Introduction
Breast cancer is the most frequent malignancy
diagnosed in women in the western world. Oxidative stress is one of
the important pathogenic factors of cancer development (1). Both in vitro and in
vivo studies have shown that curcumin and its analogs target
critical genes associated with angiogenesis, apoptosis, cell cycle,
and metastasis (1). Among the
antioxidants, curcumin
(1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione;
(diferuloylmethane) is a dietary natural yellow pigment derived
from the rhizome of the turmeric herb known as Curcuma longa
(Zingiberaceae) originating from India and South Asia. It is a
polyphenol derived from several curcumin species, commonly known as
turmeric which has been shown to inhibit carcinogen activation,
modulate cell survival and apoptosis, with anti-invasive and
anti-metastatic effects on breast, lung, colon and prostate cancer
(1).
Epidemiological and experimental data demonstrated
the efficacy of curcumin in chemoprevention and reversing
chemoresistance of tumors of certain cancers. It possesses
anti-proliferative and anti-carcinogenic potential (2,3).
Curcumin interferes with multiple genes that promote
carcinogenesis. It is a pleiotropic molecule with
anti-proliferative, antioxidant and chemopreventive properties.
Alteration of gene expressions involved in key signaling pathways
render this model an important tool for monitoring effects of
natural dietary compounds in breast carcinogenesis (2).
One important concept of epithelial-mesenchymal
transition (EMT), which has been recognized for several decades as
a fundamental process of embryogenesis, is currently considered a
pivotal event in the initial step of the metastatic cascade that
allows cells to acquire migratory, invasive and stem-like
properties (4).
During EMT of cancer cells in situ,
epithelial cell layers lose polarity together with cell-to-cell
contacts and then undergo a dramatic remodeling of the
cytoskeleton. Changes in gene expression that promote cell-to-cell
contact, such as E-cadherin and γ-catenin, may be
lost and the cells may acquire mesenchymal characteristics such as
changes at N-cadherin, vimentin, fibronectin levels
resulting in an enhanced ability for cell migration and invasion
(5). Cadherins are glycoproteins
responsible for homotypic and calcium-dependent cell-cell adhesion
(6). E-cadherin is a
membrane glycoprotein that plays an essential role in maintaining
the integrity of cell-to-cell adhesion, which is significantly
associated with tumor invasiveness and metastatic dissemination
(7). Dysfunction or loss of
E-cadherin is associated with an increased tendency for
tumor metastasis (8). In addition,
degradation of extracellular matrix and basement membranes by the
tumor cells is a critical step and occurs at several stages of the
metastatic cascade (9,10). N-cadherin (cadherin-2 or
CDH-2) is a mesenchymal cadherin overexpressed in many cancers and
associated with cancer cell migration and FGF receptor signaling in
breast cancer metastasis (11).
β-catenin is a 92 kDa protein that plays a role in both cell
adhesion and intracellular signaling (12) as the classical cadherins which play
fundamental roles in the development of multicellular organisms
(13).
Smad interacting protein 1 (SIP1; also known as
ZEB2, for zinc finger E-box-binding protein 2 and ZFHX1B)
belongs to the δEF-1 or ZEB protein family (14) and are transcriptional factors
characterized by containing a homeo domain flanked by two separated
zinc finger clusters (15). It is
expressed in various types of human tumors, such as breast cancer,
gastric cancer and ovarian cancer (15). ZEB2 is a potent repressor of
E-cadherin through its direct binding to the E-cadherin promoter
and a key player in tumor cell invasion and metastasis (16,17).
Twist1 induces EMT and extracellular matrix
degradation in tumor progression (18–20).
Slug is a member of the SNAI family of C2H2-zinc finger family of
transcriptional repressors (21–23).
It is involved in the EMT during development (22), acts as an inhibitor of apoptosis
(24), and causes tubulogenesis
during breast and kidney developments (21,22).
AXL is a member of the TAM (Tyro3, Axl, Mer) family of receptor
tyrosine kinases (RTK), originally identified as a transforming
gene in cells of chronic myelogenous leukemia patients. It is
activated through several mechanisms, including binding of its
ligand, growth arrest specific 6 (Gas6), and extracellular
domain-mediated dimerization or crosstalk with HER2/neu (25–27).
Vimentin belongs to intermediate filaments that with microfilaments
and microtubules are the major cytoskeletal elements of the cell
(28,29).
STAT-3, a versatile member of the family known as
signal transducers and activators of transcription (STAT) mediates
the axial responses of cytokines. STAT-3 is involved in normal
cellular responses, as well as oncogenesis (30). Fibronectin is a component of the
extracellular matrix and exerts multiple effects in vitro
and in vivo including stimulation of cell proliferation,
migration, differentiation and survival (31–34).
It affects cell behavior through activation of various cell surface
receptors most notably integrins (35). Fibronectin is required for the
development of fibrillar structures (36) and for the storage and activation of
various growth factors (37).
The p53 gene is known as the guardian of the
genome (38). A major biological
function of p53 is to respond to stress signals and activate the
transcription of downstream target genes involved in important
cellular mechanisms like cell cycle control, DNA repair and
apoptosis. Caveolin is a specialized lipid raft on the plasma
membrane found in mesenchymal cells. The caveolin family consists
of three members, caveolin-1 (Cav-1), caveolin-2 and caveolin-3.
Cav-1 is widely expressed in various tissues and plays an essential
role in a number of human diseases including cancer (39).
Apoptosis is genetic death program (40). The balance between pro- and
anti-apoptotic signals maintain biological homeostasis and its
imbalance is related to malignant transformation (40). The central component of the
apoptotic machinery is the family of caspases (41,42).
The members of the caspase family can be divided into two groups:
i) upstream initiator caspases, such as caspase-8 and ii)
downstream effector caspases, such as caspase-3 (43,44).
Cyclins, cyclin-dependent kinases (cdks) have been
identified as regulatory subunits (cyclins) and catalytic subunits
(cdks) of cell cycle-regulated kinases involved in the control of
mitosis. It is involved in regulating the G1 to S transition
(45,46). Abnormalities involving cyclin D1
may deregulate control of the G1-S transition and, therefore,
contribute to genomic instability and tumor development (45,46).
Cyclin D1 along with its binding partners CDK 4/6 partially mediate
G1 to S-phase transition of the cell cycle through phosphorylation
and inactivation of retinoblastoma (Rb) protein with subsequent
release of E2F transcription factors (47–49).
It is believed that the oncogenic properties of cyclin D1 depend to
a large extent on its ability to activate cyclin-dependent kinases
4 or 6 (Cdk4/6) (50,51).
Nuclear Factor κB (NFκB) is a complex of
transcription factors that function in the development of acquired
resistance to several other targeted agents (52). NFκB signaling has two major
pathways, one is the canonical pathway that mainly modulates cell
proliferation, inflammation or anti-apoptosis, and the other one is
the non-canonical pathway that mainly controls lymphogenesis
(53).
