Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide (1). Non-small
cell lung cancer (NSCLC), with the two major pathological subtypes
of adenocarcinoma (Ad) and squamous cell carcinoma (SCC) accounts
for approximately 85% of all lung cancers. NSCLC patients have low
overall survival (OS) rate, and there has been little change in the
OS rate over the past three decades (1). Chemotherapy can be used as the
primary treatment for advanced NSCLC or as an adjuvant treatment
after surgery for the patients with lymph node metastasis.
Initially chemotherapy has efficacy in the NSCLC patients, but
chemoresistance finally occurs (2).
Cancer stem cells (CSCs) are defined as a small
subpopulation, showing properties of self-renewal, differentiation,
tumorigenesis and drug resistance (3). CSCs have been documented in several
types of cancers including breast, prostate, colon, and lung
cancers (4–7). Recent studies have provided evidence
that CSCs are responsible for the poor prognosis of lung cancer
patients and contribute to chemoresistance for the lung cancer
cells (8–10). CD133, CD44, ALDH1A1, CD90, and
CD166 were verified to be effectively used as CSC markers in NSCLC
(11–13). Notch pathway has been implicated in
CSC control and cell fate determination (14). In mammals, there are four Notch
receptors Notch1, Notch2, Notch3 and Notch4. Notch interacts with
its ligand that give rise to release of Notch intracellular domain
(NICD). NICD then translocates to the nucleus to regulate target
gene transcription (15,16). It has been reported that high
activation of Notch was associated with poor outcome for lung
cancer patients (17). Notch3 has
been found to be associated with lung CSCs and is a potential
therapeutic target in lung cancer (18). In addition, when Notch3 expression
was knocked down using small interfering RNA (siRNA), the
proliferation was also reduced in lung cancer cells (19).
However, how Notch3 influences CSCs and regulate
chemoresistance in the lung cancer cells are not fully elucidated.
In the current study, cisplatin was found to induce clonogenicity
of stem-like property and this effect could be inhibited when
Notch3 was knocked down in the lung cancer cells. The Notch3
overexpression was associated with poor outcome of NSCLC patients.
CSC markers CD44 and ALDH1A1 were shown to be positively related to
Notch3 expression. Particularly, γ-secretase inhibition (GSI) could
suppress stem-like features in lung cancer cells with
chemoresistance and also led to decreased expression levels of CD44
and ALDH1A1. Moreover, autophagy was involved in cisplatin
resistance of lung cancer that might be through Notch3 signal.
Materials and methods
NSCLC patients
Frozen tissues were obtained from 104 NSCLC patients
surgically resected for stage I–IIIA NSCLC at Peking University
Cancer Hospital, from 2006 to 2009. The formalin-fixed tissues
derived from NSCLC patients who received platinum-based neoadjuvant
chemotherapy were studied in the current investigation. The study
was carried out in accordance with the approved guidelines of the
local ethics committee of Peking University Cancer Hospital, and
informed consent was obtained from all the subjects.
Immunohistochemistry (IHC)
IHC was carried out on formalin-fixed sections
(4-mm). After deparaffination of the sections, endogenous
peroxidase activity was blocked by 3% H2O2
solution for 10 min. Then, antigen retrieval was achieved by
microwave in citrate buffer (pH 6.0) followed by blocking in 5%
goat serum in PBS for 15 min. The primary antibodies including
Notch3 (Santa Cruz Biotechnology, sc-5593, 1:400 dilution), CD44
(Abcam, ab51037, 1:400 dilution) and ALDH1A1 (Abcam, ab52492, 1:400
dilution) were used to incubate the sections at 4°C overnight.
HRP-conjugated secondary antibody was added on the sections for 30
min. Then, substrate-chromogen (DAB) solution was employed to
incubate the tumor tissues for 10 min and automated hematoxylin was
used to finally counterstain the slides for 5 min. The
immunostaining was microscopically evaluated by two independent
pathologists.
Cell culture and cell treatment
Human NSCLC cell lines A549 and H520 were maintained
in the RPMI-1640 medium (Hyclone) containing 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin in a humidified 5%
CO2 incubator at 37°C. A549 and H520 cells were treated
with GSI (5 μg/ml) obtained from Sigma-Aldrich Corp. Cisplatin (1
μg/ml) (Qilu Pharmaceutical, China) was added to treat the A549 and
H520 cells for 72 h and the cells were recovered in normal medium
for additional 24 h.
Colony formation assay
The single cells were prepared and planted into the
6-well plates. After 10 days, the cells were fixed with 4%
formaldehyde for 15 min followed by PBS washing. After that, the
cells were stained with crystal for 15 min and the cells were
washed with PBS at least twice. The colonies were evaluated by
inverse microscopy. Colony formation efficiency was determined as:
colony formation efficiency = colony/input cells ×100%.
Sphere formation assay
The cells were digested and the single cells were
seeded in ultralow attachment 96-well plates (Corning Inc., Life
Sciences). The DMEM/F-12 (Invitrogen) serum-free medium including
B27 (Invitrogen), 20 ng/ml epidermal growth factor (Peprotech,
Rocky Hill, NJ, USA), 20 ng/ml basic fibroblast growth factor
(Peprotech), 10 ng/ml hepatocyte growth factor (Peprotech), and 1%
methylcellulose (Sigma) were used to cultivate the cells for 10
days. The formed spheres were counted by inverse microscopy. Sphere
formation efficiency was calculated as: sphere formation efficiency
= sphere/input cells ×100%.
Transient transfection
The specific siRNA834 and siRNA3408 to know down
Notch3 were obtained from Suzhou GenePharma Corp., China. The
siRNA834 and siRNA3408 and scramble siRNA were transfected to the
A549 and H520 cells by X-tremeGENE siRNA transfection reagent
according to the manufacturer's instructions (Roche). After 48 h,
the cells were collected for further experiments.
Western blotting
Cell pellets were lysed in RIPA lysis buffer added
with phenylmethylsulfonylfluoride (PMSF), Complete Mini protease
inhibitor cocktail, and phosphatase inhibitor cocktail (Roche). The
protein was measured and equal amount of protein was separated on a
10% SDS-PAGE gel, and then transferred to nitrocellulose membrane
(Millipore). 5% fat-free milk was used to block the membrane, and
primary antibodies of Notch3 (Santa Cruz Biotechnology, sc-5593,
1:500 dilution), LC3 (MBL International Corp., 1:1000 dilution) and
GAPDH (Cell Signaling Technology, #2118, 1:5000 dilution) were
added on the membrane for 1 h at room temperature. After washing
three times in Tris Buffered Saline plus Tween (TBST), the
membranes were added with the corresponding secondary antibody
conjugated with HRP. The signal was visualized using Immobilon
Western Chemiluminescent HRP Substrate (Millipore).
