Introduction
It is common knowledge that not only steroid sex
hormones are implicated in the development and progression of
cancer of reproductive and non-reproductive tissues. Deregulation
of certain peptide hormones, such as angiotensin and relaxin, has
been linked to a higher risk of certain cancers or a poor prognosis
in patients (1–3). Changes in selected components of the
renin-angiotensin system (RAS) and relaxin family peptide system
have been observed in pathological states of the prostate,
including the chronic inflammatory state, prostatic hyperplasia
(BPH) or prostate cancer (PC). It has been shown that these
peptides are able to modulate cell proliferation and apoptosis,
invasion and spread of many types of cells. High expression of
relaxin 2 and the relaxin receptor LGR7/RXFP1 as well as
angiotensin II and angiotensin receptor type 1 (AT1) was observed
in tumor tissue compared to normal prostate tissue. Studies suggest
that both the expression of the LGR7/ RXFP1 and AT1 receptors can
be new early markers of meta-static potential in primary tumors
(4–8).
Interactions between RAS and the relaxin family
peptide system have been reported in organ fibrosis (9), blood pressure, in the cardiovascular
system (10,11), and osmoregulation, for the central
nervous system (12,13) but so far, never in the
carcinogenesis of reproductive tissues. However, this study is the
first to present the influence of the combined action of
angiotensin II and relaxin 2 on various aspects of prostate cancer
development and progression.
The effects of these peptide hormones, alone and in
combination, were investigated on cell viability and proliferation
in both two-dimensional (2D) and three-dimensional (3D) Geltrex™
cultures. The 3D culture system allows complex interactions to take
place between normal cell-cell and cell-extracellular matrices
which are not possible under monolayer growth conditions (14). Survival pathway analysis included
the expression of the anti-apoptotic genes BCL2, survivin
and pro-apoptotic BAX. Studies indicate that survivin
(BIRC5), an inhibitor of apoptosis, mediates resistance to
anti-androgen therapy in prostate cancer (15). High levels of BIRC5 mRNA
expression and decreased BAX to BCL2 ratio are
predictors of poor prognosis in many cancer types. These results
suggest that BIRC5 expression or the BCL2/BAX
expression ratio may be a potential molecular marker for the
progression and aggressiveness of prostate cancer (15–17).
Adhesion plays an important role in both the
migration and invasion of cancer cells (14). Therefore, we examined the influence
of the peptides on the ability of prostate cancer cells to
proliferate without adhering to the substratum, as well as their
ability to adhere to extracellular matrix (ECM) proteins. The
potential relationship between angiotensin II and relaxin 2
regarding the secretion levels of metalloproteinases-2 and
metalloproteinase-9 was also assessed. The action of matrix
metalloproteinases (MMPs), enzymes which mediate the degradation of
basement membrane proteins, was found to strictly correlate with
the invasion and metastasis of various malignancies. Significantly
higher plasma levels of MMP-2 and MMP-9 were diagnosed in patients
with metastatic prostate cancer than patients with organ-confined
carcinomas and healthy controls (18).
Materials and methods
Cell lines and 2D/3D culture
conditions
LNCaP (American Type Culture Collection; CRL-1435),
is a hormone-dependent line with high androgen receptor (AR)
activity. The cells of the LNCaP line are characterised by
relatively slow growth and low invasiveness. PC3 cells (American
Type Culture Collection; CRL-1740) display high metastatic
potential and low AR expression (19). Cell lines were authenticated by
short-tandem repeat (STR) DNA profiling (LGC Standards Cell Line
Authentication Service, Germany). The cells were grown in
two-dimensional (2D) cell cultures or three-dimensional (3D)
basement membrane cultures. The cells were seeded in plates or
flasks (BD Biosciences) and were maintained in Advanced RPMI-1640
supplemented with 5% fetal bovine serum (FBS), 2 mM L-glutamine, 1
mM sodium pyruvate and antibiotics. For 3D culture, plates were
coated with Geltrex LDEV-FreeBasement Membrane Matrix, according to
the manufactures instructions Gibco® (Thermo Fisher
Scientific, Inc.). Moreover, the culture medium additionally
contained 2% Geltrex. Both cell lines were incubated at 37°C, with
5% carbon dioxide. The cells were passaged at least twice after
thawing from liquid nitrogen. For further experiments, cells
passaged between 9 and 18 times were used.
Reagents
Angiotensin II (H-1705) and relaxin 2 (H-6784) were
obtained from Bachem. The peptides were used at a final
concentration of 10−8 M for all experiments. This
concentration was selected on the basis of earlier research work
and trial experiments (6). The
medium, FBS and other culture supplements / reagents were purchased
from Gibco (Thermo Fisher Scientific, Inc.) unless otherwise
specified.
Two-dimensional (2D) MTT and Alamar blue
assay
The cells were seeded in a 96-well flat bottom plate
and were maintained overnight in complete medium at 37°C and 5%
CO2. The cells were then incubated for 48 h with RLN2,
Ang II alone or with a combination of both. Four hours prior to the
end of the incubation period, 5 mg/ml MTT solution was diluted to a
final concentration of 0.5 mg/ml and added to each well; this would
clarify the difference in the concentrations. The formazan crystals
formed by viable cells were dissolved in DMSO (100 μl) and
measured using a microplate reader (BioTek®) at 570 nm.
Alamar Blue® (Molecular Probes®, Thermo
Fisher Scientific, Inc.) was added to each well and was incubated
for 1 h. During incubation, the color of Alamar blue changed from
blue to red. Absorbance was monitored at 570 and 600 nm. The cell
viability (%) was calculated relative to control cells cultured in
complete growth medium without RLN2 and Ang II.
Three-dimensional (3D) WST-1 assay
LNCaP and PC3 cells were cultured in a 3D
environment. Following a 5-day 3D culture, the medium was removed
and fresh medium, along with peptide hormones (Ang II, RLN2 or Ang
II + RLN2) were added to the cultures. After 48 h of incubation,
WST-1 was added to each well and incubated for another 1–2 h. A
colorimetric indicator changed color from red to yellow depending
on the number and metabolic activity of live cells. The colored
reduction product was quantified using a microplate reader (BioTek)
at 450 nm. The effect of peptide hormones on cell viability was
calculated as a percentage of the metabolic activity measured in
the non-treated cells.
BrdUrd proliferation assay
Detailed procedures for the colorimetric BrdUrd
proliferation assay (Roche Diagnostics; 11647229001) have been
previously described (20). The
cells were seeded in a 96-well flat bottom plate and were
maintained overnight in complete medium at 37°C and 5%
CO2. Then cells were treated with indicated
concentration of Ang II and/ or RLN2 for 48 h. Bromodeoxyuridine
(BrdUrd) was added to the culture medium in the last 4 h of
incubation. Absorbance was measured using a microplate reader
(BioTek) at 450 nm. Cell proliferation (% of control) was
calculated in relation to untreated controls.
Adhesion assay
Cells were seeded in a 6-well plate, with or without
peptide hormones at a final concentration of 10−8 M. The
cells were then collected after 24 or 48 h of incubation time. The
populations of cells that detached from the substrate were added at
concentrations of 1×105 cells/well to ECM-coated wells
in RPMI/FBS-free medium, using 24-well tissue culture plates coated
with human plasma fibronectin, laminin, collagen I or IV (BD
Biosciences). After 90 min incubation at 37°C in a humidified
atmosphere of 95% air - 5% CO2, the cells were gently
washed with PBS to remove non-adherent cells. Samples were fixed
and stained with a 0.1% solution of crystal violet in 25% ethanol.
