Corp.) with 10 high magnification fields each group.
Statistical analysis
The SPSS 13.0 software system (SPSS, Inc., Chicago,
IL, USA) was used for statistical evaluation. Data are expressed as
mean ± standard deviation (SD) for at least 3 independent
experiments. Statistical significance was evaluated by analysis of
variance (ANOVA) with Tukey's for post-hoc test. A probability
value of <0.05 (P<0.05) was considered to indicate a
statistically significant result.
Results
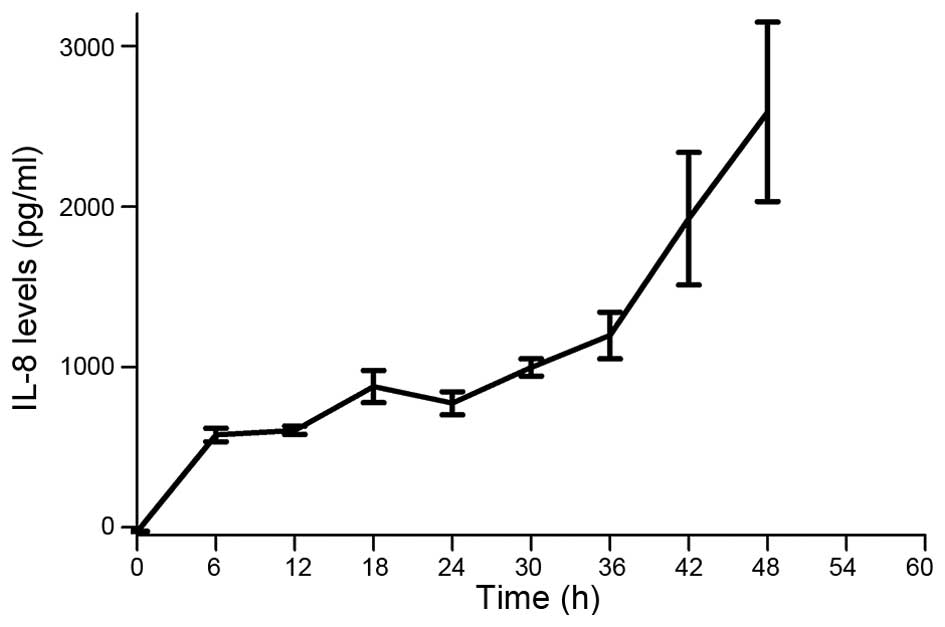
MKN45 cells secrete IL-8
Previously it was established that some tumor cells
secrete IL-8 as an autocrine growth factor, which can bind CXCR1/2
receptors and promote tumor growth, invasion and metastatic spread
(40). We performed ELISA analysis
and found that the gastric cancer cell line MKN45 secretes
significant levels of IL-8 over time (Fig. 1).
Repertaxin combined with 5-FU inhibits
MKN45 cell proliferation and enhances apoptosis
We investigated the effects of repertaxin both alone
and in combination with 5-FU on the proliferation of MKN45 gastric
cancer cells. Cells were treated with multiple concentrations of
repertaxin alone (50, 25, 2.5 0.25 and 0.025 μg/ml) and 5-FU alone
(20, 10, 1, 0.1 and 0.01 μg/ml). Both compounds inhibited
proliferation in a dose-dependent and time-dependent manner
(Fig. 2A and B). Based on these
initial experiments, we selected 25 μg/ml repertaxin and 10 μg/ml
