Introduction
Human cancers harbor elevated levels of reactive
oxygen species and possess increased oxidative stress due to
enhanced metabolism, peroxisomal and inflammatory activities
(1). Such a heightened metabolic
stress has been strongly linked to carcinogenesis via the oxidative
and nitrosative damage to DNA, protein and lipids. On the contrary,
it has become increasingly apparent that the redox-stress and redox
regulatory mechanisms prevalent in cancers may have significant
therapeutic implications (2). A
huge endeavor is being undertaken to exploit the redox imbalance in
cancers for developing novel therapeutic strategies and
preferentially eliminating the tumor cells (1,2).
Evidence indicates that human malignancies respond to even slight
oscillations in their redox milieu with an array of adaptive
protein modifications, signaling and gene expression changes, which
can render them vulnerable to drugs and natural compounds (1). Piperlongumine (PL) obtained from the
fruits and roots of the long pepper plant is a pyridine alkaloid
that has been reported to selectively exert cytotoxicity in a wide
variety of tumor cell types both in vitro and in
vivo, while sparing non-cancerous normal cell types (3). Although the exact mechanism has not
been elucidated, the anticancer effects of PL have been attributed
to its pharmacophore containing two active double bonds (C2–C3 and
C7–C8 olefins) that act as Michael acceptors and increase the
levels of reactive oxygen species (4). PL directly binds to and inhibits the
antioxidant enzyme glutathione S-transferase π (GSTP1) resulting in
a decrease in glutathione levels and subsequent promotion of
cancer-selective cell death by increasing the ROS levels (3). In addition, PL was found to suppress
NF-κB transcription factor by inhibiting cys179 indicating that
thiol manipulation might play a crucial role in its anticancer
effects (5).
p53, also known as the guardian of the genome is
found to be mutated in >50% of all human cancers and these
mutations drive the emergence of oncogenic genomes and aggressive
malignancies (6,7). p53 mutations are highly diverse in
their type, position, sequence context and structural impact and
can be broadly classified into groups, namely, the DNA contact
mutants (p53R273H) where p53 contact with its recognition sequence
is disrupted, and the conformational mutants (p53R175H) where
structural alterations in the protein mediate the loss of binding
to DNA (8). Most p53 mutations
confer drug resistance to cancer cells through the impairment of
apoptosis by inducing the expression of anti-apoptotic proteins
like Bcl-2 and reducing the expression of proapoptotic proteins
like Bax and PUMA (9).
A number of reports have been published addressing
the reactivation of mutant p53 into its functional form for
improved and efficient anticancer therapies (10). Among them, the gene therapy to
express the wild-type tumor suppressor and oncolytic adenovirus
Onyx 015 has been successful to some extent but are still under
investigation due to lack of suitable delivery systems (11). Inhibitors of MDM2-p53 interaction
showed promising effects and one of them, nutlin-3 is undergoing
clinical trial (12). Several
small-molecule screening studies have led to the identification of
compounds such as PRIMA-1 (13),
MIRA-1 (14), CP-31398 (15), STIMA-1 (16), SCH529074 (17), and NSC319726 (18) that demonstrated the ability to
reactivate the mutant p53 protein and confer biological functions
such as the activation of the target gene expression. Many of these
compounds share a unique feature of possessing chemically active,
highly electrophilic double bonds that participate in Michael
addition reactions with the nucleophilic thiols in p53. Thus, they
are potent electrophiles acting as Michael acceptors that readily
react with nucleophilic thiols. Such an interaction also supports
the notion that intramolecular or intermolecular disulfide bond
formation might be inhibited by thiol modification that could
result in the proper folding of the protein core (19).
PL appears to be cytotoxic against tumor cell lines
irrespective of their p53 status. Further, PL has demonstrated
marked tumor regression in a number of murine cancer models without
tumor recurrence incidents or specific toxicities (3). Previously, we reported that human p53
was a substrate for glutathionylation which interferes with the
tumor suppressor protein binding with DNA (20,21).
Putting these observations together, we hypothesized that PL may
promote the conversion of mutant p53 protein into its DNA-bindable
and functional form. This study shows indeed that PL possesses the
ability to reactivate the R273H mutant form of p53, albeit with a
lower efficiency.
Materials and methods
Cell lines, chemicals and antibodies
Human colon carcinoma cell lines HT29, SW620, and
HCT116 were obtained from the American Type Culture Collection and
were grown in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum and antibiotics. Cell cultures were
maintained at 37°C in 5% CO2 and 95% air. Monoclonal
antibodies to actin, GSH, and p53 were purchased from Millipore
Corp. 1620 (wt-p53) and 240 (mt-p53) were purchased from
Calbiochem. Piperlongumine was purchased from Cayman Chemical (CAS
registry no. 20069-09-4, ≥98%, Ann Arbor, MI, USA). Stock solutions
of PL were prepared in DMSO.
Western blotting
After trypsinization, the cell pellets were washed
with cold phosphate-buffered saline (PBS) and subjected to
sonication in 40 mM Tris-HCl (pH 8.0) containing 1% glycerol, 1 mM
EDTA, 0.5 mM DTT, 1 mM PMSF and 1 mM benzamidine and centrifuged.
Equal protein amounts from different treatments were
electrophoresed on 12% sodium dodecyl sulfate (SDS)-polyacrylamide
gels and transferred onto PVDF membranes (Millipore). The membranes
were blocked with 5% non-fat dry milk in Tris-buffered saline (pH
8.0) containing 0.1% Tween-20 for 3 h, and subsequently incubated
with appropriate primary antibodies. Antigen-antibody complexes
were visualized by enhanced chemiluminescence.
Animal studies
Female Nu/Nu mice were obtained from Charles River
Laboratories (Wilmington, MA, USA) and fed ad libitum.
Female mice are traditionally used for developing xenografts
because the males often tend to be aggressive and bite their
littermates. They may also destroy the tumors present on their
fellow cage mates or kill them. The animals were allowed to
acclimatize to a 12 h light/12 h dark cycle, and all procedures
were performed under the guidelines of the institutional animal
care and use committee (IACUC). HT29 cells (5×106) were
injected subcutaneously into the right and left flanks. Tumor
volume was calculated every 2–3 days with a caliper using the
following formula: Volume = [length × (width)2]/2. The
mice weighing 25 g were randomized into three groups and were
administered i.p. injections of 5 and 10 mg/kg/day of PL dissolved
in 1X PBS saline (6 animals per groups), when the agent was used
alone. In the second study, the mice were randomized into six
groups and they received 7.5 mg/kg/day PL, 1 mg/kg/day cisplatin,
0.75 mg/kg/day doxorubicin and the combinations of PL with
cisplatin or doxorubicin. In both cases control mice received only
PBS. The animals were sacrificed on day 21 after drug
administration. The tumors were excised, washed and homogenized
using a polytron in 40 mM Tris-HCl buffer (pH 8.0) containing 5%
glycerol, 1 mM EDTA, 20 μM spermidine, 0.5 mM DTT, 1 mM PMSF, 1 mM
benzamidine, 0.5% Triton X-100, and 1 mM sodium vanadate. All
samples were centrifuged, and the resulting extracts were used for
western blot analyses.
Electrophorectic mobility shift
assay
Nuclear extracts were prepared using the nuclear
extract lysis buffer containing 25 mM Tris-HCl (pH 8.0), 5%
glycerol, 1 mM EDTA, 0.5 mM DTT, 1 mM PMSF and 1 mM benzamidine and
centrifuged. Five milligrams of each nuclear extract was used in a
binding assay with 20 ng/μl of a biotin-labeled p53 probe (5′
Biotin AGACATGCCTAGACATGCCT-3′) (Signosis, Inc.). The nuclear
extract was incubated with p53 probe at room temperature (20–23°C)
for 30 min and the protein/DNA complexes were separated on a
non-denaturing 6.5% polyacrylamide gel. The gel was transferred to
a nylon membrane, and the biotin-labeled oligonucleotides were
detected using streptavidin-HRP and a chemiluminescent substrate
according to the manufacturer's protocol.
Binding of p53 to DNA: quantitation by
DNA-affinity immunoblotting (DAI)
Binding of p53 proteins to their respective
consensus recognition sequences was determined by the
biotin-labeled oligonucleotide pull-down assays followed by western
blotting in a procedure called DNA affinity immunoblotting
(22). Nuclear extracts were
prepared, and DNA-binding reactions were performed according to our
published procedure (20). The
following double-stranded consensus recognition sequences were
prepared by annealing a strand labeled with biotin at 5′ end with
an unlabeled complementary sequence; p53 used were
5′-TACAGAACATGTCTAAG CATGCTGGGGACT-3′. The duplex oligonucleotides
(50 nM) were bound to streptavidin magnetic beads in TE buffer (pH
8.0) containing 100 mM NaCl. The beads were washed with TE buffer
containing 1 M NaCl and then without salt. The oligo-bound beads
were subsequently suspended in a binding buffer [20 mM Tris-HCl (pH
8.0), 75 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 2.5 mM DTT, 20
mM KCl, 1 μg poly(deoxyinosinic-deoxycytidylic) acid and 5%
glycerol] and incubated with nuclear extracts at room temperature
for 30 min. The beads were then washed with 300 mM NaCl and the
DNA-bound protein was eluted by boiling the beads in SDS sample
buffer, electrophoresed on 10% SDS-polyacrylamide gel and western
blotting using appropriate antibodies.
