Introduction
Colorectal cancer (CRC) has one of the highest
prevalence rates of solid tumor world-wide and thus represents a
significant financial burden for society (1,2). It
is a practical issue to identify effective diagnostic and
prognostic biomarkers. The more we understand about the innate
biological mechanism of CRC, the better the optimal clinical
outcome will be to benefit patients and reduce the burden of this
disease.
In the past, research and clinical practice paid
most attention to protein coding gene alteration and subsequently
effect in CRC initiation and progression (3,4).
Nowadays, a class of small (18–24 nucleotide) non-coding RNAs,
microRNAs (miRNAs), are focussed on considering their role in
regulating the transcription of multiple target mRNAs (5). A decade ago, it was found in chronic
leukemia studies that more and more miRNA molecules were confirmed
to be involved in the occurrence and development of a variety of
cancers including CRC (6–8). A previous study explored a panel of
altered miRNAs in CRC and reported differential expression levels
in patient samples (9). Both
elevated miRNAs and decreased miRNAs were found from multiple study
resources. Thus indicating the role of miRNAs in CRC progression
(10–12).
Extensive research is being conducted in order to
clarify the direct target of a specific miRNA, evaluating the
feasibility of a specific miRNA as diagnostic and prognostic
biomarker and regulation of the process. miR-181b was recognized as
one of the most important miRNAs that regulate tumor initiation and
progression (13,14). A recently released case-control
study reported downregulation of miR-181b in 30 CRC tissue samples
compared to paired control. In contrast, earlier study reported
high expressed miR-181b in CRC samples (15,16).
This controversial result indicates that more research should be
conducted to explore the role of miR-181b in CRC as well as the
involved mechanisms.
A relatively large study group was recruited in the
present study to further determine the expression of miR-181b in
CRC tissues and paired normal tissues, and to evaluate the
association between the miR-181b expression level and the
clinicopathological factors. Epigenetic status of miR-181b was also
investigated. In vitro functional study, including target
genes identification, cell proliferation and invasion and
metastasis ability, was also conducted in multiple colorectal
cancer cell lines.
Patients and methods
Patients and tissue samples
Ethics Committee from the First Affiliated Hospital
of Xinxiang Medical University approved the ethical requirements
for this study. All the patients consented to the procedures of the
molecular analysis. Moreover, tissues samples and adjacent normal
tissues from 97 CRC patients, who underwent curative surgery
without the use of chemotherapy in the First Affiliated Hospital of
Xinxiang Medical University from July 2010 to August 2014, were
analyzed and compared. The American Joint Committee on Cancer TNM
system was used in order to determine the tumor burden. The CRC
patient group consisted of 32 females and 65 males with a mean age
of 61.34±11.27 years. All tissue samples were frozen in liquid
nitrogen at −80°C.
Cell lines and 5-aza-CdR treatment
Four commonly used colorectal cancer cell lines
HCT-29, HCT116, SW1116, SW480 and the normal colon cell line
CCD-18co were obtained from the Chinese Academy of Sciences
(Beijing, China) and supplemented with RPMI-1640 containing 10%
fetal bovine serum (FBS) (both from Gibco, Grand Island, NY, USA)
in 5% CO2 incubator until 70% confluence. 5-Aza-CdRr
(Sigma-Aldrich, St. Louis, MO, USA) diluted with DMSO was
administered to the cultured media at concentrations of 5 μmol/l,
whereas, the control group comprising of untreated plates were only
incubated with equal amounts of DMSO similarly to the cultured
media. After 3 days of treatment, the cells were collected for
further analysis.
Cell proliferation and colony
formation
To evaluate cell viability, 1×104 cells
from mock control and transfected groups were seeded in the 96-well
plates for 48 h. The plates were added with 100 μl MTT
(Sigma-Aldrich) for each well and incubated for 4 h. Surviving
cells developed purple formazan crystals and were air dried at room
temperature for 30 min. The cell viability was quantified at the
absorbance value A490 using iMark Microplate absorbance reader
(Bio-Rad, Hercules, CA, USA).
Colony formation assay was performed in transfected
and mock control groups. Cells (1×103) were seeded in a
6-well plate in each well, and incubated for 72 h at 37°C. The
colonies were then fixed with 20% methanol for 10 min at room
temperature following 0.1% crystal violet (Sigma-Aldrich) staining.
The number of colonies for each group was counted.
DNA methylation analysis
DNA was bisulfite modified using the commercial DNA
methylation kit (Invitrogen, Carlsbad, CA, China). PCR primers were
designed using MethPrimer software miR-181b primer, forward,
5′-TAAGCGATAGAAGGTAGTG-3′ and reverse,
5′-CAAACTACCTTATTATAATTATAACA-3′.
The PCR cycle conditions were as follow: initial
denaturation at 94°C for 7 min, 45 cycles at 94°C for 30 sec, 52°C
for 30 sec, and 72°C for 30 sec.
A total of 6 μl amplified PCR was used for
electrophoresis in a 2% gel. To increase the efficacy of this
procedure, MSPs were analytically validated using DNA methylated
with CpG Methylase (M.SssI) (New England Biolabs, Hitchin, Herts,
UK) from placental tissue as positive control, and normal human
lymphocyte DNA as unmethylated control.
Apoptosis analysis
Cell apoptosis analysis was performed by flow
cytometry with commercial Annexin V-FITC apoptosis detection kit I
(BD Pharmingen, San Diego, CA, USA). The nuclear apoptosis was
evaluated by Hoechst 33342 (Qiagen, Hilden, Germany) staining
according to the instructions of the supplier. The morphology was
detected and recorded by a fluorescent microscope (Nikon 3200;
Nikon, Tokyo, Japan) at excitation of 350 nm and absorbance at 460
nm.
Exogenous miRNA transfection
Cells were seeded into cell culture plates at
density of 1×106 and harvested following 48 h incubation
when cells reached 70% confluence. Exogenous miRNA-181b
(GenePharma, Shanghai, China) was transfected in to cells by using
Lipofectamine 2000 (Invitrogen) at final concentration of 50 nM
according to the manufacturer's protocol. Mock control was also set
up with same procedure, and quantitative PCR (qPCR) was performed
48 h post-transfection.
