Introduction
Mucins belong to a heterogeneous group of
high-molecular-weight O-glycosylated proteins that participate in
the protection, lubrication, and acid resistance of the epithelial
surface (1). In cancer, mucins
influence cell adhesion and contribute to tumor invasiveness
(2). There is a body of evidence
indicating that mucins, not only constitute cancer biomarkers, but
also play an active role in cancer progression by regulating cell
growth, adhesion, invasion and signaling (2,3).
Mucins are also involved in chemo-resistance to drugs (4). Thus, the implication of mucins and
their associated carbohydrate antigens in the metastatic process of
tumor cells makes them relevant targets for the prevention of
metastasis and recurrence of cancers by immunotherapy in
combination with effective chemotherapy (5–7).
Up to date, 21 human mucins have been described and
are expressed on a tissue specific basis (1). There is a variety of evidence
describing the role of mucins in relation to cancer cell behavior
and cell signaling pathways associated with tumorigenesis. For
instance, the membrane-bound mucins MUC1 and MUC4 promote both
cellular differentiation and proliferation (2,8) and
inhibit apoptosis (9). Similarly,
secreted mucins MUC5B (10) and
MUC5AC (11,12) and membrane-bound mucins MUC13
(13,14) and MUC16 (15) have also been associated with
aggressive behavior of cancer cells.
Mucins produced by tumor cells can also modulate
tumor immunity, affecting both the innate and adaptive immune
response (16). Mucin
overexpression in cancer can create an immunosuppressive barrier by
decreasing the accessibility of immune cells (such as NK cells) or
molecules (such as complement) as well as therapeutic drugs
(4). Mucins can also exert
immunosuppressive roles on dendritic cells (DCs) (17). DCs are potent antigen presenting
cells that possess the ability to stimulate naive T cells. In
response to infectious agents DCs undergo a maturation process
during which they migrate to secondary lymphoid organs where they
present captured antigens to naive T cells, for the triggering of
specific immunity (18). This
process is associated to an upregulation of the expression of MHC
molecules, adhesion molecules and co-stimulatory molecules as well
as a downregulation of their endocytotic capacity (18). Mucins, however, can modulate DC
function or maturation. For instance, tumoral MUC1 is chemotactic
to immature DCs, and maturation of DC in its presence subverts DC
function by negatively affecting their ability to simulate type-1
helper T cell responses (19,20).
In other settings, mucins can, through their glycans, impair
DC-maturation and antigen processing and presentation (19,21,22).
Indeed, we have previously shown that MUC6 carrying the
tumor-associated Tn antigen impairs antigen presentation by DCs
(23). Last, mucins can also
induce DC death by apoptosis through interaction with Siglec-3
(24).
Among mucins, MUC5B is a secreted mucin found at
high levels in the normal respiratory tract, submandibular glands,
endocervix, pancreas and the hepatobiliary system (1,25).
MUC5B is the major mucin in the respiratory tract mucus where it is
essential for mucociliary clearance that controls bacterial
infection, providing protection against pathogens (26). However, MUC5B is aberrantly
expressed in different cancers and may constitute a target antigen
itself. Indeed, MUC5B has been detected in breast (27), gastric (28) and colon (29) adenocarcinomas, whereas it is not
expressed in their respective normal tissues. Our group has
reported the expression of MUC5B in breast tissues, showing that
MUC5B apomucin was detected in >80% of primary breast tumors
while it was absent in normal control breast samples (27). Furthermore, the detection of MUC5B
mRNA could constitute a specific marker applicable to the molecular
diagnosis of breast cancer cell dissemination (30). Using different approaches we have
demonstrated that the human breast cancer cell line MCF-7 expresses
MUC5B (27). In this study we
evaluated the role of MUC5B in breast cancer by gene silencing the
MUC5B with short hairpin RNA (shRNA) using MCF-7 cells as a
model.
Materials and methods
Generation of MUC5Bsi and mock MCF-7
cells
The human breast cancer cell line MCF-7 (ATCC) was
cultured in complete culture medium, consisting of RPMI-1640 with
glutamine (PAA Laboratories, GE Healthcare, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS), 50 μM
2-mercaptoethanol, 100 U/ml penicillin, 100 mg/ml streptomycin
(Sigma-Aldrich, USA), at 37°C and 5% CO2. To generate
the pCMV-MUC5B plasmid we amplified by PCR the CMV promoter from
the pCMV-RL (Promega, USA) using primers CMV_Fw (5′-CTCACATGGCTCG
ACAGATCTTCAATATTGGCC-3′) and CMV_Rev (5′-GCG
GGATCCTACTCTAGCCTTAAGAGCTGTAATTG-3′). The product was then digested
with BglII and BamHI and cloned at the 5′ end of the
eGFP coding region of peGFP-1 (BD, Biosciences, USA). The resultant
plasmid was modified by PCR inserting an XhoI site at the
3′UTR region of eGFP. The shRNA directed to MUC5B was then cloned
in the XhoI site using the following primers: shRNA5B_Fw
(5-ATCTCGAG ATCTAGCGACCTGATCCTGTTTCTGACTAAATCGGTG AAGCCACAGATGG-3′)
and shRNA5B_Rev (5′-CGCTCG AGGATCCGGCAGCCTGATCCTGTTTGACCAAATCCC
ATCTGTGGCTTCACCG-3′). The shRNA5B targeted the sequence
ATTTGGTCAAACAGGATCAGGC that corresponds to positions 15,534–15,552
of the mRNA of MUC5B (NM_002458.2). Presence of the inserted
sequence was confirmed by sequencing. The mock cells consisted of
cells transfected with the plasmid without any shRNA sequences.
MCF-7 cells were transfected with the plasmids using FuGENE (Roche,
France) and placed under antibiotic selection (geneticin at 0.75
mg/ml).
Quantitative RT-PCR for MUC5B
Total cellular RNA was isolated from cells grown to
70% confluence by use of the Tri-reagent (Sigma-Aldrich) from
1-5×106 cells, according to the manufacturer's
protocols. For cDNA synthesis, 5 μg of total RNA was used as
template in a 20-μl reverse transcription (RT) reaction using the
M-MLV reverse transcriptase (Thermo Scientific Fermentas, USA)
following the manufacturer's instructions. For real-time PCR, a
Corbett Rotor Gene 6000 Real-Time PCR Machine and the SYBR Green 1
dye (Applied Biosystems, USA) were used according to the
manufacturer's protocol. Standard amplification conditions were 3
min at 95°C and 35 cycles of 10 sec at 95°C, 30 sec at 52°C, and 30
sec at 72°C. After each PCR reaction, the corresponding
dissociation curves were analyzed to ensure that the desired
amplicon was being detected and to discard contaminating DNA or
primer dimers. For GAPDH detection, sense and antisense
primers were 5′-TCGGAGTCAACGGATTG-3′ and 5′-CCTGGAAGATGGTGATGG-3′,
respectively. For detection of MUC5B, sense and antisense
primers were 5′-GGGCCT CGAGTGCCGTG-3′ and 5′-CACACGGATTCATAGTT
GAA-3′, respectively, generating a 152-bp fragment. Samples were
analyzed in duplicates, and product purity was checked through
dissociation curves at the end of quantitative PCR (qPCR) cycles.
Control reactions were performed to verify the absence of genomic
DNA and cross-contamination of any other DNA source. PCR
specificity was checked by melting curves, and in the case of
MUC5B, the qPCR product was verified by sequencing. Relative
quantity of gene expression normalized to GAPDH was analyzed
with Chromas 2.4 or REST 2009 V2.0.13 software.
MUC5B expression by
immunofluorescence
For immunofluorescence (IF) assays, cells were fixed
for 30 min at room temperature with 4% paraformaldehyde in PBS.
