Introduction
Berberine, an alkaloid found in many plant species
[e.g., Berberis aquifolium (Oregon grape) and Hydrastis
canadensis (goldenseal)], exhibits antiproliferative or
cytotoxic effects against cancerous cells of different origins
(1–3). Berberine inhibits the proliferation
of MCF-7 breast cancer cells through upregulation of p53 and a
mitochondria-dependent apoptotic pathway (4). Berberine also strongly induces
cytotoxicity and apoptosis in ALL cells that express p53 and MDM2
oncoprotein (5). However, previous
studies did not clearly identify the target of activated p53
protein involved in inducing or controlling the apoptosis pathway
after treatment with berberine.
It has long been recognized that p53 is the most
commonly mutated tumor suppressor gene and that loss of p53
expression or function is associated with an increased risk of
tumor development (6). XAF1, a
novel target gene of p53, is downregulated by wild-type p53, but
not by mutant p53, at both mRNA and protein levels (7). XAF1 was previously identified as a
binding partner of XIAP that directly interacts with endogenous
XIAP (8). XAF1 was reported to
antagonize the anticaspase activity of recombinant XIAP and reverse
the protective effect of XIAP overexpression in various cancer cell
lines (9). Epigenetic silencing of
XAF1 by aberrant promoter methylation is associated with cancer
development and progression (10).
Restoration of XAF1 expression induces cancer cell apoptosis in
vitro and in vivo (11,12).
p53 binds to the core promoter of the XAF1 gene with
high affinity. p53 knockdown or abrogation of this element by
site-specific mutation inhibits this binding and abolishes the
inhibitory effect of p53 on XAF1 transcription (12). Overexpression of XAF1 leads to
activation of wild-type p53 via phosphorylation of gastric or colon
cancer cells, resulting in nuclear accumulation of p53 and enhanced
p53-dependent apoptosis (7).
However, the exact role of activated p53 in XAF1 expression and
function remains unclear, especially in hematologic cancers.
GADD45α gene is one of the numerous downstream
targets of p53 (13). The role of
GADD45α in the regulation of apoptosis is controversial and may
depend on cell type and the nature of the environmental stimulus
that triggers apoptosis. GADD45 family members are able to activate
the p38/JNK MAPK pathway (14,15),
and p38/JNK can contribute to the activation of p53 (16,17).
Moreover, the p53-responsive genes GADD45 and p21WAF1
are significantly induced in adult T-cell leukemia/lymphoma cells
after treatment with ionizing radiation (18). Based on these reports, we predicted
that GADD45α might play some role in the regulation of XAF1 by p53
in berberine-induced apoptosis of EBV-transformed B cells.
In this study we examined whether the functional p53
status of EBV-transformed B cells or cancerous B cells affects
their response to berberine. We also explored the interactions
between MAPK-activated p53 and XAF1 in the induction of
mitochondrial apoptosis signaling after treatment with
berberine.
Materials and methods
Preparation of EBV-infectious supernatant
and generation of EBV-transformed B cells
Preparation of cell-free EBV virions and generation
of EBV-transformed B cells were carried out as described previously
(19). Human Burkitt's lymphoma
Raji and Daudi cells and multiple myeloma IM-9 cells were purchased
from the American Type Culture Collection (Manassas, VA, USA).
These cells were maintained in RPMI-1640 medium (Hyclone, Logan,
UT, USA) supplemented with 10% FBS (Hyclone) and antibiotics under
a humidified atmosphere with 5% CO2. This study was
approved by the Institutional Bioethics Review Board at the Medical
College of Inje University, and all donors gave informed consent
for the study.
Analysis of apoptotic cells via flow
cytometry
The percentage of cells undergoing apoptosis in
human EBV-transformed B cells (4 weeks, 2.5×105
cells/ml) and normal PBMCs was determined via flow cytometry using
FITC-conjugated Annexin V (BD Biosciences, San Diego, CA, USA) and
7-AAD (BD Biosciences). To determine optimal conditions,
experiments were performed using different concentrations (0, 10,
25, 50, 75 and 100 μM) of berberine (Sigma-Aldrich, St. Louis, MO,
USA) and different periods of incubation (2, 4, 8, 16 and 24 h).
DMSO was used as a vehicle control. To examine the role of
caspases, cells were pretreated with the caspase-3 inhibitor
z-DEVD-fmk (N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone,
20 μM in DMSO; Calbiochem, San Diego, CA, USA), the caspase-9
inhibitor z-LEHD-fmk
(z-Leu-Glu(OMe)-His-Asp-(OMe)-fluoremethylketone, 20 μM;
Calbiochem), or the broad spectrum caspase inhibitor z-VAD
(N-benzyloxycarbonyl-Val-Ala-Aspfluoromethylketone, 20 μM in DMSO;
Calbiochem) for 2 h before treatment with berberine. To inhibit the
production of ROS, cells were pretreated with the antioxidant NAC
(10 mM; Sigma-Aldrich) for 1 h. To block activation of p38-MAPK or
JNK, cells were pretreated with SB203580 (10 μM, Calbiochem) or
SP600125 (25 μM, Calbiochem) respectively, for 2 h. To block
activation of p53, cells were pretreated with PFTα (50 μM, Santa
Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h. Cells were
harvested, washed in PBS, and incubated with Annexin V and 7-AAD in
Annexin V binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl,
2.5 mM CaCl2) at room temperature for 15 min in the
dark. The stained cells were analyzed using a FACSCalibur flow
cytometer (BD Biosciences) equipped with CellQuest Pro software (BD
Biosciences).
Measurement of mitochondria membrane
potential (ΔΨm) and intracellular reactive oxygen
species production
Changes in mitochondrial membrane potential were
measured using DiOC6 (3, 3′-dihexyloxacarboxyanine
iodide; Molecular Probes, Eugene, OR, USA). Cells were treated with
berberine or DMSO for 24 h, harvested, washed twice in PBS,
resuspended in PBS supplemented with DiOC6 (20 nM),
incubated at 37°C for 15 min in the dark, and immediately analyzed
with flow cytometry. Intracellular accumulation of ROS was
monitored via flow cytometry after staining with the fluorescent
probe, DCFH-DA (10 μM, 2′, 7′-dichlorodihydro-fluorescein
diacetate; Molecular Probes). DCFH-DA is deacetylated in cells by
esterase to a non-fluorescent compound, DCFH, which remains trapped
within the cell and is cleaved and oxidized by ROS in the presence
of endogenous peroxidase to a highly fluorescent compound, DCF (2′,
7′-dichlorofluorescein). Briefly, cells were preincubated with 10
μM DCFH-DA for 30 min at 37°C. The cells were seeded in 6-well
plates (5×105 cells/ml) and treated with or without
berberine. Cells were washed, resuspended in PBS, and ROS levels
were determined using a FACSCalibur flow cytometer.
Western blot analysis
After drug treatment, cells were harvested and lysed
in NP-40 buffer (Elpis Biotech, Daejeon, Korea) supplemented with a
protease inhibitor cocktail (Sigma-Aldrich). To address
phosphorylation events, a set of phosphatase inhibitors (Cocktail
II, Sigma-Aldrich) was added to the NP-40 buffer. Protein
concentration was determined using a Pierce BCA assay kit (Thermo
Scientific, Rockford, IL, USA). An equal volume of 2× Laemmli
sample buffer (Elpis Biotech) was added to each sample of lysate
containing 10 μg protein and immediately boiled for 5 min at 100°C.
The insoluble material was spun down at 13,000 rpm. Total cell
lysates (~5×106 cells/sample) were subjected to SDS-PAGE
on 10–15% (w/v) acrylamide gels under reducing conditions.
Separated proteins were transferred to nitrocellulose membranes
(Millipore Corp., Billerica, MA, USA), the membranes were blocked
with 5% skim milk and western blot analysis was performed using a
commercial kit. Chemiluminescence was detected using an ECL kit
(Advansta Corp., Menlo Park, CA, USA) and the multiple Gel DOC
system (Fujifilm, Tokyo, Japan). Primary antibodies against the
following proteins were used: caspase-3, caspase-9, PARP, β-actin,
Bcl-2, Bax, phospho-Bim (Ser69), PUMA, XIAP, phospho-p53
(Ser15), p53, phospho-JNK
(Thr183/Tyr185), JNK, phospho-p38-MAPK
(Thr180/Tyr182), p38-MAPK, phospho-ERK1/2
(Thr202/Tyr204), ERK1/2, phospho-Akt
(Ser473), and Akt (Cell Signaling Technology, Beverly,
MA, USA); XAF1 (Abcam, Cambridge, UK); GADD45α, Ref-1, and COX-IV
(Santa Cruz Biotechnology); phospho-Bax (Ser184) (Bioss
Inc., Woburn, MA, USA); Bim and β-tubulin (BD Biosciences).
