Introduction
Fibulin-5, also known as DANCE, EVEC or UP50, is a
66-kDa secreted glycoprotein belonging to the fibulin family, whose
members play essential roles in growth and tissue development
(1,2). Fibulins can act as molecular bridges
within the ECM, and as mediators of cellular processes including
cell adhesion and motility, cell-cell and cell-ECM communications
and elastogenesis. In particular, Fibulin-5 is an elastogenic short
fibulin that interacts with different ECM proteins such as
tropoelastin, fibrillin-1, EMILIN-1, lysyl oxidase-like I or
apolipoprotein A (3,4). It is also noteworthy that Fibulin-5
is the only member of the family that contains an RGD motif within
its structure, which allows its interaction with a subset of
integrins (5). Functional
importance of Fibulin-5 is also highlighted when performing the
phenotypic characterization of the mice deficient in Fbln5,
the gene coding for murine fibulin-5 (6). Although these mice live through
adulthood, they exhibit anatomical abnormalities related to elastic
fiber assembly with resulting emphysematous lungs, vascular
disorders and cutis laxa. Notably, mutations indentified in human
FBLN5 gene have been also associated to age-related macular
degeneration and cutix laxa (7,8).
In addition to their role in tissue architecture and
function, fibulins have also been involved in tumorigenesis. Thus,
different fibulins have been found overexpressed or downregulated
in tumor or stromal cells showing both oncogenic and
tumor-protective properties (9,10).
In this regard, Fibulin-5 elicits tumor-promoting effects in
nasopharyngeal carcinoma as its overexpression significantly
correlates with advanced tumor metastasis (11); and Fibulin-5 is also involved in
pulmonary metastasis of a rat model of renal cell carcinoma
(12). Moreover, Fibulin-5
mediates EMT in mouse 4T1 breast tumor cells (13), and in human HeLa cervical cancer
cells (14). By contrast, a
growing number of studies highlight the antitumor effects caused by
Fibulin-5. For instance, Fibulin-5 inhibits invasion and
proliferation of the human bladder cancer cell line 5637 (15), and hampers cancer invasion and
tumor metastasis of lung cancer cell lines (16,17).
Furthermore, Fibulin-5 can inhibit angiogenesis induced by HT1080
(18) and MCA102 (19) fibrosarcoma cell lines.
Additionally, FBLN5 gene has been found epigenetically silenced in
prostate (20) and lung cancer
(16), which reinforces the
tumor-suppressive roles of Fibulin-5.
In the present study, we have employed well-known
human breast cancer cell models to overexpress Fibulin-5 and to
investigate changes in cell phenotype using different cell-based
approaches including cell proliferation, migration and invasion
assays, as well as mammosphere formation. Overall, our data suggest
that Fibulin-5 induces antitumor effects by suppression of
β-catenin phosphorylation. Immunohistochemical analysis of tumor
samples of breast cancer patients indicated that a high Fibulin-5
expression level is concomitant with a low expression of the
proliferative marker Ki-67 (21),
which suggest that Fibulin-5 may influence breast cancer cell
proliferation.
Materials and methods
Cell culture and transfection
MCF-7, T47D and MDA-MB-231 breast cancer cells and
A549 lung cancer cell lines were routinely maintained in Dulbecco's
Modified Eagle's medium (DMEM) containing 10% heat-inactivated
fetal bovine serum and 50 μg/ml streptomycin and 100 U/ml
penicillin (Life Technologies, Carlsbad, CA, USA). These cell lines
were kindly provided by Dr Carlos López-Otín (Universidad de
Oviedo). pcDNA3 plasmid containing full-length cDNA for
FBLN5 (22) was kindly
provided by Dr William P. Schiemann (Case Western Reserve
University, Cleveland, OH, USA). This expression vector was
transfected into cells using TransIT-X2 Dynamic Delivery System
(Mirus Bio LLC, Madison, WI, USA) as recommended by the
manufacturer. Cells stably expressing exogenous FBLN5 were selected
in the presence of 500 μg/ml G418 (Sigma-Aldrich). In all
experiments cells transfected with an empty vector were employed as
a control.
Western blot analysis and cell
staining
Cell extracts were resolved by 10% polyacrylamide
gel electrophoresis, transferred to a PVDF membrane and then probed
with a rabbit anti-Fibulin-5 antibody (Origene Technologies Inc.,
Rockville, MD, USA). Rabbit anti-β-catenin antibodies were from
Cell Signaling Technology (Danvers, MA, USA), rabbit anti-Ki-67
antibody was from Santa Cruz Biotechnology (Dallas, TX, USA) and
mouse monoclonal anti-β-actin antibody (AC-40) was from
Sigma-Aldrich Química S.A. (Madrid, Spain). Immunoreactive proteins
were visualized using HPR-peroxidase labeled anti-rabbit antibody
(Pierce, Dorchester, MA, USA). For immunocytochemical analysis,
breast cancer cells expressing Fibulin-5 were fixed with 3.7
paraformaldehyde in phosphate-buffered saline buffer and cells were
then blocked with 10% fetal bovine serum. To detect Ki-67, blocked
slides were incubated overnight with the H-300 antibody from Santa
Cruz Biotechnologies, followed by 1-h incubation with a secondary
Alexa 546-conjugated antibody (Life Technologies, Carlsbad, CA,
USA). In all samples, DAPI was added at 100 ng/ml to visualize DNA
in the cell nucleus. Images were obtained using a fluorescence
microscope (Axiovert).
Cell proliferation assay
Cell proliferation was assayed using the CellTiter
96 Non-radiactive Cell Proliferation assay kit from Promega Biotech
Ibérica SL (Madrid, Spain). MCF-7, T47D and MBA-MB-231 cells
(5×103/well) were seeded in 96-well plates and six
replicates per condition. Cell proliferation rates were measured on
four days using an automated microtiter plate reader Power WaveWS
(Bio-Tek Instruments, Inc., Winooski, VT, USA). Additionally, cell
proliferation was also estimated as an average of Ki-67-positive
nuclei in relation to the total number of nuclei per microscopic
field (n=4) (23).
Invasion assay
In vitro invasion potential was examined by
using 24-well Matrigel-coated invasion chambers with an 8-μm pore
size (BD Biosciences, San Jose, CA, USA). To this end,
5×104 MCF-7 or T47D cells were allowed to migrate for 96
h using 10% fetal bovine serum as a chemoattractant. In the case of
MDA-MB-231 cells, migration time was 24 h. At least three
independent experiments were made for each condition. Non-invading
cells on the upper surface were removed from the chambers using a
cotton swab and cells that reached the lower surface were fixed
with 100% methanol and stained with 0.5% crystal violet in 2%
ethanol. Cells were counted in four randomly selected microscopic
fields.
Mammosphere assay
To evaluate mammosphere formation capacity of breast
tumor cells overexpressing Fibulin-5, 4×104 MCF-7 and
T47D cells were plated in 6-well ultralow attachments plates
(Corning Costar, Corning, NY, USA) and grown in MammoCult Basal
Medium (Stemcell Technologies, Vancouver, Canada) supplemented with
10% MammoCult proliferation supplement, 4 μg/ml heparin and 0.5
μg/ml hydrocortisone. After 7 days, cells were collected and
enzymatically dissociated as previously described (24). Then, individual dissociated cells
were cultured in 96-well ultralow attachment (Corning Costar) at a
density of 20 cells/well. Mammosphere formation was daily monitored
to ensure that they derived from single cells. Number of
mammospheres were determined after 7 days and mammospheres were
again counted and graphically presented.
