Introduction
Lung cancer is one of the most aggressive types of
cancer, and the leading cause of cancer-related mortality.
Approximately 85% of all diagnosed cases of lung cancer are
non-small cell lung cancer (NSCLC). Extremely low survival rates in
patients with lung cancer are attributable to lack of potent
therapeutic targets and drugs against recurrent and metastatic
phenotypes (1,2). Matrix metalloproteinases (MMPs) have
been known to control cancer metastasis-related processes including
cell detachment, invasion, proliferation and angiogenesis by
degrading all components of extracellular matrix (ECM) and cell
surface molecules. High expression and activity of MMPs are well
correlated with aggressive phenotypes and poor survival of a
variety of cancers (3–6). However, strategy to regulate MMP
activity for the treatment of cancer has been disappointing in
clinical trials (7,8). Therefore, further understanding
molecular mechanisms of lung cancer growth and progression is
required for the identification of therapeutic targets and
development of potent anticancer agents.
Ligularia fischeri (L. fischeri)
(Ledeb.) Turcz. (Compositae) has been used as a traditional
medicine for the treatment of rheumatoid arthritis, scarlet fever
and jaundice in eastern Asia including Korea, China and Japan.
Previous investigations demonstrate that L. fischeri extract
and its bioactive components such as caffeic acid and chlorogenic
acid isomers possess anti-oxidant, anti-inflammatory,
anti-angiogenic and anticancer properties (9–11).
Chlorogenic acid isomers including 5-caffeoylquinic acid (5-CQA),
which have been identified in a variety of plants including green
coffee beans, walnut (Juglans regia L.) leaves, yerba-mate
(Ilex paraguariensis) extract, Petasites japonicus
extract and Marrubium vulgare extract as well as L.
fischeri extract, have been known to exert a variety of
biological functions such as anti-oxidant, anti-microbial,
anti-diabetic, anti-inflammatory and anticancer activities
(12–19). However, no detailed mechanisms of
5-CQA responsible for regulation of NSCLC cell fate has been
clearly elucidated to date. In the present study, the regulatory
effects and action mechanisms of 5-CQA on cell proliferation and
differentiation were investigated in p53 wild-type A549 and
p53-deficient H1299 NSCLC cells.
Materials and methods
Cell culture conditions
Human NSCLC cell lines (A549 and H1299) from the
American Type Culture Collection (ATCC; Manassas, VA, USA) were
grown in 10% fetal bovine serum-Dulbecco's modified Eagle's medium
(FBS-DMEM; Hyclone Laboratories, Logan, UT, USA).
Reagents
5-Caffeoylquinic acid (5-CQA) was isolated from the
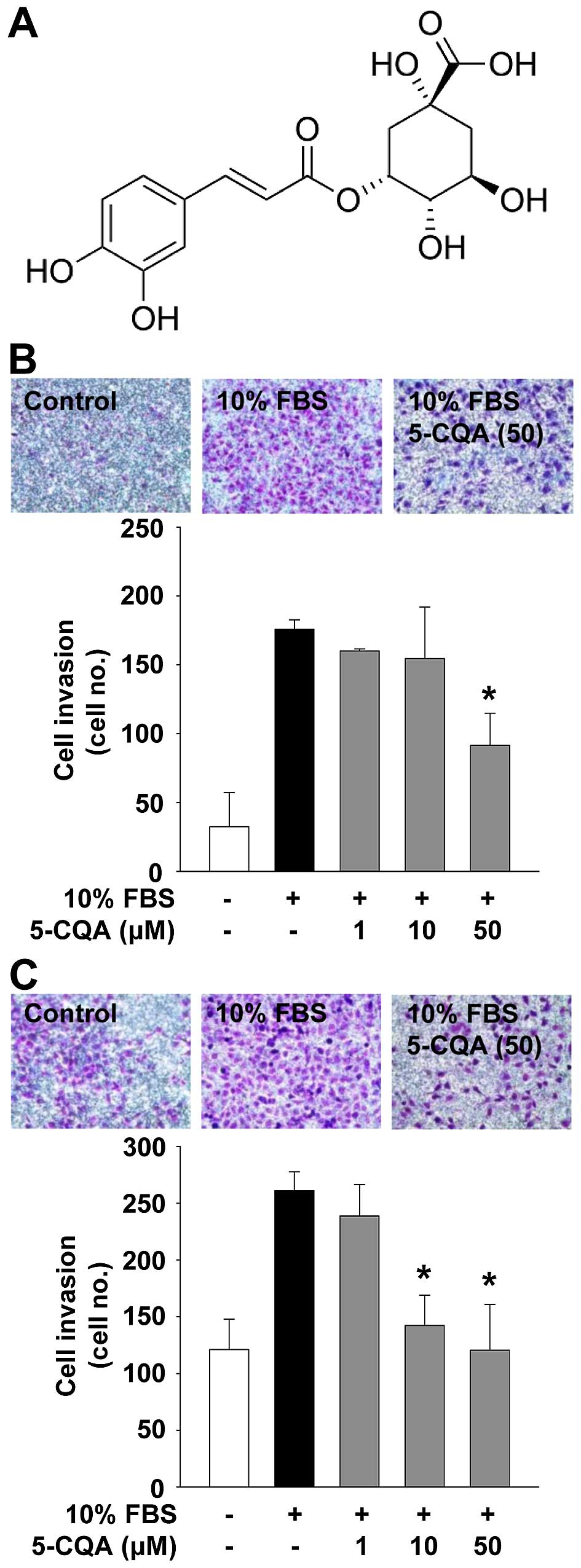
ethanolic extract of L. fischeri. The structure of 5-CQA is
presented in Fig. 1A. The
following pharmacological agents and antibodies were purchased from
commercial sources: mTOR/p70S6K inhibitor, rapamycin
(Sigma-Aldrich, St. Louis, MO, USA); PI3K/Akt inhibitor, LY294002
(Merck Millipore, Billerica, MA, USA); anti-phospho-ERK
(T202/Y204), anti-phospho-Akt (S473),
anti-phospho-p70S6K (T421/S424),
anti-phospho-p38MAPK (T180/Y182) and
anti-p38MAPK (Cell Signaling Technology, Beverly, MA,
USA); anti-integrin β1 (BD Biosciences, Bedford, MA, USA);
anti-ERK, anti-Akt, anti-p70S6K, anti-EGFR,
anti-integrin α3, anti-ILK, anti-actin antibodies, and mouse and
rabbit IgG-horseradish peroxidase conjugates (Santa Cruz
Biotechnology, Santa Cruz, CA, USA).