Finally, cells were rinsed in water and solubilized by adding 10%
acetic acid. The intensity of the violet color was measured by a
microplate reader (BioTek) at 570 nm. The data are presented as
percentage relative to the untreated control.
Transwell invasion assay
In a similar way to the adhesion assay, the cells
were seeded in a 6-well plate, with or without peptide hormones,
and collected after 24–48 h of incubation time by typsinization.
The cell suspension and standard medium, or experimental medium
with peptides, were mixed at a final concentration 1×106
cells/ml, and added to the upper chambers. The undersides of the
filters were made of 8 μm pores which were coated with
Matrigel (BD Biosciences). The lower chamber contained standard
medium with 10% FBS. The cells were incubated for 48 h at 37°C with
5% CO2. After this time, the incubated non-invading
cells that remained on the upper surface of the filter were
mechanically removed. Cells which migrated to the lower surface of
the filter were fixed and stained with 1 mg/ml crystal violet
solution prepared in 10% ethanol and then dissolved in 10% acetic
acid. The cell invasion potential was quantified by measuring the
absorbance at 570 nm (BioTek). The results are expressed as the
percentage ratio over untreated, control cells.
Soft agar colony assay
The assay was performed in a 6-well plate coated
with 0.9% agar in growth medium. Cells were prepared by
trypsinization and suspended at a final concentration of
1×104 cells/ml of 0.3% agarose in complete medium. The
cells were then plated on the top of the agar and maintained at
37°C in a humidified incubator with 5% CO2. Every 2–3
days, the cells were treated with another dose of the peptide
hormones: Ang II, RLN2 or Ang II + RLN2. After 21 days, the
colonies were stained with 0.05% crystal violet for at least 1 h.
The results were recorded and documented by scanning the plates.
The number of colonies in each well were counted using ImageJ, a
free image processing program from Wayne Rasband at the National
Institutes of Health (http://imagej.nih.gov/ij).
Gelatin zymography assay
The matrix metalloproteinase activity (MMP-2 and
MMP-9) in normal prostate epithelial cells, was determined by
gelatin zymography, before and after peptide hormone treatment, as
previously described (21). The
prostate cancer cells were plated onto 6-well plates and incubated
until 80% confluence, after which the medium was changed to fresh
serum-free medium with or without peptide hormones at a
concentration of 10−8 M. After 24 h of incubation, the
conditioned culture medium was collected and stored at −80°C. The
protein concentration was measured by Qubit® Protein
Assay kit (Invitrogen™ Thermo Fisher Scientific, Inc.). Protein
extracts (5–10 μg) were subjected to electrophoresis in a
10% polyacrylamide gel in the presence of 4% gelatin. After the
electrophoresis runs, the gels were washed for 1.5 h in 2.5% Triton
X-100 at room temperature. The gels were then incubated overnight
at 37°C in the reaction buffer and sequentially stained with
Coomassie brilliant blue and destained in a solution of 10% acetic
acid and 30% methanol. Areas of enzymatic activity appeared as
clear bands over a dark background. The density of gel bands was
analyzed using ImageJ software.
Real-time RT-PCR
The prostate cancer cells were exposed to peptide
hormones (Ang II, RLN2 and Ang II + RLN2) at a concentration of
1×10−8 M, for 24 h. The total RNA from cells was then
extracted using a Universal kit (A&A Biotechnology). The
quantity of recovered RNA and its purity was determined by reading
the absorbance at 260 and 280 nm. Reverse transcriptase was used to
create cDNA libraries from mRNA. Amplification reactions were
performed with a LightCycler 480 Real-time PCR. The expression
levels of investigated genes were normalized to two reference genes
(H3F3A and RPLPO). All quantitative PCR data were analyzed using
the relative expression software tool REST 2009 (version 2.0.13)
which uses a hypothesis test to determine significant differences
between control and sample groups. The software provides proper
error propagation and robust statistical analysis by using a random
reallocation algorithm with 2,000 iterations. The real-time RT-PCR
primers and reaction conditions are presented in Table I (22).
 | Table IReal-time RT-PCR primers and reaction
conditions (22). |
Table I
Real-time RT-PCR primers and reaction
conditions (22).
| Genes | Gene primers
(5′-3′) | Annealing
temp. | Detection
temp. |
|---|
| H3F3A (H3 histone,
family 3A) | F:
5′-AGGACTTTAAAAGATCTGCGCTTCCAGAG-3′
R: 5′-ACCAGATAGGCCTCACTTGCCTCCTGC-3′ | 65°C | 72°C |
| RPLPO (Ribosomal
phosphoprotein) | F:
5′-ACGGATTACACCTTCCCACTTGCTAAAAGGTC-3′
R: 5′-AGCCACAAAGGCAGATGGATCAGCCAAG-3′ | 65°C | 72°C |
| BAX | F:
5′-AGAGGTCTTTTTCCGAGTGGCAGC-3′
R: 5′-TTCTGATCAGTTCCGGCACCTTG-3′ | 56°C | 81°C |
| BCL2 | F:
5′-TTGGCCCCCGTTGCTTTTCCTC-3′
R: 5′-TCCCACTCGTAGCCCCTCTGCGAC-3′ | 56°C | 81°C |
| BIRC5
(Survivin) | F:
5′-AGTGTTTCTTCTGCTTCAAGGAGCTGGAAG-3′
R: 5′-ACCGGACGAATGCTTTTTATGTTCCTCTATG-3′ | 56°C | 81°C |
| AR (Androgen
receptor) | F:
5′-AAGGCTATGAATGTCAGCCCA-3′
R: 5′-CATTGAGGCTAGAGAGCAAGGC-3′ | 60°C | 72°C |
Statistical analysis
The data are presented as mean ± SD of at least
three independent experiments. The measurements were subjected to
analysis of variance (One-Way ANOVA) and Tukey's test using
GraphPad Prism 5 (GraphPad Software, La Jolla, CA, www.graphpad.com). Relationships were regarded as
statistically significant at p<0.05.