Migration assay
To evaluate the migratory capacity on ECM
components, we employed the Radius™ 24-Well Cell Migration assay
kit (Cell Biolabs Inc., San Diego, CA, USA), following the
manufacturer' instructions. Briefly, 1.25×104 MCF-7 and
MDA-MB-231 cells overexpressing Fibulin-5 or transfected with an
empty vector were seeded on wells coated with fibronectin and
type-I collagen. Migration was monitored for 12 h using a
time-lapse Zeiss Axio Observer Microscopy. Experiments were
performed in triplicates and covered area was quantified at
different times using ImageJ software.
Human breast cancer tissue array
A breast cancer tissue array containing 78 samples
of different types of human breast tumors and 3 normal tissues was
obtained from the Institute of Oncology of Asturias Tumor Bank.
Study subjects were newly diagnosed with breast cancer and
histopathologically confirmed at the Hospital Universitario Central
de Asturias (HUCA). Tumor-free samples were selected from healthy
tissue areas and immediately frozen. Written informed consent was
obtained from all patients prior to sample collection. The study
was approved by the appropriate institutional review board
according to national and EU guidelines. To perform the
histological analysis, deparaffinized and rehydrated sections were
processed for detection of Fibulin-5 and Ki-67 using the EnVision
antibody complex kit (Dako Denmark A/S, Copenhagen, Denmark)
following the manufacturer's recommendations. The rabbit
anti-Fibulin-5 antibody (Abcam) was diluted 1:200 and the anti-Ki67
was a mouse monoclonal antibody (Dako, clone MIB-1) diluted 1:100.
For control purposes, representative sections were processed as
above but rabbit or goat non-immune serum, or blocking buffer, were
used instead of the primary antibodies. For simultaneous detection
of Fibulin-5 and Ki-67 double immunofluorescence coupled to laser
confocal microscope was used. Non-specific binding was reduced by
incubation for 30 min with a solution of 1% bovine serum albumin in
TBS (Tris-buffered saline buffer). Sections were incubated
overnight, at 4°C in a humid chamber with a 1:1 mixture of
anti-fibulin-5 and anti-Ki-67 antibodies, diluted 1:200 and 1:100,
respectively. After rinsing with TBS, sections were incubated for 1
h with Alexa Fluor 488-conjugated goat anti-rabbit IgG (AbD
Serotec, Kidlington, UK), diluted 1:1,000 in TBS containing 5%
mouse serum (AbD Serotec), then rinsed again, and incubated for
another hour with CyTM3-conjugated donkey anti-mouse antibody
(Jackson ImmunoResearch) diluted 1:50 in TBS. Double fluorescence
was detected using a Leica DMR-XA automatic fluorescence microscope
(Photonic Microscopy Service, University of Oviedo) coupled with a
Leica Confocal software, version 2.5 (Leica Microsystems, Wetzlar,
Germany) and the images captured were processed using ImageJ
version 1.43g software, McMaster Biophotonics Facility (www.macbiophotonics.ca).
Survival analysis
To evaluate the effect of Fibulin-5 on breast cancer
prognosis, a Kaplan-Meier log-rank test survival plot was performed
using the data available at www.kmplot.com (affy
ID 203088_at) (25).
Statistical analysis
To perform statistical analysis, we employed
GraphPad Prism 5.0 software and data are presented as means ± SE.
Significant differences were determined with the Student-Welch
t-test and P-values <0.05 were considered statistically
significant (in the figures as: *P<0.05,
**P<0.01, ***P<0.005).
Results
Recombinant Fibulin-5 reduces breast
cancer cell invasion
In the present study we wanted to examine if
Fibulin-5 induces changes in the behavior of three commonly
employed human breast cancer cells, the poorly invasive T47D and
MCF-7 and the highly invasive MDA-MB-231 cell lines. Following
transfection, production of recombinant Fibulin-5 was analyzed by
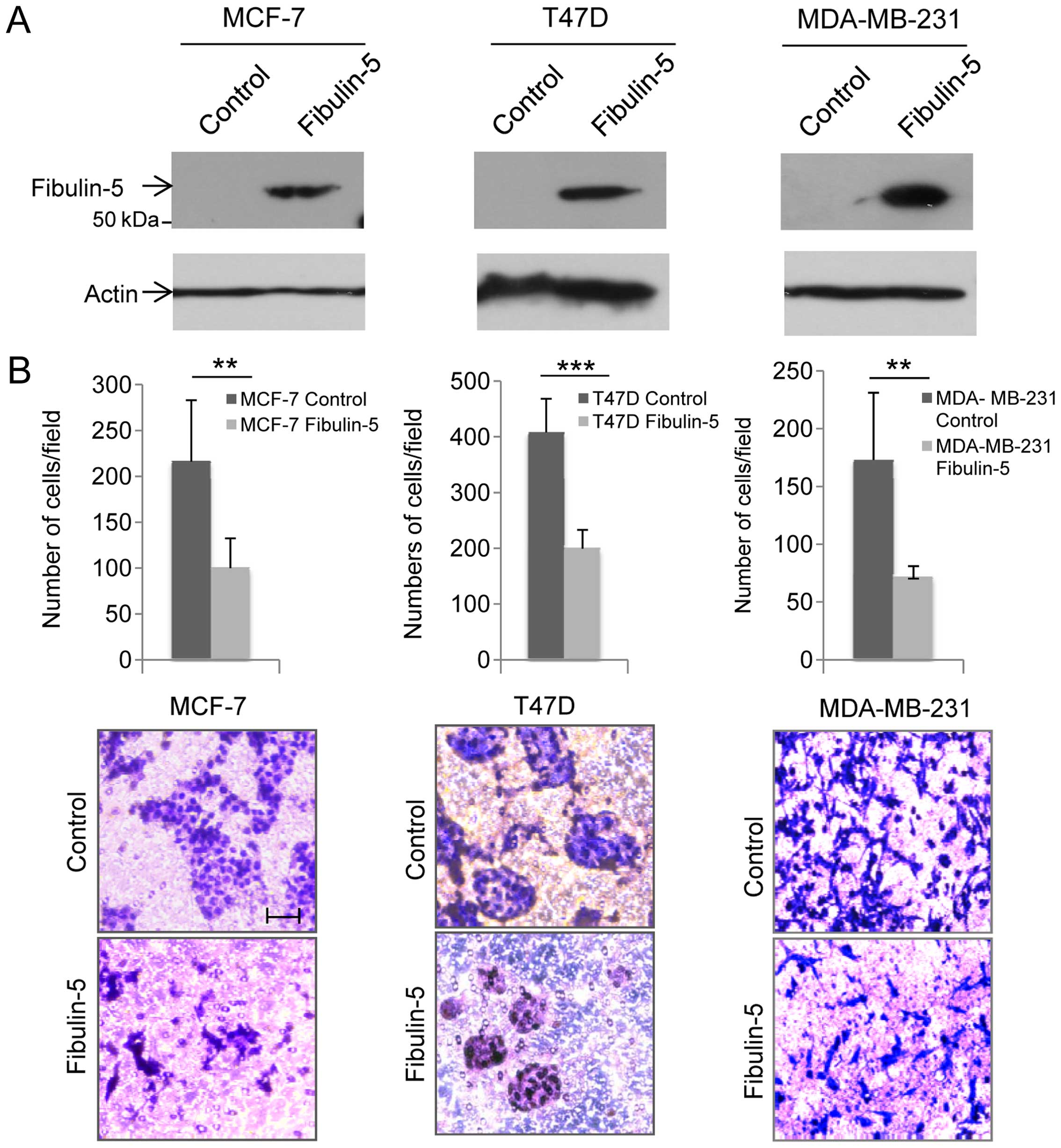
western blot analysis using an anti-Fibulin-5 antibody (Fig. 1A). An immunoreactive band
corresponding to Fibulin-5 was detected in cell extracts containing
Fibulin-5, but not in control cells transfected with an empty
vector. To assess whether Fibulin-5 could affect cell invasion,
cells were allowed to invade using Matrigel-coated invasion
chambers (Fig. 2B). After 96 h,
invasion of T47D and MCF-7 was analyzed with the finding that the
presence of Fibulin-5 reduced the invasive potential of both cell
lines in comparison with control cells (45.5% reduction in the case
of T47D cells; and 49.8% reduction in MCF-7 cells). MDA-MB-231
cells were allowed to invade for 24 h and cells producing
recombinant Fibulin-5 showed 40.4% reduction in their invasive
capacity when compared to control cells (Fig. 1B). These results suggest that
Fibulin-5 strongly affects invasive capacity of breast cancer
cells.
