Introduction
Prostate cancer (PCa) is the most commonly diagnosed
cancer and the second leading cause of cancer-related death in
American men (1). Androgen
deprivation therapy (ADT) is currently the standard therapeutic
modality for advanced, metastatic PCa. ADT initially induces an
antitumor response in more than 90% of patients. However, the
efficacy of androgen withdrawal is temporary, and tumors in the
majority of these patients eventually relapse and evolve into an
androgen-insensitive stage, known as castration-resistant prostate
cancer (CRPC), which is commonly fatal (2). Chemotherapy plays an increasingly
important role in the management of CRPC patients. Recently,
taxanes (paclitaxel or docetaxel) in combination with various
adjuvants, have been shown to induce effective antitumor responses
and improve the overall survival of CRPC patients (3,4).
Originating from the bark of a yew tree, paclitaxel acts as an
antitumor agent that promotes apoptosis in cancer cells by
stabilizing the microtubule cytoskeleton (5). Paclitaxel currently serves as the
first-line chemotherapeutic agent for CRPC patients. However,
acquired paclitaxel-resistance almost always occurs after initial
responses, which is essentially incurable.
One of the hallmarks of cancer cell adaption to the
micro-environmental stresses, such as chemotherapeutics, heat and
radiation, is alterations in the nuclear architecture (6–8). In
concordance with these changes are alterations in nuclear matrix
proteins (NMPs) which comprise the structural elements of the
nucleus and likely act as a signature for tumorigenesis. The NMPs
are a well-structured and dynamic network of filaments that
functions in the nucleus similarly to that of microtubules and
tubulin in the cytoplasm (9). Some
specific NMP patterns associated with cancers, including bladder,
prostate and colon cancers have been identified. Assessment of
these NMP changes resulted in further elucidation of functional
implications as well as the identification of biomarkers (6,7,10–13).
To date, the mechanisms of acquired
paclitaxel-resistance remain largely unknown. Presently, the main
identified mechanisms relate to the expression of β-tubulin
isoforms/mutations and the activation of drug efflux pumps
(14). However, in spite of these
advances, treatment of paclitaxel-resistant PCa patients remains a
critical clinical challenge. We have previously established a
stable paclitaxel-resistant DU145-TxR cell line from the
androgen-independent DU145 cell line, which mimics to a certain
extent the progression of paclitaxel-resistance in PCa patients
(8,15). In the present study, we aimed to
identify differentially expressed protein(s) associated with
paclitaxel-resistance by comparing the NMP patterns of DU145-TxR
cells with that of DU145 cells and further explore the potential
mechanisms involved in the drug resistance, which may help develop
novel therapeutic strategies as well as clinically useful
biomarker(s) for PCa patients.
Materials and methods
Cell lines and cell culture
The CRPC cell line DU145 was purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA). DU145
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco, Shanghai, China) at 37ºC in incubator with
humidified air and 5% carbon dioxide. The stable
paclitaxel-resistant DU145-TxR cells were generated by culturing
DU145 cells with stepwise increasing concentrations of paclitaxel
(8,15). The initial culture was at 10 nM
paclitaxel (Sigma-Aldrich, St. Louis, MO, USA) for 2 days. Then the
medium was changed to fresh one without paclitaxel until the cells
grew well. Subsequently, the surviving DU145 cells were cultured
with paclitaxel in a dose-escalation manner using 48-h exposure to
establish stable paclitaxel-resistant DU145-TxR cells. The process
of acquired paclitaxel-resistance took 10 months. The DU145-TxR
cells were routinely cultured in normal DMEM medium as described
above supplemented with 10 nM paclitaxel to maintain their
drug-resistant phenotypes. Prior to each experiment, these cells
were grown for a minimum of two passages in normal DMEM medium.
Human prostate cancer tissue samples
Formalin-fixed paraffin-embedded human primary
(n=38) and metastatic (n=22) PCa tissue samples were obtained from
patients undergoing transrectal ultrasound-guided biopsy, from 2012
to 2015, at Shengjing Hospital of China Medical University. This
study protocol was approved by the Institutional Review Board of
Shengjing Hospital of China Medical University. Informed consent
was obtained from each patient.
Cell viability assay
Cells (2,500) per well were seeded in 96-well
plates. After culturing for 24 h, cells were treated with the
indicated concentrations of paclitaxel and cultured for an
additional 48 h. At the end of the culture period, cell
proliferation reagent WST-1
(4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene
disulfonate) was added to each well, as specified by the supplier
(Roche, Nutley, NJ, USA). After 3-h incubation, WST-1 absorbance at
450 nm was measured.
Isolation of nuclear matrix proteins
(NMPs), two-dimensional (2D) gel electrophoresis and mass
spectrometry
NMP isolation and high resolution, two-dimensional
(2D) electrophoresis was performed as previously described
(6,7,10–13).
Multiple gels were run for each sample and at least three samples
for each cell line were run at different times. The comparison of
NMP patterns was done by image analysis software together with
visual inspection (6,7). Protein spots significantly
differentially expressed were excised for mass spectrometry
analysis. Mass spectrometry analysis was performed at the Mass
Spectrometry/Proteomics Core Facility, The Johns Hopkins University
School of Medicine (6,7,16).