Flow cytometry
The lung cancer cells A549 and H520 were washed in
PBS and collected by centrifrugation. The cells were stained with
primary antibodies including Notch3 (Santa Cruz Biotechnology,
sc-5593, 1:50 dilution), CD44 (Abcam, ab51037, 1:100 dilution) and
ALDH1A1 (Abcam, ab52492, 1:100 dilution) at 4°C for 1 h. After
washing with PBS twice, the cell suspension was incubated with
secondary antibody conjugated with FITC at 4°C for 30 min in the
dark. The cells were filtered through a 70-μm nylon mesh and
carried out on a flow cytometer (BD Biosciences). The viable and
single cells were gated for analyses. FlowJo 7.6 software was used
to analyze the data.
RNA extraction and quantitative real
time-PCR (qRT-PCR)
Total RNA was isolated from human NSCLC tissues
using TRIzol Reagent (Invitrogen) according to the manufacturer's
instructions. Total RNA (2 μg) was reverse-transcribed to cDNA with
Moloney murine leukemia virus (MMLV, Invitrogen). The genes
expression levels were measured by qRT-PCR with the SYBR Green PCR
kit (Roche) using Light-Cycler®480 Real-Time PCR System
(Roche). The reactions were incubated in a 96-well optical plate at
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min. β-actin was used as internal control and Notch3
expression level was calculated according to 2−ΔCt, in
which ΔCt=Ct target − Ct control. All PCR
assays were carried out in triplicate, and the mean of triplicates
is reported.
Immunofluorescence staining
Briefly, the cells were washed with PBS and fixed in
cold 10% paraformaldehyde. The cells were washed twice in PBS and
incubated with the primary antibody LC3 (MBL International Corp.,
1:100 dilution) at 4°C overnight. After washing with PBS, the cells
were added with secondary antibody at room temperature for 1 h.
Finally, slides were washed with PBS, mounted with DAPI in mounting
media. Cells were examined using a laser scanning confocal
microscope (Leica, Wetzlar, Germany).
Statistical analyses
Data are shown as mean ± SEM and the experiments
were independently done at least three times. Student's t-test was
used to analyze the mean between different groups. Spearman's
correlation test was carried out to assess correlation between
Notch3 and CSC markers expression. Survival curves were analyzed by
the log-rank Kaplan-Meier method. GraphPad Prism 5 was conducted
for data analysis and p<0.05 was considered to indicate a
statistically significant difference.
Results
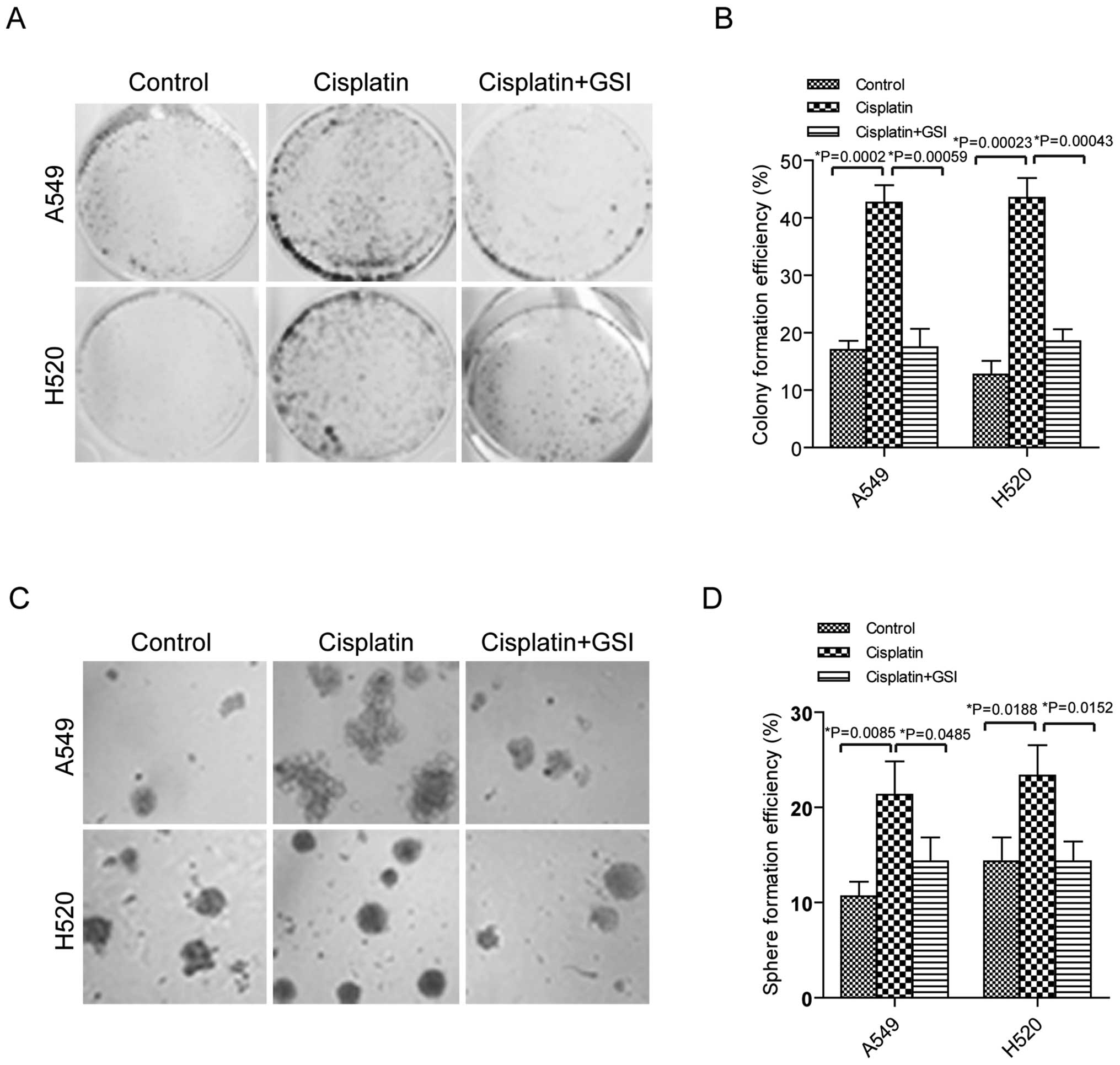
Cisplatin promotes clonogenicity in A549
and H520 cells
It has been reported that CSCs have ability to be
related to drug resistance in lung cancer (2). We investigated how cisplatin as
first-line chemotherapy influences cell clonogenicity of stem-like
property. To obtain drug resistant cells, the lung cancer cells
A549 and H520 were treated with cisplatin for 72 h and the dead
cells were removed by washing in PBS. After that, the single cells
were seeded into 6-well plates or low attachment plates for further
cultivation. Ten days later, more colonies were observed in the
lung cancer cells with cisplatin treatment than the control cells
with significant statistical difference in both A549 and H520 cells
(Fig. 1A and B p<0.05). In
addition, more spheres were formed in the A549 and H520 cells
stimulated with cisplatin compared to the cells without cisplatin
(Fig. 1C and D, p<0.05). These
data suggested that cisplatin induced clonogenicity of stem-like
ability in lung cancer cells.
