Introduction
Oral squamous cell carcinoma (OSCC) is the most
common cancer of the oral cavity; it accounts for more than 90% all
oral neoplasms (1). Oral cancers
account for 2–4% of all cancer cases worldwide and OSCC is the 8th
most common cancer in humans (2,3,4). For
several decades increasing trends of oral cancer incidences have
been observed in either gender in the general population all over
the world (5,6). Current approaches of treatment of
OSCC include surgery, radiation therapy and/or chemotherapy.
However, despite the progress in research and therapy, survival has
not improved considerably in recent years (7). If detected during its early stage,
the 5-year survival rate of oral cancer is 60–80% (1,8).
However, the overall survival rate of OSCC is approximately 50% in
the advanced stage of the disease (9,10).
Moreover, these therapeutic approaches usually cause adverse
effects that reduce quality of life. Therefore, it is necessary to
identify novel, effective and less cytotoxic therapeutic agents for
OSCC treatment.
Proliferating tumor cells do not exploit the full
capacity of oxidative metabolism of glucose to produce ATP, instead
it shows enhanced lactate production during glucose metabolism even
in the presence of abundant oxygen (11). This phenomenon is known as the
Warburg effect or aerobic glycolysis, which is a common metabolic
characteristic of cancer cells and is related to tumor
proliferation, progression and drug-resistance in cancer (12,13).
The Warburg effect has been directly associated with the
upregulation of HIF-1α and lactate dehydrogenase (LDH) as well as
downregulation of pyruvate dehydrogenase (PDH) (14,15).
Several members of PI3K/AKT/mTOR signaling pathway are recognized
as the major control points to support the metabolic autonomy of
tumor cells and the Warburg effect (14–16).
Herein, Akt acts as a key enzyme of the Warburg effect in tumor
cells by favoring the glucose-to-lactate metabolic pathway. PI3K
and mTOR are located up- and down-stream of Akt respectively, and
also act as active players (16).
On the contrary, it was reported that, silencing AMPK in tumor
cells results in a metabolic shift towards aerobic glycolysis.
HIF-1α has a significant role in this metabolic shift, therefore
AMPK acts as a negative regulator of the Warburg effect (17). Targeting the regulator molecules of
Warburg effect might be a useful strategy to overcome
drug-resistance and effectively kill cancer cells.
Metformin, a low cost antidiabetic drug has been
reported to be effective in the treatment of different types of
cancers including oral cancer and head and neck cancer, and it is
well tolerated by patients (18–27).
Metformin exerts inhibitory effects on multiple pathways involved
in the initiation of carcinogenesis, as well as proliferation,
survival and metastasis of cancer cells (28). It targets cancer stem cells and
several regulatory molecules of Warburg effect, and it also induces
apoptosis in cancer cells (29).
Merformin inhibits cell proliferation by activating AMPK, and
inhibiting mTOR and HIF-1α (30).
It can also ameliorate the cytotoxicity of some drugs and several
studies demonstrated the combined effect of metformin and
5-fluorouracil (5-FU) against esophageal and colon cancer cells
(31,32).
In this study, we evaluated the efficacy of combined
therapy with metformin and 5-FU against human OSCC cell lines in
vitro and in vivo.
Materials and methods
Cell lines and cell culture
OSCC cell lines (HSC2, HSC3 and HSC4) were obtained
from Cell Bank, RIKEN BioResource Center (Ibaraki, Japan). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS) (Thermo Fisher Scientific Inc., Waltham, MA,
USA), 100 μg/ml streptomycin, 100 U/ml penicillin (Thermo Fisher
Scientific) in a humidified atmosphere containing 5%
CO2.
In vitro cell growth assay
Cells (5×103 per well) were seeded on
96-well plates (Becton Dickinson Labware, Franklin lakes, NJ, USA)
in DMEM supplemented with 10% FBS. Twenty-four hours later, the
cells were treated with 5-FU (0.5–10 μg/ml) (Kyowa Hakko Kirin Co.,
Ltd, Tokyo, Japan), Metformin hydrochloride (metformin; 1–10 mg/ml)
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) or both for 24,
48 or 72 h. Then, 3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) was added to each well (25
μl/well) and incubated for 4 h. The blue dye taken up by cells was
dissolved in dimethyl sulfoxide (100 μl/well), and the absorbance
was measured with a spectrophotometer (Bio-Rad Laboratories,
Hercules, CA, USA) at 490 nm. All assays were run in
triplicate.
TUNEL (terminal deoxynucleotidyl
transferase (Tdt)-mediated nick end labeling) assay
To detect apoptotic cells in cell lines and in mouse
tumor tissues, TUNEL assay was performed by labeling 3′-OH DNA ends
generated by DNA fragmentation. Cells (5×103 cells per
well) were seeded on cover glass (Matsunami Glass Ind. Ltd., Osaka,
Japan) in DMEM containing 10% FBS. After incubation for 24 h, cells
were treated with 5-FU (2.5 μg/ml) and/or metformin (4 mg/ml) and
incubated for 48 h. Then, the cells on the cover glass were washed
twice with phosphate buffered saline (PBS), air dried, and fixed in
4% paraformaldehyde at room temperature for 30 min. The TUNEL assay
was performed using a DeadEnd™ Colorimetric TUNEL System according
to the manufacturer's instructions (Promega Corp., Madison, WI,
USA). Briefly, the cells on the cover glass were incubated in 20
μg/ml proteinase K for 15 min. Endogenous peroxidase of cells on
the cover glass was blocked by incubating in a 3% hydrogen peroxide
solution for 5 min after cells were rinsed in distilled water.
After being washed with PBS, the cells were incubated with
equilibration buffer (0.05 M phosphate buffer containing 0.145 M
sodium chloride, pH 7.4) and then Tdt enzyme in a humidified
chamber at 37°C for 60 min. They were subsequently put into
pre-warmed working strength stop wash buffer for 10 min. After
being rinsed in PBS, the cells were incubated with
anti-digoxigenin-peroxidase conjugate for 30 min. Peroxidase
activity in each cell was demonstrated by the application of
diaminobenzidine. Hematoxylin was used as a counterstain. At least
1000 cells were counted under a microscope in three random fields
of each cover glass. The number of apoptotic cells was calculated
by dividing the number of TUNEL positive cells by the total number
of counted cells and the result was expressed as a percentage.
In the same manner, TUNEL assay was performed in 4
μm paraffin sections of mouse tumor tissues using a DeadEnd
Colorimetric TUNEL System according to the manufacturer's
instructions (Promega Corp.).