5-FU for follow-up experiments.
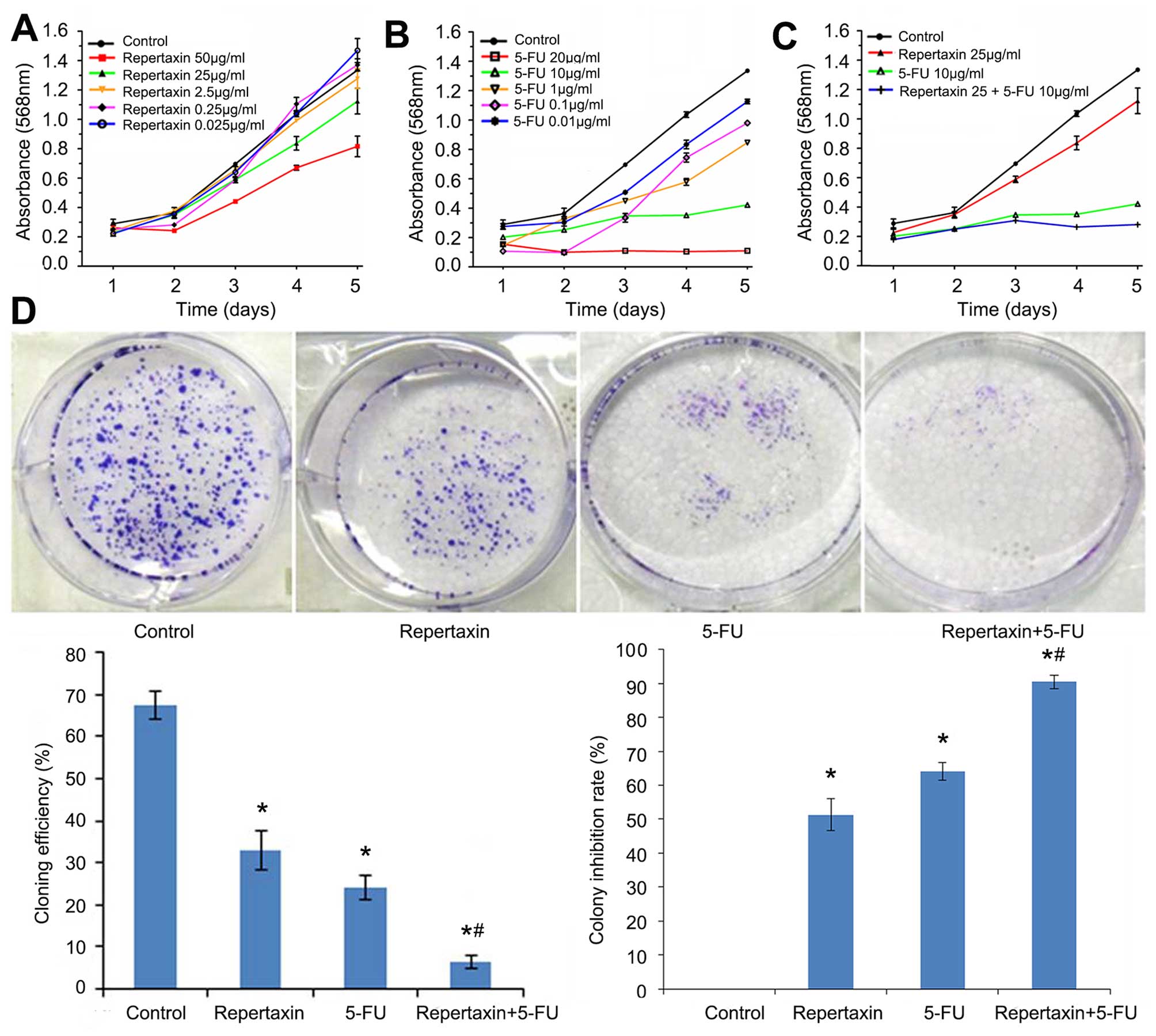 | Figure 2Effects of repertaxin and repertaxin
combined with 5-FU on MKN45 cell proliferation and growth. Cell
proliferation was analyzed by MTT assay. MKN45 cells were treated
with (A) different concentrations of repertaxin alone (0.025, 0.25,
2.5, 25 and 50 μg/ml), (B) 5-FU alone (0.01, 0.1, 1, 10 and 20
μg/ml), and (C) repertaxin (25 μg/ml) in combination with 5-FU (10
μg/ml) for 1, 2, 3, 4 and 5 days. (D) Cell growth was determined by
colony forming assay. MKN45 cells were treated with repertaxin (25
μg/ml) and 5-FU (10 μg/ml) alone or in combination. After two
weeks, colonies of >50 cells were identified under an inverted
microscope. The colony forming efficiency and the colony inhibition
rate were calculated. Data are shown as mean ± SD.
*P<0.05 vs. control group (0.9% normal saline);
#P<0.05 repertaxin+5-FU vs. 5-FU. |
As shown in Fig.
2C, the anti-proliferative effect of repertaxin in combination
with 5-FU was better than that of repertaxin or 5-FU alone. Similar
effects were observed in colony formation assays. That is, the
combined treatment of repertaxin and 5-FU more significantly
(P<0.05) inhibited colony formation compared to either compound
administered individually (Fig.
2D).
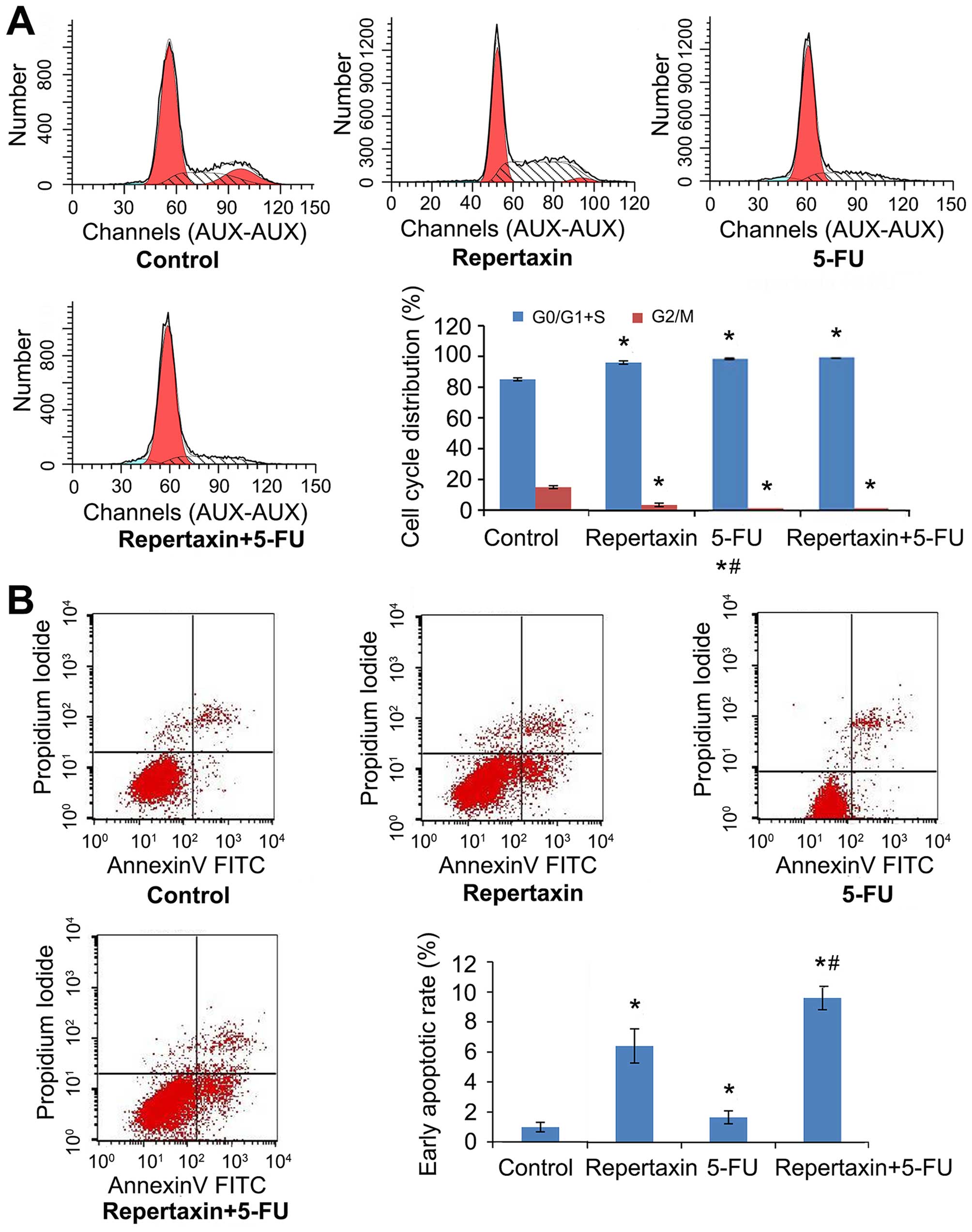
We next examined the effect of repertaxin and 5-FU
on cell cycle progression by performing flow cytometric analysis.