To gain insights into the effects of curcumin on
breast carcinogenesis an established in vitro experimental
breast cancer model (Alpha model) (54) was used. It was developed with the
immortalized human breast epithelial cell line, MCF-10F (55) that was exposed to low doses of high
LET (linear energy transfer) α-particles (150 keV/μm) of radiation,
values comparable to α-particles emitted by radon progeny, and
subsequently cultured in presence or absence of 17β-estradiol
(estrogen). MCF-10F was exposed to low doses of high LET
α-particles (150 keV/microm) and subsequently cultured in the
presence or absence of 17beta-estradiol (E) for periods of up to 10
months post-irradiation. MCF-10F cells irradiated with either a
single 60 cGy dose or 60/60 cGy doses of α-particles showed gradual
phenotypic changes including altered morphology, increase in cell
proliferation relative to the control, anchorage-independent growth
and invasive capability before becoming tumorigenic in nude mice.
In addition, these cells present all the characteristics of breast
epithelium in their ultra structural features (56–58).
However, only those MCF-10F cells treated with a 60 cGy dose of
α-particles followed by estrogen treatment and exposed to a second
dose of 60 cGy dose of α-particles followed by estrogen (60 cGy +
E/60 cGy + E), named Alpha5 became tumorigenic in both SCID and
nude mice (54). Tumor2 developed
from Alpha5 injected in the athymic mice. The aim of this work was
to evaluate the effect of curcumin on epithelial mesenchymal
transition and other related genes in breast cancer cells
transformed by low doses of α-particles and estrogen.
Materials and methods
Breast cancer cell lines
The spontaneously immortalized breast epithelial
cell line, MCF-10F cells was grown in DMEM/F-12 (1:1) medium
supplemented with antibiotics 100 U/ml penicillin, 100 μg/ml
streptomycin, 2.5 μg/ml amphotericin B (all from Life Technologies,
Grand Island, NY, USA) and 10 μg/ml and 5% equine serum (Biofluids,
Rockville, MD, USA), 0.5 μg/ml hydrocortisone (Sigma, St. Louis,
MO, USA) and 0.02 μg/ml epidermal growth factor (Collaborative
Research, Bedford, MA, USA). An in vitro experimental breast
cancer model developed by exposure of the immortalized human breast
epithelial cell line was used. MCF-10F was exposed to low doses of
high LET (linear energy transfer) α-particles radiation (150
keV/μm) and subsequent growth in the presence or absence of
17β-estradiol at 10−8 M (E or Estrogen) (Sigma-Aldrich,
St. Louis, MO, USA). The following cell line consisted of human
breast epithelial cells in different stages of transformation: i) a
control cell line, MCF-10F; ii) Alpha5 and iii) Tumor2 (54).
RNA extraction and cDNA synthesis
RNA from cells were obtained using TRIzol reagent
(Invitrogen Corp., Carlsbad, CA, USA) following to the
manufacturer's protocol. RNA (2 μg) were reverse-transcribed to
cDNA with kit High capacity cDNA Reverse Transcription (Applied
Biosystems, Carlsbad, CA, USA).
RT-qPCR
Synthesized cDNA (2 μl) was mixed in 20 μl qPCR
reaction containing SYBR Green PCR Master Mix (Agilent, La Jolla,
CA, USA) and 5 μM of primers for the target genes such as
E-cadherin, N-cadherin, β-catenin, ZEB2, Twist, Slug, Axl,
vimentin, STAT-3, fibronectin, p53, caveolin-1, caspase-3,
caspase-8, cyclin D1 and NFκB. Table I shows the primers to develop cDNA
probes. CFX 96 Touch Real-time PCR Detection Systems (Bio-Rad
Laboratories, Hercules, CA, USA) was used to perform the reaction
with the following conditions: 95°C for 10 min and 40 cycles of a
2-step program of 95°C for 10 sec and 61°C for 45 sec. Reactions
were performed in triplicate. Threshold cycle (Ct) was obtained
using Bio-Rad CFX Manager 2.1 software. Gene expression was
normalized using β-actin. Relative expression was always normalized
to the average breast cells and its counterparts.
 | Table IPrimers for genes selected to develop
cDNA probes. |
Table I
Primers for genes selected to develop
cDNA probes.
| Gene name | Product length
(bp)a | Primer
sequenceb |
|---|
|
E-cadherin | 93 | F:
AGTGGGCACAGATGGTGTGA
R: TAGGTGGAGTCCCAGGCGTA |
|
N-cadherin | 67 | F: TCG ATT GGT TTG
ACC ACG G
R: GAC GGT TCG CCA TCC AGA C |
|
β-catenin | 94 | F:
GCAGAGTGCTGAAGGTGCTA
R: TCTGTCAGGTGAAGTCCTAAAGC |
| ZEB2 | 128 | F:
CAAGAGGCGCAAACAAGC
R: GGTTGGCAATACCGTCATCC |
| Twist1 | 118 | F:
TCCGCGTCCCACTAGCA
R: AGTTATCCAGCTCCAGAGTCTCTAGAC |
| Slug | 72 | F:
GACCCTGGTTGCTTCAAGGA
R: TGTTGCAGTGAGGGCAAGAA |
| AXL | 121 | F:
GTTTGGAGCTGTGATGGAAGGC
R: CGCTTCACTCAGGAAATCCTCC |
|
Vimentin | 117 | F:
TGTCCAAATCGATGTGGATGTTTC
R: TTGTACCATTCTTCTGCCTCCTG |
| STAT-3 | 163 | F:
GGTTGGACATGATGCACACTAT
R: AGGGCAGACTCAAGTTTATCAG |
|
Fibronectin | 105 | F:
GGAGGAAGCCGAGGTTTTAAC
R: ACGCTCATAAGTGTCACCCA |
| mp53 | 128 | F:
CCTCAGCATCTTATCCGAGTGG
R: TGGATGGTGGTACAGTCAGAGC |
|
Caveolin-1 | 79 | F:
AACGATGACGTGGTCAAGATTG
R: TCCAAATGCCGTCAAAACTGT |
|
Caspase-3 | 192 | F:
CAGAACTGGACTGTGGCATTG
R: GCTTGTCGGCATACTGTTTCA |
|
Caspase-8 | 128 | F:
CATCCAGTCACTTTGCCAGA
R: GCATCTGTTTCCCCATGTTT |
| Cyclin
D1 | 60 | F:
GTGGCCTCTAAGATGAAGGA
R: GGTGTAGATGCACAGCTTCT |
| NFκB | 114 | F:
ATCTGCCGAGTGAACCGAAACT
R: CCAGCCTGGTCCCGTGAAA |
Cell migration and invasion assays
Migration and invasiveness were performed using
modified Boyden's chambers (Corning, Inc., Corning, NY, USA)
constructed with multiwell cell culture plates and cell culture
inserts. The upper chambers of Transwells with 8-μm membrane pores
were pre-coated with 60 μl Matrigel matrix gel (BD Biosciences) at
least 1 h before seeding of the tested cells (54). A total of 3×105 in 100
μl of medium without fetal bovine serum was added into the upper
chambers and 600 μl of medium with 10% FBS was placed to lower
chambers as chemoattractant. Cells were cultured for 48 h following
treatment. Curcumin (30 μM) was added to the cell culture. Normal
culture medium was added at the bottom chamber to induce the cancer
cell lines. Cells which were pretreated were seeded in the top
chamber. The matrigel invasion chamber was incubated for 16 h in a
humidified tissue culture incubator. Then, the upper chambers were
removed from lower chambers and then wiped using cotton swabs. The
invaded and migrated cells were fixed using 100% methanol at room
temperature for 15 min, visualized and quantified using DAPI. Three
fields of each chamber were photographed (x40 magnification). This
experiment was independently repeated at least twice.