Cell invasion assay
The upper side of the Transwell insert (Costar, 6.5
mm diameter insert, 8 μm pore size; Corning Inc., Corning, NY, USA)
was coated with 50 μl of 1 mg/ml Matrigel® (BD
Biosciences) diluted in serum-free DMEM. Aliquots (100 μl) of cells
(5×105 cells/ml) resuspended in serum-free DMEM were
added to the upper compartment of the Matrigel-coated Transwell and
600 μl of serum-free DMEM were added to the lower compartment.
After serum starvation for 2 h, cells were pretreated with 5-CQA
(1–50 μM) for 30 min in the presence or absence of rapamycin (50
nM) or LY294002 (10 μM), followed by 10% FBS stimulation for 16 h.
The inserts were fixed with methanol and using a cotton-tipped swab
the non-invasive cells were removed from the top of the membrane.
After staining with 0.04% Giemsa staining solution (Sigma-Aldrich),
the numbers of invasive cells (mean ± standard deviation) were
determined from six different fields using x200 objective
magnification (20).
Zymogram analysis
Activities of MMPs were measured by zymography
(21). Aliquots of conditioned
medium were diluted in sample buffer, and applied to 10%
polyacrylamide gels containing 1 mg/ml gelatin (Sigma-Aldrich) as a
substrate. After electrophoresis, the gels were incubated in 2.5%
Triton X-100 for 1 h to remove SDS and allow re-naturalization of
MMPs, and further incubated in developing buffer containing 50 mM
Tris-HCl (pH 7.5), 10 mM CaCl2, and 150 mM NaCl for 16 h
at 37°C. The gels were stained with 0.5% Coomassie brilliant blue
R-250 in 30% methanol-10% acetic acid for 2 h, and followed by
destaining with 30% methanol-10% acetic acid. Gelatinolytic
activities were detected as unstained bands against the background
of the Coomassie blue-stained gelatin.
Cell viability and proliferation
assay
Subconfluent A549 and H1299 cells, plated on 6-well
plates (5×104 cells/well; SPL Life Sciences Co., Ltd.,
Gyeonggi-do, Korea), were serum-starved for 24 h in basal DMEM to
synchronize cells in the G1/G0 phase of the
cell cycle, and treated with 5-CQA (1–50 μM) for 30 min prior to
10% FBS stimulation for 24 h. Following culture for 24 h, cell
viability was determined by a Muse™cell analyzer using
cell count and viability assay kit (Merck Millipore), and the cell
proliferation was quantified as previously described (22). The results from triplicate
determinations (mean ± standard deviation) are presented as the
percentage of viable cells of total cell count or the fold-increase
of the untreated controls.
Cell cycle analysis
Quiescent cells were pretreated with 5-CQA (50 μM)
for 30 min, and further incubated with 10% FBS for 24 h. Cells were
harvested with trypsin-EDTA, rinsed with phosphate-buffered saline
(PBS, pH 7.4) and then fixed with ice-cold 70% ethanol for 3 h.
After washing with PBS, cells were stained with Muse™
cell cycle reagent (Merck Millipore). The profile of cells in the
G1/G0, S and G2/M phases of the
cell cycle was analyzed with a Muse cell analyzer (23).
Western blot analysis
Quiescent cells were pretreated with 5-CQA for 30
min, followed by 10% FBS stimulation for 15 min or 24 h. Cells were
rinsed twice with ice-cold PBS and lysed by incubation in 50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM
EDTA, 100 μg/ml AEBSF, 10 μg/ml aprotinin, 1 μg/ml pepstatin A, 0.5
μg/ml leupeptin, 80 mM β-glycerophosphate, 25 mM sodium fluoride
and 1 mM sodium orthovanadate for 30 min at 4°C. Cell lysates were
clarified at 13,000 x g for 20 min at 4°C, and the supernatants
were subjected to western blot analysis as previously described
(24). All western blots are
representative of at least three independent experiments. Bands of
interest were integrated and quantified by the use of National
Institutes of Health (NIH) ImageJ version 1.34s software.
Cell adhesion assay
Subconfluent cells were detached with trypsin-EDTA
and allowed to recover in 10% FBS-DMEM for 1 h at 37°C with gentle
rocking. After recovery, the cells were collected by low-speed
centrifugation and resuspended in serum-free DMEM. The cell
suspension was pretreated with 5-CQA for 30 min, and followed by
10% FBS stimulation. The cells were plated on 96-well plates
(1.5×104 cells/well), and further incubated for 1 h at
37°C. Following incubation unattached cells were removed by washing
the wells three times with PBS. Attached cells were fixed with
methanol, and then stained with 0.04% Giemsa staining solution. The
cells were photographed and counted. The results (mean ± standard
deviation) are presented as the number of adherent cells (25,26).
