Introduction
Non-Hodgkin lymphoma (NHL) is the most common
hematologic malignancy arising from lymphoid tissue worldwide
(1), ~85% of which derive from B
lymphocytes. The overall survival of B-cell lymphomas has
significantly improved since the FDA approval and clinical use of
rituximab, a monoclonal antibody targeting CD20-positive malignant
B cells (2). However, relapse and
resistance are obstacles to the cure of B-NHL patients,
underscoring the need for more effective therapies. Immunotherapy
is one promising new direction for lymphoma as it is for many other
malignancies (2).
The B7 family is a group of molecules important for
the regulation of tumor immunity, and can profoundly affect tumor
progression and elimination (3–8).
Monoclonal antibodies targeting B7 molecules including CD80
(9), PD-L1 (10), PD-L2 (11) and B7-H3 (12), have shown promising results in
lymphomas, melanoma, non-small cell lung cancer and other cancers.
B7-H6, also called NCR3L1 or DKFZp686O24166, is a new member of the
B7 family identified by Brandt et al in 2009 (3). B7-H6 is a 454-aa-long type I
transmembrane protein that shows considerable homology with PD-L1
and B7-H3 (3), both of which
contribute to the oncogenesis and chemoresistance of lymphomas
(13,14). The receptor of B7-H6, NKp30, is an
activating NK-cell receptor. The interaction of tumor
membrane-bound B7-H6 and NKp30 leads to the activation of NK cells
and to the lysis of tumor cells (3,15). A
fusion protein of B7-H6 ectodomain and CD20 single-chain fragment
stimulates NKp30-mediated NK cell cytotoxicity (7). Moreover, the NKp30-based chimeric
antigen receptor promotes T-cell effector functions and antitumor
efficacy through recognizing and killing B7-H6-positive tumor cells
(16). These results indicate that
B7-H6 may be a potential therapeutic target in cancer therapy.
It is reported that B7-H6 is widely and specifically
expressed on tumor cells including melanomas (3,17),
cervical carcinomas (3), ovarian
carcinomas (3,4), liver cancer (18), gastric cancer (19), lung cancer (20), neuroblastoma (6), T and B lymphomas and myeloid leukemia
(3,8), but is absent from normal tissues
(3). B7-H6 is associated with
tumor progression and metastasis in ovarian cancers (4). To date, however, little is known
about the oncogenic role of B7-H6 in lymphomas. Hence, we
established a B7-H6 knockdown cell line for the first time to
investigate the impact of B7-H6 on cell proliferation, apoptosis,
cell cycle, migration, invasion and chemosensitivity. The
underlying mechanisms were also investigated, with the aim of
providing a basis for clinical therapy.
Materials and methods
Cell lines and cell culture
Raji, CA46, Z138, Maver and HeLa cell lines were
obtained from the American Type Culture Collection (ATCC,
Rockville, MD, USA). Raji, CA46 and Z138 cells were cultured in
RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 2
mM L-glutamine, 10% FBS (Hyclone, South Logan, UT, USA), 100 U/ml
penicillin and 100 μg/ml streptomycin. Maver and HeLa cells were
respectively cultured in IMDM and DMEM medium. Cells were routinely
cultured at 37°C in a humidified incubator containing 5%
CO2 (14). The primary
tumor proteins were obtained from Peking University Third Hospital.
The study was approved by the local ethics committee and all
patients provided written informed consent.
Lentivirus-based RNA interference
transfection and generation of stable cell lines
The human B7-H6 targeting small hairpin RNA (shRNA)
was designed and synthesized by Life Technologies (Lifetech,
Beijing, China). The sequence was 5′-CGGCACAGTCTTTCTGAAACT-3′. A
negative non-target control shRNA was also used with the sequence
5′-CAAC AAGATGAAGAGCACCAA-3′. These shRNAs were used to generate
recombinant lentiviral particles as described (14). CA46 cells were infected in the
presence of 8 μg/ml of polybrene (Sigma, Milwaukee, WI, USA) and
selected by flow cytometry 72 h later. The B7-H6 knockdown was
confirmed by RT-PCR, western blotting and flow cytometry. The
infected cells comprised B7-H6shRNA (CA46shB7-H6) and non-targeted
control (CA46shCtrl) groups, while non-infected cells (CA46)
constituted an additional control group. These three cell lines
were used for the following experiments.
RT-PCR and quantitative PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and reverse transcribed to cDNA
using Reverse Transcription System (Promega, Madison, WI, USA)
according to the manufacturer's instructions. cDNA (100 ng) was
subjected to PCR amplification in a total volume of 25 μl according
to the manufacturer's protocols. The primer sequences of the PCR
were as follows: B7-H6 forward, 5′-ACAGTAAATGCCTGATGGACCT-3′; B7-H6
reverse, 5′-ATTGGGTATGTGAATGCTGGT-3′; β-actin forward,
5′-TGACGTGGACATCCGCAAAG-3′; β-actin reverse,
5′-CTGGAAGGTGGACAGCGAGG-3′. The PCR products were run on agarose
gels and visualized under ultraviolet. Quantitative PCR was
conducted with SuperReal PreMix Plus kit (Tiangen, Beijing, China)
in a volume of 20 μl according to the manufacturer's instructions.
The primer sequences of quantitative PCR were as follows: B7-H6
forward, 5′-GCACTTCCTCACCGCTAATG-3′; B7-H6 reverse,
5′-AGCCTGTTTCCTTTCGCTATT-3′. The ratio of expression of B7-H6 to
β-actin mRNA was calculated.
Western blotting
Cytoplasmic proteins were extracted from primary and
culture cells by lysis buffer composed of 50 mmol/l Tris-HCl (pH
7.5), 150 mmol/l NaCl, 5 mmol/l EDTA, 0.5% Nonidet P-40, 5 mmol/l
dithiothreitol and 10 mmol/l NaF, and containing protease inhibitor
cocktail (Applygen, Beijing, China). Proteins were separated by
SDS-PAGE and transferred onto nitrocellulose membranes. After
blocking, the membranes were sequentially incubated with primary
antibodies, including anti-B7-H6, anti-c-Myc, anti-Rb, and
anti-phospho-Rb (Abcam, Cambridge, MA, USA); anti-Survivin,
anti-CDK4, anti-CDK6, anti-p21, anti-Bad, anti-MMP-2, anti-MMP-9,
and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA);
and anti-Cyclin D1, anti-Bcl-2, anti-Bcl-xL, anti-Bax,
anti-Caspase-3, anti-Caspase-8, anti-STAT3, anti-phospho-STAT3
(Ser727 and Tyr705), anti-ERK1/2 and phospho-ERK1/2 (CST, Beverly,
MA, USA), and then with anti-rabbit or mouse secondary antibodies
(LI-COR, Lincoln, NE, USA). Fluorescent bands were visualized using
an Odyssey infrared imaging system (LI-COR).
Flow cytometry
Single cell suspensions were stained with B7-H6-APC
or IgG1-APC (R&D, Minneapolis, MN, USA) antibodies on ice for
30 min, cells were analyzed using a flow cytometry system
(FACSCalibur; BD Biosciences, San Jose, CA, USA).
Colony formation assay
An 800 single-cell suspension was resuspended in 1
ml medium with 20% FBS and 0.9% methyl-cellulose medium (Sigma, St.
Louis, MO, USA). Samples were plated in 24-well plates and
incubated for 14 days. A colony with >50 cells was counted as a
positive colony. The colony-forming ability was calculated to be
the number of colonies in the test group expressed as a percentage
of the number of colonies in the control group (13).
CCK-8 assay
The Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo,
Japan) was used to study cell viability according to the
manufacturer's instructions. A cell suspension was inoculated into
a 96-well plate (2×104 cells/well) and incubated for 0,
24, 48 or 72 h. At every time-point, 10 μl CCK-8 was added to each
well and the plate was incubated for 3 h at 37°C and 5%
CO2. Absorbance was measured at 450 nm using a
microplate reader.