Real-time reverse transcription-PCR
Total RNA was isolated and treated with DNase I
(Invitrogen) following the supplier's protocol (Qiangen, Valencia,
CA, USA). cDNA was synthesized using the iScript cDNA synthesis kit
(Bio-Rad Laboratories, Hercules, CA, USA). Real-time PCR was done
in triplicate on an iCycler iQ Multicolor Real-time PCR detection
system (Bio-Rad Laboratories). Target gene expression was related
to TATA box binding protein (TBP) for normalization. The sequences
of primers used for PCR analyses are as follows: CK18, forward,
5′-TAGATGCCCCCAAATCTCAG-3′ and reverse, 5′-CACTGTGGTGCTCTCCTCAA-3′;
TBP, forward, 5′-GAATATAATCCCAAGCGGTTTG-3′ and reverse,
5′-ACTTCACATCACAGCTCCCC-3′.
Immunoblotting
Briefly, protein (25 μg) was separated on a 4–15%
SDS-PAGE and transferred onto PVD filters (Millipore, Bedford, MA,
USA). Membranes were incubated with primary antibodies overnight at
4ºC followed by horse-radish peroxidase-conjugated secondary
antibodies for 2 h at room temperature, and developed with the
SuperSignal West Dura Extended Duration Substrate kit (Pierce,
Rockford, IL, USA) (17). Mouse
monoclonal anti-CK18, rabbit polyclonal anti-actin and mouse
monoclonal anti-lamin A/C antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Secondary antibodies including
sheep anti-mouse antibody linked with horseradish peroxidase and
donkey anti-rabbit antibody linked with horseradish peroxidase were
purchased from GE Healthcare (Little Chalfont, UK). Lamin A/C was
used as a loading control for NMPs, and actin for total cellular
protein.
Immunofluorescence
Double immunofluorescence was applied simultaneously
to detect the expression of CK18 and actin. Briefly, cells were
fixed with 4% paraformaldehyde in PBS (Affymetrix Inc., Santa
Clara, CA, USA) for 15 min at room temperature, washed three times
with PBS, and blocked with 1% bovine serum albumin (BSA; Thermo
Fisher Scientific, Waltham, MA, USA) in PBST (0.3% Triton X-100 in
PBS) for 1 h. Slides were then incubated with the mixture of two
primary antibodies including anti-CK18 antibody and anti-actin
antibody in 1% BSA in PBST overnight at 4ºC. The mixture of two
secondary antibodies including Alexa Fluor-555 labeled goat
anti-mouse (Molecular Probes, Eugene, OR, USA) and Alexa Fluor 488
labeled goat anti-rabbit (Molecular Probes) antibodies in 1% BSA in
PBST was applied and incubated for 2 h at room temperature in the
dark. The cells were also washed three times with PBS and mounted
with ProLong Gold antifade reagent (Invitrogen) with
4′,6-diamidino-2-phe-nylindole (DAPI) to detect the nuclei. Slides
were observed with a Nikon Eclipse TE2000E using the GFP-BP Filter
(Ex 460-500, DM 5005, DA: 510-560).
Xenograft transplantation
All mouse procedures were carried out in accordance
with the institutional protocol guidelines at Shengjing Hospital of
China Medical University. Xenograft experiments were performed with
6- to 8-week-old female nonobese diabetic/severe combined
immunodeficient (NOD/SCID) mice provided by the Model Animal
Research Center of China Medical University, Shenyang, China.
DU145-TxR and DU145 cells were resuspended in 50 μl PBS in
quantities ranging from 103 to 105 cells.
Cells were then mixed with 50 μl Matrigel (BD Biosciences, San
Jose, CA, USA) and injected into the subcutaneous space of the back
of NOD/SCID mice (5 mice per group). Tumor growth was monitored
weekly and quantified by caliper measurements. Tumor volume was
calculated using the formula: V= (a2 × b)/2, where a and
b are the minimal and maximal diameter in millimeters,
respectively.
Immunohistochemistry
Immunohistochemical staining was conducted using
formalin-fixed paraffin-embedded tissue samples (18). Briefly, tissue sections were
deparaffinized, rehydrated and microwaved for antigen retrieval.
Subsequently, the sections were incubated with mouse anti-CK18
monoclonal antibody (Santa Cruz Biotechnology) at 1:150 overnight
at 4ºC, followed by detection using PowerVision Two-Step
histostaining reagent (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China).
The results of immunohistochemical staining were
scored by two pathologists, who were blinded to clinical data. CK18
expression was scored using a semi-quantitative method by
evaluating the number of positive tumor cells over the total number
of tumor cells. CK18 staining was scored according to the intensity
and proportion of positive cells as follows: −, no positive
staining cells; +, weak intensity with <25% positive staining
cells; ++, moderate intensity with 26–50% positive staining cells;
and +++, strong intensity with >50% positive staining cells.
Statistical analysis
Statistical analysis was done with the SPSS version
16.0 (SPSS, Inc., Chicago, IL, USA). Experimental data are
presented as means ± SD and analyzed by the Student's t-test for
determining statistical significance between groups. Pearson's
chi-squared test (χ2) was performed to compare the
correlation of clinicopathological characteristics with CK18
expression. P<0.05 was considered statistically significant.
