Introduction
Breast cancer is one of the leading causes of cancer
deaths among women worldwide (1).
Even with all the attention towards prevention and latest advances
in screening, diagnosis, treatment modalities and methods to
follow-up, 1 out of 8 US women will have invasive breast cancer,
either at the time of diagnosis or developed during disease
progression (2,3). Particularly interesting is the
diversity and complexity with which advanced breast cancer occurs
(4). The intricate variety is not
only related to the distinct breast cancer subtypes but is even
present within a single subtype. Specifically, triple-negative
breast cancers (TNBC), represent a major challenge. The treatment
options for this breast cancer subtype, which is classified as
estrogen receptor (ER) and progesterone receptor (PR) negative and
human epidermal growth factor 2 (HER2) negative, are extremely
limited, because of the absence of specific targets and the
heterogeneity with which TNBC presents itself (5). While standard systemic chemotherapy
may have some positive impact and lead to the eradication of the
disease, patients with recurrent disease or other TNBC patients for
which this therapy is not effective, may experience an aggressive
cancer type and a high incidence of metastases, resulting in
overall poor patient outcomes with high mortality (5–7).
Over the years, TNBC has been characterized by high
cell proliferation, poor cellular differentiation, several
recurrent gene copy number imbalances, and in numerous cases
mutations in the p53 tumor suppressor gene and BRCA-1 (8). In addition, more recent studies
aiming at the discovery of effective therapies for TNBC, indicated
specific roles for poly(ADP-ribose) polymerase-1 (PARP), mainly
against BRCA-mutant TNBC cancers (9), and other molecular targets involved
in growth factor receptor signaling, such as the insulin-like
growth factor receptor (IGFR), epidermal growth factor receptor
(EGFR), vascular endothelial growth factor-α (VEGF-α) and
non-receptor tyrosine kinases src, phosphoinositide 3-kinase (PI3K)
and Akt as well as mTOR. While many of these signal transducers
play indeed a role in growth factor receptor-mediated signaling,
potential roles for these signal transducers in stimulating
metastases are described as well (10,11).
From the current scientific literature, it is suggested that the
heterogeneity of TNBC is characterized by the activation of
distinct signaling pathways and is supported by suggestions,
concerning the changes occurring in biological characteristics in
primary tumors as compared to their metastatic counterparts. The
latter has been ascribed as a consequence of progressed disease or
exposure to treatment (12).
Although new avenues have been opened there is a
lack of effective targeted therapies for TNBC breast cancer
patients. Therefore, an improved and profound understanding of the
mechanisms underlying the metastatic and aggressive nature of TNBC,
and this at all levels of the invasion-associated cellular
behaviors are needed. This will aid the identification of new
biomarkers for diagnosis as well as molecular targets for the
development of novel and better targeted therapeutic strategies
that can reduce the number of deaths related to this cancer
subtype, specifically since TNBC is known to affect a younger age
group in addition to specific ethnic groups (13). A drawback of the current trend in
research studies and clinical trials, and especially in TNBC
studies, is the fact that these studies are specifically designed
for the overall TNBC patient population, using a variety of cell
lines or patient samples. Results are often disappointing and
suggest an overall negative effect, which may not always reflect
the usefulness of these identified targets given the heterogenic
character of TNBC, and rather underscore the value of the new
findings for a subset of patients, with particular TNBC
characteristics (14).
To address this problem, this study explores the
changes in invasion-associated cellular events in the Hs578T TN
breast cancer cell line and its more invasive subclone
Hs578Ts(i)8, representing an in vitro cell system
of TNBC breast cancer progression which embodies an elegant
experimental model for studying the metastatic nature of TNBC
(15) or at least may provide
valuable insight on novel predictive biomarkers and therapeutic
targets for a subset of TNBC patients. Moreover, with the movement
towards personalized therapies, biochemically-oriented
comprehensive approaches linking active targets or full aberrant
signaling pathways, which do not always signify mutations or
amplifications (16), and their
(sub)cellular localization to biological functions may propose new
directions for classification of metastatic TNBC and consequently
may suggest new targeted therapeutic approaches.
Signal transduction molecules, such as the
cytoplasmic non-receptor tyrosine kinases (NRTK), focal adhesion
kinase (FAK) and src have been found to play an important role in
tumorigenesis. Increased catalytic activity of FAK and src has been
observed in numerous invasive and metastatic tissues, such as
breast, prostate and thyroid cancers and in TNBC as well as in TNBC
cell lines (17,18). FAK is linked to signaling events
between cells and the extracellular matrix (ECM), thereby affecting
cell-adhesion, cell motility and invasion (19) and may be an important prognostic
biomarker and therapeutic target for metastatic TNBC tumors.
In this study, we investigated differences in
invasion-associated cellular activities, as cell-matrix adhesion
and migration, between the mesenchymal-like Hs578T TNBC cell line,
which is considered moderately aggressive, and the more invasive
and isogenic Hs578Ts(i)8 cells. The invasive
Hs578Ts(i)8 cells demonstrated important
invasion-associated cellular changes as compared to the parental
Hs578T cells. The cellular behavior could be correlated to the
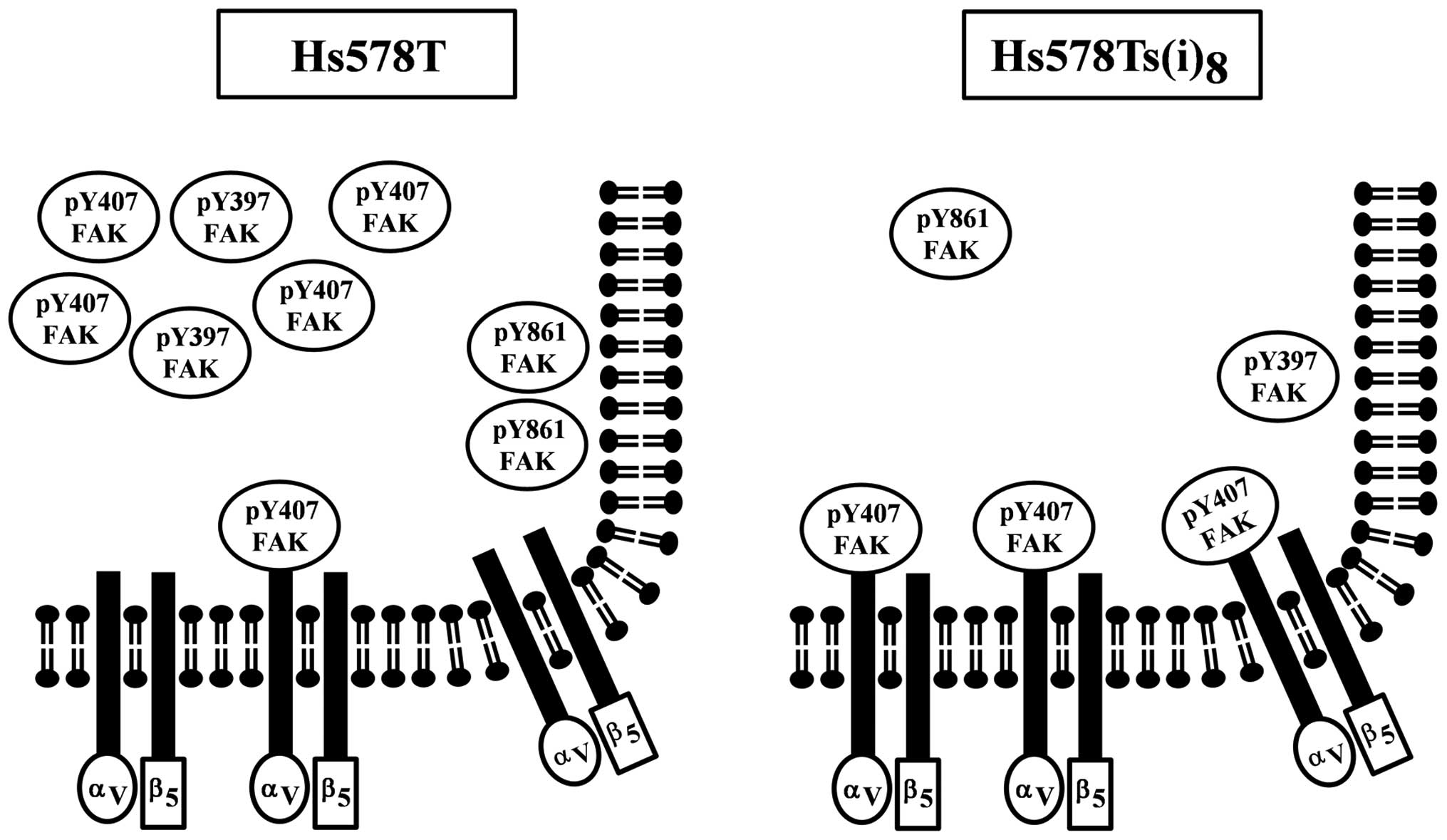
organization of integrin αVβ5 receptors in the cell membrane with
FAK Y407. Our results revealed an unusual activation pattern, yet a
common activation pattern when taking into account the dynamic
spatio-temporal organization.