Statistical analysis
Statistical analysis was performed using the
Student's t-test, and was based on at least three different
experiments. The results were considered to be statistically
significant at P<0.05.
Results
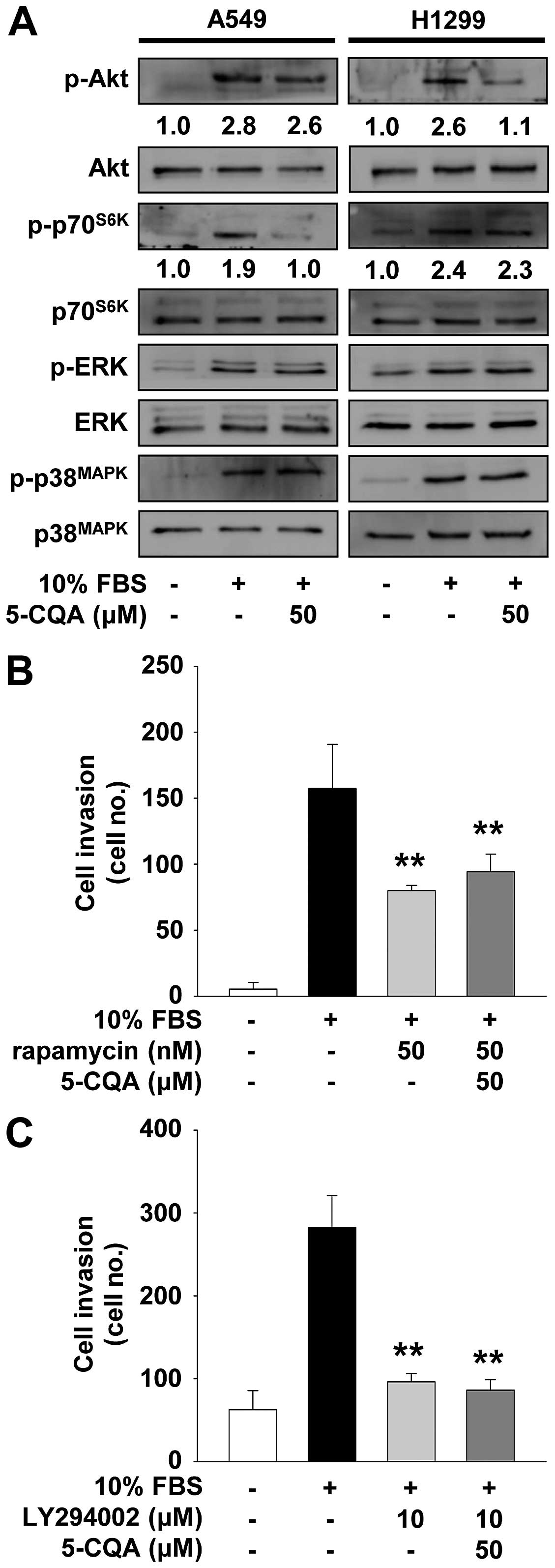
5-CQA inhibits NSCLC cell invasion
We first analyzed the effect of 5-CQA on cell
invasion which plays pivotal roles in cancer progression. 5-CQA
treatment dose-dependently blocked mitogen-stimulated cell invasion
in p53 wild-type A549 and p53-deficient H1299 NSCLC cells (Fig. 1B and C). H1299 cells appeared to be
more responsive to 5-CQA-mediated inhibition of cell invasion, as
compared with A549 cells, indicating that anti-invasive activity of
5-CQA might be dependent on p53 expression status. We next examined
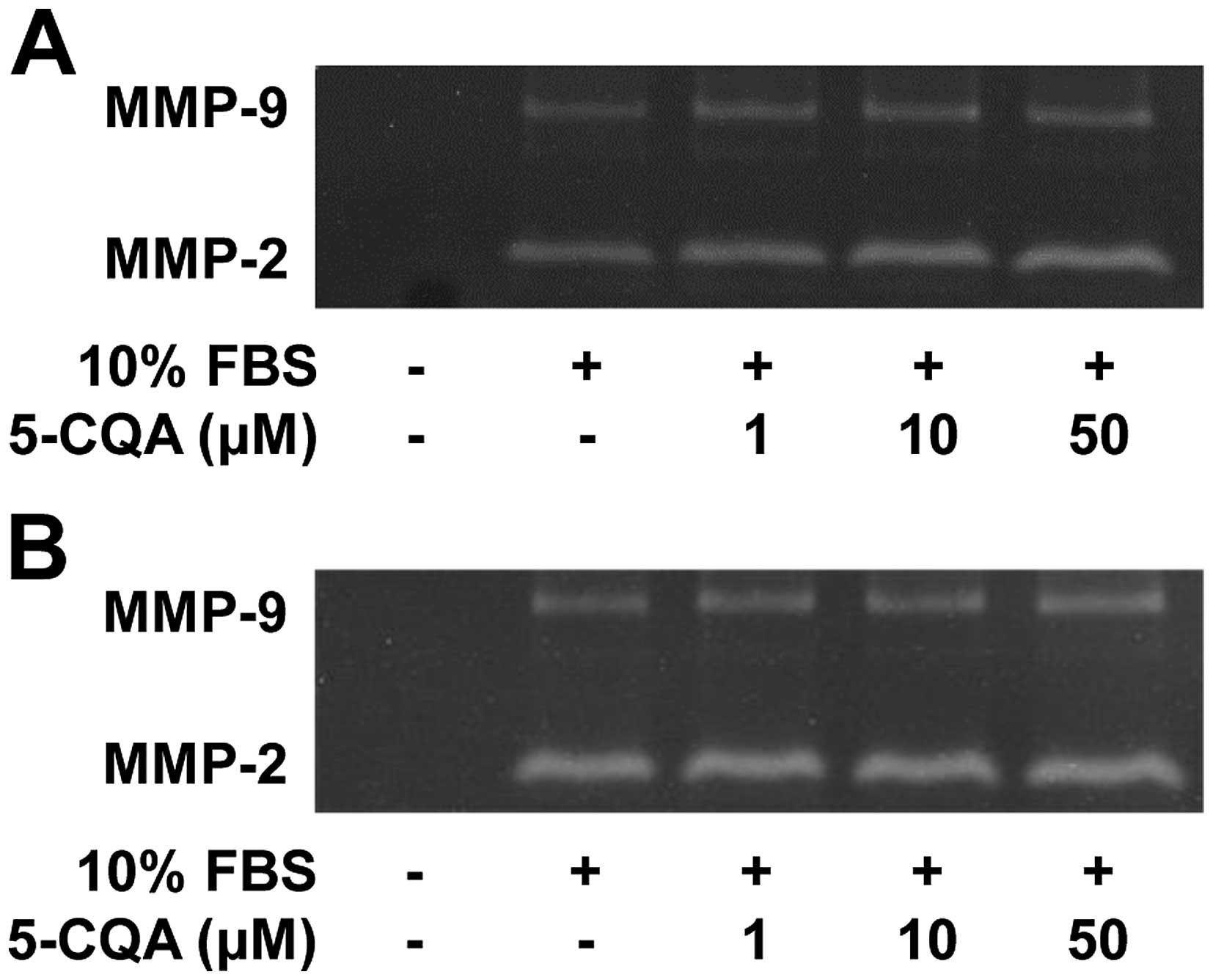
the activity of matrix metalloproteinases (MMPs) in 5-CQA-treated
NSCLC cells. As shown in Fig. 2,
the conditioned media from cell cultures had high levels of MMP-2
activity relative to those of MMP-9. 5-CQA treatment did not alter
activity of MMP-2 and MMP-9 in response to mitogenic stimulation.
In addition, the levels of MMP-2, MMP-9 or tissue inhibitor of