Detection of translocated GADD45α, XAF1,
Bax, Bim, and Puma
Mitochondrial and cytosol cellular fractions were
prepared using a Cytosol/Mitochondria Fractionation kit
(Calbiochem). Untreated or berberine-treated cells
(1×107 cells) were harvested by centrifugation at 600 ×
g for 5 min at 4°C, washed twice with cold PBS, and resuspended in
250 μl Cytosol Extraction buffer containing a protease inhibitor
cocktail and 1 mM dithiothreitol (DTT). After incubation on ice for
10 min, the cells were homogenized on ice using a Dounce tissue
homogenizer and centrifuged at 700 × g for 10 min at 4°C. The
supernatants were collected and centrifuged again at 10,000 × g for
30 min at 4°C. The resulting supernatants were harvested as
cytosolic fractions and the pellets were resuspended in 50 μl
Mitochondria Extraction buffer containing a protease inhibitor
cocktail and 1 mM DTT and designated the mitochondrial fractions.
The nuclear fraction was prepared using a Nuclear/Cytosol
Fractionation kit (Biovision, Mountain View, CA, USA). Briefly,
2×106 cells were harvested and resuspended in 200 μl
cytosol extraction buffer A. After incubation on ice for 10 min,
the cell suspension was added to cytosol extraction buffer B and
incubated on ice for 1 min. After centrifugation the pellets were
resuspended in 100 μl nuclear extraction buffer mix and designated
the nuclear fraction.
Co-immunoprecipitation (co-IP) assay
For the protein binding assay, cells were treated
with berberine for 24 h. Cells were harvested (5×106
cells/sample) and lysed in NP-40 buffer containing a protease
inhibitor cocktail. To reduce non-specific binding of protein, we
performed pre-clearing on equal amounts of cell lysates by
incubating the samples with washed protein G PLUS-agarose beads
(Santa Cruz Biotechnology). For IP, pre-cleared lysate was
incubated with the optimal amount of antibody against XAF1, Puma,
GADD45α, or p53 antibody at 4°C for 2 h on a rotator. The
immunoprecipitates were harvested using protein G PLUS-agarose
beads and incubated at 4°C for 2 h under rotary agitation. The
supernatant was removed and the beads were washed four times in
lysis buffer. Finally, the immunoprecipitates were eluted by
boiling the beads in Laemmli sample buffer for 10 min and
characterized by western blotting with the appropriate
antibodies.
Results
Berberine induces apoptosis in
EBV-transformed B cells through the disruption of mitochondria
The viability of EBV-transformed B cells was
significantly decreased in a dose-dependent manner after treatment
with different concentrations of berberine. However, inhibition of
proliferation and induction of apoptosis was not observed at the
same doses in normal PBMCs (data not shown). To confirm that
berberine induced apoptosis in EBV-transformed B cells we used a
7-AAD and Annexin V staining method. Treatment with different
concentrations of berberine resulted in time- and dose-dependent
induction of apoptosis and altered mitochondria membrane potential
in EBV-transformed B cells (Fig. 1A
and B). Treatment with berberine induced activation of capase-3
and -9, which was detected by generation of the cleaved products.
Appearance of cleaved PARP, a target of caspase-3, was also
observed 12 h after treatment of EBV-transformed B cells or
cancerous B cells with berberine (Fig.
1C and E). EBV-transformed B cells were pretreated with several
caspase inhibitors including z-VAD (pan-caspase inhibitor), z-DEVD
(caspase-3 inhibitor), and z-LEHD (caspase-9 inhibitor) 2 h before
treatment with berberine. These inhibitors reduced caspase activity
and effectively blocked berberine-induced apoptosis in
EBV-transformed B cells (Fig. 1D).
These data suggest that berberine induces apoptosis in
EBV-transformed B cells or cancerous B cells, but in not normal
PBMCs, through a mitochondria-dependent pathway.
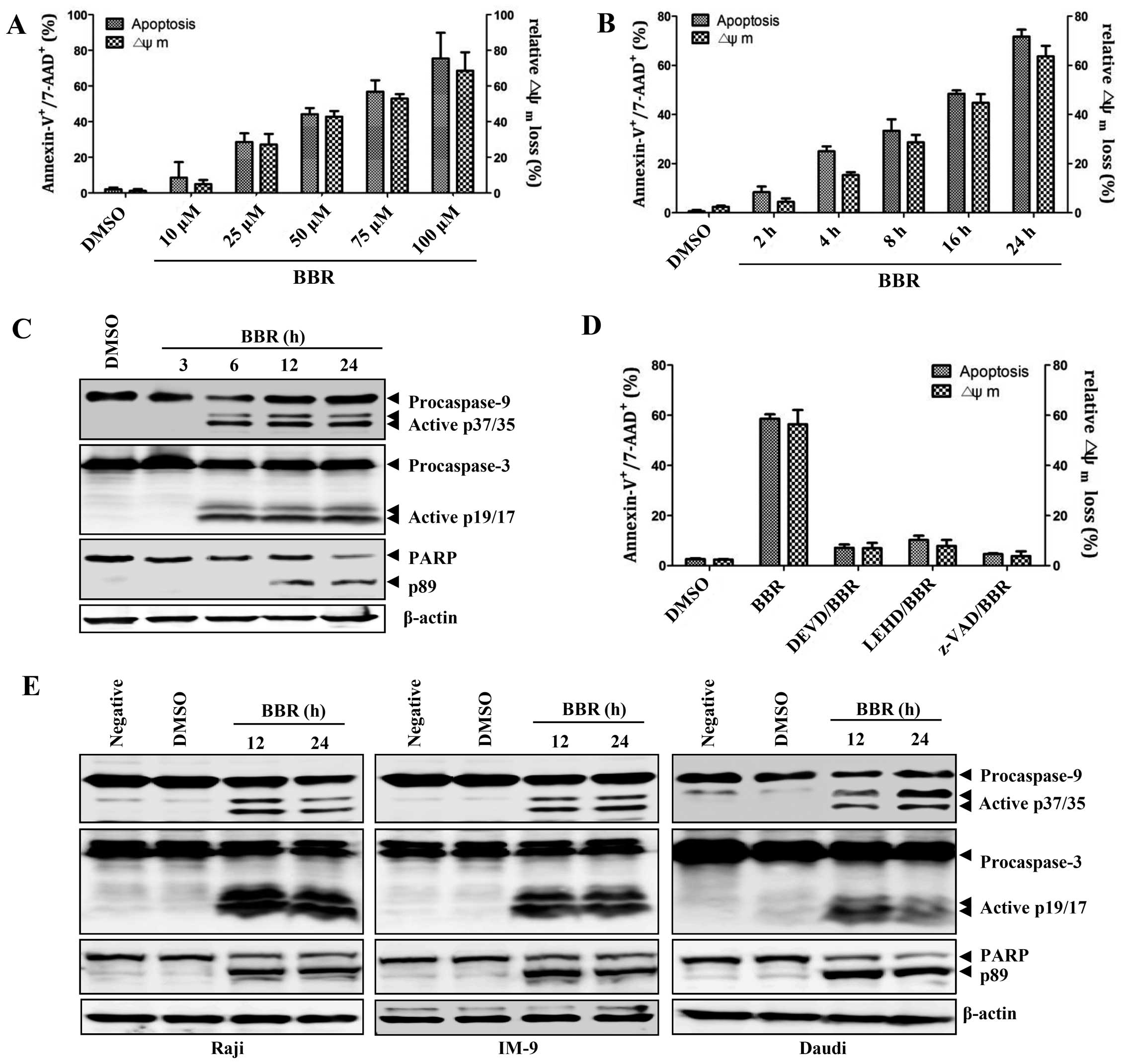 | Figure 1Berberine induces caspase-dependent
apoptosis in EBV+ B lymphoma cells. (A) EBV-transformed
B cells (2.5×105 cells/ml) were cultured in 6-well
plates and treated with 10, 25, 50, 75 and 100 μM berberine
overnight. (B) EBV-transformed B cells (2.5×105
cells/ml) were treated with 50 μM berberine for 2, 4, 8, 16 and 24
h. To detect the degree of apoptosis, cells were stained with
Annexin V-FITC and 7-AAD and analyzed with flow cytometry. The
percentage of apoptotic cells is determined by Annexin
V+/7-AAD+ staining. To measure disruption of
ΔΨm, the cells were stained with
DiOC6; diminished DiOC6 fluorescence (%)
indicates ΔΨm disruption. Each value was
expressed as the mean ± SD of three determinations. (C)
EBV-transformed B cells were treated with 50 μM berberine for the
indicated times. Western blot analysis of active caspase-9, -3 or
PARP cleavage was performed to characterize the apoptotic response.