For cell viability assay, cells were seeded in
96-well plates and exposed to various concentrations of VCR (0,
1.5, 3, 6, 12.5, 25, 50, 75 and 100 μg/ml) or Dex (0, 1.5, 3, 6,
12.5, 25, 50, 100, 200, 400, 800, 1,200, 1,600 and 2,400 μg/ml) for
24 h. A CCK-8 assay was used to detect the chemotherapeutic
sensitivity of cells. The assay was performed with six replicates
(n=6) for each group and repeated at least three times.
Cell apoptosis analysis
To explore the effect of B7-H6 knockdown on cell
apoptosis, cells were serum starved for 24 h. To explore the B7-H6
knockdown on the sensitivity to VCR and Dex, cells were seeded into
6-well plates at a cell density of 1×106 cells/well and
treated with or without VCR (25 μg/ml) or Dex (200 μg/ml) for 24 h.
Cells were then harvested and apoptosis was analyzed by FACSCalibur
cytometer using Annexin V-APC and 7-AAD (BD Pharmingen, San Jose,
CA, USA) according to the manufacturer's instructions. CellQuest
software was used for data analysis.
Cell cycle analysis
To explore the effect of B7-H6 knockdown on the cell
cycle, cells were serum starved for 24 h. Cells were then
collected, washed and fixed in 70% ethanol overnight at 4°C.
Cellular DNA was stained with propidium iodide (PI) (Beyotime,
Nangtong, China) in the dark for 10 min at room temperature. DNA
content and cell number were determined using a FACSCalibur
cytometer. The data were analyzed using the ModFit program (Verify
Software House, Cambridge, UK).
Cell migration and invasion assay
Migration and invasion assays were performed using
24-well Transwell cell culture chambers (Corning, NY, USA) or
Matrigel-coated invasion chambers. Cells (5×105
cells/well) were serum starved for 24 h and placed in the upper
chamber in serum-free medium. Medium containing 10% FBS was placed
in the lower chamber as a chemoattractant. After 24 h of
incubation, the migrated cells in the lower chamber were collected
and resuspended. Non-invading cells were removed with a
cotton-tipped swab from the top of Matrigel and invading cells were
fixed and stained with 0.1% crystal violet. Cells were photographed
and counted in six random microscopic fields.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (Chicago, IL, USA). Data are shown as mean± SD of
triplicate for each experiment. Differences between groups were
evaluated using SPSS 18.0 software. Data are shown as mean ± SD of
all replicates for each experiment. Differences between groups were
evaluated using ANOVA. P<0.05 was considered to be statistically
significant.
Results
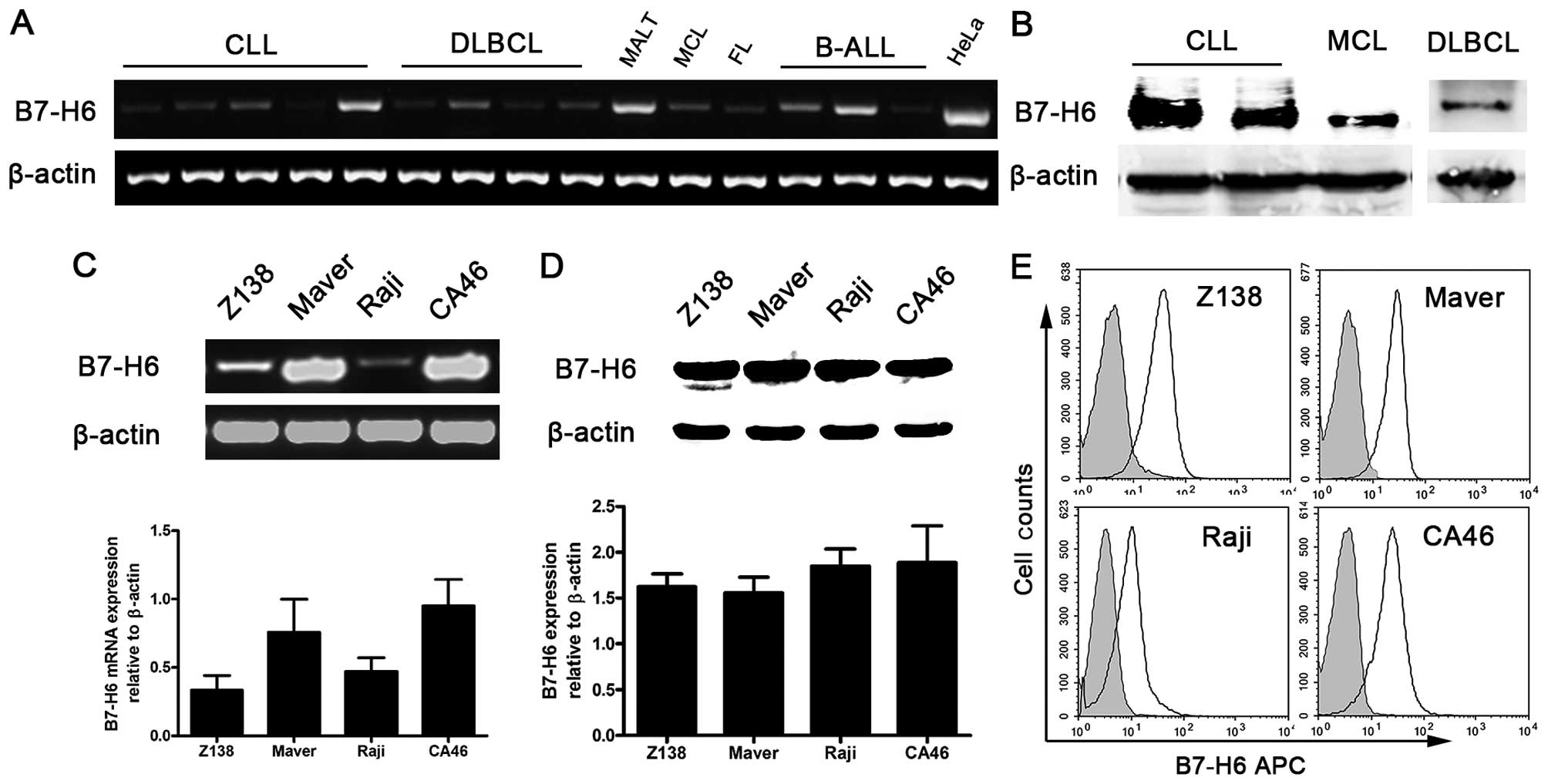
B7-H6 is expressed in B-cell non-Hodgkin
lymphomas
Studies have shown that B7-H6 mRNA is absent in
normal tissues and relatively abundant in tumor cells (3). To investigate the role of B7-H6 in
B-cell NHL, we first detected B7-H6 expression at the mRNA level in
patients with B-cell NHL and healthy donors. Using the HeLa cell
line as a positive control, we found that B7-H6 was widely and
heterogeneously expressed in B-cell NHLs including chronic
lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL),
mucosa-associated lymphoid tissue (MALT) lymphoma, mantle cell
lymphoma (MCL), follicular lymphoma (FL) and B-cell acute
lymphocytic lymphoma (B-ALL) (Fig.
1A), but was absent in healthy donors' peripheral mononuclear
cells (PMNC) at the mRNA level (data not shown). We also measured
the expression of B7-H6 at nuclear/cytosolic level in 8 B-cell NHL
patients including CLL (n=3), DLBCL (n=3), MCL (n=1) and B-ALL
(n=1). B7-H6 expression was observed in tumor cells from 4 patients
(2 patients with CLL, 1 patient with MCL and 1 patient with DLBCL;
Fig. 1B). However, we did not
detect B7-H6 in PMNC of 4 healthy donors (data not shown).
Furthermore, human B-cell lymphoma cell lines (Raji, CA46, Z138 and
Maver) expressed B7-H6 at the level of mRNA (Fig. 1C), nuclear/cytosolic protein
(Fig. 1D) and membrane protein
(Fig. 1E). These results indicated
that B7-H6 is widely expressed in B-cell lymphoma specimen and cell
lines.