Results
Confirmation of paclitaxel-resistance in
DU145-TxR cells
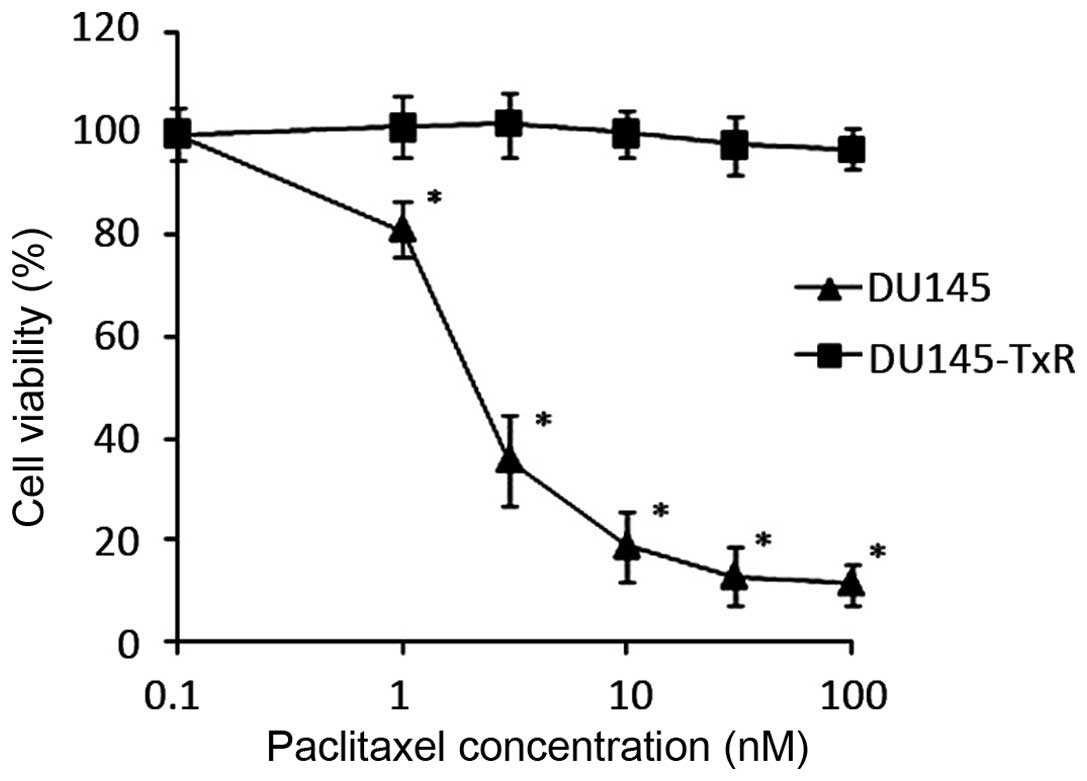
We confirmed the paclitaxel-resistance of the
DU145-TxR cells as compared to their parental DU145 cells by
evaluating cell viability at different concentrations of paclitaxel
(Fig. 1). DU145 and DU145-TxR
cells were treated with increasing concentrations of paclitaxel for
48 h. At the end of the culture period, cell viability was
determined by WST-1 assay. The IC50 of the DU145 cells
was 4.75 nM and the IC50 of the DU145-TxR cells
increased dramatically to >100 nM (Table I). The DU145-TxR cells are at least
20-fold more paclitaxel resistant than the parental DU145
cells.
 | Table IIC50 values of DU145 and
DU145-TxR cells. |
Table I
IC50 values of DU145 and
DU145-TxR cells.
| Drug | DU145 | DU145-TxR | Fold change |
|---|
| Paclitaxel
(nM) | 4.75 | >100 | >20 |
Cytokeratin 18 (CK18) is downregulated in
DU145-TxR cells
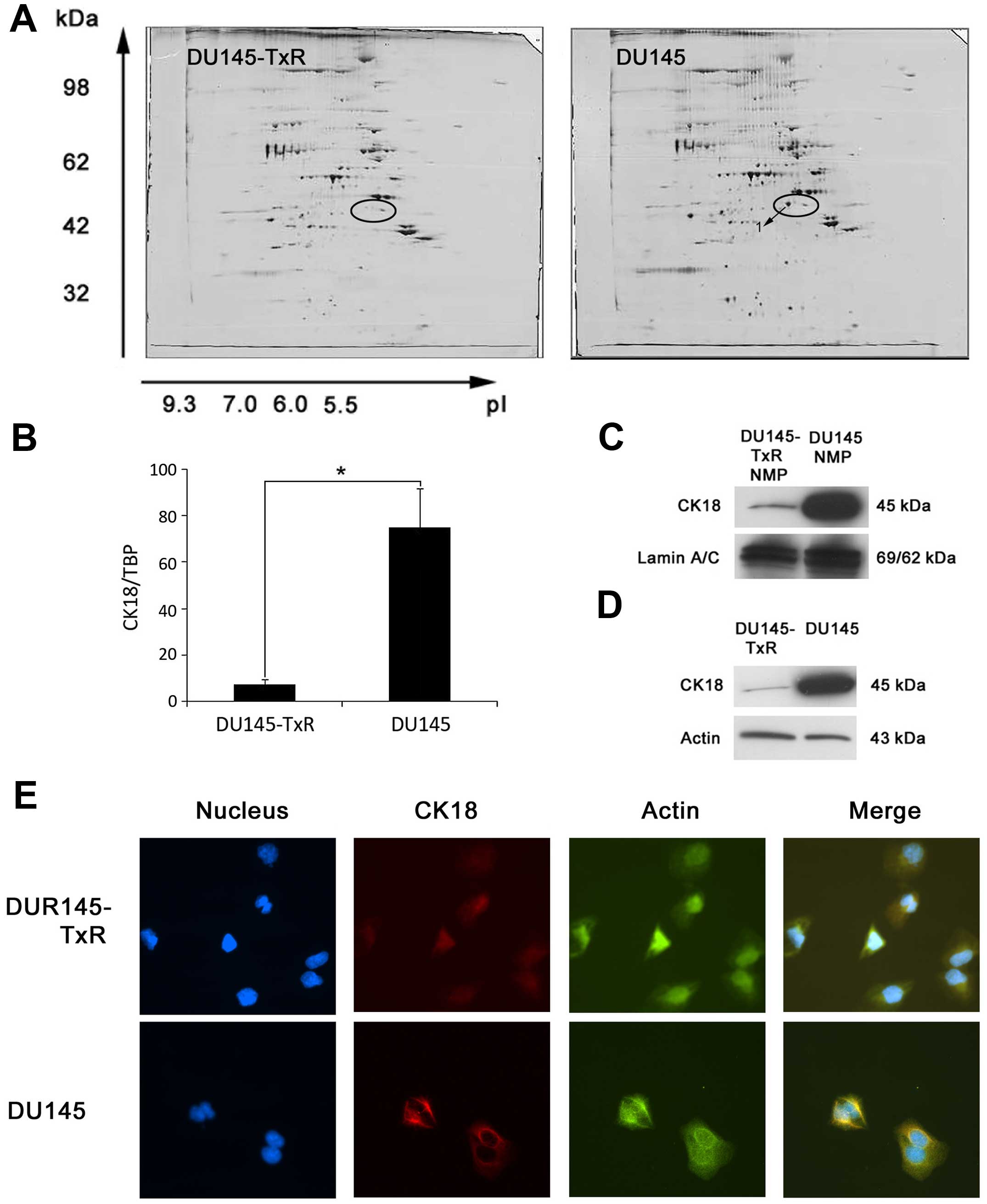
To identify differentially expressed protein(s)
associated with paclitaxel-resistance, we investigated the NMP
patterns of DU145-TxR cells compared to that of the parental DU145
cells by high-resolution two-dimensional gel electrophoresis. As
shown in Fig. 2, our data were
similar with our previous results (15). Analyses of the high-resolution
two-dimensional gels showed that several protein spots were present
in DU145 cells, but significantly downregulated in DU145-TxR cells.
Of note, protein spot 1 (Fig. 2A)
was reproducibly observed to be downregulated in DU145-TxR cells in
comparison with the parental DU145 cells. This spot was excised
from the two-dimensional gels and analyzed by mass spectrometry,
which suggested this protein as cytokeratin 18 (CK18) (Table II).
 | Table IIMass spectrometry analysis of NMP
from DU145 cells. |
Table II
Mass spectrometry analysis of NMP
from DU145 cells.
| Protein spot | MW (kDa) | pI | Protein
identity | Accession
number | Peptide sequence
coverage (%) | No. of (%)unique
peptides |
|---|
| 1 | 47 | 5.27 | Cytokeratin 18