Immunoprecipitation
Equal protein amounts in lysates from different
treatments were precleaned with 5 μl protein-A agarose beads, and
immunoprecipitated using 240 p53 mutant (PAb240) and 1620 p53 wt
(PAb1620) antibodies. The immuno-complexes were solubilized in
non-reducing SDS-sample buffers, subjected to electrophoresis on
12% gels followed by western blotting with D-1 antibodies for p53
which recognizes both the wt and mutant proteins.
Detection of ROS generation
Intracellular ROS production was determined by
2′,7′-dichlorofluorescin diacetate (DCFDA) staining followed by
fluorescence detection using a Biotek plate reader (Model-Synergy
2SL) with excitation and emission wavelengths set at 485 and 535
nm, respectively. Briefly, cells were incubated with 10 μM DCF-DA
solution at 37°C for 0.5 h, washed with PBS twice, treated with
different concentrations of PL for 1, 3 and 6 h followed by
fluorescence measurement.
Cell viability assays
For cell viability assays, the yellow tetrazolium
dye [(3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyl tetrazolium
bromide) (MTT)] was used. HT29 cells were seeded at a density of
7,000 cells per well in 96-well plates and were treated post-24 h
with PL at concentrations specified. In some cases, cells were
treated with PL for 24 h. They were treated with PL alone or with
PL followed by exposure to BCNU or temozolomide or doxorubicin. The
cells were washed, and MTT (10 μl of 5 mg/ml) in 1X PBS was added.
The plates were incubated for 4 h followed by the addition of DMSO,
stored in the dark for 2 h before reading the absorbance at 570
nm.
Statistical analysis
All experiments were performed three times
independent of each other. Results were assessed by Student's
t-test. Significance was defined as P<0.05. Power analysis was
used to calculate the number of animals required to achieve a
statistical power of >80%.
Results and Discussion
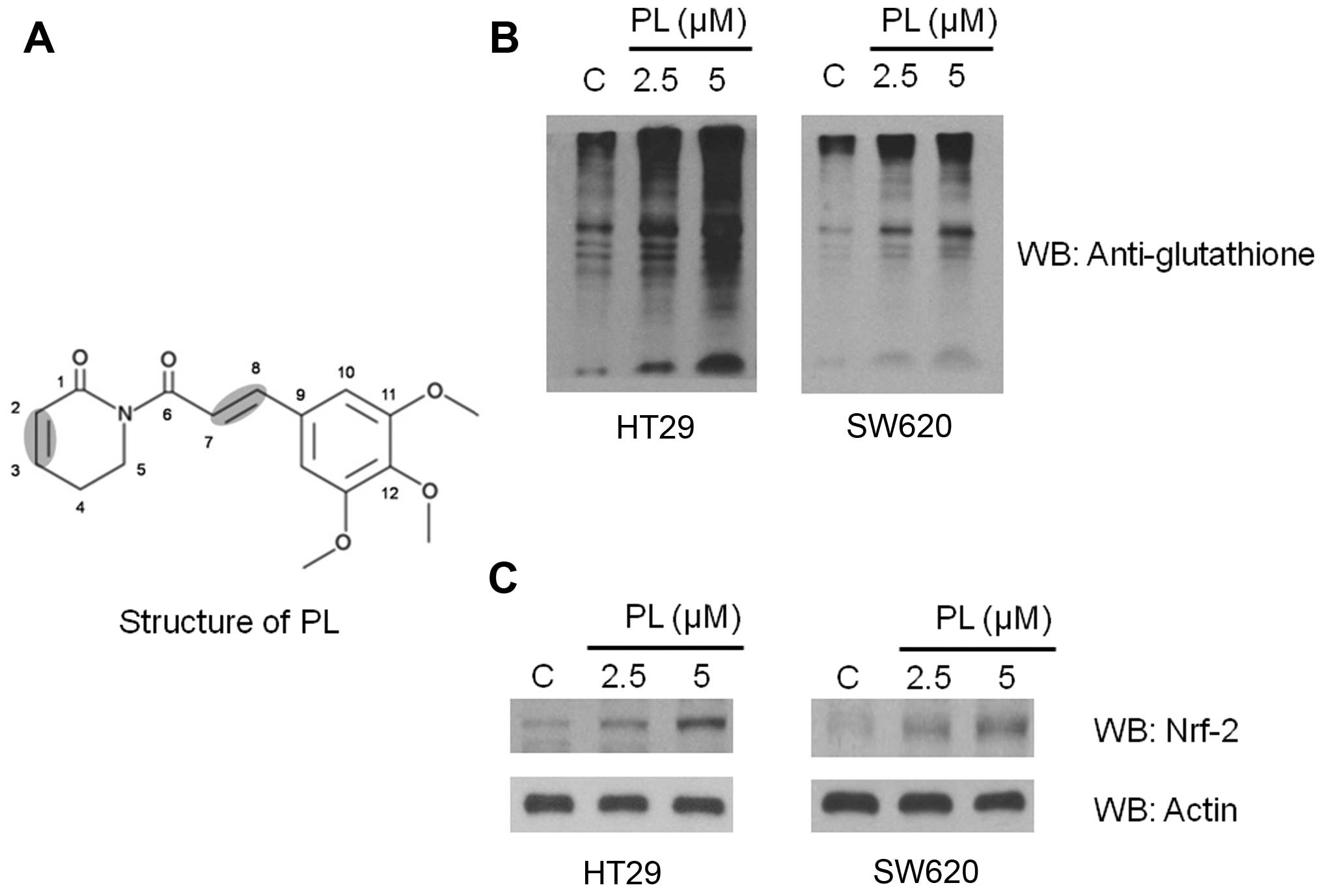
Chemistry of piperlongumine
PL, an electrophilic small molecule identified in
cell-based, high-throughput screening assays was shown to
selectively kill cancer cells without harming the normal epithelial
cells (3). Structurally, PL has
two active double bonds (Fig. 1A)
and can conjugate with small-molecule thiols. Adams et al
synthesized 80 structural analogs of PL to investigate some lead
compounds from which they concluded that C2–C3 olefin is critical
for the electrophilicity of the molecule. They observed that
elimination of C2–C3 olefin did not result in ROS elevation or
decreased viability of cancer cells while removal of the C7–C8
olefin was associated with decreased toxicity without affecting the
generation of ROS. Several analogs of piperlongumine such as
piperine and piperlonguminine also have active double bonds that
are located in proximity to the carbonyl group but they lack the
crucial C2–C3 olefin in their structures. As a result, although
these analogs still possess the ability to elevate cellular ROS,
they exhibit reduced toxicities (4). Therefore, the therapeutic properties
of PL can be attributed to both the olefins present in the
molecule. These structural features of PL also appear to underlie
the feeble p53 reactivating properties described in this
report.
Protein glutathionylation induced by
PL
Glutathionylation is a posttranslational
modification where low molecular weight thiols such as the
glutathione (GSH or GSSG) form mixed disulfides with protein-bound
reactive (anionic) cysteines, apparently, as a defensive/protective
strategy during oxidative stress (23). This modification is reversible
through an increase in GSH/GSSG ratio or enzymatic reactions
involving glutare-doxin, thioredoxin or sulfiredoxins which restore
the protein sulfhydryl groups back to their reduced state (24). Similar to phosphorylation,
glutathionylation is known to inhibit enzyme activities and
transcription protein functions and alter protein-protein
interactions (25). Our previous
study demonstrated that human p53 is a substrate for
glutathionylation in vitro and in cells, and p53 function
may be modulated by glutathionylation (20,21).
Another study suggested that the thiolation induced by PL, and its
analogs might play an important role in inducing cancer cell death
(4). Because PL induces a redox
imbalance, we examined the bulk glutathionylation of proteins as a
biochemical marker of oxidative stress. Conjugation of glutathione
or glutathione disulfide with bulk proteins was determined by
western blotting using a monoclonal antibody against glutathione.
In HT29 and SW620 cells, we observed a large increase in protein
glutathionylation after PL treatment where a de novo or
increased glutathionylation of numerous proteins was observed as
reflected by enhanced band densities when compared with the
untreated cells (Fig. 1B).
PL treatment upregulates Nrf-2 expression
and generates ROS
The Nrf-2 (nuclear factor erythroid 2-related
factor) is a cytoprotective transcription factor activated during
oxidative stress and functions to restore the redox homeostasis.