Compared to control, repertaxin increased the percentage of cells
in G0/G1 and S phases (96.33 compared to 85.07% in controls) and
decreased the percentage of cells in G2/M phase (3.67 compared to
14.93% in controls) (Fig. 3A).
These observations were made 24 h after repertaxin (25 μg/ml)
treatment. Additionally, the combination of repertaxin and 5-FU
performed better than either compound alone (Fig. 3A). We also performed Annexin V/PI
staining to assess the effect of repertaxin and 5-FU on apoptosis
of MKN45 cells. Repertaxin alone (25 μg/ml) increased the
percentage of cells undergoing early (Annexin
V+/PI−) apoptosis (Fig. 3B). Importantly, the early apoptotic
rates of repertaxin alone, 5-FU alone, and repertaxin plus 5-FU
were 6.43±1.14, 2.21±0.33 and 9.63±0.78%, respectively. Although
all treatment groups were significantly different from controls
(1.00±0.32%) (P<0.05), the combination performed significantly
better than either agent alone (P<0.05).
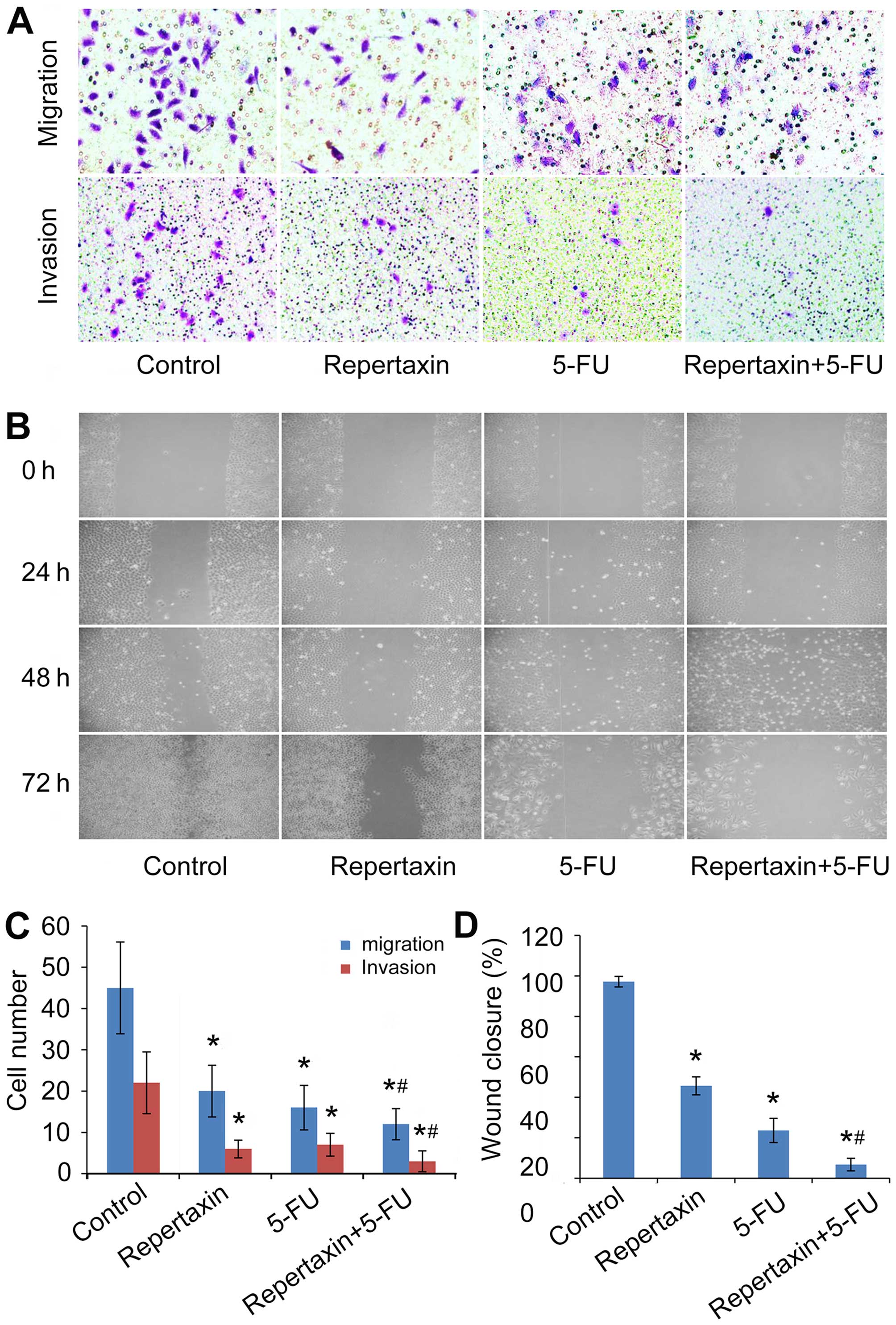
Repertaxin combined with 5-FU inhibits
MKN45 cell migration and invasion
In addition to effects on cellular proliferation,
CXCR1/2 can also influence the migration and invasion of certain
tumor cells (41). In the present
study, we examined the effect of repertaxin and 5-FU on the
migratory and invasive behavior of MKN45 cells. Repertaxin (25
μg/ml) significantly attenuated (P<0.05) chemotaxis migration of
MKN45 cells compared to controls (Fig.
4A and C). Similarly, the number of invading cells was also
decreased by repertaxin in an in vitro Matrigel invasion
assay (Fig. 4A and C). Even
further inhibition was observed when repertaxin was combined with
5-FU (10 μg/ml) (Fig. 4A and C).
In agreement with results from the Transwell assay, data from the
wound healing assay also showed significantly improved inhibition
of wound closure in the repertaxin-5-FU combination treatment group
compared to either the control group or the individual treatments
alone (P<0.05) (Fig. 4B and
D).
