Introduction
Glioma is the most common malignant primary brain
tumor; thus, it is treated aggressively with surgery and
chemotherapy and radiotherapy. The poor survival of patients with
glioma reflects the prevalence of cancer recurrence after surgery,
invasion into other sites, and intrinsic or acquired resistance to
chemotherapy and radiotherapy (1).
Ionizing irradiation (IR) is the most commonly employed treatment
modality for various human cancers and regional cancer disease. IR
is often used in combined treatments with chemotherapy and surgery
(2). Concomitant combination
therapy is used to treat patients with non-small lung, head and
neck, cervical cancer, and glioblastoma (3–7),
~50% of cancer patients receive radio-therapy (8). The goal of radiotherapy is to provide
a suitable dose to the primary tumor, while minimizing IR
side-effects to surrounding normal tissues. Notably, one of the
problems with lower dose (0.1–0.6 Gy) or high dose (10 Gy and
higher) fractionated radiotherapy is radio-resistance and bystander
factors released among treated cancer cells and in an animal model
(9). Patients undergoing
radiotherapy for local tumor control show better survival rates
than patients with radiation-induced secondary tumors, which is
attributed primarily to the observation that local treatment
failure increases the probability of developing metastatic disease
at distant organ sites (10,11).
The first study on the effect of local tumor IR on metastatic
frequency in a transplantable mouse carcinoma model was reported in
1949 by Kaplan and Murphy (12).
Since then, various research groups have reported that the
incidence of metastasis increases after IR of the primary tumor
(13). Radio-therapeutic effects
are considered because of the induction of DNA damage causing cell
cycle arrest, apoptosis and senescence (14). IR induces modifications in the
tumor microenvironment, which have a profound impact on tumor
biology (15) and the incurred
tumor hypoxic conditions can promote metastasis by recurrence in
untreated hypoxic cells (16). It
is now becoming evident that IR can also result in cancer cells
acquiring a stemness state characterized by increased stemness gene
expression and a cancer stem cell-like phenotype (17). Some studies indicate that
irradiated tumors contain a stemness population and that growth of
distant metastasis is driven by cancer stem-like cells.
Furthermore, several studies have shown that the
epithelial-mesenchymal transition (EMT) has a crucial role in IR
resistance of cancer cells (18).
EMT was initially recognized as an important process during the
morphogenesis of epithelial tissue in embryonic development, and is
now shown to be one of the key steps promoting tumor metastasis.
The EMT maintains cancer stemness and is induced by various
factors. Several effectors, including transforming growth factor
(TGF)-β, fibronectin (Fn1), metalloproteinases (MMPs), vimentin
(Vim) and cadherin, mediate the EMT. Acquisition of stemness
results in metastasis along with CD24, CD133, β-catenin, Oct-4 and
Sox-2 expression in non-small cell lung cancer cells (19). Although the relationships between
PI3K/Akt/mTOR signaling, EMT and cancer stem cells are known, the
regulation mechanisms of metastasis in irradiated tumor are still
unclear.
Regular follow-up examinations, such as imaging and
tumor marker tests, may be required after radiotherapy to detect
metastasis at an early stage. Therefore, many investigators utilize
advance imaging techniques to monitor neoplastic progression to
metastasis, such as bioluminescence imaging (BLI), computed
tomography (CT) and positron emission tomography (PET) (20). These imaging modalities can
evaluate progression, such as tumor growth, metabolism, or
metastasis in vivo, in a longitudinal manner. A non-invasive
imaging method is indispensable for detecting tumor lesions in an
internal organ, such as the lungs, liver or brain, and diagnosing
and determining the size of tumors in a preclinical animal model
(21–24). Other ways to detect cancer involved
in distance metastasis include immunohistochemical staining of
ex vivo biopsies; however, these often lack reproducibility
and accuracy (25).
In the present study, metastatic tumors in C6-L
xenografted mice were studied after local treatment with
fractionated γ-IR. To accurately detect the metastatic nodules
after γ-IR, we observed the effect of γ-IR on distant metastatic
tumor growth using different imaging modalities, such as BLI and
PET/CT. A non-invasive longitudinal imaging study with repeated
measurements of metastatic nodules after γ-IR indicated extensive
colonization of C6-L cells in the lungs within 6 weeks after γ-IR.
We also identified and described the molecular events occurring
after γ-IR through gene expression profiling to elucidate genetic
changes. We identified the differentially expressed genes between
the γ-IR primary tumors vs. non-γ-IR primary tumors and metastatic
lung nodules vs. γ-IR primary tumors using an Agilent Expression
microarray contained ~30,003 Entrez Gene RNAs. In particular, we
found known cancer stem cell markers and detected EMT among the
differentially expressed genes.
Materials and methods
Cell culture
C6 rat glioma cells and C6-L infected cells
containing the firefly luciferase (fLuc) gene in lentiviral vectors
were used with selected blasticidin treatment (5 mg/ml), as
previously described by Park et al (26).
Xenograft model and local Agilent
Expression microarray-IR
BALB/c nu/nu mice (females, 5–6 weeks of age) were
purchased from Orient Bio, Inc. (Seoul, Korea). C6-L cells
(5×105/head) were implanted subcutaneously into the
right thigh of mice. When the tumors reached ≥80 mm3 (15
days after inoculation), we randomly assigned them to the C6-L
γ-irradiated (γ-IR) and non-IR groups. C6-L tumor-bearing mice were
treated locally with 50 Gy γ-IR in five 10-Gy fractions every day
using a 60Co γ-IR source (Theratrom 780; AECL, Ltd.,
Mississauga, ON, Canada; n=35), but not the control group (n=5).