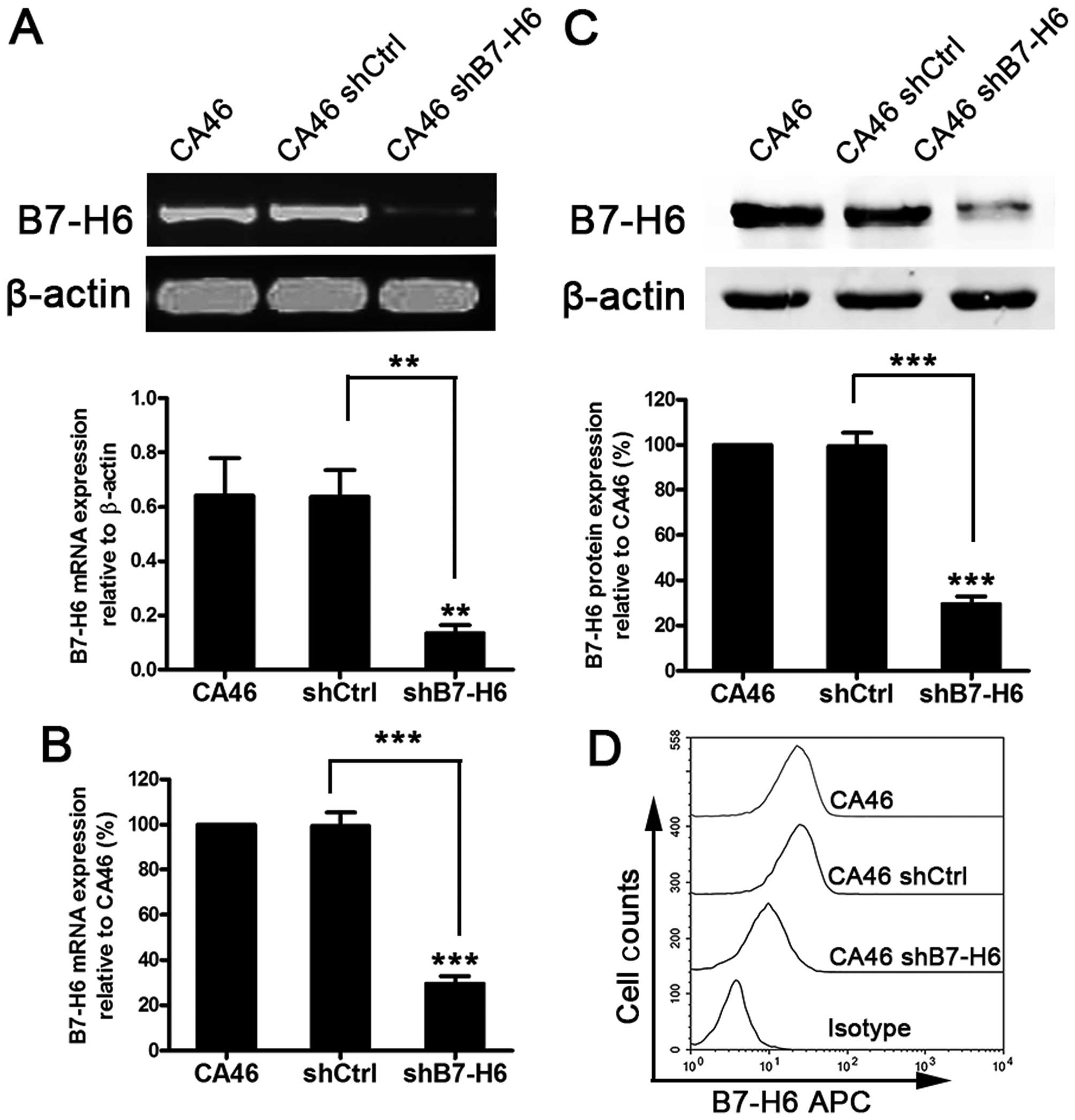
The establishment of a B7-H6 stable
knockdown cell line
To investigate the role of B7-H6 in B-cell lymphoma,
CA46 cells were selected for in vitro studies as they
expressed a high and steady level of endogenous B7-H6 (Fig. 1C–E). B7-H6 knockdown in CA46 cells
was performed using lentivirus transduction to stably express shRNA
targeting B7-H6. There was no significant difference of B7-H6
expression between the CA46 and CA46shCtrl (non-target control
shRNA) groups at the mRNA and protein level (P>0.05). However,
B7-H6 expression in the CA46shB7-H6 group containing the
B7-H6-targetting shRNA, was significantly decreased compared to the
CA46 group (P<0.01). The inhibition rates of mRNA expression in
CA46 cells was 74.4% (Fig. 2A and
C), whereas the nuclear/cytosolic protein was reduced by 77.9%
(Fig. 2B) and the membrane protein
was inhibited by 60.1% (Fig.
2D).
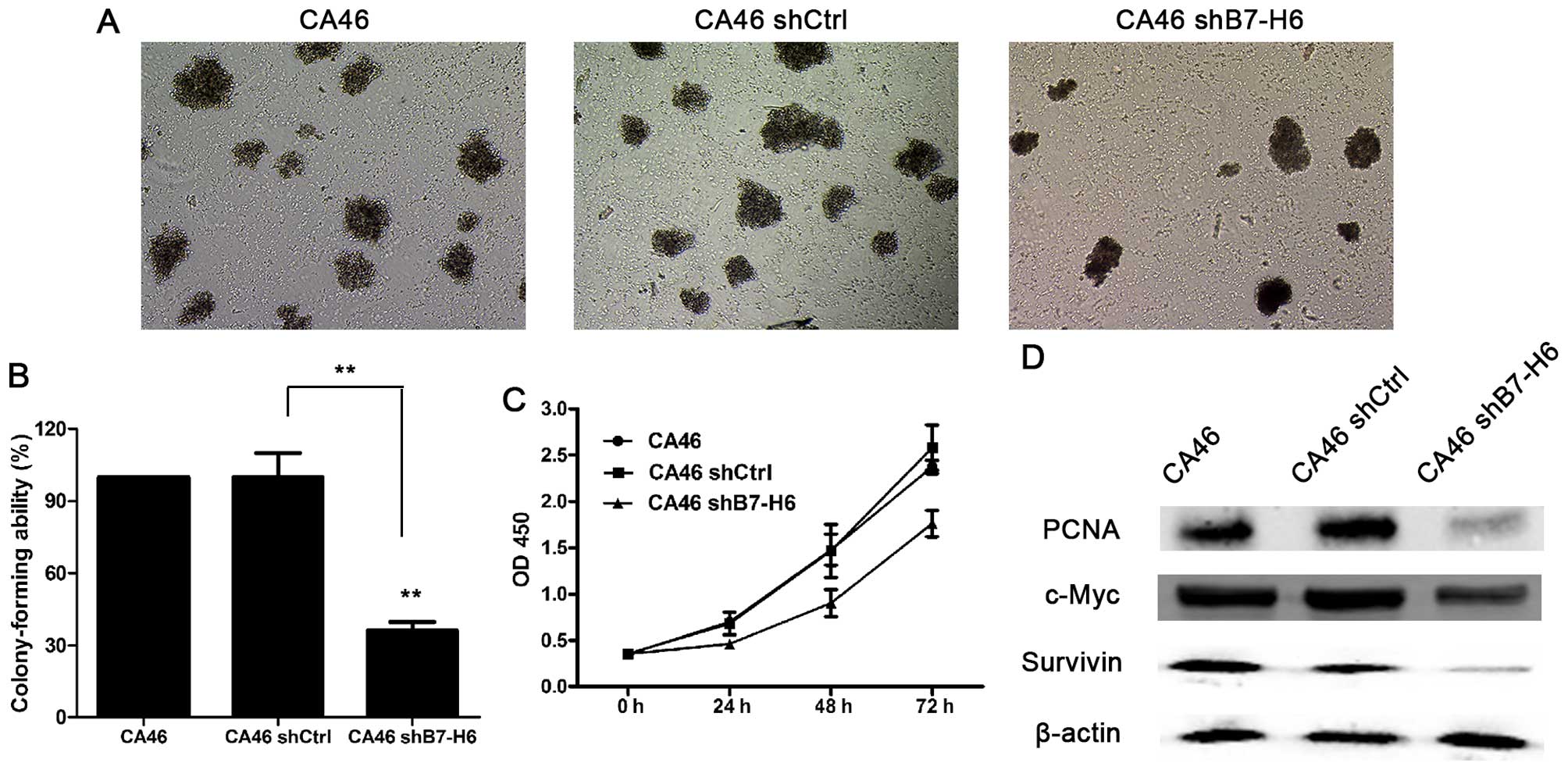
B7-H6 knockdown inhibits tumor cell
proliferation
To evaluate the effects of B7-H6 knockdown on tumor
cell proliferation, colony forming and CCK-8 assays were performed.