RNA interference
For siRNA, the following sequences were used:
5′-GACCUCUGUGGCGACUUCA-3′, 5′-GGUUUUGGAUCUUGAAUGU-3′ and
5′-GGAUAUCCUUAUCAGAGCU-3′. All oligonucleotides (Thermo Fisher
Scientific, Waltham, MA, USA) were delivered into HeLa cells and
were harvested 48 h after transfection. Knockdown efficiency was
validated with RT-PCR.
Luciferase report system identifies
miR-181b target gene
Transfection of wild and mutated miR-181b binding
sequences (Sangon, Shanghai, China) in the 3′UTR of RASSF1A were
carried out in SW480 cells with pMIR-Report Luciferase plasmid
(Life Technologies, Carlsbad, CA, USA) at final concentration of
500 ng for 24 h incubation, and 50 nM miR-181b or miR-control
vector were next transfected into cells for further 24 h
incubation. Comparison of luciferase activity from different groups
was then conducted.
Real-time qPCR
TRIzol (Invitrogen) was used to extract total
cellular RNA from the CRC tissue samples and cell pellets as per
instructions of the manufacturer. UV spectrophotometer was used to
detect the purity and concentration of total RNA, which was diluted
with water treated pyrocarbonate (DEPC). Then, liquid nitrogen was
used to freeze and store the total RNA at −80°C. M-MLV Rtase cDNA
Synthesis kit (Invitrogen) was used to synthesize complementary DNA
(cDNA) stands with RNA as a template. In order to evaluate
transcript expression, gene-specific primers were used in a 20 μl
reaction which contained 2 μl of template cDNA, 10 μl of 2X
SYBR-Green Master Mix, 1 μl of 10 μmol/l primers, 2 μl of
ddH2O and 4 μl of 25 mmol/l Mg2+. Then the
solution was administered to a ABI Prism 7500HT sequence detection
system (Applied Biosystems, Foster City, CA, USA) for the following
sequence of cycles: 3 min at 95°C which was immediately followed by
40 consecutive cycles at 95°C for 10 sec and lastly 30 sec at 60°C.
Moreover, comparative cycle threshold (CT) method was applied to
calculate the level of transcripts by using RNU48 as an endogenous
control. The final results were calculated by using
2−ΔΔCt method. The method involved estimating ΔCt for
each sample by subtracting the Ct value of genes from the Ct value
of RNU48, which served as a controlled variable. However, Ct values
of triplicates having a standard deviation of <0.20 were
acceptable. The product of MSP was kept at 4°C, and analyzed by gel
electrophoresis.
Western blot analysis
Manufacturer's instructions were followed throughout
the procedures (Abcam, Cambridge, UK). Briefly, cells were
centrifuged and lysed in solution. Proteins were loaded into the
wells of the SDS-PAGE gel along with molecular weight markers.
Afterwards, electro-transference of proteins on nitrocellulose
membrane took place, which was immediately followed by blocking the
membrane overnight at 4°C or for 1 h at room temperature using 5%
blocking solution. Moreover, the membranes were subjected to
overnight incubation (at 4°C) with various primary antibodies
alongside horseradish peroxidase-conjugated secondary antibodies
for 1 h (at room temperature). Anti-β-actin antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA)
while anti-RASSF1A antibodies were from Cell Signaling Technology,
Inc. (Danver, MA, USA).
Statistical analysis
SPSS 13.0 (SPSS Inc., Chicago, IL, USA) was used for
all data in statistical analysis. Numerical data were performed
with mean ± standard deviation (SD). Statistical analysis was
performed using the Student's t-test. Category data are displayed
with actual number and percentage. Depending on sample size,
Chi-square test or Fisher's exact test were performed. Each
experiment was carried out in triplets or repeated at least twice.
P-value <0.05 was adopted as significant.
Results
Declined expression of miR-181b in CRC
cell lines and tumor tissues
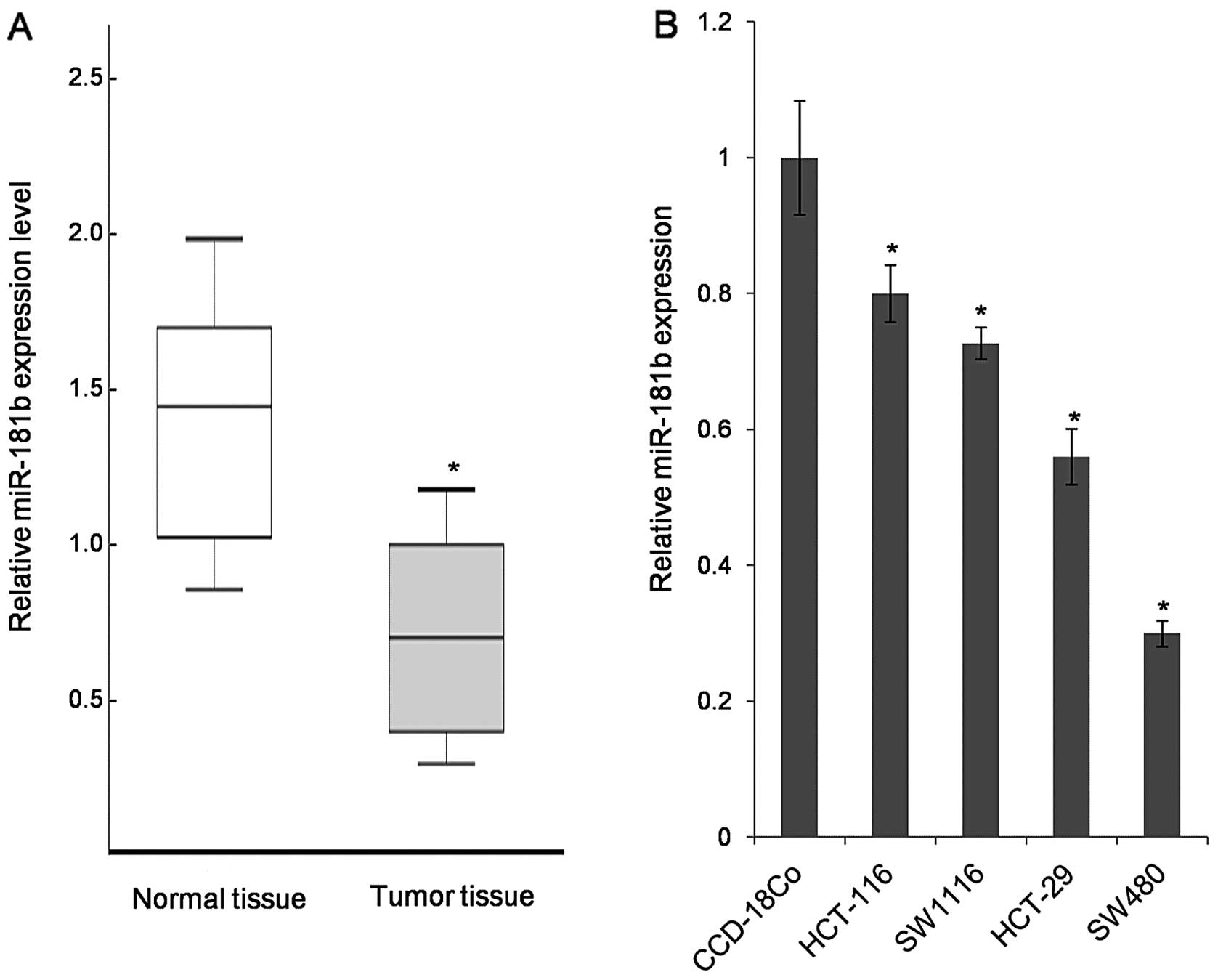
The expression of miR-181b was first detected in 97
pairs of CRC tissues and adjacent normal tissue samples. Compared
to normal tissue samples, the tumor tissues exhibited lower levels
of miR-181b in 65 out of 97 samples (Fig. 1A). Furthermore, four primary
colorectal carcinoma cell lines (HCT-29, HCT116, SW480 and SWS116)
were analyzed revealing the universal downregulation of miR-181b in
the tested CRC cell lines (Fig.