β-actin was used to normalize protein content. (D) EBV-transformed
B cells were pre-incubated with z-LEHD-fmk (20 μM), z-DEVD-fmk (20
μM) or z-VAD-fmk (20 μM) for 2 h and then treated with 50 μM
berberine for 24 h. The number of apoptotic cells (Annexin
V+/7-AAD+) and ΔΨm
(DiOC6) were determined as described in Materials and
methods. Each value was expressed as the mean ± SD of three
determinations. (E) Raji, IM-9 or Daudi cells were exposed to 50 μM
berberine for the indicated times. Western blot analysis of active
caspase-9, -3 or PARP cleavage was performed to characterize the
apoptotic response. Results are representative of three independent
experiments. |
Berberine-induced apoptosis mainly
targets the p53-related signaling pathway in EBV-transformed B
cells
As berberine-induced apoptosis is associated with
activation of p53 in breast cancer (MCF-7) cells (4,20),
we next examined whether XAF1 (an antagonist of XIAP) and GADD45α,
both downstream targets of p53, and the Bcl-2 protein family are
associated with mitochondrial apoptosis signaling after treatment
with berberine in EBV-transformed B cells or cancerous B cells.
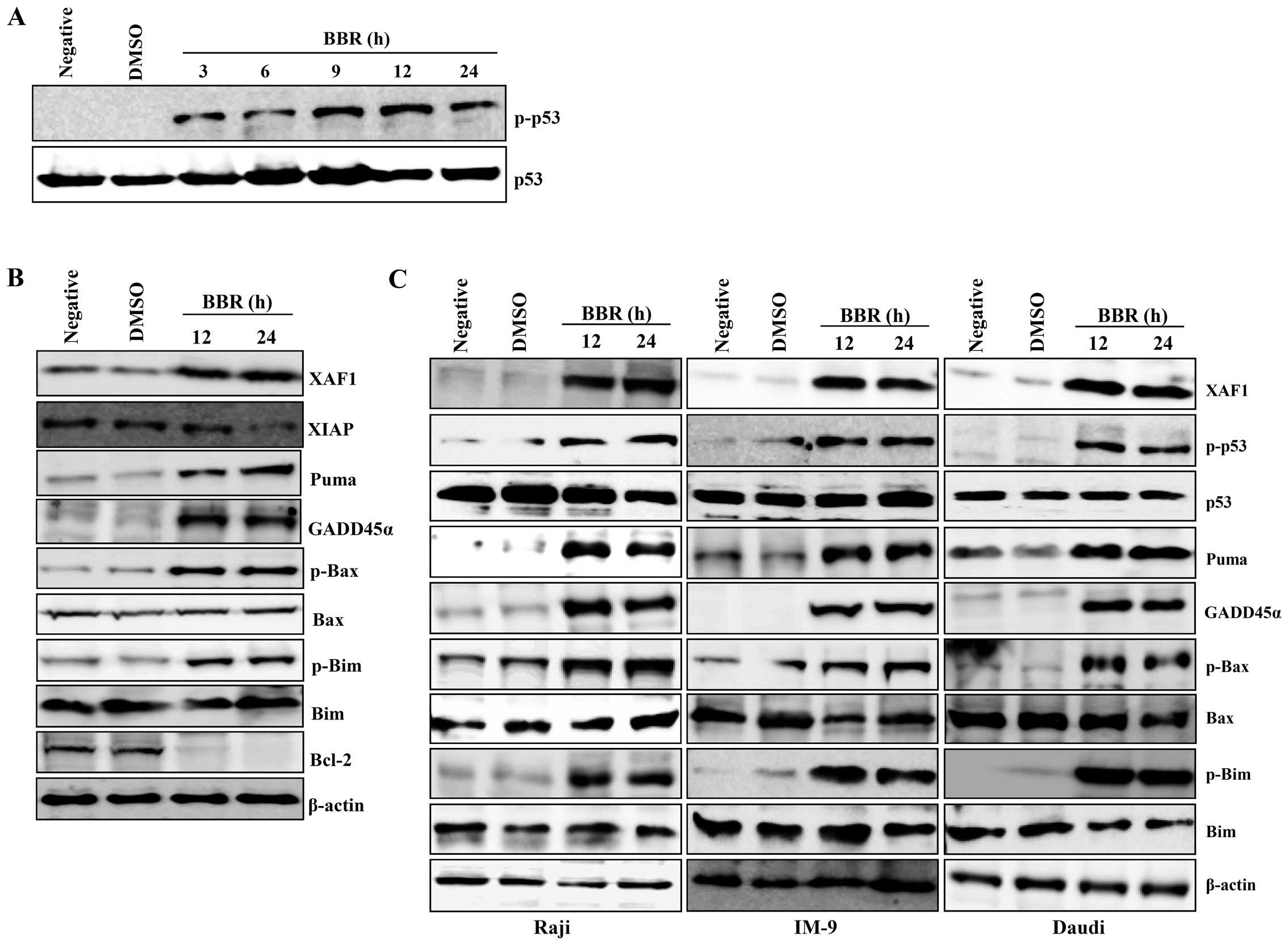
Berberine increased the level of phosphorylated p53 in a
time-dependent manner (Fig. 2A).
Expression of XAF1 and GADD45α were induced in EBV-transformed B
cells or cancerous B cells. However, expression of XIAP and the
antiapoptotic protein Bcl-2 significantly decreased to almost
undetectable levels 24 h post-treatment in EBV-transformed B cells.
Similar to GADD45α and XAF1, we observed induction of PUMA,
phosphorylated Bax, and phosphorylated Bim after berberine
treatment in EBV-transformed B cells and cancerous B cells
(Fig. 2B and C). These data
suggest that berberine-induced apoptosis in EBV-transformed B cells
or cancerous B cells is not only related to phosphorylated p53 and
its downstream targets, XAF1 and GADD45, but also involves
apoptotic members of the Bcl-2 protein family disrupting the
mitochondria membrane.
Re-located XAF1 in the cytosol binds to
p53 and GADD45α to regulate berberine-induced mitochondrial
apoptosis signaling
Next, we further examined the interaction between
XAF1 and apoptosis-related proteins to elucidate the role of XAF1
in regulating the apoptotic signaling induced by berberine. XAF1
was not detected in cytosol before treatment with berberine,
whereas, XAF1 in the nuclear fraction disappeared after treatment
with berberine. However, GADD45α was observed in both nuclear and
cytosolic fractions of EBV-transformed B cells treated with
berberine (Fig. 3A).
Interestingly, XAF1 in the mitochondrial fraction were increased in
berberine-treated EBV-transformed B cells. Bax and Bim disappeared
from the cytosol and were accumulated in mitochondria after
treatment with berberine; furthermore pro-apoptotic protein, PUMA
was detected in the mitochondria (Fig.
3B). To further characterize the relationship between p53 and
XAF1 and/or GADD45α, we investigated the potential interactions of
these proteins using immunoprecipitation and immunoblotting. After
treatment of EBV-transformed B cells with berberine, XAF1
translocated from the nucleus and interacted with p53 and GADD45α
in the cytosol in addition relocated XAF bound to PUMA, Bax, and
Bim in mitochondria (Fig. 3C–F).
These data suggest that re-localization of XAF1 from the nucleus to
the cytoplasm might be one of the key processes in the induction of
apoptosis through mitochondrial signaling in cancerous B cells
after treatment with berberine.