Nrf2 controls the basal and induced expression of an array of
antioxidant response element-dependent genes such as the
γ-glutamylcysteine synthase, glutathione S-transferases, and
NAD(P)H oxidoreductase 1 to regulate the physiological and
pathophysiological outcomes of oxidant exposure (26). Consistent with the properties known
for PL, we observed an increase in the Nrf-2 protein levels in HT29
and SW620 cells (Fig. 1C).
Therefore, an increase in Nrf-2 demonstrates the development and
maintenance of oxidative stress by PL in cancer cells. The ability
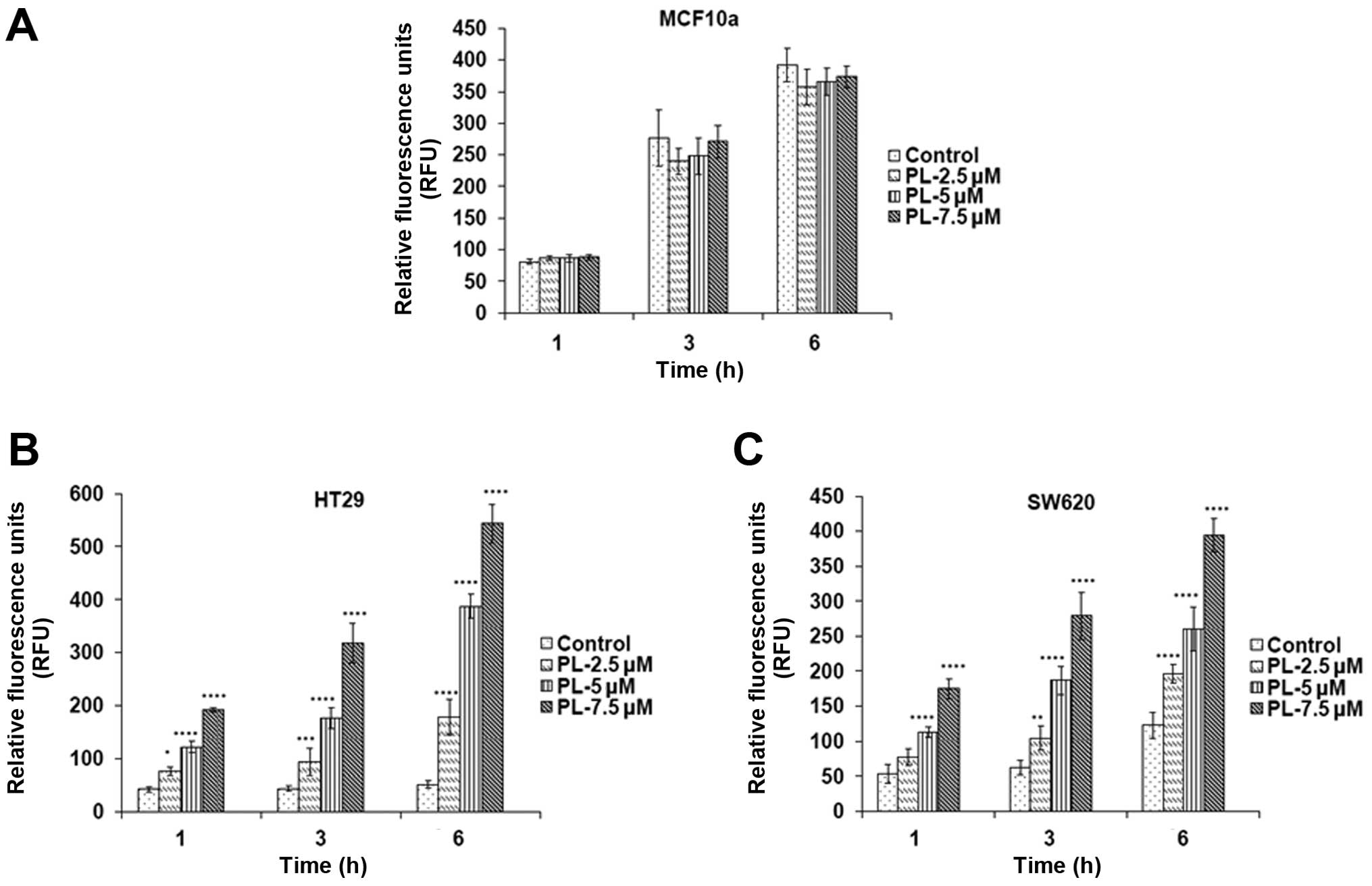
of PL to induce oxidative stress in tumor cell lines with mutant
p53 was further explored using the redox probe DCF-DA. DCF-DA
oxidizes rapidly to a highly fluorescent 2′,
7′-dichlorodihydrofluorescein (DCF) in stressed cells and the
fluorescence intensity is proportional to the cytosolic ROS levels
(27). The increase in ROS levels
by PL was confirmed when the HT29 and SW620 cells were incubated
with DCF-DA for 30 min, followed by PL treatment for 1, 3 and 6 h
(Fig. 2B and C). However, there
was no significant difference in ROS levels in MCF10a cells after
PL treatment for the same time periods (Fig. 2A) indicating that ROS production is
entirely selective for cancer cells, not for normal epithelial
cells.
Restoration of functional status to
mutant p53 by piperlongumine
p53 is the most commonly mutated gene in human
cancers and hence has emerged as a huge target for novel cancer
therapies (28). These mutations
occurring in the DNA-binding domain abrogate binding of p53 protein
to its target sequences, leading to a suppression of target gene
products involved in cell cycle arrest, apoptosis and a multitude
of oncogenic pathways (29).
Additionally, p53 mutations are also associated with the inhibition
of two other p53 family members, p63 and p73 through
oligomerization, and this leads to the inhibition of apoptosis
(30). Reactivation or restoration
of functional properties to the mutant p53 proteins is expected to
sensitize the human tumors to therapy-induced DNA damage and
facilitate a greater antitumor efficacy through a pronounced
apoptosis. Most compounds reported to impart at least some
wild-type properties to mutant p53 proteins are all electrophilic
with great affinity for nucleophilic groups such as the protein
bound cysteines or glutathione present in abundance (19). PL is a reactive compound as well
(4); therefore, we hypothesized
that anticancer effects of PL may stem at least partially from the
oxidative stress-induced modification of the p53 protein. Our own
findings implicating the reactive cysteines 124, 141 and 182 in the
DNA-binding domain of p53 as targets for oxidation and thiolation
further supported the above hypothesis (20,21).
We used a number of approaches to validate the
functionalization of mutant p53. Chief among these were the
conformation specific monoclonal antibodies for p53. pAb 1620 can
recognize the wild-type structure, and many cancer mutations
perturb this conformation (31).
The epitope comprising Arg156, Leu209, Arg 209, and Gln/Asn210 in
p53 DNA-binding domain is recognized by pAb1620 (32). On the other hand, a highly
conserved, denaturation-resistant epitope on p53 is targeted by the
antibody pAb240; it binds to p53 in the mutant conformation and
also to denatured p53. Both antibody epitopes are located away from
the p53-DNA interface (33).