metalloproteinase-2, an endogenous inhibitor of MMP, were not
changed in 5-CQA-treated cells (data not shown), suggesting the
inhibitory effect of 5-CQA on cell invasion might be mediated
through an MMP-independent mechanism. However, the possibility that
5-CQA may regulate the expression and activity of other MMPs and
their endogenous inhibitors cannot be excluded (3,27–29).
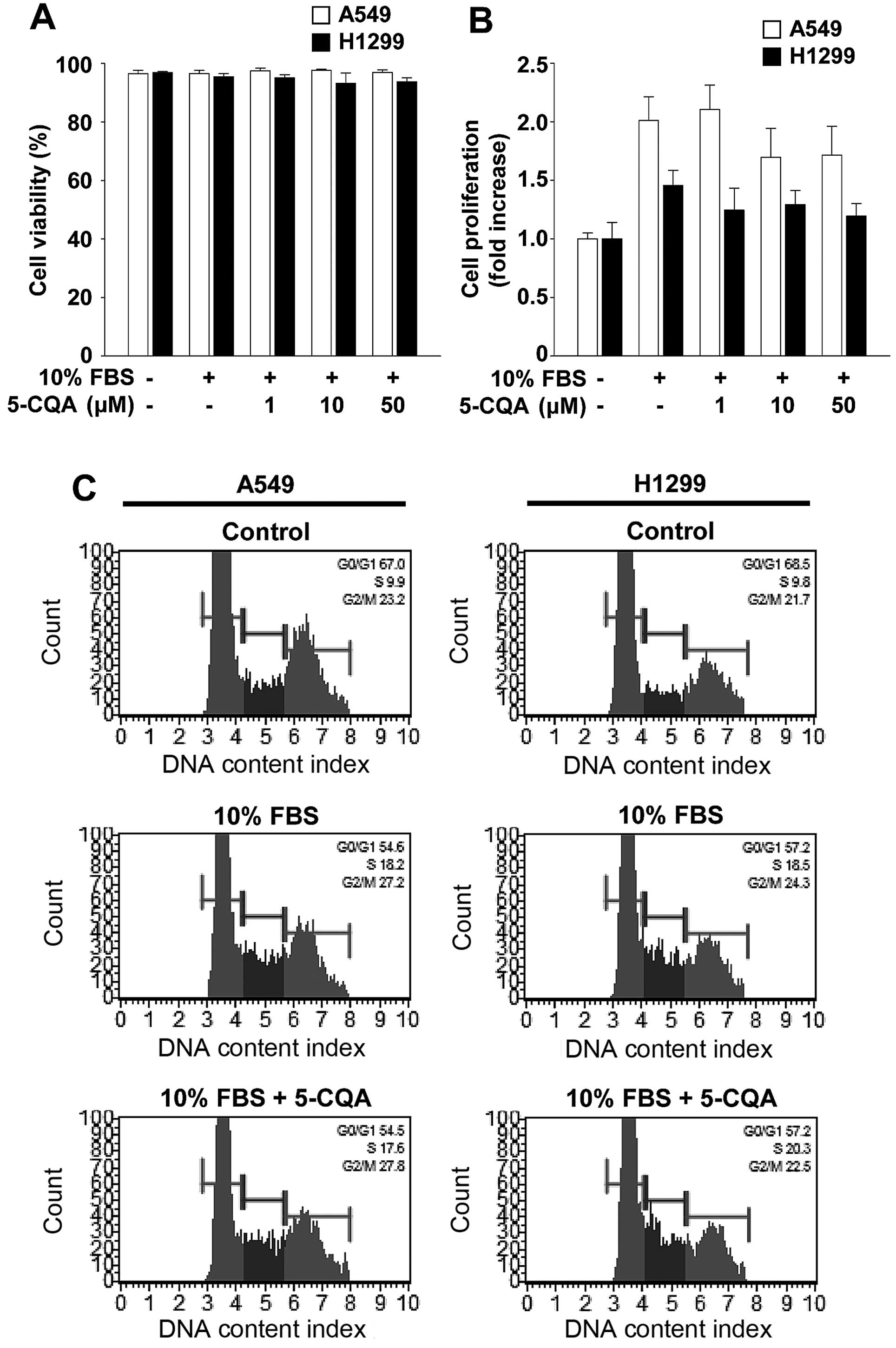
5-CQA does not alter viability and
proliferation in NSCLC cells
Based on 5-CQA-mediated inhibition of cell invasion,
we investigated the possibility that cytotoxicity or
anti-proliferative effect of 5-CQA might mediate anti-invasive
activity. 5-CQA treatment did not significantly alter cell
viability and proliferation in A549 or H1299 cells (Fig. 3A and B). To ascertain that 5-CQA
had little or no effect on cell proliferation, we next examined the
ability of 5-CQA to regulate cell cycle progression (Fig. 3C). Mitogenic stimulation for 24 h
increased the percentage of cells in S and G2/M phases,
and simultaneously decreased the percentage of cells in
G1 phase, compared with untreated controls. 5-CQA
treatment did not alter the percentage of G1, S and
G2/M phases of the cell cycle associated with mitogenic