After fixation, cells were incubated for 10 min at room temperature
with 0.05 M ammonium chloride and permeabilized in 0.2% Triton
X-100 for 1 h at room temperature. Then slides were incubated with
a goat polyclonal anti-MUC5B IgG antibody (Y-20, Santa Cruz
Biotechnology) or PBS overnight at 4°C. After 3 washes with 0.1%
Tween-20 in PBS, slides were incubated with the secondary antibody
coupled to rhodamine (Pierce, USA) for 1 h at room temperature
followed by incubation with DAPI and mounted with 80% glycerol and
analyzed in a fluorescence microscope.
Cell viability assays
Cells were plated in triplicates on 96-well plates
at a density of 5-160×103 cells/well. After incubation
for 24, 48 or 72 h, 3-(4,5 dimethyl-2 thiazolyl)-2,5 diphenyl-2H
tetrazolium (MTT) bromide solution (Sigma-Aldrich) was added to
each well, containing both adherent and non-adherent cells, at a
final concentration of 0.5 mg/ml and incubated for 4 h. The
formazan crystals were dissolved in a solution containing 0.1 N HCl
in isopropanol and absorbance was measured at 570 nm with a plate
spectrophotometer (Thermo Scientific Labsystems Multiskan).
Alternatively, cells were incubated with cis-diammine-platinum
dichloride (cisplatin) or 5-fluorouracil (5-FU) at 10 μg/ml.
Evaluation of apoptosis by flow
cytometry
Cells were harvested and incubated for 6 h in
complete medium in 96-well plates (0.5×106 cells/ml).
Then, cells (both adherent and non-adherent) were incubated with
Annexin V-APC (Invitrogen, Carlsbad, CA, USA) for 30 min and
positive cells were quantified using a CyAn ADP Analyzer (Beckman
Coulter, USA). For each analysis sample a minimum of 10,000 counts
were recorded.
Cell cycle analysis by flow
cytometry
Mock and MUC5Bsi cells were seeded in 60-mm Petri
dishes (5×105 cells/dish) and cultured for 36 h in
complete culture medium. Subsequently, the culture medium from each
dish was collected and cell monolayers were then trypsinized,
gently resuspended in 3 ml of complete medium and immediately mixed
with the previously collected culture medium. Cells were
centrifuged (800 rpm, 5 min), washed two times in 5 ml cold PBS,
fixed in cold 70% ethanol and stored at −20°C. Prior to flow
cytometry analyses, cells were centrifuged, resuspended in 0.5 ml
PBS, passed through a 50-μm nylon mesh filter, treated with 25 μl
RNase (1 mg/ml) and finally stained with 25 μl propidium iodide
(PI, 1 mg/ml) for 10 min. Flow cytometry cell cycle estimations
were performed on a FACSVantage flow cytometer (BD Biosciences, CA,
USA) equipped with a 70-μm nozzle and an argon laser emitting at
488 nm (100 mW). Fluorescence emitted from PI was collected in FL2
channel using a 575/26 band pass filter. The DNA QC Particles kit
(BD Biosciences) was used to check the calibration and linearity of
the equipment. Data were acquired and processed using CellQuest
software (BD Biosciences). Side scatter versus forward scatter
(SSC/FSC), side scatter versus FL2 pulse area (SSC/FL2-A) and FL2
pulse width versus FL2 pulse area (FL2-W/FL2-A) plots as well as
FL2-A histograms were used for the analysis of DNA measurements.
Experiments were performed in sextuplicate. In all cases, a total
of 5,000 cells were analyzed per experimental point.
Colony forming assay
MUC5B knock-down or mock MCF-7 cells were seeded in
complete media at a density of 500 cells in 10-cm dishes. The
plates were incubated at 37°C for two weeks and then stained with
0.1% crystal violet. Colonies of >50 cells were counted
manually.
Cell adhesion
Ninety-six-well plates were coated with Matrigel (BD
Biosciences) at 4°C for 8 h and then blocked with 1% (w/v) bovine
serum albumin at room temperature for 30 min. Cells were
trypsinized, washed with complete medium and recovered in RPMI
containing 2% FBS. Cells (5, 10 and 20×104) were allowed
to attach for 2 h at 37°C in a humidified 5% CO2
incubator. Attached cells were first fixed with 1% glutaraldehyde
for 10 min at room temperature and then stained with 0.1% crystal
violet for 30 min at room temperature. Then they were lysed with
10% acetic acid. Absorbance was read in a spectrophotometer at 570
nm.
Preparation of tumor cell-derived
conditioned media (CM)
CM were prepared by seeding 1×106 tumor
cells in 5 ml of complete medium placed in a 25 cm3
culture flask and collected after 72 h. The effects of CM on DCs
were examined by replacing 50% of the culture medium with CM at the
initiation of DC differentiation or maturation.
Generation of monocyte-derived DCs
PBMC were obtained following Ficoll-hypaque
density-gradient centrifugation and stored in liquid nitrogen
before use, as described (31).
Monocytes from PBMC were purified by plastic adherence. For DC
differentiation, monocytes were incubated for 2 days in RPMI
supplemented with 10% (v/v) heat-inactivated FBS, 1000 U/ml GM-CSF
(PeproTech, USA) and IL-4 (1% of a conditioned supernatant from the
IL-4 transfected J588L cell line). Monocyte-derived DCs were
harvested and cultured in 12-well tissue culture plates for
experiments. Cells with the following phenotype: CD11c+
CD14− MHC IIlow CD86low
CD80low CD83low CD40low were
considered immature DC.
DC differentiation and maturation assays
with CMs
To test the effect of CM on DC-differentiation,
monocytes were cultured as described above in the presence of 50%
of CM derived from MUC5Bsi or mock MCF-7 cells. After 2 days,
medium was renewed with the same conditions and further incubated
for 24 h. Then, DCs were stimulated with LPS (1 μg/ml) from
Escherichia coli serotype O26:B6 (Sigma-Aldrich) for 18 h
and incubated at 37°C in 5% CO2. To test the effect of
CM on the maturation of DCs, differentiated DCs
(1.25×105 cells/well) were cultured with CM (50%) in
12-well culture plates and incubated for 24 h. Then, LPS (1 μg/ml)
was added and incubated for 18 h at 37°C in 5% CO2.
Culture supernatants were collected, clarified by centrifugation
and stored at −80°C for a maximum period of 1 month until cytokine
analysis. Monocyte-derived DCs were harvested and stained for flow
cytometry analyses.
Flow cytometry and antibodies for DC
staining
The following antibodies purchased from BD
Biosciences were used for flow cyto-metry staining: anti-human CD86
(clone 2,331, phycoerythrin (PE)-conjugated), anti-human HLA-DR
(clone TU36, fluorescein isothiocyanate (FITC)-conjugated),
anti-human CD19 (clone HIB19, PE-conjugated), anti-human CD3 (clone
HIT3a, FITC-conjugated), anti-human CD14 (clone M5E2,
FITC-conjugated). The anti-human CD11c (clone 3.9,
PerCP-eFluor® 710-conjugated) was purchased from
eBioscience (USA). DCs were stained and later analyzed using a
CyAn™ ADP (Beckman Coulter) flow cytometer and Summit 4.3 software.
For each sample the following parameters were studied: forward
scatter (FSC) versus side scatter (SSC) to define the acquisition
gates for intact cells, FSC versus Pulse Width dot plot were used
for doublet discrimination and FSC versus FL5 channel (CD11c) for
dendritic cells. For each analysed sample a minimum of 10,000
counts, gated on a FSC vs SSC dot plot, single intact DC were
recorded. The median fluorescence intensity (MFI) was used to
compare results.
Cytokine measurement
IL-10, IL-6, IL-12, IL-8, TNFα, and IL-1β
concentrations were determined by FlowCytomix™ technology
(eBioscience) and analyzed by flow cytometry. BMS FlowCytomix
software version 2.2.1 was used for the analysis of the
results.