Results
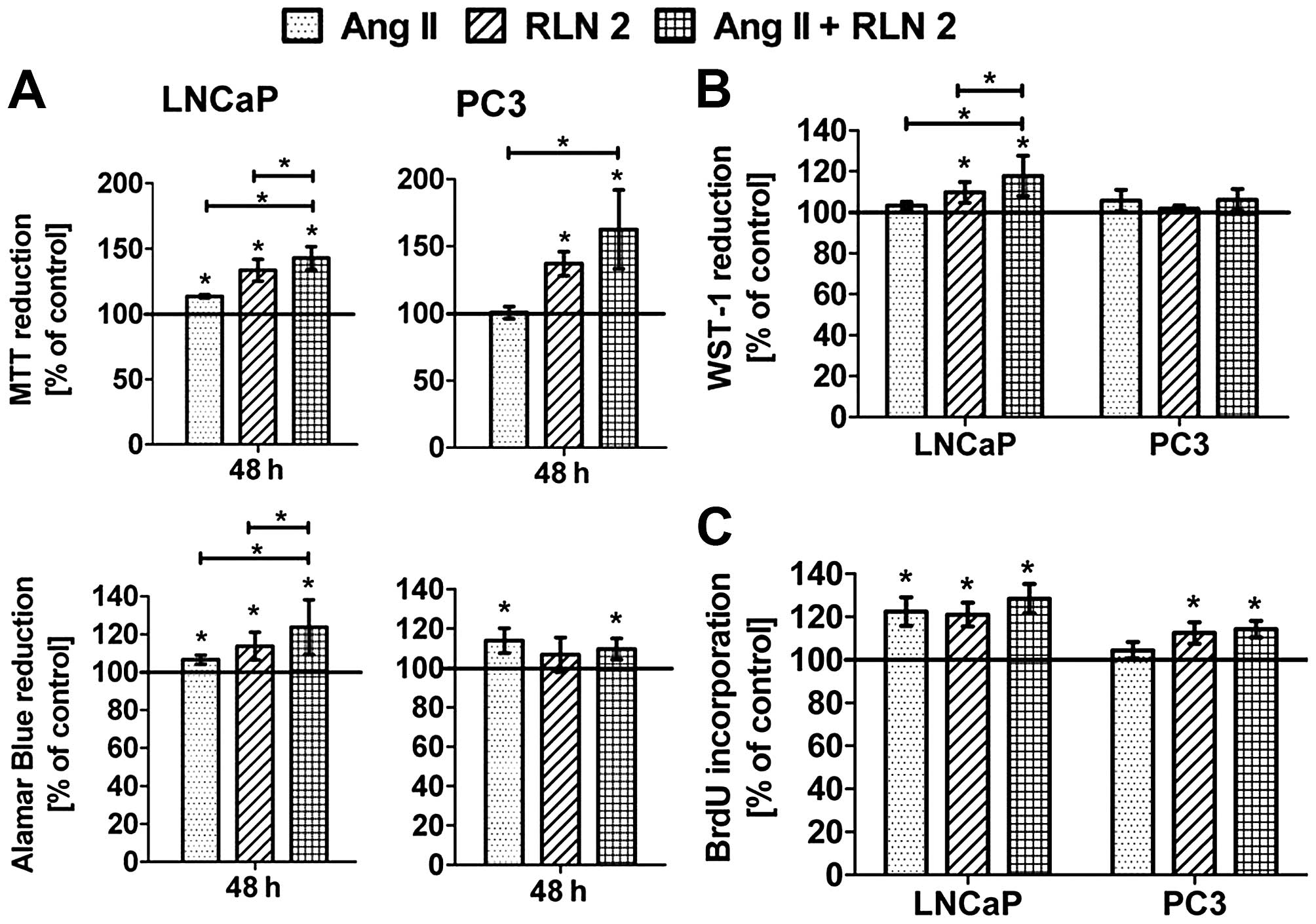
Effect of Ang II and/or RLN2 on cell
viability and proliferation of prostate cancer cells
In 2D cultures (Fig.
1A), both peptides Ang II and RLN2 indeed improved the
viability androgen-dependent prostate cancer cell lines. In
androgen-independent prostate cancer cells, the results were
unambiguous for example the Alamar blue assay showed a significant
increase in PC3 cell viability after treatment with Ang II, the MTT
method did not. The stimulating impact of the combination of
peptide hormones on cell viability was more evident in LNCaP cells
than PC3. Treatment of prostate cancer cells with both peptide
hormones induced BrdU incorporation into DNA during the S-phase of
the cell cycle (Fig. 1C). Ang II
promoted cell division of LNCaP to a much greater degree than PC3,
where the result was not statistically significant. In any case, no
changes in cell proliferation were noted between Ang II and RLN2
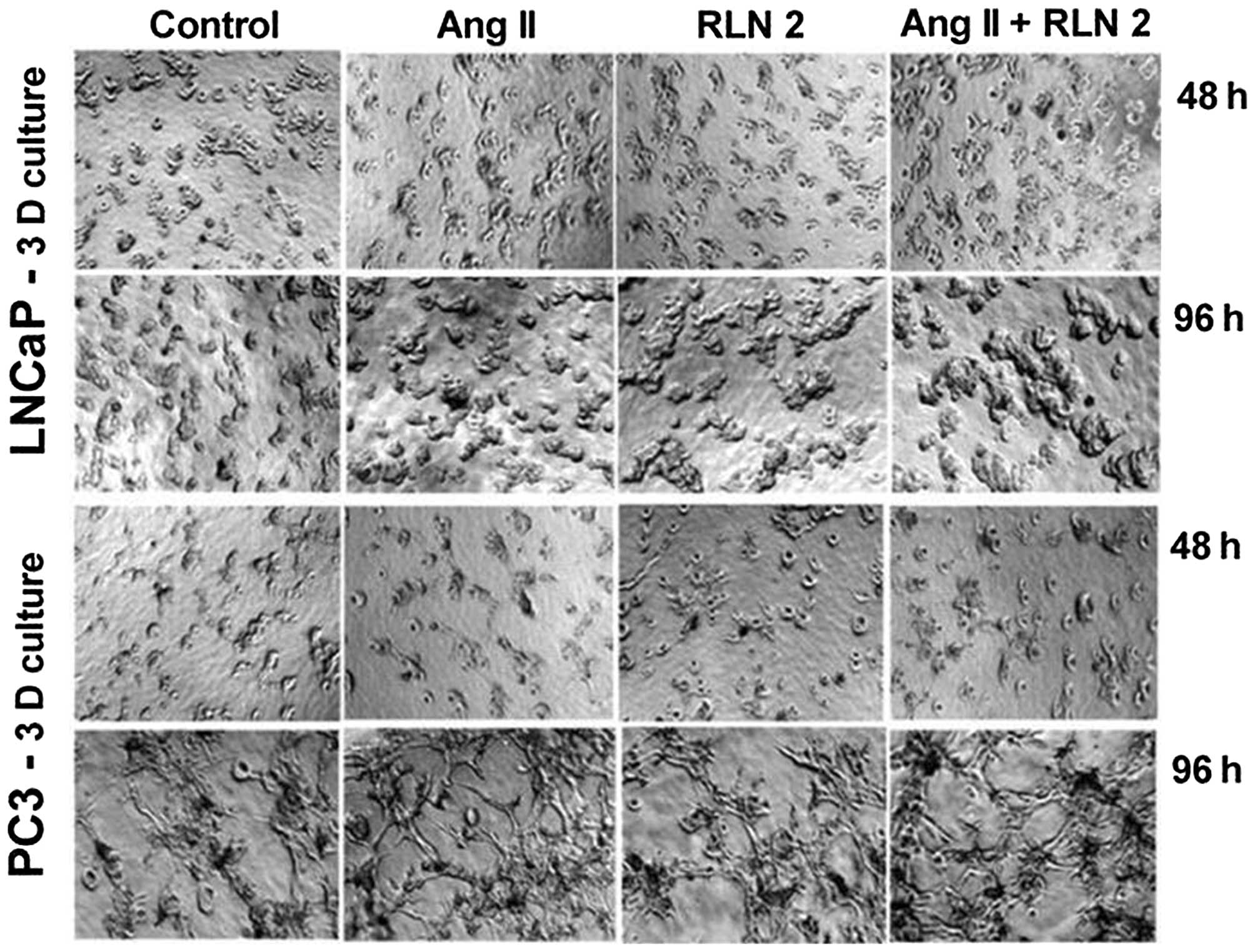
used alone or a combination of both. The results of the 3D cultures
(Fig. 1B) showed a similar trend
to those of the 2D cell culture experiments (Alamar blue), although
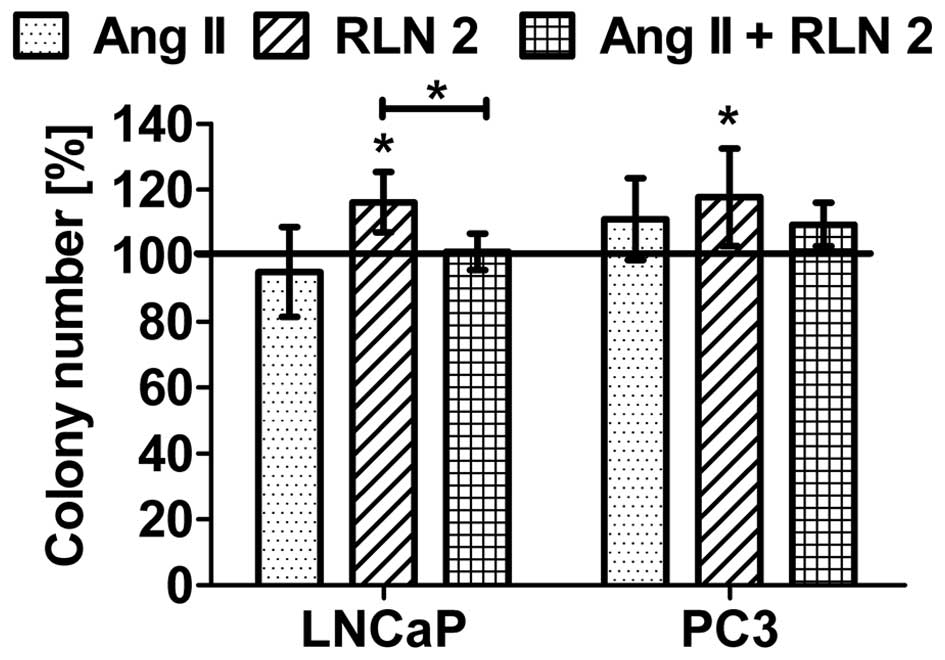
with less marked differences. Furthermore, the prostate cells were
found to exhibit greater colony formation after 5-day treatment
with Ang II and RLN2 (Fig. 2),
especially after exposure to a combination of both peptide
hormones.