The mice were anesthetized with an intraperitoneal injection of a
mixture of zolazepam/tiletamine (50 mg/kg; Zoletil 50®;
Virbac, Magnyen-Vexin, France) and xylazine (10 mg/kg;
Rompun®; Bayer Healthcare, Berlin, Germany) fixed on an
acryl plate. All experiments with animals were carried out
according to the guidelines for the care and the use of
experimental animals and were approved by the Korea Institute of
Radiological and Medical Sciences.
BLI acquisition
BLI was performed with a highly sensitive, optical
CCD camera mounted in a light-tight specimen chamber (IVIS200;
Xenogen, Alameda, CA, USA). Animals were given the firefly
substrate D-luciferin potassium salt diluted to 2.5 mg/100 μl in
saline. The mice were injected intraperitoneally with 100 μl of
this D-luciferin solution and were anesthetized (2% isoflurane) for
in vivo imaging. The mice were placed on the stage inside
the light-tight camera box with continuous exposure to 0.5%
isoflurane. Image acquisition time was 10 min. Bioluminescence
signals were expressed in units of photons per cm2 per
second per steradian (p/cm2/s/sr). Imaging and signal
quantification were controlled by the acquisition and analysis
software (Living Image v. 2.50; Xenogen).
PET/CT image acquisition
Mice were imaged using a small animal PET/CT system
(INVEON™; Siemens Preclinical Solutions, Knoxville, TN, USA).
[18F] Fluordeoxyglucose (FDG) (7.4 MBq, 200 μCi) was
injected via tail vein 1 h prior to PET/CT scanning.
[18F] fluorothymidine (FLT) (same dose) was injected 2 h
prior. Mice were anesthetized using 2% isoflurane. PET and CT
images were acquired using small animal PET/CT scanner. The mice
were moved to the PET scanner on the same bed and scanned for 30
min after CT acquisition. Tissue radioactivity was expressed as the
percentage of injected radioactivity dose per gram of tissue
(%ID/g). Visualization and analyses of PET images were carried out
using AsiPRO™ software (Siemens Preclinical Solutions).
Radioactivity concentration in the local region was calculated from
the PET images using maximum pixel values.
Evaluation of fLuc expression for reverse
transcription-polymerase chain reaction (RT-PCR) analysis with
tissue
Total RNA was isolated from metastatic tissue and
used as a template to produce cDNA using SuperScript III
First-Strand Synthesis for RT-PCR (Invitrogen, Carlsbad, CA, USA).
The synthesized cDNA was amplified using Taq DNA polymerase (iNtRON
Biotechnology, Inc., Daejeon, Korea) with the fLuc primer: forward,
5′-CGC CTT GAT TGA CAA GGA TGG-3′, and reverse, 5′-GGC CTT TAT GAG
GAT CTC TCT-3′. The forward rat GADPH primer was 5′-CAG TGC CAG CCT