(Homo-sapiens) | gi|30311 | 44 | 16 |
To validate the identification of CK18 in
two-dimensional gels, we further examined the expression of CK18 in
DU145 and DU145-TxR cells. Firstly, we determined the mRNA level of
CK18 by real-time RT-PCR. As shown in Fig. 2B, we found downregulation of CK18
mRNA in DU145-TxR cells as compared to the parental DU145 cells.
Then we determined the protein level of CK18 by immunoblotting. As
expected, CK18 protein was significantly downregulated in the NMPs
of DU145-TxR cells upon normalization with lamin A/C (Fig. 2C).
In order to understand whether the difference of
CK18 expression in NMPs was a result of alterations in protein
compartmentalization, we extracted and analyzed the total cellular
protein by immunoblotting. These immunoblotting studies
demonstrated that CK18 protein was constitutively downregulated in
the total cellular protein of DU145-TxR cells in comparison with
the parental DU145 cells upon normalization with Actin (Fig. 2D). Considered together, CK18 was
downregulated in DU145-TxR cells at both NMP and total cellular
protein levels, indicating a global effect rather than a
redistribution of CK18 away from the nucleus in the
paclitaxel-resistant DU145-TxR cells.
Furthermore, immunofluorescence was applied to
examine the expression and intracellular location of CK18 in DU145
and DU145-TxR cells. As shown in Fig.
2E, more intense CK18 fluorescence was observed in DU145 cells,
whereas only faint CK18 fluorescence was observed in DU145-TxR
cells. CK18 appeared to be localized mainly at the cytoskeleton
predominantly in the cytoplasm. These results further suggested
that the downregulation of CK18 was a global effect in DU145-TxR
cells due to paclitaxel-resistance.
DU145-TxR cells possess cancer stem
cell-like properties
Some recent studies have shown that cancer stem
cells (CSCs) may preferentially survive exposure to chemotherapy,
providing an attractive rationale for relapse following initial
tumor shrinkage with standard therapy (19,20).