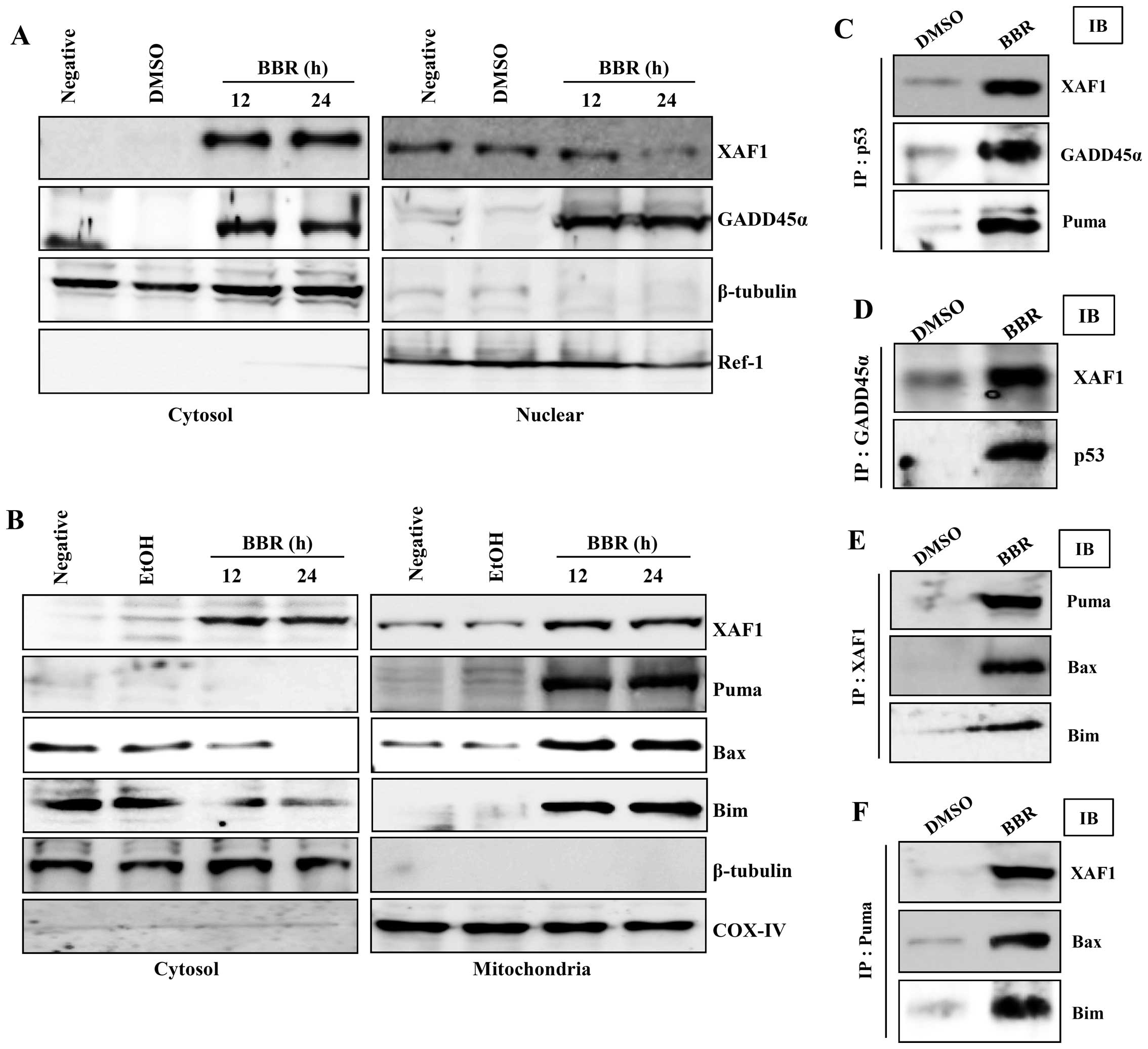 | Figure 3Berberine-induced p53 is involved in
GADD45α translocation to the nucleus and XAF1 and Puma
translocation to mitochondria. (A and B) EBV-transformed B cells
were treated with 50 μM berberine for the indicated times. Cells
were harvested and the amount of XAF1 and GADD45α in cytosolic and
nuclear fractions (A), and the amount of XAF1, Puma, Bax and Bim in
cytosolic and mitochondrial fractions (B) was determined.
Fractionation was performed as described in Materials and methods
and the mitochondria marker, COX-IV, the cytosol marker, β-tubulin,
and the nuclear marker, Ref-1, were used to verify the purity of
each fraction. (C-F) For the binding assay, p53 (C), GADD45α (D),
Puma (E) or XAF1 (F) was immunoprecipitated using specific antibody
followed by immunodetection of XAF1, GADD45α, Puma, p53, Bax and
Bim in the immunoprecipitate as detailed in Materials and methods.
Results are representative of three independent experiments. IP,
immunoprecipitation; IB, immunoblotting. |
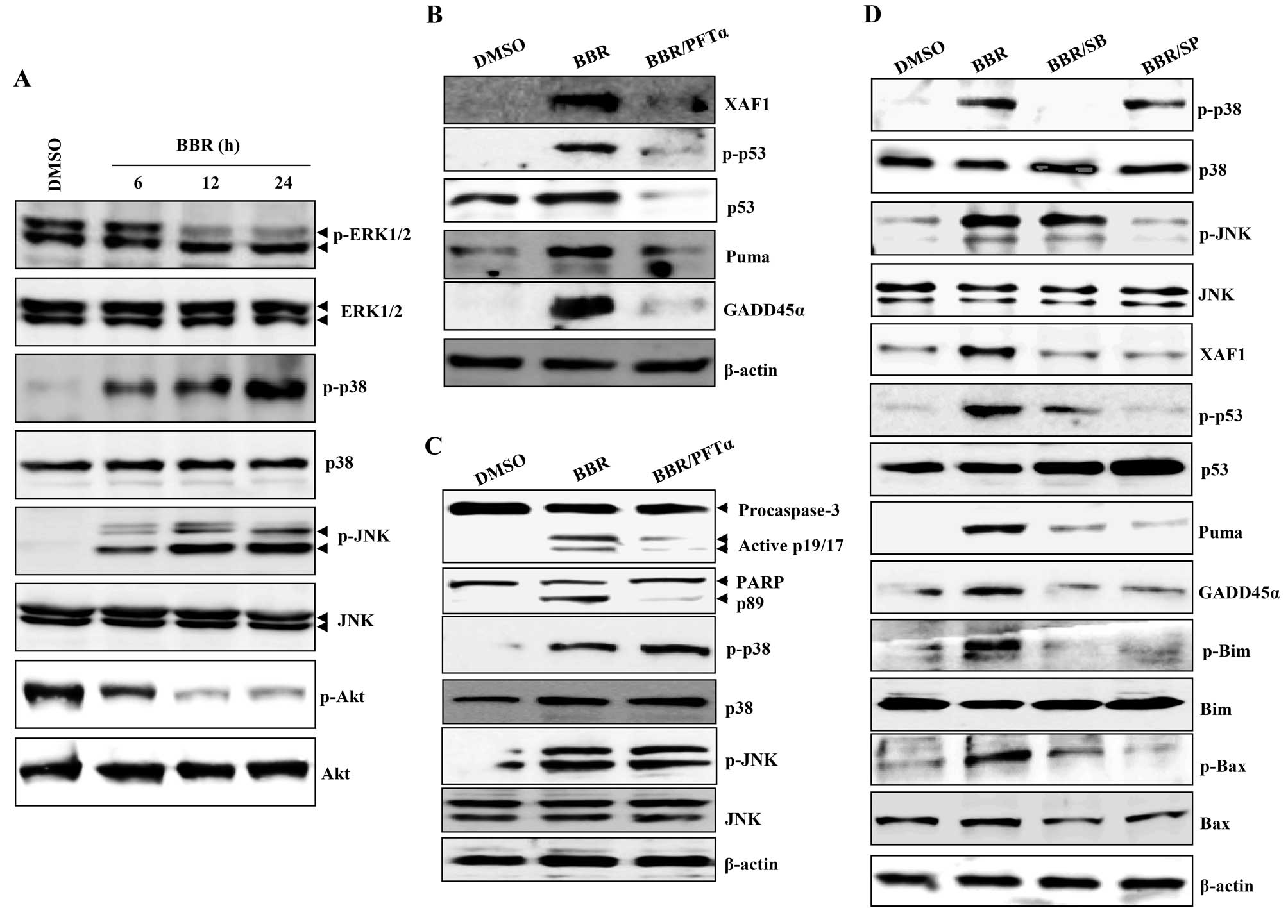
p38/JNK-mediated p53 activation regulates
XAF1-mediated mitochondrial apoptosis signaling
Death receptors and the mitogen-activated protein
kinase (MAPK) pathway promote apoptosis in HeLa cells after
treatment with 300 μM berberine (20,21).