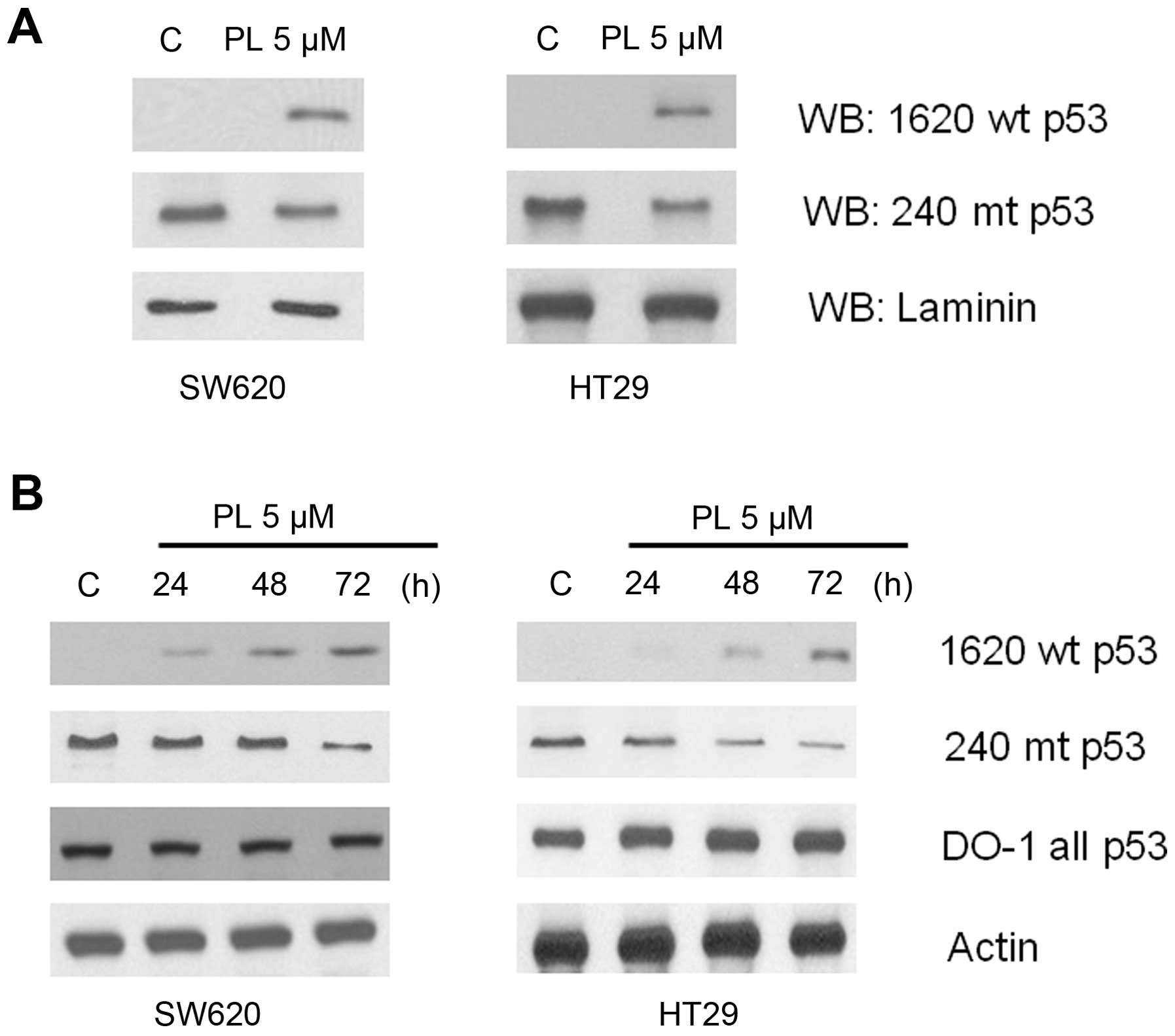
As a first step to verify the reactivation of mutant
p53, the cell lysates from PL-treated and untreated HT29 and SW620
cells were immunoprecipitated with pAb1620 or pAb240 followed by
immunoblotting with the DO1 anti-p53 antibodies. The western blots
in Fig. 3A show that in both cell
lines, the bands corresponding to the wt-like p53, which were
absent in control cells appeared after PL-treatment. Further, a
concomitant decrease of the mutant p53 protein was evident in
extracts immunoprecipitated with pAb240. Next, the above results
were verified by direct western blotting using the p53 conformation
specific antibodies. The untreated HT29 and SW620 cells which
harbor a mutant p53 exhibited a very weak immunoreactivity for pAb
1620, which, however, increased significantly in a time-dependent
manner after PL treatment. On the contrary, the intrinsic mutant
p53 in these cells decreased gradually as evident from the
diminished pAb240 immunoreactivity (Fig. 3B).
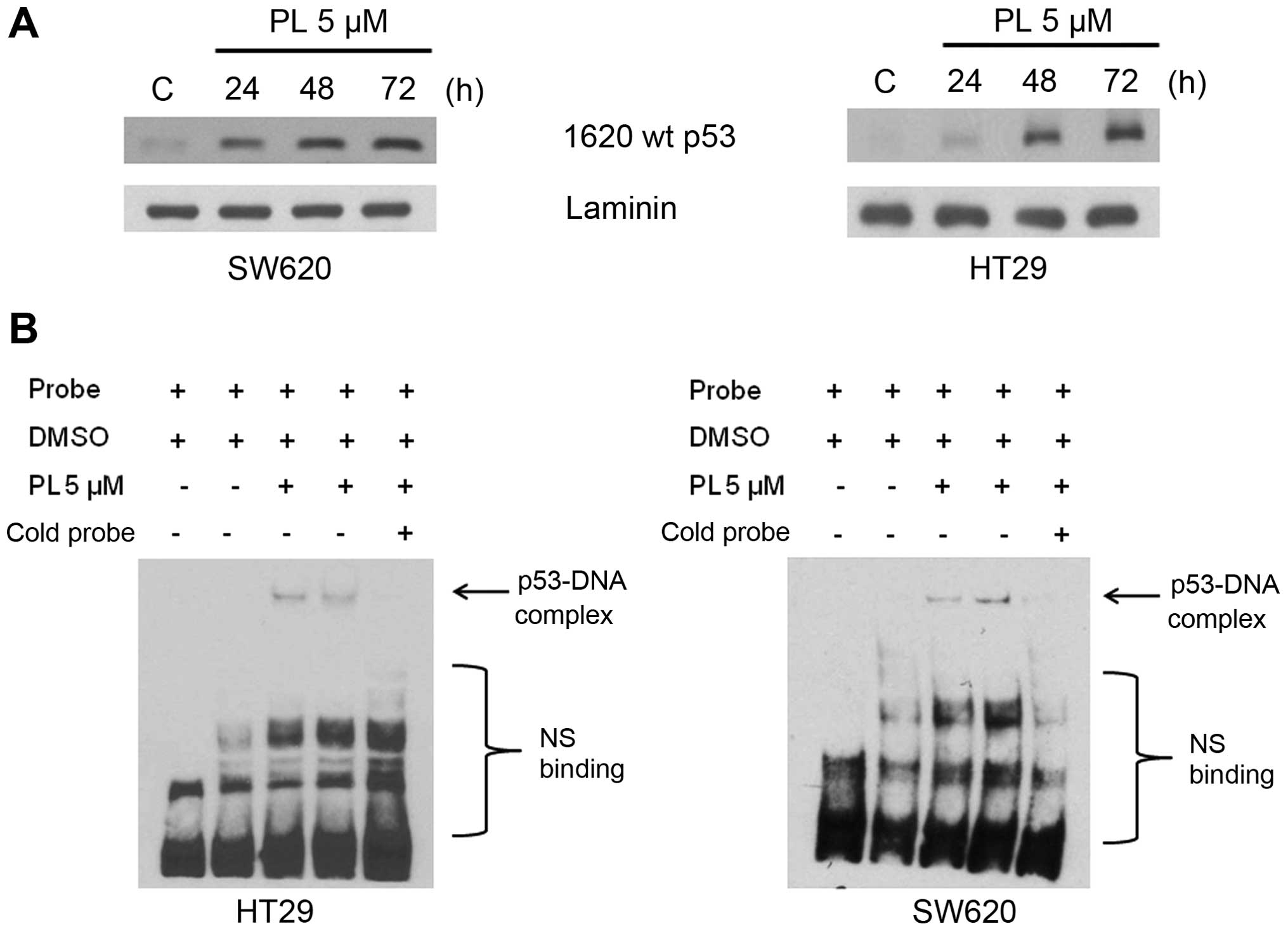
To further establish the conformational transition
and acquisition of DNA binding of the R273H mutant p53 present in
HT29 an SW620 cells, we performed DNA-affinity immunoblotting (DAI)
and EMSA, both of which detect the protein specifically associated
with DNA. The representative results from these experiments are
shown in Fig. 4. The nuclear
extracts (NE) from control cells failed to bind with DNA, whereas
the NE from PL-treated cells showed a time-dependent increase in
DNA binding in the DAI assays (Fig.
4A). Similar data showing the binding of p53 with DNA in both
HT29 and SW620 cells were obtained in EMSAs; the bands
corresponding to DNA-bound proteins were diminished by the addition
of the non-biotinylated cold probe, demonstrating the specificity
of interaction (Fig. 4B).