Materials and methods
Antibodies and other reagents
Antibodies directed against the distinct tyrosine
phosphorylation sites of FAK (Y397, Y407, Y576, Y861, and Y925),
src (Y416, Y527), p-JNK, p-paxillin and p-rac as well as anti-FAK,
anti-src, non-phospho-src (Y416 and Y527), anti-rac, anti-JNK and
anti-paxillin and anti-β-actin antibodies were from Cell Signaling
Technology as well as the mouse and rabbit monoclonal IgG isotype,
Alexa Fluor® 555 Phalloidin and Alexa Fluor®
antibodies (Danvers, MA, USA). The antibodies against the different
integrin subunits and receptors were from EMD Millipore (Billerica,
MA, USA). Secondary biotinylated anti-rabbit and anti-mouse,
FITC-labeled anti-mouse and FITC-labeled anti-rabbit secondary
antibodies and Vectashield mounting medium were obtained from
Vector Laboratories (Burlingame, CA, USA). Antibodies against
serine phosphorylation sites of FAK (S732 and S910), anti-mouse and
anti-rabbit alkaline phosphatase-labeled secondary antibodies and
the BCA protein assay reagent kit were from ThermoFisher Scientific
(Waltham, MA, USA).
Cell culture
The human mesenchymal breast cancer Hs578T cells and
the derivative cell line Hs578Ts(i)8 were a kind gift
from Dr S. McDonnell (UCD School of Chemical and Bioprocess
Engineering, University College Dublin, Ireland) (15) and were grown in DMEM-medium
supplemented with 10% (v/v) FBS, 100 IU/ml penicillin, 100 μg/ml
streptomycin and 0.1 U/ml bovine insulin (Thermo Fisher Scientific)
at 37°C equilibrated with 5% (v/v) CO2 in humidified
air. The breast cancer cells used in this study were frozen in
liquid nitrogen when not in use and were not passaged in our
laboratory for >10 weeks.
Cell adhesion assay
Cells were detached with 0.2% (w/v) EDTA, to avoid
proteolytic degradation of cell surface proteins, and washed with
serum-free medium. Next, 5×105 cells were resuspended in
1 ml DMEM supplemented with 2% (v/v) FBS. Cell suspensions (100
μl), in the presence or absence of function blocking antibodies
(5–10 μg/ml) were added to collagen I, collagen IV, fibronectin and
laminin precoated 96-well plates (BD Biosciences, San Jose, CA,
USA), and centrifuged for 1 min at 115 g. After 90-min incubation
at 37°C, wells were washed four times with PBS to remove
non-adherent cells. The adherent cells were then detected and
quantified by measuring the acid phosphatase activity, through
solubilization of the remaining cells with 0.2% Triton X-100 and by
the addition of the substrate, PNPP (p-nitrophenyl
phosphate; Sigma, St. Louis, MO, USA). Absorbance values of the
lysates were determined on a microplate reader at 405 nm (Biotek,
Synergy H1, Winooski, VT, USA) and expressed as relative absorbance
(%). Mouse and rabbit IgG isotype control antibodies were included
to estimate the non-specific binding.
Wound healing assay
Cells were grown in 24-well plates until confluency
and washed twice with PBS. A scratch was made using a P-200 pipette
tip and 3 ml of medium in the presence or absence of function
blocking integrin antibodies (5–10 μg/ml) was added. Cell migration
was monitored and images were collected, after 10 and 17 h, with a
Leica DMIL microscope with a CCD camera (Leica, Buffalo Grove, IL,
USA). Leica software was used to estimate the cell free area of the
wounds. The distances over which the cells migrated were measured
and expressed as migratory velocity (μm/h) or as % compared to
control Hs578T cells.
Flow cytometry analysis
Cells were detached with 0.2% (w/v) EDTA, to avoid
proteolytic degradation of cell surface proteins, neutralized with
cold PBS and resuspended in PBS containing 0.1% (w/v) BSA (Sigma).
Next, 2.5×105 cells were incubated with the relevant
primary antibodies for 90 min at 4°C, followed by secondary FITC or
Alexa Fluor®-labeled antibodies for 45 min at 4°C. After
washing, 1×104 stained cells were analyzed for
fluorescence using the Cell Lab Quanta™ SC MPL (Beckman Coulter,
Miami, FL, USA) and BD FACSCalibur™ (BD Biosciences). Stainings
without primary antibodies and IgG isotype antibodies were used as
controls.
Western blotting
Cell lysates were made from 80 to 90% confluent
cultures using 0.5 ml lysis buffer containing 1% (v/v) Triton
X-100, 1% (v/v) Nonidet P-40 substitute (Roche, Indianapolis, IN,
USA) and the following inhibitors: aprotinin (10 μg/ml), leupeptin
(10 μg/ml), PMSF (1.72 mM), NaF (1 mM), NaVO3 (500 μM),
and Na4P2O7 (500 μg/ml) (Sigma).
Aliquots of lysates, containing 30 μg of protein, were boiled for 5
min in SDS-PAGE sample buffer containing 5% (v/v)
β-mercaptoethanol, electrophoresed on 7.5 or 10% TGXTM
precast gels and transferred to PVDF membranes (Bio-Rad
Laboratories, Hercules, CA, USA). After transfer, membranes were
blocked and incubated with relevant primary antibodies followed by
incubation with secondary biotinylated antibodies and developed by
using an ECL (Vectastain ABC-AmP) detection kit. Some of the
membranes were stripped at 50°C for 30 min in 100 mM
β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl (pH 6.8), and reblotted
with appropriate antibodies, for control of equal loading.
Membranes were imaged on the BioChemi System and analysis software
(UVP, Upland, CA, USA). Alternatively, the proteins of interest
were detected using alkaline phosphatase-labeled secondary
antibodies and developed using NBT/BCIP substrate (Roche, 1:50 in
0.1 M Tris-HCl, 0.05 M MgCl2 and 0.1 M NaCl at pH
9.5).
Fluorescence immunostaining
Cells were grown on glass cover slips (diameter, 12
mm) placed in 24-well plates. The glass cover slips were removed,
washed and fixed with ice-cold methanol or 4% paraformaldehyde
(EMS, Hatfield, PA, USA). Next, fixed cells were washed, blocked
and incubated with Alexa Fluor® 555 Phalloidin or
primary antibodies, followed by incubation with Alexa
Fluor®-labeled secondary antibodies or for intracellular
staining, prior to the antibody incubation, cells were treated with
Triton X-100 (2%). Stained cells were mounted with Vectashield
mounting medium and images were acquired using a Leica DMI 3000B
fluorescence microscope and a DFC310 FX digital color camera
(Leica). Control stainings were performed without primary
antibodies and with IgG isotype control antibodies to measure
possible cross-reaction or non-specific binding.
Co-immunoprecipitation of FAK Y407
Cells at 80–90% confluency were lysed, as described
in ‘Western blotting’. Lysates, containing 1,000–1,500 μg
protein, were mixed with protein G-Sepharose beads (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) to preclear non-specific
binding. Site-specific p-FAK Y407 antibody (1:500) was added to the
collected supernatant and rotated at 4°C overnight. Subsequently,
protein G-Sepharose beads were added to recover the
immunocomplexes. The immunoprecipitates were resolved in 150 μl
SDS-PAGE sample buffer and heated to 95°C for 5 min. The
supernatants were then electrophoresed on 7.5 or 10%
TGXTM precast gels and transferred to PVDF membranes.
After transfer, the membranes were analyzed and developed as
described in ‘Western blotting’.
Statistics
All treatments were matched and carried out at least
five times. Data were analyzed using Excel, for determination of
mean, SD, and Student's t-test (95%). Intensity of the
immunoblotted bands was quantified by densitometry, using
statistical software Scion image (Scion Corp., Frederick, MD,
USA).