Lactate colorimetric assay
To detect the lactate production from 5-FU (2.5
μg/ml) and/or metformin (4 mg/ml) treated cells, lactate
colorimetric assay was carried out to measure the total lactate
content in the cell culture supernatant of the untreated control,
5-FU (2.5 μg/ml) and/or metformin (4 mg/ml) treated cells for 48 h
using a Lactate Assay kit according to the manufacturer's
instructions (BioVision Inc., Milpitas, CA, USA).
Western blot analysis
After the cells were treated with 5-FU (2.5 μg/ml)
and/or metformin (4 mg/ml) for 48 h, they were collected and lysed.
Whole cell lysates were subjected to electrophoresis on 10%
SDS-polyacrylamide gels, and then transferred to a PVDF membrane.
The membranes were incubated with the anti-HIF-1α mouse monoclonal
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
anti-mTOR rabbit monoclonal antibody (Cell Signaling Technology
Inc., Danvers, MA, USA), anti-Akt1 mouse monoclonal antibody (Santa
Cruz), and anti-AMPKα rabbit monoclonal antibody (Cell Signaling
Technology Inc.) followed by Novex® alkaline-phosphatase
conjugated (goat) anti-rabbit or (goat) anti-mouse immunoglobulin G
(IgG) secondary antibody (Thermo Fisher Scientific). The antibody
was detected using a chromogenic immunodetection system,
WesternBreeze (Thermo Fisher Scientific) according to the
manufacturer's instructions. Moreover, anti-α-tubulin monoclonal
antibody (Santa Cruz Biotechnology, Inc.) was used for
normalization of western blot analysis.
Nude mice and tumor inoculations
Female athymic nude mice with
CAnN.Cg-Foxnlnu/CrlCrlj genetic background (CLEA Japan, Inc. Tokyo,
Japan) were purchased at 4 weeks of age and kept under sterile
conditions in a pathogen-free environment. The mice were provided
with sterile water and food. In addition, all manipulations were
carried out aseptically inside a laminar flow hood. Cells were used
as a xenograft model in the nude mice. Briefly, cells
(1×106) were suspended in 0.1 ml of serum-free medium
and injected into the subcutaneous tissue of 5-week-old nude mice
(average weight 20.0 g) using a 27-gauge needle. Tumors were
allowed to grow for 14 days before treatment. The mice were then
divided into 4 groups, 5 mice in each group with similar mean tumor
volumes (600–800 mm3). All in vivo experiments
were approved by the Institutional Animal Care and Use Committee of
Yamaguchi University.
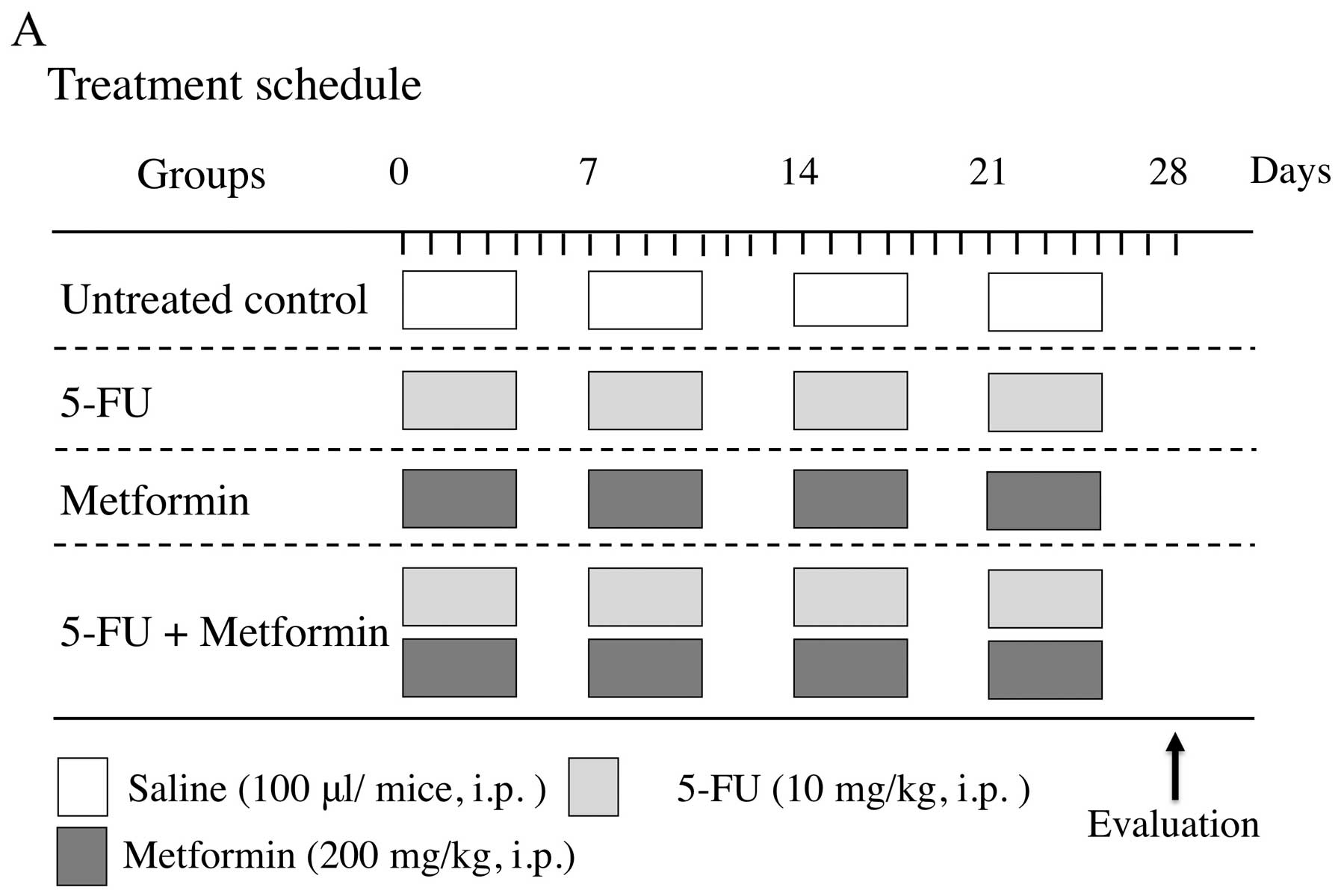
In vivo treatment protocol
For this experiment, suitable doses for 5-FU and
metformin were selected as 10 and 200 mg/kg, respectively, as these
doses were reported to be effective in tumor xenograft models of
other cancer types (33,34). The treatment protocol of the four
experimental groups of mice is shown in Fig. 5A. After tumor formation, these mice
were treated with sterile saline (100 μl), 5-FU (10 mg/kg) and/or
metformin (200 mg/kg) by intraperitoneal (i.p.) injection for 4
weeks (5 days/week).