GADD family proteins are known to be upstream activators of p38/JNK
MAPK (15,22). Next, we examined whether
berberine-induced apoptosis involves activation of these signaling
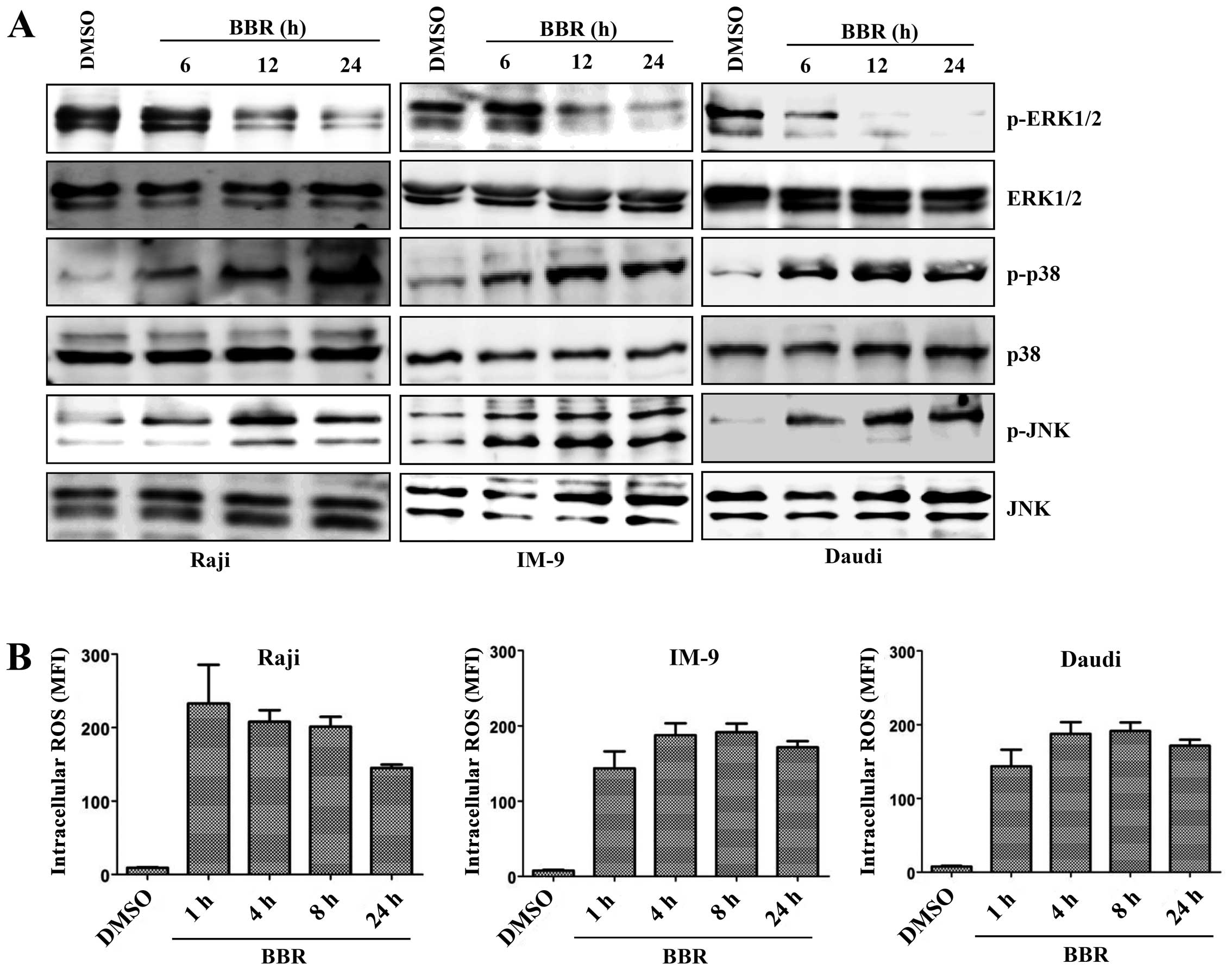
pathways. Levels of the phosphorylated forms of JNK and p38
increased in a time-dependent manner after treatment of
EBV-transformed B cells and cancerous B cells with berberine,
whereas expression of the phosphorylated forms of ERK1/2 and Akt
decreased (Figs. 4A and 6A). PFT-α inhibits p53 apoptotic
signaling by interfering with its nuclear translocation and the
transcriptional activation of p53 inducible genes (23,24).
PFT-α blocked the berberine-induced upregulation of phosphorylated
p53, PUMA, GADD45α, and XAF1 in EBV-transformed B cells (Fig. 4B). PFT-α also abrogated the
cleavage and activation of caspase-3 and -9, but did not prevent
the phosphorylation of JNK and p38-MAPK (Fig. 4C). To investigate the role of
p38/JNK MAPK in p53-mediated apoptosis after treatment with
berberine, EBV-transformed B cells were pretreated with SB203580
(p38-MAPK inhibitor) or SP600125 (JNK MAPK inhibitor) 2 h before
treatment with berberine. These inhibitors effectively prevented
the upregulation of phosphorylated p53, XAF1, GADD45α, and
proapoptotic Bcl-1 family proteins including PUMA, phosphorylated
Bim, and phosphorylated Bax (Fig.
4D). These data suggest that functional p53 is not only
required for berberine-induced apoptosis through regulation of
XAF1-GADD45α, but is also regulated by the p38/JNK MAPK signaling
pathway.
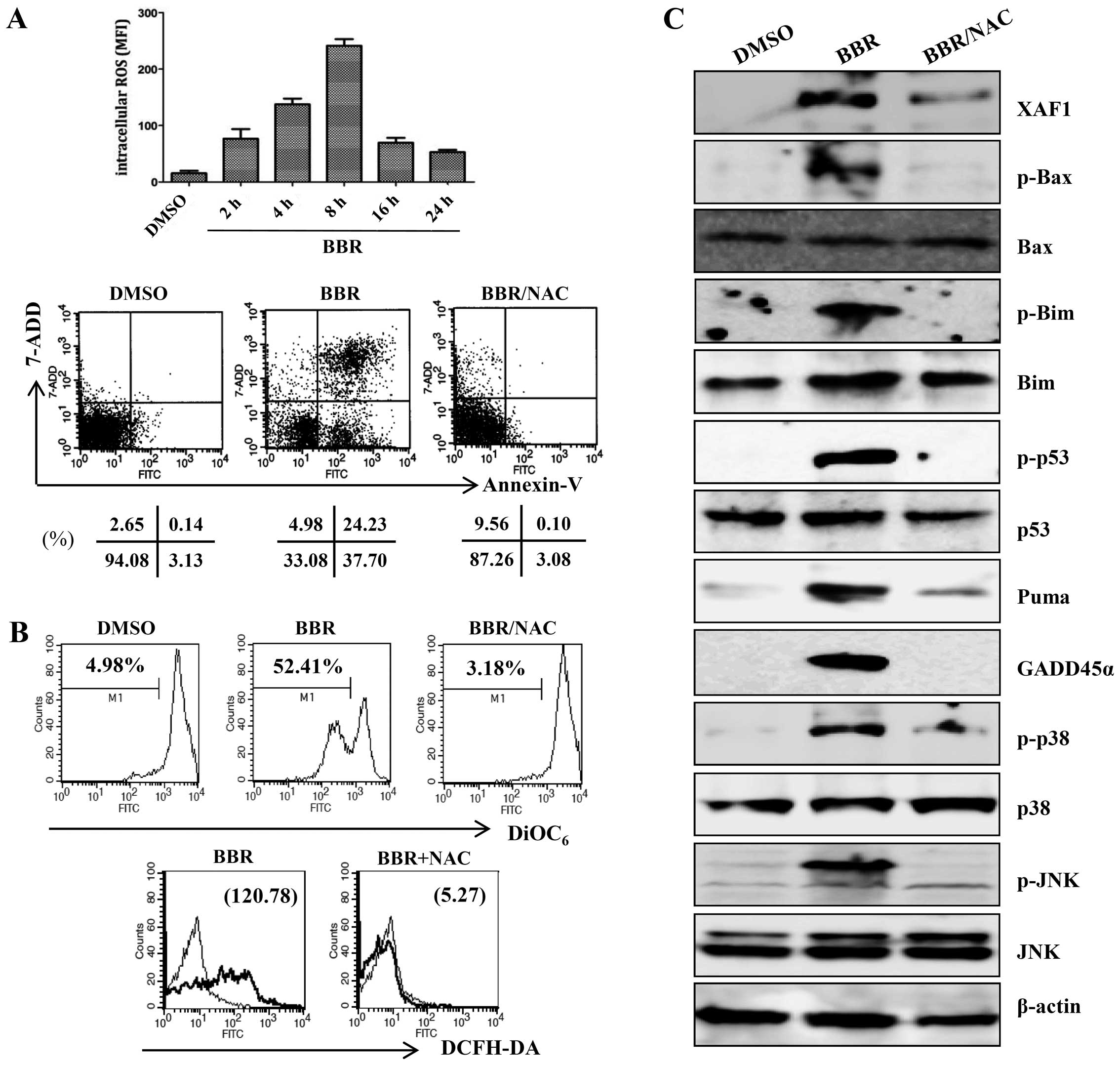
Generation of ROS is an initial step in
berberine-induced mitochondrial apoptosis signaling via the
MAPK/p53/XAF1 pathway
ROS are associated with the activation of MAPKs and
caspases in apoptosis (25). To
investigate whether ROS were involved in the MAPK/p53/XAF1-mediated
apoptosis induced by treatment with berberine, EBV-transformed B
cells were treated with berberine for the indicated times and the
intracellular ROS level was measured by addition of DCFH-DA. The
ROS level was significantly higher in the berberine-treated
EBV-transformed B cells than in the control group at 2 h after
treatment and peaked at 8 h after treatment, after which ROS levels
were downregulated. N-acetylcysteine (NAC), a ROS quencher,
effectively blocked berberine-induced apoptosis (Fig. 5A). NAC also suppressed ROS
production by berberine and the berberine-induced mitochondria
disruption (Fig. 5B).