Statistical analysis
Numerical data were expressed as the average ±
standard error of the mean (SEM). Comparison between treated groups
and controls was carried out by ANOVA and Dunnet's test. A
P<0.05 was considered to be significant.
Results
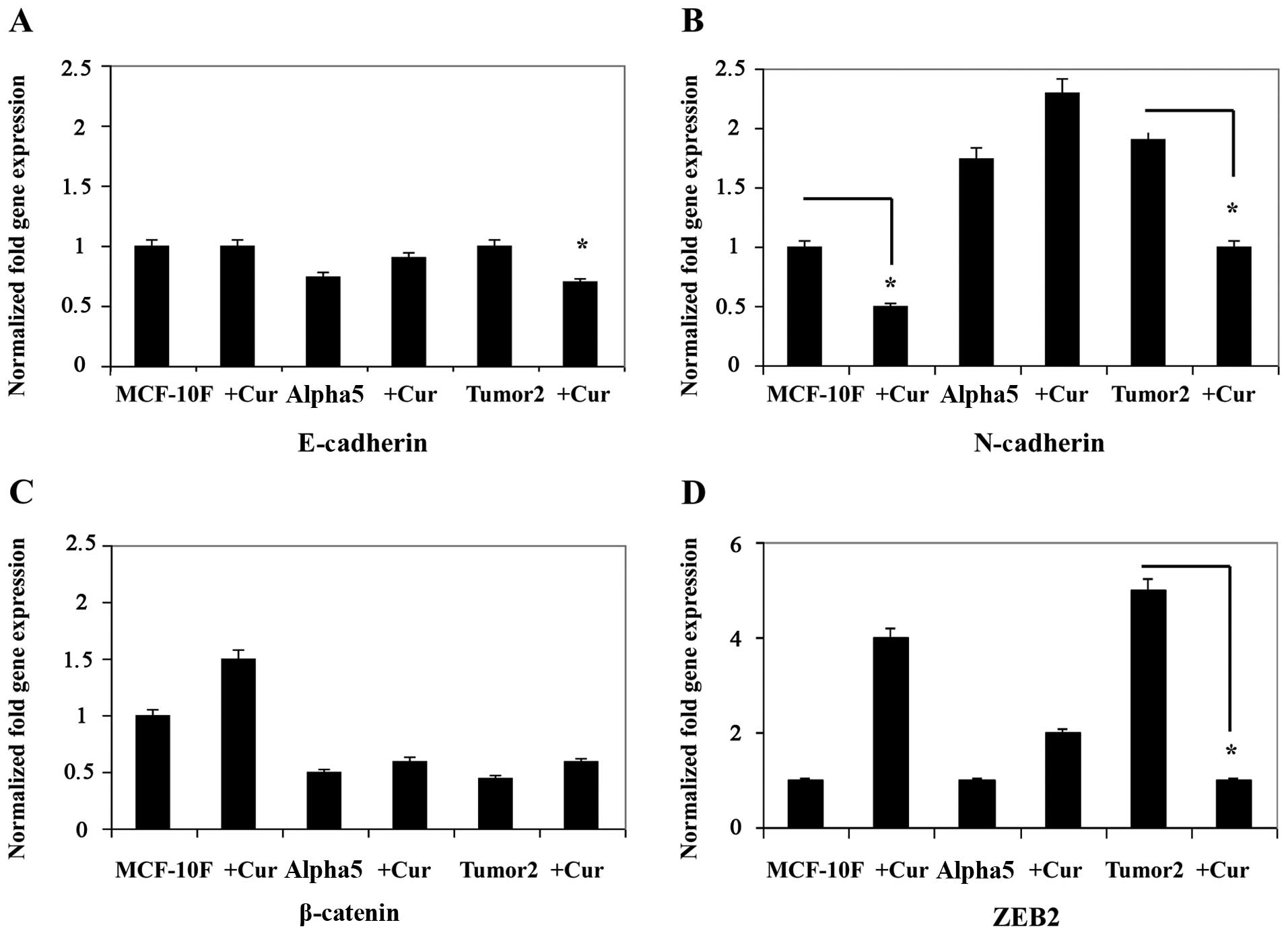
Curcumin inhibited the expression of markers of EMT
such as E-cadherin, N-cadherin, β-catenin and ZEB2 in breast cancer
cells (Fig. 1A–D). Results in
Fig. 1A–D show a decrease in
E-cadherin (Fig. 1A),
N-caherin (Fig. 1B),
β-catenin (Fig. 1C), and
ZEB2 (Fig. 1D) gene
expression in MCF-10F and Tumor2 (P<0.01) in comparison with its
counterparts. However, there was no difference in Alpha5 cell line.