Statistical analysis
Data were expressed as median and range or mean and
SD, as specified. Statistic calculations were performed using the
Student's t-test or two-way ANOVA with GraphPad Prism software
version 5.01. Differences were considered statistically significant
when the obtained value was <0.01 or <0.001.
Results
MUC5B shRNA silencing decreases
cell-adhesion, cell growth and clonogenic ability of breast cancer
cells
In order to evaluate the biological role of MUC5B in
breast cancer, we silenced its expression in the human breast
cancer cell line MCF-7, that constitutively expresses this mucin
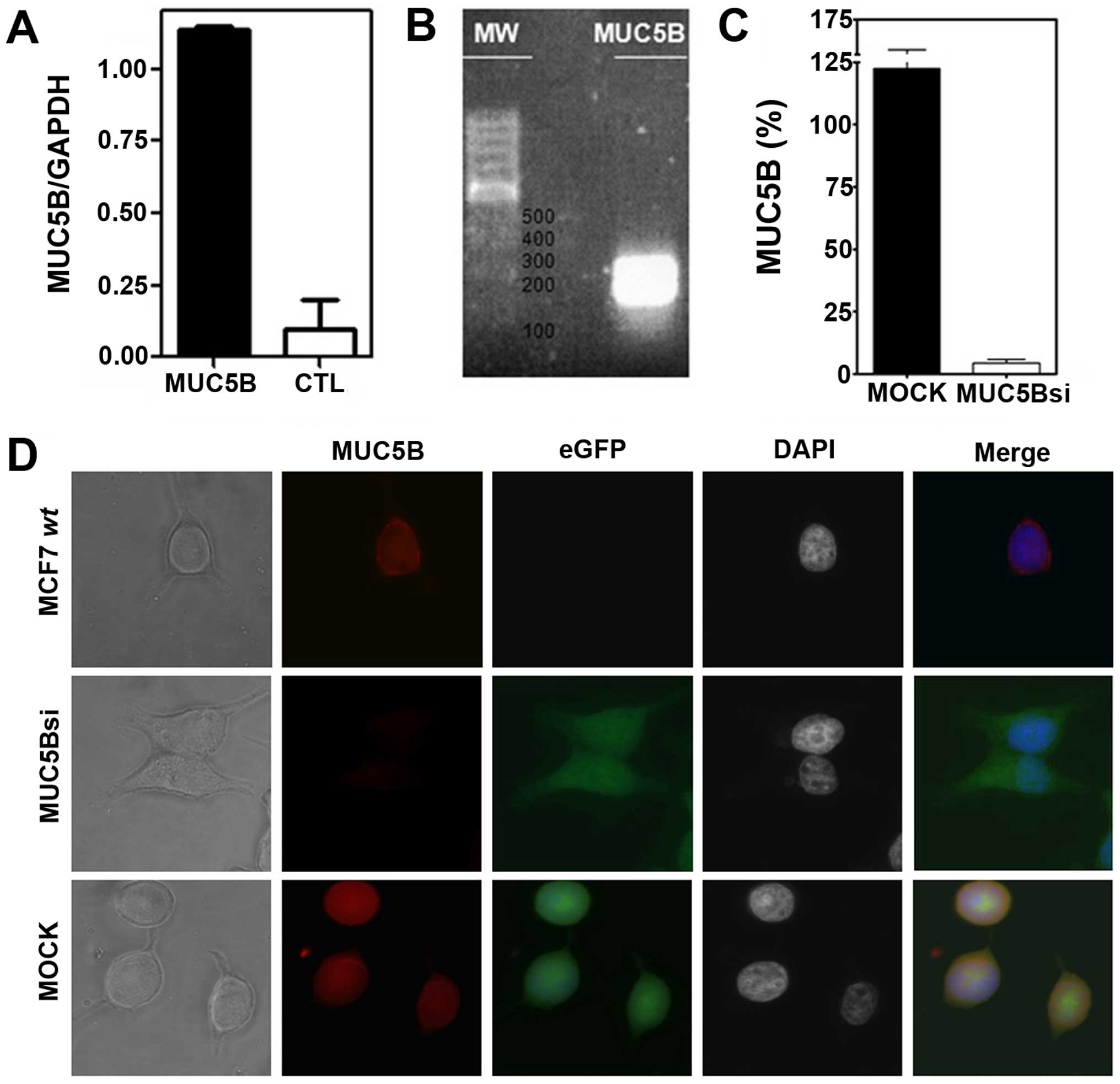
(30). The expression of MUC5B in
MCF-7 cells was evaluated by qRT-PCR (Fig. 1A) and the sequence of the amplified
segment (Fig. 1B) was confirmed by
DNA sequencing. MUC5B expression was also evaluated by
immunofluorescence on MCF-7 cells revealing a cytoplasmic and
surface staining and suggesting an active biosynthesis of this
mucin (Fig. 1C). To obtain a
MUC5B-silenced or knock-down clone from these cells (referred here
as MUC5Bsi) we transfected them with a plasmid coding for a
MUC5B-specific shRNA sequence and selected them by incubation in
geneticin-containing medium. MUC5Bsi cells showed a 96% inhibition
rate of MUC5B expression by qRT-PCR compared to mock cells
(Fig. 1C). As expected, both
transfected cells expressed the eGFP protein, while only the mock
cells expressed MUC5B apomucin (Fig.
1D).
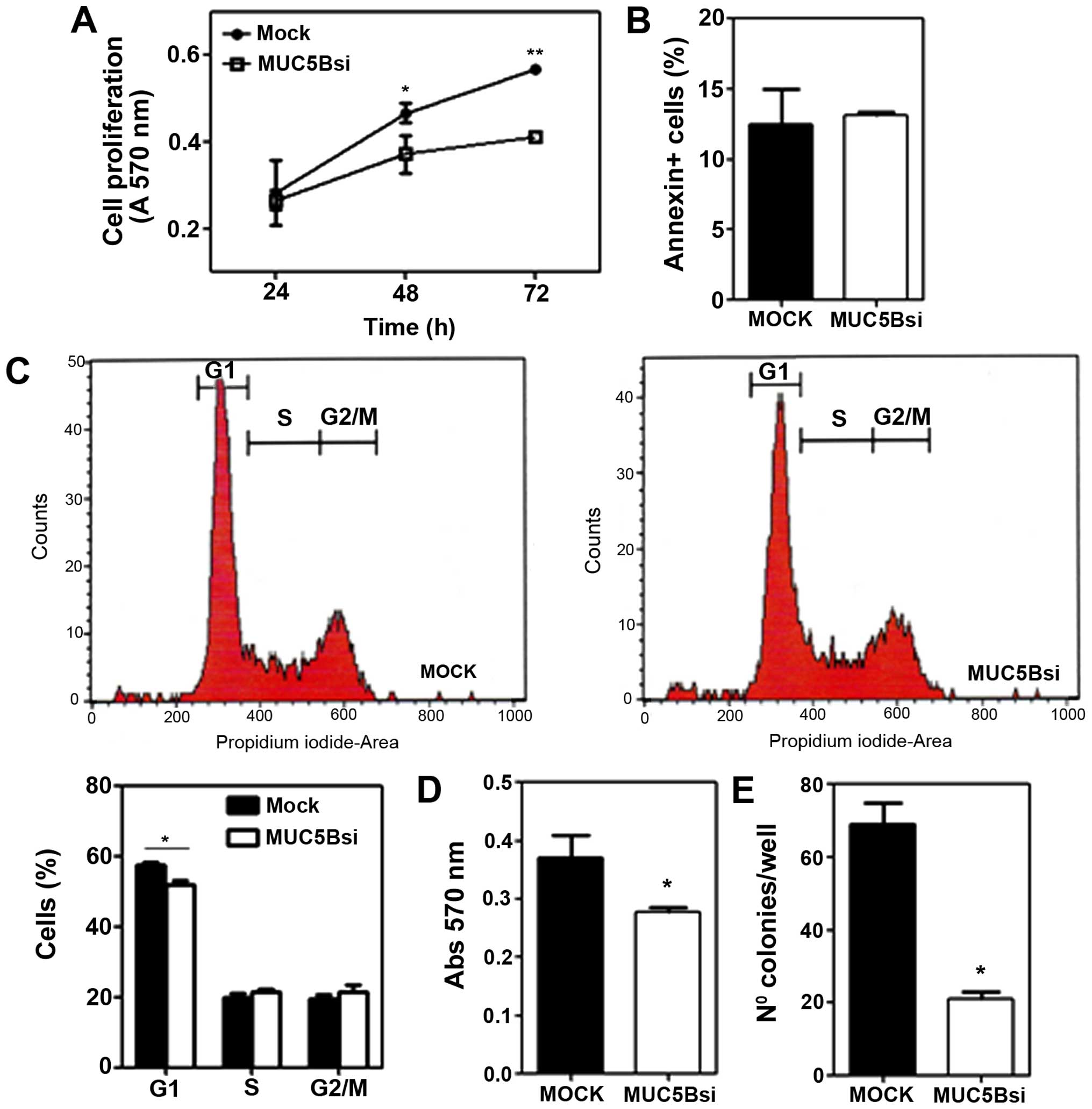
Next, we evaluated the properties of
MUC5B-suppressed cells, in terms of cell growth and adhesion.