Pre-incubation with NAC almost completely blocked the activation of
p38/JNK MAPK and the change in mitochondria-related apoptotic
proteins (Bax, Bim, and PUMA). The phosphorylation of p53 and
expression levels of its downstream targets (XAF1, GADD45α) were
restored to control levels after preincubation of EBV-transformed B
cells with NAC (Fig. 5C). ROS
level was also significantly higher in the berberine-treated
EBV-positive cancerous B cells (Raji, IM-9, Daudi cells) than in
the DMSO-treated control cells at even 1 h after treatment and was
maintained up at 24 h after treatment (Fig. 6B). These data suggest that ROS
generation might be one of the predisposing events promoting
mitochondria-mediated apoptosis through MAPK/p53 signaling after
treatment of EBV-transformed B cells with berberine.
Discussion
Berberine has various pharmacological activities
against infection and inflammation, and even antiarrhythmic
activities (26). Although
berberine has been reported to inhibit the cell growth and induce
apoptosis of various cancer cells (3,27,28),
there is insufficient evidence for its anticancer effects,
especially in lymphoma. Berberine induces apoptosis in human
promonocytic U937 cells through a mitochondrial/caspase pathway
(27). Our results suggest that
activation of the MAPK-p53 signaling pathway by intracellular ROS
might be the key event inducing berberine-mediated apoptosis in
EBV-transformed B cells through disruption of the mitochondrial
potential. Moreover, berberine-induced apoptosis involves
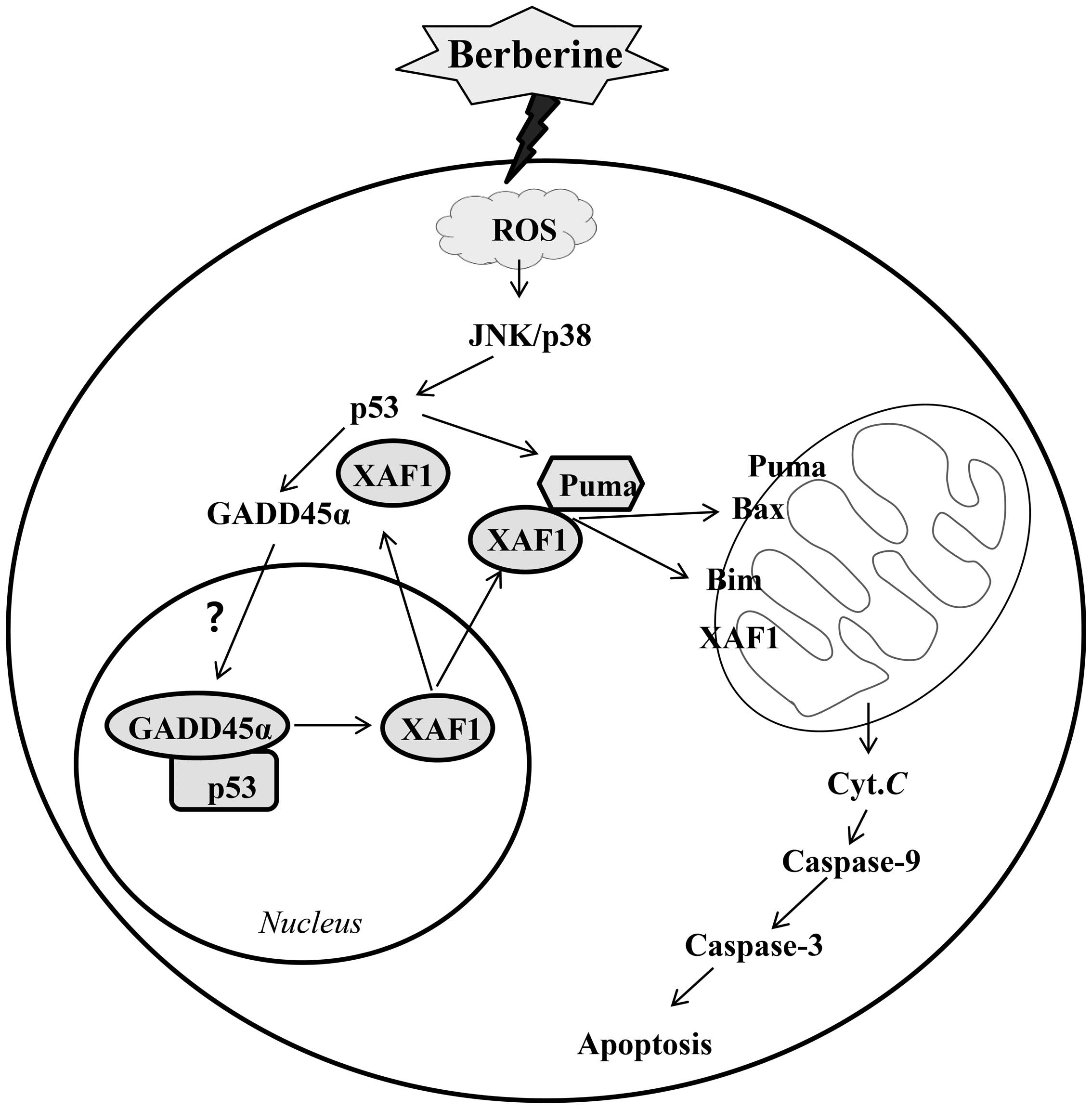
activation of the p53 target proteins XAF1 and GADD45α (Fig. 7).
p53 mutations are the most common genetic changes
found in human cancers (29).
Mutations of p53 often contribute to aggressive tumor behavior and
therapeutic resistance (30,31).
Some studies have shown that the expression of wild-type p53 is
necessary for the cytotoxic response to chemotherapeutic agents
(31,32); however, others have reported
opposite results (33,34). Berberine induces apoptosis in human
epidermoid carcinoma cells associated with increased expression of
Bax, reduced expression of Bcl-2 and Bcl-xL, and disruption of the
mitochondrial membrane potential (3). Transcriptional activation of
proapoptotic Bax and Bak by functional p53 enhances the
permeability of the mitochondrial membrane, which in turn results
in repression of antiapoptotic members of this family including
Bcl-2 and Bcl-x (35,36). These findings are consistent with
the results of our study. We first showed that the activity of
proapoptotic Bcl-2 family members, including Bax, Bim, and PUMA,
was regulated by interaction with the XAF1-GADD45α complex during
berberine-induced apoptosis of EBV-transformed B cells. We also
observed that XAF1 co-existed with Bax, Bim, and PUMA proteins in
the mitochondrial membrane fraction by co-immunoprecipitation
analyses.
XIAPs belong to an important family of antiapoptotic
proteins involved in resistance to cancer therapy (37). XIAPs can bind and inactivate key
caspases involved in the initiation (caspase-9) and execution
(caspase-3 and -7) of the apoptosis cascade (38,39).
Thus, XIAPs represent critical regulatory factors in mitochondrial
apoptosis signaling. XAF1 was first defined as a XIAP interacting
protein that antagonized the effect of XIAP in inhibiting caspase
activation (9). Low expression of
XAF1 correlates with promoter hypermethylation and is strongly
associated with tumor stage (40,41).
Restoration of XAF1 expression induces cancer cell apoptosis and
inhibits tumor growth in multiple types of cancers, including
gastric, colon, liver, pancreatic, and prostate cancers (11,42,43).
Byun et al first reported that 7 of 9 (78%) primary tumors
with reduced XAF1 expression carried wild-type p53 and 13 of 15
(87%) primary tumors with mutant p53 showed normal expression of
XAF1 (12). Once activated, p53
translocates and accumulates in the nucleus, where it can either
cause cell cycle arrest or induce cell apoptosis (44). Berberine was shown to induce
p53-independent, XIAP-mediated apoptotic cell death in p53-null
leukemia cells (45). However, the
functions of XAF1 as a tumor suppressor in lymphoma are not fully
understood. XAF1 can activate p53-mediated apoptosis in cancer
cells through posttranslational modification (7) although the exact mechanism of the
interaction between XAF1 and functional p53 in the control of
apoptosis is still unclear. XAF1 may enhance the protein stability
of phosphorylated p53, thus leading to prolonged activation of p53
and expression of its target gene p21 under stress condition
(41). p53 provokes cell cycle
arrest in G0/G1 and G2/M phases by upregulating p21 and
downregulating cyclin B1 (46).