Inhibition of Notch3 suppresses
clonogenicity in A549 and H520 cells
GSI was reported to prevent Notch3 activation and
reduce proliferation in human lung cancers (19). GSI was added in A549 and H520 cells
before cisplatin treatment, and then colony and sphere formation
were examined. As shown in Fig. 1A and
B, colonies were reduced in the cells with cisplatin plus GSI
compared to the cells with cisplatin. Similarly, decreased sphere
number was identified in the cells stimulated with cisplatin plus
GSI than the cells treated with cisplatin, showing statistically
significant difference (Fig. 1C and
D).
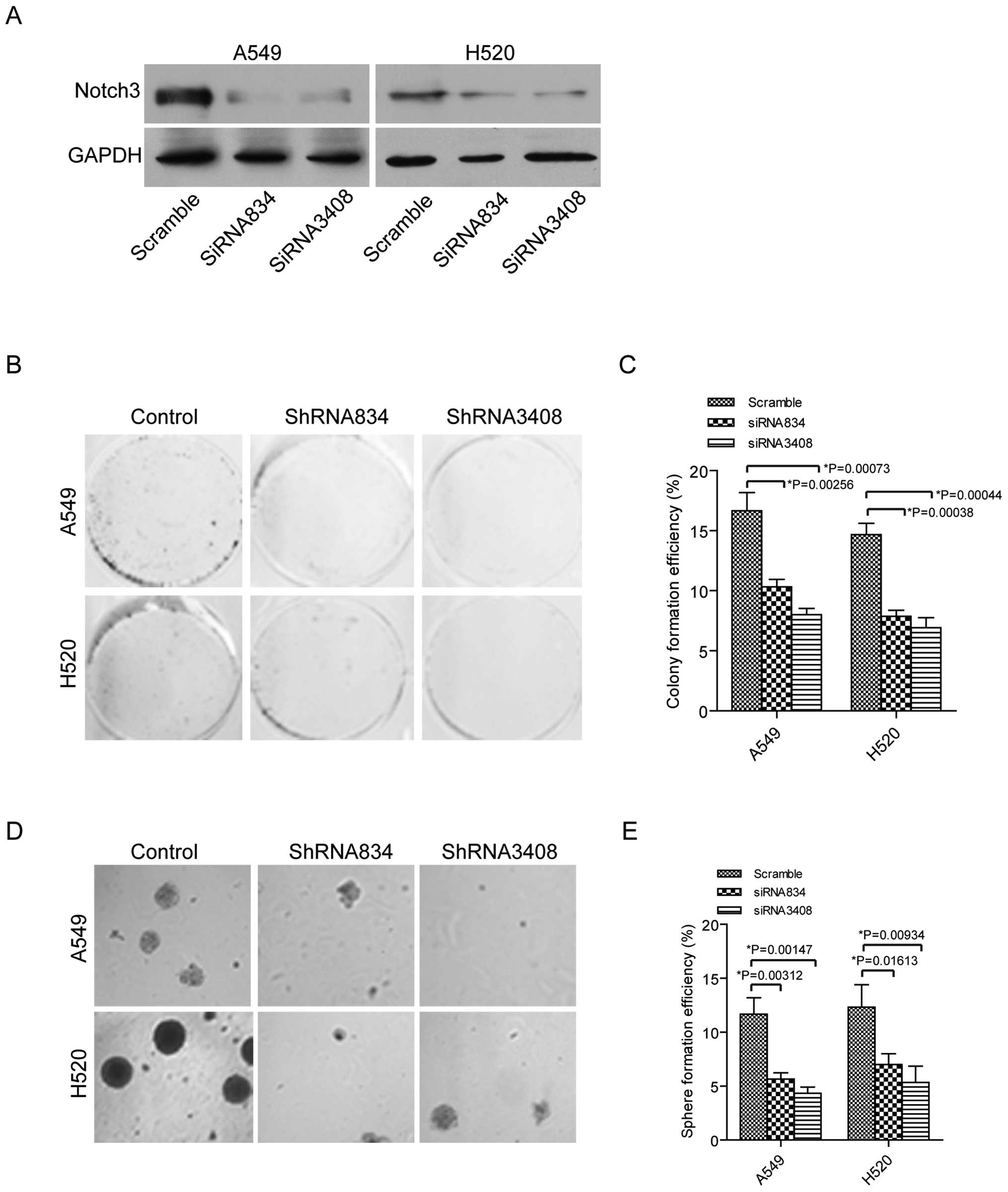
We have mainly studied the role of Notch3 in
regulating stem-like ability in lung cancer cells of A549 and H520.
Thus, we used specific siRNAs to knock down Notch3 expression in
these lung cancer cells. Firstly, the siRNA834 and siRNA3408 were
used to knock down Notch3 expression in A549 and H520 cells and
lower expression of Notch3 was found in these cells by western
blotting (Fig. 2A). Next,
clonogenic ability was tested in the cells transfected with
scramble siRNA, siRNA834 or siRNA3408. As we expected, colony
formation was decreased in the lung cancer cells with siRNA834 or
siRNA3408 treatment compared to the cells with scramble siRNA
treatment (Fig. 2B and C).
Moreover, sphere formation was downregulated in the A549 and H520
cells transfected with siRNA834 and siRNA3408 compared to the cells
with scramble control (Fig. 2D and
E). Altogether, our data indicated that blocking of Notch3 was
able to inhibit stem-like features in lung cancer cells.