MUC5Bsi cells showed a cell growth rate in vitro that was
significantly lower than the one observed for mock cells (Fig. 3A). One possible explanation for
this slowed tumor cell growth could be an increase in the
steady-state levels of apoptosis of breast tumor cells or an arrest
in the cell cycle. However, the downregulation of MUC5B in the
breast tumor cells did not correlate to an increase in the
steady-state apoptosis (Fig. 3B).
On the contrary, when analyzing the percentage of mock and MUC5Bsi
cells in the various phases of cell cycle, we only observed a
slight decrease in the percentage of MUC5Bsi cells in the G1 phase
compared to mock cells (Fig.
2C).
Suppression of MUC5B also altered the adhesive
properties of breast cancer MCF-7 cells, decreasing the capacity of
MUC5Bsi cells to adhere to components of the extracellular matrix
(Fig. 2D). We also evaluated the
clonogenic ability of both transfected cells by plating efficiency.
Cancer cells, in general, have higher plating efficiency than
normal cells. MUC5Bsi cells showed a significant lower clonogenic
efficiency as compared with the mock control (Fig. 2E).
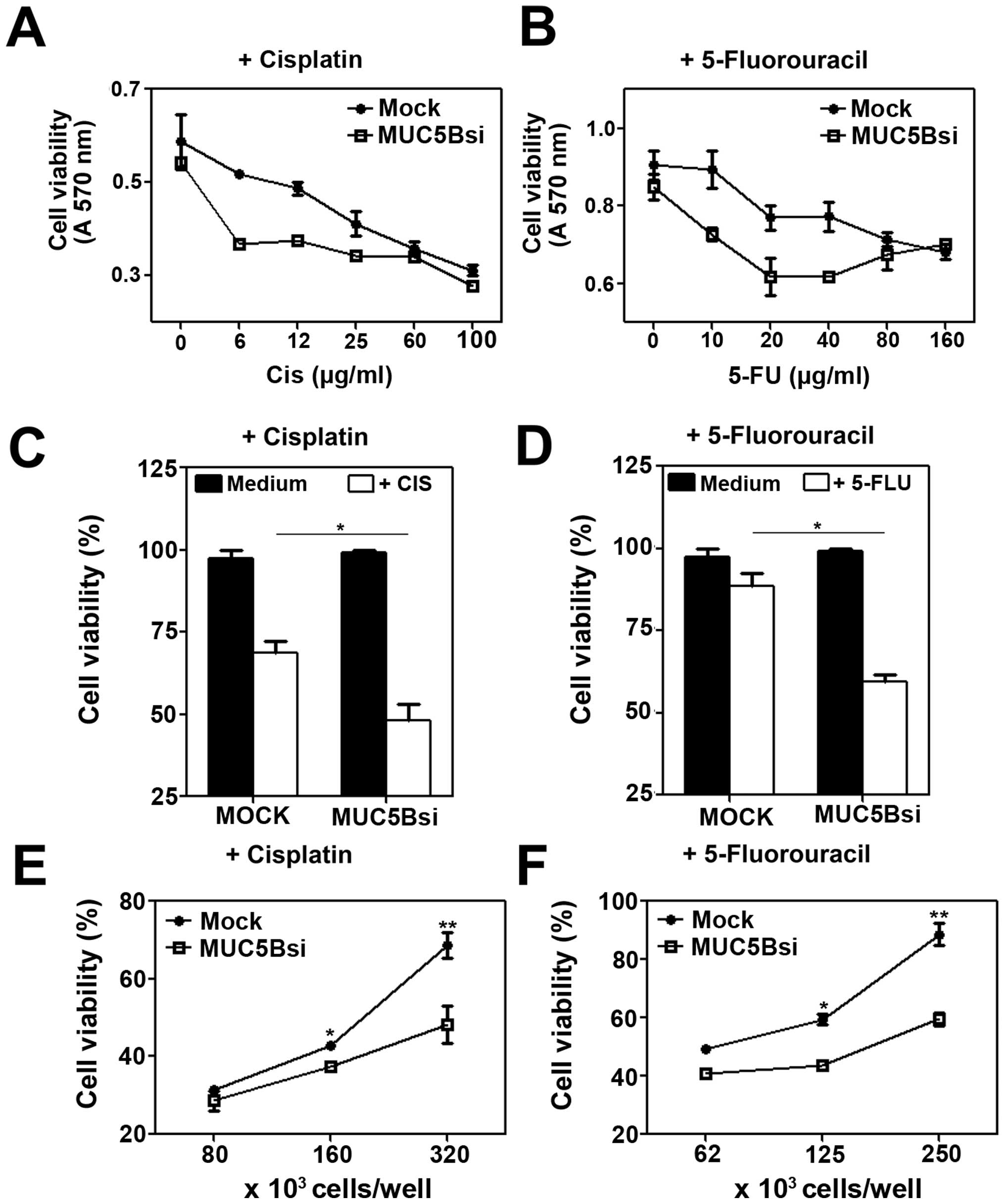
Downregulation of MUC5B expression
increases chemosensitivity of breast cancer cells
Different independent reports have shown that cancer
cells may develop resistance to cell death induced by anticancer
agents (32). To investigate
whether MUC5B contributes to the resistance of breast cancer cells
to chemotherapeutic drug-induced death, we exposed both MUC5Bsi and
mock cells to 5-fluorouracil (5-FU) or cisplatin treatment. As
shown in Fig. 3, MUC5B in MCF-7
cells was associated with decreased sensitivity to 5-FU or
cisplatin induced-death (Fig. 3A and
B). Furthermore, MUC5Bsi cells exposed to cisplatin (at 10
μg/ml) showed a 2-fold increase in the percentage of cell death as
compared to mock cells (Fig. 3C).
In the same line, the percent viability with 5-FU treatment at 10
μg/ml for mock cells was 90%, which reduced significantly to 60% in
MUC5Bsi cells, indicating a 4-fold increase of cell death in
MUC5B-downregulated tumor cells (Fig.
3D). The augmented chemo-sensitivity to both drugs in MUC5Bsi
cells was also confirmed at different cell concentrations (Fig. 3E and F).