Results
Differences in cell-matrix adhesive and
migratory behavior
The group of S. McDonnell established this isogenic
Hs578T/Hs578Ts(i)8 highly invasive triple-negative
breast cancer model. Such a matched breast cancer cell line pair,
with the same genetic background, is a unique preclinical model and
allows for the investigation of the underlying mechanisms involved
in the tendency these cells have to form metastasis, in this
subtype of breast cancer. Their previous study showed that the
Hs578Ts(i)8 cell line is 3-fold more invasive and
2.5-fold more migratory in the BD Matrigel™ invasion chamber assay
than the parental Hs578T cells and produces tumors in mice
(15). Here, we show additional
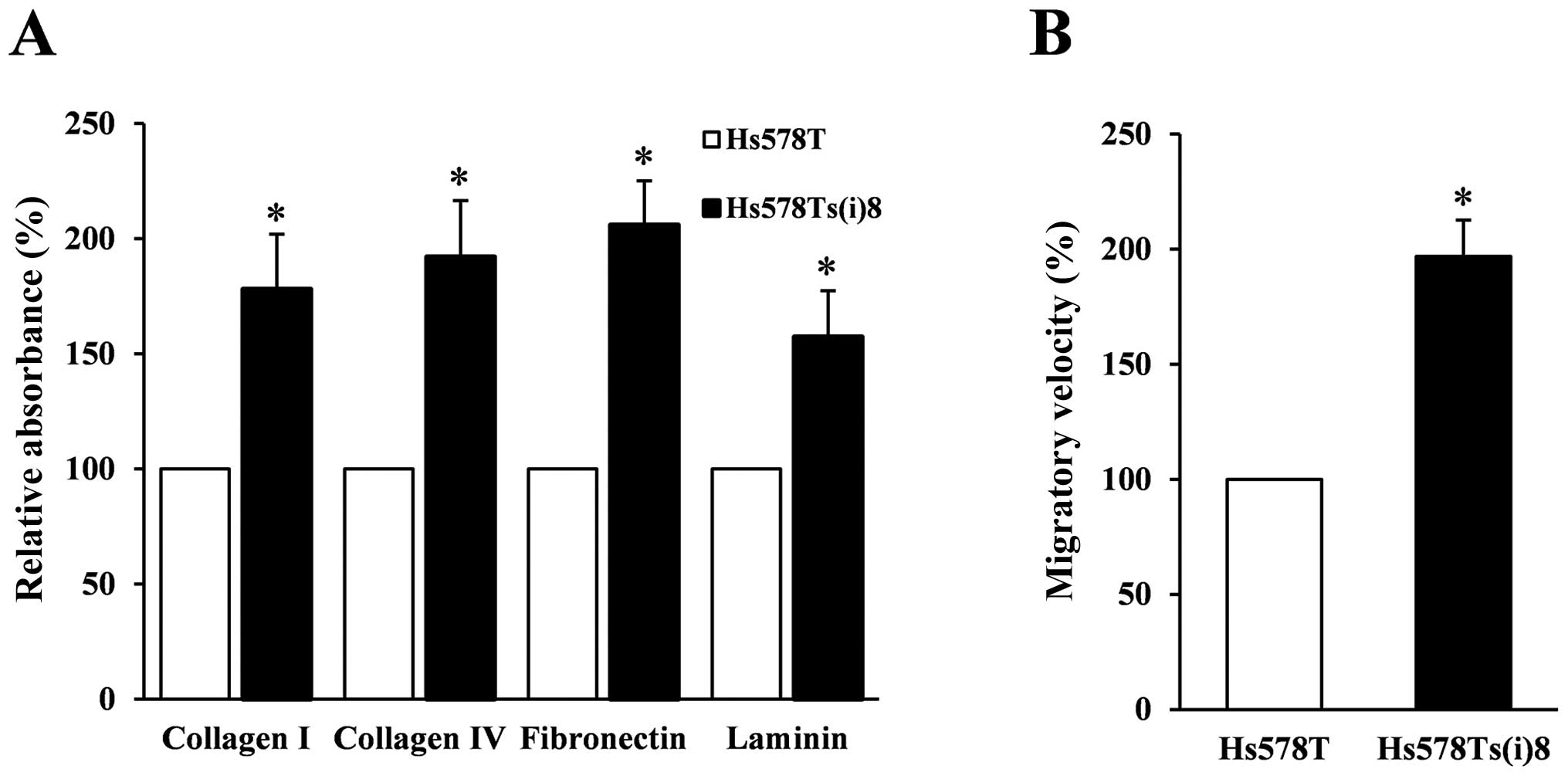
differences in invasion-associated cellular behaviors. Fig. 1A illustrates that the invasive
subclone, Hs578Ts(i)8, interacts significantly more with
several extracellular matrices, including collagen I and IV,
fibronectin and laminin. Furthermore, Fig. 1B displays results of wound-healing
assays under normal growth conditions, and reveals that the
Hs578Ts(i)8 migrate twice as fast compared to the
parental Hs578T cells which is in line with the previously
published increased migratory capacity of the more invasive
Hs578Ts(i)8 cell line (15). Overall, these results indicate
noteworthy differences between the two cell lines related to
increased metastatic behavior and provide a solid basis for further
investigation into the underlying mechanisms.
Identification of mediators of
cell-matrix adhesion and migration
To identify the mediators involved in the observed
increased adhesion to extracellular matrix proteins and migratory
capacity of the Hs578Ts(i)8 cells, we analyzed the
potential roles of integrins, the major class of cell-matrix
adhesion molecules mediating cell-matrix adhesion and migration.
Using a panel of available integrin function blocking antibodies,
at concentrations suggested by the manufacturer, ranging from 5 to
10 μg/ml, we found that the antibody against the integrin α5β1
receptor was the sole antibody significantly inhibiting the
adhesion of Hs578Ts(i)8 cells to fibronectin (Fig. 2A), while little or no reduced
effect was observed when using other function blocking integrin
antibodies in adhesion experiments using collagen I and IV or
laminin (data not shown). Furthermore, the integrin αVβ5 and α5β1
antibodies, where found to reduce the migratory velocity of
Hs578Ts(i)8 cells by almost 50 and 40%, respectively,
while no such effect was observed on the parental Hs578T cells
(Fig. 2B). Next, we examined the
cell surface and total expression levels of the identified integrin
receptors by western blotting and flow cytometry. Fig. 2C shows the cell surface expression
levels of the α5β1 and αVβ5 integrin receptors, detected using a
Cell Lab Quanta™, and confirmed on a BD FACSCalibur™. A slight
decrease of α5β1 integrin receptors at the cell surface of the more
invasive subclone Hs578Ts(i)8 was found, while no
significant differences were observed for the integrin αVβ5
receptor at the surface of the two cell lines. The total expression
level experiments, with quantification, displayed in Fig. 2D, reveal that the expression levels
of the integrin α5 and β1 subunits, as well as the α5β1 integrin
receptor are significantly reduced in the invasive subclone as
compared to the expression levels in the parental Hs578T cells,
whereas no significant differences were found for the total
expression levels of the integrin αV and β5 subunits and integrin
αVβ5 receptors.