Fibulin-5 affects proliferation of breast
cancer cells
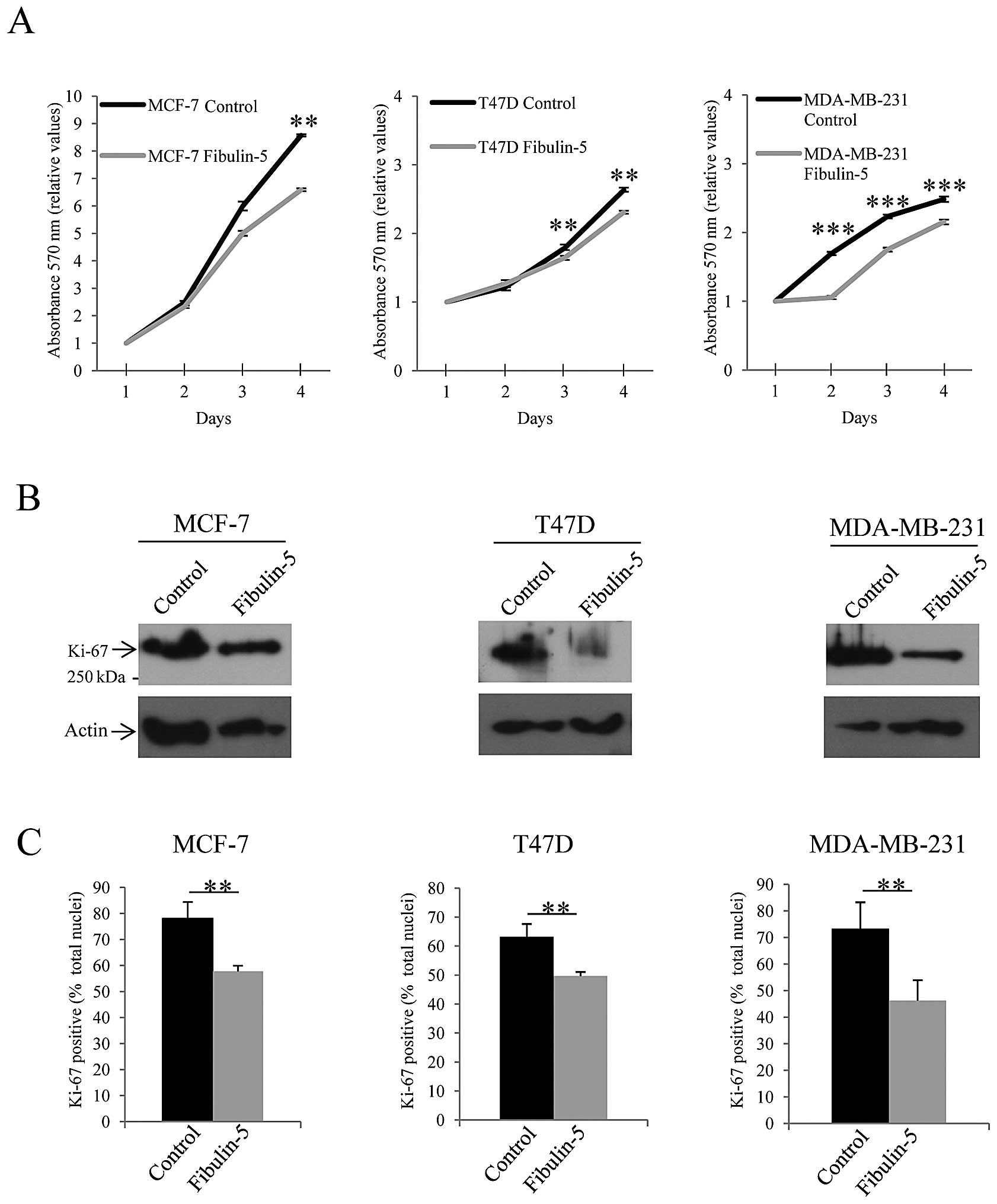
To assess the potential effect of Fibulin-5 on
breast cancer cell proliferation we performed two different
approaches. First, we measured cell proliferation on 4 consecutive
days employing a MTT assay. As shown in Fig. 2A, presence of Fibulin-5 reduced the
number of T47D, MCF-7 and MDA-MB-231 proliferating cells. It is
noteworthy that the highly invasive MDA-MB-231 cells seemed to be
more affected than the other two since difference in cell
proliferation between MDA-MB-231 cells expressing recombinant
Fibulin-5 and control cells was already evident on the second day
of the assay. Second approach was to examine the presence of the
proliferation marker Ki-67 in Fibulin-5-transfected breast cancer
cells. Western blot analysis showed that expression level of Ki-67
was considerably reduced in breast cancer cell lines expressing
Fibulin-5 when compared to control cells (Fig. 2B). Moreover, nuclear staining of
the cells using an anti-Ki-67 antibody revealed that the number of
nuclei showing Ki-67-positive staining was lower in cells
expressing recombinant Fibulin-5 than in control cells. Thus,
Ki-67-positive nuclei represented an average of 78, 64 and 72% in
control MCF-7, T47D and MDA-MB-231 cells respectively, whereas 58,
50 and 44% of total nuclei showed positive staining in cells
expressing exogenous Fibulin-5 (Fig.
2B). These data indicate that Fibulin-5 has an effect on breast
cancer cell proliferation.