1B). Because of the lowest expression level of miR-181b in
SW480, it was selected for further functional study.
Association of reduced miR-181b
expression and clinicopathological features in CRC
Based on relative expression levels in tumor and
normal tissue (tumor/normal <0.05), patients were divided into
lower expression and higher expression (Table I). Results revealed a tightly
linked association between the expression of miR-181b and CRC
clinical stage. The patients with downregulation of miR-181b
expression were statistically associated with high differential
grade (P=0.042) and advanced stage (P=0.028, Fisher's exact
test).
 | Table IAssociation between miR-181b mRNA
expression and methylation status and clinicopathological features
in colorectal carcinomas. |
Table I
Association between miR-181b mRNA
expression and methylation status and clinicopathological features
in colorectal carcinomas.
| Clinical and
pathological features | N | miR-181b | P-value |
|---|
|
|---|
| Low | % | Higher | % |
|---|
| All cases | 97 | 65 | 67.0 | 32 | 33.0 | |
| Age (years) | | | | | | 0.24 |
| <50 | 29 | 17 | 26.2 | 12 | 37.5 | |
| ≥50 | 68 | 48 | 73.8 | 20 | 62.5 | |
| Gender | | | | | | 0.37 |
| Female | 42 | 24 | 36.9 | 18 | 56.3 | |
| Male | 55 | 41 | 63.1 | 14 | 43.7 | |
| TNM stage | | | | | | 0.028 |
| T1N0M0 | 33 | 17 | 26.2 | 16 | 50.0 | |
| T2N0M0 | 38 | 26 | 40.0 | 12 | 37.5 | |
| T3N0M0 | 26 | 22 | 33.8 | 4 | 12.5 | |
| Tumor
differentiation | | | | | | 0.042 |