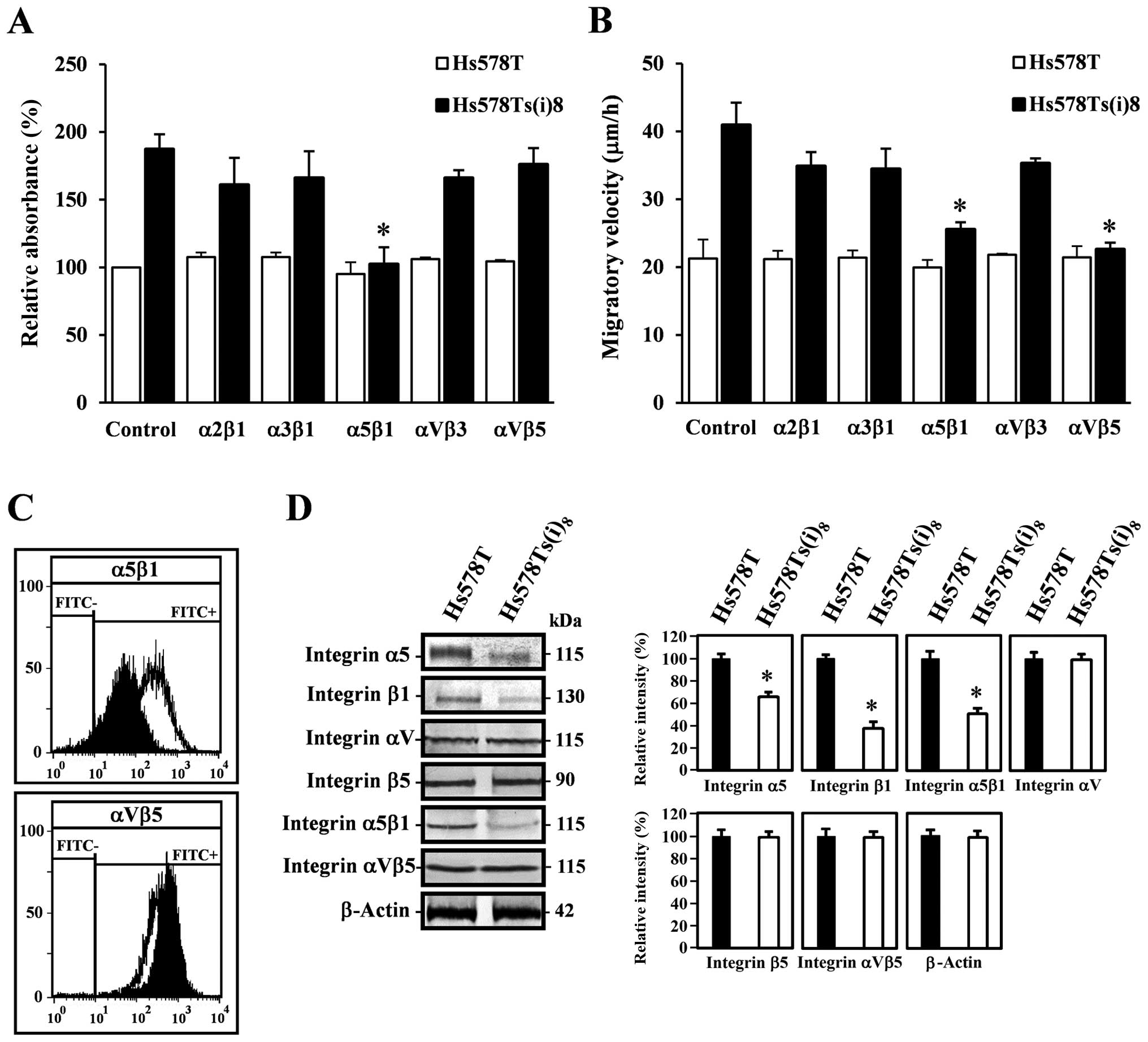 | Figure 2Integrins mediate cell-matrix
adhesion and migration of Hs578Ts(i)8 cells. (A)
Blocking the α5β1 integrin receptor in Hs578T (open bars) and
Hs578Ts(i)8 cells (closed bars), reduced the binding of
Hs578Ts(i)8 to fibronectin. 104 cells/100 μl
were seeded in fibronectin precoated 96-well plates, in the
presence (5–10 μg/ml) or absence of integrin function blocking
antibodies and incubated for 90 min. The adherent cells were washed
and determined by measuring acid phosphatase activities. The
adhesion of these cells to fibronectin is represented as relative
absorbance (%), after subtracting non-specific binding. Mouse IgG
isotype control antibodies were included to estimate non-specific
binding. Eight wells were analyzed for each condition, in each
experiment and compared to Hs578T cells, under similar conditions.
(B) Migratory differences between Hs578T and Hs578Ts(i)8
cells. Cells grown in 24-well plates until confluency, were wounded
and allowed to grow in the absence or presence of function blocking
integrin (5–10 μg/ml) antibodies. After 10 and 17 h, the distances
over which the cells migrated were measured and results are
expressed as migratory velocity (μm/h) as compared to the
respective Hs578T and Hs578Ts(i)8 cells, and the
particular conditions. Asterisks in (A) and (B) indicate
statistical difference from control Hs578Ts(i)8 cells
(p<0.05). (C) Cell surface expression levels of integrin α5β1
(upper panel) and αVβ5 receptors (lower panel) in Hs578T (open
area) and Hs578Ts(i)8 cells (closed area). Single cell
suspensions of Hs578T and Hs578Ts(i)8 cells were
incubated with relevant primary integrin receptor antibodies,
followed by FITC-labeled secondary antibodies and analyzed on the
Cell Lab Quanta™ or BD FACSCalibur™. Control stainings were
performed without primary antibody or with mouse IgG isotype
control antibodies. (D) Western blot analysis for total expression
levels of integrin α5, β1, αV and β5 subunits and α5β1 and αVβ5
receptors in Hs578T and Hs578Ts(i)8 cells. Cell lysates,
containing 30 μg protein, were analyzed by 7.5% TGX™ precast gels,
transferred to PVDF membranes and blotted with the corresponding
primary antibodies (left panel). Scion image densitometry analysis
of bands comparing the expression levels of the subunits or
receptors in Hs578Ts(i)8 cells against the control
Hs578T cells (right panel). β-actin was used as a loading control.
Bar graphs are means ± SD from at least five independent
experiments. Asterisks indicate statistical difference from control
Hs578T cells (p<0.05). |
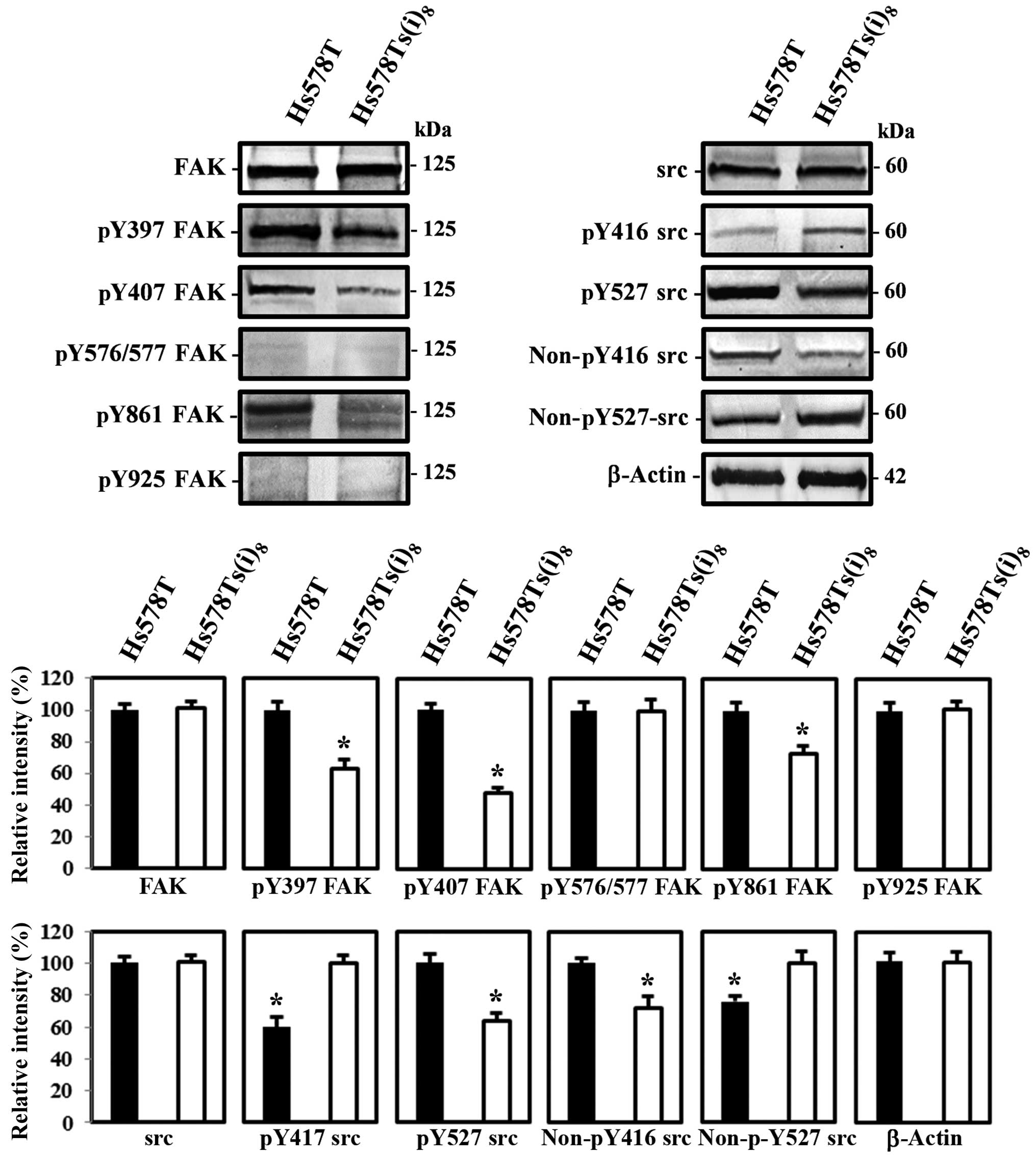
Phosphorylation of FAK and its additional
sites
It is well known that integrin receptor engagement
results in the activation of FAK. FAK becomes phosphorylated at
Y397 upon integrin ligation. Subsequent binding of FAK to src leads
to the formation of an active and transient signaling complex,
which promotes further FAK kinase activity and signaling through
phosphorylations on several tyrosine sites, including Y407 near the
N-terminal FERM (band 4.1-ezrin-radixin-moesin) domain, the kinase
domain activation loop (Y576 and Y577), as well as phosphorylation
of FAK at the C-terminal domain residues, Y861 and Y925.