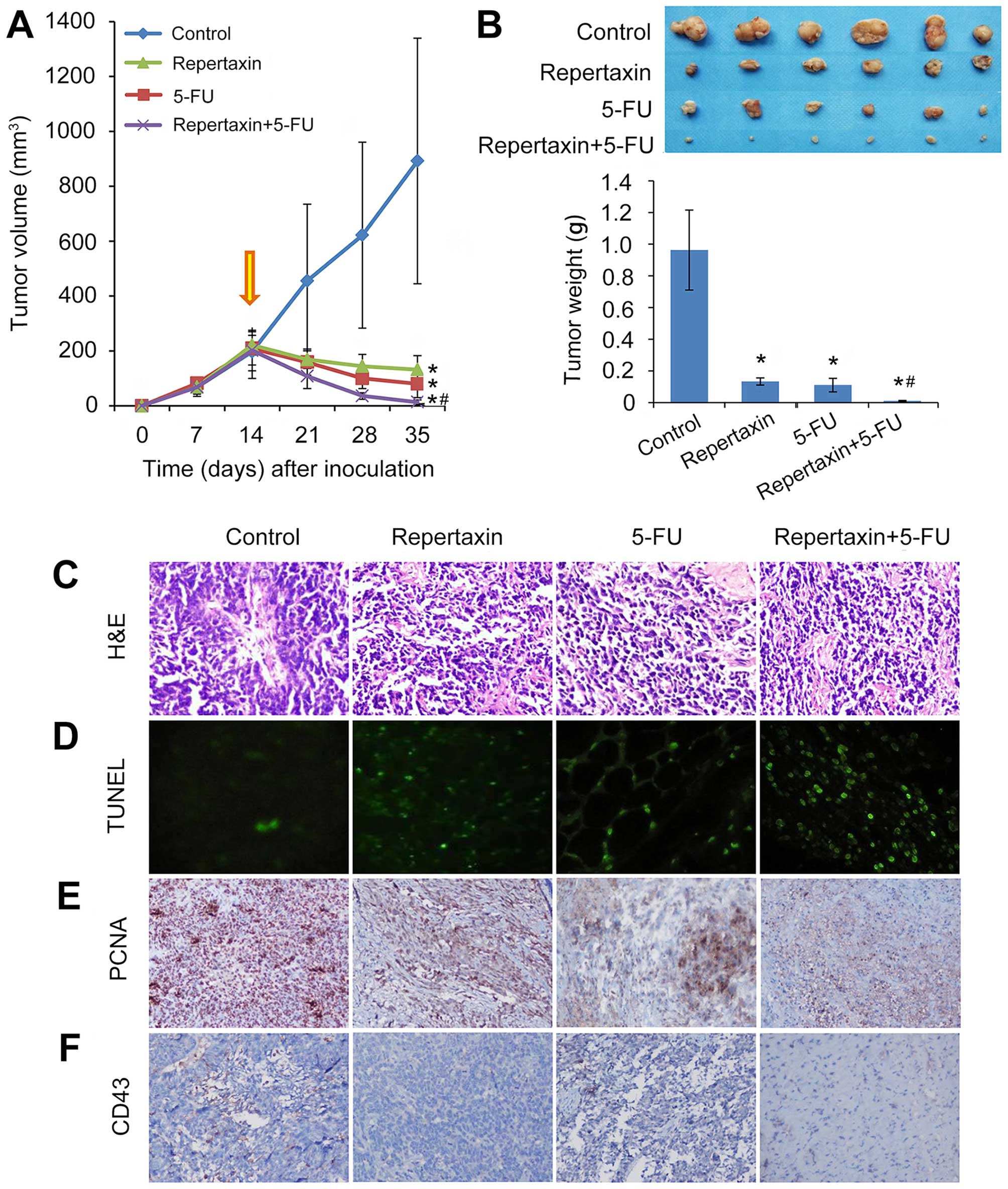
Repertaxin combined with 5-FU
significantly reduces gastric cancer cell tumorigenicity and
angiogenesis in nude mouse xenografts
To characterize the in vivo effects of
repertaxin alone and in combination with 5-FU, we established in
vivo MKN45 xenograft models in nude mice. Mice treated with
either repertaxin (30 mg/kg) or 5-FU (10 mg/kg) alone showed
significant reduction in tumor volume and weight compared to
control-treated mice (Fig. 5A and
B). Importantly, combined administration of repertaxin and 5-FU
performed better at reducing xenograft tumor growth compared to
either agent alone (both P<0.05) (Fig. 5A and B). All treatments were well
tolerated, and we did not observe any signs of general toxicity or
body weight loss during therapy. Taken together, our findings
suggest that combination therapy of repertaxin and 5-FU may
cooperate to effectively reduce gastric cancer tumor growth in
vivo.
This ability of combination treatment to repress
tumor growth was followed up with immunohistochemical staining of
tumor sections isolated from each of the treatment groups.
Thirty-five days following treatment, tumors were excised and
subject to molecular analysis. H&E staining showed large number
of tumor cells, more heterogeneity and pathological mitosis in the
controls, but repertaxin and 5-FU alone or in combination
significantly reduced the number of tumor cells, showing less
heterogeneity and pathological mitosis (Fig. 5C).
TUNEL staining showed that repertaxin and 5-FU were
both individually capable of inducing apoptosis (Fig. 5D); importantly, this effect was
further increased when both compounds were administered
together.
We next performed PCNA staining to assess the
effects on proliferation in vivo (42). Repertaxin alone significantly
reduced the number of PCNA-positive cells, and combined treatment
with 5-FU may have even further decreased the number of
proliferating cells (Fig. 5E).
Analysis of apoptosis and proliferation was
complemented by examination of angiogenesis, a critical component
of gastric cancer growth and metastasis (43,44).