The colony forming ability of the CA46shB7-H6 group was decreased
by 73.8% compared to the CA46 group (P<0.01; Fig. 3A and B). The cell proliferation of
the CA46shB7-H6 group was decreased by 33.8, 38.9 and 25.8% after
24, 48 and 72 h respectively, compared to the CA46 group
(P<0.05; Fig. 3C). Furthermore,
we assessed markers reflecting tumor cell proliferation ability.
The expressions of PCNA, c-Myc and Survivin in CA46 cells were
significantly decreased by 74.1, 21.6 and 55.2% compared to the
CA46shB7-H6 group (P<0.05; Fig.
3D).
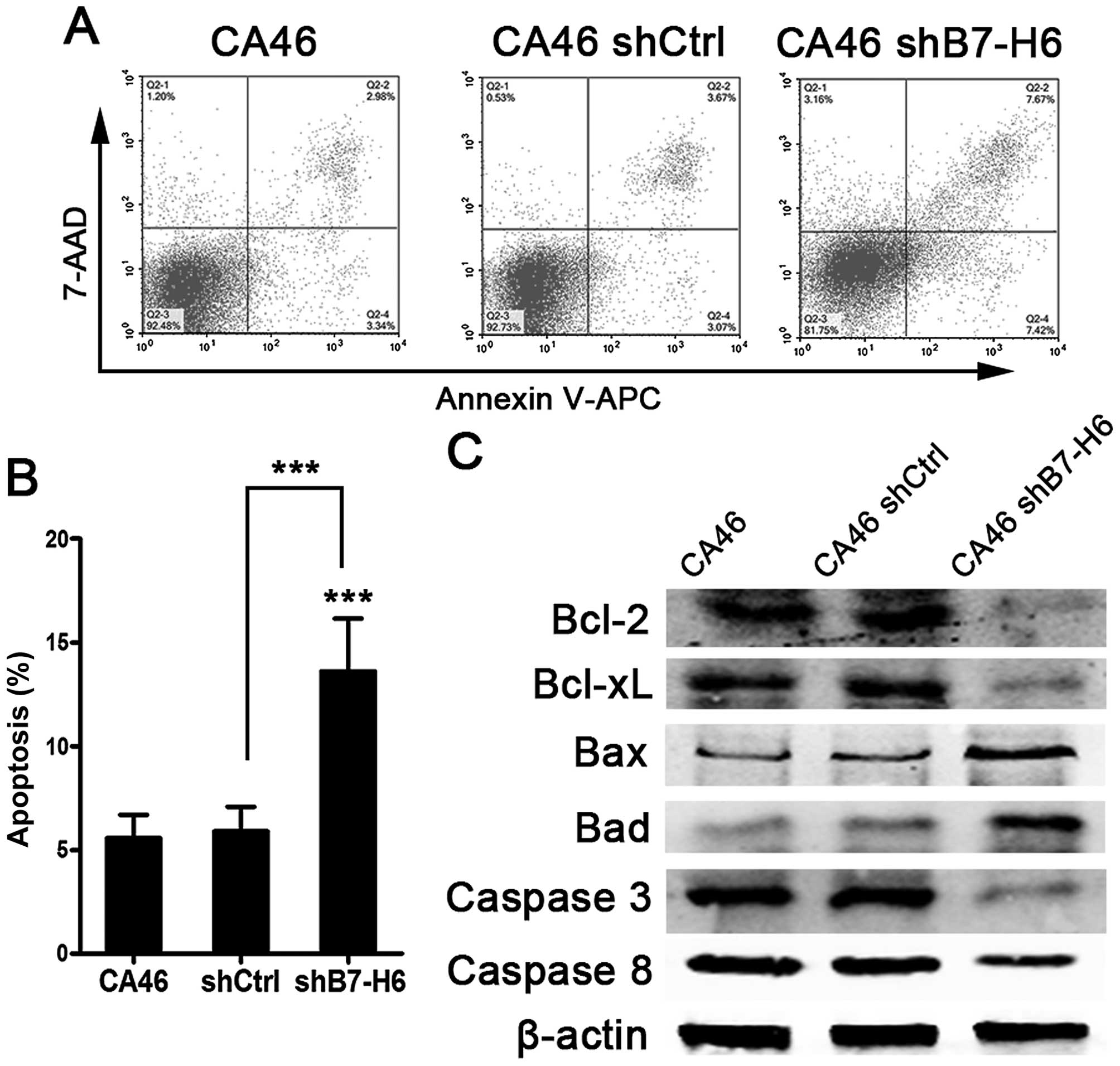
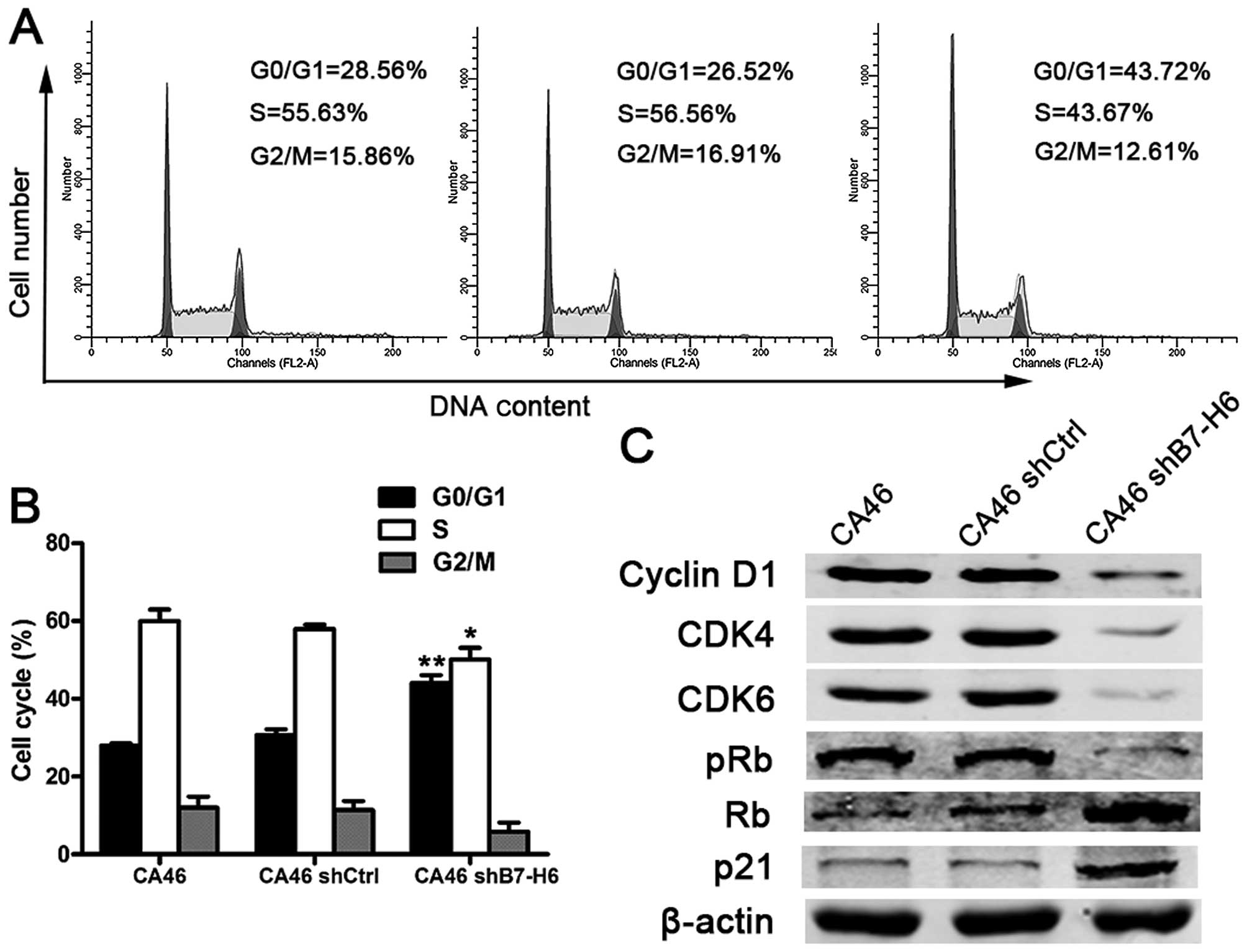
B7-H6 knockdown induces apoptosis and
inhibits cell cycle progression
To explore the effects of B7-H6 knockdown on cell
apoptosis and cell cycle, we analyzed apoptotic rates and cell
cycle distribution by flow cytometry. Our results showed that there
were 15.09% apoptotic cells in CA46shB7-H6 cells compared to only
6.32% in CA46 cells (P<0.001; Fig.