MUC5B expression in the culture medium
enhances the production of LPS-induced cytokine levels by DCs,
while MUC5B silencing impairs DC-maturation
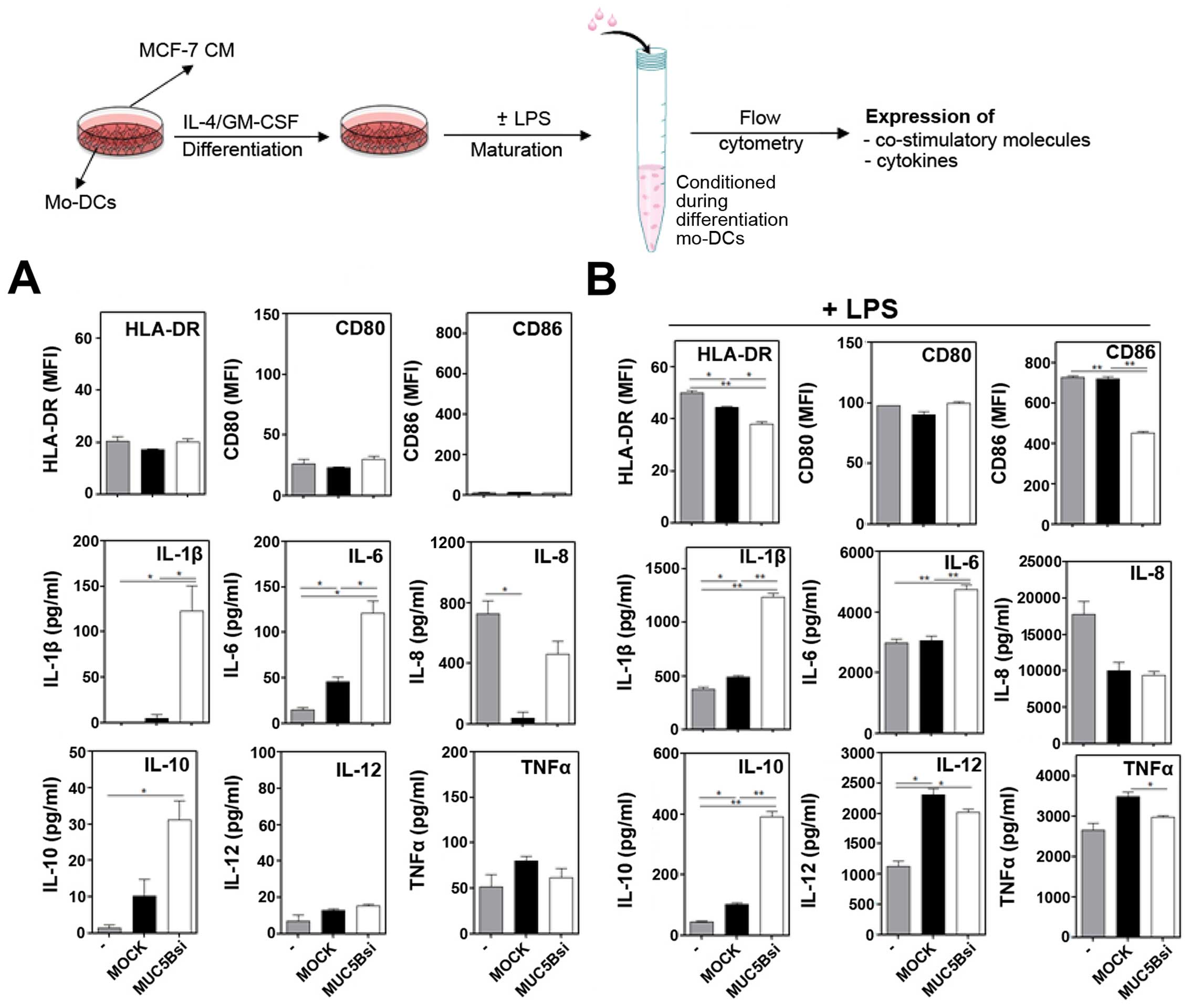
Then, we investigated whether the silencing of MUC5B
could affect the maturation of DCs, the only antigen presenting
cells capable of priming naïve T cells. Although their essential
role in cancer immunosurveillance (33), function of DCs can be compromised
with tumor growth (34). In order
to evaluate whether MUC5B silencing has an effect on the maturation
of DCs we cultured them with the conditioned medium (CM) derived
from MUC5Bsi or mock cells in the presence or absence of LPS. The
expression of different surface molecules as well as the production
of pro- and anti-inflammatory cytokines was evaluated by flow
cytometry and compared to DCs incubated with CM from mock cells.
Cultured DCs in presence of CM derived from tumor cells did not
induce significant changes on the expression of co-stimulatory or
the production of cytokines, except for the fact that incubation
with CM from MUC5Bsi cells resulted in a decreased expression of
HLA-DR (Fig. 4A).
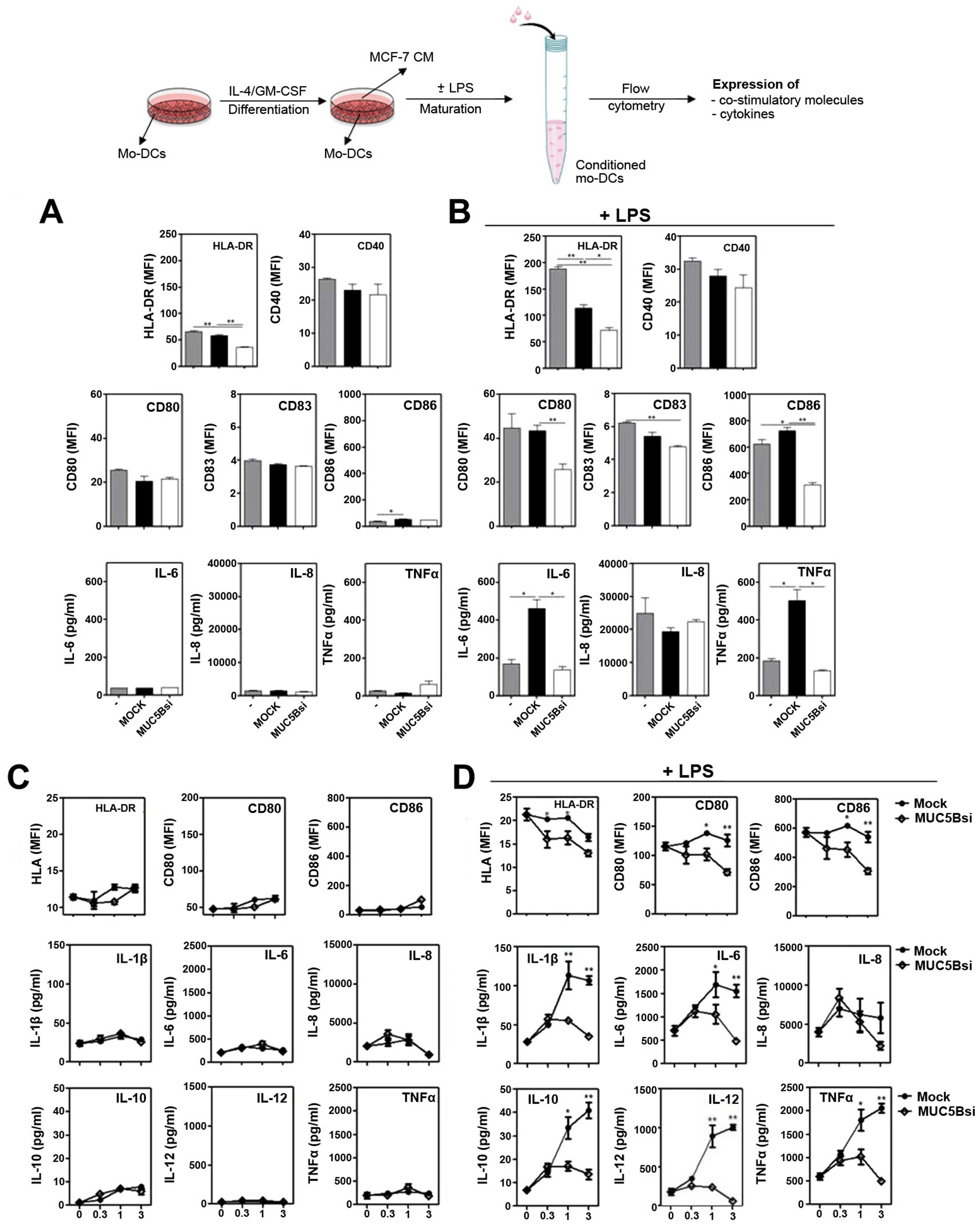 | Figure 4DCs incubated with conditioned medium
(CM) from MUC5Bsi tumor cells downregulate HLA-DR, co-stimulatory
molecules, as well as IL-6 and TNFα upon LPS stimulation. DCs were
incubated with CM from MUC5Bsi or mock MCF-7 cells (ratio basal
medium: CM, 1:1) in the absence (A) or presence (B) of LPS for 18 h
at 37°C. Expression of surface markers (HLA-DR, CD40, CD80, CD83
and CD86) and secreted cytokines (IL-6, IL-8 and TNFα was
evaluated. Alternatively, DCs were incubated with CM from MUC5Bsi
or mock MCF-7 cells (ratio basal medium:CM, 1:0, 1:0.3, 1:1 or 1:3)
in the absence (C) or presence (D) of LPS. Then, cells were stained
with anti-HLA-DR, CD80 or CD86 antibodies and evaluated by flow
cytometry. Culture supernatants were collected for IL-1β, IL-6,
IL-8, IL-10, IL-12 and TNFα quantification. Data are expressed as
mean ± SD and are representative of, at least, two independent
experiments performed with two different donors. Statistical
differences were calculated by the Student's t-test (A and B) or
the two-way ANOVA test (C and D) (*p<0.01 and
**p<0.001). |
On the contrary, when DCs were incubated in the
presence of mock CM and LPS, HLA-DR expression was significantly
reduced and associated to an upregulation of IL-6 and TNFα
(Fig. 4B). Nevertheless, when
silencing MUC5B a decrease of HLA-DR, CD80, CD83 and CD86, as well
as the IL-6 and TNFα production was observed when compared to mock
cells (Fig. 4B). To further
analyze the effect of CM on DCs, these experiments were carried out
in the presence of different doses of CM and a broader panel of
cytokines was evaluated. In the absence of a maturation stimulus we
did not observe significant changes in the expression either of
surface molecules or cytokines (Fig.
4C). However, in the presence of LPS and CM from mock cells,
not only IL-6 and TNFα production was increased, but also IL-1β,
IL-10 and IL-12 levels were augmented, while IL-8 remained
unchanged (Fig. 4D) and this
effect was dependent on the doses of CM added. The increase in the
cytokine levels was not observed with CM from MUC5Bsi cells,
indicating that MUC5B silencing on MCF-7 cells significantly
decreased the production of cytokines induced by MCF-7
derived-factors upon LPS stimulation. MUC5B silencing also resulted
in significant decrease of HLA-DR, CD80 and CD86 by DCs incubated
in presence of CM and LPS (Fig.
4D).
MUC5B silencing is associated with an
increase of IL-1β, IL-6 and IL-10 production by DCs differentiated
in the presence of tumor conditioned media
Mucins produced or shed by tumors can drain to
regional lymph nodes and enter the peripheral circulation, where
they could potentially modulate immune responses or differentiation
of DCs (22). In order to evaluate
whether MUC5B affects DC-differentiation, we incubated CM from
MUC5Bsi MCF-7 cells with monocytes in the presence of GM-CSF and
IL-4. After differentiation, LPS-maturation was induced, and the
expression of HLA-DR and co-stimulatory molecules was evaluated by