CGT CTC AT-3′ and the reverse primer was 5′-AGG GGC CAT CCA CAG TCT
TC-3′.
Microarray analysis
Total RNA from primary tumors and IR-induced
metastatic tissue for each model were used for expression
profiling. Total RNA was purified using the Easy-spin Total RNA
Extraction kit (iNtRON Biotechnology) according to the
manufacturer's recommendations with the Agilent SurePrint G3 Rat
Gene Expression 8×60K microarrays (Agilent Technologies, Inc.,
Santa Clara, CA, USA). The Agilent expression microarray contained
~30,003 Entrez Gene RNAs. The microarray analysis was done by
Macrogen (Seoul, Korea). The arrays were scanned using the Agilent
Technologies G2600D SG12494263. Array data export processing and
analysis were performed using Agilent Feature Extraction software
v11.0.1.1.
Results
Detection of metastatic tumors by BLI
after γ-IR
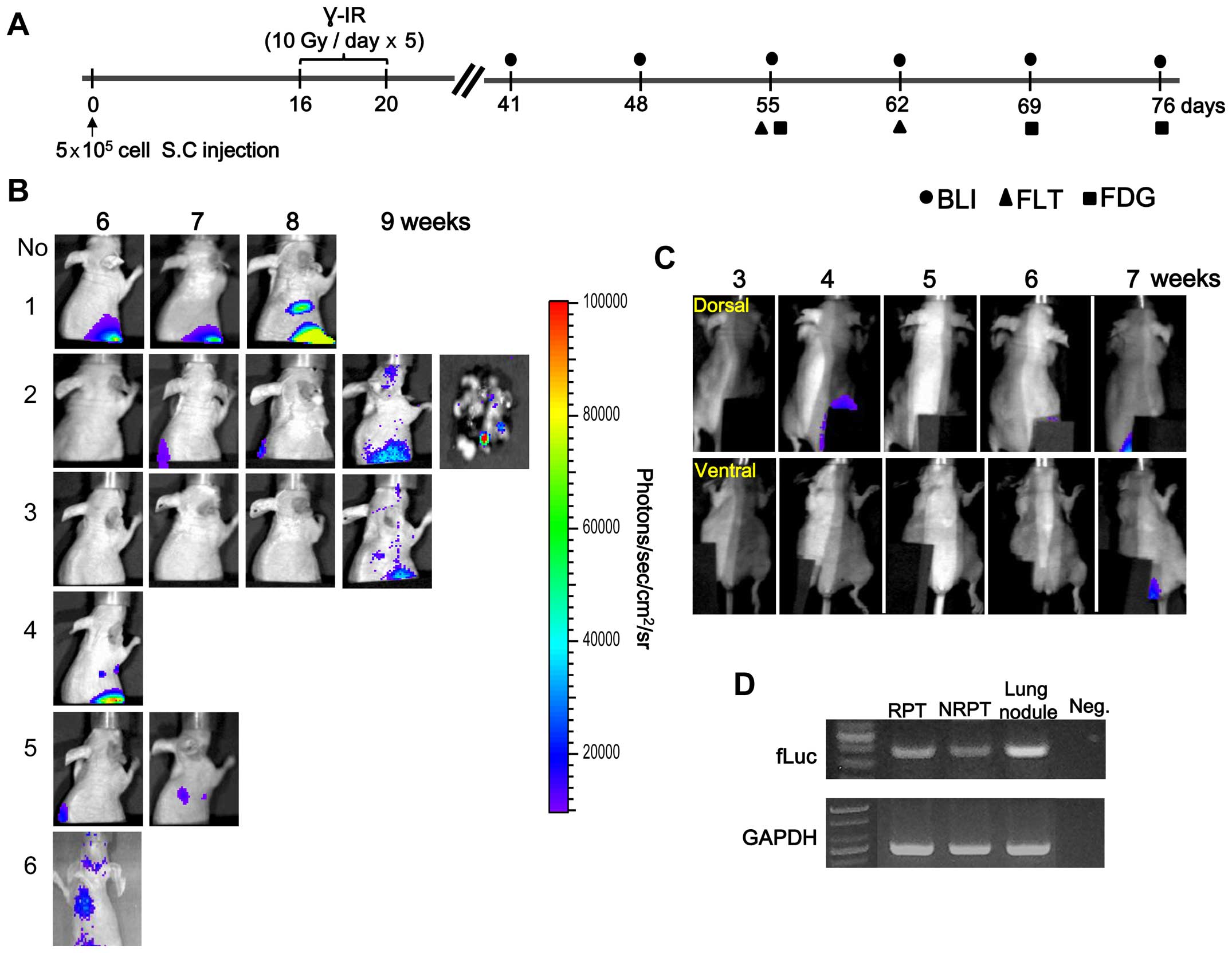
The schedule for obtaining the BLI and nuclear
medicine images beginning 4 weeks after γ-IR is presented in
Fig. 1A. The BLI results of a
longitudinal study of primary tumor growth and distant metastasis
at a C6-L secondary site are shown in Fig. 1B and C. We detected metastatic
tumors 6–9 weeks after γ-IR in the lungs by BLI and confirmed fLuc
gene expression in the tissues (Fig.
1B and D). However, no distant metastasis was detected at the
secondary site in the non-IR primary tumor (NRPT) model by BLI
(Fig. 1C). Light emission of the
lungs removed from sacrificed animal was examined at 9 weeks to
confirm that the metastatic nodules were from the C6-L primary
tumor (Fig. 1B, No. 2). This
result suggests that the BLI signal from the lung originated from a
γ-IR primary tumor (RPT) and was confirmed by RT-PCR (Fig. 1D). A fLuc-specific RT-PCR DNA band
of 399 bp was detected in the metastatic lung nodules after γ-IR.
However, survival of γ-IR treated mice was longer than that of the
non-IR group because of the relatively low growth rate of the
primary tumor mass after γ-IR (26).
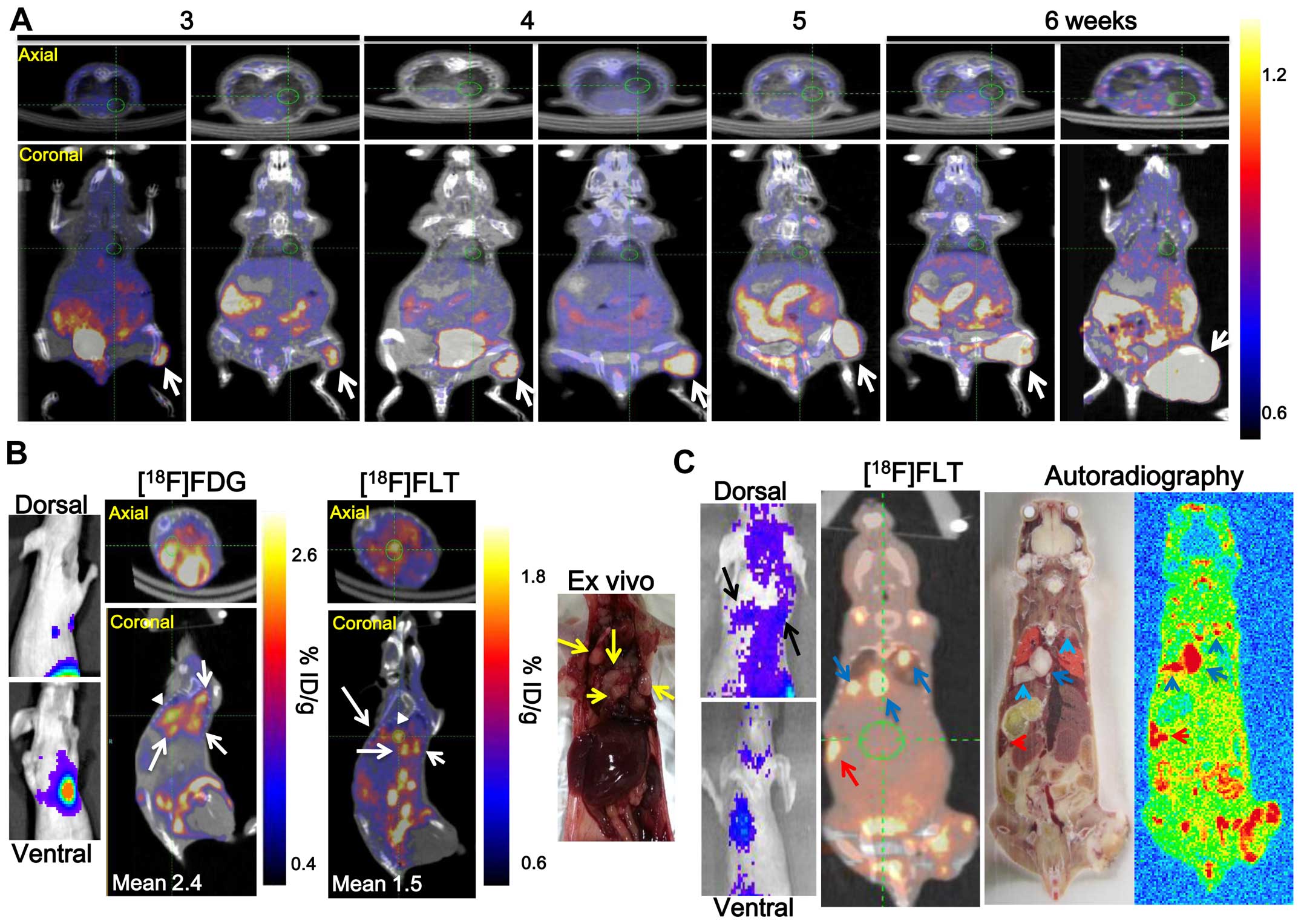
Confirmation of metastatic tumor after
γ-IR by nuclear medicine imaging
Metastatic nodules at secondary sites in the non-IR
tumor model were monitored for 6 weeks by [18F]FLT-PET
(Fig. 2A), however, no metastatic
nodules were detected at secondary sites. Mouse (No. 4 of Fig. 1B) underwent PET/CT 6 weeks after
γ-IR and the administration of 7.4 MBq [18F]FLT and
[18F]FDG to confirm the metastatic lung nodules after
γ-IR detected by BLI via fLuc gene expression (Fig. 2B). [18F]FLT and
[18F]FDG activity in the four metastatic lung nodules
was high (Fig. 2B, white arrows).