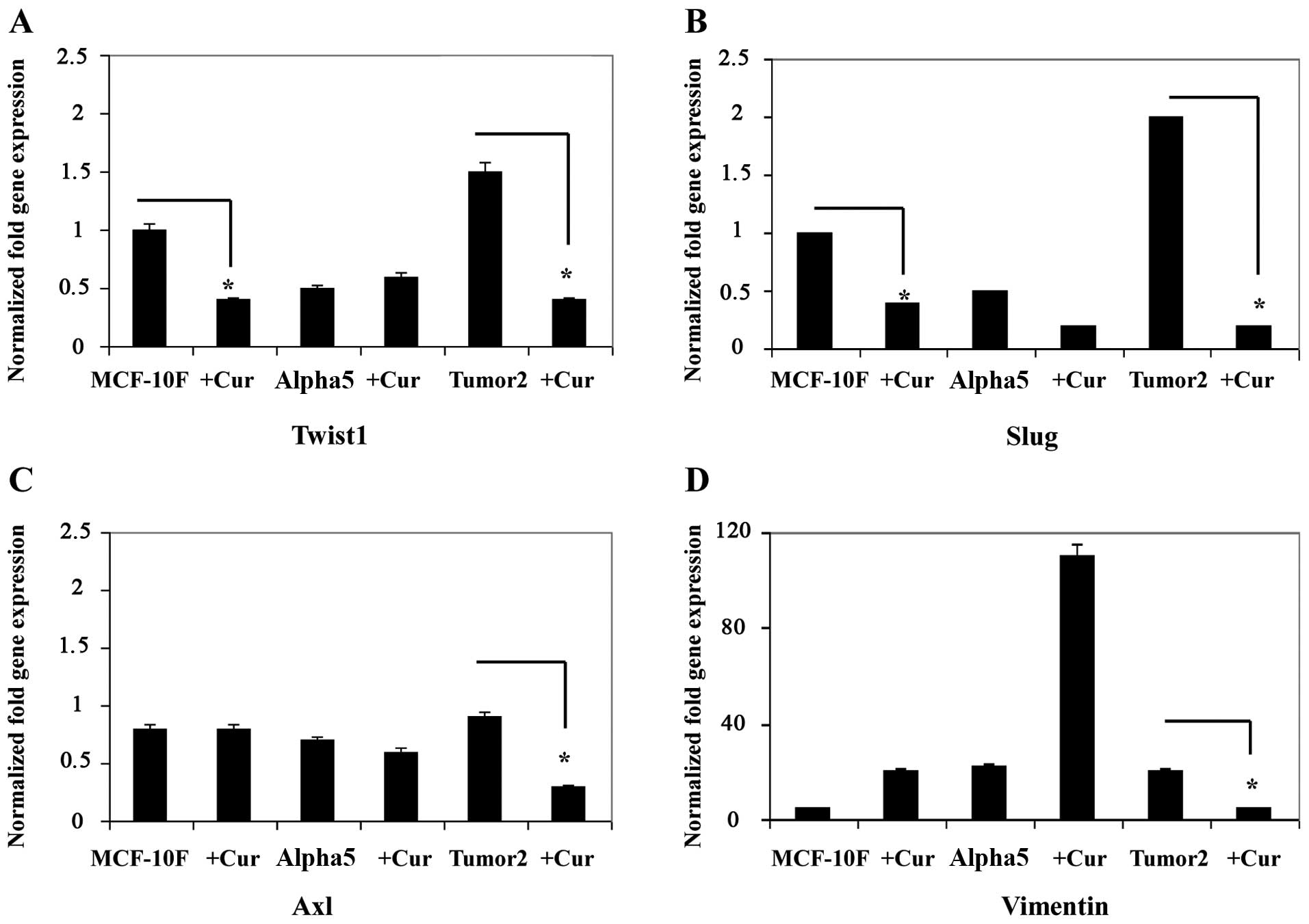
Results in Fig. 2A–D show a
decrease in other EMT-related genes Twist (Fig. 2A), Slug (Fig. 2B), Axl (Fig. 2C), and vimentin (Fig. 2D) gene expression in MCF-10F and
Tumor2. However, there was no difference in Alpha5 cell line.
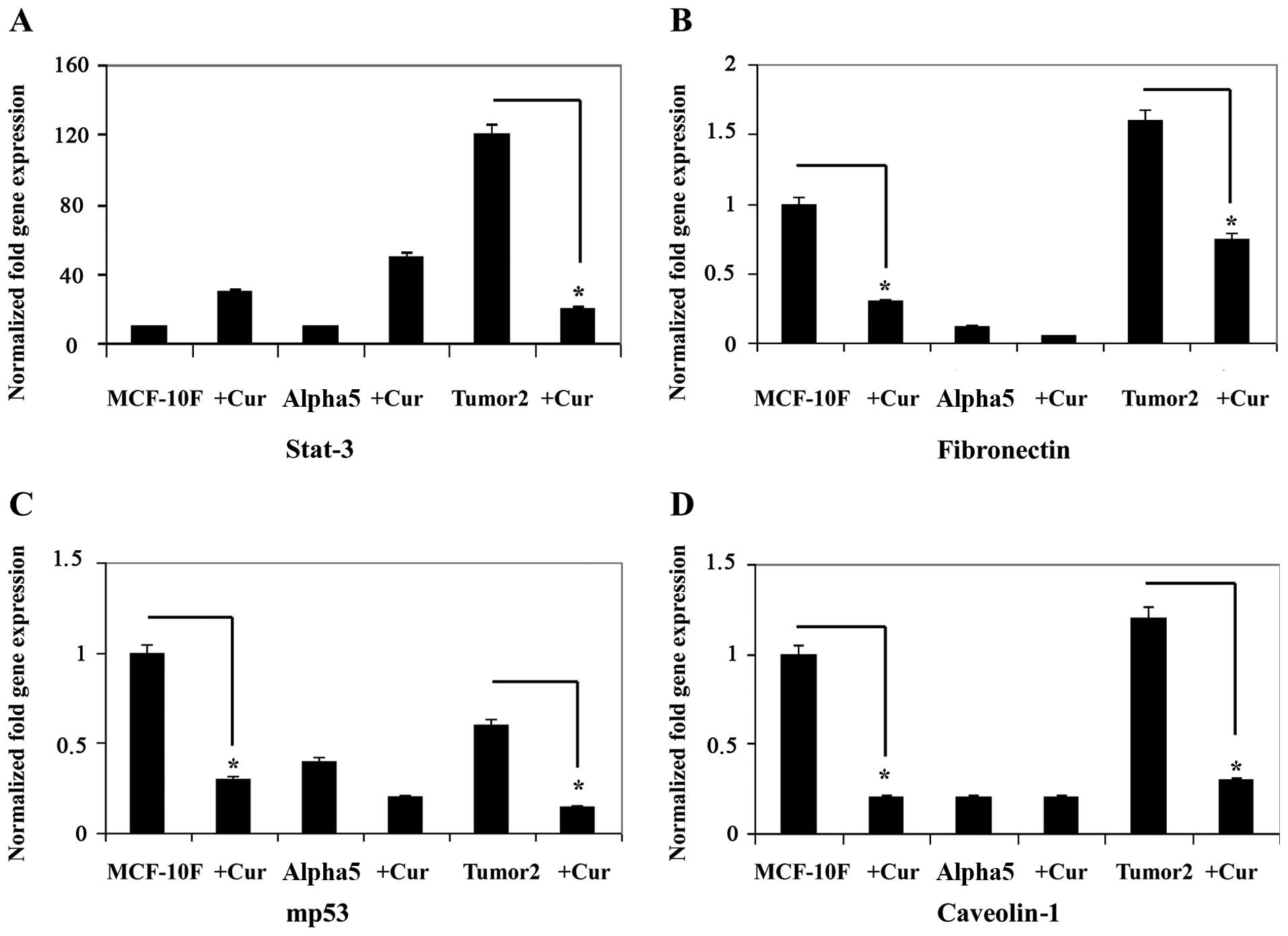
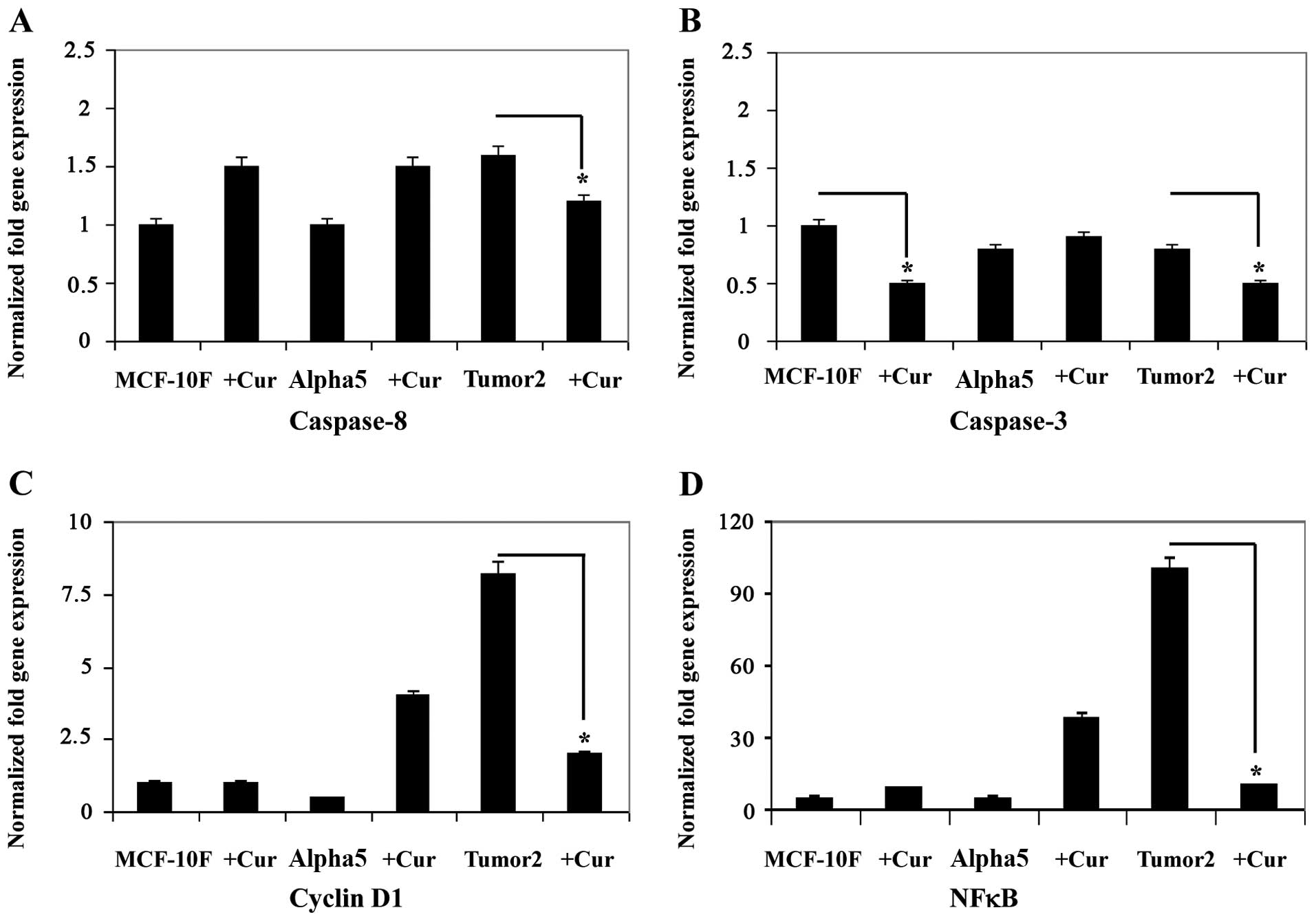
Curcumin induced a decrease in gene expression as
shown in Figs. 3 and 4A–D for STAT-3 (Fig. 3A), fibronectin (Fig. 3B), mp53 (Fig. 3C), and Cav1 (Fig. 3D), caspase-8 (Fig. 4A), caspase-3 (Fig. 4B), cyclin D1 (Fig. 4C) and NFκB (Fig. 4D) in the malignant and tumorigenic
cell line Tumor2 (P<0.01) in comparison with its counterpart.
However, there was no difference in Alpha5 cell line in comparison
with its counterpart. Since EMT is associated with cellular
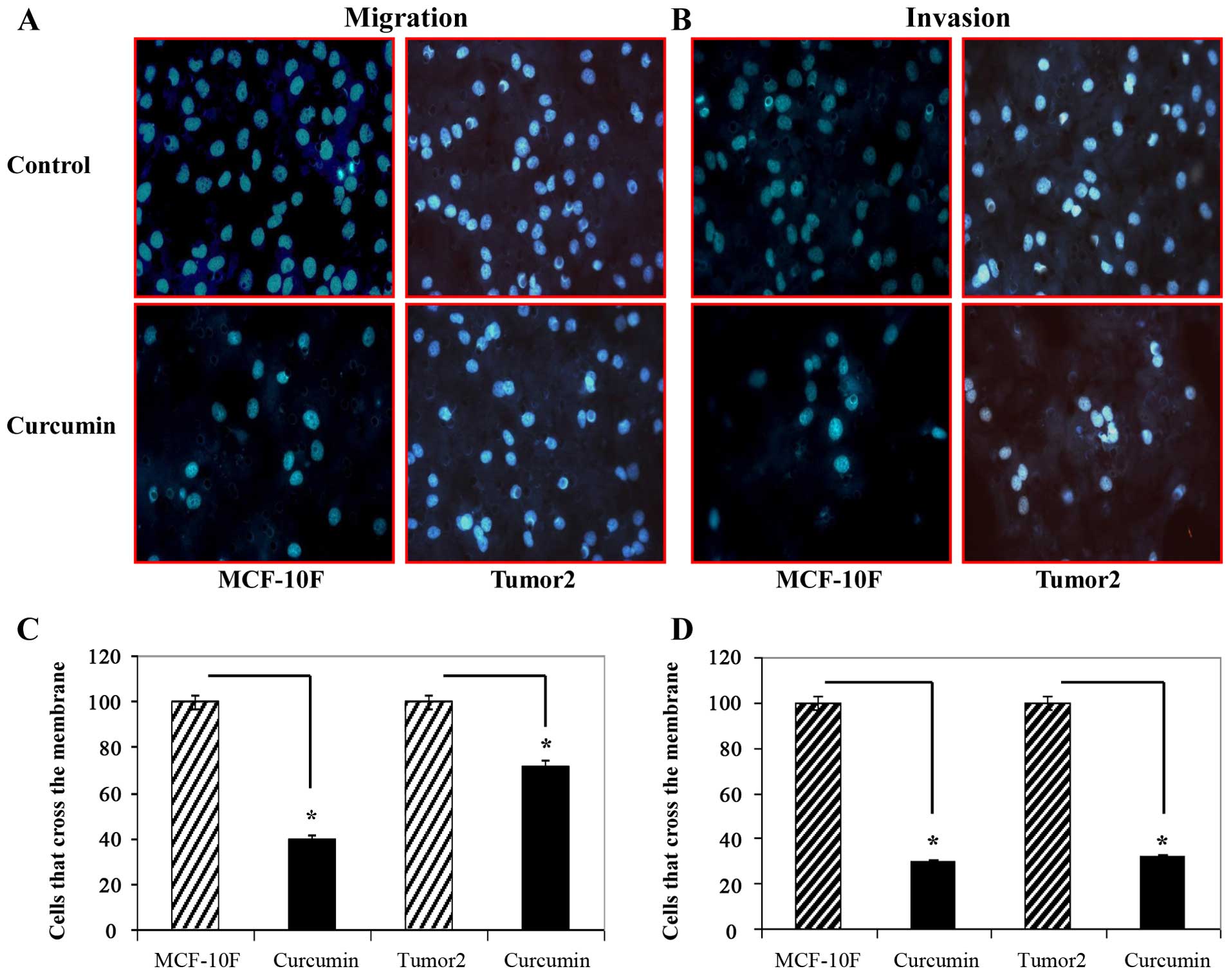
progression we studied the effect on migration and invasion of
breast cancer cells. Curcumin decreased the number of migratory and
invasive cells significantly (P<00.5) compared to the untreated
cells as can be observed in Fig.