Fibulin-5 decreases the number of
mammosphere formation units
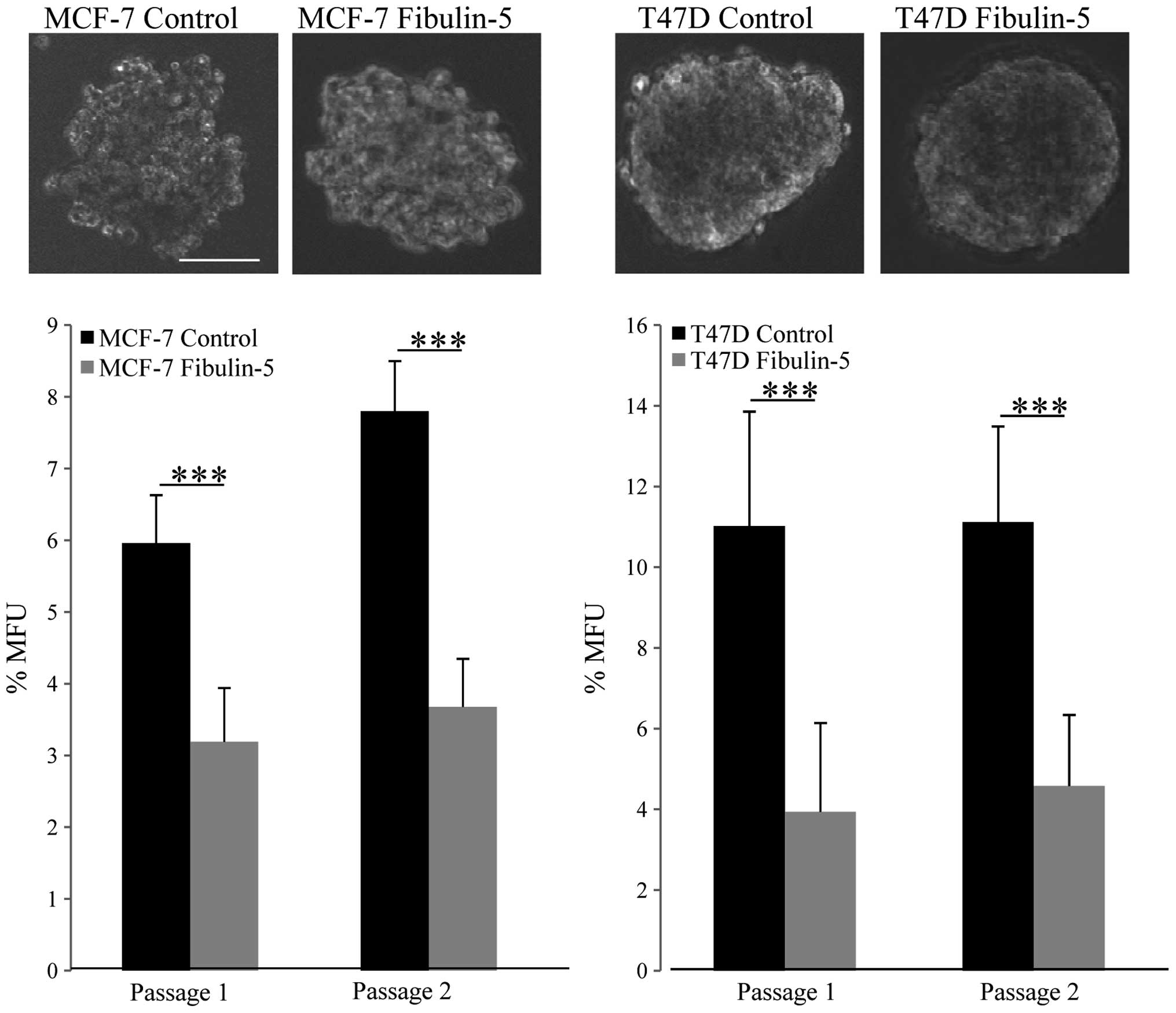
MCF-7 and T47D are breast tumor cell lines that show
a strong ability to form mammospheres (26). To evaluate whether the presence of
Fibulin-5 could alter this ability, we carried out mammosphere
forming assays that showed that the presence of Fibulin-5 did not
seem to affect the size or morphology of the mammospheres (Fig. 3). However, we found a significant
decrease in the number of mammospheres derived from MCF-7 and T47D
cells expressing recombinant Fibulin-5. Indeed, Fibulin-5 decreased
~50% the mammosphere forming units (MFU) in the case of MCF-7 cells
and ~64% in the case of T47D cells in comparison with control cells
and in two consecutive passages (Fig.
3). Overall, these data indicate that capacity for in
vitro self-renewal of MCF-7 and T47D cells is constrained by
the presence of Fibulin-5.
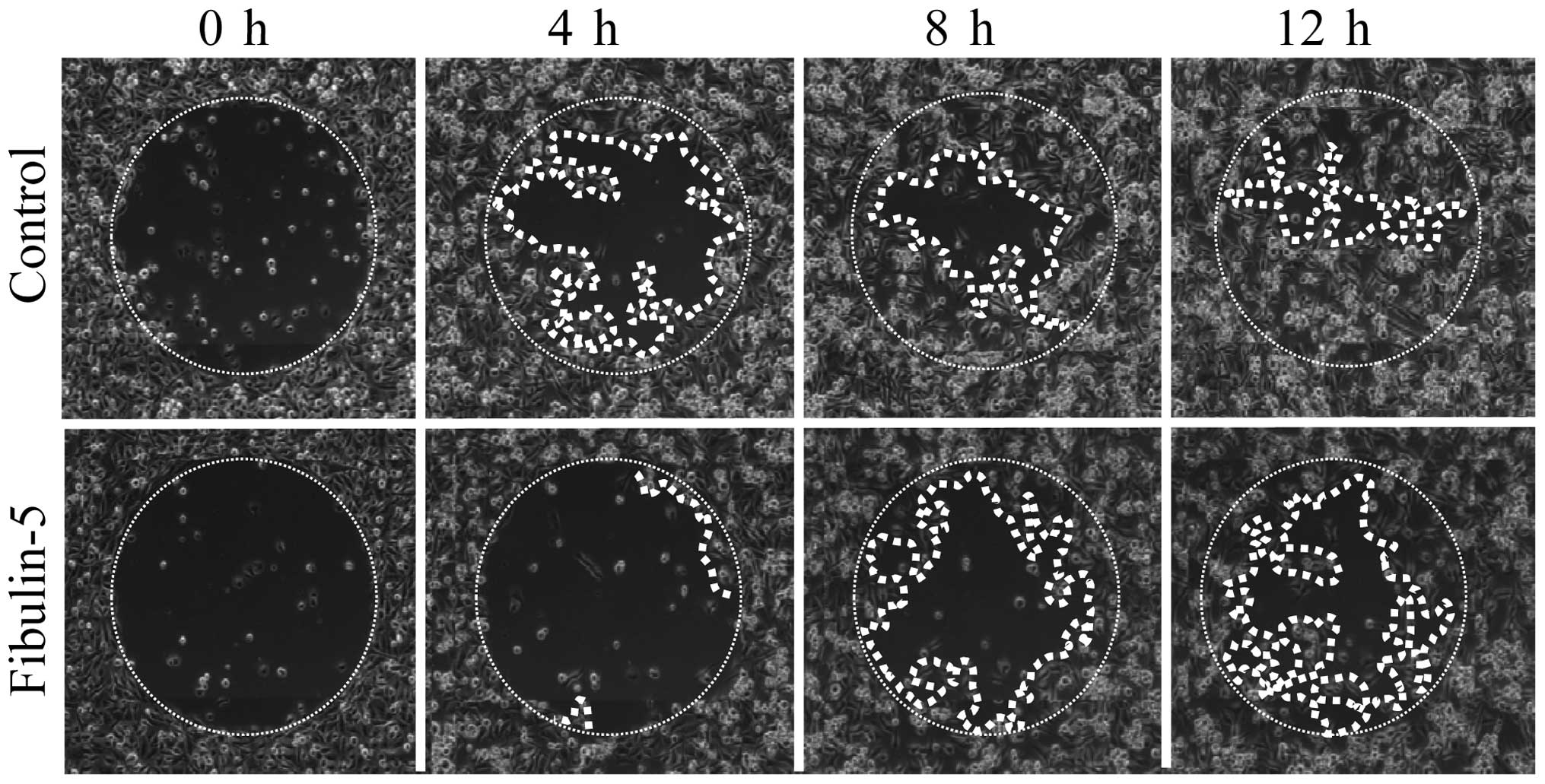
Effect of Fibulin-5 on breast cancer cell
migration
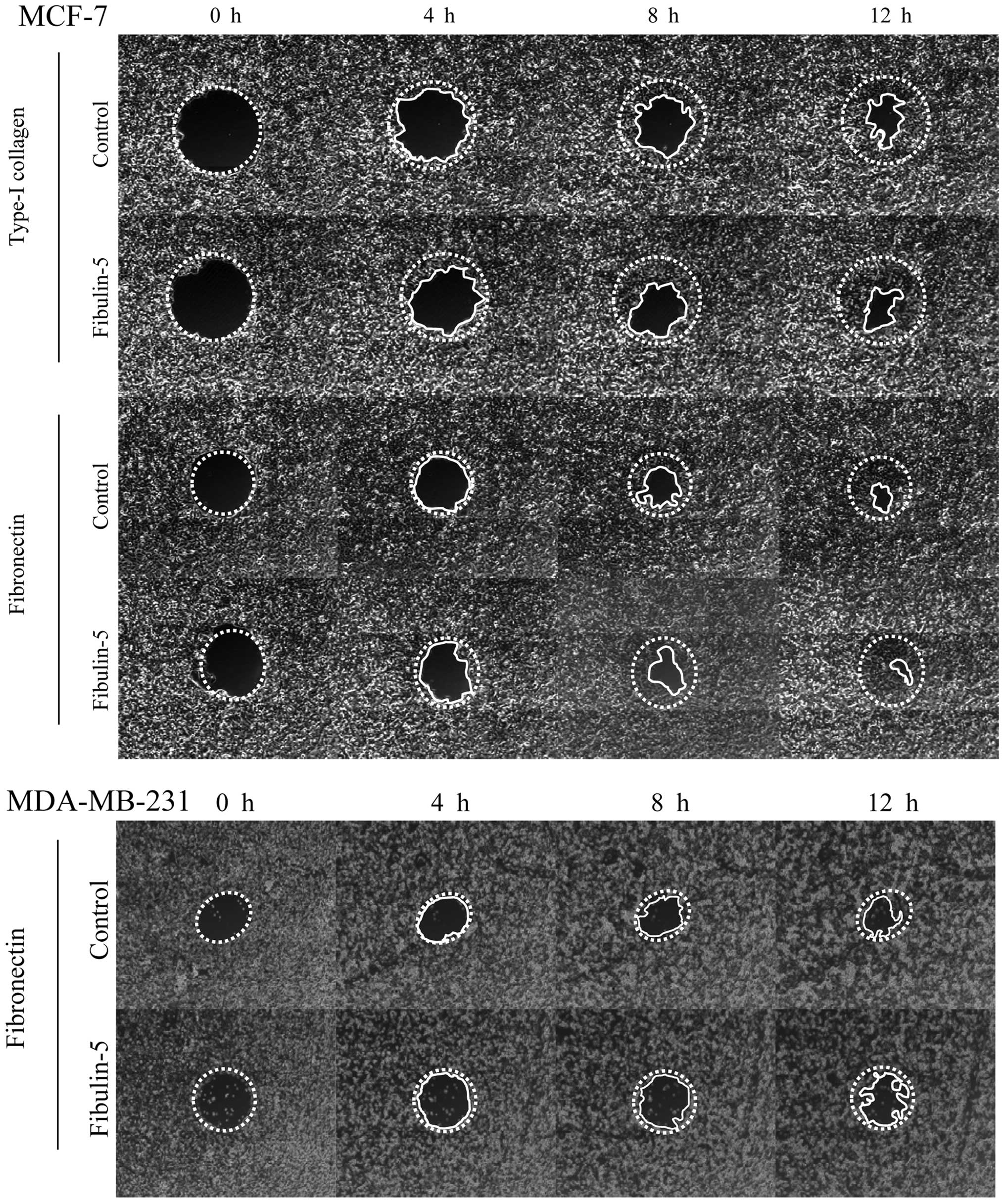
Next, we wanted to explore the possibility that
Fibulin-5 affected breast cancer cell migration on type-I collagen
and fibronectin, two extracellular matrix proteins that influence
cell adhesion and migration properties of both the poorly invasive
MCF-7 cells and the highly invasive MDA-MB-231 cells (27). After 12 h, MCF-7 expressing
Fibulin-5 and control MCF-7 cells did not show differences in cell
migration using wells coated with type-I collagen or fibronectin
(Fig. 4). MDA-MB-231 cells also