Furthermore, the relationship between CXCR1/2 and tumor
angiogenesis is well-established (45). The extent of neovascularization of
transplanted tumor in nude mice was examined by staining tumor
sections with an anti-CD34 antibody and determining microvessel
density (MVD) (Fig. 5F). Treatment
with repertaxin or 5-FU alone decreased MVD, and the combination of
these two compounds may have further decreased the number of
MVD/each high-power field (MVD: repertaxin +5-FU: 3.1±1.7;
repertaxin: 3.7±1.6; 5-FU: 4.1±1.4 and controls: 6.1±1.9) (Table II).
 | Table IIThe number of MVD in transplanted
tumor of nude mice. |
Table II
The number of MVD in transplanted
tumor of nude mice.
| Groups | Control | 5-FU | Repertaxin | Repertaxin +
5-FU |
|---|
| Mean | 6.1 | 4.1 | 3.7 | 3.1 |
| SD | 1.9 | 1.4 | 1.6 | 1.7 |
Repertaxin treatment inhibits AKT and
ERK1/2 phosphorylation in gastric cancer MKN45 cells
We next sought to determine which signaling pathways
were activated downstream of CXCR1/2. Since AKT and ERK1/2 are well
characterized regulators of cell growth, survival and invasion
(46,47), we examined the effect of the
CXCR1/2 inhibitor repertaxin on phosphorylation of these kinases.
Treatment of gastric cancer MKN45 cells with either repertaxin
alone or in combination with 5-FU for 48 h significantly
downregulated phosphorylation of both AKT and ERK1/2 compared to
control cells (Fig. 6E). These
findings suggest that AKT and ERK1/2 signaling may be key
downstream mediators of CXCR1/2 signaling in gastric cancer cells,
and that inhibition of these kinases may be responsible, at least
in part, for the anti-proliferative, anti-invasive and
anti-angiogenic activity of repertaxin.
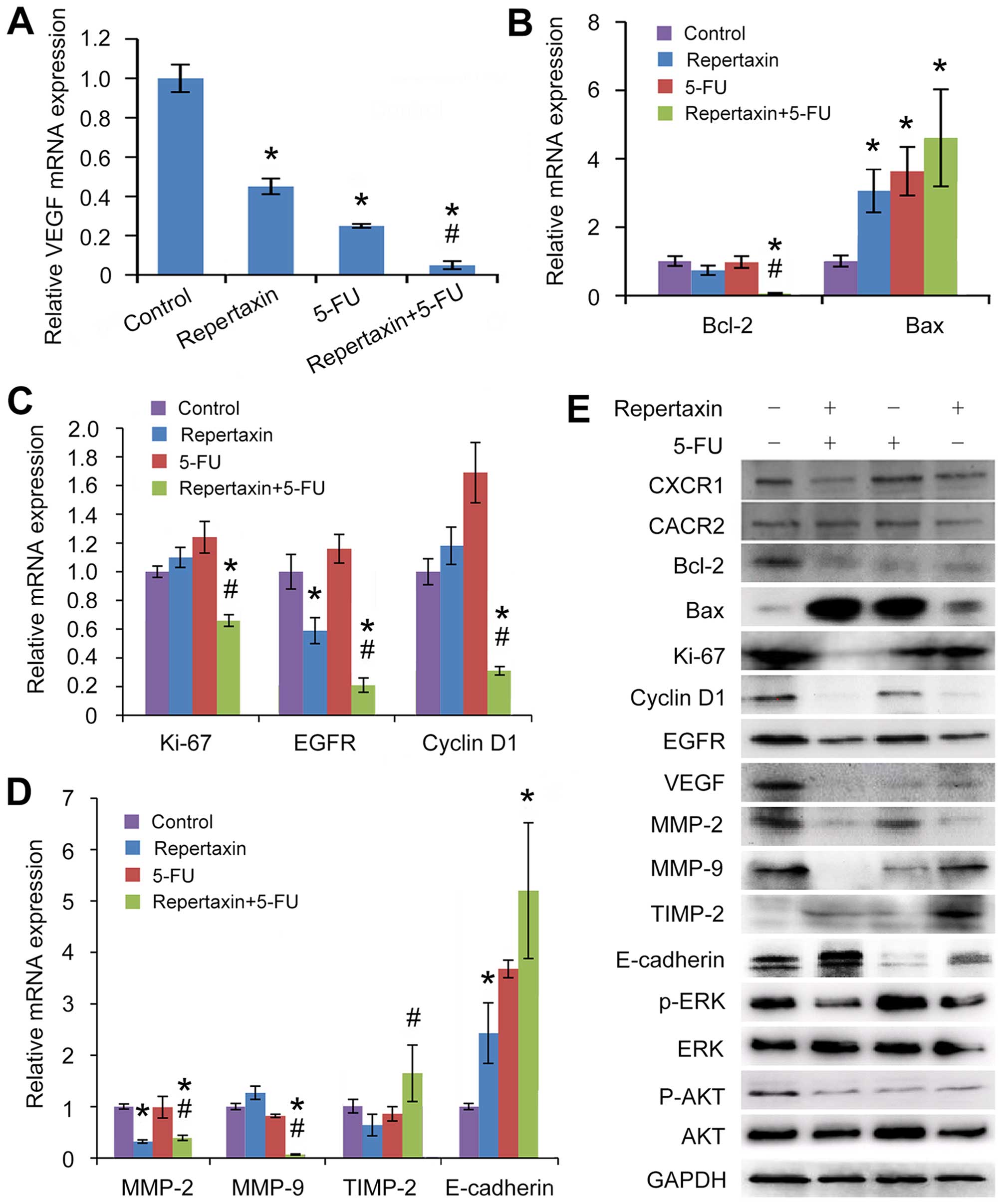 | Figure 6Effects of repertaxin and repertaxin
combined with 5-FU on cell proliferation, cell cycle, cell
apoptosis, cell migration and invasion-related signaling molecules
in the MKN45 cells. MKN45 cells were treated with repertaxin (25
μg/ml) alone, 5-FU (10 μg/ml) alone, or combined repertaxin and
5-FU for 48 h. (A) angiogenesis (VEGF), (B) apoptosis (Bcl-2 and
Bax), (C) proliferation and growth (cyclin D1, EGFR and Ki-67), (D)
invasion and metastasis (MMP-9, MMP-2, TIMP-2 and E-cadherin). mRNA
expression was determined by real-time RT-PCR. GAPDH was used as an
internal control. (E) Cell lysates (50 μg) were fractionated by
SDS-PAGE and subject to western blot analysis; GAPDH was used as a
loading control. Data are shown as mean ± SD. *P<0.05
vs. control group; #P<0.05 combined treatment group
vs. 5-FU alone group. |
Repertaxin combined with 5-FU alters
expression of several proteins involved in cell cycle progression,
apoptosis, migration and angiogenesis
We previously showed that repertaxin in combination
with 5-FU induces apoptosis of MKN45 cells. To gain insight into
this regulation, we examined the effect of these compounds on
expression of key apoptosis regulators Bax and Bcl-2 (48). We found that repertaxin
significantly decreased expression of the anti-apoptotic molecule
Bcl-2 at both the mRNA and protein levels (Fig. 6B and E). In contrast, repertaxin
increased expression of the pro-apoptotic molecule Bax (Fig. 6B and E). These findings suggest
that repertaxin may influence apoptosis of gastric cancer cells by
regulating the Bax/Bcl-2 ratio.