The activities were re-calculated using a region of interest
analysis from a three-dimensional reconstruction encompassing the
[18F]FLT and [18F]FDG uptake region. The
[18F]FLT and [18F]FDG uptake values into one
metastatic lung nodule was 1.5±0.13 and 2.4±0.24 %ID/g,
respectively (white arrowhead). Three metastatic lung nodules (blue
arrow) and one metastatic spleen nodule (red arrow) were detected
by [18F]FLT-PET and [18F]FLT autoradiography
in another γ-IR treated C6-L bearing mouse (No. 6 of Figs. 1B and 2C). However, no splenic metastatic
nodules were detected by BLI (Fig.
2C, left panel). Metastatic nodules were detected by BLI or
nuclear medicine imaging in 6 (17.14%) of the 35 C6-L bearing mice
from 6 weeks after γ-IR (Table I).
RNA isolated from RPT, 3 meta-static lung nodules of mouse No. 6 of
Fig. 1B, and NRPT of a
non-irradiated mouse was analyzed by microarray.
 | Table ISummary of the incidence of
metastatic nodules after γ-irradiation (IR). |
Table I
Summary of the incidence of
metastatic nodules after γ-irradiation (IR).
| No. | Images | Detection time
(weeks) |
|---|
| 1 | WB BLI, nuclear
imaging (FDG) | 8 |
| 2 | WB BLI, ex
vivo BLI | 9 |
| 3 | WB BLI, nuclear
imaging (FDG) | 9 |
| 4 | WB BLI, nuclear
imaging (FDG, FLT), ex vivo BLI | 6 |
| 5 | WB BLI, nuclear
imaging (FLT) | 7 |
| 6a | WB BLI, nuclear
imaging (FLT), autoradiography | 6 |
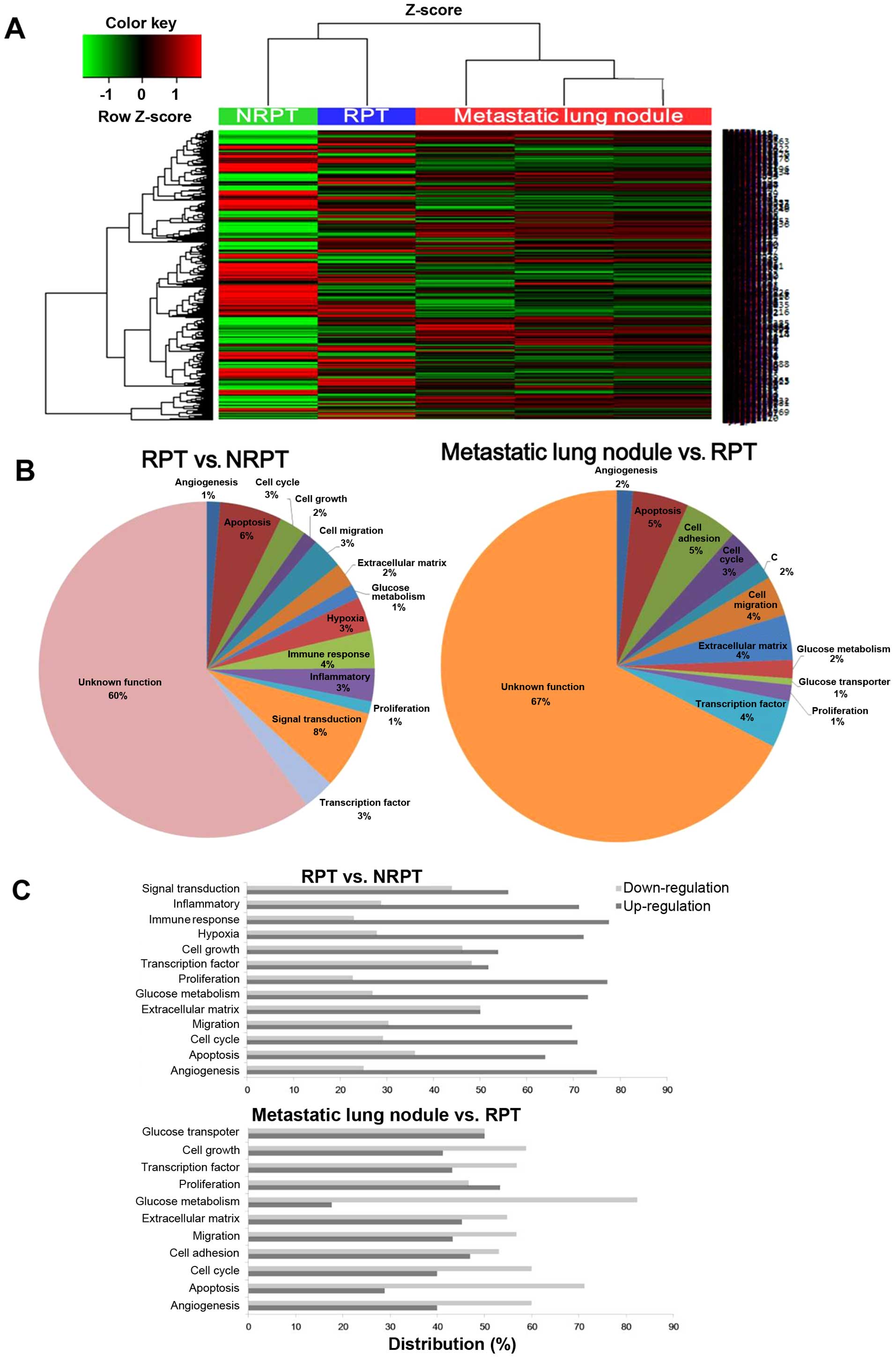
Overview of metastatic tumors after
γ-IR-related gene expression
The expression patterns of whole mRNAs were analyzed
by microarray to elucidate the changes in NRPT, RPT and metastatic
lung nodules. A hierarchical clustering analysis of 3,881 genes
(≥2-fold change, P-value <0.05) indicated differentially
expressed genes between the NRPT, RPT and three metastatic lung
nodules (Fig. 3A). RPT and NRPT
are closely clustered together and showed a similar heat map
pattern of mRNA expression, which is different from that of the
meta-static lung nodules. As shown in Fig. 3B, the biological process terms
differ between the RPT vs. NRPT and the metastatic lung nodules vs.
RPT that reflects their known functions. The RPT enriched genes
have linked biological process terms: hypoxia (3%), immune response
(4%), inflammatory (3%) and signal transduction (8%). In contrast,
transcription factor (4%) and glucose metabolism (2%) are linked by
biological process terms for the metastatic lung nodules. Gene
Ontology (GO) analysis was performed using DAVID to gain a
comprehensive understanding of the gene classes that were
differentially regulated in the RPT vs. NRPT and the metastatic