4A and B). We also detected the proteins involved in apoptosis
by western blotting. Expression of anti-apoptotic proteins
including Bcl-2, Bcl-xL, Caspase-3 and -8 was decreased, and
pro-apoptotic proteins including bax and bad were increased in
CA46shB7-H6 cells compared to CA46 and CA46shCtrl cells (P<0.05;
Fig. 4C). The percentage of cells
in G0/G1 phase was 43.72% in CA46 shB7-H6 cells and 28.56% in CA46
cells (P<0.001; Fig. 5A and B).
To further understand the effect of B7-H6 on the cell cycle
progression, we measured expression of cell cycle regulatory
proteins. Cyclin D1, CDK4, CDK6 and phospho-Rb expression was
significantly decreased, while Rb and p21 expression was increased
in CA46shB7-H6 cells in comparison to CA46 or CA46shCtrl cells
(P<0.05; Fig. 5C). The data
indicate that knockdown of B7-H6 in CA46 cells increases apoptosis
and induces G0/G1 cycle arrest.
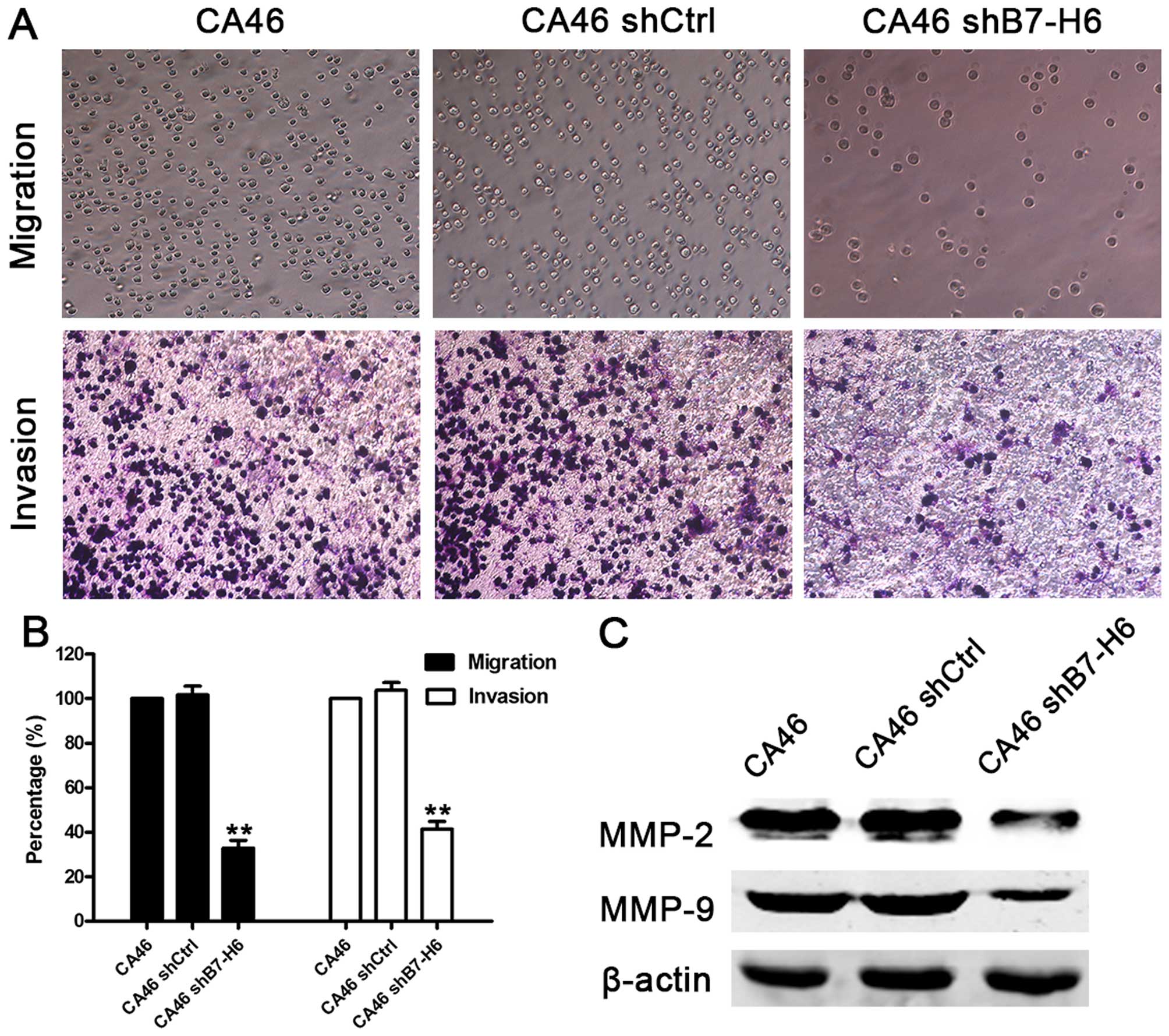
B7-H6 knockdown inhibits tumor cell
migration and invasion
We performed Transwell migration and invasion assays
to determine whether B7-H6 acts as a regulator of cell migration
and invasion. The cell migration assay showed that the migration
ability was reduced by 67.3% in CA46shB7-H6 cells compared to CA46
cells (P<0.01). Furthermore, the invasion assay showed that
knockdown of B7-H6 reduced cell invasion ability by 59.4%
(P<0.01; Fig. 6A and B). We
also measured the expression of crucial proteins involved in cell
migration and invasion, and found that expression of MMP-2 and
MMP-9 was lower in CA46shB7-H6 cells than in CA46 cells (Fig. 6C). These results indicate that
knockdown of B7-H6 could impair cell migration and invasion ability
via downregulating the expression of MMP-2 and MMP-9.