The size of the tumors was measured every three days
and tumor volumes were calculated as 0.5 × length ×
width2. At 28 days, mice were sacrificed by an overdose
of Somnopentyl (200 mg/kg; Merck & Co., Inc., Whitehouse
Station, NJ, USA), and the tumors were dissected, fixed in
neutral-buffered formalin and embedded in paraffin for further
study.
Immunohistochemistry
The avidin-biotin complex immunohistochemical
technique was used to detect Warburg effect related factors
(HIF-1-α, mTOR, Akt1 and AMPKα) in mouse tissue specimens, using
the EnVision™ kit (Dako, Glostrup, Denmark). Paraffin-embedded 4 μm
tissue sections were deparaffinized in xylene and rehydrated
through graded alcohols. Endogenous peroxidase was quenched with a
0.3% hydrogen peroxide/methanol mixture for 30 min. Sections were
rinsed and pre-incubated with 2% blocking serum for 30 min,
followed by incubation with the anti-HIF-1α mouse monoclonal
antibody (Santa Cruz Biotechnology, Inc.), anti-mTOR rabbit
monoclonal antibody (Cell Signaling Technology Inc.), anti-Akt1
mouse monoclonal antibody (Santa Cruz Biotechnology, Inc.), and
anti-AMPKα rabbit monoclonal antibody (Cell Signaling Technology
Inc.) for 8 h at 4°C. After rinsing the tissue sections in
phosphate buffered saline (PBS) for 10 min, the antibody was
detected using the EnVision kit according to the manufacturer's
instructions. Tissues were finally rinsed in PBS for 5 min followed
by tap water for 5 min, and then counterstained with hematoxylin
for 1 min. The tissue sections were subsequently dehydrated in
graded ethanol followed by xylene and mounted with glass coverslips
using DPX.
Statistical analysis
All statistical significance was set at P<0.05.
Statistical analyses were performed using the StatView software
(version 5.0J, SAS Institute Inc., Cary, NC, USA).
Results
Effects of 5-FU and/or metformin on the
growth of OSCC cells in vitro
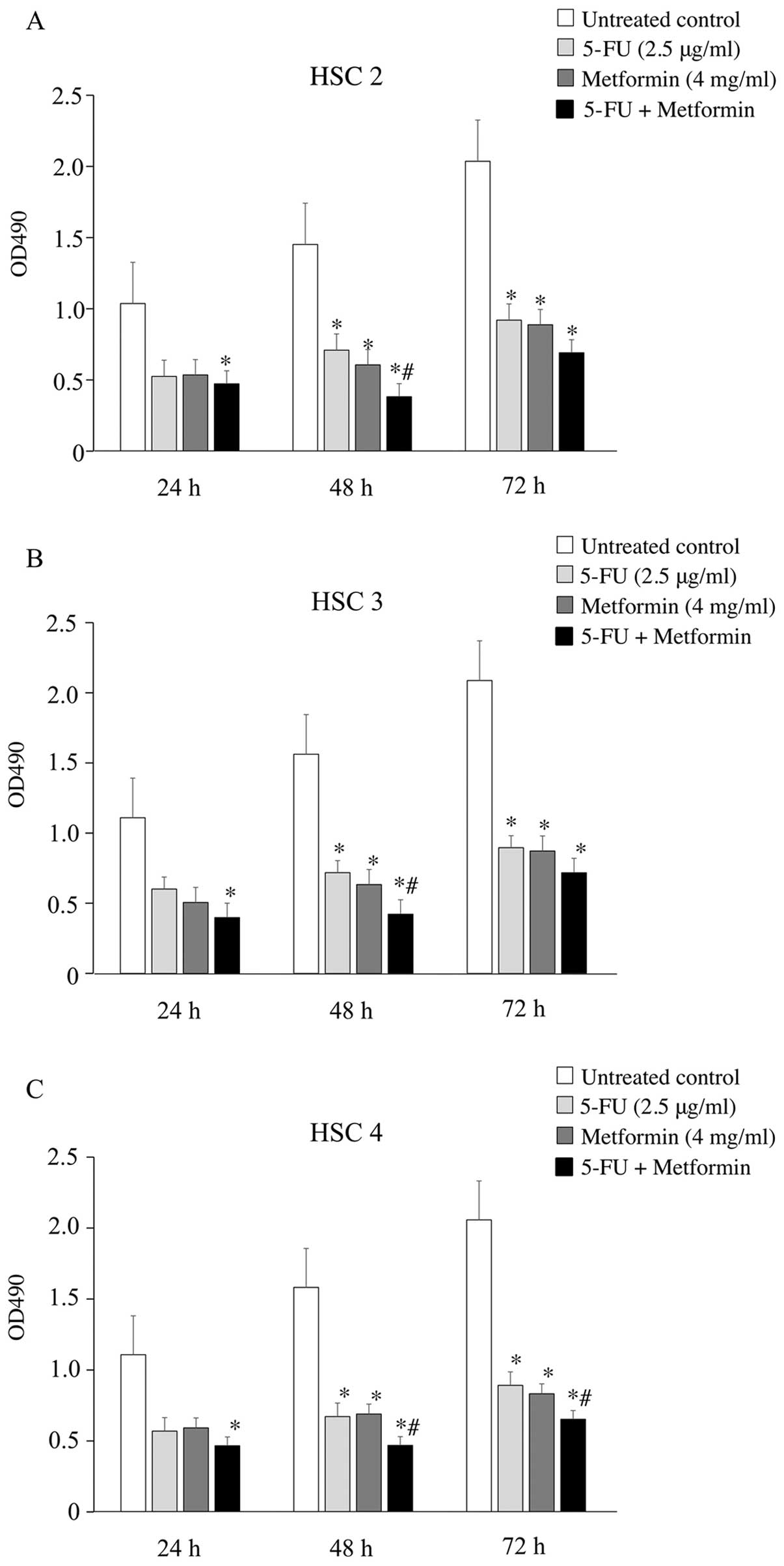
The growth inhibitory effect of 5-FU and metformin
on HSC2, HSC3 and HSC4 cells was analyzed by the MTT assay. Cells
were treated with 5-FU (0.5–10 μg/ml) and/or metformin (1–10 mg/ml)
for 24, 48 and 72 h. 5-FU or metformin inhibited cell growth in a
dose-dependent manner, and single treatment with 2.5 μg/ml 5-FU or
4 mg/ml metformin inhibited ≥50% cell growth in all three cell
lines (data not shown). Therefore, these concentrations of 5-FU and
metformin were chosen for all in vitro experiments as single
or combination treatments. As shown in Fig. 1, 5-FU and metformin combination
significantly inhibited the growth of HSC2, HSC3 and HSC4 cells
compared to 5-FU or metformin alone, or the untreated control. In
addition, 48 h treatment was the most effective (growth inhibition
ratio: 70–73%) for all three cell lines.