lung nodules vs. RPT (Fig. 3C). We
found upregulated expression of angiogenesis, migration, and
proliferation-related genes in RPT but downregulated expression in
glucose metabolism-related genes and apoptosis in the metastatic
lung nodules. The molecular mechanisms and therapeutic targets
underlying metastatic tumors after γ-IR remain unclear. Identifying
metastatic tumors using γ-IR-related molecular target will be
helpful to identify useful therapeutic targets, developing novel
treatment approaches, and overcome recurrence after γ-IR in
patients with glioma. We present novel insight into the EMT and
enhanced stemness in RPT based on our total gene expression
analysis in γ-IR tumor tissue and metastatic lung nodules of the
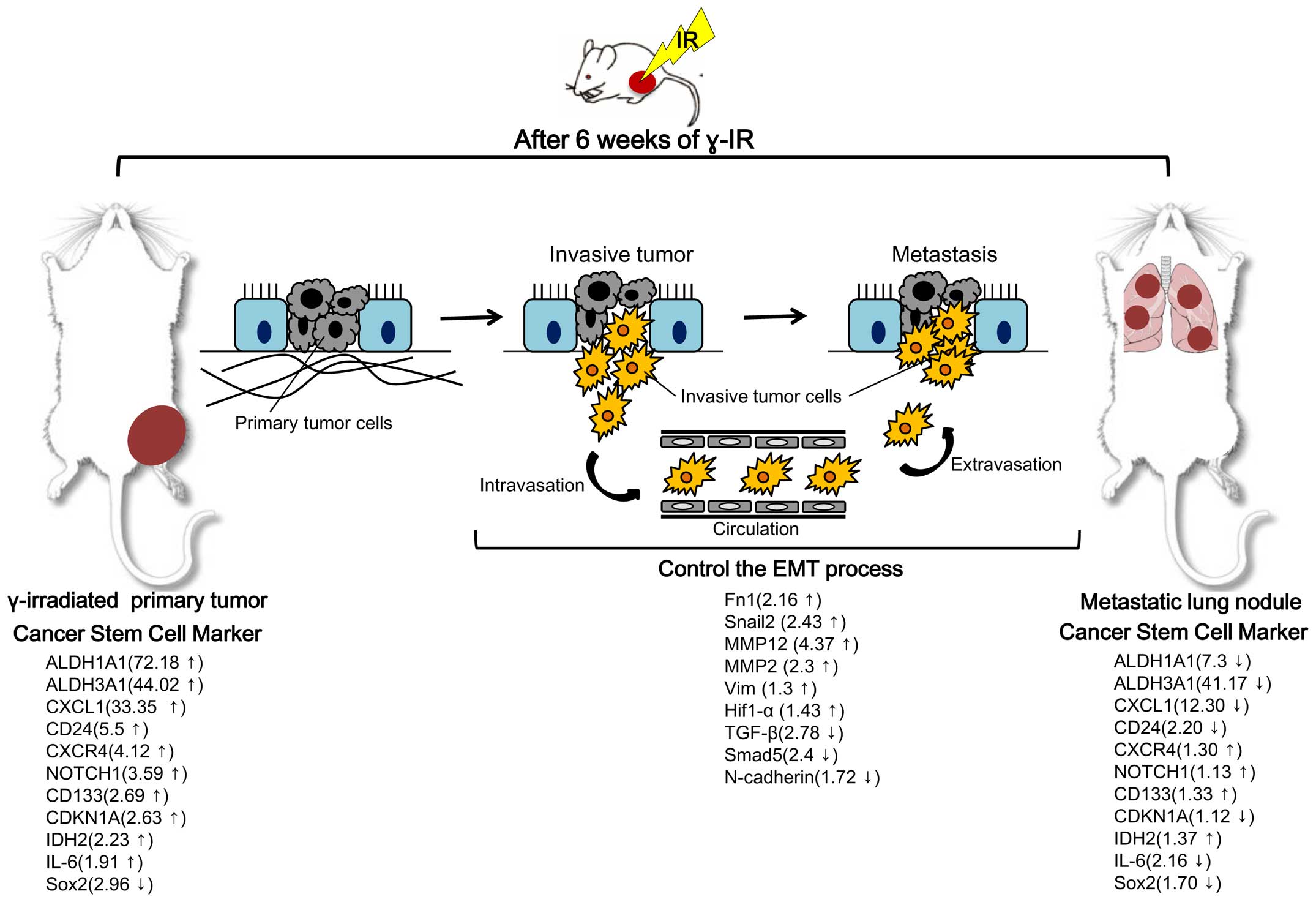
genes of interest. Our findings are summarized in Fig. 4. In particular, expression changes
in RPT with cancer stem cell markers were highly significant. For
example, aldehyde dehydrogenases 1A1 and 3A1 (ALDH1A1 and ALDH3A1),
which are members of the human aldehyde dehydrogenase superfamily,
constitute novel candidate cancer stem cell markers in various
solid tumors in the testis, brain, lens, liver, lung and retina
(27). These cytoplasmic enzymes
act during the oxidative stress response (28), differentiation (29) and drug resistance (30). ALDH1A1 has been reported as a novel
marker for glioblastoma cells with stem cell characteristics
(31) and showed the highest level
change (72.18-fold higher in RPT than in NRPT) in the present
study. The cancer stem cell marker CD24 was also differentially
expressed (5.5-fold higher in RPT than in NRPT). In contrast to the
finding on RPT and NRPT, expression of cancer stem cell markers,
such as ALDH1A1, ALDH3A1, CD24, CXCL1 and IL-6 was mostly
downregulated in metastatic lung nodules after γ-IR compared to RPT
(Fig. 4).
Discussion
In the present study, we observed distant metastasis
after local γ-IR using BLI of fLuc gene expressing rat glioma and
[18F]FLT and [18F]FDG-PET. Next, we observed
that γ-IR, particularly fractionated local γ-IR, increased stem
cell marker expression in γ-IR primary tumors by microarray. We
used the γ-IR dose and schedule for C6-L tumor-bearing mice as
described by Camphausen et al (32), who used Lewis lung carcinoma cells
to confirm that γ-IR promotes metastasis in a mouse model.
BLI has relatively low cost and high throughput
capability, but the depth dependence of the signal is a major
disadvantage in small animals. The other major limitation is that