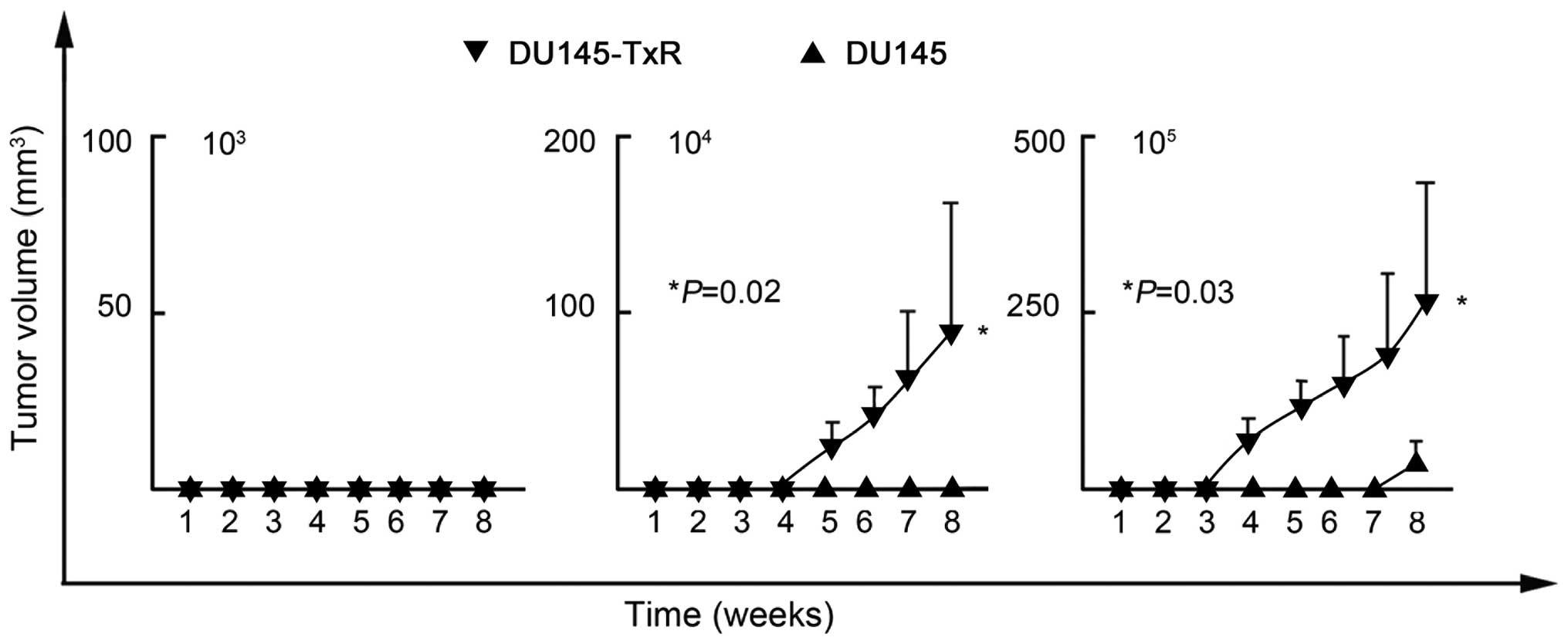
Thus, we further investigated the CSC-like properties of these
DU145-TxR cells. Because efficient xenograft transplantation is a
major criterion for the validation of CSCs (21), we inoculated serial dilutions of
DU145-TxR and DU145 cells into NOD/SCID mice. As shown in Table III and Fig. 3, DU145-TxR cells initiated tumor
formation with 104 (2 of 5 mice) and 105 (4
of 5 mice) cells, whereas DU145 cells needed 105 (1 of 5
mice) to initiate tumor formation. Therefore, DU145-TxR cells have
much more efficient tumorigenicity than DU145 cells. These data
validated that DU145-TxR cells possessed potent CSC-like properties
and eventually developed paclitaxel-resistance.
 | Table IIITumor initiating ability of DU145-TxR
and DU145 cells. |
Table III
Tumor initiating ability of DU145-TxR
and DU145 cells.