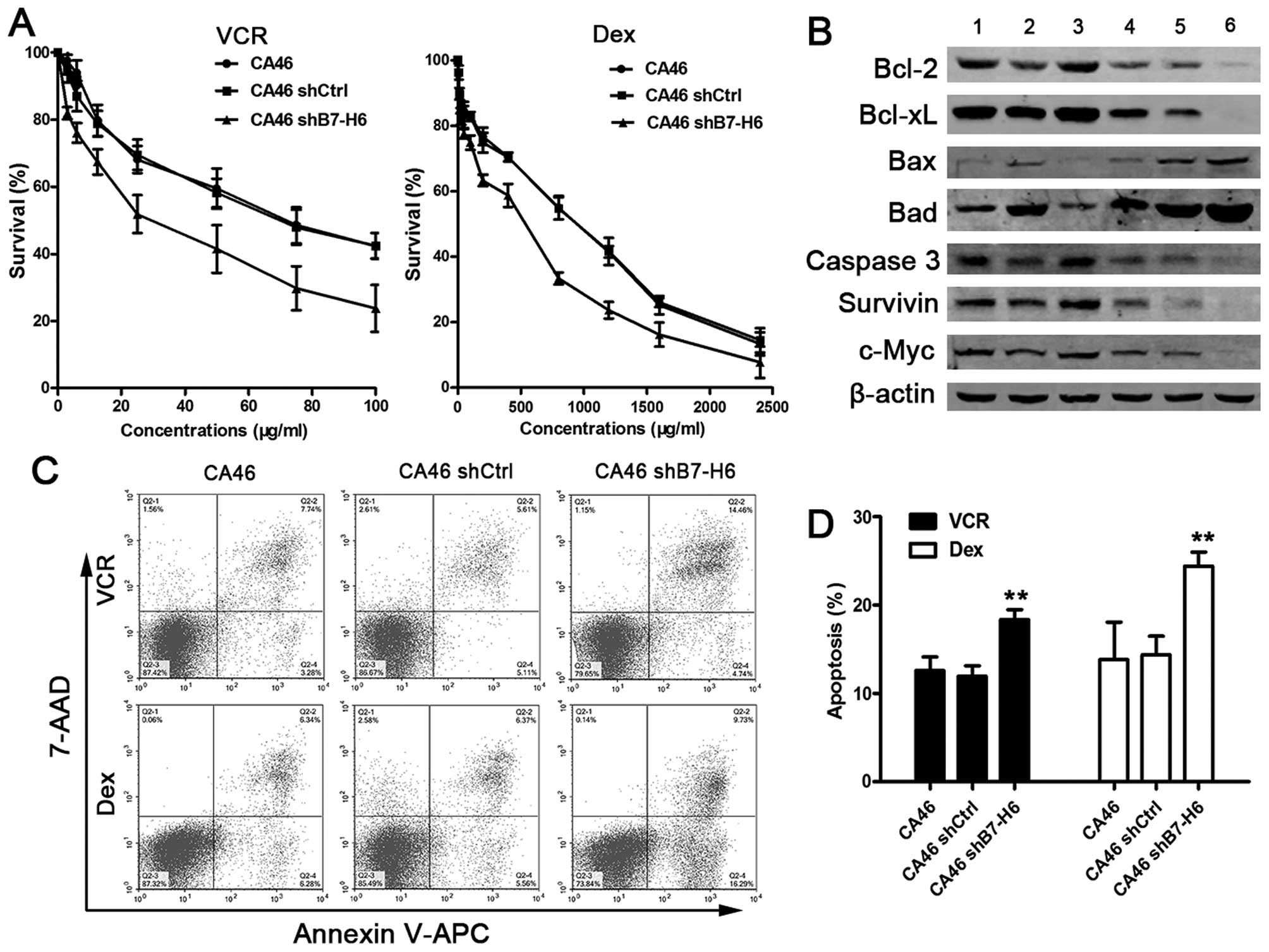
B7-H6 knockdown enhances the sensitivity
of tumor cells to chemotherapy
To explore whether knockdown of B7-H6 would
sensitize lymphoma cells to chemotherapeutic drugs, we chose the
frontline drugs in the clinical regimen for NHL, namely VCR and
Dex. Cell viability was measured after cells were treated with
different concentrations of VCR and Dex for 24 h. The cell survival
rates in the CA46shB7-H6 group were significantly decreased
compared with CA46 or CA46shCtrl group (P<0.01; Fig. 7A). We also detected apoptosis
induced by VCR and Dex. The data showed that the proportion of
apoptotic cells was significantly increased in CA46shB7-H6 cells
compared to CA46 cells (19.2 vs. 11.0%, P<0.01 and 26.0 vs.
12.6%, P<0.01; Fig. 7C and D).
Our previous studies showed that knockdown of B7-H6 inhibited
induced cell apoptosis and influenced expression of
apoptosis-associated proteins. To verify whether the sensitization
of cells to chemotherapeutic drugs was also associated with these
proteins, we analyzed the expressions of representative proteins by
western blotting. We observed that the expression of Bcl-2, Bcl-xL,
Caspase-3, Survivin, and c-Myc was decreased more in drug-treated
CA46shB7-H6 group compared to drug-treated CA46 or CA46shCtrl
groups (lane 6 vs. lane 2 and lane 6 vs. lane 4; Fig. 7B). Our findings support the notion
that sensitization to chemotherapeutic drugs is mediated by
apoptosis-related proteins. These results suggest that knockdown of
B7-H6 improves the chemosensitivity of B-cell lymphoma cells.
Knockdown of B7-H6 leads to inactivation
of STAT3
STAT3 is often constitutively activated in lymphoma
cells (21–23), and is involved in oncogenesis. To
evaluate whether loss of STAT3 activation was involved in the
effects induced by B7-H6 knockdown, we reactivated STAT3 with IL-6,
an activator of STAT3 pathway (24), and detected the expression of its
target genes. We observed that expression of pSTAT3, pERK1/2,
Cyclin D1 and Bcl-xL was decreased in CA46shB7-H6 cells, but
increased by IL-6 (P<0.05; Fig.
8), thus indicating that knockdown of B7-H6 leads to
inactivation of STAT3.
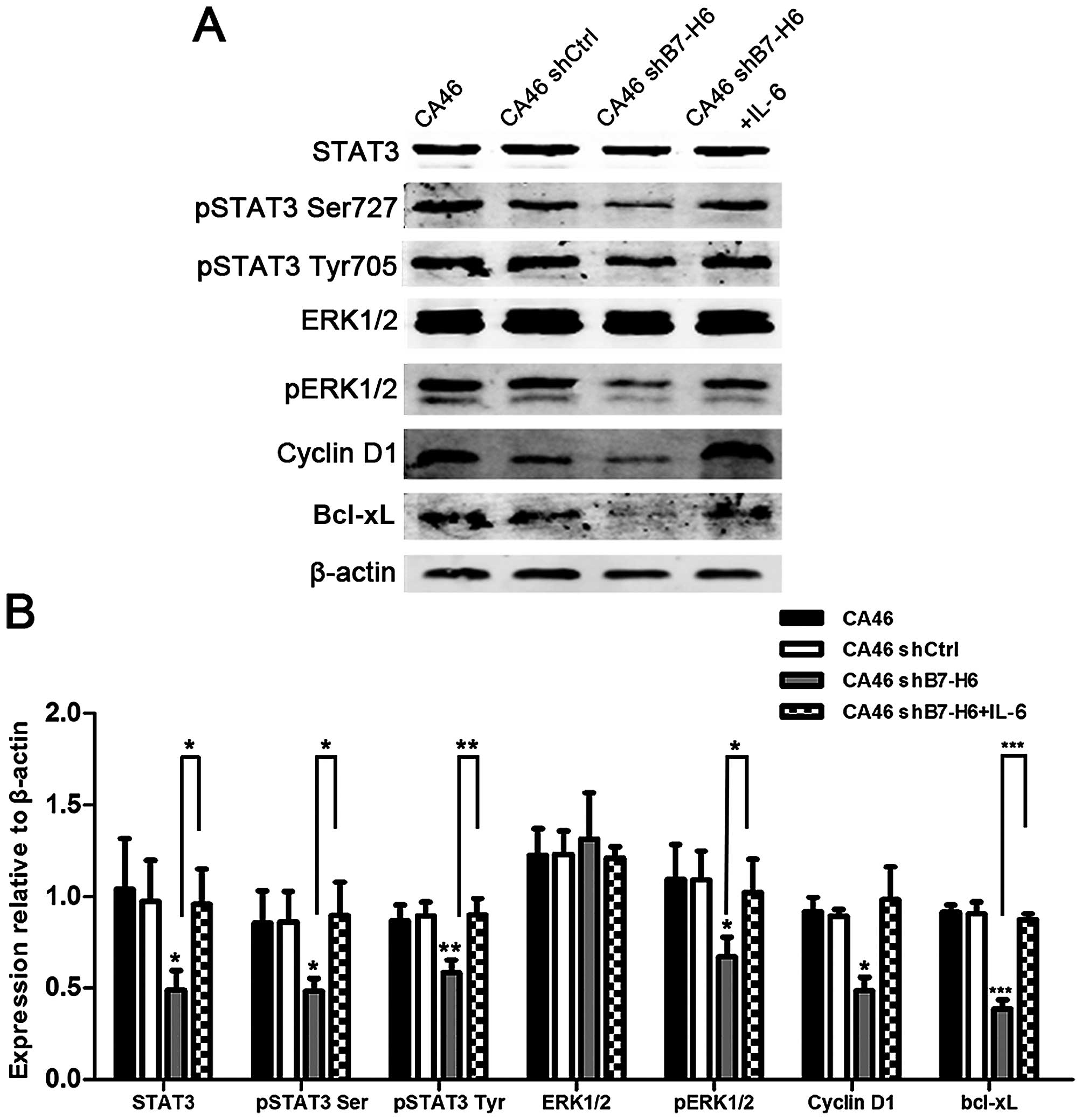 | Figure 8Effects of B7-H6 knockdown on the
expression of STAT3 and STAT3 downstream targets. (A) Cellular
proteins were analyzed by western blotting in CA46, CA46shCtrl,
CA46shB7-H6 and CA46shB7-H6+IL-6 groups with antibodies specific
for STAT3, pSTAT3 Ser727, pSTAT3 Tyr705, ERK1/2, pERK1/2, Cyclin
D1, Bcl-xL and β-actin. (B) The ratio of colorimetric density of
STAT3 and downstream genes. β-actin served as loading control.