Subsequently, this results in the activation of downstream
signaling events (17). Moreover,
recent studies have shown upregulated FAK and src expression as
well as FAK Y397 phosphorylation in tumor samples of patients with
advanced breast cancer, including triple-negative breast cancer
(18). We therefore examined and
compared the total expression and phosphorylation levels of FAK and
src in Hs578T and Hs578Ts(i)8 cells by western blotting.
Fig. 3 demonstrates that both cell
lines express similar protein levels of FAK and src, while
differences were found in levels of phosphorylated FAK at tyrosine
residues Y397, Y407 and Y861 and src Y416, but not at FAK Y576-577
and Y925. Interestingly, we observed a pronounced increase in FAK
Y397, Y407 and Y861 activation in the parental Hs578T cell line,
while it was expected in the more invasive Hs578Ts(i)8
cells, as increased FAK activation correlates with advanced stages
of breast cancer (18). The src
phosphorylation studies on the other hand, showed increased
phosphorylation levels in the Hs578Ts(i)8 cells at Y416
in the activation loop of the kinase domain and is associated with
increased src activity, which is in agreement with previous studies
demonstrating increased src activity in human breast cancer tissues
(13), and the corresponding
decreased phosphorylation levels at Y527 in the carboxy-terminal
domain, associated with decreased src activity. Furthermore, levels
of src when dephosphorylated at Y416 and Y527 were found elevated
in the parental Hs578T and more invasive Hs578Ts(i)8
cells respectively, confirming the src results described above.
Additionally, we noted that downstream signal transducers, such as
JNK and ERK, that correlate with the adhesive and migratory
behavior were also more phosphorylated in the less invasive Hs578T
cells, which again represents an unexpected activation pattern and
this in an opposite way (data not shown). Given that numerous
studies indicate FAK activation during epithelial to mesenchymal
transition (EMT) and further augmentation during cell migration,
one particular study by Nakamura et al (19) suggest that FAK
Y407 is a unique phosphorylation site to FAK and demonstrated that
this tyrosine phosphorylated form is predominantly localized to
focal adhesions and the cell periphery in motile cells. Given our
observations, we opted in this study to continue further and try to
elucidate the unanticipated decreased FAK Y407 activation as well
as the phosphorylation of tyrosine residues 397 and 861 in the
migratory subclone Hs578Ts(i)8.
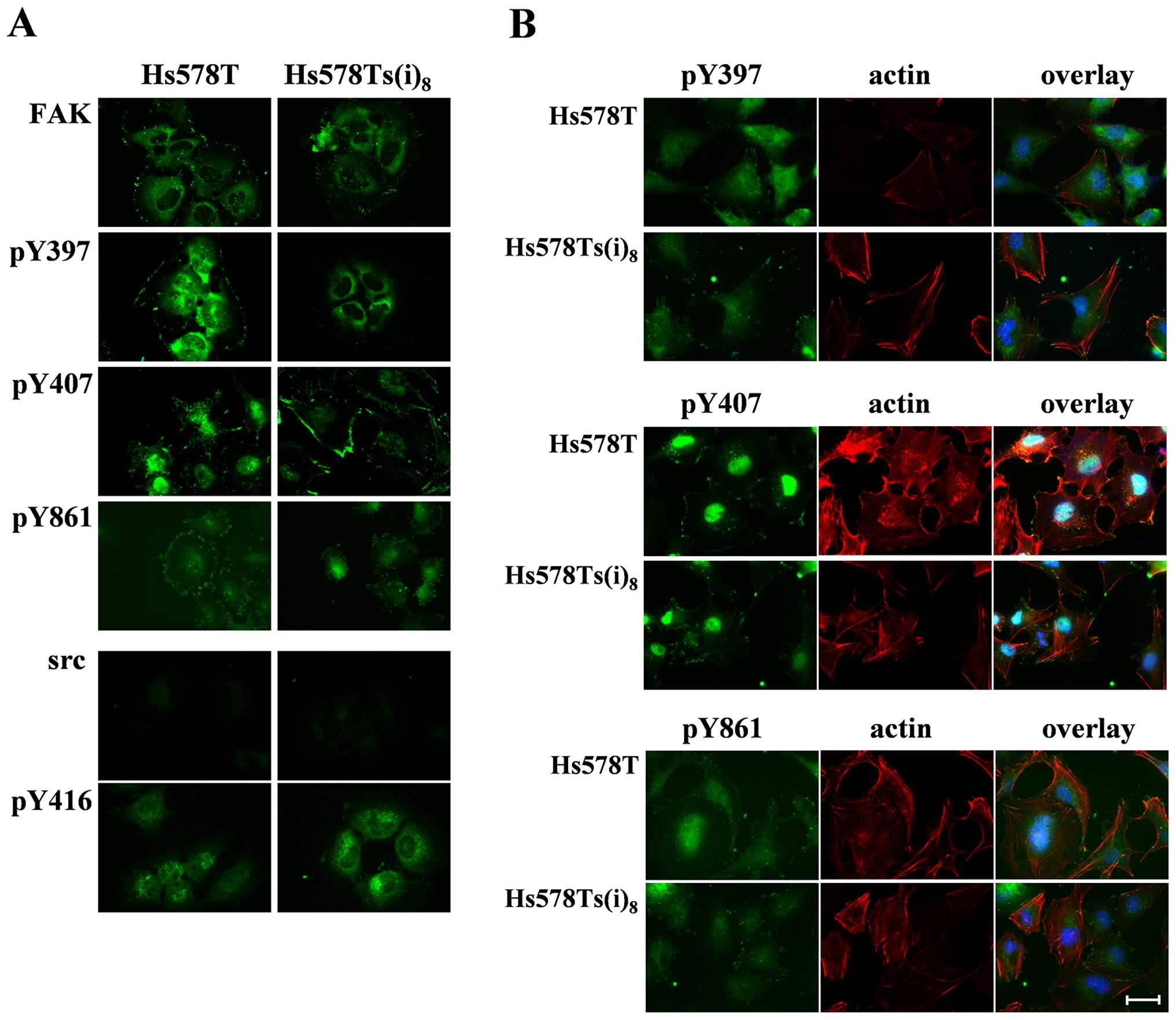
Localization of FAK Y407 at focal
adhesion sites in more invasive Hs578Ts(i)8
Our previously described results revealed that FAK
is well phosphorylated at Y397, Y407 and Y861 in Hs578T cells, as
compared to the more invasive Hs578Ts(i)8 cells. The
autophosphorylation site of FAK, Y397, has been shown to be
activated and to function primarily at focal adhesions. Several
studies describe the importance of FAK Y861 and invasiveness
(17), however, limited studies
demonstrate a role for FAK Y407. We therefore, examined the
(sub)cellular localization of FAK and src as well as their tyrosine
phosphorylated forms in Hs578T and Hs578Ts(i)8 cells
cultured on glass substrate under normal growth conditions.
Fig. 4A, shows that there are no
significant differences in FAK location between the two cell lines.
FAK was predominantly found in the perinuclear region and partially
at the focal adhesions. FAK Y397, on the contrary, was more present
in the perinuclear area and the cytosol in the invasive
Hs578Ts(i)8 cells and weakly at the focal adhesions,
while it was found in the nucleus and to a lesser extent in the
perinuclear region and focal contact sites in Hs578T cells.
Furthermore, FAK Y407 staining occurred weakly in the nuclear
region, and was more pronounced at the cell periphery particularly
at focal adhesions, at the leading and trailing edges of the more
invasive Hs578Ts(i)8. In addition to this major
staining, a filamentous pattern that extended into the cytosol was
also detected. Slight differences in distribution were observed for
FAK Y861 fluorescence, which was seen in a weak punctate pattern
throughout the cytosol of Hs578Ts(i)8 cells and more
intensely at the cell periphery in Hs578T cells. Next, the
organization of src and src Y416 was examined. The src fluorescence
staining was weak and showed a diffuse distribution throughout the
cytosol and concentrated in a one-sided perinuclear pattern in both
cell lines. src Y416 staining was punctate at the cell periphery
and extended in the cytoplasm in both cell lines, with a more
prominent one-sided perinuclear pattern in the more invasive
Hs578Ts(i)8 cells.