Effects of 5-FU and/or metformin on
induction of apoptosis in vitro
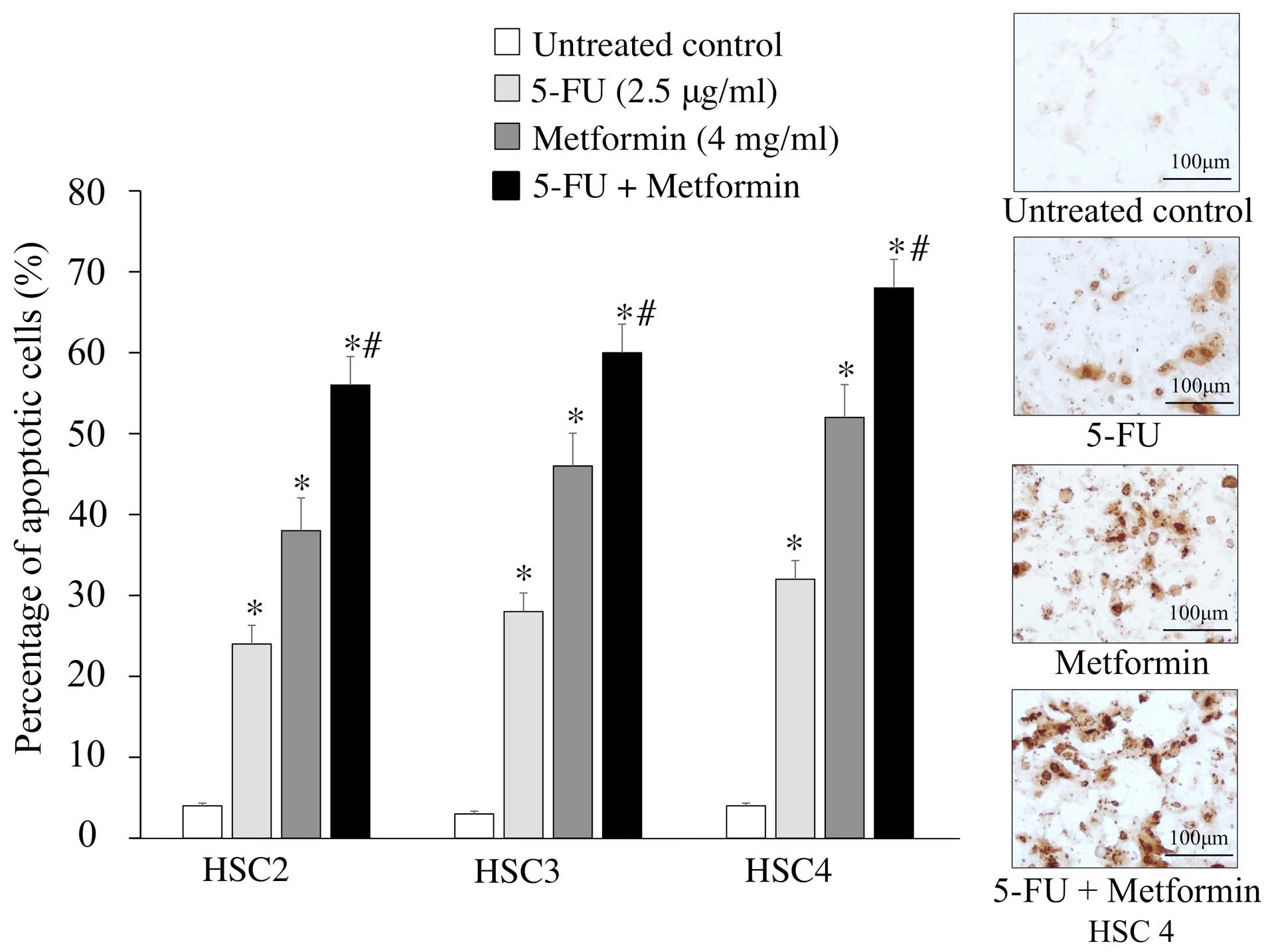
To understand whether the enhanced cell growth
inhibitory effect of 5-FU and metformin combined treatment was due
to apoptosis, we performed TUNEL assay to detect DNA fragmentation
and chromatin condensation in treated cells. TUNEL assay showed
that 5-FU (2.5 μg/ml) and metformin (4 mg/ml) combination treatment
for 48 h induced apoptosis (56–68%) more strongly in cells compared
to single agent chemotherapy. Briefly, the numbers of apoptotic
cells were significantly increased after 5-FU and metformin
combined treatment than treatment with either agent alone (Fig. 2).
Effects of 5-FU and/or metformin on the
production of lactate in vitro
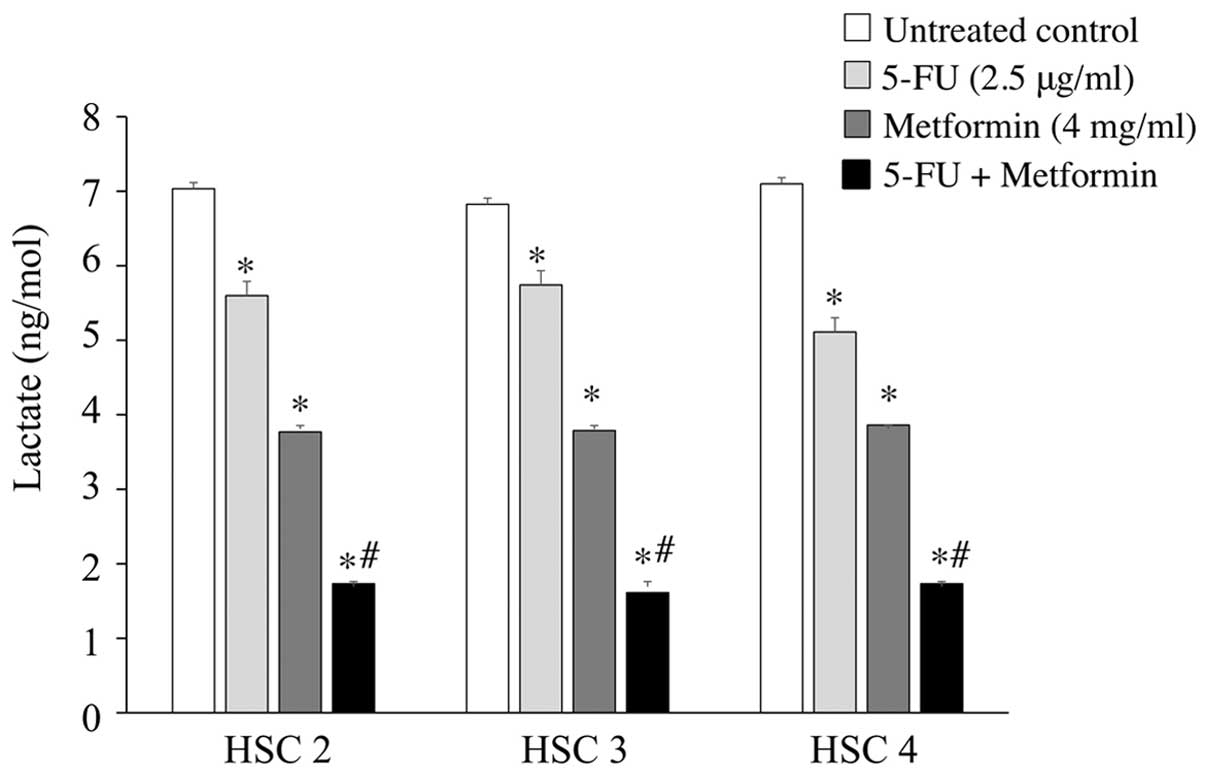
To clarify the mechanisms of the antitumor activity
of 5-FU and metformin combined treatment, we examined the
production of lactate in cells that was relevant to the Warburg
effect. Lactate colorimetric assay detected a decreased level (≤1.7
ng/mol) of lactate in the supernatants of 5-FU (2.5 μg/ml) and
metformin (4 mg/ml) treated cells compared to untreated control
cells (≤7ng/mol), or cells treated with 5-FU (≤5.7ng/mol) or
metformin (≤3.9 ng/mol) alone. Briefly, metformin reduced the
production of lactate in the three cell groups compared to 5-FU,
whereas 5-FU and metformin combined treatment markedly reduced the
production of lactate in the three cell groups compared to either
agent alone (Fig. 3).