| Cell type | Cell dose | Tumor
incidence |
|---|
| DU145-TxR | 103 | 0/5a |
| 104 | 2/5 |
| 105 | 4/5 |
| DU145 | 103 | 0/5 |
| 104 | 0/5 |
| 105 | 1/5 |
Downregulation of CK18 in prostate cancer
tissues is associated with tumor aggressiveness
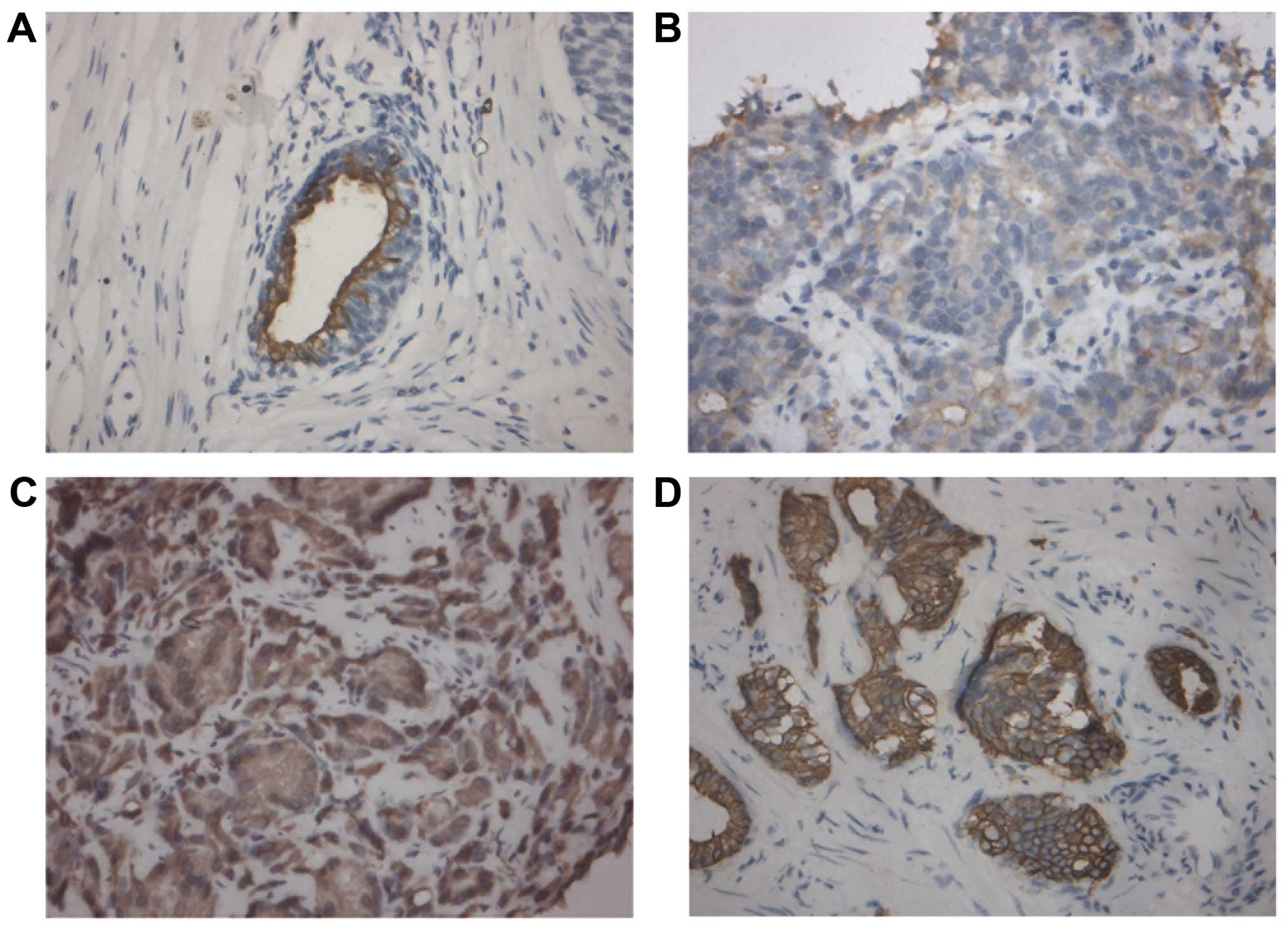
Immunohistochemistry was performed to investigate
the correlation between CK18 expression and clinicopathological
characteristics of human PCa tissue samples in terms of tumor stage
and grade. As shown in Fig. 4 and
Table IV, CK18 protein was
predominantly expressed in the cytoplasm of cancer cells and the
expression of CK18 was inversely correlated with tumor grade in a
statistically significant fashion (P=0.028): tissue samples with
higher tumor grades (Gleason score ≥7) showed gradually decreased
immunostaining intensity. We did not detect any significant
correlation between CK18 expression and tumor stage (P>0.05,
data not shown). These data suggested a potential association
between the downregulation of CK18 and tumor aggressiveness of
PCa.
 | Table IVCK18 expression by IHC correlated
with tumor grade of PCa. |
Table IV
CK18 expression by IHC correlated
with tumor grade of PCa.
| Tumor grade | Total number | CK18
expression | P-value |
|---|
|
|---|
| − to + | ++ to +++ |
|---|
| Gleason score |
| <7 | 25 | 10 | 15 | 0.028 |
| ≥7 | 35 | 24 | 11 | |
| Total number | 60 | 34 | 26 | |
Discussion
In the present study, we determined that CK18 is
downregulated in the paclitaxel-resistant DU145-TxR cells as
compared to the parental DU145 cells and is associated with the
acquisition of paclitaxel-resistance. CK18 is a member of
cytokeratins which are the products of a large gene family of the
intermediate filament genes. The family is divided into six types
or subclasses based on the sequence characteristics of the genes
and their products, of which cytokeratins make up type I (acidic,
CK9-CK20) and type II (neutral-basic, CK1-CK8) groups (22). CK18 is the representative of the
acidic type I cytokeratins and has been recognized for >30 years
as an epithelial marker in diagnostic histopathology (23). Furthermore, CK18 is one of the most
prevalent and often downregulated cytokeratins in malignant cell
lines and many types of carcinomas (24).
Various regulatory changes in cytokeratin expression
at the transcriptional and post-transcriptional levels have been
described in experimental studies on epithelial tumor cells,
challenging the view that cytokeratins are merely marker protein
(25,26). Although the recognized function of
cytokeratins, in general, has been defined historically as
structural because of their interactions with the extracellular
matrix and their role in cell-cell contact and adhesion (27), these structurally related proteins
also influence protein synthesis and modulate intracellular
cytokine signaling cascades (28).