To better understand how repertaxin regulates
proliferation of MKN45 cells, we examined expression of cyclin D1,
EGFR and Ki-67. While repertaxin significantly decreased mRNA
expression of EGFR (Fig. 6C),
repertaxin alone had no effect on transcript levels of Ki-67 or
cyclin D1 (Fig. 6C); in contrast,
repertaxin was able to decrease expression of all three molecules
at the protein level (Fig. 6E).
Notably, repertaxin plus 5-FU significantly decreased expression of
EGFR, cyclin D1, and Ki-67 at both the mRNA and protein levels
(Fig. 6C and E). These results
suggest that repertaxin alone or in combination with 5-FU may
induce cell cycle arrest and inhibit proliferation by reducing
levels of cyclin D1, EGFR and Ki-67.
MMP-9, MMP-2, TIMP-2 and E-cadherin are all
regulators of cellular migration and invasion. Repertaxin alone
significantly decreased mRNA expression of MMP-2 and increased
transcript levels of E-cadherin (Fig.
6D). Combination treatment with repertaxin and 5-FU
significantly altered mRNA levels of MMP-2, MMP-9, TIMP-2 and
E-cadherin (Fig. 6D). Consistent
with these findings, protein levels of the molecules were similarly
regulated by combined administration of repertaxin and 5-FU
(Fig. 6E). Taken together, our
data suggest that repertaxin and 5-FU may regulate gastric cancer
cell migration and invasion via regulation of MMP-2, MMP-9, TIMP-2
and E-cadherin.
Finally, we examined the effect of repertaxin and
5-FU on expression of the key angiogenesis regulator VEGF.
Expression of VEGF at both the mRNA (Fig. 6A) and protein (Fig. 6E) levels was most significantly
decreased by combined treatment of repertaxin and 5-FU, suggesting
that these compounds regulate angiogenesis by altering levels of
VEGF in these cells.
Discussion
CXCR1/2 are expressed at high levels in a number of
solid tumors, including gastric cancer. Chemokine signaling has
been shown to regulate a number of tumorigenic processes, including
proliferation, angiogenesis, survival, invasion and metastasis
(13,20,49).
However, the exact role of CXCR1/2 signaling in gastric cancer
remains poorly characterized. In the present study, we show that
gastric cancer MKN45 cells secrete IL-8, the ligand for CXCR1/2.
Additionally, we show that small molecule inhibition of CXCR1/2
with repertaxin block many tumorigenic phenotypes of gastric cancer
cells. Moreover, repertaxin can synergize with 5-FU, a commonly
administered chemotherapy for gastric cancer treatment. Our data
presented suggest that combined administration of repertaxin and
5-FU may be a useful therapeutic regimen for certain gastric cancer
patients.
We found that repertaxin treatment decreases
proliferation and colony-forming ability of MKN45 cells. This is
consistent with other reports describing a role for chemokine
signaling in various tumor types. We also find potentiated
inhibition when repertaxin is combined with 5-FU. Although the
exact mechanism of the enhanced effect of these compounds is
unknown, it likely involves a combination of disrupted RNA
synthesis (5-FU's primary mechanism of action) and CXCR1/2
blockade. However, much more work is required to completely
understand this interaction. Interference with transcription is
toxic to the cell, and we provide evidence here that inhibition of
CXCR1/2 with repertaxin decreases expression of several molecules
that regulate growth and survival. Thus, the combination of these
two inhibitors is likely very toxic to cancer cells.
To begin to better understand the mechanism of
action of these compounds, we examined expression of key downstream
mediators of CXCR1/2 signaling. Previous study has shown that cells
overexpressing CXCR1/2 exhibit increased levels of AKT
phosphorylation (50). These
findings suggest that AKT may act downstream of CXCR1/2 in gastric
cancer cells as well. Additionally, IL-8-mediated activation of
CXCR1/2 has been shown to activate phosphatidylinositol-3-kinase,
which is upstream of AKT (51).
CXCR1/2 signaling has also been shown to regulate the activity of
MAPK signaling (51–53). Our previous data also verified that
strong CXCR1/2 expression was positively associated with the
phosphorylation of AKT and ERK1/2 (37). Thus, we examined the effect of
repertaxin and 5-FU on phosphorylation of these molecules. We find
that combined treatment of repertaxin and 5-FU significantly
inhibited phosphorylation and activation of both AKT and ERK.
Increased AKT expression is correlated with poor prognosis in
tumors (47). Taken together, our
data suggest that repertaxin and 5-FU may slow gastric cancer cell
growth via inhibition of AKT and ERK signaling. This is in line
with previous study, and also raises the possibility that AKT or
ERK inhibitors may potentially be useful also in combination with
repertaxin or 5-FU.
Furthermore, we found that repertaxin and 5-FU
altered expression of several other molecules, such as Bax, Bcl-2,
MMP-2, MMP-9, VEGF and E-cadherin, all of which are important
regulators of apoptosis, migration and angiogenesis. It is
currently unclear whether which, if any, of these downstream
pathways are the essential mediators downstream of CXCR1/2
signaling in gastric cancer. More work is required to identify
which pathways are most critical at mediating the inhibitory
effects of repertaxin in these cells. The fact that we observed
similar effects in mouse xenograft models treated in vivo
suggests that this may represent a useful therapeutic strategy.