flow cytometry, as well as the production of a variety of
cytokines. As shown in Fig. 5, DCs
differentiated with CM from MUC5Bsi cells produced higher levels of
IL-1β, IL-6 and IL-10 than DCs incubated with CM from mock cells,
either in absence or presence of LPS. Interestingly, the production
of IL-8 was completely abrogated when DCs were differentiated in
the presence of CM from mock cells, while it was significantly
augmented in the presence of CM from MUC5Bsi cells (Fig. 5A). In the presence of LPS, IL-8 and
IL-12 expression was decreased and increased, respectively, with
both CM. However, TNFα, although augmented when DCs were
differentiated in the presence of CM from mock cells, was decreased
when incubated with CM from MUC5Bsi cells (Fig. 5B). Additionally, in response to
LPS, the expression of HLA-DR, CD86 and TNFα was reduced on DCs
differentiated in the presence of CM from MUC5Bsi cells as compared
to incubation with CM from mock cells (Fig. 5B).
Discussion
An altered expression of mucin has been reported to
be associated with cancer progression and can influence cell
growth, differentiation, transformation, adhesion, invasion, and
immune surveillance. In this study we evaluated the role of MUC5B
in MCF-7 breast cancer cells by knocking down its expression with
shRNA. MUC5B-silenced cells were characterized by a reduced cell
growth rate, clonogenicity and capacity to adhere to components of
the extracellular matrix, suggesting that MUC5B could participate
in the metastatic process by providing tumor cells with a more
aggressive behavior. Furthermore, the altered cell growth and
adhesion did not correlate with an increase of apoptotic cells or a
cell cycle arrest, suggesting that these processes might be under
the control of other mechanisms. Interestingly, it has been
recently reported that siRNA-mediated silencing of the secreted
mucin MUC5AC on pancreatic cells led to a decrease in the
expression of a set of α- and β-integrins (11) and the vascular endothelial growth
factor (VEGF) (12), explaining at
least in part, the reduced cell-adhesion and cell growth,
respectively, of silenced cells (11). In this context it would be
interesting to investigate whether the silencing of MUC5B in MCF-7
cells modifies the expression of these or other molecules
implicated in tumor adhesion or invasion. On the other hand, the
lower clonogenic efficiency of MUC5B-silenced MCF-7 cells could
indicate that MUC5B silencing affected the ability of single cells
to proliferate which could reduce the capacity of cancer cells to
metastasize (35). Consistent with
the results presented here, a previous study showed a significant
increase in the growth rates of MCF-7 cells overexpressing a
recombinant form of MUC5B, compared with clones expressing
endogenous amounts of MUC5B (10).
Mucins can also contribute to an augmented
aggressive behavior by conferring resistance to drug-induced death.
Our results show that MUC5B silencing in MCF-7 cells was associated
with an increase sensitivity to 5-FU or cisplatin induced-death. In
support of these findings, the ectopic overexpression of MUC4 or
the knockdown of MUC1 and MUC4 on tumor cells was associated with a
decreased sensitivity of these cells to various chemotherapeutic
drugs (9,36–40).
Since mucins are high-molecular weight glycoproteins, they could
restraint the drug accessibility to the tumor cell, limiting their
effectiveness in inducing cell-killing (4). The steric hindrance of membrane-bound
mucins is closely linked to the number and length of O-glycosidic
chains they bear. In fact, the inhibition of O-glycosylation using
benzyl-α-GalNAc both in vitro and in vivo resulted in
improved antitumor effects of 5-FU (37,41).
A more likely mechanism that could explain the drug-resistance
conferred by mucins would be through the enhancement of the
expression of multi-drug resistance genes. This was described for
MUC1 in pancreatic cancer cells, and found that the cytoplasmic
tail motif of MUC1 was associated with the promoter region of a
multi-drug resistance gene, indicating that MUC1 would act as a
transcriptional regulator (42).