BLI does not provide anatomical information. Therefore, metastatic
tumors in lung and spleen after γ-IR were confirmed by small-animal
PET/CT. [18F]FDG is the most widely used PET tracer and
is indispensable for diagnosing and staging PET tracer for a
variety of cancers. Several research groups have suggested that
[18F]FLT is useful as a PET tracer to monitor
proliferation and other biological response of tumors to
chemotherapy and radiotherapy (33–36).
In the present study, we evaluated
[18F]FDG and [18F] FLT-PET as a potential
diagnostic tool for monitoring the response to metastatic tumors
after γ-IR in a tumor-bearing mouse model. We found high uptake of
[18F]FLT and [18F] FDG in metastatic lung
nodules after γ-IR. The four nodules that were discriminated by
[18F]FLT and [18F]FDG-PET were detected as
one spot on BLI (Fig. 2B). The
other limitation of BLI is that it does not discriminate focal
signals due to spill-over. Three metastatic lung nodules and one
metastatic splenic nodule in another γ-IR C6-L bearing mouse were
detected by [18F]FLT-PET and autoradiography, but the
splenic nodule was not detected by BLI due to a light penetration
problem into deep tissue (Fig.
2C). Therefore, BLI and nuclear medicine imaging may be
suitable for metastatic tumor screening after γ-IR and to more
precisely locate metastatic tumors, respectively.
Recent studies have shown that a decreased cellular
proliferation capacity is an early event in response to 20-Gy IR
(37). We wondered whether
proliferation had recovered in IR primary tumor lesions at 6 weeks
γ-IR. Cancer stem cell markers were upregulated in γ-IR primary
tumor lesions compared to that in non-IR primary tumors. We also
found downregulation in a proportion of cancer stem cell markers in
metastatic lung nodules. The proportion of cancer cell markers,
particularly the ALDH family and CD24, increasing in γ-IR primary
tumors may be important for distant metastasis in glioma. In
particular, we revealed that upregulation of ALDH1A1 in γ-IR C6-L
primary tumors may be a cancer stemness property. ALDH1A1 is a
predominant isoform of the ALD family located in the cytoplasm
(38) and has gained attention as
a putative cancer stem cell and progenitor cell marker (39). Our data show that the small number
of C6-L cells that survived in γ-IR C6-L primary tumors may have
high ALDH1A1 expression, suggesting that cells surviving γ-IR are a
source for distant metastasis. CD44 and CD90 have also been
proposed as cancer stem-like cell markers in esophageal squamous
cell carcinoma but cell heterogeneity limits their application
(40,41).