Although repertaxin and 5-FU had a more pronounced
effect when administered together, the exact mechanism underlying
this interaction is unknown. One possibility is that 5-FU when
administered alone resulted in apoptosis and concomitant increase
in IL-8 secretion. This secreted IL-8 could act through CXCR1/2 to
protect tumor cells against chemotherapy (23). However, this would sensitize the
cells to CXCR1/2 inhibition. Thus, in this setting, repertaxin
would be more effective at inhibition of gastric cancer cell
tumorigenesis. Although this represents one possible mechanism of
action, further experimentation is necessary to determine the
precise mechanism of action.
In summary, repertaxin alone or repertaxin in
combination with 5-FU inhibits gastric cancer cell proliferation,
survival, and migration both in vitro and in vivo.
The present study suggests that targeting CXCR1/2 may represent a
novel strategy for gastric cancer therapy, and that combined
CXCR1/2 inhibition and 5-FU chemotherapy may be a useful
therapeutic approach for certain gastric cancer patients.
Acknowledgements
The present study was partially supported by the
China Postdoctoral Science Foundation (no. 2014M562137), the Hunan
Provincial Innovation Foundation for Postgraduate (no. CX2011B046),
and the Science and Technology Program Foundation of Changsha City
(nos. K1005005-31 and K1106041-31).
References
|
1
|
Alberts SR, Cervantes A and van de Velde
CJ: Gastric cancer: Epidemiology, pathology and treatment. Ann
Oncol. 14(Suppl 2): ii31–ii36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
|
|
3
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.PubMed/NCBI
|
|
4
|
Shah MA and Kelsen DP: Gastric cancer: A
primer on the epidemiology and biology of the disease and an
overview of the medical management of advanced disease. J Natl
Compr Canc Netw. 8:437–447. 2010.PubMed/NCBI
|
|
5
|
Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW,
Lah KH, Lee JH, Choi SH and Min JS: Prognostic significance of
meta-static lymph node ratio in T3 gastric cancer. World J Surg.
26:323–329. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nathan C and Ding A: Nonresolving
inflammation. Cell. 140:871–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani A: Cancer: Inflaming metastasis.
Nature. 457:36–37. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Righi E, Kashiwagi S, Yuan J, Santosuosso
M, Leblanc P, Ingraham R, Forbes B, Edelblute B, Collette B, Xing
D, et al: CXCL12/CXCR4 blockade induces multimodal antitumor
effects that prolong survival in an immunocompetent mouse model of
ovarian cancer. Cancer Res. 71:5522–5534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wendel C, Hemping-Bovenkerk A, Krasnyanska
J, Mees ST, Kochetkova M, Stoeppeler S and Haier J: CXCR4/CXCL12
participate in extravasation of metastasizing breast cancer cells
within the liver in a rat model. PLoS One. 7:e300462012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balkwill FR: The chemokine system and
cancer. J Pathol. 226:148–157. 2012. View Article : Google Scholar
|
|
12
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: New insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ali S and Lazennec G: Chemokines: Novel
targets for breast cancer metastasis. Cancer Metastasis Rev.
26:401–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vindrieux D, Escobar P and Lazennec G:
Emerging roles of chemokines in prostate cancer. Endocr Relat
Cancer. 16:663–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Colmone A, Amorim M, Pontier AL, Wang S,
Jablonski E and Sipkins DA: Leukemic cells create bone marrow
niches that disrupt the behavior of normal hematopoietic progenitor
cells. Science. 322:1861–1865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh S, Varney M and Singh RK: Host
CXCR2-dependent regulation of melanoma growth, angiogenesis, and
experimental lung metastasis. Cancer Res. 69:411–415. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitamura T, Kometani K, Hashida H,
Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M,
Takabayashi A, et al: SMAD4-deficient intestinal tumors recruit
CCR1+ myeloid cells that promote invasion. Nat Genet.
39:467–475. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buonamici S, Trimarchi T, Ruocco MG,
Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng JC, Sen
F, et al: CCR7 signalling as an essential regulator of CNS
infiltration in T-cell leukaemia. Nature. 459:1000–1004. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murphy PM: The molecular biology of
leukocyte chemoattractant receptors. Annu Rev Immunol. 12:593–633.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin BR, Chang CC, Chen LR, Wu MH, Wang MY,
Kuo IH, Chu CY, Chang KJ, Lee PH, Chen WJ, et al: Cysteine-rich 61
(CCN1) enhances chemotactic migration, transendothelial cell
migration, and intravasation by concomitantly up-regulating
chemokine receptor 1 and 2. Mol Cancer Res. 5:1111–1123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitadai Y, Haruma K, Mukaida N, Ohmoto Y,
Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ,
et al: Regulation of disease-progression genes in human gastric
carcinoma cells by interleukin 8. Clin Cancer Res. 6:2735–2740.
2000.PubMed/NCBI
|
|
24
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Wang L, Zhang M, Jin M, Bai C and
Wang X: Potential mechanism of interleukin-8 production from lung
cancer cells: An involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J
Cell Physiol. 227:35–43. 2012. View Article : Google Scholar
|
|
26
|
Ginestier C, Liu S, Diebel ME, Korkaya H,
Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum
D, et al: CXCR1 blockade selectively targets human breast cancer
stem cells in vitro and in xenografts. J Clin Invest. 120:485–497.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh S, Nannuru KC, Sadanandam A, Varney
ML and Singh RK: CXCR1 and CXCR2 enhances human melanoma
tumourigenesis, growth and invasion. Br J Cancer. 100:1638–1646.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varney ML, Singh S, Li A, Mayer-Ezell R,
Bond R and Singh RK: Small molecule antagonists for CXCR2 and CXCR1
inhibit human colon cancer liver metastases. Cancer Lett.