stimulation. Moreover, our initial experiments indicate that 5-CQA
treatment did not change the percentage of live, apoptotic or dead
cells in either cell line at the highest concentration used in this
study (data not shown). Collectively, these findings demonstrate
that 5-CQA directly regulates cell invasion without any effect on
cell cycle progression, cell proliferation or cell viability in
NSCLC cells.
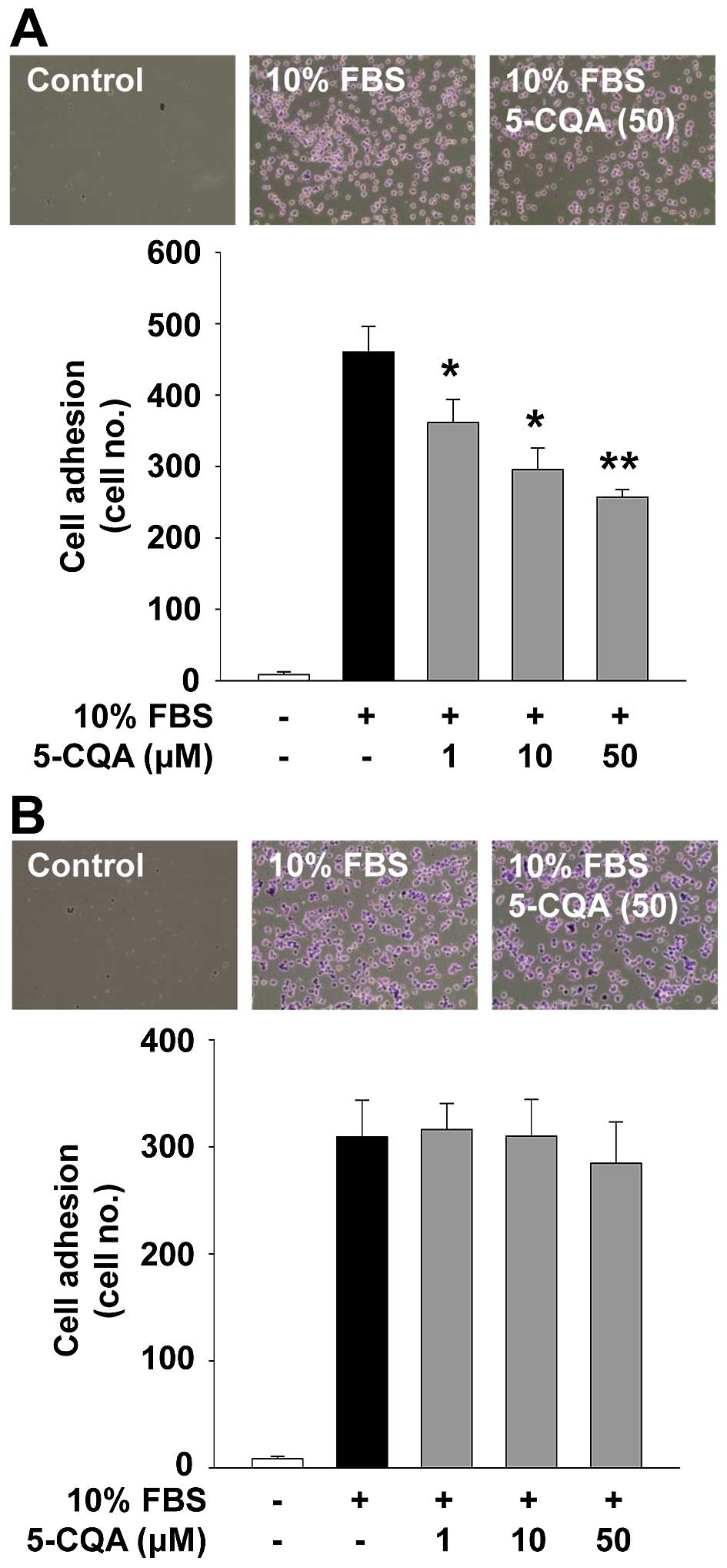
5-CQA inhibits adhesion in A549
cells
Cell adhesion and migration associated with cancer
cell growth and progression are controlled by interactions with ECM
molecules and cellular components (4). We next investigated the ability of
5-CQA to regulate cell adhesion. As shown in Fig. 4, 5-CQA treatment dose-dependently
suppressed mitogen-stimulated cell adhesion in A549 cells, but not
in H1299 cells. Although the inhibitory effect and functional
consequences of 5-CQA on cell adhesion in A549 cells remain to be
addressed, 5-CQA-mediated inhibition of cell adhesion did not
appear to affect the cell viability and proliferation (Fig. 3A and B), and may contribute to the
modulation of cell invasion (Fig. 1B
and C).