Some authors have observed the association of downregulation of
CK18 with anticancer drug resistance. Parekh et al (29) reported that the cisplatin-resistant
human ovarian 2008/13 cell line contained markedly lower level of
CK18 when compared with the sensitive parental cells. Transfection
of full-length CK18 cDNA into this cell line increased sensitivity
to cisplatin. Liang et al (30) reported that the
paclitaxel-resistant human nasal RPMI-2650Tx cell line displayed a
decrease in CK18 expression compared with the parental RPMI-2650
cells. Our data also suggested that CK18 is downregulated in the
paclitaxel-resistant DU145-TxR cells. We have been investigating
the potential role of CK18 in the development of
paclitaxel-resistance in these DU145-TxR cells. However,
transfection of full-length CK18 cDNA into this cell line did not
reverse the paclitaxel-resistance (data not shown), indicating that
another mechanism might be involved in the acquisition of
paclitaxel-resistance in these PCa cells.
Accumulating evidence indicates that CSCs, with the
abilities of tumor-initiating, self-renewal and differentiation,
are responsible for the origin, progression and relapse of cancer.
CSCs are thought to cause post-chemotherapeutic recurrence due to
the resistance to chemotherapy by various mechanisms (31,32).
Thus, we examined the CSC phenotype of these DU145-TxR cells.
Higher tumorigenicity is the fundamental phenotype of CSCs and can
be confirmed functionally by serial xenograft transplantation of a
small number of putative CSCs in immunodeficient mice (21,33).
Our findings demonstrated that DU145-TxR cells had higher
tumorigenicity than DU145 cells by xenograft transplantation,
suggesting that these DU145-TxR cells possessed potent CSC-like
properties and eventually developed the paclitaxel-resistance.
Therefore, further study is warranted to elucidate the potential
role of CK18 in the maintenance of CSC-like properties as well as
the acquisition of paclitaxel-resistance in these prostate cancer
cells in order to obtain more insight into the pathobiology of PCa
and to develop novel therapeutics.
Moreover, the downregulation of CK18 is associated
with progression of cancer and is clinically important for
detecting and monitoring neoplastic disease (26,34).
Our data demonstrated that CK18 was downregulated in poorly
differentiated (Gleason score ≥7) PCa tissue samples, indicating a
potential association of the downregulation of CK18 with tumor
aggressiveness. CK18 has also been introduced as a potentially
useful serum biomarker for the determination of tumor cell death of
epithelial-derived tumors (carcinomas) (35). Its prime utility is in monitoring
treatment and in providing early indications on recurrence and
tumor progression. Encouraging data have been reported by various
groups with regard to the potential use of CK18 as a clinically
useful biomarker for monitoring treatment efficacy in cancer
patients (24,35,36).
We are currently investigating the expression of CK18 in PCa
tissues and serum to predict and monitor paclitaxel responsiveness.
Since only 60% of CRPC patients respond to initial paclitaxel-based
chemotherapy, it will be helpful to predict the paclitaxel
responsiveness prior to treatment to assist in decision making for
chemotherapy in these patients. It is also very important to
monitor the paclitaxel responsiveness during its administration for
earlier regimen adjustment and more favorable treatment
outcome.
In conclusion, we demonstrated herein that the
down-regulation of CK18 is associated with the acquisition of
paclitaxel-resistance and tumor aggressiveness in PCa. Therefore,
further study to define the potential role of CK18 may lead to
novel therapy strategies as well as a clinically useful biomarker
for PCa patients.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81372725 and
81372766) and the Liaoning Provincial Natural Science Foundation
(no. 2013021007).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Debes JD and Tindall DJ: Mechanisms of
androgen-refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al; TAX 327 Investigators. Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer. N Engl J
Med. 351:1502–1512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madan RA, Pal SK, Sartor O and Dahut WL:
Overcoming chemotherapy resistance in prostate cancer. Clin Cancer
Res. 17:3892–3902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue T, Leman ES, Yeater DB and
Getzenberg RH: The potential role of purine-rich element binding
protein (PUR) alpha as a novel treatment target for
hormone-refractory prostate cancer. Prostate. 68:1048–1056. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng Y, Kulkarni P, Inoue T and Getzenberg
RH: Down-regulating cold shock protein genes impairs cancer cell
survival and enhances chemosensitivity. J Cell Biochem.
107:179–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Zeng Y, Mooney SM, Yin B, Mizokami
A, Namiki M and Getzenberg RH: Resistance to paclitaxel increases
the sensitivity to other microenvironmental stresses in prostate
cancer cells. J Cell Biochem. 112:2125–2137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pederson T: Half a century of ‘the nuclear
matrix’. Mol Biol Cell. 11:799–805. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Getzenberg RH, Konety BR, Oeler TA,
Quigley MM, Hakam A, Becich MJ and Bahnson RR: Bladder
cancer-associated nuclear matrix proteins. Cancer Res.
56:1690–1694. 1996.PubMed/NCBI
|
|
11
|
Partin AW, Getzenberg RH, CarMichael MJ,
Vindivich D, Yoo J, Epstein JI and Coffey DS: Nuclear matrix
protein patterns in human benign prostatic hyperplasia and prostate
cancer. Cancer Res. 53:744–746. 1993.PubMed/NCBI
|
|
12
|
Brünagel G, Vietmeier BN, Bauer AJ, Schoen
RE and Getzenberg RH: Identification of nuclear matrix protein
alterations associated with human colon cancer. Cancer Res.