Given the significant differences in distribution of
the phosphorylated forms of FAK in the two cell lines, we
investigated the organization of actin with each of these
phosphorylated FAKs in Hs578Ts(i)8 cells. Results in
Fig. 4B reveal that
Y407-phosphorylated FAK, which was already shown in Fig. 4A, to be mainly localized to the
focal adhesions in Hs578Ts(i)8 cells, is prevalent at
each end of actin stress fibers in this more invasive subclone as
compared to the location in the parental counterpart. Moreover,
fluorescence stainings of the organization of actin and FAK Y397 or
Y861, indicate that there are no major dissimilarities between the
parental and more invasive cell lines, which correlates with the
minor changes found when both cell lines were compared for
distribution of FAK Y397 and Y861 alone. Here, the more diffuse and
punctate staining pattern in the cytosol suggests that, unlike Y407
phosphorylation, phosphorylation of Y397 and Y861 can take place in
the cytosol, and that this cytosolic phosphorylation is not
associated with the formation of focal adhesions and stress fibers
in Hs578Ts(i)8 cells.
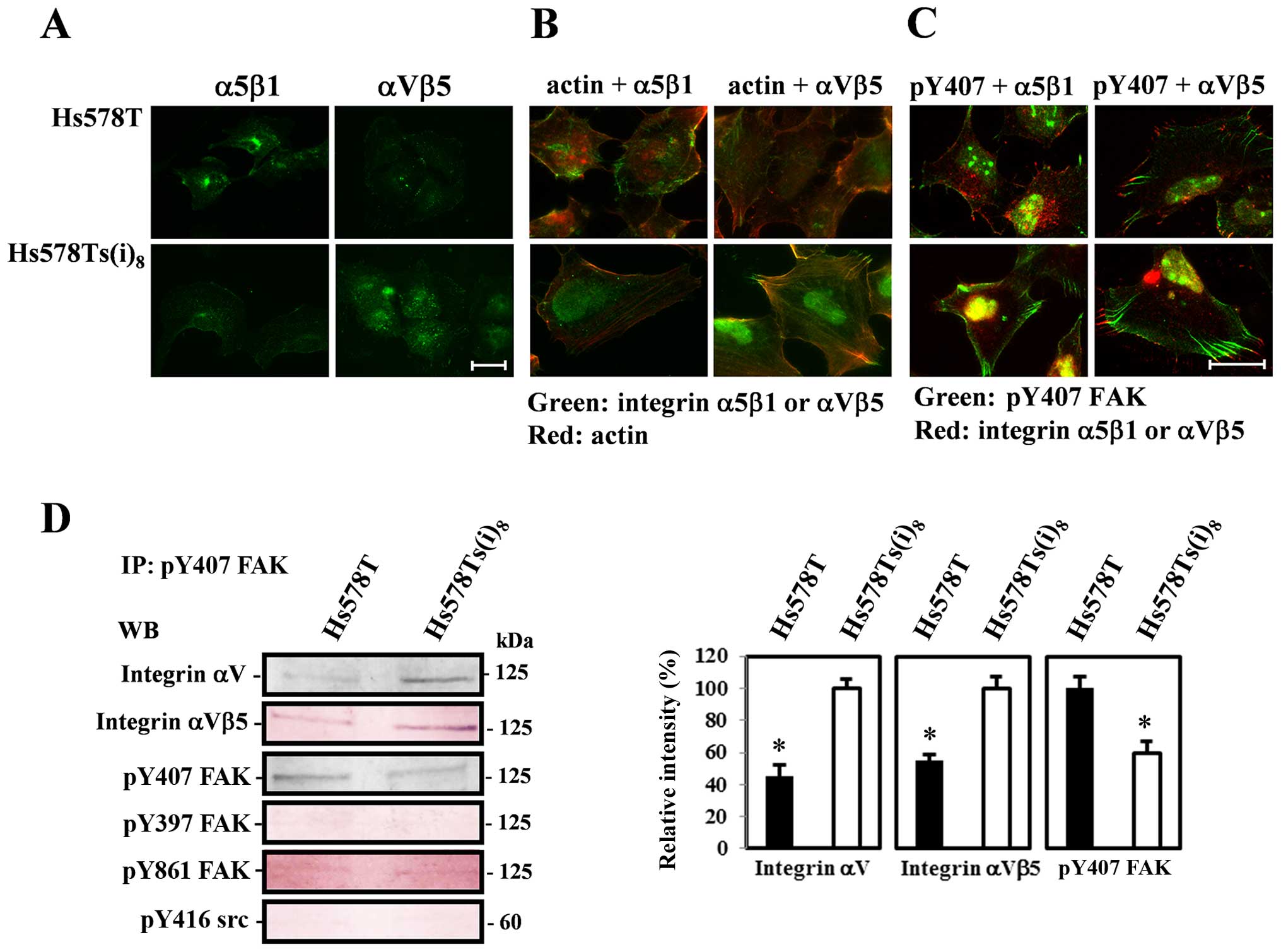
Next, we investigated the distribution of the α5β1
and αVβ5 receptors in this cell model of breast cancer progression,
in an attempt to link the observed integrin-mediated changes in
cellular behavior to altered FAK phosphorylation and in particular
the phosphorylation of FAK Y407. The fluorescence images in
Fig. 5A demonstrate a weaker
staining pattern for α5β1 receptors at the cell periphery of Hs578T
cells and a more intense staining in the perinuclear region, as
compared to the Hs578Ts(i)8 cells, in which also a very
faint network of α5β1 integrin receptors was observed. In contrast,
αVβ5 receptors were found at the cell periphery of both cell lines,
with a more pronounced staining in the focal adhesions and a
punctate cytosolic pattern with a vague filamental distribution, in
the more invasive cell variant. Dual fluorescence stainings of
actin and integrins (Fig. 5B)
disclosed that the distribution of integrin α5β1 receptors did not
localize to actin stress fibers in Hs578T and
Hs578Ts(i)8 cells, while co-staining of actin and αVβ5
integrin receptors revealed that these integrin receptors
colocalized with actin in Hs578Ts(i)8 cells. Such a
noticeable pattern was absent in Hs578T cells. Moreover, integrin
αVβ5 receptor staining was prominent at each end of the actin
stress fibers. Visualization of Y407-phosphorylated FAK and these
integrin receptors further indicate that in Hs578Ts(i)8
cells, FAK Y407 staining extends inward from αVβ5 receptors at the
cell periphery in focal adhesions, as much more highly bundled
fiber-like structures. Similar stainings in the parental Hs578T
cells show fewer focal adhesions to which integrin αVβ5 receptors
are localized and are less organized with FAK Y407. Furthermore,
such an organizational pattern was not observed for α5β1 integrin
receptors and FAK Y407 in these cells, rather they showed limited
focal complexes containing α5β1 integrin receptors and
phosphorylated FAK Y407 (Fig.
5C).
In conclusion, these observations suggest that for
each phosphorylated form of FAK, there seems to be some differences
in intensities of the signal, depending on the subcellular
localization, in particular for Y407 phosphorylation which can be
correlated to the migratory behavior and organization of
microfilaments and integrin αVβ5 receptors. Subsequently,
co-immunoprecipitation experiments using FAK Y407 antibody,
confirmed the increased phosphorylation at FAK Y407 in the parental
Hs578T cell line and the distribution and close connection of FAK
Y407 with integrin αV subunits and αVβ5 receptors, but not with the
β5 subunits, in the invasive Hs578Ts(i)8 cells (Fig. 5D). Moreover, the levels of FAK Y397
and Y861 as well as src Y416 were almost not detectable. These
results indicate that increased phosphorylation of FAK Y407 in the
parental Hs578T cell line does not correlate with the formation of
focal adhesions and subsequently its behavior.
Discussion
Several studies and reviews have shown the
importance of FAK in processes as tumor cell adhesion, cell
motility, invasion and ultimately metastases, in several types of
cancers, including breast cancer and also in the triple-negative
subtype (17,18). Although the knowledge on FAK has
increased over the last years, there is still much knowledge to
gain. In this study, we present important findings on differences
between the Hs578T triple-negative breast cancer cell line and the
more invasive variant Hs578Ts(i)8, a model which
represents the metastatic nature of TNBC and eliminates confounding
results due to cell line heterogeneity (15). We document for the first time the
occurrence of FAK when phosphorylated at Y407 in triple-negative
breast cancer cell lines, and provide a rationale for the
controversial data previously described in the literature (19–24)
and in the findings presented in this report.