Differential regulation of 5-CQA in
mitogen-stimulated signaling pathways in NSCLC cells
To investigate the molecular mechanisms and
therapeutic targets of 5-CQA in regulating cell invasion, we first
examined the changes in the expression of cell surface
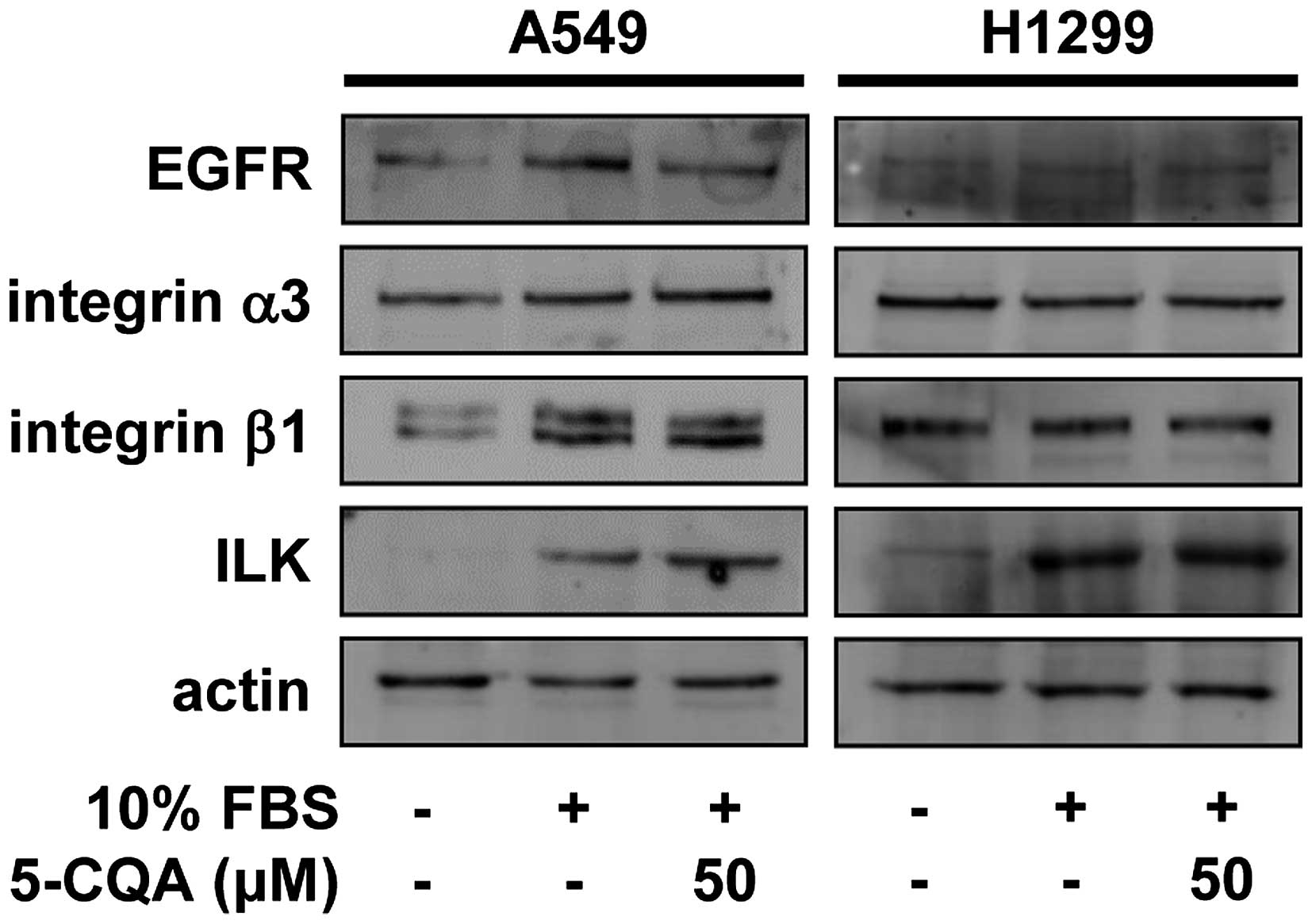
signaling-related molecules such as epidermal growth factor
receptor (EGFR), integrin α3β1 and integrin-linked kinase (ILK) in
5-CQA-treated NSCLC cells (30,31).