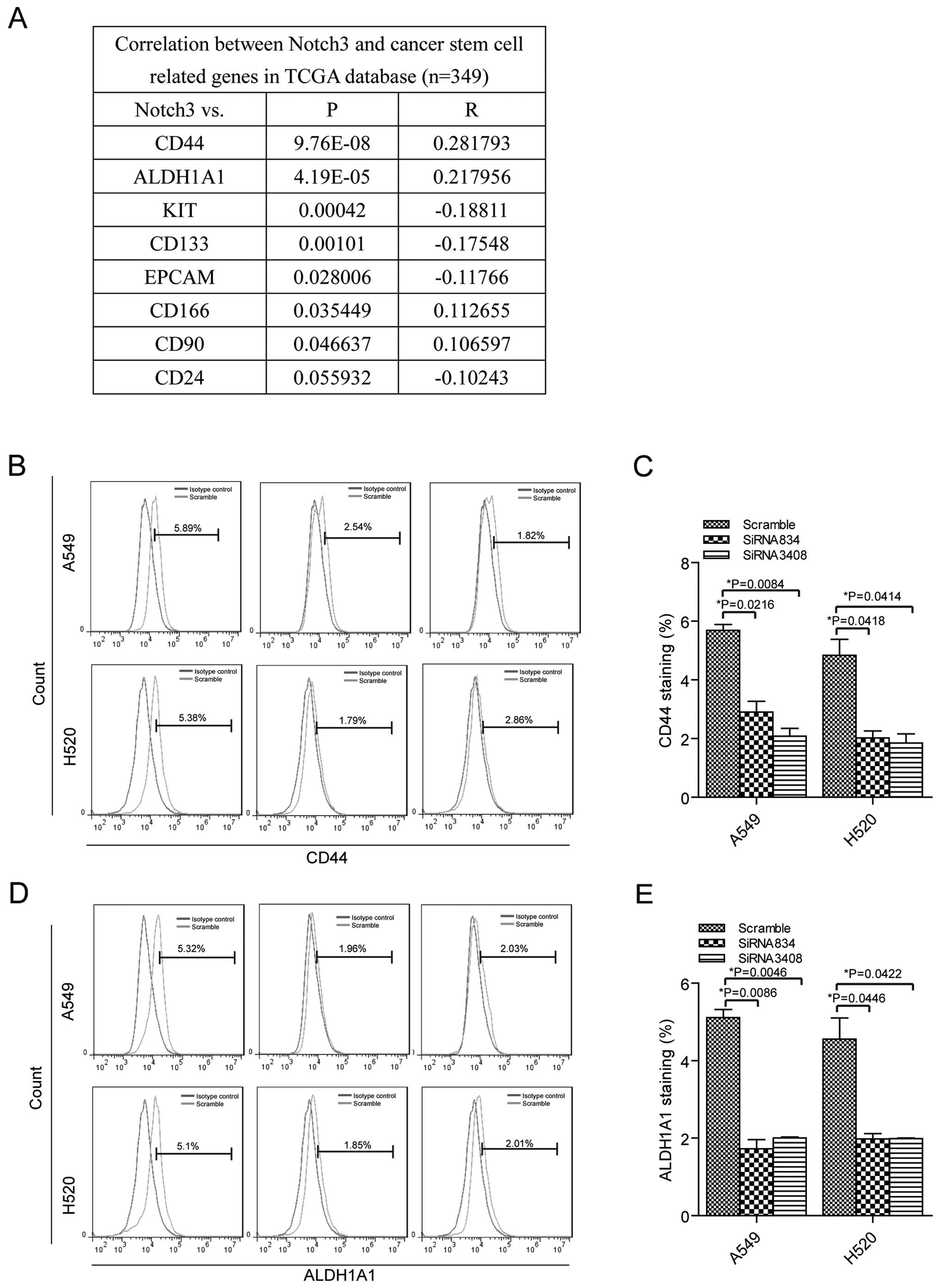
Notch3 is associated with CD44 and
ALDH1A1
Next, we identified the correlation between Notch3
and the CSC markers CD44, ALDH1A1, KIT, CD133, EPCAM, CD166, CD90
and CD24 in lung cancer tissues from RNA-seq of TCGA database
(https://tcga-data.nci.nih.gov/tcga/,
n=349). The analysis showed that Notch3 expression was positively
related to CD44 and ALDH1A1 expression levels with statistically
significant difference (Fig. 3A).
Then, we tested CD44 and ALDH1A1 alterations when the Notch3 was
knocked down in the lung cancer cells. Silenced of Notch3 resulted
in downregulation of CD44 in A549 cells transfected with siRNA834
(2.91±0.52%) and siRNA3408 (2.09±0.37%) compared to the control
cells (5.69±0.28%), (Fig. 3B and
C). H520 cells showed decreased expression of CD44 in siRNA834
transfected cells (2.03±0.33%) and siRNA3408 transfected cells
(1.85±0.44%) than the cells with scramble siRNA (4.83±0.77%).
ALDH1A1 expression was reduced when Notch3 expression was blocked
in A549 cells transfected with siRNA834 (1.73±0.33%) and siRNA3408
(2.01±0.04%) compared to the control cells (5.11±0.3%), (Fig. 3D and E). In addition, there was
decreased expression of ALDH1A1 in siRNA834 transfected H520 cells
(1.99±0.19%) and siRNA3408 transfected cells (1.99±0.04%) compared
to the cells with scramble siRNA (4.56±0.77%). Our results
suggested that Notch3 was positively associated with CD44 and
ALDH1A1 in lung cancer cells.