showed no differences between cells expressing Fibulin-5 and
control cells in wells coated with fibronectin (Fig. 4). However, presence of Fibulin-5
affected the migratory capacity of MDA-MB-231 cells in wells coated
with type-I collagen (Fig. 5).
Thus after 12 h, 87.7% of area was covered by control MDA-MB-231
cells while only 53.9% was covered by MDA-MB-231 cells expressing
Fibulin-5 (P<0.01). Differences in migration on type-I collagen
were already evident at 4 h (48.2 vs. 5.7%; P<0.01). These data
indicate that Fibulin-5 may act as a modulator of metastatic breast
cancer cell migration through type-I collagen.
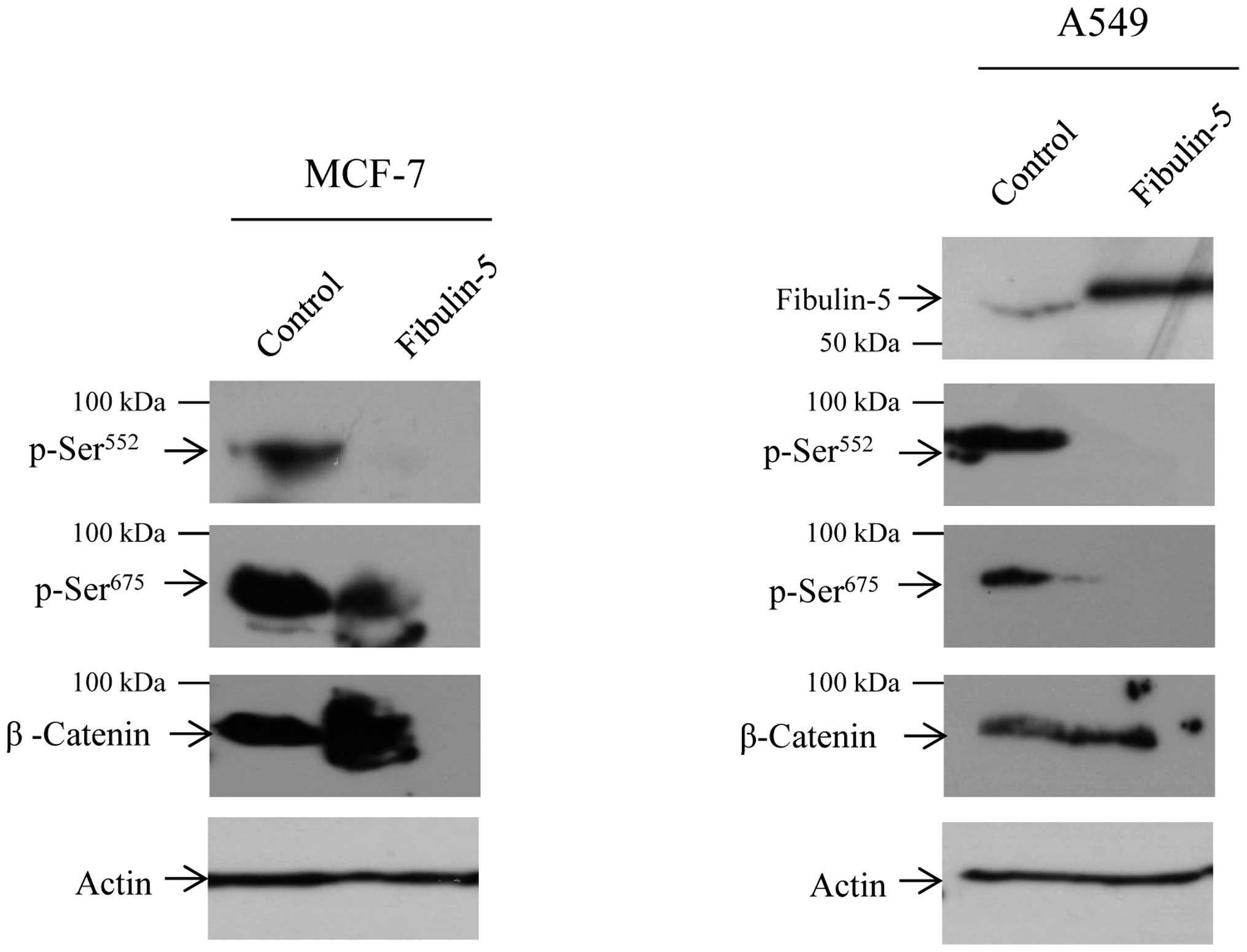
Presence of Fibulin-5 inhibits β-catenin
phosphorylation
Then we asked whether the functional changes due to
Fibulin-5 could be attributed to alterations in β-catenin
phosphorylation. Western blot analysis revealed a decrease in the
phosphorylation levels of the β-catenin residues Ser552
and Ser675 in MCF-7 cells (Fig. 6). Attending to previous results on
the effect of Fibulin-5 in Wnt/β-catenin signaling pathway in lung
cancer (17), we evaluated whether
the human lung carcinoma cell line A549 undergoes the same effect,
with the finding that Fibulin-5 also affects phosphorylation of
Ser552 and Ser675 in this cell line (Fig. 6). Ser552 and
Ser675 residues are involved in the translocation of
β-catenin from cell-cell contacts into the nucleus. In consequence,
decrease in β-catenin phosphorylation could underlie the changes
observed in the phenotype of breast cancer cells employed in the
present study.