Adeno-XAF1 transduction similarly induces cell cycle G2/M arrest
and upregulates the expression of p21 and downregulates the
expression of cyclin B1 and cdc2 (42). Exposure to 5-aza-cytidine induces
the upregulation of p53 and its downstream effectors
p21WAF1 and GADD45 (47).
p53-dependent and -independent mechanisms have been
reported to play a role in the regulation of GADD45α expression
(48). However, the expression of
GADD45α for induction of apoptosis did not change after
translocation of XAF1 into the cytosol following treatment with
berberine. Using immunoprecipitation and immunoblotting analysis we
showed that XAF1 formed a complex with p53 and GADD45α. Although
direct interaction between GADD45α and p53 or XAF1 was detected in
the present study, we still need to investigate how the
p53-XAF1-GADD45α complex is formed in the cytosol or is
translocated into nucleus to determine its exact role in
apoptosis.
In this study, co-incubation with the selective p53
inhibitor PFT-α not only blocked phosphorylation of p53, but also
restored expression of XAF1, GADD45, and Puma, and cleavage of
caspase-3 and PARP to control levels. This result is consistent
with previous reports that PFT-α blocks apoptosome-mediated
processing and activation of caspase-9 and -3 and inhibits the
induction of p53, p21, and Bax (49,50).
GADD45α induces apoptosis through induction of the G2/M phase
arrest in response to nerve growth factor stimulation independent
of the p38 and JNK pathways (51,52),
although GADD45α also plays some role in the regulation of p53
through p38/JNK (16,17). ROS act as an early signal activator
to provoke apoptosis in vinca alkaloid-treated lung adenocarcinoma
cells (53). In our previous
report, we proposed that ROS generation might be the initiating
event in the translocation of XAF1 and phosphorylation of ERK1/2 in
dexamethasone-induced apoptosis of EBV-transformed B cells
(54). Likewise, NAC, p38
inhibitor, and JNK inhibitors significantly inhibited
berberine-induced apoptosis by restoration of proapoptotic
proteins, including PUMA, phosphorylated Bax, and phosphorylated
Bim to control levels and completely blocked the upregulation of
XAF1, GADD45α, and phosphorylation of p53.
Based on our findings, the interaction of XAF1 with
functional p53 appears to have novel and important clinical
potential in chemotherapy against cancers originating from B cells.
Although the exact mechanism underlying the regulation of p53 by
XAF1 is still unclear, our data suggest that the role of XAF1 as a
promoter of the mitochondrial apoptosis pathway might provide a
novel target for cancer therapy, particularly for cancers with
expression of wild-type p53. The induction of XAF1 translocation
and GADD45α activation by phosphorylated p53 can be considered a
new target pathway, as exemplified by berberine-induced
mitochondrial apoptosis signaling in EBV-transformed B cells and
cancerous B cells.
Acknowledgements
This study was supported by a grant from the Korea
Healthcare Technology R&D Project, Ministry of Health and
Welfare Affairs, Republic of Korea (grant no. HI15C2800), the 2015
Inje University research grant (R08) and the National R&D
Program for Cancer Control, Ministry for Health, Welfare and Family
Affairs, Republic of Korea (grant no. 0920040).
Abbreviations:
|
EBV
|
Epstein-Barr virus
|
|
GADD45α
|
growth arrest and DNA damage inducible
alpha
|
|
MDM2
|
mouse double minute 2 homolog
|
|
NAC
|
N-acetylcysteine
|
|
PUMA
|
p53 upregulated modulator of
apoptosis
|
|
XAF1
|
X-linked inhibitor of apoptosis
protein-associated factor 1
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
References
|
1
|
Katiyar SK, Meeran SM, Katiyar N and
Akhtar S: p53 cooperates berberine-induced growth inhibition and
apoptosis of non-small cell human lung cancer cells in vitro and
tumor xenograft growth in vivo. Mol Carcinog. 48:24–37. 2009.
View Article : Google Scholar
|
|
2
|
Iizuka N, Miyamoto K, Okita K, Tangoku A,
Hayashi H, Yosino S, Abe T, Morioka T, Hazama S and Oka M:
Inhibitory effect of Coptidis Rhizoma and berberine on the
proliferation of human esophageal cancer cell lines. Cancer Lett.
148:19–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine inhibits growth, induces G1 arrest and apoptosis in human
epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin
cascade, disruption of mitochondrial membrane potential and
cleavage of caspase 3 and PARP. Carcinogenesis. 27:2018–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patil JB, Kim J and Jayaprakasha GK:
Berberine induces apoptosis in breast cancer cells (MCF-7) through
mitochondrial-dependent pathway. Eur J Pharmacol. 645:70–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Gu L, Li J, Shah N, He J, Yang L,
Hu Q and Zhou M: Degradation of MDM2 by the interaction between
berberine and DAXX leads to potent apoptosis in MDM2-overexpressing
cancer cells. Cancer Res. 70:9895–9904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donehower LA, Harvey M, Slagle BL,
McArthur MJ, Montgomery CA Jr, Butel JS and Bradley A: Mice
deficient for p53 are developmentally normal but susceptible to
spontaneous tumours. Nature. 356:215–221. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou B, Chim CS, Pang R, Zeng H, Dai Y,
Zhang R, Lam CS, Tan VP, Hung IF, Lan HY, et al: XIAP-associated
factor 1 (XAF1), a novel target of p53, enhances p53-mediated
apoptosis via post-translational modification. Mol Carcinog.
51:422–432. 2012. View
Article : Google Scholar
|
|
8
|
Lin CC, Yang JS, Chen JT, Fan S, Yu FS,
Yang JL, Lu CC, Kao MC, Huang AC, Lu HF, et al: Berberine induces
apoptosis in human HSC-3 oral cancer cells via simultaneous
activation of the death receptor-mediated and mitochondrial
pathway. Anticancer Res. 27A:3371–3378. 2007.
|
|
9
|
Liston P, Fong WG, Kelly NL, Toji S,
Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW and Korneluk
RG: Identification of XAF1 as an antagonist of XIAP anti-caspase
activity. Nat Cell Biol. 3:128–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fong WG, Liston P, Rajcan-Separovic E, St
Jean M, Craig C and Korneluk RG: Expression and genetic analysis of
XIAP-associated factor 1 (XAF1) in cancer cell lines. Genomics.
70:113–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arora V, Cheung HH, Plenchette S, Micali
OC, Liston P and Korneluk RG: Degradation of survivin by the
X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem.
282:26202–26209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Byun DS, Cho K, Ryu BK, Lee MG, Kang MJ,
Kim HR and Chi SG: Hypermethylation of XIAP-associated factor 1, a
putative tumor suppressor gene from the 17p13.2 locus, in human
gastric adenocarcinomas. Cancer Res. 63:7068–7075. 2003.PubMed/NCBI
|
|
13
|
Hildesheim J, Bulavin DV, Anver MR, Alvord
WG, Hollander MC, Vardanian L and Fornace AJ Jr: Gadd45a protects
against UV irradiation-induced skin tumors, and promotes apoptosis
and stress signaling via MAPK and p53. Cancer Res. 62:7305–7315.
2002.PubMed/NCBI
|
|
14
|
Mita H, Tsutsui J, Takekawa M, Witten EA
and Saito H: Regulation of MTK1/MEKK4 kinase activity by its
N-terminal autoinhibitory domain and GADD45 binding. Mol Cell Biol.
22:4544–4555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takekawa M and Saito H: A family of
stress-inducible GADD45-like proteins mediate activation of the
stress-responsive MTK1/MEKK4 MAPKKK. Cell. 95:521–530. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bulavin DV, Saito S, Hollander MC,
Sakaguchi K, Anderson CW, Appella E and Fornace AJ Jr:
Phosphorylation of human p53 by p38 kinase coordinates N-terminal
phosphorylation and apoptosis in response to UV radiation. EMBO J.
18:6845–6854. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buschmann T, Potapova O, Bar-Shira A,
Ivanov VN, Fuchs SY, Henderson S, Fried VA, Minamoto T,
Alarcon-Vargas D, Pincus MR, et al: Jun NH2-terminal kinase
phosphorylation of p53 on Thr-81 is important for p53 stabilization
and transcriptional activities in response to stress. Mol Cell
Biol. 21:2743–2754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takemoto S, Trovato R, Cereseto A, Nicot
C, Kislyakova T, Casareto L, Waldmann T, Torelli G and Franchini G:
p53 stabilization and functional impairment in the absence of
genetic mutation or the alteration of the p14(ARF)-MDM2 loop in ex
vivo and cultured adult T-cell leukemia/lymphoma cells. Blood.