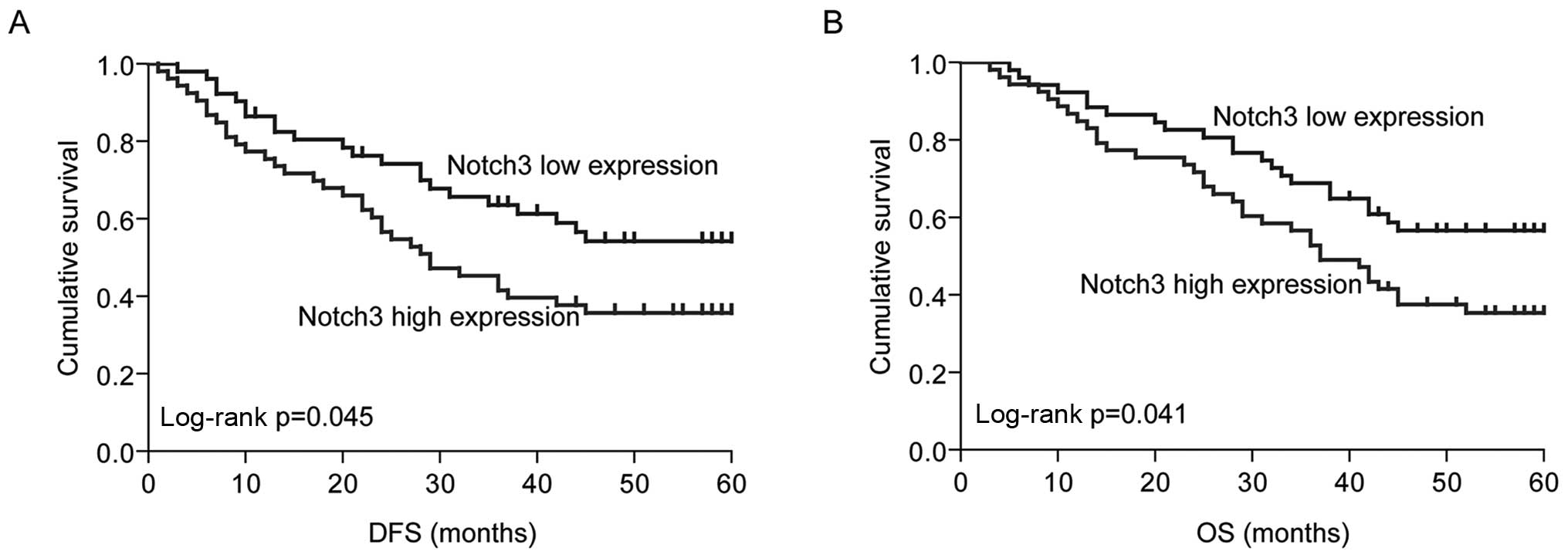
Notch3 is a prognostic factor
Since our data indicated that Notch3 promoted
stem-like property, we tested the relationship between Notch3
expression and progression-free survival (DFS) and OS. Notch3
expression was investigated by qPR-PCR in the primary tumor tissues
from 104 cases of NSCLC patients. According to the median value,
Notch3 expression levels were divided into low expression (n=52)
and high expression (n=52) in these NSCLC cases. Thus, we verified
that high expression of Notch3 was associated with shorter PSF
(median PSF in low expression group, 54.89 months and median PSF in
high expression group, 42.11 months, p=0.045) and OS (median OS in
low expression group, 58.09 months and median OS in high expression
group, 46.23 months, p=0.041), (Fig.
4A and B). The results suggested that Notch3 may be used as a
prognostic factor in NSCLC patients.
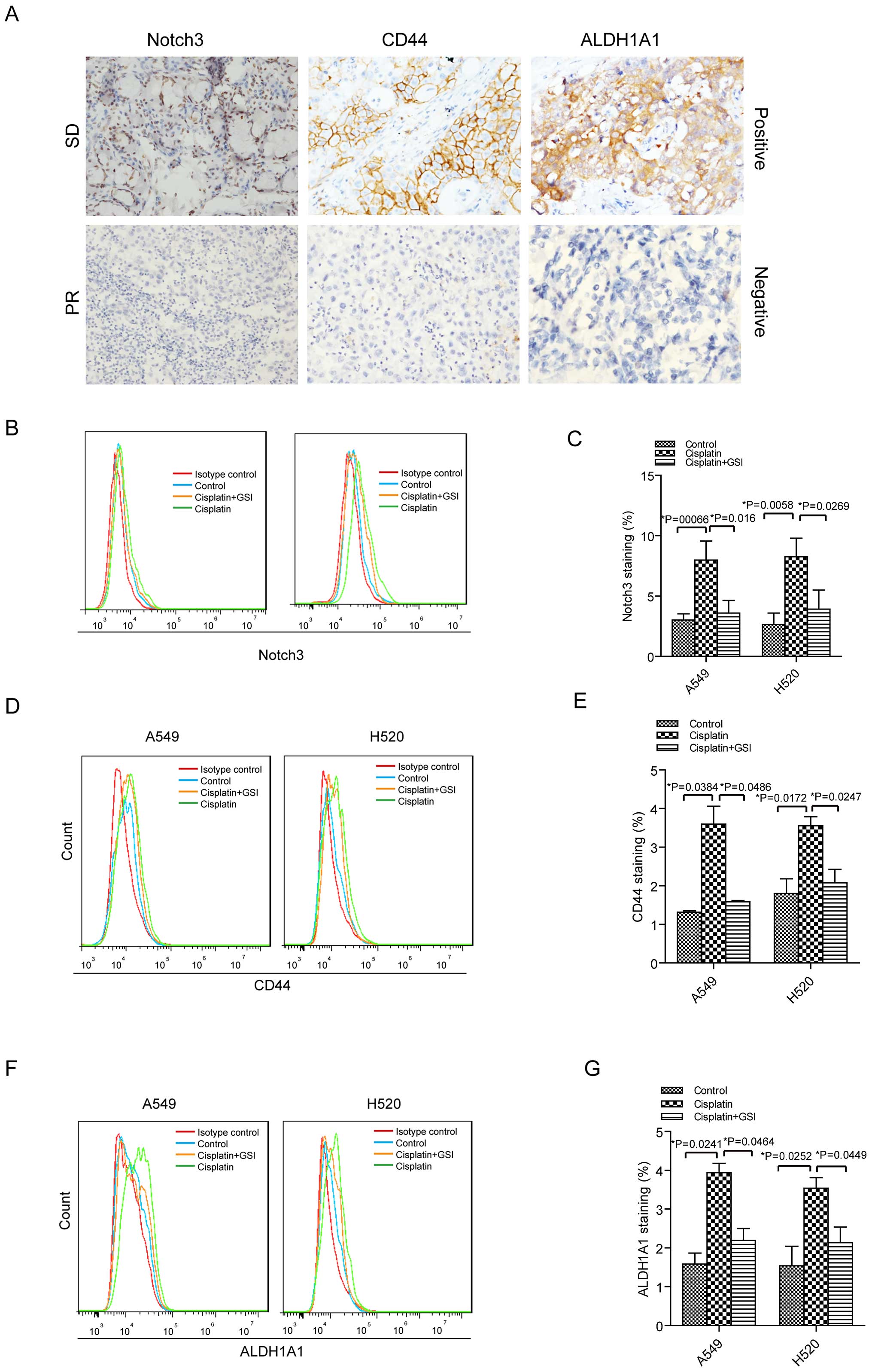
Cisplatin induces Notch3, CD44 and
ALDH1A1 expression levels and GSI suppresses this induction in A549
and H520 cells
Then, we investigated Notch3, CD44 and ALDH1A1
expression in 12 NSCLC patients who received neoadjuvant
chemotherapy. For Notch3 expression, there were 4 out of 6 cases
with chemoresistance showing positive expression and 1 out of 6
cases with chemosensitivity demonstrating positive staining by IHC
assay (Fig. 5A). Among 6 NSCLC
patients with chemoresistance, 5 patients displayed positive
staining for CD44 and ALDH1A1 expression (Fig. 5A). Whereas, there were 3 cases with
positive expression of CD44 and 2 cases with positive staining of
ALDH1A1 in 6 NSCLC patients with chemosensitivity as assessed by
IHC analysis (Fig. 5A).