Effect of Ang II and/or RLN2, on
expression of AR, BCL2, BAX, BIRC5 genes in prostate cancer
cells
The results of the real-time reverse transcription
PCR indicated that relative BIRC5 (survivin) expression to
be more than 6 times higher in the PC3 cells than in LNCaP cells
(data not shown). The results present increased levels of BIRC5
transcript in RNA extracts from prostate cancer cells treated with
peptide hormones (Table II).
However these results were statistically significant only in
androgen-dependent cells. In both prostate cancer cell lines,
expression of BCL2 and BAX was unchanged, the only
exception being the LNCaP cells, which were incubated with RLN2 for
24 h. In this case, a poor but significant increase was observed in
the expression of the pro-apoptotic BAX gene. The androgen
receptor was expressed in both androgen-dependent and -independent
prostate cancer lines. However, levels of AR were found to
be more than 30 times higher in LNCaP cells than PC3 cells (data
not shown). The clear upward trend in androgen receptor expression
was observed in androgen-dependent prostate cancer cells after
exposure to Ang II and/or RLN2, this tendency was not noted in
androgen-independent cells.
 | Table IIExpression of the genes BCL2, BAX,
BIRC5 and AR in prostate cancer cells after 24 h
incubation with Ang II and/or RLN2.a |
Table II
Expression of the genes BCL2, BAX,
BIRC5 and AR in prostate cancer cells after 24 h
incubation with Ang II and/or RLN2.a
| LNCaP |
|---|
|
|---|
| Treatment | Gene | Type | Reaction
efficiency | Expression | Std. Error | 95% CI | P(H1) | Result |
|---|
| Ang II | H3F3A | REF | 0.92 | 1.261 | | | | |
| RPLPO | REF | 0.98 | 0.793 | | | | |
| BCL | TRG | 1.0 | 1.638 | 0.875–2.651 | 0.651–4.639 | 0.120 | ↔ |
| BAX | TRG | 0.84 | 1.373 | 0.979–2.057 | 0.904–2.499 | 0.086 | ↔ |
| BIRC5 | TRG | 0.9 | 1.784 | 1.512–2.060 | 1.335–2.289 | 0.002 | ↑ |
| AR | TRG | 0.97 | 1.968 | 1.603–2.228 | 1.420–2.718 | 0.002 | ↑ |
| RLN2 | H3F3A | REF | 0.92 | 1.592 | | | | |
| RPLPO | REF | 0.98 | 0.628 | | | | |
| BCL | TRG | 1.0 | 1.174 | 0.564–2.128 | 0.416–3.665 | 0.612 | ↔ |
| BAX | TRG | 0.84 | 1.540 | 1.133–2.144 | 0.926–2.881 | 0.048 | ↑ |
| BIRC5 | TRG | 0.9 | 2.043 | 1.678–2.694 | 1.355–2.902 | 0.001 | ↑ |
| AR | TRG | 0.97 | 2.185 | 1.959–2.462 | 1.752–2.936 | 0.002 | ↑ |
| Ang II + RLN2 | H3F3A | REF | 0.92 | 1.324 | | | | |
| RPLPO | REF | 0.975 | 0.755 | | | | |
| BCL | TRG | 1.0 | 0.986 | 0.682–1.532 | 0.583–2.015 | 0.950 | ↔ |