| Moderate | 60 | 49 | 75.4 | 11 | 34.4 | |
| Well | 37 | 16 | 24.6 | 21 | 65.6 | |
| Dukes' stage | | | | | | 0.037 |
| A | 38 | 21 | 32.3 | 17 | 53.1 | |
| B | 59 | 44 | 67.7 | 15 | 46.9 | |
| miR-181b | | | | | | <0.01 |
| Unmethylation | 36 | 5 | 7.7 | 31 | 96.9 | |
| Methylation | 61 | 60 | 92.3 | 1 | 3.1 | |
miR-181b is epigenetically silenced in
colorectal cancer
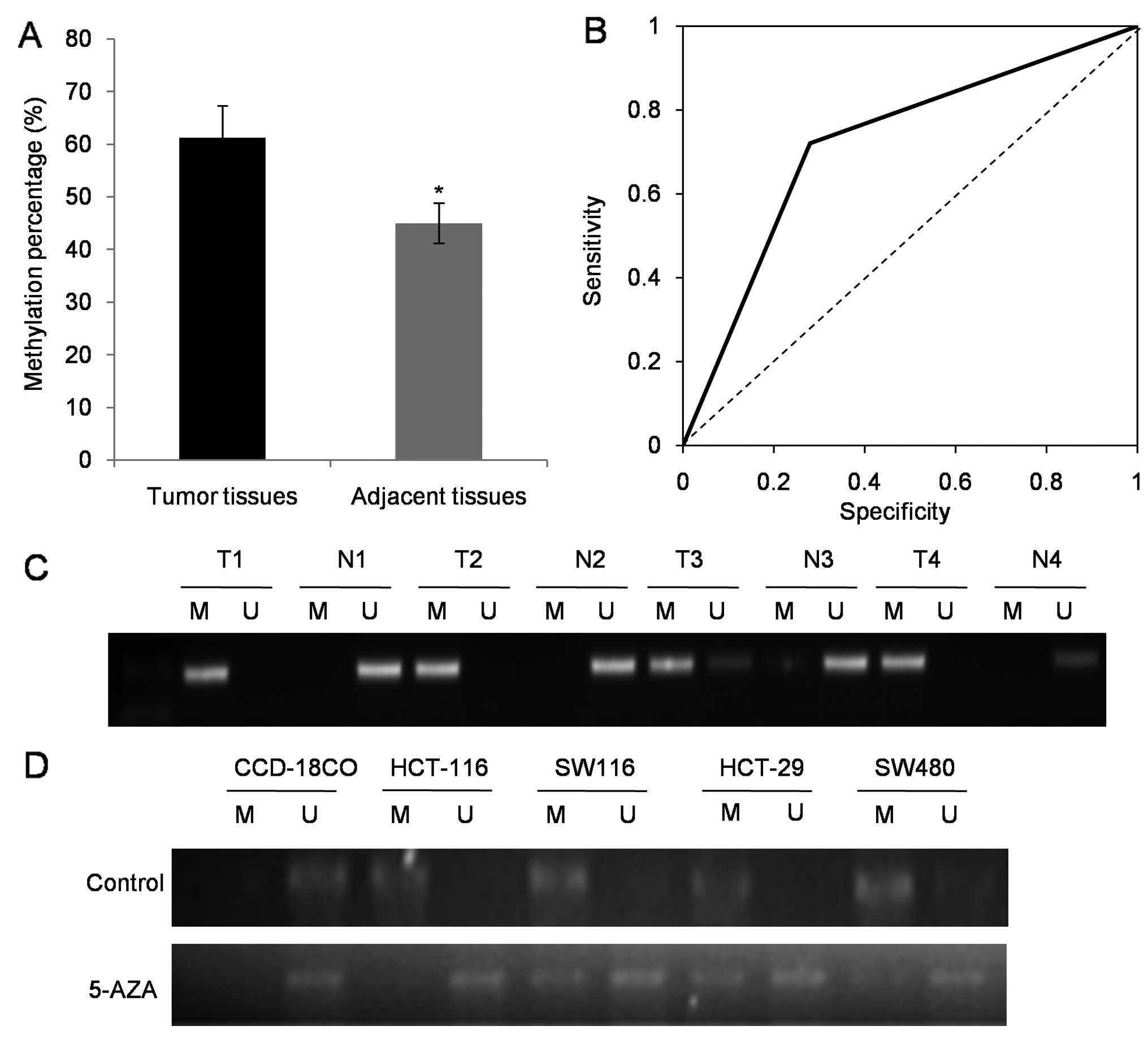
Compared to adjacent non-tumor tissues, our MSP
results indicated more frequent miR-181b epigenetic silencing in
the 97 tumor tissues (P<0.05) (Fig.
2A). Receiver operating characteristics and the area under the
curve (AUC) derived from receiver operating characteristics further
confirmed higher methylation of miR-181b in CRC than that of the
null hypothesis (P<0.05; AUC=0.72; 95% CI, 0.54–0.86) (Fig. 2B).
The randomly selected representative results of MSP
analysis in CRC tumor tissues and paired adjacent non-tumor tissues
are presented in Fig. 2C.
Furthermore, the universal methylated status in the four CRC cell
lines was detected. Following treatment with 5 nmol/ml 5-AZA,
significant demethylation occurred in all cell lines (Fig. 2D).
miR-181b inhibits colorectal cancer cell
proliferation
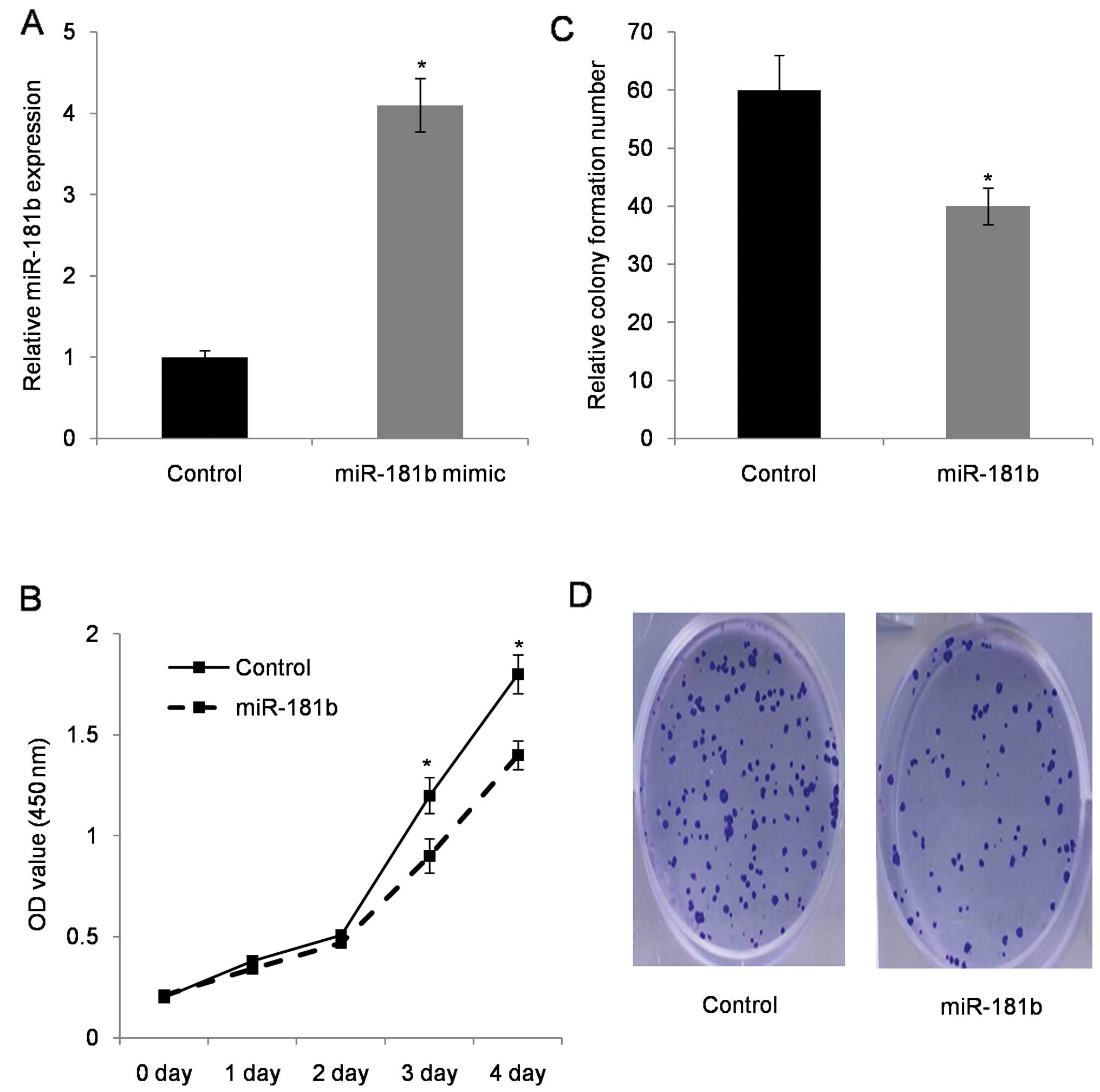
In the next cell functional study, miR-181b mimic
was introduced into cells. Transfected efficiency evaluation with
qRT-PCR revealed that miR-181b increased by >4-fold in miR-181b
transfected SW480 cells (Fig. 3A).