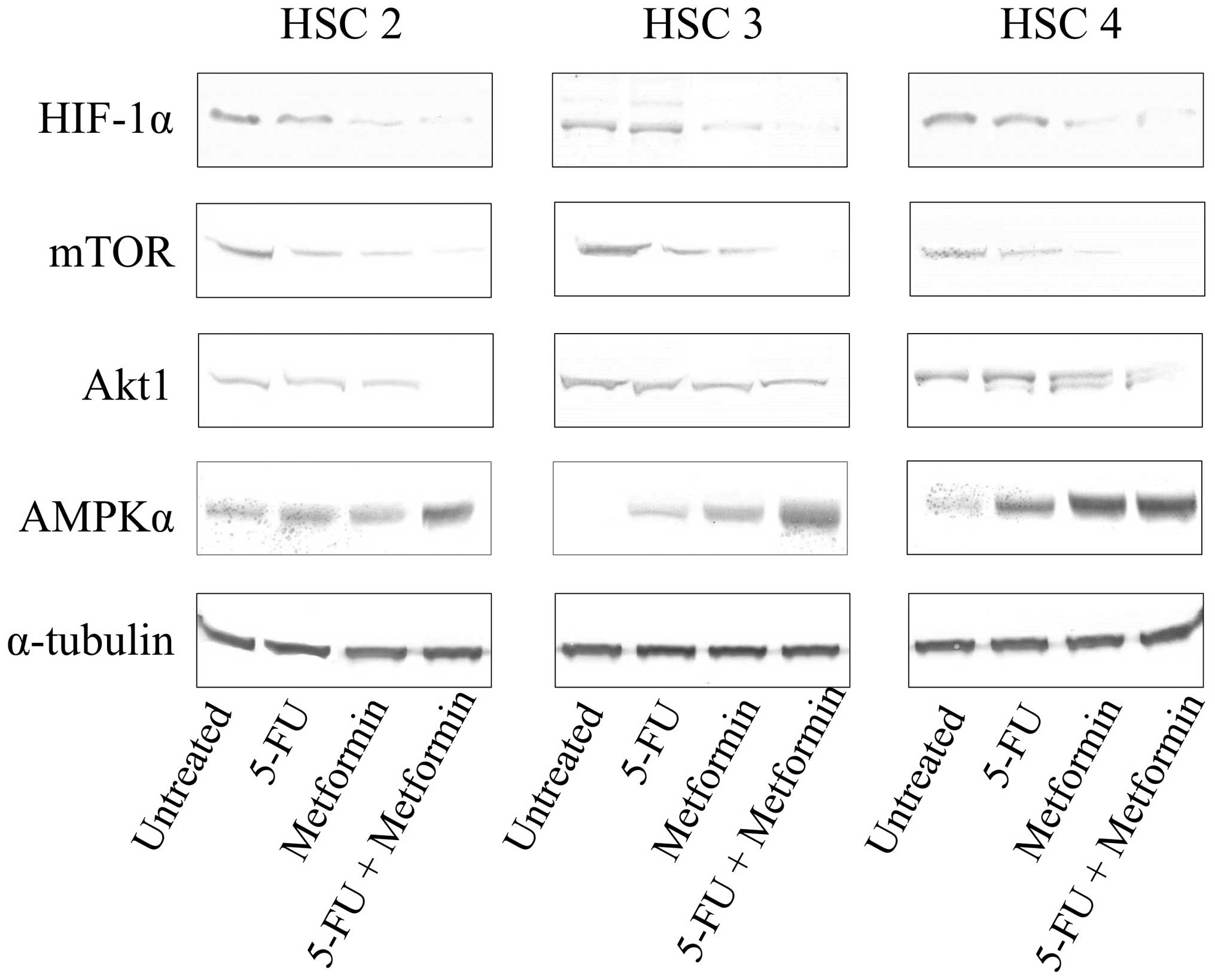
Effects of 5-FU and/or metformin on the
expression levels of Warburg effect related factors in vitro
In order to clarify whether or not 5-FU and/or
metformin treatment modulates the Warburg effect related factors in
tumor cells, we examined the expression levels of HIF-1-α, mTOR,
Akt1 and AMPKα in 5-FU and/or metformin treated (48 h) cells by
western blotting. Metformin markedly reduced the expression of
HIF-1-α and mTOR, and slightly reduced the expression of Akt1 in
all three cell lines and induced the expression of AMPKα in HSC3
and HSC4. In addition, 5-FU and metformin combined treatment
reduced the expression of HIF-1-α, mTOR and Akt1, and induced the
expression of AMPKα markedly in all three cell lines (Fig. 4).