5.
Discussion
Accumulating evidences suggest that curcumin has a
diverse range of molecular targets, supporting the concept that it
acts upon numerous biochemical and molecular cascades. Despite our
increasing knowledge on this interesting substance there still
remain many unknown effects that deserve intense investigation. The
multi-targeting of curcumin comes from its structure, chemistry and
influence on multiple signaling molecules as well as its ability to
bind directly to carrier proteins that improves its solubility and
bioavailability. It binds to DNA and RNA. Both in vitro and
in vivo studies have shown that curcumin and its analogs
target critical genes associated with angiogenesis, apoptosis, cell
cycle, and metastasis.
The MCF-10F is unique in the sense that it retains
all the characteristics of normal breast epithelium in vitro
including dome formation in confluent cultures, three-dimensional
growth in collagen gel (55),
dependence upon hormones and growth factors for growth in
vitro, lack of anchorage-independence or invasive capabilities
and non-tumorigenic in the nude or SCID mice (54). It was previously shown (59,60)
that among all the various transformed human breast cell lines only
Alpha5 cell line and Tumor2 increased cell proliferation, adhesion,
presented anchorage-independency, invasive capabilities and tumor
formation in nude mice. These cell lines were also positive for
estrogen receptor, progesterone receptor and HER, c-Ha-ras and
Rho-A gene and protein expression.
EMT is associated with enhanced cellular
progression. Curcumin inhibited EMT gene expression in breast
cancer cells as E-cadherin, N-cadherin, β-catenin and
ZEB2 in Tumor2 in comparison with its counterparts. Curcumin
also inhibited Twist1, Slug, Axl and vimentin gene
expression in the same cell line. It is known that Twist1 promotes
stationary epithelial cells to lose cell-cell junctions and gain
migratory and invasive capacities (61); Slug acts as an inhibitor of
apoptosis (24) and Axl is
overexpressed in a wide variety of human cancers with significant
correlation with tumor stage in breast cancer patients playing an
important role in cancer progression and metastases (62–64).
Curcumin decreased STAT-3 and fibronectin gene
expression in Tumor2 in comparison to its counterpart. It is known
that STAT-3 mediates the axial responses of cytokines
involved in normal cellular responses and oncogenesis (30).
The antioxidant inhibited Cav-1 gene
expression of malignant and tumorigenic cell line Tumor2. It has
been reported that inhibition of the tumor promoter Cav-1
expression in Hca-F cells prevents EMT formation by increasing
stabilization of Cav-1 with β-catenin (65,66).
Curcumin induced tumor cell apoptosis since it decreased
caspase-3, caspase-8 and cyclin D1 expression in
Tumor2 in comparison to its control. Abnormalities involving cyclin
D1 may alter G1-S transition and contribute to genomic instability
and tumor development (45,46).
The ability of curcumin to induce apoptosis in tumor cells and/or
potentiate apoptosis induction by classical chemotherapeutic drugs,
support its potential in anticancer therapies (67,68).
This substance also inhibited the mp53 gene expression of
malignant and tumorigenic cell line Tumor2 in comparison to its
control. Of note, curcumin has been found to inhibit proliferation
of normal, non-selectively, as well as malignant cells, although
its apoptogenic effect is more profound in malignant cells since it
selectively induced apoptosis in deregulated cyclin D1-expressed
cells at G2 phase of cell cycle in a p53-dependent manner (69,70).
The possibility that p53-mediated apoptosis may be associated with
the activation of caspase-3 and caspase-8 is
suggested by the ability of p53 to activate both the extrinsic and
intrinsic apoptotic pathways (71). It has been reported that p53
activates effector caspases by possibly inducing the release of
mitochondrial cytochrome-c, including caspase-3, and
caspase-8 and through the apoptotic effector machinery
engaged by p53 (72,73).
It has been reported that p53 enhances cancer cell apoptosis and
prevents cell replication by stopping the cell cycle at G1 or
interphase (74).
Curcumin induced a reduction of tumor cell invasion
and metastasis along with apoptosis. The motile phenotypes of cells
treated with curcumin were evaluated by migration and invasion
assay. After treatment with curcumin the number of migratory and
invasive cells decreased significantly compared to the untreated
cells. Curcumin has been reported to inhibit cell proliferation and
promote accumulation of cells in the G2/M phase of the cell cycle
(75).
Thus, the mechanism of apoptosis induced by curcumin
seems to be through reduction of tumor cell invasion and metastasis
by NFκB. The authors (76) showed
that NFκB, a transcription factor in the cell was altered by
curcumin. Curcumin plays an important role in the inhibition of EMT
in breast cancer cells through the downregulation of NFκB-Snail
activity (77). These data provide
a new perspective of the anti-invasive mechanism of curcumin,
indicating that the effect is partly due to its ability to
intervene in the EMT process (77). The inhibition of human breast
cancer cell growth by curcumin is mediated via certain signaling
cascades including the modulation of the NFκB signaling
pathway.
Several studies in vitro and first clinical
investigations confirm the antitumor effects of curcumin, either as
an isolated chemoprevention substance or in combination with
chemotherapeutic agents as supportive measure reducing
pharmaceutical resistance of tumor cells to certain
chemotherapeutics. The ability of curcumin to induce apoptosis in
tumor cells by a classical chemotherapeutic drug, or an antioxidant
such as curcumin supports its potential in anticancer therapies.
Despite our increasing knowledge on this interesting substance
there still remain many unknown effects that deserve intense
investigation. These studies reveal the inhibitory effect of
curcumin with emphasis on multi-targeted biological and molecular
effects in a breast cancer model. Thus, it seems that curcumin may
impinge upon several processes including apoptosis and metastatic
properties of the malignant cells exerting antitumor activity in
breast cancer cells transformed by low doses of α-particles and
estrogen in vitro.
Acknowledgements
The technical support of Guiliana Rojas, Georgina
Vargas Marchant and Leodán A. Crispin and helpful suggestions given
by Richard Ponce-Cusi are greatly appreciated. This study was
supported by Grant support FONDECYT #1120006 (G.M.C.) and
MINEDUC-UTA (G.M.C.).
References
|
1
|
Kelloff GJ, Crowell JA, Steele VE, Lubet
RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R,
Lawrence JA, et al: Progress in cancer chemoprevention: Development
of diet-derived chemopreventive agents. J Nutr. 130(Suppl):
467S–471S. 2000.PubMed/NCBI
|
|
2
|
Khar A, Ali AM, Pardhasaradhi BV,
Varalakshmi CH, Anjum R and Kumari AL: Induction of stress response
renders human tumor cell lines resistant to curcumin-mediated
apoptosis: Role of reactive oxygen intermediates. Cell Stress
Chaperones. 6:368–376. 2001. View Article : Google Scholar
|
|
3
|
Ramachandran C, Rodriguez S, Ramachandran
R, Raveendran Nair PK, Fonseca H, Khatib Z, Escalon E and Melnick
SJ: Expression profiles of apoptotic genes induced by curcumin in
human breast cancer and mammary epithelial cell lines. Anticancer
Res. 25:3293–3302. 2005.PubMed/NCBI
|
|
4
|
Cowin P and Welch DR: Breast cancer
progression: Controversies and consensus in the molecular
mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia.