*P<0.05,
**P<0.01,***P<0.001 compared to the
control group. |
Discussion
Immunotherapy has shown some glimmers of hope for
the treatment of lymphomas (2).
Monoclonal antibodies targeting B7 family checkpoint regulators
including PD-L1, B7-H3 and B7-H4 have beneficial effects in
reducing tumor burden in melanoma, lung cancer and renal cancer
(10,11). B7-H6 is a new member of B7 family
and is highly expressed in various tumors (3,5,18)
while being absent from normal tissue (3). Such differential expression makes
B7-H6 an attractive therapeutic target. B7-H6 expression is
negatively associated with overall survival in ovarian cancer
(4) and of modest value as a
prognostic marker in non-small cell lung cancer and gastric cancer
(19,20). Our results also showed that B7-H6
is widely expressed in various hematologic malignancies (data not
shown) including B-cell lymphomas, in both primary tumor cells and
B-cell lymphoma cell lines. However, it remains to be determined
whether B7-H6 expression is associated with outcome in a large
cohort of patients with lymphoma.
In this study, we first generated and validated a
B-cell lymphoma cell line with B7-H6 knockdown using lentivirus
transduction of CA46. B7-H6 expression was significantly decreased
at the mRNA and protein level in CA46 cells. The B7-H6 knockdown
was specific and efficient, and the B-cell lymphoma cell model was
used in the subsequent studies.
Uncontrolled proliferation is one of the important
hallmarks of tumor cells (25).
Previous studies found that B7-H6 expression is positively
correlated with ovarian cancer progression (4), but to date no similar study has been
published in B-cell lymphoma. We found that B7-H6 silencing
inhibited the proliferation and colony formation of lymphoma cells
and the expression of Survivin, PCNA and c-Myc was decreased in
CA46shB7-H6 cells. Survivin and PCNA are important molecular
markers for proliferation and expressed in G2/M and S phase,
respectively (26,27). Translocation involving c-Myc is
characteristic of Burkitt lymphomas (28). The inhibition of cell proliferation
might be through downregulation of PCNA, Survivin and c-Myc,
however, the molecular mechanisms need further investigations.
Resistance to cell death is also a characteristic of
tumor cells (25). Previous
studies suggested that high soluble B7-H6 was associated with
chemoresistance in neuroblastoma (6). To determine whether B7-H6 silencing
influences apoptosis in CA46 cells, we measured the apoptotic rates
induced by serum starving and chemotherapeutic drugs. We measured
an increased rate of apoptosis in the B7-H6 knockdown group.
Bcl-2/Bcl-xL and Bax/Bad are important members of Bcl-2 family,
which plays a role in cell apoptosis (29,30).
The main function of Bcl-2/Bcl-xL is to prevent the release of
pro-apoptotic molecules leaking into cytosol to block apoptosis,
and the role of Bax/Bad is quite the opposite (30). The induction of apoptosis is
influenced by the ratio of pro-apoptotic to anti-apoptotic
molecules. Our data showed that the expression of Bcl-2 and Bcl-xL
were decreased, and the expression of Bax and Bad was increased in
CA46shB7-H6 group. Moreover, the expression of Caspase-8 and -3,
which act as initiator and executor of apoptosis, respectively, was
decreased after B7-H6 silencing (31). Thus, our study indicates that
knockdown of B7-H6 induces apoptosis and chemotherapeutic
susceptibility in lymphoma cells through downregulation of
anti-apoptotic proteins, upregulation of pro-apoptotic proteins and
activation of Caspase-8 and -3.
Disturbance of cell cycle regulation has been shown
to be responsible for the initiation and development of solid and
hematologic tumors (32–34). We assessed cell cycle distribution
and found that B7-H6 silencing induced cell cycle arrest in G0/G1
phase. The Cyclin D1-CDK4/6-Rb pathway is a key regulator of the
G1/S transition (35,36). Cyclin D1 interacts with CDK4/6 to
form a complex, which phosphorylates and inactivates Rb (34). The phosphorylated Rb protein is
released from the transcription factor E2F to promote G1/S
transition (34). We found that
knockdown of B7-H6 led to decreased expressions of Cyclin D1, CDK4,
CDK6, pRb proteins and to increased expression of Rb. The p21
protein is a CDK inhibitor that binds to the Cyclin D1-CDK4/6
complex and mediates cell G1 cycle arrest (37). As shown in Fig. 5C, the expression of p21 was reduced
after B7-H6 silencing. These data suggest that the cell cycle might
be regulated by B7-H6 through the Cyclin D1-CDK4/6-Rb axis and
p21.
Invasion and metastasis are important hallmarks of
tumors (25). Several studies
reported that B7-H6 is associated with metastasis in ovarian cancer
(4) and neuroblastoma (6). In our study, we observed that B7-H6
silencing inhibited the migratory and invasive properties of CA46
cells. Since MMP-2 and MMP-9 are correlated with invasion and
metastasis in lymphomas and solid tumors (38,39),
we measured the expressions of these proteins and found them to be
decreased in CA46shB7-H6 cells. This observation indicates that
inhibition of cell migration and invasion after B7-H6 silencing
might be related to downregulation of MMP-2 and MMP-9.
STAT3 plays a critical role in cancer progression
(40) and is a promising target
for the treatment of multiple malignancies including lymphoma
(22,23,40).
Inhibition of STAT3 results in decreased proliferation and
increased apoptosis in cancer cells (40). In this study, B7-H6 silencing
inhibited the activation of STAT3 and ERK1/2. We reactivated the
STAT3 pathway with IL-6 and measured downstream targets of STAT3,
including Cyclin D1, Bcl-xL and ERK1/2 (40). The data indicated that knockdown of
B7-H6 led to dephosphorylation of STAT3 and downregulation of its
target proteins. Thus, our data support a model in which B7-H6
inhibition is responsible for decreased tumor cell oncogenicity and
increased apoptosis via abrogation of the STAT3 pathway.
Above all, this study confirmed that B7-H6 was
widely expressed in lymphomas. Knockdown of B7-H6 not only
suppressed cell proliferation, migration and invasion ability, but
also enhanced G0/G1 cycle arrest, apoptosis and chemo-sensitivity
in lymphoma cells. This study indicated that B7-H6 inhibition
exerts antitumor effects via the inactivation of the STAT3 pathway.
These findings suggest that B7-H6 may serve as a therapeutic target
in lymphomas.
Abbreviations:
|
B7-H6
|
homologue 6
|
|
NHL
|
non-Hodgkin lymphoma
|
|
FBS
|
fetal bovine serum
|
|
VCR
|
vincristine
|
|
Dex
|
dexamethasone
|
|
PCNA
|
proliferating cell nuclear antigen
|
References
|
1
|
Forman D, Bray F and Brewster D: Cancer
Incidence in Five Continents Vol X (eletronic version). IARC; Lyon:
2013, http://ci5.iarc.fr/Default.aspx.
Accessed January 2, 2014
|
|
2
|
Zappasodi R, de Braud F and Di Nicola M:
Lymphoma immunotherapy: Current status. Front Immunol. 6:4482015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brandt CS, Baratin M, Yi EC, Kennedy J,
Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, et
al: The B7 family member B7-H6 is a tumor cell ligand for the
activating natural killer cell receptor NKp30 in humans. J Exp Med.