We evaluated the metastatic tumors after γ-IR and
found invasive/migration ability after local treatment of C6-L
xeno-graft mice, suggesting that the small number of C6-L cells
that survived in locally γ-IR treated tumors have more potential to
metastasize, which is the main reason for recurrence of glioma
after radiotherapy. The microarray study revealed more surviving
cancer cells with cancer stem cell markers in the γ-IR primary
tumors compared with those in the non-IR primary tumors. After
formation of metastatic lung nodules in our experiments, expression
of cancer stem cell markers may be downregulated as shown in our
microarray data (Fig. 4). Recent
studies in patients with glioma observed that the EMT may affect
the ability of biomarkers to predict radio-resistant glioma
(42). We observed downregulation
of TGF-β and Smad5 and upregulation of MMPs, Fn1 and Snail2 in RPT
compared with NRPT.
In summary, metastatic tumors were detected after
fractionated γ-IR with 60Co by non-invasive longitudinal
imaging and repeated measurements of the metastatic tumors after
γ-IR. We demonstrated that metastatic tumors after γ-IR are
associated with several genes, including the EMT and enhanced
cancer stem cell markers which result in cancer cell growth,
survival, invasion and proliferation.
Acknowledgements
The present study was supported by the Korea Science
and Engineering Foundation (KOSEF) grant funded by the Korea
government (MEST) (NRF-2012M2A2A7013480).
References
|
1
|
Dirks PB: Brain tumor stem cells: Bringing
order to the chaos of brain cancer. J Clin Oncol. 26:2916–2924.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence TS, Haffty BG and Harris JR:
Milestones in the use of combined-modality radiation therapy and
chemotherapy. J Clin Oncol. 32:1173–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Govindan R, Bogart J and Vokes EE: Locally
advanced non-small cell lung cancer: The past, present, and future.
J Thorac Oncol. 3:917–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cognetti DM, Weber RS and Lai SY: Head and
neck cancer: An evolving treatment paradigm. Cancer. 113(Suppl):
1911–1932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gold KA, Lee HY and Kim ES: Targeted
therapies in squamous cell carcinoma of the head and neck. Cancer.
115:922–935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al; European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group. Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prasanna A, Ahmed MM, Mohiuddin M and
Coleman CN: Exploiting sensitization windows of opportunity in
hyper and hypo-fractionated radiation therapy. J Thorac Dis.
6:287–302. 2014.PubMed/NCBI
|
|
10
|
Suit HD: Local control and patient
survival. Int J Radiat Oncol Biol Phys. 23:653–660. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balasubramaniam A, Shannon P, Hodaie M,
Laperriere N, Michaels H and Guha A: Glioblastoma multiforme after
stereo-tactic radiotherapy for acoustic neuroma: Case report and
review of the literature. Neuro Oncol. 9:447–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaplan HS and Murphy ED: The effect of
local roentgen irradiation on the biological behavior of a
transplantable mouse carcinoma; increased frequency of pulmonary
metastasis. J Natl Cancer Inst. 9:407–413. 1949.PubMed/NCBI
|
|
13
|
von Essen CF: Radiation enhancement of
metastasis: A review. Clin Exp Metastasis. 9:77–104. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Núñez MI, McMillan TJ, Valenzuela MT, Ruiz
de Almodóvar JM and Pedraza V: Relationship between DNA damage,
rejoining and cell killing by radiation in mammalian cells.
Radiother Oncol. 39:155–165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barcellos-Hoff MH, Park C and Wright EG:
Radiation and the microenvironment - tumorigenesis and therapy. Nat
Rev Cancer. 5:867–875. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moulder JE and Rockwell S: Hypoxic
fractions of solid tumors: Experimental techniques, methods of
analysis, and a survey of existing data. Int J Radiat Oncol Biol
Phys. 10:695–712. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghisolfi L, Keates AC, Hu X, Lee DK and Li
CJ: Ionizing radiation induces stemness in cancer cells. PLoS One.
7:e436282012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou YC, Liu JY, Li J, Zhang J, Xu YQ,
Zhang HW, Qiu LB, Ding GR, Su XM, Mei-Shi, et al: Ionizing
radiation promotes migration and invasion of cancer cells through
transforming growth factor-beta-mediated epithelial-mesenchymal
transition. Int J Radiat Oncol Biol Phys. 81:1530–1537. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomez-Casal R, Bhattacharya C, Ganesh N,
Bailey L, Basse P, Gibson M, Epperly M and Levina V: Non-small cell
lung cancer cells survived ionizing radiation treatment display
cancer stem cell and epithelial-mesenchymal transition phenotypes.
Mol Cancer. 12:942013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adseshaiah PP, Patel NL, Ileva LV, Kalen
JD, Haines DC and McNeil SE: Longitudinal imaging of cancer cell
metastases in two preclinical models: A correlation of noninvasive
imaging to histopathology. Int J Mol Imaging. 102702:20142014.
|
|
21
|
Kang JH and Chung JK: Molecular-genetic
imaging based on reporter gene expression. J Nucl Med. 49(Suppl 2):
164S–179S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JH, Kim KI, Lee YJ, Lee TS, Kim KM,
Nahm SS, Park YS, Cheon GJ, Lim SM and Kang JH: Non-invasive
monitoring of hepatocellular carcinoma in transgenic mouse with
bioluminescent imaging. Cancer Lett. 310:53–60. 2011.PubMed/NCBI
|
|
23
|
Kim KI, Park JH, Lee YJ, Lee TS, Park JJ,
Song I, Nahm SS, Cheon GJ, Lim SM, Chung JK, et al: In vivo
bioluminescent imaging of α-fetoprotein-producing hepatocellular
carcinoma in the diethylnitrosamine-treated mouse using recombinant
adeno-viral vector. J Gene Med. 14:513–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JH, Kang JH, Lee YJ, Kim KI, Lee TS,
Kim KM, Park JA, Ko YO, Yu DY, Nahm SS, et al: Evaluation of
diethylnitrosamine- or hepatitis B virus X gene-induced
hepatocellular carcinoma with 18F-FDG PET/CT: A
preclinical study. Oncol Rep. 33:347–353. 2015.