12:99–102. 2007. View Article : Google Scholar
|
|
5
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yap AS, Brieher WM, Pruschy M and Gumbiner
BM: Lateral clustering of the adhesive ectodomain: A fundamental
determinant of cadherin function. Curr Biol. 7:308–315. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carneiro P, Figueiredo J, Bordeira-Carriço
R, Fernandes MS, Carvalho J, Oliveira C and Seruca R: Therapeutic
targets associated to E-cadherin dysfunction in gastric cancer.
Expert Opin Ther Targets. 17:1187–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pal S, Ganguly KK and Chatterjee A:
Extracellular matrix protein fibronectin induces matrix
metalloproteinases in human prostate adenocarcinoma cells PC-3.
Cell Commun Adhes. 20:105–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang
L, Zhang H, Chen X, Yang Y and Liu G: miRNA-29c suppresses lung
cancer cell adhesion to extracellular matrix and metastasis by
targeting integrin β1 and matrix metalloproteinase2 (MMP2). PLoS
One. 8:e701922013. View Article : Google Scholar
|
|
11
|
Hulit J, Suyama K, Chung S, Keren R,
Agiostratidou G, Shan W, Dong X, Williams TM, Lisanti MP, Knudsen
K, et al: N-cadherin signaling potentiates mammary tumor metastasis
via enhanced extracellular signal-regulated kinase activation.
Cancer Res. 67:3106–3116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandal A, Bhatia D and Bishayee A:
Simultaneous disruption of estrogen receptor and Wnt/β-catenin
signaling is involved in methyl amooranin-mediated chemoprevention
of mammary gland carcinogenesis in rats. Mol Cell Biochem.
384:239–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nichols SA, Roberts BW, Richter DJ,
Fairclough SR and King N: Origin of metazoan cadherin diversity and
the antiquity of the classical cadherin/β-catenin complex. Proc
Natl Acad Sci USA. 109:13046–13051. 2012. View Article : Google Scholar
|
|
14
|
Verschueren K, Remacle JE, Collart C,
Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT,
Bodmer R, et al: SIP1, a novel zinc finger/homeodomain repressor,
interacts with Smad proteins and binds to 5′-CACCT sequences in
candidate target genes. J Biol Chem. 274:20489–20498. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katoh M and Katoh M: Integrative genomic
analyses of ZEB2: Transcriptional regulation of ZEB2 based on
SMADs, ETS1, HIF1α, POU/OCT, and NF-κB. Int J Oncol. 34:1737–1742.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nam EH, Lee Y, Park YK, Lee JW and Kim S:
ZEB2 upregulates integrin α5 expression through cooperation with
Sp1 to induce invasion during epithelial-mesenchymal transition of
human cancer cells. Carcinogenesis. 33:563–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao C, Qiao Y, Jonsson P, Wang J, Xu L,
Rouhi P, Sinha I, Cao Y, Williams C and Dahlman-Wright K:
Genome-wide profiling of AP-1-regulated transcription provides
insights into the invasiveness of triple-negative breast cancer.
Cancer Res. 74:3983–3994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eckert MA, Lwin TM, Chang AT, Kim J, Danis
E, Ohno-Machado L and Yang J: Twist1-induced invadopodia formation
promotes tumor metastasis. Cancer Cell. 19:372–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hemavathy K, Guru SC, Harris J, Chen JD
and Ip YT: Human Slug is a repressor that localizes to sites of
active transcription. Mol Cell Biol. 20:5087–5095. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu WS, Heinrichs S, Xu D, Garrison SP,
Zambetti GP, Adams JM and Look AT: Slug antagonizes p53-mediated
apoptosis of hematopoietic progenitors by repressing puma. Cell.
123:641–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Bryan JP, Frye RA, Cogswell PC, Neubauer
A, Kitch B, Prokop C, Espinosa R III, Le Beau MM, Earp HS and Liu
ET: axl, a transforming gene isolated from primary human myeloid
leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell
Biol. 11:5016–5031. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bose R, Molina H, Patterson AS, Bitok JK,
Periaswamy B, Bader JS, Pandey A and Cole PA: Phosphoproteomic
analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci
USA. 103:9773–9778. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hafizi S and Dahlbäck B: Gas6 and protein
S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase
subfamily. FEBS J. 273:5231–5244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duprey P and Paulin D: What can be learned
from intermediate filament gene regulation in the mouse embryo. Int
J Dev Biol. 39:443–457. 1995.PubMed/NCBI
|
|
29
|
Stewart M: Intermediate filament structure
and assembly. Curr Opin Cell Biol. 5:3–11. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takeda K and Akira S: STAT family of
transcription factors in cytokine-mediated biological responses.
Cytokine Growth Factor Rev. 11:199–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manabe R, Oh-e N and Sekiguchi K:
Alternatively spliced EDA segment regulates fibronectin-dependent
cell cycle progression and mitogenic signal transduction. J Biol
Chem. 274:5919–5924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohnishi T, Hiraga S, Izumoto S, Matsumura
H, Kanemura Y, Arita N and Hayakawa T: Role of
fibronectin-stimulated tumor cell migration in glioma invasion in
vivo: Clinical significance of fibronectin and fibronectin receptor
expressed in human glioma tissues. Clin Exp Metastasis. 16:729–741.
1998. View Article : Google Scholar
|
|
33
|
Sakai T, Johnson KJ, Murozono M, Sakai K,
Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP and
Fässler R: Plasma fibronectin supports neuronal survival and
reduces brain injury following transient focal cerebral ischemia
but is not essential for skin-wound healing and hemostasis. Nat
Med. 7:324–330. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moursi AM, Damsky CH, Lull J, Zimmerman D,
Doty SB, Aota S and Globus RK: Fibronectin regulates calvarial
osteoblast differentiation. J Cell Sci. 109:1369–1380.
1996.PubMed/NCBI
|
|
35
|
Johansson S, Svineng G, Wennerberg K,
Armulik A and Lohikangas L: Fibronectin-integrin interactions.
Front Biosci. 2:d126–d146. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sottile J and Hocking DC: Fibronectin
polymerization regulates the composition and stability of
extracellular matrix fibrils and cell-matrix adhesions. Mol Biol
Cell. 13:3546–3559. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goerges AL and Nugent MA: pH regulates
vascular endothelial growth factor binding to fibronectin: A
mechanism for control of extracellular matrix storage and release.
J Biol Chem. 279:2307–2315. 2004. View Article : Google Scholar
|
|
38
|
Rao B, Lain S and Thompson AM: p53-Based
cyclotherapy: Exploiting the ‘guardian of the genome’ to protect
normal cells from cytotoxic therapy. Br J Cancer. 109:2954–2958.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma X, Liu L, Nie W, Li Y, Zhang B, Zhang J
and Zhou R: Prognostic role of caveolin in breast cancer: A
meta-analysis. Breast. 22:462–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reed JC: Apoptosis mechanisms:
Implications for cancer drug discovery. Oncology (Williston Park).