Effects of 5-FU and/or metformin on tumor
growth inhibition in vivo
Nude mice with HSC2 tumor xenografts were used to
examine the antitumor activity of 5-FU and metformin
single/combination treatment. Control group received saline 200 μl
only, while treatment groups were treated with either 5-FU (10
mg/kg/day, 5 times/week) or metformin (200 mg/kg/day, 5 times/week)
alone, or in combination for 4 weeks (Fig. 5A). Fig. 5B shows the result of the in
vivo experiment. All the treatment groups significantly
inhibited tumor growth compared to the untreated control. Antitumor
effect of 5-FU alone (52%) was in the same range to metformin alone
(59.9%). However, the maximum reduction (77.6%) of tumor growth was
observed with 5-FU and metformin combination therapy, which is
significantly different than treatment with either agent alone.
Compared to the control, mice in all treatment groups showed no
toxicity or significant weight loss during the treatment (Fig. 5C).
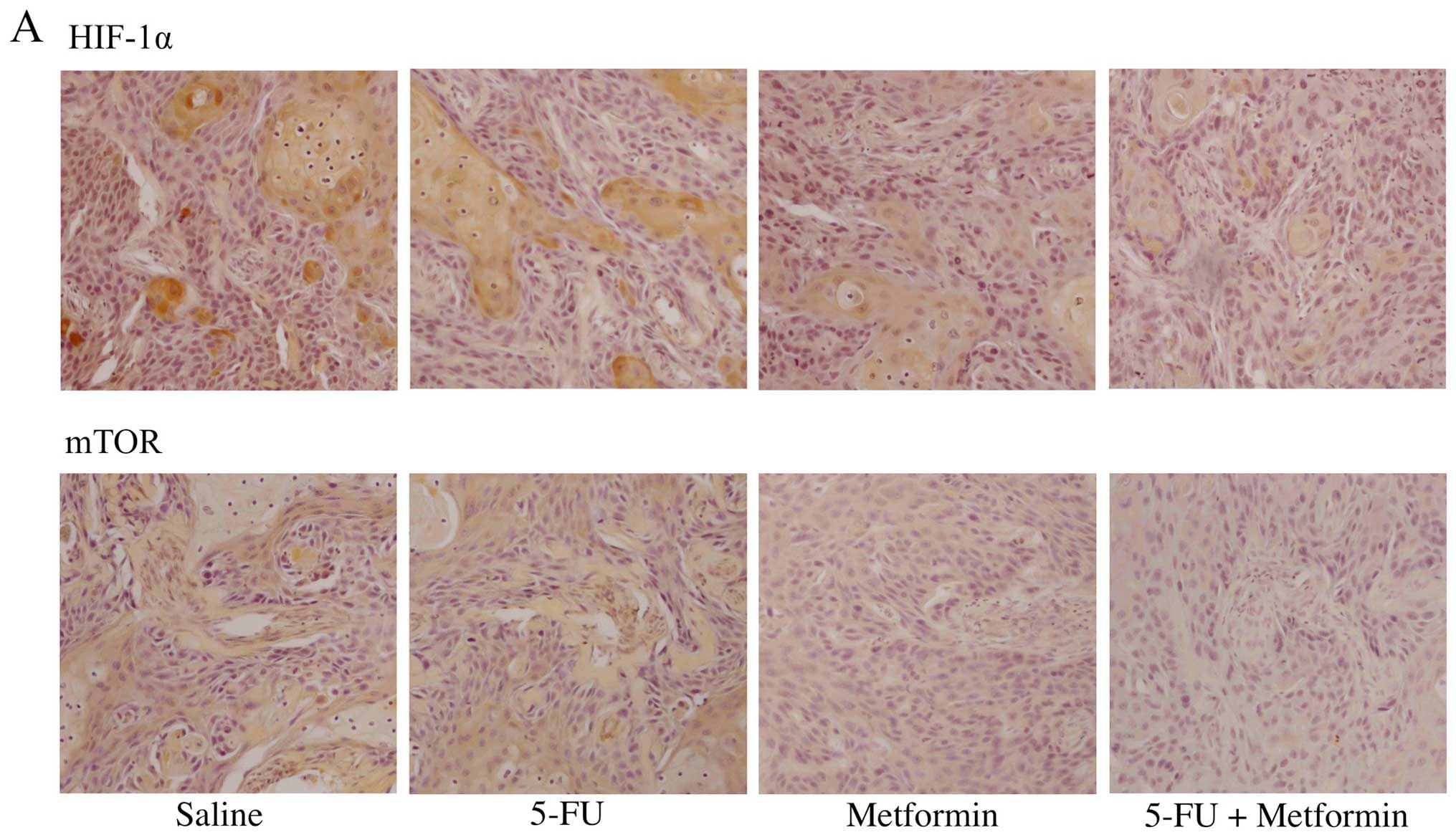
Effects of 5-FU and/or metformin on the
expression of Warburg effect related factors in vivo
We examined the expression levels of Warburg effect
related factors (HIF-1-α, mTOR, Akt1 and AMPKα) in mouse tumors by
immunohistochemistry. The expression of HIF-1-α was detected in the
nucleus of untreated HSC2 tumor cells and 5-FU treated HSC2 tumor
cells. However, the expression of HIF-1-α was not detected in the
nucleus of metformin treated tumor cells and 5-FU plus metformin
treated tumor cells. Similar result was observed in case of mTOR,
except mTOR expression was detected in the cytoplasm of tumor
cells. The expression of Akt1 was detected strongly in both the
nucleus and the cytoplasm of untreated HSC2 tumor cells. However,
the expression of Akt1 was detected weakly in 5-FU treated tumor
cells, but it was not detected in metformin treated tumor cells or
5-FU plus metformin treated tumor cells. The expression of AMPKα
was not detected in cytoplasm of untreated HSC2 tumor cells, but it
was detected weakly in 5-FU treated tumor cells, moderately in
metformin treated tumor cells, and strongly in 5-FU plus metformin
treated tumor cells (Fig. 6).