| BAX | TRG | 0.84 | 1.240 | 0.789–1.878 | 0.690–3.254 | 0.385 | ↔ |
| BIRC5 | TRG | 0.9 | 1.894 | 1.607–2.428 | 1.308–2.503 | 0.000 | ↔ |
| AR | TRG | 0.97 | 1.319 | 1.045–1.687 | 1.006–2.067 | | ↔ |
|
| PC3 |
|
| Ang II | H3F3A | REF | 0.92 | 1.236 | | | | |
| RPLPO | REF | 0.98 | 0.809 | | | | |
| BCL | TRG | 1.0 | 0.606 | 0.258–1.589 | 0.136–3.029 | 0.303 | ↔ |
| BAX | TRG | 0.84 | 0.899 | 0.601–1.347 | 0.444–2.108 | 0.658 | ↔ |
| BIRC5 | TRG | 0.9 | 1.064 | 0.661–1.588 | 0.600–2.180 | 0.783 | ↔ |
| AR | TRG | 0.97 | 0.300 | 0.095–1.341 | 0.027–2.002 | 0.141 | ↔ |
| RLN2 | H3F3A | REF | 0.92 | 1.223 | | | | |
| RPLPO | REF | 0.98 | 0.818 | | | | |
| BCL | TRG | 1.0 | 0.527 | 0.198–1.389 | 0.096–2.570 | 0.196 | ↔ |
| BAX | TRG | 0.84 | 0.798 | 0.487–1.263 | 0.357–1.876 | 0.327 | ↔ |
| BIRC5 | TRG | 0.9 | 1.162 | 0.580–2.338 | 0.373–3.297 | 0.681 | ↔ |
| AR | TRG | 0.97 | 0.269 | 0.072–0.967 | 0.027–1.433 | 0.107 | ↔ |
| Ang II + RLN2 | H3F3A | REF | 0.92 | 1.329 | | | | |
| RPLPO | REF | 0.98 | 0.753 | | | | |
| BCL | TRG | 1.0 | 0.659 | 0.278–1.603 | 0.238–1.855 | 0.360 | ↔ |
| BAX | TRG | 0.84 | 0.818 | 0.464–1.310 | 0.340–2.272 | 0.479 | ↔ |
| BIRC5 | TRG | 0.9 | 1.216 | 0.804–1.740 | 0.640–2.490 | 0.329 | ↔ |
| AR | TRG | 0.97 | 0.354 | 0.076–0.927 | 0.058–1.355 | 0.158 | ↔ |
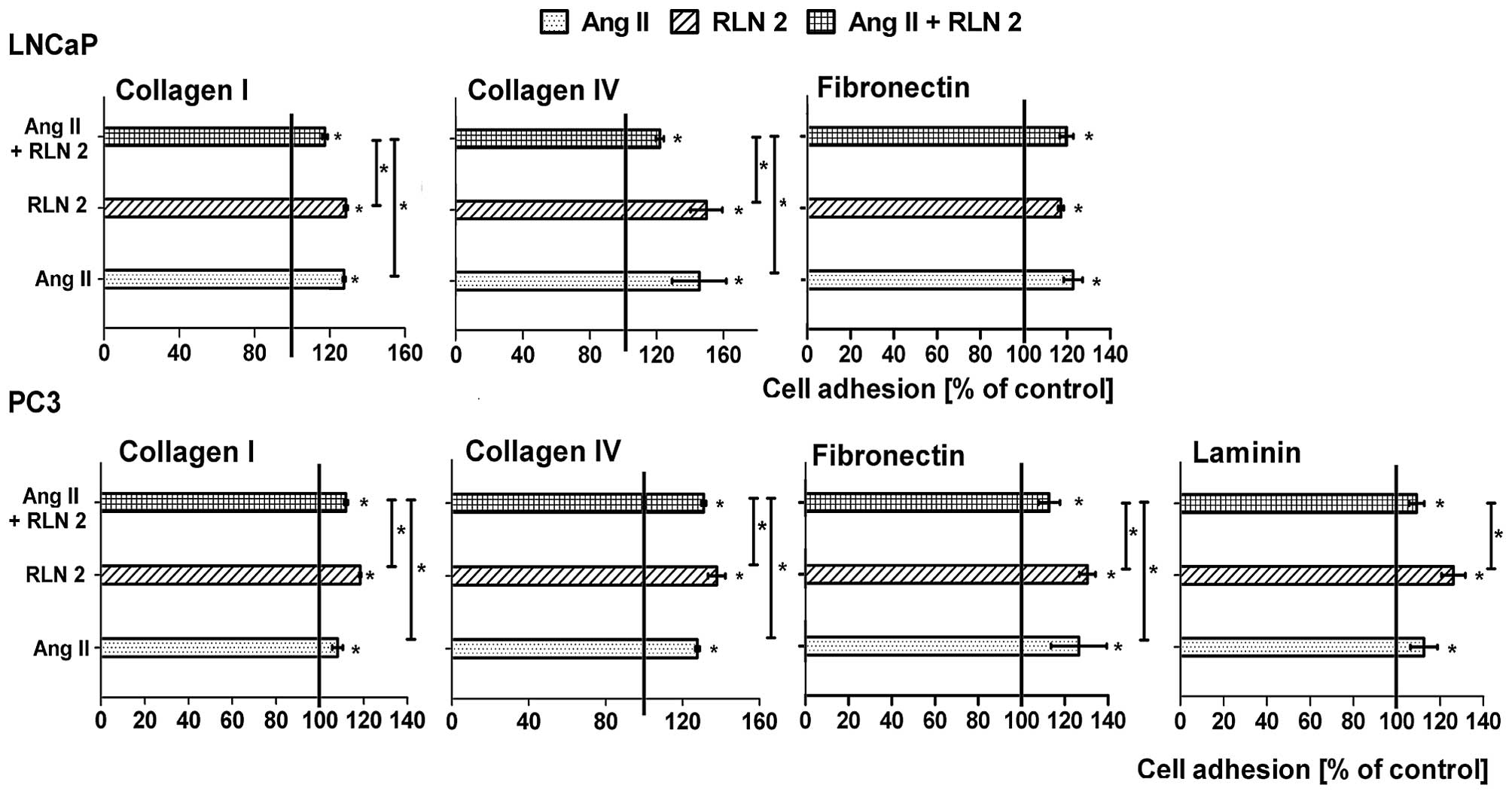
Effects of Ang II and/or RLN2 on adhesion
of prostate cancer cells
The LNaP cells that were suspended in serum-free
medium adhered efficiently to fibronectin, collagen I and IV, but
not to laminin. Whereas the PC3 cells best adhered to fibronectin
after 90 min of incubation, they adhered less effectively to both
types of collagens and very weakly to laminin. In most cases, no
differences were noted between the adhesion of the control cells
and adhesion of the experimental group after 24-h incubation (date
not shown). Prolongation of the incubation period to 48 h leads to
increased cell adhesion in all tested variations (Fig. 3). In LNCaP cells, much more
adhesion to collagen I and IV was observed after long-term
treatment with Ang II and RLN2 alone than in combination. However,
the adhesion of PC3 cells were frequently intermediate (collagens)
or lower (fibronectin) after 48 h treatment with combined Ang II
and RLN2 than the individual peptides.
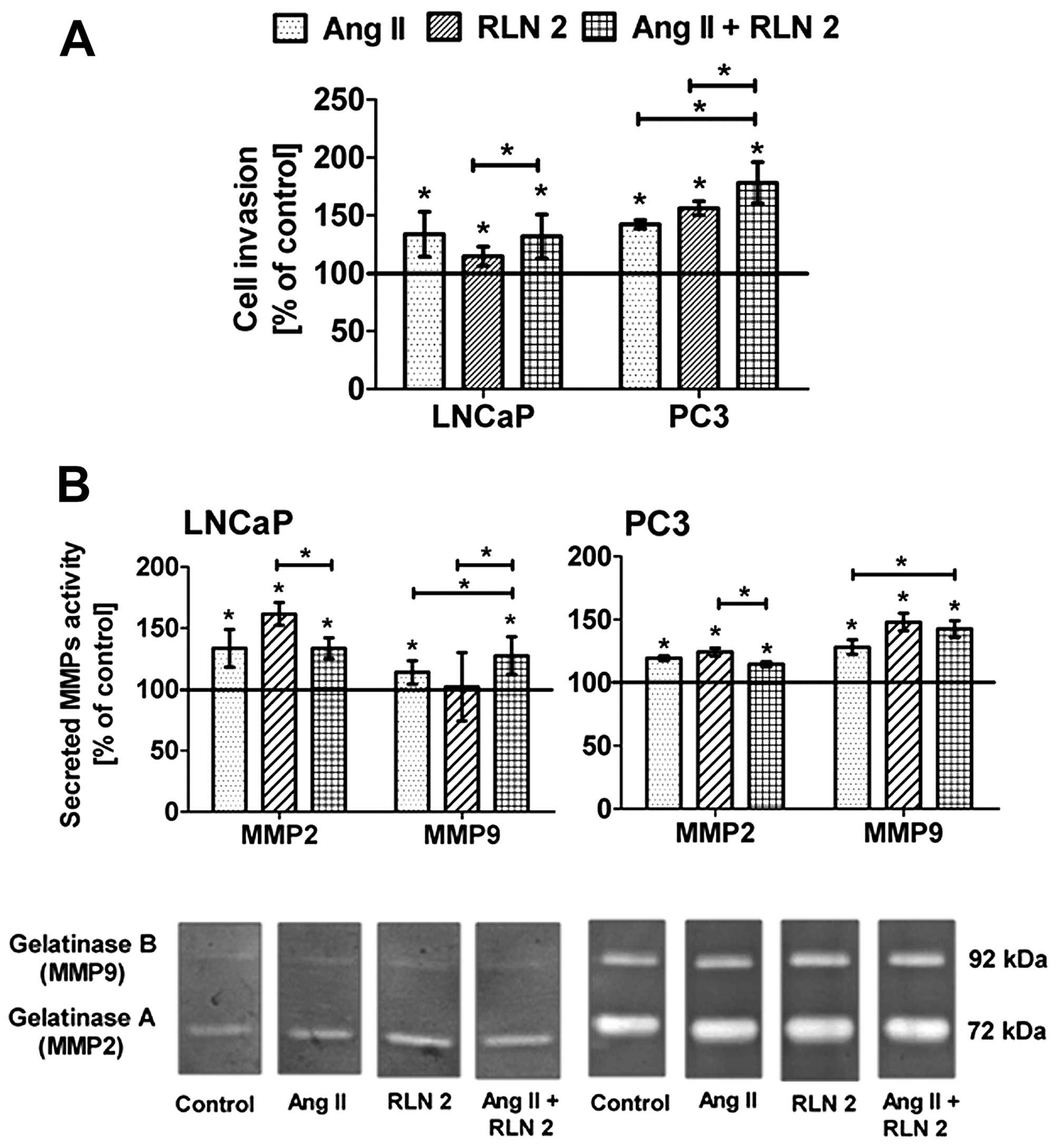
Effects of Ang II and/or RLN2 on cell
invasion of prostate cancer cells
Both LNCaP and PC3 cells have greater potential to
invade through Matrigel-coated 8 μm inserts in response to
RLN2 and/or Ang II (Fig. 4A).