Moreover, we investigated how Notch3, CD44 and
ALDH1A1 altered in the cisplatin treatment in A549 and H520 cells.
Cisplatin led to 2.64-fold increase of Notch3 expression in A549
cells and 3.12-fold increase of this protein in H520 cells
(Fig. 5B and C). Again, GSI was
used to inhibit the Notch3 signal in the cells with cisplatin
treatment. We observed that Notch3 expression was decreased in A549
and H520 cells with cisplatin plus GSI compared to this protein
expression in the cells with cisplatin treatment. Similarly,
increased expression levels of CD44 and ALDH1A1 were observed in
the lung cancer cells treated with cisplatin compared to the cells
without cisplatin treatment (Fig.
5D–G). There were 2.67-fold increase of CD44 in A549 cells
treated with cisplatin and 1.98-fold increase of CD44 in H520 cells
with cisplatin stimulation. In addition, we obtained 2.48-fold
increase of ALDH1A1 in cisplatin-treated A549 cells and 2.3-fold
increase of ALDH1A1 in cisplatin-treated H520 cells. CD44 and
ALDH1A1 were downregulated in the cells treated with both cisplatin
and GSI compared to the cells administered with cisplatin (Fig. 5D–G).
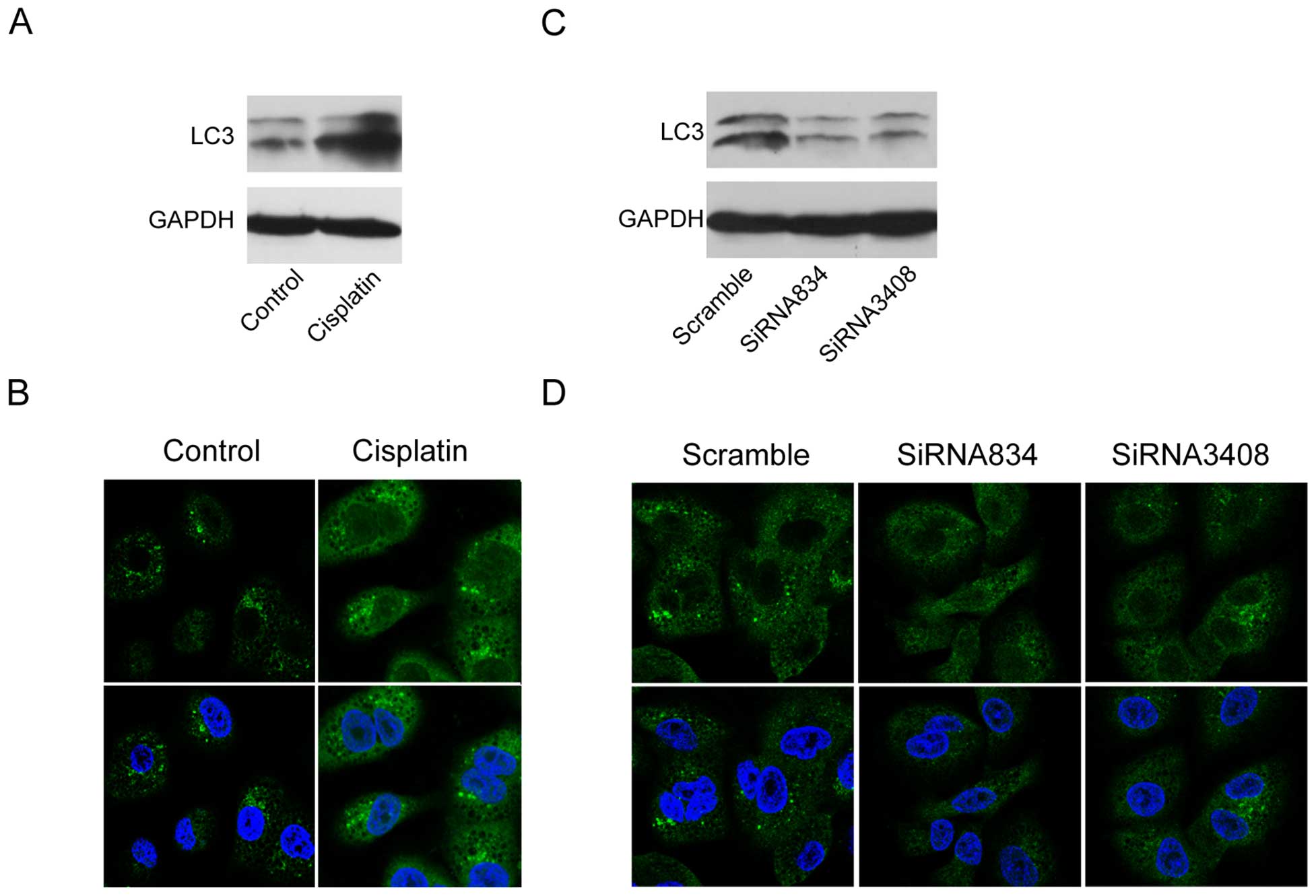
Cisplatin induces autophagy, and blockade
of Notch3 suppresses this effect in A549 cells
Since autophagy is an adaptive response to a
conventional chemotherapy and radiation therapy (20), we determined how autophagy changed
in lung cancer cells with cisplatin treatment. It has been reported
that the cells undergo autophagy, LC3I expression decreased and
LC3II expression increased (21).
Then, we tested LC3II expression in A549 cells with or without
cisplatin treatment by the methods of western blotting and
immunofluorescence. The results showed increase of LC3II in A549
cells with cisplatin treatment compared to the control cells
(Fig. 6A and B). Next, we asked
whether autophagy was associated with Notch3 in the lung cancer
cells. When Notch3 was blocked, LC3II expression was downregulated
in the cells as evaluated by western blot and immunofluorescence
analyses (Fig. 6C and D).
Discussion
Currently, chemoresistance is considered as a major
obstacle for the clinical treatment of advanced and metastatic
NSCLC patients. It is well known that CSCs have a capacity to
escape from chemotherapy and radiotherapy. However, the mechanisms
for the involvement of CSCs in chemoresistance have not been well
elucidated in NSCLC. In the present study, we investigated how
Notch3 influenced CSCs to regulate drug resistance in NSCLC cancer
cells.