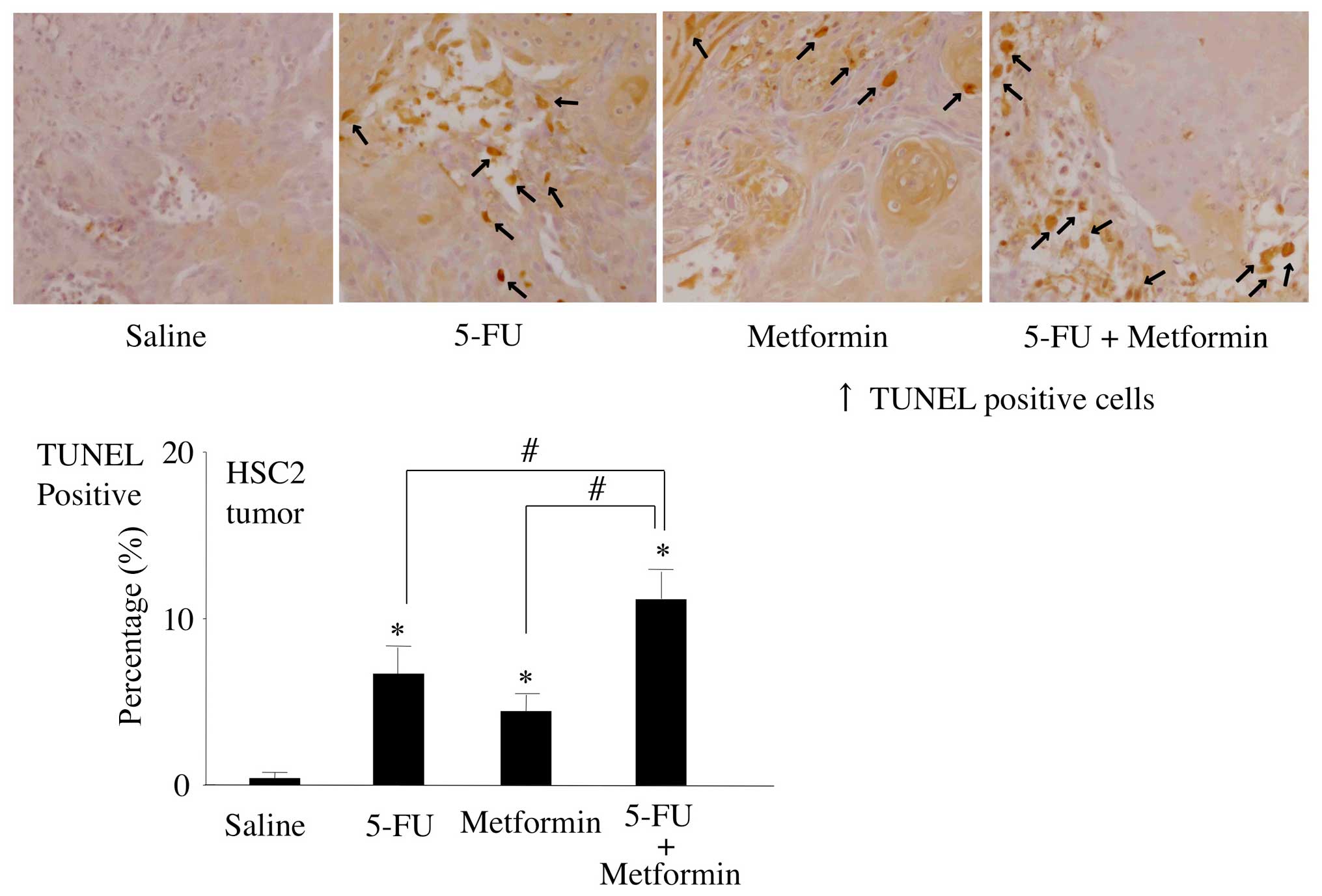
Effects of 5-FU and/or metformin on
induction of apoptosis in vivo
To detect the degree of apoptosis induced by 5-FU
and/or metformin in vivo, the number of apoptotic cells in
mouse tumor tissue sections was quantified by the TUNEL assay.
Although treatment with 5-FU or metformin alone moderately induced
apoptosis in mouse tumors compared to the untreated control, 5-FU
and metformin combined treatment significantly upregulated the
expression levels of TUNEL-positive cells in mouse tumors than all
other treatment groups or control (Fig. 7).
Discussion
The efficacy of 5-FU in combination with metformin
on OSCC both in vitro and in vivo was shown. In
addition, this study suggests that metformin may enhance the effect
of 5-FU on OSCC through the inhibition of the Warburg effect.
Metformin is an oral hypoglycemic drug that has been
used to treat type 2 diabetes mellitus, and belongs to the
biguanide class. Because of its low cost compared to insulin,
metformin is used worldwide. Epidemiologic studies and
meta-analyses have suggested that type 2 diabetes mellitus patients
have a higher incidence of malignancies in recent years (35–37).
However, a Taiwanese study demonstrated that the increasing trends
of oral cancer may not be ascribed to the increasing incidence of
diabetes over the same period (5).
The type 2 diabetes mellitus patients who received metformin have
not only showed lowered cancer-associated mortality but also
demonstrated decreased tumor incidence (38–41).
Several studies indicated the preventive effects of metformin
against thyroid, bladder, colon, prostate, breast, endometrial,
ovarian and oral cancer in patients with type 2 diabetes mellitus
(20–27). Therefore, effectiveness of
metformin against oral cancer should be evaluated. Available in
vitro and in vivo studies demonstrated that, metformin
could exert growth inhibitory effects on various human cancer cell
types, such as head and neck, pancreas, prostate, breast, stomach
and liver (12,42–46).
It was reported that carcinogenesis can be directly promoted by
insulin resistance and resultant hyperinsulinemia in diabetic
patients and metformin could reduce risk of cancer by maintaining
insulin resistance, blood glucose and insulin levels (47,48).
However, other antidiabetic drug, e.g. pioglitazone improves
insulin resistance in diabetic patients but has a neutral effect
against oral cancer. Therefore, metformin might have other
additional mechanisms of actions against cancer and unlike other
antidiabetic drug it might be useful in the treatment of cancer
(49).
The anticancer effects of metformin are its direct
pleiotropic inhibitory effects on several pathways involved in
survival and metastasis of cancer cells (48). At the cellular level, main
mechanisms of metformin against cancer cells are activation of
AMPK, mediating the PI3K/Akt signaling pathway, inducing G1-phase
arrest with induction of cyclin-dependent kinase inhibitor 1B (p27)
and inhibition of mTOR and HIF-1α (19,50,51).
Metformin can reduce the anti-senescence effects of EMT program in
cancer cells and can inhibit proliferation of CSCs (28). Furthermore, metformin can
potentiate the effect of chemotherapeutic agents or reverse drug
resistance in cancer cells (13,38,52).
Therefore, we examined the possibility of metformin combination
therapy with chemotherapeutic agents available for OSCC in Japan
pre-experimentally. In the preliminary experiment, we observed that
the growth inhibitory effect of 5-FU in combination with metformin
against OSCC cells was significantly higher when compared to
cisplatin, docetaxel or paclitaxel. However, the mechanism of the
growth inhibitory effect of 5-FU in combination with metformin is
still unclear.
In this study, we focused our attention on
inhibition of the Warburg effect by 5-FU in combination with
metformin. In accordance with our expectation, metformin alone
exerted marked inhibitory effect on Warburg effect in OSCC cells,
and in combination with 5-FU it showed more prominent inhibitory
effect on Warburg effect. Briefly, 5-FU in combination with
metformin significantly suppressed the production of lactate
(Fig. 3), and it could reduce the
expression of HIF-1-α, mTOR and Akt1 and induce the expression of
AMPKα than either agent alone (Figs.