95:3939–3944. 2000.PubMed/NCBI
|
|
19
|
Park GB, Kim YS, Lee HK, Song H, Cho DH,
Lee WJ and Hur DY: Endoplasmic reticulum stress-mediated apoptosis
of EBV-transformed B cells by cross-linking of CD70 is dependent
upon generation of reactive oxygen species and activation of p38
MAPK and JNK pathway. J Immunol. 185:7274–7284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chidambara Murthy KN, Jayaprakasha GK and
Patil BS: The natural alkaloid berberine targets multiple pathways
to induce cell death in cultured human colon cancer cells. Eur J
Pharmacol. 688:14–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu B, Hu M, Liu K and Peng J: Cytotoxicity
of berberine on human cervical carcinoma HeLa cells through
mitochondria, death receptor and MAPK pathways, and in-silico
drug-target prediction. Toxicol In Vitro. 24:1482–1490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu B, Yu H, Chow C, Li B, Zheng W, Davis
RJ and Flavell RA: GADD45gamma mediates the activation of the p38
and JNK MAP kinase pathways and cytokine production in effector TH1
cells. Immunity. 14:583–590. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komarov PG, Komarova EA, Kondratov RV,
Christov-Tselkov K, Coon JS, Chernov MV and Gudkov AV: A chemical
inhibitor of p53 that protects mice from the side effects of cancer
therapy. Science. 285:1733–1737. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy PJ, Galigniana MD, Morishima Y,
Harrell JM, Kwok RP, Ljungman M and Pratt WB: Pifithrin-alpha
inhibits p53 signaling after interaction of the tumor suppressor
protein with hsp90 and its nuclear translocation. J Biol Chem.
279:30195–30201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuzawa A and Ichijo H:
Stress-responsive protein kinases in redox-regulated apoptosis
signaling. Antioxid Redox Signal. 7:472–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lau CW, Yao XQ, Chen ZY, Ko WH and Huang
Y: Cardiovascular actions of berberine. Cardiovasc Drug Rev.
19:234–244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jantova S, Cipak L and Letasiova S:
Berberine induces apoptosis through a mitochondrial/caspase pathway
in human promonocytic U937 cells. Toxicol In Vitro. 21:25–31. 2007.
View Article : Google Scholar
|
|
28
|
Lin CC, Kao ST, Chen GW, Ho HC and Chung
JG: Apoptosis of human leukemia HL-60 cells and murine leukemia
WEHI-3 cells induced by berberine through the activation of
caspase-3. Anticancer Res. 26A:227–242. 2006.
|
|
29
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perdomo JA, Naomoto Y, Haisa M, Fujiwara
T, Hamada M, Yasuoka Y and Tanaka N: In vivo influence of p53
status on proliferation and chemoradiosensitivity in non-small-cell
lung cancer. J Cancer Res Clin Oncol. 124:10–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai SL, Perng RP and Hwang J: p53 gene
status modulates the chemosensitivity of non-small cell lung cancer
cells. J Biomed Sci. 7:64–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perez-Soler R, Kemp B, Wu QP, Mao L, Gomez
J, Zeleniuch-Jacquotte A, Yee H, Lee JS, Jagirdar J and Ling YH:
Response and determinants of sensitivity to paclitaxel in human
non-small cell lung cancer tumors heterotransplanted in nude mice.
Clin Cancer Res. 6:4932–4938. 2000.
|
|
34
|
Oizumi S, Isobe H, Ogura S, Ishida T,
Yamazaki K, Nishimura M, Kawakami Y and Dosaka-Akita H:
Topoisomerase inhibitor-induced apoptosis accompanied by
down-regulation of Bcl-2 in human lung cancer cells. Anticancer
Res. 22C:4029–4037. 2002.
|
|
35
|
McCurrach ME, Connor TM, Knudson CM,
Korsmeyer SJ and Lowe SW: bax-deficiency promotes drug resistance
and oncogenic transformation by attenuating p53-dependent
apoptosis. Proc Natl Acad Sci USA. 94:2345–2349. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryan KM, Phillips AC and Vousden KH:
Regulation and function of the p53 tumor suppressor protein. Curr
Opin Cell Biol. 13:332–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
LaCasse EC, Baird S, Korneluk RG and
MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging
role in cancer. Oncogene. 17:3247–3259. 1998. View Article : Google Scholar
|
|
38
|
Holcik M, Gibson H and Korneluk RG: XIAP:
Apoptotic brake and promising therapeutic target. Apoptosis.
6:253–261. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferreira CG, van der Valk P, Span SW,
Jonker JM, Postmus PE, Kruyt FA and Giaccone G: Assessment of IAP
(inhibitor of apoptosis) proteins as predictors of response to
chemotherapy in advanced non-small-cell lung cancer patients. Ann
Oncol. 12:799–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang J, Yao WY, Zhu Q, Tu SP, Yuan F,
Wang HF, Zhang YP and Yuan YZ: XAF1 as a prognostic biomarker and
therapeutic target in pancreatic cancer. Cancer Sci. 101:559–567.
2010. View Article : Google Scholar
|
|
41
|
Lee MG, Huh JS, Chung SK, Lee JH, Byun DS,
Ryu BK, Kang MJ, Chae KS, Lee SJ, Lee CH, et al: Promoter CpG
hypermethylation and downregulation of XAF1 expression in human
urogenital malignancies: Implication for attenuated p53 response to
apoptotic stresses. Oncogene. 25:5807–5822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tu SP, Liston P, Cui JT, Lin MC, Jiang XH,
Yang Y, Gu Q, Jiang SH, Lum CT, Kung HF, et al: Restoration of XAF1
expression induces apoptosis and inhibits tumor growth in gastric
cancer. Int J Cancer. 125:688–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tu SP, Sun YW, Cui JT, Zou B, Lin MC, Gu
Q, Jiang SH, Kung HF, Korneluk RG and Wong BC: Tumor suppressor
XIAP-Associated factor 1 (XAF1) cooperates with tumor necrosis
factor-related apoptosis-inducing ligand to suppress colon cancer
growth and trigger tumor regression. Cancer. 116:1252–1263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Harris SL and Levine AJ: The p53 pathway:
Positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu J, Zhang X, Liu A, Liu S, Zhang L, Wu
B and Hu Q: Berberine induces apoptosis in p53-null leukemia cells
by down-regulating XIAP at the post-transcriptional level. Cell
Physiol Biochem. 32:1213–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dubrez L, Coll JL, Hurbin A, de Fraipont
F, Lantejoul S and Favrot MC: Cell cycle arrest is sufficient for
p53-mediated tumor regression. Gene Ther. 8:1705–1712. 2001.
View Article : Google Scholar
|
|
47
|
Schneider-Stock R, Diab-Assef M, Rohrbeck
A, Foltzer-Jourdainne C, Boltze C, Hartig R, Schönfeld P, Roessner
A and Gali-Muhtasib H: 5-Aza-cytidine is a potent inhibitor of DNA
methyltransferase 3a and induces apoptosis in HCT-116 colon cancer
cells via Gadd45- and p53-dependent mechanisms. J Pharmacol Exp
Ther. 312:525–536. 2005. View Article : Google Scholar
|
|
48
|
Lakin ND and Jackson SP: Regulation of p53
in response to DNA damage. Oncogene. 18:7644–7655. 1999. View Article : Google Scholar
|
|
49
|
Kaji A, Zhang Y, Nomura M, Bode AM, Ma WY,
She QB and Dong Z: Pifithrin-alpha promotes p53-mediated apoptosis
in JB6 cells. Mol Carcinog. 37:138–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Proietti De Santis L, Balajee AS, Lorenti
Garcia C, Pepe G, Worboys AM and Palitti F: Inhibition of p53, p21
and Bax by pifithrin-alpha does not affect UV induced apoptotic
response in CS-B cells. DNA Repair (Amst). 2:891–900. 2003.
View Article : Google Scholar
|
|
51
|
Chou TT, Trojanowski JQ and Lee VM: p38
mitogen-activated protein kinase-independent induction of gadd45
expression in nerve growth factor-induced apoptosis in
medulloblastomas. J Biol Chem. 276:41120–41127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mak SK and Kültz D: Gadd45 proteins induce
G2/M arrest and modulate apoptosis in kidney cells exposed to
hyperosmotic stress. J Biol Chem. 279:39075–39084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chiu WH, Luo SJ, Chen CL, Cheng JH, Hsieh
CY, Wang CY, Huang WC, Su WC and Lin CF: Vinca alkaloids cause
aberrant ROS-mediated JNK activation, Mcl-1 downregulation, DNA
damage, mitochondrial dysfunction, and apoptosis in lung
adenocarcinoma cells. Biochem Pharmacol. 83:1159–1171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park GB, Choi Y, Kim YS, Lee HK, Kim D and
Hur DY: ROS and ERK1/2-mediated caspase-9 activation increases XAF1
expression in dexamethasone-induced apoptosis of EBV-transformed B
cells. Int J Oncol. 43:29–38. 2013.PubMed/NCBI
|