Furthermore, the MTT proliferation assay showed that cell growth
rate was reduced in miR-181b mimic-transfected SW480 cells when
compared with control cells (Fig.
3B). Colony formation assay provided visible evidence in which
miR-181b mimic-transfected SW480 cells had less colony formation
compared to the control parental cells (Fig. 3C).
miR-181b targets 3′UTR of the tumor
suppressor RASSF1A
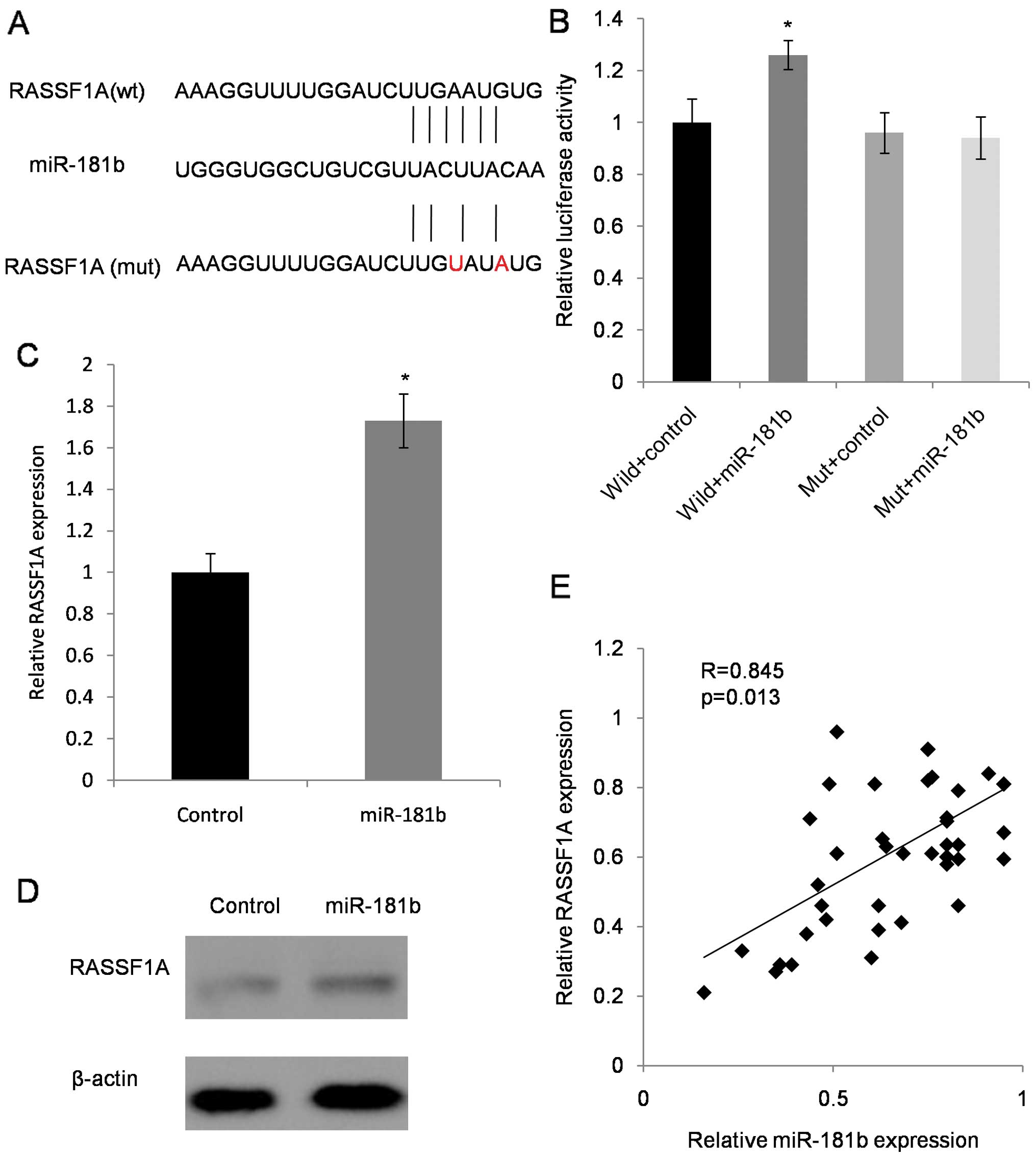
We searched miR-181b putative targets using online
databases (TargetScan, microRNA.org and PicTar). To validate this
interaction, we introduced a mutant 3′UTR fragment of RASSF1A in
our experiment (Fig. 4A). Results
showed that the relative luciferase activity of RASSF1A wild-type
was significantly elevated by miR-181b compared to RASSF1A mutation
type and control mimics (Fig. 4B).
The mRNA expression and protein levels of RASSF1A, after
transfecting SW480 cells with miR-181b, significantly increased
compared with control transfection (Fig. 4C and D). miR-181b and RASSF1A were
positively expressed in CRC cell lines and specimens. Fifty
patients were randomly chosen to evaluate the clinical relevance of
the expression of miR-181b and RASSF1A. We found that the
downregulated miR-181b was statistically correlated with decreased
expression of RASSF1A in the CRC tissues (Fig. 4E).