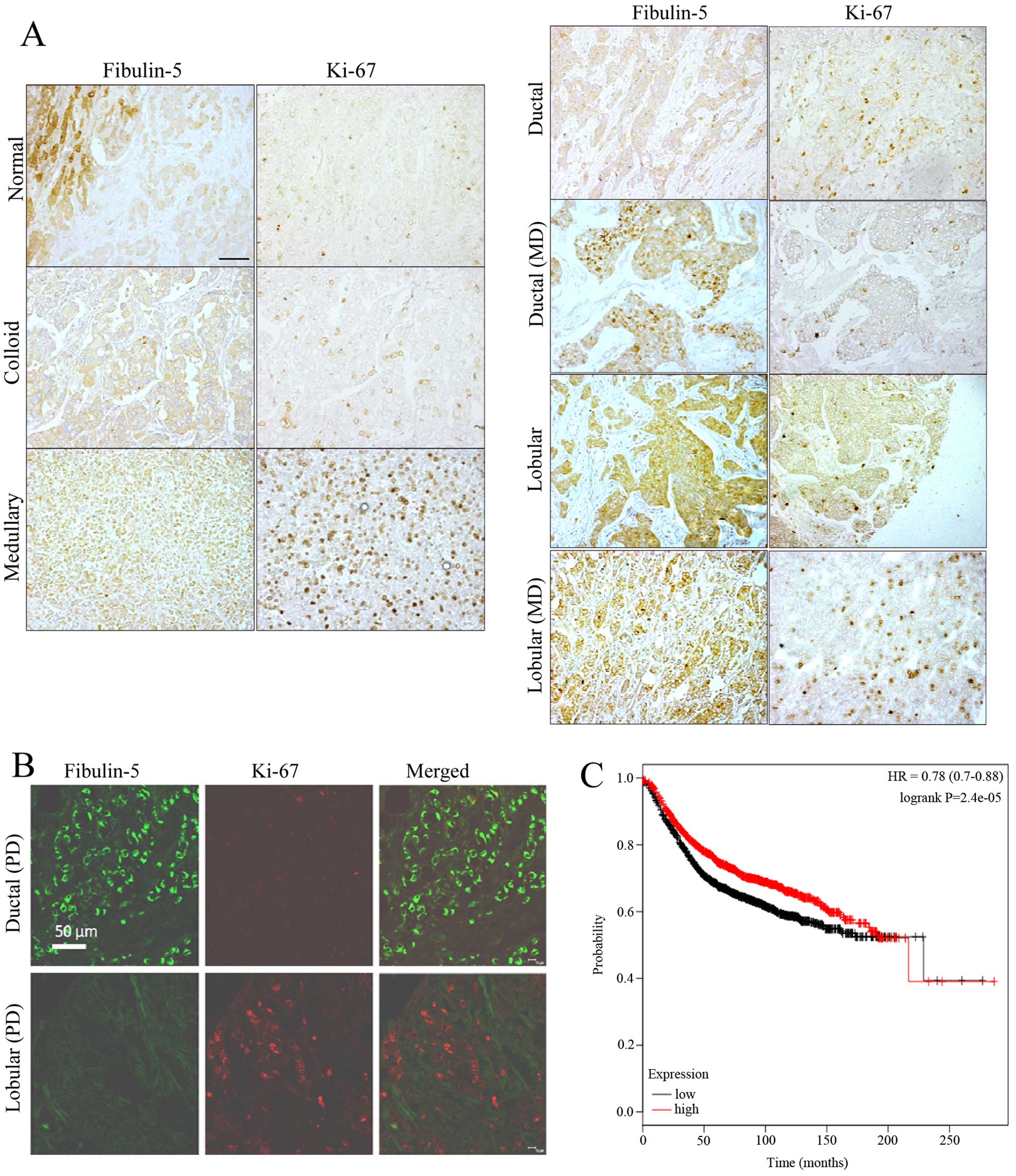
Immunostaining of Fibulin-5 and Ki-67 in
breast tumors
Taking into account our previous results on
Fibulin-5 and Ki-67 in breast cancer cells, we next performed an
immunohistochemical analysis to evaluate the simultaneous detection
of Fibulin-5 and Ki-67 in breast cancer samples. To this end we
employed a tissue array containing samples of the most common types
of breast tumors as well as 3 normal breast tissues (Fig. 7). In normal samples, Fibulin-5 was
present in both lobular and ductal epithelial cells, and the
intensity of immunostaining varied among different segments of the
organ. The density of Ki-67 positive nuclei profiles was very low
or undetectable in wide segments of the tissue. In tumor samples,
immunoreactivity of Fibulin-5 was very low or absent in those
samples showing medium or high expression of Ki-67 independently of
tumor type (Fig. 7A). In ductal
carcinoma, Fibulin-5 showed high immunoreactivity in the most
advance stages of the disease. In parallel, the density of Ki-67
immunoreactive nuclei profiles decreased. By contrast, in lobular
carcinoma, the expression of Fibulin-5 and Ki-67 showed an opposite
evolution. Double immunofluorescence for simultaneous detection of
Fibulin-5 and Ki-67 was used to ascertain these results (Fig. 7B). In a representative section of
poorly differentiated ductal carcinoma, abundant tumor cells
displaying strong Fibulin-5 immunofluorescence were found which
were devoid of Ki-67 immunofluorescence. Conversely, in sections of
poorly differentiated lobular carcinoma Fibulin-5 was absent in
tumor cells while they showed numerous Ki-67 positive nuclei
profiles. This analysis also indicated that Fibulin-5 was not
colocalized with Ki-67 in most samples examined. Moreover,
Kaplan-Meier analysis using the data available at www.kmplot.com revealed that breast cancer patients
with high levels of Fibulin-5 showed better prognosis than patients
expressing low levels (Fig. 7C).
This analysis suggests that FBLN5 gene expression is associated
with better outcome in breast cancer patients.
Discussion
The number of studies supporting the importance of
fibulins in tumorigenesis has considerably grown in recent years
(10). Role of these secreted
glycoproteins in cancer is still controversial as they can induce
both tumor-promoting or tumor-suppressive effects depending on
multiple factors, and Fibulin-5 is not an exception. For instance,
the interaction between Fibulin-5 and β1 integrins regulates the
levels of reactive oxygen species (ROS) in tumors, which has been
shown to be increased in
Fbln5(−/−)
mice in comparison with the wild-type littermates (28). Nogo-B, an isoform of reticulon-4,
is also an interacting partner of Fibulin-5 and the consequence of
this interaction is induction of EMT as well as the promotion of
cell migration and invasion of HeLa cervical cancer cells (14). Fibulin-5 also enhances EMT and
stimulates dedifferentiation of mammary epithelial cells upon
induction by TGF-β (13).