However, it should be emphasized that this effect was observed only
after long-term incubation. Relaxin 2 exerted the strong impact on
the invasiveness of androgen-independent cell lines, whereas in
androgen-dependent lines, the most notable was Ang II. In both
lines, the effect of combined RLN2 and Ang II was not significantly
different than that of the individual effect of more powerful
peptide hormone for this line.
Effect of Ang II and/or RLN2 on MMP-2 and
MMP-9 secretion in prostate cancer cells
All of the samples contained two prominent
gelatinolytic bands corresponding to monomeric pro-MMP-9 (92 kDa)
and pro-MMP-2 (72 kDa) (Fig. 4B).
Additionally, two slight complexes of MMPs (240 kDa and 130 kDa)
were also observed in PC3 cells (data not shown). The levels of
both MMPs significantly rose after exposure to peptide hormones,
the only exception being the sample from the LNCaP cells exposed to
RLN 2, where no increase was observed. Furthermore, the results of
the densitometry analysis of MMP zymographic activities show that
changes of gelatinase A were more pronounced than in gelatinase B
in the PC3 cells, while in LNCaP cells, greater changes were noted
for MMP-2.
Effects of Ang II and/or RLN2 on
anchorage-independent cell growth
The ability to exhibit anchorage-independent cell
growth was observed in both prostate cancer lines. As shown in
Fig. 5, RLN2 increased the growth
of both androgen-dependent and androgen-independent prostate cancer
cells in soft agar. Furthermore, these results demonstrated that
Ang II has no influence on the anchorage-independent growth of
LNCaP. In the case of PC3 cells, Ang II was observed to have a poor
stimulatory effect which was not statistically significant. Results
for combination of Ang II and RLN2 oscillated within the control
samples.
Discussion
It is common knowledge that local hormones such as
angiotensin and relaxin regulate a wide range of prostatic
functions. Nevertheless, detailed role of Ang II and RLN2 peptides
in prostate physiology and pathology is still not clear. The
present study examines the common effects of angiotensin II or
relaxin 2 administration on various aspects of prostate cancer
growth and progression. The weakly tumorigenic LNCaP prostate
cancer cell line and the aggressively tumorigenic PC3 cell line
were used as models of early stage androgen-dependent prostate
cancer and late androgen-independent stage disease,
respectively.
In this study, it was observed that relaxin 2
improved maintenance of cellular viability and proliferation of
both the LNCaP and PC3 prostate cancer cell lines. These results
confirm earlier studies showing that this peptide hormone promotes
the division of prostate cancer cells (6). Experimental studies report that
greater changes of cell proliferation take place in the high AR
expression cells (LNCaP) than low AR expression cells (PC3). Liu
et al (23) noted that H2
relaxin induces the transcriptional activity of AR and the growth
of prostate cancer cells through increased expression of β-catenin.
Furthermore PI3K/Akt, factors of the Wnt pathway, can facilitate
H2-relaxin-mediated activation of the AR pathway (23). Vinall et al (24) report that the cAMP/PKA and NF-κB
pathways play an important role in facilitating the H2
relaxin-mediated castration resistant growth of prostate cancer
cells.
The findings of the present study reveal a
significant increase in androgen receptor expression in LNCaP cells
but an insignificant decrease in PC3. Some of the data generated in
PC3 cells seem to contradict the conventional idea that the AR
functions as a stimulator in prostate tumor growth and metastasis.
For example Niu et al (25)
noted that restoring AR in PC3 cells could suppress their ability
to invade in vitro and in vivo. Whereas Lin et
al (26) hypothesize that PC3
cells possess cellular machinery that turns the AR in PC3-AR cells
into a growth suppressor. In another study, an equally interesting
observation is that RLN2 is negatively regulated by androgens in
prostate cancer (in vitro and in vivo) through the
AR, implying that RLN2 levels increase in the both the absence of
androgens and their ablation (27). It seems that this polypeptide
hormone is involved in the mechanism of transformation from the
androgen-dependent to androgen-independent state of prostate cancer
cells.
Using a BrdUrd incorporation assay as an indicator
of cell proliferation, it was noted that Ang II increased the DNA
synthesis much more strongly in LNCaP cells than in PC3 cells. Our
earlier studies found that Ang II significantly enhanced cell
viability and proliferation in both prostate cancer cell lines
(21,28). Uemura et al (29) found that AT1 receptor mRNA was
strongly expressed in LNCaP cells and very poorly in PC3, which may
explain the better response of the first line to angiotensin
II-induced cell proliferation. Our observation seems compatible
also with finding of Pawlikowski et al (30) presenting that expression of Ang II
type 1 and 2 receptors in neoplastic epithelium of Gleason grate 2
is higher than in more advanced cancerous structures of Gleason
grades 3–5. On the contrary, Chow et al (31) demonstrated that Ang II
dose-dependently stimulated DNA synthesis in LNCaP but not PC3
cells suggesting that only LNCaP has the functional state of
AT1.
However, the above observation is contradictory to
our earlier findings, which identified the presence of AT1 receptor
mRNA, and the expression of its protein in PC3 cells. The
angiotensin II type 1 receptor antagonist candesartan revealed that
Ang II has a stimulatory effect on metabolic activity and prostate
cancer cell division in PC3 cells (21). This difference in the results may
also be due to variables such as differences in type of antagonist,
peptide concentration and time of incubation or differences in
research techniques. The findings of the present study highlight
some differences between the results of the MTT and Alamar blue
assays: for example, the former showed a significant increase in
PC3 cell viability after incubation with Ang II (48 h), whereas the
latter did not. Both tests measure the metabolic activity of cells
but in different ways. The first method measures the mitochondrial
metabolic activity in the whole cells while the second assay
indicates the total metabolic activity (mitochondrial and
cytoplasmatic) in the entire cell culture. It is worth noting that
while MTT can be reduced by NADPH, FADH, FMNH or NADH, Alamar blue
can also be reduced by cytochromes (32,33).
Discrepancies between results from MTT and Alamar blue have also
been reported by other workers in cytotoxicity studies (34).
Many molecules are known to regulate and modulate
the cellular pathways related to cell division and survival, among
which are survivin (BIRC5) and members of the BCL2
family. BIRC5 is rarely expressed in most terminally
differentiated normal tissues but is abundantly and ubiquitously
expressed during fetal and embryonic development. Of note, survivin
is up-regulated in almost all human malignant tumors and recently
has been considered as a diagnostic and prognostic marker for human
PCa (35,36). An examination of clinical samples
has established that the expression of survivin gradually rises
from the low levels observed in normal prostate tissue, through
low-grade primary carcinoma, to high-grade primary carcinoma, and
is highest in lymph node metastases (15,37).