It is more likely for MUC5B to confer chemo-resistance by reducing
intracellular drug uptake and hence its antitumor drug effects,
since it is a secreted mucin that could form a protection mucus web
around the cell (4).
Mucins can also modulate the immune system in the
benefice of tumor growth. For instance, MUC16 protects cancer cells
from cytotoxic responses of natural killer cells (43), probably by interacting with the
inhibitory receptor Siglec-9 (44). MUC1, on the contrary, impairs the
differentiation and function of DCs (19,22).
For example, it has been well evidenced that tumor-derived products
can selectively chemoattract immature DCs to the tumor by producing
the MIP-3α chemokine (17).
Moreover, MUC1 produced by breast cancer cells attracts immature
DCs that lack the capacity to activate Th1 cell immunity (19). Despite all the outstanding studies
performed on MUC1, and to a lesser extent on MUC4 and MUC16, very
few studies have focused on the elucidation of the role of secreted
mucins in breast cancer, and in particular, on MUC5B. We found that
MUC5Bsi-derived soluble factors impaired DC-maturation by LPS,
while those from mock cells enhanced the production of LPS-induced
cytokines. This could indicate that MUC5B produced by MCF-7 may
induce a pro-inflammatory phenotype of DCs. However, additional
experiments performed on DC conditioned with purified MUC5B are
needed to establish the direct role of MUC5B on maturation of DCs.
One possibility is that MUC5B interacts with DCs through its
glycans, since when expressed on mucins, they can modulate immunity
(45). Thus, it would be of
interest to study the glycan structures carried by MUC5B produced
by MCF-7 tumor cells and elucidate whether they mediate some of the
effects evidenced when downregulating MUC5B. Alternatively, the
silencing of MUC5B could alter the expression of other molecules
that might influence DC-maturation.
Regarding the effect of mucins on DC
differentiation, tumor-derived MUC1 has been shown to inhibit
DC-differentiation or induce an altered function of DC
differentiated in the presence of MUC1 (20,22).
It has been demonstrated that DC differentiated in the presence of
MUC1-rich CM from pancreatic cancer cells express lower levels of
MHCII and CD40 while they produce higher levels of IL-10. These
treated-DCs had an impaired capacity to trigger Th1 responses and
the ability to promote T cell regulatory activity (20). These properties have also been
described for a recombinant MUC1 glycosylated protein carrying
sialylated carbohydrate antigens, which impairs DC-differentiation
and antigen presentation, through a mechanism involving MUC1
O-glycans (22). Here, we show
that CM derived from MUC5Bsi MCF-7 tumor cells increases the
production of IL-1β, IL-6 or IL-10 by DCs during their
differentiation, in absence or presence of a maturation stimulus,
while this was not caused by mock MCF-7 cells. In this context, it
is suggested that the silencing of MUC5B could alter the expression
of other molecules that could eventually be responsible for the
immunomodulatory effects of MUC5Bsi CM.
In conclusion, we provide evidence showing that
MUC5B expression in cancer cells contributes to certain tumorigenic
properties of breast cancer cells, such as cell growth, adhesion,
clonogenic ability and drug chemo-resistance, suggesting that
targeting MUC5B expression by gene therapy could constitute a tool
of choice in the future. On the contrary, when evaluating the
effects MUC5B on differentiation and maturation of DCs, we show
that MUC5B silencing impaired LPS-maturation of DCs, and production
of cytokines. Furthermore, MUC5B silencing also influenced
DC-differentiation since it resulted in an upregulation of IL-1β,
IL-6 and IL-10. The immunomodulatory properties described in this
study could help to develop a rational design of MUC5B-based cancer
vaccines.
Acknowledgements
This study was supported by ‘Comisión Honoraria de
Lucha contra el Cáncer’ (Uruguay), Programa Grupos de Investigación
(CSIC, Universidad de la República, Uruguay) and partially financed
by FOCEM (MERCOSUR Structural Convergence Fund), COF 03/11. E.P.
García was supported by ‘Agencia Nacional de Investigación e
Innovación’ and ‘Comisión Académica de Posgrado’ (CAP, CSIC,
Uruguay). We specially acknowledge helpful advice of Dr M.G.
Kramer, Dr R. Sapiro and Dr G. Peluffo.
Abbreviations:
|
MUC5B
|
mucin 5B
|
|
DCs
|
dendritic cells
|
|
CM
|
conditioned media
|
|
MUC5Bsi
|
MUC5B-silenced cells
|
References
|
1
|
Corfield AP: Mucins: A biologically
relevant glycan barrier in mucosal protection. Biochim Biophys
Acta. 1850:236–252. 2015. View Article : Google Scholar
|
|
2
|
Kufe DW: Mucins in cancer: Function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rachagani S, Torres MP, Moniaux N and
Batra SK: Current status of mucins in the diagnosis and therapy of
cancer. Biofactors. 35:509–527. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jonckheere N, Skrypek N and Van Seuningen
I: Mucins and tumor resistance to chemotherapeutic drugs. Biochim
Biophys Acta. 1846:142–151. 2014.PubMed/NCBI
|
|
5
|
Beatson RE, Taylor-Papadimitriou J and
Burchell JM: MUC1 immunotherapy. Immunotherapy. 2:305–327. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura T and Finn OJ: MUC1 immunotherapy
is here to stay. Expert Opin Biol Ther. 13:35–49. 2013. View Article : Google Scholar
|
|
7
|
Häuselmann I and Borsig L: Altered
tumor-cell glycosylation promotes metastasis. Front Oncol.
4:282014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaur S, Kumar S, Momi N, Sasson AR and
Batra SK: Mucins in pancreatic cancer and its microenvironment. Nat
Rev Gastroenterol Hepatol. 10:607–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Workman HC, Sweeney C and Carraway KL III:
The membrane mucin Muc4 inhibits apoptosis induced by multiple
insults via ErbB2-dependent and ErbB2-independent mechanisms.
Cancer Res. 69:2845–2852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valque H, Gouyer V, Gottrand F and Desseyn
JL: MUC5B leads to aggressive behavior of breast cancer MCF7 cells.
PLoS One. 7:e466992012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoshi H, Sawada T, Uchida M, Saito H,
Iijima H, Toda-Agetsuma M, Wada T, Yamazoe S, Tanaka H, Kimura K,
et al: Tumor-associated MUC5AC stimulates in vivo tumorigenicity of
human pancreatic cancer. Int J Oncol. 38:619–627. 2011.PubMed/NCBI
|
|
12
|
Yamazoe S, Tanaka H, Sawada T, Amano R,
Yamada N, Ohira M and Hirakawa K: RNA interference suppression of
mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of
human pancreatic cancer cells. J Exp Clin Cancer Res. 29:532010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chauhan SC, Ebeling MC, Maher DM, Koch MD,
Watanabe A, Aburatani H, Lio Y and Jaggi M: MUC13 mucin augments
pancreatic tumorigenesis. Mol Cancer Ther. 11:24–33. 2012.
View Article : Google Scholar
|
|
14
|
Maher DM, Gupta BK, Nagata S, Jaggi M and
Chauhan SC: Mucin 13: Structure, function, and potential roles in
cancer pathogenesis. Mol Cancer Res. 9:531–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lakshmanan I, Ponnusamy MP, Das S,
Chakraborty S, Haridas D, Mukhopadhyay P, Lele SM and Batra SK:
MUC16 induced rapid G2/M transition via interactions with JAK2 for
increased proliferation and anti-apoptosis in breast cancer cells.
Oncogene. 31:805–817. 2012. View Article : Google Scholar :
|
|
16
|
Tinder TL, Subramani DB, Basu GD, Bradley
JM, Schettini J, Million A, Skaar T and Mukherjee P: MUC1 enhances
tumor progression and contributes toward immunosuppression in a
mouse model of spontaneous pancreatic adenocarcinoma. J Immunol.