18(Suppl 10): 11–20. 2004.
|
|
41
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thornberry NA: Caspases: Key mediators of
apoptosis. Chem Biol. 5:R97–R103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng TS, Hunot S, Kuida K and Flavell RA:
Caspase knockouts: Matters of life and death. Cell Death Differ.
6:1043–1053. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou P, Jiang W, Weghorst CM and Weinstein
IB: Overexpression of cyclin D1 enhances gene amplification. Cancer
Res. 56:36–39. 1996.PubMed/NCBI
|
|
46
|
Arnold A and Papanikolaou A: Cyclin D1 in
breast cancer pathogenesis. J Clin Oncol. 23:4215–4224. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kato J, Matsushime H, Hiebert SW, Ewen ME
and Sherr CJ: Direct binding of cyclin D to the retinoblastoma gene
product (pRb) and pRb phosphorylation by the cyclin D-dependent
kinase CDK4. Genes Dev. 7:331–342. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lundberg AS and Weinberg RA: Functional
inactivation of the retinoblastoma protein requires sequential
modification by at least two distinct cyclin-cdk complexes. Mol
Cell Biol. 18:753–761. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Malumbres M and Barbacid M: To cycle or
not to cycle: A critical decision in cancer. Nat Rev Cancer.
1:222–231. 2001. View Article : Google Scholar
|
|
51
|
Malumbres M and Barbacid M: Is Cyclin
D1-CDK4 kinase a bona fide cancer target? Cancer Cell. 9:2–4. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Calaf GM and Hei TK: Establishment of a
radiation- and estrogen-induced breast cancer model.
Carcinogenesis. 21:769–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Soule HD, Maloney TM, Wolman SR, Peterson
WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF and Brooks
SC: Isolation and characterization of a spontaneously immortalized
human breast epithelial cell line, MCF-10. Cancer Res.
50:6075–6086. 1990.PubMed/NCBI
|
|
56
|
Calaf G, Russo J and Alvarado ME:
Morphological phenotypes in neoplastic progression of
benz(alpha)pyrene-treated breast epithelial cells. J Submicrosc
Cytol Pathol. 32:535–545. 2000.
|
|
57
|
Calaf G, Russo J, Tait L, Estrad S and
Alvarado ME: Morphological phenotypes in neoplastic progression of
human breast epithelial cells. J Submicrosc Cytol Pathol. 32:83–96.
2000.PubMed/NCBI
|
|
58
|
Calaf G and Hei TK: Oncoprotein expression
in human breast epithelial cells transformed by high-LET radiation.
Int J Radiat Biol. 77:31–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Calaf GM, Alvarado ME and Hei TK: Beta
catenin is associated with breast cancer progression in vitro. Int
J Oncol. 26:913–921. 2005.PubMed/NCBI
|
|
60
|
Calaf GM, Alvarado ME and Hei TK:
Oncoprotein expression and morphological phenotypes of human breast
epithelial cells transformed by the c-Ha-ras oncogene. Oncol Rep.
14:885–893. 2005.PubMed/NCBI
|
|
61
|
Rodrigues CO, Nerlick ST, White EL,
Cleveland JL and King ML: A Myc-Slug (Snail2)/Twist regulatory
circuit directs vascular development. Development. 135:1903–1911.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hutterer M, Knyazev P, Abate A, Reschke M,
Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, et
al: Axl and growth arrest-specific gene 6 are frequently
overexpressed in human gliomas and predict poor prognosis in
patients with glioblastoma multiforme. Clin Cancer Res. 14:130–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J,
Kallop D, Ludlam MJ and Pei L: Axl as a potential therapeutic
target in cancer: Role of Axl in tumor growth, metastasis and
angiogenesis. Oncogene. 28:3442–3455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang YX, Knyazev PG, Cheburkin YV, Sharma
K, Knyazev YP, Orfi L, Szabadkai I, Daub H, Kéri G and Ullrich A:
AXL is a potential target for therapeutic intervention in breast
cancer progression. Cancer Res. 68:1905–1915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang S, Yu S, Shi W, Ge L, Yu X, Fan J and
Zhang J: Curcumin inhibits the migration and invasion of mouse
hepatoma Hca-F cells through down-regulating caveolin-1 expression
and epidermal growth factor receptor signaling. IUBMB Life.
63:775–782. 2011. View Article : Google Scholar
|
|
66
|
Sun LN, Chen ZX, Liu XC, Liu HY, Guan GJ
and Liu G: Curcumin ameliorates epithelial-to-mesenchymal
transition of podocytes in vivo and in vitro via regulating
caveolin-1. Biomed Pharmacother. 68:1079–1088. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Limtrakul P: Curcumin as chemosensitizer.
Adv Exp Med Biol. 595:269–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Reuter S, Eifes S, Dicato M, Aggarwal BB
and Diederich M: Modulation of anti-apoptotic and survival pathways
by curcumin as a strategy to induce apoptosis in cancer cells.
Biochem Pharmacol. 76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ruby AJ, Kuttan G, Babu KD, Rajasekharan
KN and Kuttan R: Anti-tumour and antioxidant activity of natural
curcuminoids. Cancer Lett. 94:79–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Choudhuri T, Pal S, Das T and Sa G:
Curcumin selectively induces apoptosis in deregulated cyclin
D1-expressed cells at G2 phase of cell cycle in a p53-dependent
manner. J Biol Chem. 280:20059–20068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Salim LZ, Mohan S, Othman R, Abdelwahab
SI, Kamalidehghan B, Sheikh BY and Ibrahim MY: Thymoquinone induces
mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in
vitro. Molecules. 18:11219–11240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Takaoka A, Hayakawa S, Yanai H, Stoiber D,
Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, et al:
Integration of interferon-alpha/beta signalling to p53 responses in
tumour suppression and antiviral defence. Nature. 424:516–523.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pekar O, Molotski N, Savion S, Fein A,
Toder V and Torchinsky A: p53 regulates cyclophosphamide
teratogenesis by controlling caspases 3, 8, 9 activation and
NF-kappaB DNA binding. Reproduction. 134:379–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fridman JS and Lowe SW: Control of
apoptosis by p53. Oncogene. 22:9030–9040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Aggarwal BB, Shishodia S, Takada Y,
Banerjee S, Newman RA, Bueso-Ramos CE and Price JE: Curcumin
suppresses the paclitaxel-induced nuclear factor-kappaB pathway in
breast cancer cells and inhibits lung metastasis of human breast
cancer in nude mice. Clin Cancer Res. 11:7490–7498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Calaf GM, Echiburú-Chau C, Roy D, Chai Y,
Wen G and Balajee AS: Protective role of curcumin in oxidative
stress of breast cells. Oncol Rep. 26:1029–1035. 2011.PubMed/NCBI
|
|
77
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-κB-Snail
signaling in breast cancer cells. Oncol Rep. 29:117–124. 2013.
|