Our results demonstrate that the increased
cell-matrix adhesion to fibronectin and the migratory behavior of
the Hs578Ts(i)8 cells, as compared to the Hs578T cells,
can be ascribed to the action of integrin receptors, α5β1 and αVβ5,
respectively. Cell surface and total expression levels of these
integrin receptors revealed that the enhanced cellular activities
could not be correlated to their expression levels, but rather
could be attributed to the location in the more invasive cells,
with some integrin α5β1 receptors at the cell's periphery and αVβ5
integrin receptors more pronounced at focal adhesions. While this
may be a weak justification at this point, it is the most plausible
explanation, given the limited accessibility of commercially
available antibodies that block the function of integrin receptors.
The latter may also be brought in connection to our findings on
other extracellular matrix proteins, for which we were not able to
identify a potential role for integrin receptors interacting with
their corresponding ECM proteins (data not shown). Moreover, it
should be noted that all the experiments involved in this study,
except for the cell matrix adhesion assays, were performed with
cells grown under normal growth conditions, on either plastic or
glass substrates, allowing for the detection of differences between
the two cell lines, displaying their specific features and
properties, and thus maintaining their phenotypes as previously
published (15). Furthermore, the
complexity expands given the overlap in substrate specificity
(25) and the growing numbers of
integrin receptors or other classes of cell surface proteins and
lipids involved in cell matrix interactions, such as
glycosphingolipids (GSLs) (26–28).
These GSLs have been linked to the regulation of cell motility and
invasion, by themselves or via particular organizations with
integrin receptors. Interestingly, these GSL-assemblies may
positively or negatively influence integrin-associated signaling
pathways (27,28). This may also provide a
justification for the subtle differences in expression levels of
the integrin receptors we detected despite their observed role in
the adhesion and migration experiments.
One of the major signaling events linking the role
of integrin receptors to cell adhesion and motility is the
activation of focal adhesion kinase (16). In this study, we show that there
are significant differences in tyrosine phosphorylation levels on
multiple sites of FAK, but not in the levels of the known serine
phosphorylations sites (data not shown), between the Hs578T and
Hs578Ts(i)8 cells. Our results demonstrate that the
autophosphorylation site Y397 and two additional tyrosine sites
(Y407 and Y861) are major activation sites and that phosphorylation
of Y576/Y577 in the kinase loop and Y925 in the C-terminal domain,
were at the detection limit. Moreover, we found higher activity
levels at tyrosine residues 397, 407 and 861 in Hs578T cells,
whereas it was expected in the more invasive Hs578Ts(i)8
cell line. The observed phosphorylation of tyrosine residues of FAK
in Hs578T is likely attributed to the mesenchymal-like character of
these cells and can be related to changes in epithelial to
mesenchymal transition, as is the case for MDA-MB-231 and BT-459
cells, representing cell line models for advanced TNBC (28). Generally, increased FAK activity
levels are linked to enhanced metastatic behavior (17,18).
Despite the fact that Hs578Ts(i)8 cells display a more
invasive phenotype as compared to Hs578T cells (15), such a correlation was not detected
in this study. To the best of our knowledge, no information is
published supporting decreased expression and activity of FAK in
advanced disease stages. There are, however, varieties in levels of
FAK mRNA and activities in different types of cancer, including
breast cancer, for which increased expression and activity is
linked to a poor prognosis (18).
Interestingly, a recent report demonstrated that src homology
phosphotyrosyl phosphatase 2 (SHP2) promotes cell migration by
dephosphorylating FAK Y397. Moreover, this study showed that FAK
Y407 was not a substrate for SHP2, and further provided evidence
that SHP2 not just acts on FAK, but likely targets additional
proteins to promote cell motility (30).
Of particular interest in this study are the levels
of FAK when phosphorylated at tyrosine 407. Yet, there are limited
studies available and some of them indicate a negative correlation,
demonstrating increased FAK Y407 phosphorylation under conditions
which normally lower FAK Y397 activity, such as serum starvation
and cell cycle arrest (21,22).
Other studies describe increased Y407 phosphorylation during
endothelial cell migration, induced by vascular endothelial growth
factor (VEGF) and requires the association of vascular endothelial
growth factor receptor 2 (VEGFR2) and heat shock protein (HSP)90.
Furthermore, the latter study reports that Y407 phosphorylation is
insensitive to src inhibition by the pharmacological inhibitor,
PP-2 (20), suggesting that
phosphorylation of FAK at Y407 is independent of src, a phenomenon
that was also observed in KM12C colon cancer cells expressing
kinase deficient src proteins (31). On the contrary, src-dependent
phosphorylation of Y407 has been described as well (32,33).
In addition, Ciccimaro et al implied an important future
role for FAK Y407, as an additional autophosphorylation site and
measure of FAK activity, next to FAK Y397 (33). Our results, at first sight, also
suggested a negative correlation, particularly concerning the
decreased FAK tyrosine kinase activities and the migration and
invasion-associated cellular activities of the
Hs578Ts(i)8 cell line. Furthermore, the inverse relation
between phosphorylation of FAK Y407 and src Y416, could be
interpreted as FAK activation occurs independently of src or may
propose that there are other, thus far unknown, FAK regulatory
mechanisms at play. In regards to this, recent reports indicate
that FAK activity is regulated by an intra-molecular interaction
between the N-terminal FERM and the catalytic domains, which may
alter Y397 autophosphorylation and its catalytic activity. These
studies highlight the importance of the FERM domain, since
truncation increases the FAK kinase activity, and additionally
demonstrate that interactions with phosphoinositide lipid and the
ECM, induce conformational changes in FAK, influencing its activity
and association with tetraspanins and growth factor receptors
(34,35). Additionally, it has been shown that
FAK activity can be affected by indirect interactions, between the
C-terminal focal adhesion targeting (FAT) domain and integrins at
focal adhesions. These linkages involve either FAK Y925 or Y861,
and are associated with paxillin and p130Cas or FAK S910 activation
(36). Our results, however, did
not confirm the constitutive activation of the involved FAK Y861
and Y925 or S910, nor paxillin and p130Cas in
Hs578Ts(i)8 cells, yet again increased phosphorylation
levels of paxillin at Y31 and Y118 phosphorylation and at Y410 of
p130Cas were detected in Hs578T cells (data not shown).
Hs578T and Hs578Ts(i)8 cells showed a
different subcellular localization of the tyrosine phosphorylated
FAKs. In the more invasive Hs578Ts(i)8 cells, FAK Y407
was mainly present at the cell periphery in focal adhesion-like
structures at each end of actin stress fibers and organized with
integrin αVβ5 receptors. These results link the αVβ5
integrin-mediated migratory behavior in these cells to FAK Y407.
Furthermore, our data suggest that localization, rather than FAK
expression and overall FAK Y407 phosphorylation levels, could be a
FAK-mediated regulatory mechanism to control migratory behavior. In
previous studies, such a mechanism has been proposed for src, with
its translocation to the cell membrane, phosphorylation and
activation of signaling pathways influencing cell adhesion and
migration (37). In our studies,
this would mean that, in the more invasive Hs578Ts(i)8
cells, αVβ5 integrin receptors at the cell periphery recruit FAK
from the cytoplasm, resulting in perhaps autophosphorylation or
indeed src-dependent phosphorylation of FAK Y407 and its
organization with these integrin receptors, to regulate cellular
migration, and this in the absence of its ligand vitronectin.
Further investigations, using vitronectin, are necessary to detect
the effect of integrin αVβ5-vitronectin engagement on FAK
phosphorylation levels. This will allow us to further explore the
atypical decrease in FAK activation observed in the
Hs578Ts(i)8 cells.
Many FAK studies focus on expression levels and to a
lesser extent on activity levels and localization. Our data
demonstrate FAK Y407 in TNBC cell lines and suggest that the
subcellular localization is more important than the overall Y407
activity levels. We propose that this spatio-temporal expression of
FAK Y407 is the key to the regulation of the αVβ5 integrin
receptor-mediated cell motility in the Hs578Ts(i)8 cells
(Fig. 6). Moreover, our results
hint that FAK as well as the other site-specific phosphorylated
FAKs are also temporally and spatially controlled and that this
regulation affects their involvement in signaling pathways
controlling other cellular functions. Our results add to some
existing evidence, supporting the importance of measuring activity
levels and determining the subcellular localization of signaling
molecules (38). The latter two
evaluations may provide a better understanding of the underlying
biochemical disease mechanisms and may more accurately reflect
tumor progression which all together represents a potentially more
suitable way for the identification of novel biomarkers for
diagnosis and therapy. In conclusion, our findings propose new
directions and ideas for studying disease mechanisms in TNBC and
warrant the need for expanding this idea to other TNBC cell lines
and metastatic tissues. These future studies may provide valuable
insight for the classification of metastatic TNBC and the
development of targeted therapeutic approaches.