Interestingly, FBLN5 is a TGF-β-inducible gene that can
modulate protein kinase activities thus affecting cell growth and
motility.
As opposed to these tumor-promoting effects,
Fibulin-5 can also support tumor-protective properties (4). Thus, Fibulin-5 has been identified as
angiostatic agent in tumors produced by MCA102 (19) and HT1080 (18) fibrosarcoma cells. Moreover, it has
been found that Fibulin-5 hampers invasion and proliferation of
human bladder, ovarian and lung cancer cells (15,17,29),
thereby inhibiting tumor growth. In the present study and using
different cell-based assays we have found that Fibulin-5 also
induces antitumor effects in three well-known human breast cancer
cell lines. In fact, overexpression of Fibulin-5 decreases invasion
of the poorly invasive T47D and MCF-7 cells, and also of the highly
invasive MDA-MB-231 cells. Moreover, MDA-MB-231 cells
overexpressing Fibulin-5 significantly reduce their in vitro
migration capacity on type-I collagen. Interestingly, deposition of
type-I collagen facilitates metastasis of breast cancer cells
(30). Collectively, our data
allow to speculate that the presence of Fibulin-5 may exert its
antitumor effects through the modulation of a signaling pathway
involved in breast cancer progression. Recently Chen et al
(17) found that Fibulin-5 affects
Wnt/β-catenin signaling pathway in lung cancer cells. To evaluate
the possibility that β-catenin is also affected in breast cancer
cells upon overexpression of Fibulin-5, we explored the
phosphorylation status in MCF-7 cells of the two forms β-catenin
involved in its nuclear translocation. We found that
phosphorylation levels of Ser675 and particularly of
Ser552 were drastically reduced in MCF-7 cells
overexpressing Fibulin-5 in comparison to control cells. Nuclear
translocation of β-catenin implies an increase of its
transcriptional activity which contributes to promote tumor
metastasis not only in breast cancer cells, including MCF-7
(31–33), but also in different types of
cancer (34). Consequently,
Fibulin-5 could function as a tumor suppressor by blocking
phosphorylation of β-catenin in breast cancer.
Two more findings highlight the antitumor effect
displayed by Fibulin-5 in breast cancer cells. First, presence of
Fibulin-5 affects the capacity of tumor cells to form mammospheres.
This effect strongly suggests that Fibulin-5 abrogates the ability
of breast cancer cells for in vitro self-renewal (24). Second finding is that Fibulin-5
inhibits proliferation of breast tumors cells. Moreover, our data
indicate that Fibulin-5 is associated with a low expression of
Ki-67, a nuclear protein related to cell proliferation and an
important parameter in breast cancer prognosis (21). To explore if this effect can be
reproduced in breast cancer samples we performed and
immunohistochemical analysis. Detection of Fibulin-5 in breast
cancer samples has been previously reported by Lee et al
(13). These authors found that
breast tumors contained abundant levels of Fibulin-5 protein
despite the FBLN5 messenger RNA levels were reduced in mammary
tumors attending to the data obtained from Oncomine Research
Database. Our immunohistochemical study confirmed that Fibulin-5 is
detected in breast cancer samples, and also revealed that its
detection is concomitant with low detection of Ki-67. Moreover,
ductal and lobular carcinoma, the two most common types of breast
tumors, have a contrary evolution as poorly differentiated ductal
carcinoma showed high Fibulin-5 and low Ki-67 detection and vice
versa. Overall, these data suggest that Fibulin-5 could influence
breast cancer cell proliferation. Previous studies have addressed
the influence of Fibulin-5 in cell proliferation and again,
Fibulin-5 showed a dual role as it can display both pro- and
anti-proliferative effects. For instance, Fibulin-5 overexpression
inhibits endothelial cell proliferation (35,36).
Moreover, Spencer et al (37) highlighted the role of Fibulin-5 as
an inhibitor of vascular smooth muscle cell proliferation employing
the Fbln5-deficient mice. By contrast, Fibulin-5 can also exert
pro-proliferative effects in nasopharyngeal carcinoma cells
(11). Based on these data, we
speculate that upon secretion, Fibulin-5 can modify the local cell
microenvironment through distinct interactions or establishing
complex networks with other ECM that could either promote or
repress a tumor microenvironment. In the present study we show new
insights into the tumor-protective functions of Fibulin-5.
Moreover, high expression levels of FBLN5 patients indicate a
better outcome of breast cancer patients. Overall, these data could
help to develop new therapeutic approaches to target tumor
microenvironment.
Acknowledgements
We thank Dr Carlos López-Otín (Universidad de
Oviedo) for providing cell lines and reagents, and Dr William P.
Schiemann (Case Western Reserve University, Cleveland, OH, USA) for
providing plasmid containing cDNA for Fibulin-5. Y.M. is supported
by a Ficyt (Gobierno del Principado de Asturias, Spain) fellowship
and T.F. is supported by the IUOPA. The present study is partially
supported by a grant from European Union FEDER funds, Principado de
Asturias (Plan de Ciencia, Tecnologia e Innovacion), FICYT (GRUPIN
14-069), to J.A.V., S.C. and A.J.O, and by a grant from the Fondo
de Investigaciones Sanitarias, FISS PI11/00371. IUOPA is supported
by the Obra Social Cajastur, Asturias, Spain.
Abbreviations:
|
DAPI
|
4′-6′-diamino-2-phenylindole
hydrochloride
|
|
ECM
|
extracellular matrix
|
|
EMT
|
epithelial-mensenchymal transition
|
|
kDa
|
kilodaltons
|
|
RGD
|
arginine-glycine-aspartic acid
|
References
|
1
|
de Vega S, Iwamoto T and Yamada Y:
Fibulins: Multiple roles in matrix structures and tissue functions.
Cell Mol Life Sci. 66:1890–1902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yanagisawa H and Davis EC: Unraveling the
mechanism of elastic fiber assembly: The roles of short fibulins.
Int J Biochem Cell Biol. 42:1084–1093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papke CL and Yanagisawa H: Fibulin-4 and
fibulin-5 in elastogenesis and beyond: Insights from mouse and
human studies. Matrix Biol. 37:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albig AR and Schiemann WP: Fibulin-5
function during tumorigenesis. Future Oncol. 1:23–35. 2005.
View Article : Google Scholar
|
|
5
|
Lomas AC, Mellody KT, Freeman LJ, Bax DV,
Shuttleworth CA and Kielty CM: Fibulin-5 binds human smooth-muscle
cells through alpha5beta1 and alpha4beta1 integrins, but does not
support receptor activation. Biochem J. 405:417–428. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagisawa H, Davis EC, Starcher BC, Ouchi
T, Yanagisawa M, Richardson JA and Olson EN: Fibulin-5 is an
elastin-binding protein essential for elastic fibre development in
vivo. Nature. 415:168–171. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stone EM, Braun TA, Russell SR, Kuehn MH,
Lotery AJ, Moore PA, Eastman CG, Casavant TL and Sheffield VC:
Missense variations in the fibulin 5 gene and age-related macular
degeneration. N Engl J Med. 351:346–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones RP, Ridley C, Jowitt TA, Wang MC,
Howard M, Bobola N, Wang T, Bishop PN, Kielty CM, Baldock C, et al:
Structural effects of fibulin 5 missense mutations associated with
age-related macular degeneration and cutis laxa. Invest Ophthalmol
Vis Sci. 51:2356–2362. 2010. View Article : Google Scholar :
|
|
9
|
Gallagher WM, Currid CA and Whelan LC:
Fibulins and cancer: Friend or foe? Trends Mol Med. 11:336–340.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Obaya AJ, Rua S, Moncada-Pazos A and Cal
S: The dual role of fibulins in tumorigenesis. Cancer Lett.