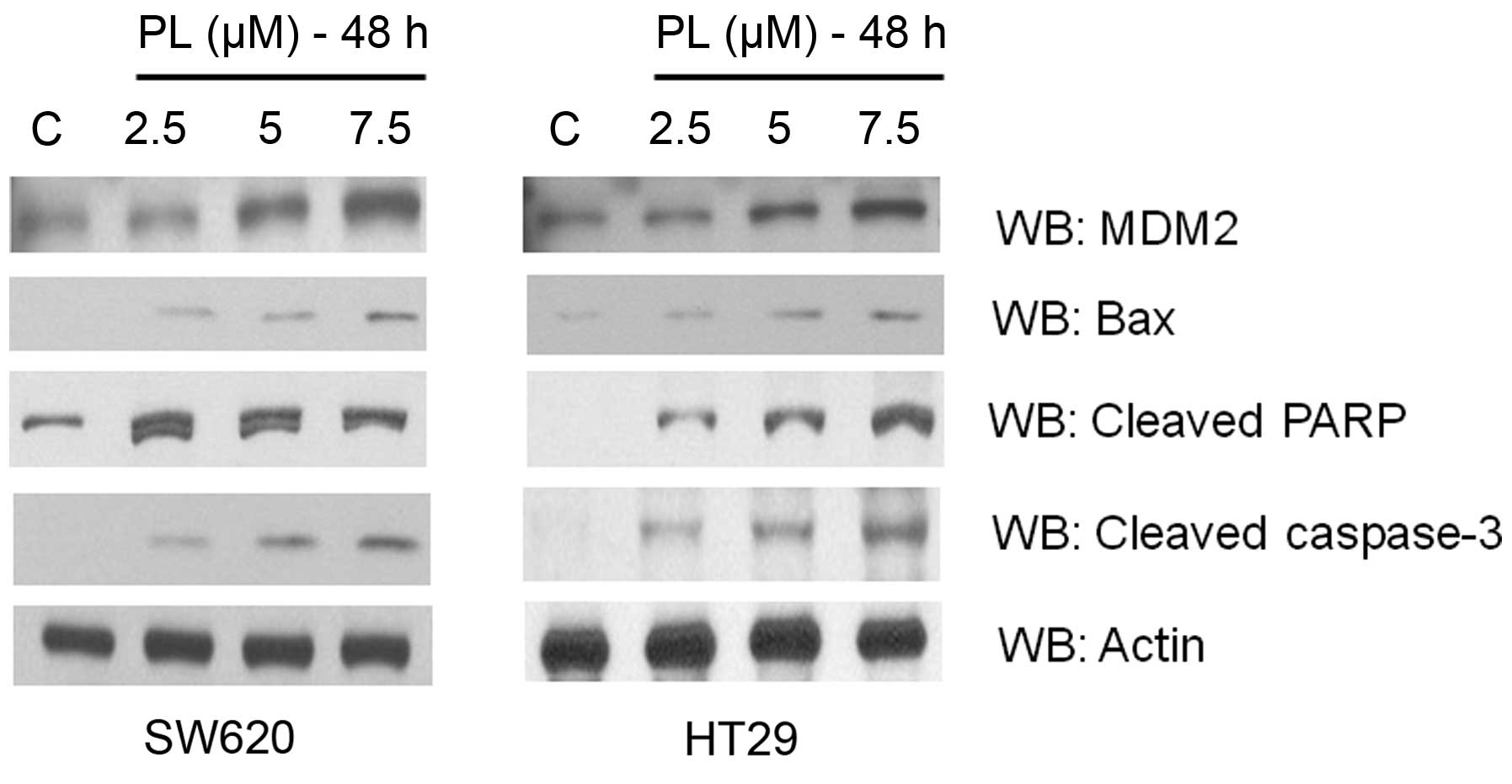
Consistent with the mutant p53 conversion to its functional form,
we observed upregulation of the target genes, MDM2 and Bax in both
cell lines after PL treatment (Fig.
5). A significant increase in the levels of apoptotic markers,
cleaved caspase-3 and cleaved PARP was also observed (Fig. 5), suggesting that the oxidative
milieu may prime and usher the tumor cells along the cell death
pathways.
Piperlongumine exerts marked cytotoxicity
by itself and enhances cell killing by anticancer drugs
To investigate the selective anticancer effects of
PL in the context of the DNA contact mutants of p53 and our
observations that PL can impart wt-like the protein conformation to
the mutant proteins (Figs.
3Figure 4–5), we performed cell survival assays with
PL alone or PL in combination with some clinically used drugs
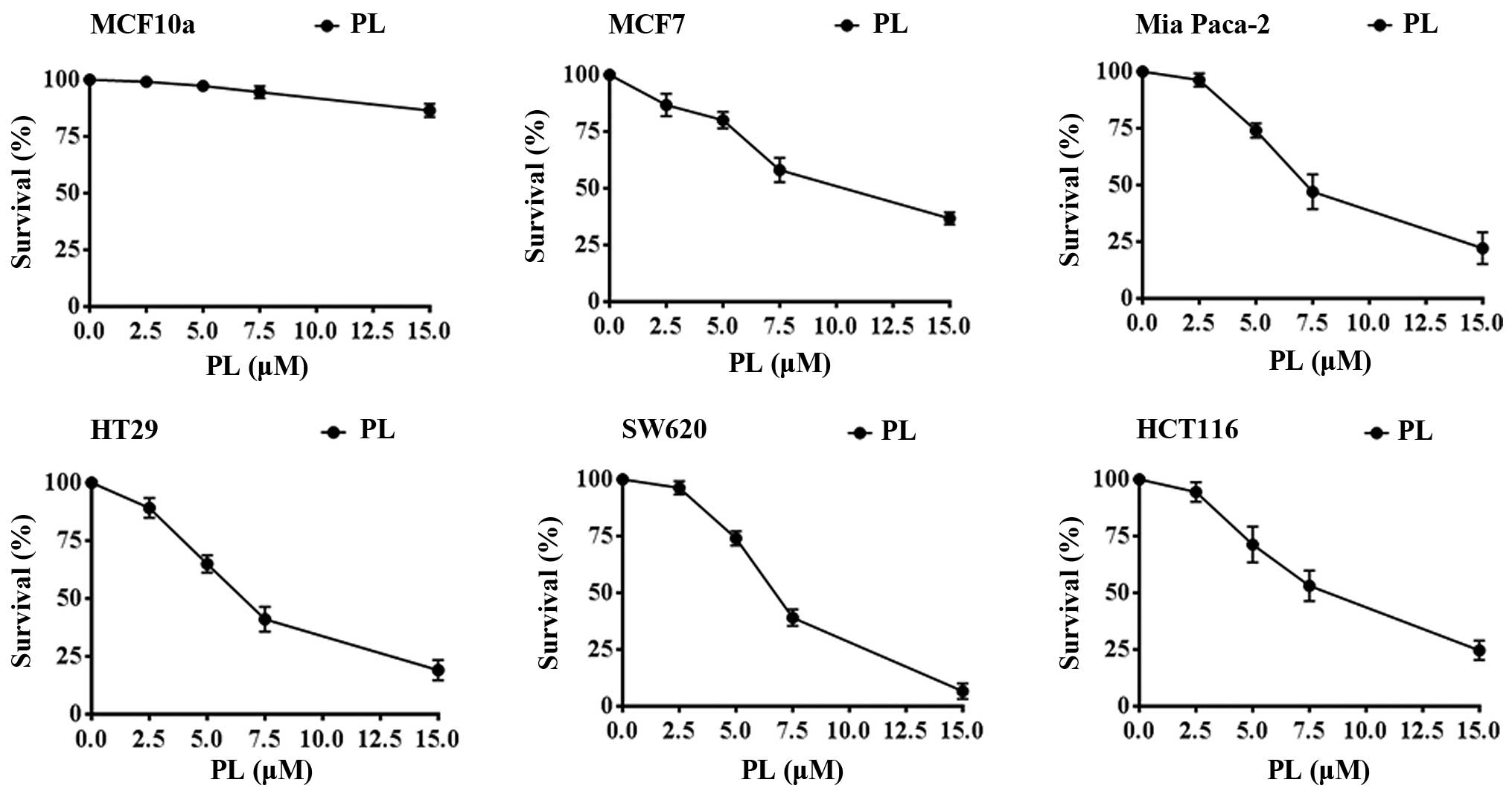
against cancers. PL by itself, over a concentration range of 2.5–15
μM for 24 h, showed significant anti-proliferative effects against
cell lines of various cancer types. These included the breast
cancer MCF-7, colon cancer HT29, HCT116, SW620, and the Mia Paca of
pancreatic origin (Fig. 6).