Firstly, we verified that cisplatin could induce
colony and sphere formation potential of lung cancer cells A549 and
H520. Consistent with a previous study (8), cisplatin improved stem-like
characteristics in lung cancer cells. Increasing evidence shows
that Notch signal is important in maintenance of tumor cell
subpopulation with great clonogenicity of stem-like property in
lung cancer (22,23). GSI hinders the final proteolytic
cleavage of Notch receptors is a major class of agents inhibiting
the Notch pathway. GSI has the ability of anti-cancer growth in
several cancer types that regulate CSC property (24–26).
We found GSI significantly reduced colony formation and sphere
formation potential in the cisplatin treated cells. Our results
were consistent with a previous study that GSI inhibited Notch
signal restraining cancer cell growth in lung cancer cells
(19). Next, we determined whether
interference with CSC clonogenicity was through blocking Notch3.
Our data showed that specific knock-down of Notch3 led to decrease
of colony and sphere formation, indicating Notch3 played an
important role in promoting stem-like features in lung cancer
cells.
Previous studies have reported several cell surface
markers such as CD133, CD166, CD44 and ALDH1A1 that could identify
CSCs for NSCLC (11–13). Then, we analyzed the relationship
between Notch3 and these CSC markers and our data showed that
Nocth3 expression was positively related to CD44 and ALDH1A1
expression levels in lung cancer tissues. CD44+ cells
showed great tumorigenicity by serial tumor transplantation in
vivo and higher expression of stem-related factors in the
positive lung cancer cells, suggesting CD44 affects the enrichment
of stem-like property (27). In
lung cancer, ALDH+ cells have been proven to be
associated with stem-like capacity and suppression of Notch3 could
decrease clonogenicity (17,23).
In the current study, we clarified that blockade of Notch3 reduced
CD44 and ALDH1A1 expression levels in lung cancer cells. These data
indicated that Notch3 may be combined with ALDH1A1 and CD44 to
regulate stem-like property in lung cancer cells.
Highly active Notch3 is generally found to be
related to poor prognosis in lung cancer patients (28,29).
Meta-analysis from Yuan et al showed high activation of
Notch3 is linked to poor outcome in lung cancer patients (30). In this meta-analysis, IHC was used
to test Notch3 from two articles, and additionally we used qRT-PCR
to analyze Notch3 expression in 104 cases of stage I–III NSCLC
tissues from Peking University Cancer Hospital. The obtained
results showed that high expression of Notch3 is associated with
shorter PFS and OS, and Notch3 could be used as a predictor for
poor prognosis of NSCLC patients.
It has been documented that Notch signal is involved
in drug resistance of cancer cells (14,31).
Shi et al demonstrated that overexpression of Notch3
predicts a significantly higher possibility of resistance to
chemotherapy in advanced NSCLC patients (32). Currently, we demonstrated that
higher expression of Notch3 was shown in the lung cancer tissues
from the patients who were resistant to chemotherapy compared to
expression of this gene in the tissues from the patients who were
sensitive to chemotherapy. Higher expression of CD44 and ALDH1A1
were observed in the lung cancer tissues from the NSCLC patients
who were resistant to chemotherapy, in comparison to the expression
levels of these two markers in the tissues from the NSCLC patients
who were sensitive to chemotherapy. Quan et al reported that
hyaluronan (HA) is a specific ligand for CD44, and HA-conjugated
cisplatin which targets CD44 has highly selective and sensitive
anticancer effects in NSCLC cells (33). The novel synthetic curcumin
analogues against ALDH1A1 and GSK-3β have functions to overcome
chemoresistance in breast cancer (34). Based on induction of the stem-like
feature in lung cancer cells by cisplatin treatment, we also
observed Notch3, CD44 and ALDH1A1 expression levels were
upregulated in the A549 and H520 cells with cisplatin treatment.
Moreover, GSI led to decrease of Notch3, CD44 and ALDH1A1
expression in the lung cancer cells treated with cisplatin to the
extent of lung cancer cells without cisplatin treatment. Thus,
Notch3 is associated with chemotherapy resistance that might be
through CD44 and ALDH1A1.
In general, autophagy refers to an evolutionarily
conserved intracellular catabolic process by consisting of several
sequential stages: initiation, nucleation, elongation, and
maturation to remove damaged cellular components under diverse
stressful conditions (35).
Autophagy can be conducted as a strategy for cancer cells to
overcome the stresses caused by radiation, chemotherapy, or other
treatments (20). Therefore, we
examined autophagy in lung cancer cells with cisplatin treatment.
We observed that autophagy was activated in the lung cancer cells
stimulated with cisplatin. In esophageal cancer, chemotherapy was
also found to induce autophagy (36), and it has been reported that cdx1
promoted CSC property that played an essential role in
chemoresistance through activation of autophagy in colon cancer
(37). We also checked whether
Notch3 was related to autophagy and verified that siRNAs of Notch3
inhibited autophagy in lung cancer cells. It was suggested that
activation of autophagy is related to chemoresistance of CSCs, a
feature that is associated with Notch3 in lung cancer cells.
In conclusion, Notch3 has the capacity to promote
colony formation and sphere formation of stem-like capacity in lung
cancer cells. After verifying induction of stem-like ability in
lung cancer cells with cisplatin, we also found that Notch3 was
related to the CSCs markers ALDH1A1 and CD44. Notch3 was shown to
be related to poor outcome of NSCLC patients. Moreover, cisplatin
promotes autophagy and inhibition of Notch3 was shown to prevent
autophagy, providing a novel therapy to target CSCs in lung
cancer.
Acknowledgements
This study was funded by National Natural Science
Foundation of China (grant no. 81502578), Peking University (PKU)
985 Special Funding for Collaborative Research with PKU Hospitals,
and Beijing Municipal Administration of Hospitals Clinical Medicine
Development of special funding support (code: ZYLX201509).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopez-Ayllon BD, Moncho-Amor V, Abarrategi
A, Ibañez de Cáceres I, Castro-Carpeño J, Belda-Iniesta C, Perona R
and Sastre L: Cancer stem cells and cisplatin-resistant cells
isolated from non-small-lung cancer cell lines constitute related
cell populations. Cancer Med. 3:1099–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012.PubMed/NCBI
|
|
4
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
5
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
6
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+
prostate cancer cells from xenograft human tumors are enriched in
tumorigenic and metastatic progenitor cells. Oncogene.
25:1696–1708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT,
Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J, et al: Cisplatin selects
for multidrug-resistant CD133+ cells in lung
adenocarcinoma by activating Notch signaling. Cancer Res.