As expected, a higher level of BIRC5 expression was observed
in aggressive androgen-independent prostate cancer cells (PC3)
compared to the androgen-dependent, non-invasive prostate cancer
cell line (LNCaP) (data not shown). Furthermore, both Ang II and
RLN2 were found to stimulate survivin mRNA synthesis, mainly in the
LNCaP cancer cells. Zhang et al (15) noted that survivin can mediate
resistance to androgen ablation in prostate cancer via activation
of the IGFR1/AKT pathway, suggesting that Ang II and RLN2 may play
a role in the development of AIPC (androgen-independent prostate
cancer) just by promoting the expression of BIRC5.
As the early stages of prostate cancer are typically
asymptomatic and that the cells acquire motility, i.e. the ability
to move to another location, this kind of cancer is very frequently
fatal (38). Migration of cancer
cells is critically regulated by physical adhesion of cells to each
other and the extracellular matrix (ECM). The present study
examines the ability of prostate cancer cells to adhere to the
major structural elements of the ECM, such as type I and IV
collagen, laminin and fibronectin. Our results are similar to those
of Moro et al (39)
indicating that carcinoma cells adhere to different degrees to
various components of the basement membrane. As shown in the
present study, extent of prostate cell adhesion was dependent
firstly on the type of prostate cell, on the kind of protein matrix
and finally on the duration of Ang II and RLN2 incubation.
Nevertheless, after 48-h incubation, increased adhesion of both
LNCaP and PC3 prostate cancer cells was observed in all tested
combinations. Feng et al (6) evaluated the effect of relaxin 2 on
adhesion of LNCaP and PC3 cells, but only to type I collagen. They
observed that pre-incubation with different concentrations of
relaxin 2 (0–300 ng/ml) resulted in a dose-dependent increase in
prostate cancer cell adhesion (6).
The modulation of cell adhesion to ECM by angiotensin II has also
been observed by other researchers (40).
Prostate cancer tends to spread to either lymph
nodes or bones (38). During this
metastasis, tumor cells must pass through structural barriers such
as basement membranes. The matrix metalloproteinases are believed
to be the most important physiological mediators of ECM
degradation. Increased expression of MMPs has been observed in
various cancers and many reports indicate their potential
prognostic value in patients with prostate cancer (18). The gelatin zymography used in the
present study revealed that both prostate cancer cell lines
secreted MMP-2 and MMP-9 into the culture medium. In controls, it
was observed that PC3 cells secrete many times more gelatinase A
and B than LNCaP cells, which certainly affects the differences in
invasiveness of both cell lines. Our findings add to an increasing
body of evidence suggesting that both Ang II and RLN2 play an
important role in the normal and pathological remodeling of ECM in
several reproductive tract tissues (1–3). The
results of the present study demonstrate the levels of gelatinases
clearly grew after exposure to Ang II and RLN2, which is supported
by the results of the Transwell invasion assay. These findings
confirm that both peptide hormones can promote a more aggressive
and invasive phenotype in prostate cancer via upregulation of MMPs.
Feng et al (6) indicated a
significant increase in invasiveness of relaxin-treated LNCaP and
PC-3 cells in the Matrigel invasion assay.
The cancer cells travel to new organs via the lymph
or blood stream. Therefore, the next critical step in the
multistage process of metastasis formation is the ability to
survive and grow in the absence of anchorage to the extracellular
matrix and their neighboring cells (14). Our results clearly show that only
relaxin 2 can induce a higher anchorage-independent growth and
non-adherent spheroid formation of both LNCaP and PC3 cells in a
soft agar medium. The angiotensin II effect, in this case, does not
play a significant role, because the number of colony formation
remains almost unaltered.
The present study is the first to consider the
impact of Ang II and RLN2, alone and in combination, on prostate
cancer cells. The obtained results confirm their influence on
prostate cancer cells, in terms of cell viability and
proliferation, cell adhesion and invasion. Nevertheless, the
stimulatory potency of either Ang II or RLN2 when used alone was
not found to increase synergistically/additively when both peptides
were used in combination; the effects of both hormones in
combination were most frequently intermediate between the two
individual values or sometimes lower than both. The obtained
results suggest that the investigated RAS and relaxin family
peptide system may have impact on cell growth/division or spread at
least in part via overlapping signal transduction pathways. Ang II
and RLN2 may well play an important role in increasing the
aggressiveness of prostate tumors by the upregulation of survivin
expression and gelatinase secretion. Furthermore, it can be
speculated that Ang II and RLN2 are involved in the transition from
the androgen-dependent to the androgen-independent phenotype via
modulation of the expression of androgen receptors. However,
further studies are necessary to confirm this hypothesis and to
elucidate the mechanisms involved.
Acknowledgements
This work was financially supported by the National
Science Center, research grant no. NN403 208 139.
References
|
1
|
Silvertown JD, Summerlee AJ and Klonisch
T: Relaxin-like peptides in cancer. Int J Cancer. 107:513–519.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domińska K and Lachowicz-Ochedalska A: The
involvement of the renin-angiotensin system (RAS) in
cancerogenesis. Postepy Biochem. 54:294–300. 2008.(In Polish).
|
|
3
|
Domińska K: Relaxin 2 - a pregnancy
hormone involved in the process of carcinogenesis. Ginekol Pol.
84:126–130. 2013.(In Polish). View
Article : Google Scholar
|
|
4
|
Dinh DT, Frauman AG, Somers GR, Ohishi M,
Zhou J, Casley DJ, Johnston CI and Fabiani ME: Evidence for
activation of the renin-angiotensin system in the human prostate:
Increased angiotensin II and reduced AT(1) receptor expression in
benign prostatic hyperplasia. J Pathol. 196:213–219. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Louis SN, Wang L, Chow L, Rezmann LA,
Imamura K, MacGregor DP, Casely D, Catt KJ, Frauman AG and Louis
WJ: Appearance of angiotensin II expression in non-basal epithelial
cells is an early feature of malignant change in human prostate.
Cancer Detect Prev. 31:391–395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng S, Agoulnik IU, Bogatcheva NV, Kamat
AA, Kwabi-Addo B, Li R, Ayala G, Ittmann MM and Agoulnik AI:
Relaxin promotes prostate cancer progression. Clin Cancer Res.
13:1695–1702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng S, Agoulnik IU, Li Z, Han HD,
Lopez-Berestein G, Sood A, Ittmann MM and Agoulnik AI:
Relaxin/RXFP1 signaling in prostate cancer progression. Ann NY Acad
Sci. 1160:379–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamp O, Honscha KU, Schweizer S, Heckmann
A, Blaschzik S and Einspanier A: The metastatic potential of canine
mammary tumours can be assessed by mRNA expression analysis of
connective tissue modulators. Vet Comp Oncol. 11:70–85. 2013.
View Article : Google Scholar
|
|
9
|
Chow BS, Kocan M, Bosnyak S, Sarwar M,
Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD,
et al: Relaxin requires the angiotensin II type 2 receptor to
abrogate renal interstitial fibrosis. Kidney Int. 86:75–85. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasser JM, Molnar M and Baylis C: Relaxin
ameliorates hypertension and increases nitric oxide metabolite
excretion in angiotensin II but not N(ω)-nitro-L-arginine methyl
ester hypertensive rats. Hypertension. 58:197–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferreira VM, Gomes TS, Reis LA, Ferreira
AT, Razvickas CV, Schor N and Boim MA: Receptor-induced dilatation
in the systemic and intrarenal adaptation to pregnancy in rats.