300:180–188. 2011. View Article : Google Scholar
|
|
29
|
Eck M, Schmausser B, Scheller K, Brändlein
S and Müller-Hermelink HK: Pleiotropic effects of CXC chemokines in
gastric carcinoma: Differences in CXCL8 and CXCL1 expression
between diffuse and intestinal types of gastric carcinoma. Clin Exp
Immunol. 134:508–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar
|
|
31
|
Ning Y, Labonte MJ, Zhang W, Bohanes PO,
Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Nagulapalli
Venkata KC, et al: The CXCR2 antagonist, SCH-527123, shows
antitumor activity and sensitizes cells to oxaliplatin in
preclinical colon cancer models. Mol Cancer Ther. 11:1353–1364.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh S, Sadanandam A, Nannuru KC, Varney
ML, Mayer-Ezell R, Bond R and Singh RK: Small-molecule antagonists
for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing
tumor cell proliferation, survival, and angiogenesis. Clin Cancer
Res. 15:2380–2386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Casilli F, Bianchini A, Gloaguen I, Biordi
L, Alesse E, Festuccia C, Cavalieri B, Strippoli R, Cervellera MN,
Di Bitondo R, et al: Inhibition of interleukin-8 (CXCL8/IL-8)
responses by repertaxin, a new inhibitor of the chemokine receptors
CXCR1 and CXCR2. Biochem Pharmacol. 69:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bertini R, Allegretti M, Bizzarri C,
Moriconi A, Locati M, Zampella G, Cervellera MN, Di Cioccio V,
Cesta MC, Galliera E, et al: Noncompetitive allosteric inhibitors
of the inflammatory chemokine receptors CXCR1 and CXCR2: Prevention
of reperfusion injury. Proc Natl Acad Sci USA. 101:11791–11796.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clarke C, Kuboki S, Sakai N, Kasten KR,
Tevar AD, Schuster R, Blanchard J, Caldwell CC, Edwards MJ and
Lentsch AB: CXC chemokine receptor-1 is expressed by hepatocytes
and regulates liver recovery after hepatic ischemia/reperfusion
injury. Hepatology. 53:261–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JP, Hu WM, Wang KS, Luo BH, Wu C,
Chen ZH, Luo GQ, Liu YW, Liu QL, Yu J, et al: Upregulation of C-X-C
chemokine receptor type 1 expression is associated with late-stage
gastric adenocarcinoma. Exp Ther Med. 4:55–60. 2012.PubMed/NCBI
|
|
37
|
Wang JP, Hu WM, Wang KS, Yu J, Luo BH, Wu
C, Chen ZH, Luo GQ, Liu YW, Liu QL, et al: Expression of C-X-C
chemokine receptor types 1/2 in patients with gastric carcinoma:
Clinicopathological correlations and significance. Oncol Lett.
5:574–582. 2013.PubMed/NCBI
|
|
38
|
Kim JG, Kang MJ, Yoon YK, Kim HP, Park J,
Song SH, Han SW, Park JW, Kang GH, Kang KW, et al:
Heterodimerization of glycosylated insulin-like growth factor-1
receptors and insulin receptors in cancer cells sensitive to
anti-IGF1R antibody. PLoS One. 7:e333222012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lecomte N, Njardarson JT, Nagorny P, Yang
G, Downey R, Ouerfelli O, Moore MA and Danishefsky SJ: Emergence of
potent inhibitors of metastasis in lung cancer via syntheses based
on migrastatin. Proc Natl Acad Sci USA. 108:15074–15078. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuo Y, Ochi N, Sawai H, Yasuda A,
Takahashi H, Funahashi H, Takeyama H, Tong Z and Guha S: CXCL8/IL-8
and CXCL12/SDF-1alpha co-operatively promote invasiveness and
angiogenesis in pancreatic cancer. Int J Cancer. 124:853–861. 2009.
View Article : Google Scholar :
|
|
41
|
Singh S, Sadanandam A, Varney ML, Nannuru
KC and Singh RK: Small interfering RNA-mediated CXCR1 or CXCR2
knock-down inhibits melanoma tumor growth and invasion. Int J
Cancer. 126:328–336. 2010. View Article : Google Scholar
|
|
42
|
Hall PA, Levison DA, Woods AL, Yu CC,
Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover
R, et al: Proliferating cell nuclear antigen (PCNA)
immunolocalization in paraffin sections: An index of cell
proliferation with evidence of deregulated expression in some
neoplasms. J Pathol. 162:285–294. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Che X, Hokita S, Natsugoe S, Tanabe G,
Baba M, Takao S and Aikou T: Tumor angiogenesis related to growth
pattern and lymph node metastasis in early gastric cancer. Chin Med
J (Engl). 111:1090–1093. 1998.
|
|
44
|
Kitadai Y: Angiogenesis and
lymphangiogenesis of gastric cancer. J Oncol. 2010:4687252010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singh S, Sadanandam A and Singh RK:
Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis
Rev. 26:453–467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Smalley KS: A pivotal role for ERK in the
oncogenic behaviour of malignant melanoma? Int J Cancer.
104:527–532. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng GZ, Park S, Shu S, He L, Kong W,
Zhang W, Yuan Z, Wang LH and Cheng JQ: Advances of AKT pathway in
human oncogenesis and as a target for anti-cancer drug discovery.
Curr Cancer Drug Targets. 8:2–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xue M, Ge Y, Zhang J, Wang Q, Hou L, Liu
Y, Sun L and Li Q: Anticancer properties and mechanisms of fucoidan
on mouse breast cancer in vitro and in vivo. PLoS One.
7:e434832012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Verbeke H, Geboes K, Van Damme J and
Struyf S: The role of CXC chemokines in the transition of chronic
inflammation to esophageal and gastric cancer. Biochim Biophys
Acta. 1825.117–129. 2012.
|
|
50
|
Lane HC, Anand AR and Ganju RK: Cbl and
Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated
chemo-taxis. Int Immunol. 18:1315–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Venkatakrishnan G, Salgia R and Groopman
JE: Chemokine receptors CXCR-1/2 activate mitogen-activated protein
kinase via the epidermal growth factor receptor in ovarian cancer
cells. J Biol Chem. 275:6868–6875. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
MacManus CF, Pettigrew J, Seaton A, Wilson
C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG and
Waugh DJ: Interleukin-8 signaling promotes translational regulation
of cyclin D in androgen-independent prostate cancer cells. Mol
Cancer Res. 5:737–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luppi F, Longo AM, de Boer WI, Rabe KF and
Hiemstra PS: Interleukin-8 stimulates cell proliferation in
non-small cell lung cancer through epidermal growth factor receptor
transactivation. Lung Cancer. 56:25–33. 2007. View Article : Google Scholar
|