5-CQA treatment did not significantly change the expression of
EGFR, integrin α3β1 and ILK in either cell line (Fig. 5), raising the possibility that
5-CQA-mediated inhibition of cell invasion might be mediated
through the regulation of downstream signaling pathways of receptor
tyrosine kinases (RTKs) and integrins. Therefore, we next analyzed
the changes in activation of mitogen-stimulated signaling pathways
including phosphatidylinositol 3-kinase (PI3K)/Akt, mammalian
target of rapamycin (mTOR)/p70S6K, extracellular
signal-regulated kinase (ERK) and p38 mitogen-activated protein
kinase (p38MAPK) in 5-CQA-treated cells (10,20,32).
As shown in Fig. 6A, mitogenic
stimulation increased the phosphorylation/activation of Akt,
p70S6K, ERK and p38MAPK, as compared with
unstimulated controls. 5-CQA treatment markedly inhibited
mitogen-stimulated activation/phosphorylation of p70S6K,
but not Akt, ERK and p38MAPK, in A549 cells.
Pretreatment of A549 cells with rapamycin, an inhibitor of
mTOR/p70S6K pathway, mimicked the suppressive effect of
5-CQA on cell invasion (Fig. 6B).
In contrast, 5-CQA treatment inhibited mitogen-stimulated
phosphorylation of Akt in H1299 cells (Fig. 6A). Inhibition of PI3K/Akt signaling
pathway by LY294002 suppressed cell invasion similarly in
5-CQA-treated H1299 cells (Fig.
6C). Co-treatment with 5-CQA did not enhance the inhibitory
effect of these chemical inhibitors on cell invasion, suggesting
that 5-CQA and these inhibitors may share similar roles and
mechanisms of action in regulating NSCLC cell invasion.
Discussion
L. fischeri has been consumed as an edible
herb and traditional medicine for the treatment of inflammatory and
infectious diseases. 5-Caffeoylquinic acid (5-CQA), a chlorogenic
acid isomer isolated from a variety of plants including L.
fischeri, has been reported to possess anti-oxidant,
anti-bacterial and anti-inflammatory activities (13–16,19).
In addition, some previous studies demonstrate that 5-CQA exerts
anticancer activity against several types of cancer cells including
breast and colon cancer (12,18,33).
However, the effects and molecular mechanism of 5-CQA on lung
cancer cell growth and progression have not yet been reported.
Overexpression or dysregulated activation of EGFR is
known to be closely correlated with malignancy and poor prognosis
in human lung cancer, suggesting the potential role of EGFR and its
downstream signaling pathways as therapeutic targets for the
treatment of lung cancer (30). In
addition, recent studies demonstrate that cross-talk between RTKs
including EGFR and cell adhesion receptors such as integrins plays
pivotal roles in cancer growth and progression (31). Therefore, identification of key
molecular targets and their roles in RTK/integrin signaling
pathways is required for the development of potential therapeutic
strategies and agents to treat cancer.
In the present study, we demonstrate that 5-CQA
exhibits strong anti-invasive activity against both p53-positive
and p53-negative NSCLC cells without any influence on cell
proliferation, apoptosis or cytotoxicity. In addition, 5-CQA does
not alter the expression of EGFR and integrin α3β1, but
differentially modulates mitogen-stimulated signaling pathways,
depending on the status of p53 expression in NSCLC cells.
Inactivation of p70S6K and Akt by 5-CQA contributes to
inhibition of cell invasion in p53 wild-type A549 and p53-deficient
H1299 cells, respectively, suggesting the possibility of p53
involvement in 5-CQA-mediated differential regulation of
mitogen-stimulated signaling components. In conclusion, this is the
first report that 5-CQA exerts anti-invasive activity against NSCLC
cells through p53-dependent regulation of signaling pathways, and
warrants further evaluation and development of 5-CQA as a potent
anticancer agent for the treatment of NSCLC.
Acknowledgements
The present study was supported by the Research Fund
of Dankook University in 2015.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SH, Cho YR, Kim HJ, Oh JS, Ahn EK, Ko
HJ, Hwang BJ, Lee SJ, Cho Y, Kim YK, et al: Antagonism of
VEGF-A-induced increase in vascular permeability by an integrin
α3β1-Shp-1-cAMP/PKA pathway. Blood. 120:4892–4902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Overall CM and Kleifeld O: Tumour
microenvironment - opinion: Validating matrix metalloproteinases as
drug targets and anti-targets for cancer therapy. Nat Rev Cancer.
6:227–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vandenbroucke RE and Libert C: Is there
new hope for therapeutic matrix metalloproteinase inhibition? Nat
Rev Drug Discov. 13:904–927. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JH, Kim HJ, Kim JK, Ahn EK, Ko HJ, Cho
YR, Lee SJ, Bae GU, Kim YK, Park JW, et al: Ligularia fischeri
inhibits endothelial cell proliferation, invasion and tube
formation through the inactivation of mitogenic signaling pathways
and regulation of vascular endothelial cadherin distribution and
matrix metalloproteinase expression. Oncol Rep. 34:221–226.
2015.PubMed/NCBI
|
|
10
|
Cho YR, Kim JK, Kim JH, Oh JS and Seo DW:
Ligularia fischeri regulates lung cancer cell proliferation and
migration through down-regulation of epidermal growth factor
receptor and integrin β1 expression. Genes Genomics. 35:741–746.