325:132–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang CF, Shiu LY, Su LJ, Yin Yu-Fang,
Wang WS, Huang SC, Chiu TJ, Huang CC, Zhen YY, Tsai HT, et al:
Oncogenic fibulin-5 promotes nasopharyngeal carcinoma cell
metastasis through the FLJ10540/AKT pathway and correlates with
poor prognosis. PLoS One. 8:e842182013. View Article : Google Scholar
|
|
12
|
Ohara H, Akatsuka S, Nagai H, Liu YT,
Jiang L, Okazaki Y, Yamashita Y, Nakamura T and Toyokuni S:
Stage-specific roles of fibulin-5 during oxidative stress-induced
renal carcinogenesis in rats. Free Radic Res. 45:211–220. 2011.
View Article : Google Scholar
|
|
13
|
Lee YH, Albig AR, Regner M, Schiemann BJ
and Schiemann WP: Fibulin-5 initiates epithelial-mesenchymal
transition (EMT) and enhances EMT induced by TGF-beta in mammary
epithelial cells via a MMP-dependent mechanism. Carcinogenesis.
29:2243–2251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao W, Zhou S, Xu H, Li H, He G, Liu Y
and Qi Y: Nogo-B promotes the epithelial-mesenchymal transition in
HeLa cervical cancer cells via Fibulin-5. Oncol Rep. 29:109–116.
2013.
|
|
15
|
Hu Z, Ai Q, Xu H, Ma X, Li HZ, Shi TP,
Wang C, Gong DJ and Zhang X: Fibulin-5 is down-regulated in
urothelial carcinoma of bladder and inhibits growth and invasion of
human bladder cancer cell line 5637. Urol Oncol. 29:430–435. 2011.
View Article : Google Scholar
|
|
16
|
Yue W, Sun Q, Landreneau R, Wu C,
Siegfried JM, Yu J and Zhang L: Fibulin-5 suppresses lung cancer
invasion by inhibiting matrix metalloproteinase-7 expression.
Cancer Res. 69:6339–6346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Song X, Yue W, Chen D, Yu J, Yao Z
and Zhang L: Fibulin-5 inhibits Wnt/β-catenin signaling in lung
cancer. Oncotarget. 6:15022–15034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie L, Palmsten K, MacDonald B, Kieran MW,
Potenta S, Vong S and Kalluri R: Basement membrane derived
fibulin-1 and fibulin-5 function as angiogenesis inhibitors and
suppress tumor growth. Exp Biol Med (Maywood). 233:155–162. 2008.
View Article : Google Scholar
|
|
19
|
Albig AR, Neil JR and Schiemann WP:
Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res.
66:2621–2629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wlazlinski A, Engers R, Hoffmann MJ, Hader
C, Jung V, Müller M and Schulz WA: Downregulation of several
fibulin genes in prostate cancer. Prostate. 67:1770–1780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inwald EC, Klinkhammer-Schalke M,
Hofstädter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schiemann WP, Blobe GC, Kalume DE, Pandey
A and Lodish HF: Context-specific effects of fibulin-5 (DANCE/EVEC)
on cell proliferation, motility, and invasion. Fibulin-5 is induced
by transforming growth factor-beta and affects protein kinase
cascades. J Biol Chem. 277:27367–27377. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rhodes LV, Antoon JW, Muir SE, Elliott S,
Beckman BS and Burow ME: Effects of human mesenchymal stem cells on
ER-positive human breast carcinoma cells mediated through
ER-SDF-1/CXCR4 crosstalk. Mol Cancer. 9:2952010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Győrffy B, Benke Z, Lánczky A, Balázs B,
Szállási Z, Timár J and Schäfer R: RecurrenceOnline: An online
analysis tool to determine breast cancer recurrence and hormone
receptor status using microarray data. Breast Cancer Res Treat.
132:1025–1034. 2012. View Article : Google Scholar
|
|
26
|
Manuel Iglesias J, Beloqui I,
Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon
A, Menendez JA, Dopazo J, et al: Mammosphere formation in breast
carcinoma cell lines depends upon expression of E-cadherin. PLoS
One. 8:e772812013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kelly T, Yan Y, Osborne RL, Athota AB,
Rozypal TL, Colclasure JC and Chu WS: Proteolysis of extracellular
matrix by invadopodia facilitates human breast cancer cell invasion
and is mediated by matrix metalloproteinases. Clin Exp Metastasis.
16:501–512. 1998. View Article : Google Scholar
|
|
28
|
Schluterman MK, Chapman SL, Korpanty G,
Ozumi K, Fukai T, Yanagisawa H and Brekken RA: Loss of fibulin-5
binding to beta1 integrins inhibits tumor growth by increasing the
level of ROS. Dis Model Mech. 3:333–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heo JH, Song JY, Jeong JY, Kim G, Kim TH,
Kang H, Kwon AY and An HJ: Fibulin-5 is a tumour suppressor
inhibiting cell migration and invasion in ovarian cancer. J Clin
Pathol. jclinpath-2015-203129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang K, Corsa CA, Ponik SM, Prior JL,
Piwnica-Worms D, Eliceiri KW, Keely PJ and Longmore GD: The
collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to
facilitate breast cancer metastasis. Nat Cell Biol. 15:677–687.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan
J, Wu J and Li M: MicroRNA-374a activates Wnt/β-catenin signaling
to promote breast cancer metastasis. J Clin Invest. 123:566–579.
2013.PubMed/NCBI
|
|
32
|
Klemm F, Bleckmann A, Siam L, Chuang HN,
Rietkötter E, Behme D, Schulz M, Schaffrinski M, Schindler S,
Trümper L, et al: β-catenin-independent WNT signaling in basal-like
breast cancer and brain metastasis. Carcinogenesis. 32:434–442.
2011. View Article : Google Scholar
|
|
33
|
Laezza C, D'Alessandro A, Paladino S,
Maria Malfitano A, Chiara Proto M, Gazzerro P, Pisanti S, Santoro
A, Ciaglia E and Bifulco M; Endocannabinoid Research Group.
Anandamide inhibits the Wnt/β-catenin signalling pathway in human
breast cancer MDA MB 231 cells. Eur J Cancer. 48:3112–3122. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang D, Hawke D, Zheng Y, Xia Y,
Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T and Lu Z:
Phosphorylation of beta-catenin by AKT promotes beta-catenin
transcriptional activity. J Biol Chem. 282:11221–11229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Preis M, Cohen T, Sarnatzki Y, Ben Yosef
Y, Schneiderman J, Gluzman Z, Koren B, Lewis BS, Shaul Y and
Flugelman MY: Effects of fibulin-5 on attachment, adhesion, and
proliferation of primary human endothelial cells. Biochem Biophys
Res Commun. 348:1024–1033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Xu H, Zeng Y and Yin ZQ:
Overexpression of fibulin-5 in retinal pigment epithelial cells
inhibits cell proliferation and migration and downregulates VEGF,
CXCR4, and TGFB1 expression in cocultured choroidal endothelial
cells. Curr Eye Res. 37:540–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Spencer JA, Hacker SL, Davis EC, Mecham
RP, Knutsen RH, Li DY, Gerard RD, Richardson JA, Olson EN and
Yanagisawa H: Altered vascular remodeling in fibulin-5-deficient
mice reveals a role of fibulin-5 in smooth muscle cell
proliferation and migration. Proc Natl Acad Sci USA. 102:2946–2951.
2005. View Article : Google Scholar : PubMed/NCBI
|