PLoS One. 4:e48452009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geddes BJ, Parry LJ and Summerlee AJ:
Brain angiotensin-II partially mediates the effects of relaxin on
vasopressin and oxytocin release in anesthetized rats.
Endocrinology. 134:1188–1192. 1994.PubMed/NCBI
|
|
13
|
Amamoo A and Wilson BC: Relaxin inhibits
central angiotensin II expression in killifish: A central
osmoregulatory role for relaxin and angiotensin II in the killifish
Fundulus heteroclitus. Ann NY Acad Sci. 1041:229–232. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Latham DE, Delaney MA and
Chakravarti A: Survivin mediates resistance to antiandrogen therapy
in prostate cancer. Oncogene. 24:2474–2482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duffy MJ, O'Donovan N, Brennan DJ,
Gallagher WM and Ryan BM: Survivin: A promising tumor biomarker.
Cancer Lett. 249:49–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin Y, Fukuchi J, Hiipakka RA, Kokontis JM
and Xiang J: Up-regulation of Bcl-2 is required for the progression
of prostate cancer cells from an androgen-dependent to an
androgen-independent growth stage. Cell Res. 17:531–536. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metal-loproteinases in cancer: Their value
as diagnostic and prognostic markers and therapeutic targets.
Tumour Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alimirah F, Chen J, Basrawala Z, Xin H and
Choubey D: DU-145 and PC-3 human prostate cancer cell lines express
androgen receptor: Implications for the androgen receptor functions
and regulation. FEBS Lett. 580:2294–2300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piastowska-Ciesielska AW, Gajewska M,
Wagner W, Dominska K and Ochedalski T: Modulatory effect of
selenium on cell-cycle regulatory genes in the prostate
adenocarcinoma cell line. J Appl Biomed. 12:87–95. 2014. View Article : Google Scholar
|
|
21
|
Piastowska-Ciesielska AW, Kozłowski M,
Wagner W, Domińska K and Ochędalski T: Effect of an angiotensin II
type 1 receptor blocker on caveolin-1 expression in prostate cancer
cells. Arch Med Sci. 9:739–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pluciennik E, Krol M, Nowakowska M,
Kusinska R, Potemski P, Kordek R and Bednarek AK: Breast cancer
relapse prediction based on multi-gene RT-PCR algorithm. Med Sci
Monit. 16:CR132–CR136. 2010.PubMed/NCBI
|
|
23
|
Liu S, Vinall RL, Tepper C, Shi XB, Xue
LR, Ma AH, Wang LY, Fitzgerald LD, Wu Z, Gandour-Edwards R, et al:
Inappropriate activation of androgen receptor by relaxin via
beta-catenin pathway. Oncogene. 27:499–505. 2008. View Article : Google Scholar
|
|
24
|
Vinall RL, Mahaffey CM, Davis RR, Luo Z,
Gandour-Edwards R, Ghosh PM, Tepper CG and de Vere White RW: Dual
blockade of PKA and NF-κB inhibits H2 relaxin-mediated
castrate-resistant growth of prostate cancer sublines and induces
apoptosis. Horm Cancer. 2:224–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke
WA, Messing EM, Yao J, Yeh S and Chang C: Androgen receptor is a
tumor suppressor and proliferator in prostate cancer. Proc Natl
Acad Sci USA. 105:12182–12187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin B, Wang J, Hong X, Yan X, Hwang D, Cho
JH, Yi D, Utleg AG, Fang X, Schones DE, et al: Integrated
expression profiling and ChIP-seq analyses of the growth inhibition
response program of the androgen receptor. PLoS One. 4:e65892009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thompson VC, Morris TG, Cochrane DR,
Cavanagh J, Wafa LA, Hamilton T, Wang S, Fazli L, Gleave ME and
Nelson CC: Relaxin becomes upregulated during prostate cancer
progression to androgen independence and is negatively regulated by
androgens. Prostate. 66:1698–1709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Domińska K, Piastowska-Ciesielska AW,
Lachowicz-Ochędalska A and Ochędalski T: Similarities and
differences between effects of angiotensin III and angiotensin II
on human prostate cancer cell migration and proliferation.
Peptides. 37:200–206. 2012. View Article : Google Scholar
|
|
29
|
Uemura H, Ishiguro H, Nakaigawa N,
Nagashima Y, Miyoshi Y, Fujinami K, Sakaguchi A and Kubota Y:
Angiotensin II receptor blocker shows antiproliferative activity in
prostate cancer cells: A possibility of tyrosine kinase inhibitor
of growth factor. Mol Cancer Ther. 2:1139–1147. 2003.PubMed/NCBI
|
|
30
|
Pawlikowski M, Minias R, Sosnowski M and
Zielinski KW: Immunohistochemical detection of angiotensin AT1 and
AT2 receptors in prosate cancer, Central Eur. J Urol. 64:252–255.
2011.
|
|
31
|
Chow L, Rezmann L, Imamura K, Wang L, Catt
K, Tikellis C, Louis WJ, Frauman AG and Louis SNS: Functional
angiotensin II type 2 receptors inhibit growth factor signaling in
LNCaP and PC3 prostate cancer cell lines. Prostate. 68:651–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rampersad SN: Multiple applications of
Alamar Blue as an indicator of metabolic function and cellular
health in cell viability bioassays. Sensors (Basel).
12:12347–12360. 2012. View Article : Google Scholar
|
|
33
|
Niles AL, Moravec RA and Riss TL: Update
on in vitro cytotoxicity assays for drug development. Expert Opin
Drug Discov. 3:655–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamid R, Rotshteyn Y, Rabadi L, Parikh R
and Bullock P: Comparison of alamar blue and MTT assays for high
through-put screening. Toxicol In Vitro. 18:703–710. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khan S, Jutzy JM, Valenzuela MM, Turay D,
Aspe JR, Ashok A, Mirshahidi S, Mercola D, Lilly MB and Wall NR:
Plasma-derived exosomal survivin, a plausible biomarker for early
detection of prostate cancer. PLoS One. 7:e467372012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arbab IA, Looi CY, Abdul AB, Cheah FK,
Wong WF, Sukari MA, Abdullah R, Mohan S, Syam S, Arya A, et al:
Dentatin Induces apoptosis in prostate cancer cells via Bcl-2,
Bcl-xL, survivin downregulation, caspase-9, −3/7 activation, and
NF-κB inhibition. Evid Based Complement Alternat Med.
2012:8560292012. View Article : Google Scholar
|
|
37
|
Shariat SF, Lotan Y, Saboorian H, Khoddami
SM, Roehrborn CG, Slawin KM and Ashfaq R: Survivin expression is
associated with features of biologically aggressive prostate
carcinoma. Cancer. 100:751–757. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arya M, Bott SR, Shergill IS, Ahmed HU,
Williamson M and Patel HR: The metastatic cascade in prostate
cancer. Surg Oncol. 15:117–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moro L, Arbini AA, Marra E and Greco M:
Up-regulation of Skp2 after prostate cancer cell adhesion to
basement membranes results in BRCA2 degradation and cell
proliferation. J Biol Chem. 281:22100–22107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rodrigues-Ferreira S, Abdelkarim M,
Dillenburg-Pilla P, Luissint AC, di-Tommaso A, Deshayes F, Pontes
CL, Molina A, Cagnard N, Letourneur F, et al: Angiotensin II
facilitates breast cancer cell migration and metastasis. PLoS One.
7:e356672012. View Article : Google Scholar : PubMed/NCBI
|