2013. View Article : Google Scholar
|
|
11
|
Lee HN, Kim JK, Kim JH, Lee SJ, Ahn EK, Oh
JS and Seo DW: A mechanistic study on the anti-cancer activity of
ethyl caffeate in human ovarian cancer SKOV-3 cells. Chem Biol
Interact. 219:151–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwai K, Kishimoto N, Kakino Y, Mochida K
and Fujita T: In vitro antioxidative effects and tyrosinase
inhibitory activities of seven hydroxycinnamoyl derivatives in
green coffee beans. J Agric Food Chem. 52:4893–4898. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pereira JA, Oliveira I, Sousa A, Valentão
P, Andrade PB, Ferreira ICFR, Ferreres F, Bento A, Seabra R and
Estevinho L: Walnut (Juglans regia L.) leaves: Phenolic compounds,
antibacterial activity and antioxidant potential of different
cultivars. Food Chem Toxicol. 45:2287–2295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Z, Shin HS, Satsu H, Totsuka M and
Shimizu M: 5-caffeoylquinic acid and caffeic acid down-regulate the
oxidative stress- and TNF-α-induced secretion of interleukin-8 from
Caco-2 cells. J Agric Food Chem. 56:3863–3868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berté KA, Beux MR, Spada PK, Salvador M
and Hoffmann-Ribani R: Chemical composition and antioxidant
activity of yerba-mate (Ilex paraguariensis A.St.-Hil,
Aquifoliaceae) extract as obtained by spray drying. J Agric Food
Chem. 59:5523–5527. 2011. View Article : Google Scholar
|
|
16
|
Kim SM, Kang SW, Jeon J-S, Jung YJ, Kim
CY, Pan CH and Um BH: Rapid identification and evaluation of
antioxidant compounds from extracts of Petasites japonicus by
hyphenated-HPLC techniques. Biomed Chromatogr. 26:199–207. 2012.
View Article : Google Scholar
|
|
17
|
Boudjelal A, Henchiri C, Siracusa L, Sari
M and Ruberto G: Compositional analysis and in vivo anti-diabetic
activity of wild Algerian Marrubium vulgare L. infusion.
Fitoterapia. 83:286–292. 2012. View Article : Google Scholar
|
|
18
|
Murad LD, Soares NC, Brand C, Monteiro MC
and Teodoro AJ: Effects of caffeic and 5-caffeoylquinic acids on
cell viability and cellular uptake in human colon adenocarcinoma
cells. Nutr Cancer. 67:532–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu SL, Peng BJ, Zhong YL, Liu YL, Song Z
and Wang Z: Effect of 5-caffeoylquinic acid on the NF-κB signaling
pathway, peroxisome proliferator-activated receptor gamma 2, and
macrophage infiltration in high-fat diet-fed Sprague-Dawley rat
adipose tissue. Food Funct. 6:2779–2786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HN, Joo JH, Oh JS, Choi SW and Seo DW:
Regulatory effects of Siegesbeckia glabrescens on non-small cell
lung cancer cell proliferation and invasion. Am J Chin Med.
42:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho YR, Kim JH, Kim JK, Ahn EK, Ko HJ, In
JK, Lee SJ, Bae GU, Kim YK, Oh JS, et al: Broussonetia kazinoki
modulates the expression of VEGFR-2 and MMP-2 through the
inhibition of ERK, Akt and p70S6K-dependent signaling pathways: Its
implication in endothelial cell proliferation, migration and
tubular formation. Oncol Rep. 32:1531–1536. 2014.PubMed/NCBI
|
|
22
|
Kim JH, Kim JK, Ahn EK, Ko HJ, Cho YR, Lee
CH, Kim YK, Bae GU, Oh JS and Seo DW: Marmesin is a novel
angiogenesis inhibitor: Regulatory effect and molecular mechanism
on endothelial cell fate and angiogenesis. Cancer Lett.
369:323–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joo JH, Hong SS, Cho YR and Seo DW:
10-Gingerol inhibits proliferation and invasion of MDA-MB-231
breast cancer cells through suppression of Akt and p38MAPK
activity. Oncol Rep. 35:779–784. 2016.
|
|
24
|
Kim HJ, Cho YR, Kim SH and Seo DW:
TIMP-2-derived 18-mer peptide inhibits endothelial cell
proliferation and migration through cAMP/PKA-dependent mechanism.
Cancer Lett. 343:210–216. 2014. View Article : Google Scholar
|
|
25
|
Yoon HJ, Cho YR, Joo JH and Seo DW:
Knockdown of integrin α3β1 expression induces proliferation and
migration of non-small cell lung cancer cells. Oncol Rep.
29:662–668. 2013.
|
|
26
|
Kim HJ, Ko HY, Choi SW and Seo DW:
Anti-angiogenic effects of Siegesbeckia glabrescens are mediated by
suppression of the Akt and p70S6K-dependent signaling pathways.
Oncol Rep. 33:699–704. 2015.
|
|
27
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): An ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seo DW, Li H, Guedez L, Wingfield PT, Diaz
T, Salloum R, Wei BY and Stetler-Stevenson WG: TIMP-2 mediated
inhibition of angiogenesis: An MMP-independent mechanism. Cell.
114:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: metalloproteinase-independent
biological activities. Sci Signal. 1:re62008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar
|
|
32
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taira J, Uehara M, Tsuchida E and Ohmine
W: Inhibition of the β-catenin/Tcf signaling by caffeoylquinic
acids in sweet potato leaf through down regulation of the Tcf-4
transcription. J Agric Food Chem. 62:167–172. 2014. View Article : Google Scholar
|