73:406–416. 2013. View Article : Google Scholar
|
|
9
|
Salnikov AV, Gladkich J, Moldenhauer G,
Volm M, Mattern J and Herr I: CD133 is indicative for a resistance
phenotype but does not represent a prognostic marker for survival
of non-small cell lung cancer patients. Int J Cancer. 126:950–958.
2010.
|
|
10
|
Kitamura H, Okudela K, Yazawa T, Sato H
and Shimoyamada H: Cancer stem cell: Implications in cancer biology
and therapy with special reference to lung cancer. Lung Cancer.
66:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sterlacci W, Savic S, Fiegl M, Obermann E
and Tzankov A: Putative stem cell markers in non-small-cell lung
cancer: a clinico pathologic characterization. J Thorac Oncol.
9:41–49. 2014. View Article : Google Scholar
|
|
12
|
Tachezy M, Zander H, Wolters-Eisfeld G,
Müller J, Wicklein D, Gebauer F, Izbicki JR and Bockhorn M:
Activated leukocyte cell adhesion molecule (CD166): An ‘inert’
cancer stem cell marker for non-small cell lung cancer? Stem Cells.
32:1429–1436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan X, Luo H, Zhou X, Zhu B, Wang Y and
Bian X: Identification of CD90 as a marker for lung cancer stem
cells in A549 and H446 cell lines. Oncol Rep. 30:2733–2740.
2013.PubMed/NCBI
|
|
14
|
Pannuti A, Foreman K, Rizzo P, Osipo C,
Golde T, Osborne B and Miele L: Targeting Notch to target cancer
stem cells. Clin Cancer Res. 16:3141–3152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Selkoe D and Kopan R: Notch and
Presenilin: Regulated intra-membrane proteolysis links development
and degeneration. Annu Rev Neurosci. 26:565–597. 2003. View Article : Google Scholar
|
|
16
|
Fortini ME: Gamma-secretase-mediated
proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell
Biol. 3:673–684. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hassan KA, Wang L, Korkaya H, Chen G,
Maillard I, Beer DG, Kalemkerian GP and Wicha MS: Notch pathway
activity identifies cells with cancer stem cell-like properties and
correlates with worse survival in lung adenocarcinoma. Clin Cancer
Res. 19:1972–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arasada RR, Amann JM, Rahman MA, Huppert
SS and Carbone DP: EGFR blockade enriches for lung cancer stem-like
cells through Notch3-dependent signaling. Cancer Res. 74:5572–5584.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: Gamma-secretase inhibitor
prevents Notch3 activation and reduces proliferation in human lung
cancers. Cancer Res. 67:8051–8057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu YL, Jahangiri A, Delay M and Aghi MK:
Tumor cell autophagy as an adaptive response mediating resistance
to treatments such as antiangiogenic therapy. Cancer Res.
72:4294–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Li Y, Banerjee S and Sarkar FH:
Emerging role of Notch in stem cells and cancer. Cancer Lett.
279:8–12. 2009. View Article : Google Scholar :
|
|
23
|
Sullivan JP, Spinola M, Dodge M, Raso MG,
Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, et
al: Aldehyde dehydrogenase activity selects for lung adenocarcinoma
stem cells dependent on notch signaling. Cancer Res. 70:9937–9948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abel EV, Kim EJ, Wu J, Hynes M, Bednar F,
Proctor E, Wang L, Dziubinski ML and Simeone DM: The Notch pathway
is important in maintaining the cancer stem cell population in
pancreatic cancer. PLoS One. 9:e919832014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grudzien P, Lo S, Albain KS, Robinson P,
Rajan P, Strack PR, Golde TE, Miele L and Foreman KE: Inhibition of
Notch signaling reduces the stem-like population of breast cancer
cells and prevents mammosphere formation. Anticancer Res.
30:3853–3867. 2010.PubMed/NCBI
|
|
26
|
Saito N, Fu J, Zheng S, Yao J, Wang S, Liu
DD, Yuan Y, Sulman EP, Lang FF, Colman H, et al: A high Notch
pathway activation predicts response to γ secretase inhibitors in
proneural subtype of glioma tumor-initiating cells. Stem Cells.
32:301–312. 2014. View Article : Google Scholar :
|
|
27
|
Leung EL, Fiscus RR, Tung JW, Tin VP,
Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung
cancer cells expressing CD44 are enriched for stem cell-like
properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andersen S, Donnem T, Al-Saad S, Al-Shibli
K, Stenvold H, Busund LT and Bremnes RM: Correlation and
coexpression of HIFs and NOTCH markers in NSCLC. Anticancer Res.
31:1603–1606. 2011.PubMed/NCBI
|
|
29
|
Westhoff B, Colaluca IN, D'Ario G,
Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G,
Viale G, et al: Alterations of the Notch pathway in lung cancer.
Proc Natl Acad Sci USA. 106:22293–22298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S,
Chen Y and Wu K: Meta-analysis reveals the correlation of Notch
signaling with non-small cell lung cancer progression and
prognosis. Sci Rep. 5:103382015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee
S, Kong D and Sarkar FH: Targeting Notch signaling pathway to
overcome drug resistance for cancer therapy. Biochim Biophys Acta.
1806:258–267. 2010.PubMed/NCBI
|
|
32
|
Shi C, Qian J, Ma M, Zhang Y and Han B:
Notch 3 protein, not its gene polymorphism, is associated with the
chemotherapy response and prognosis of advanced NSCLC patients.
Cell Physiol Biochem. 34:743–752. 2014.PubMed/NCBI
|
|
33
|
Quan YH, Kim B, Park JH, Choi Y, Choi YH
and Kim HK: Highly sensitive and selective anticancer effect by
conjugated HA-cisplatin in non-small cell lung cancer overexpressed
with CD44. Exp Lung Res. 40:475–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kesharwani RK, Srivastava V, Singh P,
Rizvi SI, Adeppa K and Misra K: A Novel approach for overcoming
drug resistance in breast cancer chemotherapy by targeting new
synthetic curcumin analogues against aldehyde dehydrogenase 1
(ALDH1A1) and glycogen synthase kinase-3 β (GSK-3β). Appl Biochem
Biotechnol. 176:1996–2017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Green DR and Levine B: To be or not to be?
How selective autophagy and cell death govern cell fate. Cell.
157:65–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
O'Donovan TR, O'Sullivan GC and McKenna
SL: Induction of autophagy by drug-resistant esophageal cancer
cells promotes their survival and recovery following treatment with
chemotherapeutics. Autophagy. 7:509–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu S, Wang X, Chen J and Chen Y: Autophagy
of cancer stem cells is involved with chemoresistance of colon
cancer cells. Biochem Biophys Res Commun. 434:898–903. 2013.
View Article : Google Scholar : PubMed/NCBI
|