206:1495–1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Y, Xu Y, Chen L, Xu B, Wu C and Jiang
J: B7-H6 expression correlates with cancer progression and
patient's survival in human ovarian cancer. Int J Clin Exp Pathol.
8:9428–9433. 2015.PubMed/NCBI
|
|
5
|
Wu MR, Zhang T, DeMars LR and Sentman CL:
B7H6-specific chimeric antigen receptors lead to tumor elimination
and host antitumor immunity. Gene Ther. 22:675–684. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semeraro M, Rusakiewicz S, Minard-Colin V,
Delahaye NF, Enot D, Vély F, Marabelle A, Papoular B, Piperoglou C,
Ponzoni M, et al: Clinical impact of the NKp30/B7-H6 axis in
high-risk neuroblastoma patients. Sci Transl Med. 7:283ra552015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kellner C, Maurer T, Hallack D, Repp R,
van de Winkel JG, Parren PW, Valerius T, Humpe A, Gramatzki M and
Peipp M: Mimicking an induced self phenotype by coating lymphomas
with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J
Immunol. 189:5037–5046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu MR, Zhang T, Gacerez AT, Coupet TA,
DeMars LR and Sentman CL: B7H6-specific bispecific T cell engagers
lead to tumor elimination and host antitumor immunity. J Immunol.
194:5305–5311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mir MA and Agrewala JN: Signaling through
CD80: An approach for treating lymphomas. Expert Opin Ther Targets.
12:969–979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Philips GK and Atkins M: Therapeutic uses
of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 27:39–46.
2015. View Article : Google Scholar
|
|
11
|
Leung J and Suh WK: The CD28-B7 family in
anti-tumor immunity: Emerging concepts in cancer immunotherapy.
Immune Netw. 14:265–276. 2014. View Article : Google Scholar :
|
|
12
|
Seliger B and Quandt D: The expression,
function, and clinical relevance of B7 family members in cancer.
Cancer Immunol Immunother. 61:1327–1341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Wang Y, Wang J, Dong F, Zhu M,
Wan W, Li H, Wu F, Yan X and Ke X: B7-H3 silencing inhibits tumor
progression of mantle cell lymphoma and enhances chemosensitivity.
Int J Oncol. 46:2562–2572. 2015.PubMed/NCBI
|
|
14
|
Li Y, Wang J, Li C and Ke XY: Contribution
of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting
therapy. Leuk Lymphoma. 53:2015–2023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaifu T, Escalière B, Gastinel LN, Vivier
E and Baratin M: B7-H6/NKp30 interaction: A mechanism of alerting
NK cells against tumors. Cell Mol Life Sci. 68:3531–3539. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang T, Wu MR and Sentman CL: An
NKp30-based chimeric antigen receptor promotes T cell effector
functions and antitumor efficacy in vivo. J Immunol. 189:2290–2299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schlecker E, Fiegler N, Arnold A, Altevogt
P, Rose-John S, Moldenhauer G, Sucker A, Paschen A, von Strandmann
EP, Textor S, et al: Metalloprotease-mediated tumor cell shedding
of B7-H6, the ligand of the natural killer cell-activating receptor
NKp30. Cancer Res. 74:3429–3440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fiegler N, Textor S, Arnold A, Rölle A,
Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M and Cerwenka A:
Downregulation of the activating NKp30 ligand B7-H6 by HDAC
inhibitors impairs tumor cell recognition by NK cells. Blood.
122:684–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen XJ, Shen J, Zhang GB and Chen WC:
B7-H6 protein expression has no prognostic significance in human
gastric carcinoma. Pathol Oncol Res. 20:203–207. 2014. View Article : Google Scholar
|
|
20
|
Zhang X, Zhang G, Qin Y, Bai R and Huang
J: B7-H6 expression in non-small cell lung cancers. Int J Clin Exp
Pathol. 7:6936–6942. 2014.PubMed/NCBI
|
|
21
|
Baran-Marszak F, Boukhiar M, Harel S,
Laguillier C, Roger C, Gressin R, Martin A, Fagard R, Varin-Blank
N, Ajchenbaum-Cymbalista F, et al: Constitutive and B-cell
receptor-induced activation of STAT3 are important signaling
pathways targeted by bortezomib in leukemic mantle cell lymphoma.
Haematologica. 95:1865–1872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Munoz J, Dhillon N, Janku F, Watowich SS
and Hong DS: STAT3 inhibitors: Finding a home in lymphoma and
leukemia. Oncologist. 19:536–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soldini D, Montagna C, Schüffler P, Martin
V, Georgis A, Thiesler T, Curioni-Fontecedro A, Went P, Bosshard G,
Dehler S, et al: A new diagnostic algorithm for Burkitt and diffuse
large B-cell lymphomas based on the expression of CSE1L and STAT3
and on MYC rearrangement predicts outcome. Ann Oncol. 24:193–201.
2013. View Article : Google Scholar
|
|
24
|
Lu S, Gao Y, Huang X and Wang X: GYY4137,
a hydrogen sulfide (H2S) donor, shows potent
anti-hepatocellular carcinoma activity through blocking the STAT3
pathway. Int J Oncol. 44:1259–1267. 2014.PubMed/NCBI
|
|
25
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He C, Liu Z, Ji J and Zhu H: Prognostic
value of survivin in patients with non-Hodgkin's lymphoma: A
meta-analysis. Int J Clin Exp Med. 8:5847–5854. 2015.PubMed/NCBI
|
|
27
|
Wang SC: PCNA: A silent housekeeper or a
potential therapeutic target? Trends Pharmacol Sci. 35:178–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Said J, Lones M and Yea S: Burkitt
lymphoma and MYC: What else is new? Adv Anat Pathol. 21:160–165.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brinkmann K and Kashkar H: Targeting the
mitochondrial apoptotic pathway: A preferred approach in
hematologic malignancies? Cell Death Dis. 5:e10982014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol.
5:a87222013. View Article : Google Scholar
|
|
31
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar
|
|
32
|
Yang WJ, Yu Z and Qiu LG: Research
advances of signal pathway in the pathogenesis of mantle cell
lymphoma. Zhonghua Xue Ye Xue Za Zhi. 34:1073–1075. 2013.(In
Chinese). PubMed/NCBI
|
|
33
|
Bonn BR, Krieger D and Burkhardt B: Cell
cycle regulatory molecular profiles of pediatric T-cell
lymphoblastic leukemia and lymphoma. Leuk Lymphoma. 53:557–568.
2012. View Article : Google Scholar
|
|
34
|
Tamura K: Development of cell-cycle
checkpoint therapy for solid tumors. Jpn J Clin Oncol.
45:1097–1102. 2015.PubMed/NCBI
|
|
35
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Migliaccio I, Di Leo A and Malorni L:
Cyclin-dependent kinase 4/6 inhibitors in breast cancer therapy.
Curr Opin Oncol. 26:568–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dutto I, Tillhon M, Cazzalini O, Stivala
LA and Prosperi E: Biology of the cell cycle inhibitor p21(CDKN1A):
Molecular mechanisms and relevance in chemical toxicology. Arch
Toxicol. 89:155–178. 2015. View Article : Google Scholar
|
|
38
|
Hazar B, Polat G, Seyrek E, Bağdatoğlğlu
O, Kanik A and Tiftik N: Prognostic value of matrix
metalloproteinases (MMP-2 and MMP-9) in Hodgkin's and non-Hodgkin's
lymphoma. Int J Clin Pract. 58:139–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suminoe A, Matsuzaki A, Hattori H, Koga Y,
Ishii E and Hara T: Expression of matrix metalloproteinase (MMP)
and tissue inhibitor of MMP (TIMP) genes in blasts of infant acute
lymphoblastic leukemia with organ involvement. Leuk Res.
31:1437–1440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiong A, Yang Z, Shen Y, Zhou J and Shen
Q: Transcription factor STAT3 as a novel molecular target for
cancer prevention. Cancers (Basel). 6:926–957. 2014. View Article : Google Scholar
|