62:2437–2442. 2002.PubMed/NCBI
|
|
13
|
Leman ES and Getzenberg RH: Nuclear
structure as a source of cancer specific biomarkers. J Cell
Biochem. 104:1988–1993. 2008. View Article : Google Scholar
|
|
14
|
Seruga B, Ocana A and Tannock IF: Drug
resistance in meta-static castration-resistant prostate cancer. Nat
Rev Clin Oncol. 8:12–23. 2011. View Article : Google Scholar
|
|
15
|
Kim JJ, Yin B, Christudass CS, Terada N,
Rajagopalan K, Fabry B, Lee DY, Shiraishi T, Getzenberg RH, Veltri
RW, et al: Acquisition of paclitaxel resistance is associated with
a more aggressive and invasive phenotype in prostate cancer. J Cell
Biochem. 114:1286–1293. 2013. View Article : Google Scholar
|
|
16
|
Shevchenko A, Wilm M, Vorm O and Mann M:
Mass spectrometric sequencing of proteins silver-stained
polyacrylamide gels. Anal Chem. 68:850–858. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin B, Zeng Y, Wang X, Liu G, Zhang M and
Song Y: Expression and clinical significance of cancer-testis genes
in clear cell renal cell carcinoma. Int J Clin Exp Pathol.
7:4112–4119. 2014.PubMed/NCBI
|
|
18
|
Yin B, Liu G, Wang XS, Zhang H, Song YS
and Wu B: Expression profile of cancer-testis genes in transitional
cell carcinoma of the bladder. Urol Oncol. 30:886–892. 2012.
View Article : Google Scholar
|
|
19
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lonardo E, Hermann PC, Mueller MT, Huber
S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S,
Rodriguez-Arabaolaza I, Ramirez JC, et al: Nodal/Activin signaling
drives self-renewal and tumorigenicity of pancreatic cancer stem
cells and provides a target for combined drug therapy. Cell Stem
Cell. 9:433–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schutte B, Henfling M, Kölgen W, Bouman M,
Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et
al: Keratin 8/18 breakdown and reorganization during apoptosis. Exp
Cell Res. 297:11–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: Patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linder S, Olofsson MH, Herrmann R and
Ulukaya E: Utilization of cytokeratin-based biomarkers for
pharmacodynamic studies. Expert Rev Mol Diagn. 10:353–359. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knapp AC and Franke WW: Spontaneous losses
of control of cytokeratin gene expression in transformed,
non-epithelial human cells occurring at different levels of
regulation. Cell. 59:67–79. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woelfle U, Sauter G, Santjer S, Brakenhoff
R and Pantel K: Down-regulated expression of cytokeratin 18
promotes progression of human breast cancer. Clin Cancer Res.
10:2670–2674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kirfel J, Magin TM and Reichelt J:
Keratins: A structural scaffold with emerging functions. Cell Mol
Life Sci. 60:56–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paramio JM and Jorcano JL: Beyond
structure: Do intermediate filaments modulate cell signalling?
BioEssays. 24:836–844. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parekh H, Wiesen K and Simpkins H:
Acquisition of taxol resistance via P-glycoprotein- and
non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma
cells. Biochem Pharmacol. 53:461–470. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang Y, Meleady P, Cleary I, McDonnell S,
Connolly L and Clynes M: Selection with melphalan or paclitaxel
(Taxol) yields variants with different patterns of multidrug
resistance, integrin expression and in vitro invasiveness. Eur J
Cancer. 37:1041–1052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin B, Yang Y, Zhao Z, Zeng Y, Mooney SM,
Li M, Xu X, Song Y, Wu B and Yang Z: Arachidonate 12-lipoxygenase
may serve as a potential marker and therapeutic target for prostate
cancer stem cells. Int J Oncol. 38:1041–1046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van der Horst G, Bos L and van der Pluijm
G: Epithelial plasticity, cancer stem cells, and the
tumor-supportive stroma in bladder carcinoma. Mol Cancer Res.
10:995–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin B, Zeng Y, Liu G, Wang X, Wang P and
Song Y: MAGE-A3 is highly expressed in a cancer stem cell-like side
population of bladder cancer cells. Int J Clin Exp Pathol.
7:2934–2941. 2014.PubMed/NCBI
|
|
34
|
Barak V, Goike H, Panaretakis KW and
Einarsson R: Clinical utility of cytokeratins as tumor markers.
Clin Biochem. 37:529–540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Olofsson MH, Ueno T, Pan Y, Xu R, Cai F,
van der Kuip H, Muerdter TE, Sonnenberg M, Aulitzky WE, Schwarz S,
et al: Cytokeratin-18 is a useful serum biomarker for early
determination of response of breast carcinomas to chemotherapy.
Clin Cancer Res. 13:3198–3206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Demiray M, Ulukaya EE, Arslan M, Gokgoz S,
Saraydaroglu O, Ercan I, Evrensel T and Manavoglu O: Response to
neoadjuvant chemotherapy in breast cancer could be predictable by
measuring a novel serum apoptosis product, caspase-cleaved
cytokeratin 18: A prospective pilot study. Cancer Invest.
24:669–676. 2006. View Article : Google Scholar : PubMed/NCBI
|