Acknowledgements
This study was supported by national and private
funding, including STEM-TRAC (grant no. P03C110190), Florida Blue
and Domingo Moreira, the National Science Foundation (grant no.
0953561), the National Science Foundation/EPSCoR Cooperative
Agreement #IIA-1355423, the South Dakota Research and Innovation
Center, BioSNTR, and by the State of South Dakota.
Abbreviations:
|
TNBC
|
triple-negative breast cancer
|
|
ECM
|
extracellular matrix
|
|
FAK
|
focal adhesion kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
American Cancer Society. Breast Cancer
Facts and Figures. 2015, http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015.
Accessed: February 9, 2016
|
|
3
|
BreastCancer.org. US Breast Cancer
Statistics. http://www.breastcancer.org/symptoms/understand_bc/statistics.
Accessed: February 9, 2016
|
|
4
|
Kimbung S, Loman N and Hedenfalk I:
Clinical and molecular complexity of breast cancer metastases.
Semin Cancer Biol. 35:85–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zardavas D, Irrthum A, Swanton C and
Piccart M: Clinical management of breast cancer heterogeneity. Nat
Rev Clin Oncol. 12:381–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peintinger F, Sinn B, Hatzis C, Albarracin
C, Downs-Kelly E, Morkowski J, Gould R and Symmans WF:
Reproducibility of residual cancer burden for prognostic assessment
of breast cancer after neoadjuvant chemotherapy. Mod Pathol.
28:913–920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Eirew P, Mullaly SC and Aparicio S:
The omics of triple-negative breast cancers. Clin Chem. 60:122–133.
2014. View Article : Google Scholar
|
|
9
|
Lee JM, Ledermann JA and Kohn EC: PARP
Inhibitors for BRCA1/2 mutation-associated and BRCA-like
malignancies. Ann Oncol. 25:32–40. 2014. View Article : Google Scholar :
|
|
10
|
Bayraktar S and Glück S: Molecularly
targeted therapies for metastatic triple-negative breast cancer.
Breast Cancer Res Treat. 138:21–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brewster AM, Chavez-MacGregor M and Brown
P: Epidemiology, biology, and treatment of triple-negative breast
cancer in women of African ancestry. Lancet Oncol. 15:e625–e634.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carey LA, Rugo HS, Marcom PK, Mayer EL,
Esteva FJ, Ma CX, Liu MC, Storniolo AM, Rimawi MF, Forero-Torres A,
et al: TBCRC 001: Randomized phase II study of cetuximab in
combination with carboplatin in stage IV triple-negative breast
cancer. J Clin Oncol. 30:2615–2623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anbalagan M, Moroz K, Ali A, Carrier L,
Glodowski S and Rowan BG: Subcellular localization of total and
activated Src kinase in African American and Caucasian breast
cancer. PLoS One. 7:e330172012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chavez KJ, Garimella SV and Lipkowitz S:
Triple negative breast cancer cell lines: One tool in the search
for better treatment of triple negative breast cancer. Breast Dis.
32:35–48. 2010.
|
|
15
|
Hughes L, Malone C, Chumsri S, Burger AM
and McDonnell S: Characterisation of breast cancer cell lines and
establishment of a novel isogenic subclone to study migration,
invasion and tumourigenicity. Clin Exp Metastasis. 25:549–557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Du F, Eckhardt BL, Lim B, Litton JK,
Moulder S, Meric-Bernstam F, Gonzalez-Angulo AM and Ueno NT: Is the
future of personalized therapy in triple-negative breast cancer
based on molecular subtype? Oncotarget. 6:12890–12908. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Golubovskaya VM, Ylagan L, Miller A,
Hughes M, Wilson J, Wang D, Brese E, Bshara W, Edge S, Morrison C,
et al: High focal adhesion kinase expression in breast carcinoma is
associated with lymphovascular invasion and triple-negative
phenotype. BMC Cancer. 14:769–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura K, Yano H, Schaefer E and Sabe H:
Different modes and qualities of tyrosine phosphorylation of Fak
and Pyk2 during epithelial-mesenchymal transdifferentiation and
cell migration: Analysis of specific phosphorylation events using
site-directed antibodies. Oncogene. 20:2626–2635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Boeuf F, Houle F and Huot J: Regulation
of vascular endothelial growth factor receptor 2-mediated
phosphorylation of focal adhesion kinase by heat shock protein 90
and Src kinase activities. J Biol Chem. 279:39175–39185. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim Y, Park H, Jeon J, Han I, Kim J, Jho
EH and Oh ES: Focal adhesion kinase is negatively regulated by
phosphorylation at tyrosine 407. J Biol Chem. 282:10398–10404.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeon J, Lee H, Park H, Lee JH, Choi S,
Hwang J, Han IO and Oh ES: Phosphorylation of focal adhesion kinase
at Tyrosine 407 negatively regulates Ras transformation of
fibroblasts. Biochem Biophys Res Commun. 364:1062–1066. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lie PP, Mruk DD, Mok KW, Su L, Lee WM and
Cheng CY: Focal adhesion kinase-Tyr407 and -Tyr397 exhibit
antagonistic effects on blood-testis barrier dynamics in the rat.
Proc Natl Acad Sci USA. 109:12562–12567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boivin B, Chaudhary F, Dickinson BC, Haque
A, Pero SC, Chang CJ and Tonks NK: Receptor protein-tyrosine
phosphatase α regulates focal adhesion kinase phosphorylation and
ErbB2 oncoprotein-mediated mammary epithelial cell motility. J Biol
Chem. 288:36926–36935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Humphries JD, Byron A and Humphries MJ:
Integrin ligands at a glance. J Cell Sci. 119:3901–3903. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hakomori SI: Structure and function of
glycosphingolipids and sphingolipids: Recollections and future
trends. Biochim Biophys Acta. 1780:325–346. 2008. View Article : Google Scholar
|
|
28
|
Hakomori SI and Handa K: GM3 and cancer.
Glycoconj J. 32:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taliaferro-Smith L, Oberlick E, Liu T,
McGlothen T, Alcaide T, Tobin R, Donnelly S, Commander R, Kline E,
Nagaraju GP, et al: FAK activation is required for IGF1R-mediated
regulation of EMT, migration, and invasion in mesenchymal triple
negative breast cancer cells. Oncotarget. 6:4757–4772. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hartman ZR, Schaller MD and Agazie YM: The
tyrosine phosphatase SHP2 regulates focal adhesion kinase to
promote EGF-induced lamellipodia persistence and cell migration.
Mol Cancer Res. 11:651–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brunton VG, Avizienyte E, Fincham VJ,
Serrels B, Metcalf CA III, Sawyer TK and Frame MC: Identification
of Src-specific phosphorylation site on focal adhesion kinase:
Dissection of the role of Src SH2 and catalytic functions and their
consequences for tumor cell behavior. Cancer Res. 65:1335–1342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Calalb MB, Polte TR and Hanks SK: Tyrosine
phosphorylation of focal adhesion kinase at sites in the catalytic
domain regulates kinase activity: A role for Src family kinases.
Mol Cell Biol. 15:954–963. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ciccimaro E, Hevko J and Blair IA:
Analysis of phosphorylation sites on focal adhesion kinase using
nanospray liquid chromatography/ multiple reaction monitoring mass
spectrometry. Rapid Commun Mass Spectrom. 20:3681–3692. 2006.
View Article : Google Scholar
|
|
34
|
Frame MC, Patel H, Serrels B, Lietha D and
Eck MJ: The FERM domain: Organizing the structure and function of
FAK. Nat Rev Mol Cell Biol. 11:802–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lietha D, Cai X, Ceccarelli DF, Li Y,
Schaller MD and Eck MJ: Structural basis for the autoinhibition of
focal adhesion kinase. Cell. 129:1177–1187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fincham VJ, Unlu M, Brunton VG, Pitts JD,
Wyke JA and Frame MC: Translocation of Src kinase to the cell
periphery is mediated by the actin cytoskeleton under the control
of the Rho family of small G proteins. J Cell Biol. 135:1551–1564.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elsberger B, Tan BA, Mitchell TJ, Brown
SB, Mallon EA, Tovey SM, Cooke TG, Brunton VG and Edwards J: Is
expression or activation of Src kinase associated with
cancer-specific survival in ER-, PR- and HER2-negative breast
cancer patients? Am J Pathol. 175:1389–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|