However, consistent with earlier results (3) PL was 10–12-fold less toxic to MCF10a
normal breast epithelial cells as also shown in Fig. 6. Based on these observations, we
chose 7.5 μM PL, which caused 35–55% cell-kill for 24 h as the
preincubation period for testing the potentiation of cytotoxicities
of temozolomide (TMZ), 1,3 bis(2-chloroethyl)-1-nitrosourea (BCNU)
and doxorubicin (Dox). Three cell lines, the HT29, HCT116 and SW620
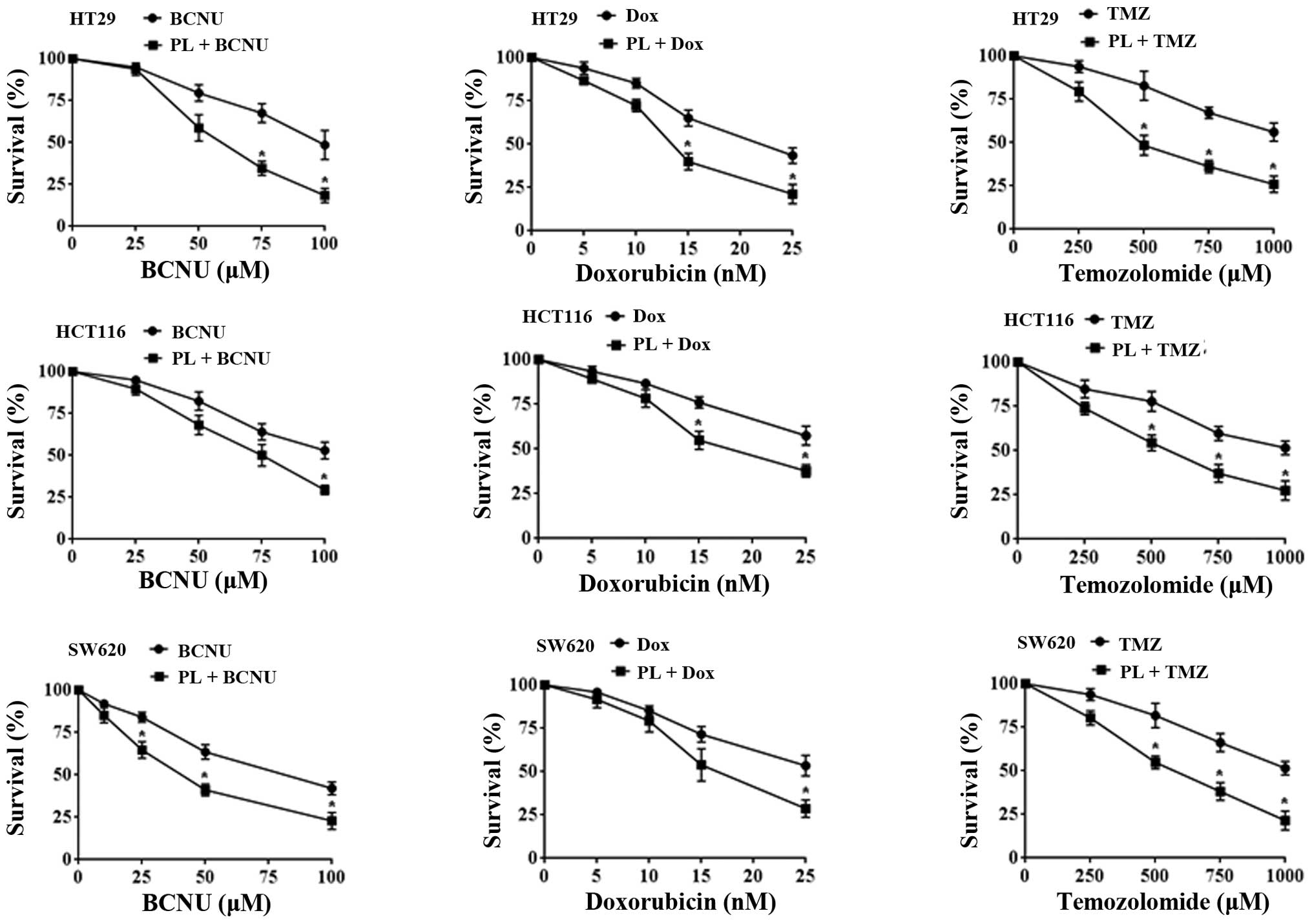
were tested to determine the drug potentiation by PL. In this
setting, the TMZ (250–1,000 μM range) + PL combination showed 2- to
3-fold increased cytotoxicity compared with TMZ alone (Fig. 7). For BCNU and doxorubicin, the
potentiation was ~2-fold in different cell lines. Our findings
suggest that addition of PL to the anticancer regimens will be
beneficial and result in increased tumor cell killing. The enhanced
anticancer effects are likely to occur at least in part through the
functional restoration of the p53 tumor suppressor observed in this
study.
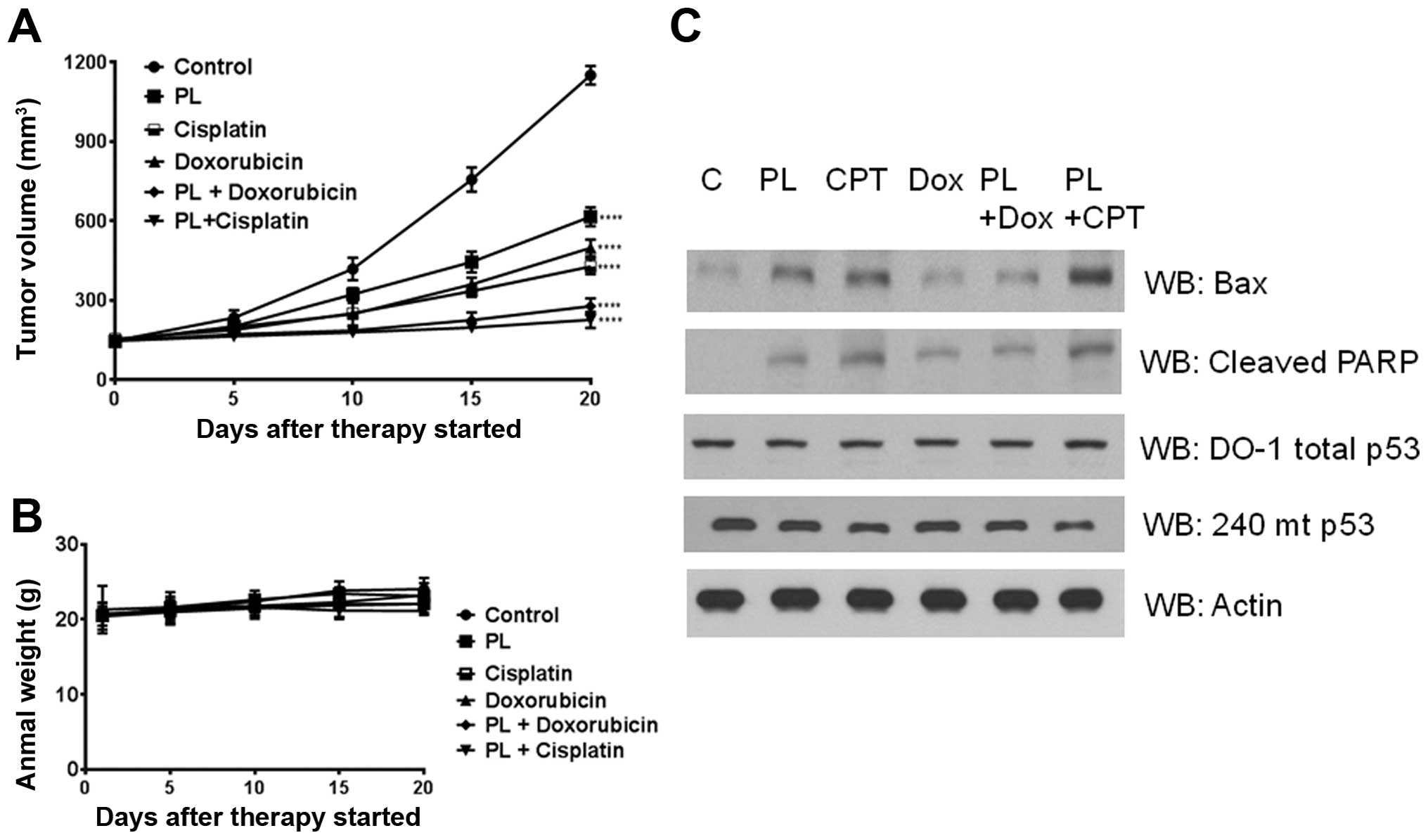
Piperlongumine induces tumor growth delay
and loss of p53 mutant protein in HT29 xenografts
To probe whether PL induces a conversion of mutant
p53 protein to its wt-like counterpart in vivo, we developed
subcutaneous xenografts by injecting the HT29 cells in nude mice.