|
|
25
|
Gown AM: Current issues in ER and HER2
testing by IHC in breast cancer. Mod Pathol. 21(Suppl 2): S8–S15.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park JK, Jang SJ, Kang SW, Park S, Hwang
SG, Kim WJ, Kang JH and Um HD: Establishment of animal model for
the analysis of cancer cell metastasis during radiotherapy. Radiat
Oncol. 7:153–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vasiliou V, Thompson DC, Smith C, Fujita M
and Chen Y: Aldehyde dehydrogenases: From eye crystallins to
metabolic disease and cancer stem cells. Chem Biol Interact.
202:2–10. 2013. View Article : Google Scholar
|
|
28
|
Marchitti SA, Brocker C, Stagos D and
Vasiliou V: Non-P450 aldehyde oxidizing enzymes: The aldehyde
dehydrogenase superfamily. Expert Opin Drug Metab Toxicol.
4:697–720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chute JP, Muramoto GG, Whitesides J,
Colvin M, Safi R, Chao NJ and McDonnell DP: Inhibition of aldehyde
dehydrogenase and retinoid signaling induces the expansion of human
hematopoietic stem cells. Proc Natl Acad Sci USA. 103:11707–11712.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muramoto GG, Russell JL, Safi R, Salter
AB, Himburg HA, Daher P, Meadows SK, Doan P, Storms RW, Chao NJ, et
al: Inhibition of aldehyde dehydrogenase expands hematopoietic stem
cells with radioprotective capacity. Stem Cells. 28:523–534.
2010.PubMed/NCBI
|
|
31
|
Rasper M, Schäfer A, Piontek G, Teufel J,
Brockhoff G, Ringel F, Heindl S, Zimmer C and Schlegel J: Aldehyde
dehydrogenase 1 positive glioblastoma cells show brain tumor stem
cell capacity. Neuro Oncol. 12:1024–1033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Camphausen K, Moses MA, Beecken WD, Khan
MK, Folkman J and O'Reilly MS: Radiation therapy to a primary tumor
accelerates metastatic growth in mice. Cancer Res. 61:2207–2211.
2001.PubMed/NCBI
|
|
33
|
Murayama C, Harada N, Kakiuchi T, Fukumoto
D, Kamijo A, Kawaguchi AT and Tsukada H: Evaluation of
D-18F-FMT, 18F-FDG, L-11C-MET, and
18F-FLT for monitoring the response of tumors to
radiotherapy in mice. J Nucl Med. 50:290–295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Molthoff CF, Klabbers BM, Berkhof J,
Felten JT, van Gelder M, Windhorst AD, Slotman BJ and Lammertsma
AA: Monitoring response to radiotherapy in human squamous cell
cancer bearing nude mice: comparison of
2′-deoxy-2′-[18F]fluoro-D-glucose (FDG) and
3′-[18F]fluoro-3′-deoxythymidine (FLT). Mol Imaging
Biol. 9:340–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YJ, Ryu JS, Kim SY, Oh SJ, Im KC, Lee
H, Lee SW, Cho KJ, Cheon GJ and Moon DH: Use of
3′-deoxy-3′-[18F]fluo-rothymidine PET to monitor early
responses to radiation therapy in murine SCCVII tumors. Eur J Nucl
Med Mol Imaging. 33:412–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sugiyama M, Sakahara H, Sato K, Harada N,
Fukumoto D, Kakiuchi T, Hirano T, Kohno E and Tsukada H: Evaluation
of 3′-deoxy-3′-18F-fluorothymidine for monitoring tumor
response to radiotherapy and photodynamic therapy in mice. J Nucl
Med. 45:1754–1758. 2004.PubMed/NCBI
|
|
37
|
Wang H, Liu B, Tian J, Xu B, Zhang J, Qu B
and Chen Y: Evaluation of 18F-FDG and 18F-FLT
for monitoring therapeutic responses of colorectal cancer cells to
radiotherapy. Eur J Radiol. 82:e484–e491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hess DA, Craft TP, Wirthlin L, Hohm S,
Zhou P, Eades WC, Creer MH, Sands MS and Nolta JA: Widespread
nonhematopoietic tissue distribution by transplanted human
progenitor cells with high aldehyde dehydrogenase activity. Stem
Cells. 26:611–620. 2008. View Article : Google Scholar
|
|
39
|
Douville J, Beaulieu R and Balicki D:
ALDH1 as a functional marker of cancer stem and progenitor cells.
Stem Cells Dev. 18:17–25. 2009. View Article : Google Scholar
|
|
40
|
Zhao JS, Li WJ, Ge D, Zhang PJ, Li JJ, Lu
CL, Ji XD, Guan DX, Gao H, Xu LY, et al: Tumor initiating cells in
esophageal squamous cell carcinomas express high levels of CD44.
PLoS One. 6:e214192011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao R, Quaroni L and Casson AG:
Identification and characterization of stemlike cells in human
esophageal adenocarcinoma and normal epithelial cell lines. J
Thorac Cardiovasc Surg. 144:1192–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng J, Li P, Zhang Q, Yang Z and Fu S: A
radiosensitivity gene signature in predicting glioma prognostic via
EMT pathway. Oncotarget. 5:4683–4693. 2014. View Article : Google Scholar : PubMed/NCBI
|