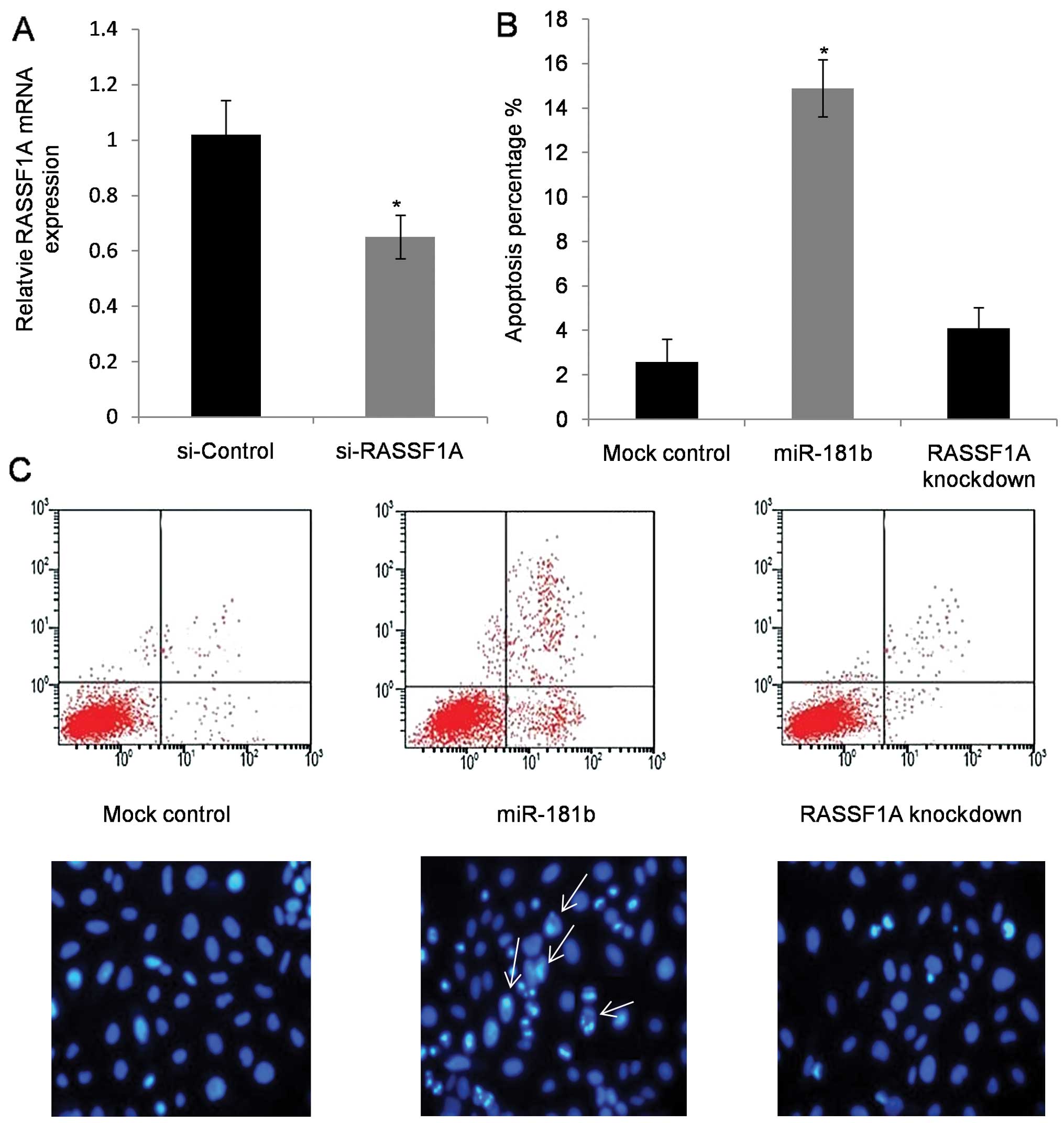
RASSF1A mediated miR-181b-induced
colorectal cancer cell apoptosis
To elucidate RASSF1A-mediated effects of miR-181b on
CRC cell apoptosis, RASSF1A knockdown cells were used (Fig. 5A). Flow cytometry was utilized to
examine the percentage of cell apoptosis in SW480 cells. As
expected, apoptotic percentage increased in cells with miR-181b
mimic treatment, which was evaluated by Annexin V staining, and no
significant apoptosis was observed in RASSF1A knockdown cells
(Fig. 5B and C). Morphology of the
Hoechst 33342 stained cells further confirmed the enhanced
apoptotic rate in miR-181b mimic-transfected cells (Fig. 5C).
Discussion
The present study found that downregulated miR-181b
was a frequent event in 97 human CRC samples. The statistical
association between lower expression of miR-181b and tumor stage
was further observed. Together, it suggested miR-181b may be
involved in colorectal cancer carcinogenesis and progression. Our
results found the methylation status of miR-181b CpG island to be
universal in colorectal cell lines and it is also responsible for
tightly regulating the miR-181b expression in tissues and cells.
This suggested that evaluation of methylation status in miR-181b is
a potential diagnostic marker for CRC. Furthermore, overexpression
of miR-181b induced apoptosis in CRC cells and cell growth
inhibition was observed. miR-181b achieved this regulation function
through direct targeting of the RASSF1A gene. The positive
correlation between miR-181b and RASSF1A in CRC tissue samples
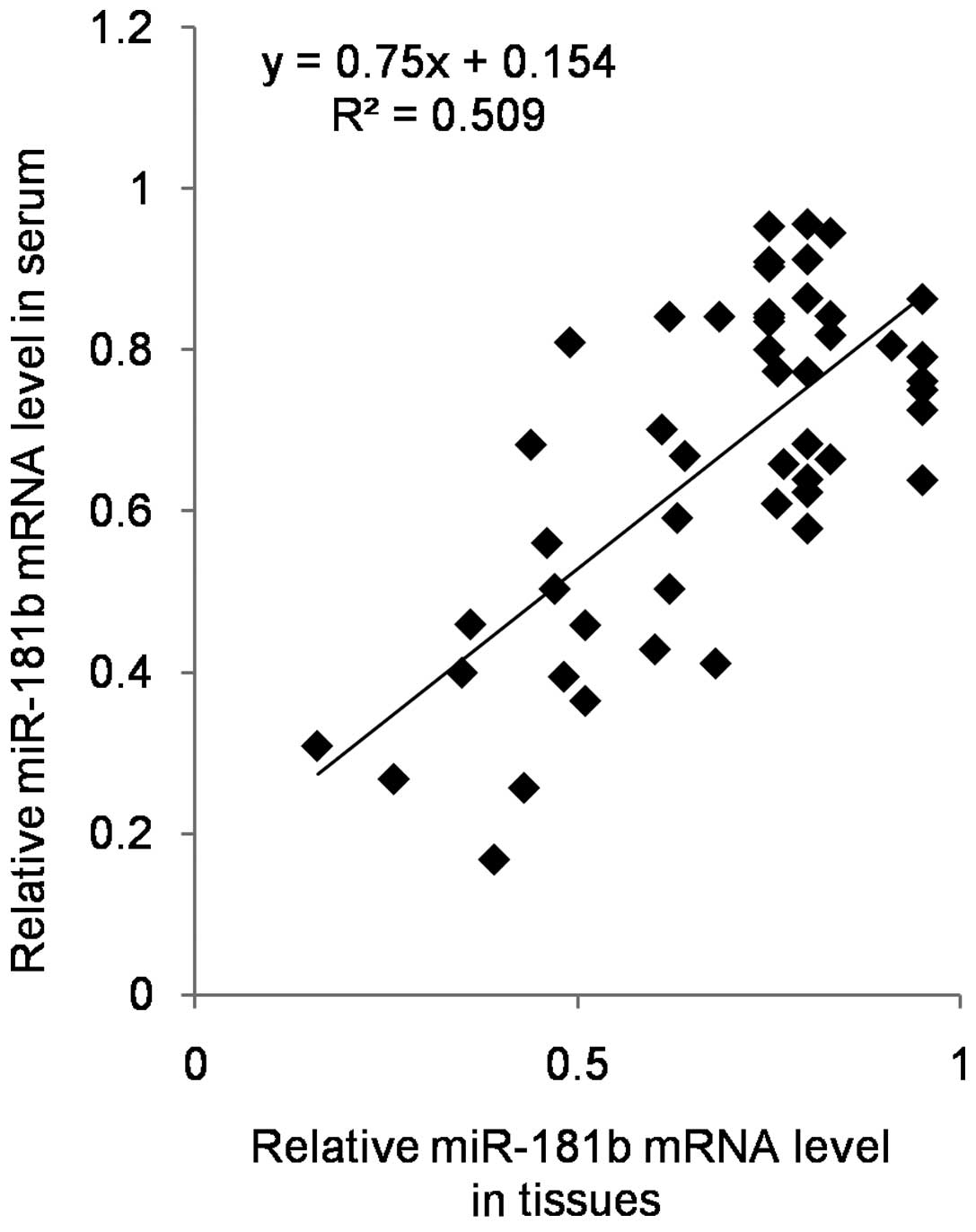
further validates this observation. Also, our results revealed the
significantly positive correlation between miR-181b in tissue and
circulating miR-181b (Fig. 6),
which suggested the potential biomarker role of miR-181b in
clinic.
Aberrant transcription of microRNAs in CRC is
diverse due to the complex regulation of miRNAs. Previous studies
reported overexpression of miRNAs such as miR-21, and let-7g in CRC
(9), while other studies provided
evidence for lower expression of miRNAs including miR-31 and
miR-92a (14). As for miR-181b,
both upregulation and downregulation was reported in CRC studies
(9,15,17).
The expression level of miR-181b was reported to correlate with
chemo-response in colorectal cancer and with poor survival in stage
III colorectal cancers (18–20).
While another miRNA study containing 113 CRC cases and 89 controls,
declared that the lower expression of miR-181b can be used as a
noninvasive biomarker for the diagnosis of CRC with relatively high
sensitivity and specificity (9).
The present study found downregulated miR-181b in 97 human CRC
samples. The different results may be explained by the diversity in
the characteristics of various types of cancers, polymorphism in
miRNA coding region or binding sites.
Epigenetic mechanisms are considered to be
responsible for aberrant microRNA expression levels (21). Multiple studies have reported the
frequency of hypermethylation of miRNAs in colon tumors and cell