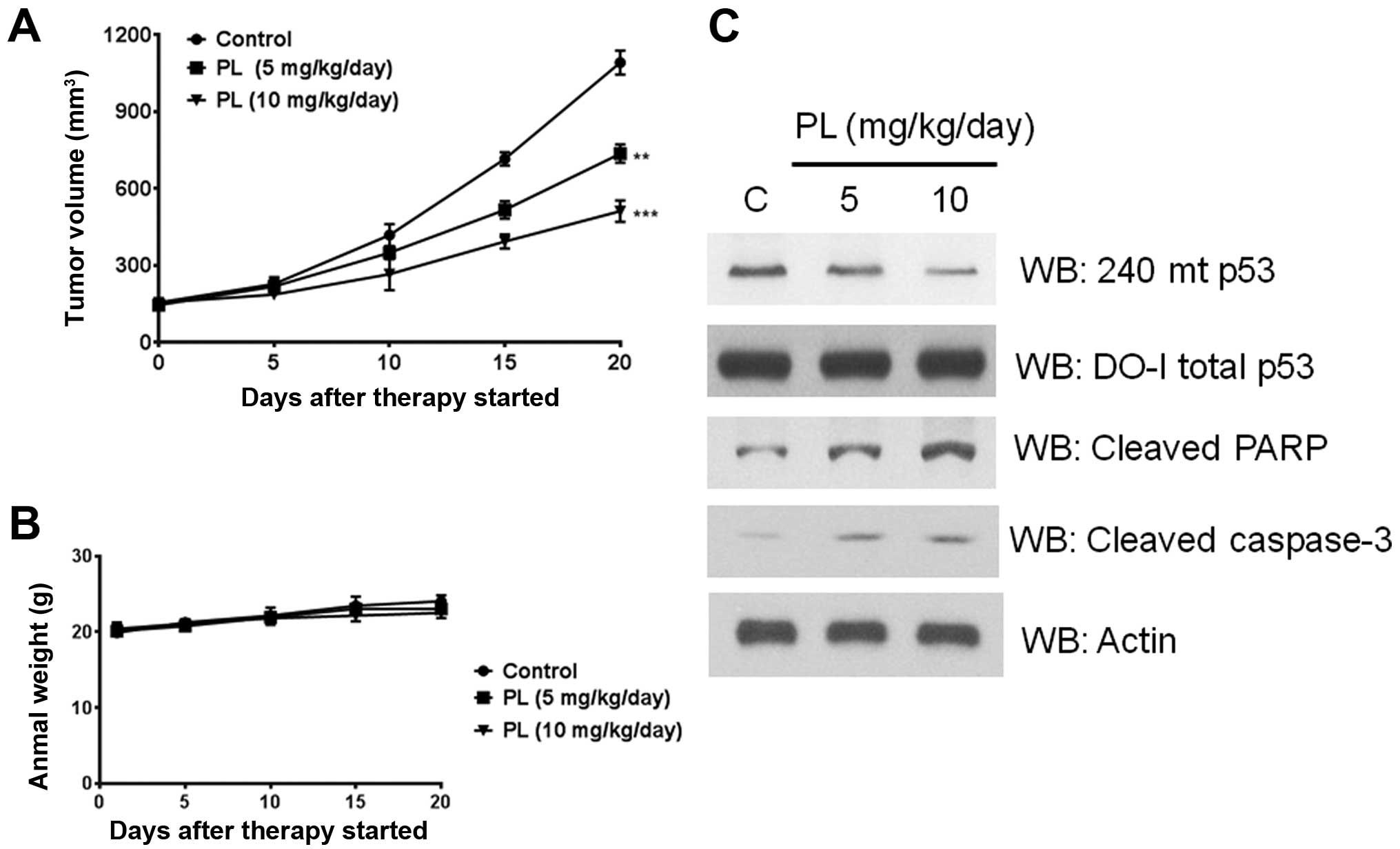
PL (5–10 mg/kg) was administered every day to the tumor bearing
mice for 20 days. A significant and PL dose-dependent delay of
tumor growth was observed (Fig.
8A). No changes were discernible in the body weight of animals
indicating a lack of toxicity (Fig.
8B). Further, when the lysates from the excised tumors were
immunoblotted, there was an apparent decrease in the R273H mutant
p53 protein levels (Fig. 8C). This
decrease was accompanied by enhanced levels of both the cleaved
PARP and cleaved caspase-3 (Fig.
8C), verifying that PL can trigger apoptotic initiation in
tumor tissues as well.
Effect of PL on the anticancer effects of
cisplatin and doxorubicin in HT29 xenografts
To explore the possibility of combining PL with
current chemotherapy drugs, we evaluated the tumor growth delay in
HT29 xenografts given cisplatin or doxorubicin along with PL (7.5
mg/kg/day). As shown in Fig. 9, PL
treatment alone produced significant tumor regression. Simultaneous
treatment of PL with cisplatin or doxorubicin resulted in a
vigorous and significant reduction of tumor volume, compared with
either agent alone. Statistical analyses indicated that the
antitumor effects were synergistic (Fig. 9A). Changes in body weights were not
significantly different between the control and PL-treated groups
(P>0.5) indicating that no apparent adverse effects were
associated with the chemotherapy (Fig.
9B). Western blot analyses again revealed a reduction in mutant
p53 levels, unaltered total p53 levels (reactive with the pan DO-1
Ab), along with a marked upregulation of apoptotic proteins such as
the Bax and cleaved PARP (Fig.
9C). Taken together, these findings suggest that oxidative
stress mediated by PL can potentiate the cytotoxic effects of many
clinically used anticancer drugs.
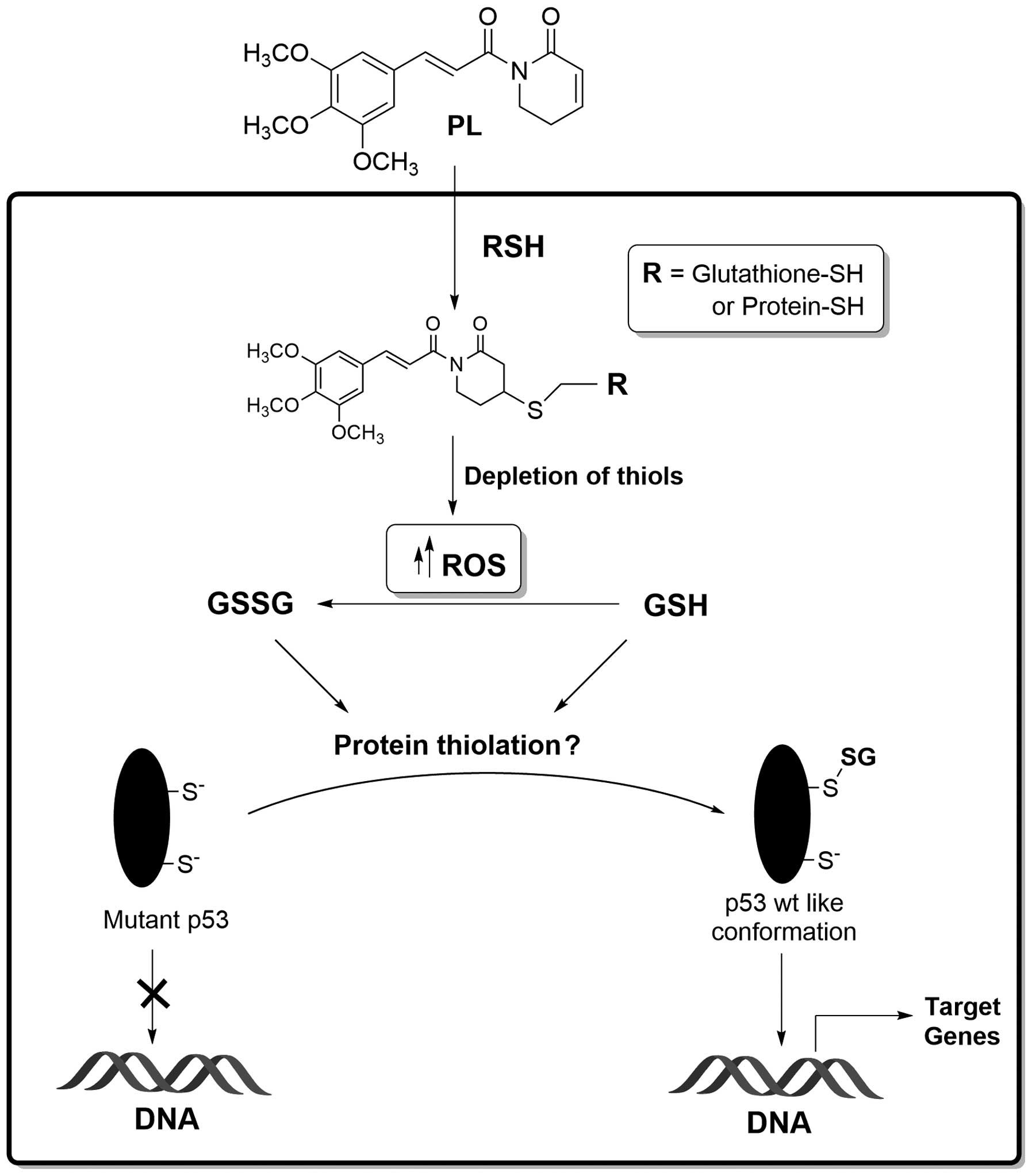
Conclusion
In continuation of our research interests in
oxidative stress signaling (34),
and regulation of p53 functions by redox imbalance in human cancers
(20,21), the present study investigated the
biochemical effects of PL on the R273H mutant p53 present in HT29
and SW620 cells and the therapeutic consequences thereof. Fig. 10 summarizes our findings. We
propose that PL, as an electrophile, can either react directly with
the protein-bound anionic cysteines or conjugate with the SH-group
of glutathione (4,5); the glutathione S-transferases may
facilitate the latter reaction. The ROS generation by PL and the
thiol conjugations are likely to deplete the cellular GSH levels
and trigger protein thiolation. We hypothesize that mutant p53
proteins are efficient substrates for glutathionylation, which in
turn can induce structural perturbations in the defective
DNA-binding domain of the tumor suppressor and restore some
functionality (Fig. 10). While
the PL-induced p53 reactivation is somewhat feeble, and the
mechanism(s) involved are yet to be proven, our recent study with a
hybrid C-7 aryl piperlongumine derivative indeed suggests that
induction of redox-imbalance may serve as a general platform to
rescue the cancer mutations in p53 (35). In conclusion, the findings made
here and the evidence that oxidative stress may prevent distant
metastasis (36) highlight the
therapeutically beneficial and exploitable aspects of tumor redox
biology.