181:3116–3125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gottfried E, Kreutz M and Mackensen A:
Tumor-induced modulation of dendritic cell function. Cytokine
Growth Factor Rev. 19:65–77. 2008. View Article : Google Scholar
|
|
18
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar
|
|
19
|
Carlos CA, Dong HF, Howard OM, Oppenheim
JJ, Hanisch FG and Finn OJ: Human tumor antigen MUC1 is chemotactic
for immature dendritic cells and elicits maturation but does not
promote Th1 type immunity. J Immunol. 175:1628–1635. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Monti P, Leone BE, Zerbi A, Balzano G,
Cainarca S, Sordi V, Pontillo M, Mercalli A, Di Carlo V, Allavena
P, et al: Tumor-derived MUC1 mucins interact with differentiating
monocytes and induce IL-10highIL-12low regulatory dendritic cell. J
Immunol. 172:7341–7349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiltbold EM, Vlad AM, Ciborowski P,
Watkins SC and Finn OJ: The mechanism of unresponsiveness to
circulating tumor antigen MUC1 is a block in intracellular sorting
and processing by dendritic cells. J Immunol. 165:3730–3741. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rughetti A, Pellicciotta I, Biffoni M,
Bäckström M, Link T, Bennet EP, Clausen H, Noll T, Hansson GC,
Burchell JM, et al: Recombinant tumor-associated MUC1 glycoprotein
impairs the differentiation and function of dendritic cells. J
Immunol. 174:7764–7772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freire T, Lo-Man R, Bay S and Leclerc C:
Tn glycosylation of the MUC6 protein modulates its immunogenicity
and promotes the induction of Th17-biased T cell responses. J Biol
Chem. 286:7797–7811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishida A, Ohta M, Toda M, Murata T, Usui
T, Akita K, Inoue M and Nakada H: Mucin-induced apoptosis of
monocyte-derived dendritic cells during maturation. Proteomics.
8:3342–3349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andrianifahanana M, Moniaux N and Batra
SK: Regulation of mucin expression: Mechanistic aspects and
implications for cancer and inflammatory diseases. Biochim Biophys
Acta. 1765:189–222. 2006.PubMed/NCBI
|
|
26
|
Roy MG, Livraghi-Butrico A, Fletcher AA,
McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK,
Song AS, Petrova YM, et al: Muc5b is required for airway defence.
Nature. 505:412–416. 2014. View Article : Google Scholar :
|
|
27
|
Sóñora C, Mazal D, Berois N, Buisine MP,
Ubillos L, Varangot M, Barrios E, Carzoglio J, Aubert JP and
Osinaga E: Immunohistochemical analysis of MUC5B apomucin
expression in breast cancer and non-malignant breast tissues. J
Histochem Cytochem. 54:289–299. 2006. View Article : Google Scholar
|
|
28
|
Buisine MP, Devisme L, Degand P, Dieu MC,
Gosselin B, Copin MC, Aubert JP and Porchet N: Developmental mucin
gene expression in the gastroduodenal tract and accessory digestive
glands. II Duodenum and liver, gallbladder, and pancreas. J
Histochem Cytochem. 48:1667–1676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Walsh MD, Clendenning M, Williamson E,
Pearson SA, Walters RJ, Nagler B, Packenas D, Win AK, Hopper JL,
Jenkins MA, et al: Expression of MUC2, MUC5AC, MUC5B, and MUC6
mucins in colorectal cancers and their association with the CpG
island methylator phenotype. Mod Pathol. 26:1642–1656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berois N, Varangot M, Sóñora C,
Zarantonelli L, Pressa C, Laviña R, Rodríguez JL, Delgado F,
Porchet N, Aubert JP, et al: Detection of bone marrow-disseminated
breast cancer cells using an RT-PCR assay of MUC5B mRNA. Int J
Cancer. 103:550–555. 2003. View Article : Google Scholar
|
|
31
|
Tiscornia I, Sánchez-Martins V, Hernández
A and Bollati-Fogolín M: Human monocyte-derived dendritic cells
from leukoreduction system chambers after plateletpheresis are
functional in an in vitro co-culture assay with intestinal
epithelial cells. J Immunol Methods. 384:164–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu HP and Chao CC: Cancer cells acquire
resistance to anticancer drugs: An update. Biomed J. 35:464–472.
2012.
|
|
33
|
Anguille S, Smits EL, Lion E, van Tendeloo
VF and Berneman ZN: Clinical use of dendritic cells for cancer
therapy. Lancet Oncol. 15:e257–e267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hegmans JP and Aerts JG: Immunomodulation
in cancer. Curr Opin Pharmacol. 17:17–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pandit TS, Kennette W, Mackenzie L, Zhang
G, Al-Katib W, Andrews J, Vantyghem SA, Ormond DG, Allan AL,
Rodenhiser DI, et al: Lymphatic metastasis of breast cancer cells
is associated with differential gene expression profiles that
predict cancer stem cell-like properties and the ability to
survive, establish and grow in a foreign environment. Int J Oncol.
35:297–308. 2009.PubMed/NCBI
|
|
36
|
Mimeault M, Johansson SL, Senapati S, Momi
N, Chakraborty S and Batra SK: MUC4 down-regulation reverses
chemoresistance of pancreatic cancer stem/progenitor cells and
their progenies. Cancer Lett. 295:69–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kalra AV and Campbell RB: Mucin impedes
cytotoxic effect of 5-FU against growth of human pancreatic cancer
cells: Overcoming cellular barriers for therapeutic gain. Br J
Cancer. 97:910–918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren J, Agata N, Chen D, Li Y, Yu WH, Huang
L, Raina D, Chen W, Kharbanda S and Kufe D: Human MUC1
carcinoma-associated protein confers resistance to genotoxic
anticancer agents. Cancer Cell. 5:163–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siragusa M, Zerilli M, Iovino F,
Francipane MG, Lombardo Y, Ricci-Vitiani L, Di Gesù G, Todaro M, De
Maria R and Stassi G: MUC1 oncoprotein promotes refractoriness to
chemotherapy in thyroid cancer cells. Cancer Res. 67:5522–5530.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Skrypek N, Duchêne B, Hebbar M, Leteurtre
E, van Seuningen I and Jonckheere N: The MUC4 mucin mediates
gemcitabine resistance of human pancreatic cancer cells via the
Concentrative Nucleoside Transporter family. Oncogene.
32:1714–1723. 2013. View Article : Google Scholar
|
|
41
|
Kalra AV and Campbell RB: Mucin
overexpression limits the effectiveness of 5-FU by reducing
intracellular drug uptake and antineoplastic drug effects in
pancreatic tumours. Eur J Cancer. 45:164–173. 2009. View Article : Google Scholar
|
|
42
|
Nath S, Daneshvar K, Roy LD, Grover P,
Kidiyoor A, Mosley L, Sahraei M and Mukherjee P: MUC1 induces drug
resistance in pancreatic cancer cells via upregulation of multidrug
resistance genes. Oncogenesis. 2:e512013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Patankar MS, Jing Y, Morrison JC, Belisle
JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A and Clark GF:
Potent suppression of natural killer cell response mediated by the
ovarian tumor marker CA125. Gynecol Oncol. 99:704–713. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Belisle JA, Horibata S, Jennifer GA,
Petrie S, Kapur A, André S, Gabius HJ, Rancourt C, Connor J,
Paulson JC, et al: Identification of Siglec-9 as the receptor for
MUC16 on human NK cells, B cells, and monocytes. Mol Cancer.
9:1182010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Freire T and Osinaga E: The sweet side of
tumor immunotherapy. Immunotherapy. 4:719–734. 2012. View Article : Google Scholar : PubMed/NCBI
|