lines such as let-7, miR-34, miR-342, miR-345, miR-9, miR-129 and
miR-137 (22). However, no study
reported methylation of miR-181b in colorectal cancer. Our results
indicate that the methylation status of miR-181b was universal in
colorectal cell lines and this methylation is thought to lead to
reduced expression in tissues and cells.
It is commonly accepted that microRNAs can
contribute to global epigenetic regulation in CRC (23–25).
For example, miR-143 is found to be a tumor suppressor which
directly targets DNA methyltransferase 3A (DNMT3A) and leads to
decreased DNMT3A in CRC tissues (26). This study chose RASSF1A as a target
candidate according to the widely used miRNA target predication
databases (27,28). The binding effect between RASSF1A
and miR-181b was confirmed by the luciferase reporter. Further
knockdown experiment revealed that RASSF1A mediated miR-181b
induced apoptosis in CRC cell lines. Their positive correlation was
also validated in tissue samples. RASSF1A is a well-known tumor
suppressor and downregulated expression of RASSF1A has been
reported in CRC (29–31). The association outcomes observed
between miR-181b and RASSF1A mRNA expression indicated that
exogenous miR-181b successfully stimulated RASS1A transcription
level and subsequently contributed to tumor suppression. Our result
therefore obtained a detailed explanation with regards to elevated
cell apoptosis following miR-181b mimic stimulation and further
provide evidence that miRNA-target interactions primarily
influenced the target mRNA translation efficiency.
In general, frequent hypermethylation at the
promoter region of miR-181b mainly contributes to the lower
expression of miR-181b and advanced clinical stage in colorectal
cancer tissues. Functionally, overexpression of miR-181b influences
tumorigenicity of CRC cells in multiple levels by directly binding
to tumor suppressor RASSF1A and stimulating its expression. This
study provides evidence for the clinical significance of miR-181b
methylation status as diagnostic biomarker and potential target in
colorectal cancer treatment.
Acknowledgements
We wish to acknowledge the evaluators, research
assistants, and particularly the patients and families who
participated in this study.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah R, Jones E, Vidart V, Kuppen PJ,
Conti JA and Francis NK: Biomarkers for early detection of
colorectal cancer and polyps: Systematic review. Cancer Epidemiol
Biomarkers Prev. 23:1712–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jimenez CR, Knol JC, Meijer GA and
Fijneman RJ: Proteomics of colorectal cancer: Overview of discovery
studies and identification of commonly identified cancer-associated
proteins and candidate CRC serum markers. J Proteomics.
73:1873–1895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xuan Y, Yang H, Zhao L, Lau WB, Lau B, Ren
N, Hu Y, Yi T, Zhao X, Zhou S, et al: MicroRNAs in colorectal
cancer: Small molecules with big functions. Cancer Lett.