Acknowledgements
This study was supported by grants from the Cancer
Prevention and Research Institute of Texas (RP130266), the
Carson-Leslie Foundation and the Association for Research of
Childhood Cancer, all to K.S.S.
Abbreviations:
|
PL
|
piperlongumine
|
|
PMSF
|
phenylmethylsulfonyl-fluoride
|
|
DTT
|
dithiothreitol
|
|
BCNU
|
1,3 bis
(2-chloroethyl)-1-nitrosourea
|
|
DAI
|
DNA-affinity immunoblotting
|
|
Dox
|
doxorubicin
|
|
TMZ
|
temozolomide
|
|
EMSA
|
electrophoretic mobility shift
assay
|
|
ROS
|
reactive oxygen species
|
|
DCF-DA
|
2, 7′-dichlorofluorescein
diacetate
|
|
wt
|
wild-type
|
|
Mut
|
mutant
|
|
WB
|
western blotting
|
References
|
1
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar
|
|
3
|
Raj L, Ide T, Gurkar AU, Foley M, Schenone
M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al:
Selective killing of cancer cells by a small molecule targeting the
stress response to ROS. Nature. 475:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams DJ, Dai M, Pellegrino G, Wagner BK,
Stern AM, Shamji AF and Schreiber SL: Synthesis, cellular
evaluation, and mechanism of action of piperlongumine analogs. Proc
Natl Acad Sci USA. 109:15115–15120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han JG, Gupta SC, Prasad S and Aggarwal
BB: Piperlongumine chemosensitizes tumor cells through interaction
with cysteine 179 of IκBα kinase, leading to suppression of
NF-κB-regulated gene products. Mol Cancer Ther. 13:2422–2435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polyak K, Xia Y, Zweier JL, Kinzler KW and
Vogelstein B: A model for p53-induced apoptosis. Nature.
389:300–305. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petitjean A, Mathe E, Kato S, Ishioka C,
Tavtigian SV, Hainaut P and Olivier M: Impact of mutant p53
functional properties on TP53 mutation patterns and tumor
phenotype: Lessons from recent developments in the IARC TP53
database. Hum Mutat. 28:622–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olivier M, Hollstein M and Hainaut P:
Hollstein M and Hainaut P: TP53 mutations in human cancers:
Origins, consequences, and clinical Use. Cold Spring Harb Perspect
Biol. 2:1–17. 2010. View Article : Google Scholar
|
|
9
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: Consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar
|
|
10
|
Bykov VJN and Wiman KG: Mutant p53
reactivation by small molecules makes its way to the clinic. FEBS
Lett. 588:2622–2627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiman KG: Strategies for therapeutic
targeting of the p53 pathway in cancer. Cell Death Differ.
13:921–926. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saha MN, Qiu L and Chang H: Targeting p53
by small molecules in hematological malignancies. J Hematol Oncol.
6:232013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bykov VJN, Issaeva N, Shilov A, Hultcrantz
M, Pugacheva E, Chumakov P, Bergman J, Wiman KG and Selivanova G:
Restoration of the tumor suppressor function to mutant p53 by a
low-molecular-weight compound. Nat Med. 8:282–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bykov VJN, Issaeva N, Zache N, Shilov A,
Hultcrantz M, Bergman J, Selivanova G and Wiman KG: Reactivation of
mutant p53 and induction of apoptosis in human tumor cells by
maleimide analogs. J Biol Chem. 280:30384–30391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang X, Zhu Y, Han L, Kim AL, Kopelovich
L, Bickers DR and Athar M: CP-31398 restores mutant p53 tumor
suppressor function and inhibits UVB-induced skin carcinogenesis in
mice. J Clin Invest. 117:3753–3764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zache N, Lambert JMR, Rökaeus N, Shen J,
Hainaut P, Bergman J, Wiman KG and Bykov VJN: Mutant p53 targeting
by the low molecular weight compound STIMA-1. Mol Oncol. 2:70–80.
2008. View Article : Google Scholar
|
|
17
|
Demma M, Maxwell E, Ramos R, Liang L, Li
C, Hesk D, Rossman R, Mallams A, Doll R, Liu M, et al: SCH529074, a
small molecule activator of mutant p53, which binds p53 DNA binding
domain (DBD), restores growth-suppressive function to mutant p53
and interrupts HDM2-mediated ubiquitination of wild-type p53. J
Biol Chem. 285:10198–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Vazquez A, Levine AJ and Carpizo DR:
Allele-specific p53 mutant reactivation. Cancer Cell. 21:614–625.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bykov VJN, Lambert JMR, Hainaut P and
Wiman KG: Mutant p53 rescue and modulation of p53 redox state. Cell
Cycle. 8:2509–2517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Velu CS, Niture SK, Doneanu CE,
Pattabiraman N and Srivenugopal KS: Human p53 is inhibited by
glutathionylation of cysteines present in the proximal DNA-binding
domain during oxidative stress. Biochemistry. 46:7765–7780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yusuf MA, Chuang T, Bhat GJ and
Srivenugopal KS: Cys-141 glutathionylation of human p53: Studies
using specific polyclonal antibodies in cancer samples and cell
lines. Free Radic Biol Med. 49:908–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Asch H and Kulesz-Martin MF:
Functional quantification of DNA-binding proteins p53 and estrogen
receptor in cells and tumor tissues by DNA affinity immunoblotting.
Cancer Res. 61:5402–5406. 2001.PubMed/NCBI
|
|
23
|
Gallogly MM and Mieyal JJ: Mechanisms of
reversible protein glutathionylation in redox signaling and
oxidative stress. Curr Opin Pharmacol. 7:381–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghezzi P, Bonetto V and Fratelli M:
Thiol-disulfide balance: From the concept of oxidative stress to
that of redox regulation. Antioxid Redox Signal. 7:964–972. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klatt P and Lamas S: Regulation of protein
function by S-glutathiolation in response to oxidative and
nitrosative stress. Eur J Biochem. 267:4928–4944. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Sawaf O, Clarner T, Fragoulis A, Kan
YW, Pufe T, Streetz K and Wruck CJ: Nrf2 in health and disease:
Current and future clinical implications. Clin Sci (Lond).
129:989–999. 2015. View Article : Google Scholar
|
|
27
|
Brandt R and Keston AS: Synthesis of
diacetyldichlorofluorescin: A stable reagent for fluorometric
analysis. Anal Biochem. 11:6–9. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z and Sun Y: Targeting p53 for novel
anticancer therapy. Transl Oncol. 3:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rainwater R, Parks D, Anderson ME,
Tegtmeyer P and Mann K: Role of cysteine residues in regulation of
p53 function. Mol Cell Biol. 15:3892–3903. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y and Prives C: Are interactions with
p63 and p73 involved in mutant p53 gain of oncogenic function?
Oncogene. 26:2220–2225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonsing BA, Corver WE, Gorsira MCB, van
Vliet M, Oud PS, Cornelisse CJ and Fleuren GJ: Specificity of seven
monoclonal antibodies against p53 evaluated with Western blotting,
immunohistochemistry, confocal laser scanning microscopy, and flow
cytometry. Cytometry. 28:11–24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang PL, Sait F and Winter G: The
‘wildtype’ conformation of p53: Epitope mapping using hybrid
proteins. Oncogene. 20:2318–2324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gannon JV, Greaves R, Iggo R and Lane DP:
Activating mutations in p53 produce a common conformational effect.
A monoclonal antibody specific for the mutant form. EMBO J.
9:1595–1602. 1990.PubMed/NCBI
|
|
34
|
Niture SK, Velu CS, Bailey NI and
Srivenugopal KS: S-thiolation mimicry: Quantitative and kinetic
analysis of redox status of protein cysteines by
glutathione-affinity chromatography. Arch Biochem Biophys.
444:174–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Punganuru SR, Madala HR, Venugopal SN,
Samala R, Mikelis C and Srivenugopal KS: Design and synthesis of a
C7-aryl piperlongumine derivative with potent antimicrotubule and
mutant p53-reactivating properties. Eur J Med Chem. 107:233–244.
2016. View Article : Google Scholar
|
|
36
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|