360:89–105. 2015. View Article : Google Scholar
|
|
6
|
Yang X, Zhong J, Ji Y, Li J, Jian Y, Zhang
J and Yang W: The expression and clinical significance of microRNAs
in colorectal cancer detecting. Tumour Biol. 36:2675–2684. 2015.
View Article : Google Scholar
|
|
7
|
Amankwatia EB, Chakravarty P, Carey FA,
Weidlich S, Steele RJ, Munro AJ, Wolf CR and Smith G: MicroRNA-224
is associated with colorectal cancer progression and response to
5-fluorouracil-based chemotherapy by KRAS-dependent and
-independent mechanisms. Br J Cancer. 112:1480–1490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taniguchi K, Sugito N, Kumazaki M,
Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama
K, et al: MicroRNA-124 inhibits cancer cell growth through
PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett.
363:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Huang SK, Zhao M, Yang M, Zhong
JL, Gu YY, Peng H, Che YQ and Huang CZ: Identification of a
circulating microRNA signature for colorectal cancer detection.
PLoS One. 9:e874512014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng W, Tu Y, Zhu Y, Wang Z, Li C, Lao L
and Wu G: Predictive power of circulating miRNAs in detecting
colorectal cancer. Tumour Biol. 36:2559–2567. 2015. View Article : Google Scholar
|
|
11
|
Vaish V, Khare T, Verma M and Khare S:
Epigenetic therapy for colorectal cancer. Methods Mol Biol.
1238:771–782. 2015. View Article : Google Scholar
|
|
12
|
Dassow H and Aigner A: MicroRNAs (miRNAs)
in colorectal cancer: From aberrant expression towards therapy.
Curr Pharm Des. 19:1242–1252. 2013.PubMed/NCBI
|
|
13
|
Ma X, Becker Buscaglia LE, Barker JR and
Li Y: MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 3:159–166.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun YC, Wang J, Guo CC, Sai K, Wang J,
Chen FR, Yang QY, Chen YS, Wang J, To TS, et al: MiR-181b
sensitizes glioma cells to teniposide by targeting MDM2. BMC
Cancer. 14:6112014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bovell LC, Shanmugam C, Putcha BD,
Katkoori VR, Zhang B, Bae S, Singh KP, Grizzle WE and Manne U: The
prognostic value of microRNAs varies with patient race/ethnicity
and stage of colorectal cancer. Clin Cancer Res. 19:3955–3965.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk
ML and Struhl K: STAT3 activation of miR-21 and miR-181b-1 via PTEN
and CYLD are part of the epigenetic switch linking inflammation to
cancer. Mol Cell. 39:493–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakajima G, Hayashi K, Xi Y, Kudo K,
Uchida K, Takasaki K, Yamamoto M and Ju J: Non-coding MicroRNAs
hsa-let-7g and hsa-miR-181b are Associated with Chemoresponse to
S-1 in Colon Cancer. Cancer Genomics Proteomics. 3:317–324.
2006.
|
|
18
|
Xi Y, Formentini A, Chien M, Weir DB,
Russo JJ and Ju J, Kornmann M and Ju J: Prognostic values of
microRNAs in colorectal cancer. Biomark Insights. 2:113–121.
2006.
|
|
19
|
Xu R, Ma N, Wang F, Ma L, Chen R, Chen R,
Kebinu M, Ma L, Han Z, Ayixiamu, et al: Results of a randomized and
controlled clinical trial evaluating the efficacy and safety of
combination therapy with Endostar and S-1 combined with oxaliplatin
in advanced gastric cancer. Onco Targets Ther. 6:925–929.
2013.PubMed/NCBI
|
|
20
|
Watanabe K, Kawahara H, Enomoto H, Toyama
Y, Akiba T and Yanaga K: Feasibility study of oxaliplatin with oral
S-1 or capecitabine as first-line therapy for patients with
metastases from colorectal cancer. Anticancer Res. 33:4029–4032.
2013.PubMed/NCBI
|
|
21
|
Jordà M and Peinado MA: Methods for DNA
methylation analysis and applications in colon cancer. Mutat Res.
693:84–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gyparaki MT, Basdra EK and Papavassiliou
AG: DNA methylation biomarkers as diagnostic and prognostic tools
in colorectal cancer. J Mol Med Berl. 91:1249–1256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
24
|
Kaur S, Lotsari JE, Al-Sohaily S,
Warusavitarne J, Kohonen-Corish MR and Peltomäki P: Identification
of subgroup-specific miRNA patterns by epigenetic profiling of
sporadic and Lynch syndrome-associated colorectal and endometrial
carcinoma. Clin Epigenetics. 7:202015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Fu J, Jiang M, Zhang X, Cheng L,
Xu X, Fan Z, Zhang J, Ye Q and Song H: MiR-410 is overexpressed in
liver and colorectal tumors and enhances tumor cell growth by
silencing FHL1 via a direct/indirect mechanism. PLoS One.
9:e1087082014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slattery ML, Wolff E, Hoffman MD, Pellatt
DF, Milash B and Wolff RK: MicroRNAs and colon and rectal cancer:
Differential expression by tumor location and subtype. Genes
Chromosomes Cancer. 50:196–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peterson SM, Thompson JA, Ufkin ML,
Sathyanarayana P, Liaw L and Congdon CB: Common features of
microRNA target prediction tools. Front Genet. 5:232014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vlachos IS and Hatzigeorgiou AG: Online
resources for miRNA analysis. Clin Biochem. 46:879–900. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fernandes MS, Carneiro F, Oliveira C and
Seruca R: Colorectal cancer and RASSF family - a special emphasis
on RASSF1A. Int J Cancer. 132:251–258. 2013. View Article : Google Scholar
|
|
30
|
Richter AM, Pfeifer GP and Dammann RH: The
RASSF proteins in cancer; from epigenetic silencing to functional
characterization. Biochim Biophys Acta. 1796:114–128.
2009.PubMed/NCBI
|
|
31
|
Volodko N, Gordon M, Salla M, Ghazaleh HA
and Baksh S: RASSF tumor suppressor gene family: Biological
functions and regulation. FEBS Lett. 588:2671–2684. 2014.
View Article : Google Scholar : PubMed/NCBI
|