4 and 6). Moreover, the level
of inhibition of Warburg effect by 5-FU in combination with
metformin seemed to be relative to the growth inhibitory effect
(Fig. 1), apoptosis inducing
effect (Figs. 2 and 7), and antitumor effect (Fig. 5B). Furthermore, it has been
reported that metformin is well tolerated by patients, it is
absorbed into the body within 1–3 h after oral administration and
90% of it is eliminated by the renal system (28). The combined treatment with 5-FU and
metformin may be an attractive option compared to other cytotoxic
therapeutic agents because in our study, mice treated with 5-FU in
combination with metformin showed no toxicity or significant weight
loss during the treatment when compared to the untreated control
mice (Fig. 5C).
As an OSCC treatment strategy, recently we tend to
select the heavy use of costly molecularly targeted drugs for OSCC
treatment. Instead, a more desirable strategy could be selecting
low cost, effective and less cytotoxic drugs against OSCC. In this
experiment, we showed that FU and metformin combination therapy
effectively suppressed the growth of OSCC cells/tumors and could
modulate the regulator molecules of the Warburg effect in cancer
cells more than our expectations. These findings suggest that 5-FU
and metformin combination treatment might be regarded as a
potential treatment strategy for human OSCC. Future studies should
aim at defining the most appropriate dose and schedule of
administration of this combination treatment.
Acknowledgements
This study was supported in part by a Grant-in-Aid
from the Japanese Ministry of Education, Science and Culture.
References
|
1
|
Yakob M, Fuentes L, Wang MB, Abemayor E
and Wong DT: Salivary biomarkers for detection of oral squamous
cell carcinoma - current state and recent advances. Curr Oral
Health Rep. 1:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Exarchos KP, Goletsis Y and Fotiadis DI: A
multiscale and multiparametric approach for modeling the
progression of oral cancer. BMC Med Inform Decis Mak. 12:1362012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
5
|
Tseng CH: Oral cancer in Taiwan: Is
diabetes a risk factor? Clin Oral Investig. 17:1357–1364. 2013.
View Article : Google Scholar
|
|
6
|
The Oral Cancer Foundation. Oral Cancer
Facts. Website. http://www.oralcancerfoundation.org/facts/.
Access date: February 28, 2016
|
|
7
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
8
|
Zini A, Czerninski R and Sgan-Cohen HD:
Oral cancer over four decades: Epidemiology, trends, histology, and
survival by anatomical sites. J Oral Pathol Med. 39:299–305. 2010.
View Article : Google Scholar
|
|
9
|
Inagi K, Takahashi H, Okamoto M, Nakayama
M, Makoshi T and Nagai H: Treatment effects in patients with
squamous cell carcinoma of the oral cavity. Acta Otolaryngol
(Suppl). 547:25–29. 2002. View Article : Google Scholar
|
|
10
|
Shingaki S, Takada M, Sasai K, Bibi R,
Kobayashi T, Nomura T and Saito C: Impact of lymph node metastasis
on the pattern of failure and survival in oral carcinomas. Am J
Surg. 185:278–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogawa T, Washio J, Takahashi T, Echigo S
and Takahashi N: Glucose and glutamine metabolism in oral squamous
cell carcinoma: Insight from a quantitative metabolomic approach.
Oral Surg Oral Med Oral Pathol Oral Radiol. 118:218–225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng
L, Bhalla KN, Keating MJ and Huang P: Inhibition of glycolysis in
cancer cells: A novel strategy to overcome drug resistance
associated with mitochondrial respiratory defect and hypoxia.
Cancer Res. 65:613–621. 2005.PubMed/NCBI
|
|
14
|
Feron O: Pyruvate into lactate and back:
From the Warburg effect to symbiotic energy fuel exchange in cancer
cells. Radiother Oncol. 92:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Courtnay R, Ngo DC, Malik N, Ververis K,
Tortorella SM and Karagiannis TC: Cancer metabolism and the Warburg
effect: The role of HIF-1 and PI3K. Mol Biol Rep. 42:841–851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Faubert B, Boily G, Izreig S, Griss T,
Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et
al: AMPK is a negative regulator of the Warburg effect and
suppresses tumor growth in vivo. Cell Metab. 17:113–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quinn BJ, Kitagawa H, Memmott RM, Gills JJ
and Dennis PA: Repositioning metformin for cancer prevention and
treatment. Trends Endocrinol Metab. 24:469–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rêgo DF, Pavan LM, Elias ST, De Luca Canto
G and Guerra EN: Effects of metformin on head and neck cancer: A
systematic review. Oral Oncol. 51:416–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng CH: Metformin reduces thyroid cancer
risk in Taiwanese patients with type 2 diabetes. PLoS One.
9:e1098522014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tseng CH: Metformin may reduce bladder
cancer risk in Taiwanese patients with type 2 diabetes. Acta
Diabetol. 51:295–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tseng CH: Diabetes, metformin use, and
colon cancer: A population-based cohort study in Taiwan. Eur J
Endocrinol. 167:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tseng CH: Metformin significantly reduces
incident prostate cancer risk in Taiwanese men with type 2 diabetes
mellitus. Eur J Cancer. 50:2831–2837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tseng CH: Metformin may reduce breast
cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer
Res Treat. 145:785–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tseng CH: Metformin and endometrial cancer
risk in Chinese women with type 2 diabetes mellitus in Taiwan.
Gynecol Oncol. 138:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tseng CH: Metformin reduces ovarian cancer
risk in Taiwanese women with type 2 diabetes mellitus. Diabetes
Metab Res Rev. 31:619–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tseng CH: Metformin may reduce oral cancer
risk in patients with type 2 diabetes. Oncotarget. 7:2000–2008.
2016.
|
|
28
|
Del Barco S, Vazquez-Martin A, Cufí S,
Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B
and Menendez JA: Metformin: Multi-faceted protection against
cancer. Oncotarget. 2:896–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen G, Xu S, Renko K and Derwahl M:
Metformin inhibits growth of thyroid carcinoma cells, suppresses
self-renewal of derived cancer stem cells, and potentiates the
effect of chemotherapeutic agents. J Clin Endocrinol Metab.
97:E510–E520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ling S, Feng T, Ke Q, Fan N, Li L, Li Z,
Dong C, Wang C, Xu F, Li Y, et al: Metformin inhibits proliferation
and enhances chemosensitivity of intrahepatic cholangiocarcinoma
cell lines. Oncol Rep. 31:2611–2618. 2014.PubMed/NCBI
|
|
31
|
Honjo S, Ajani JA, Scott AW, Chen Q,
Skinner HD, Stroehlein J, Johnson RL and Song S: Metformin
sensitizes chemotherapy by targeting cancer stem cells and the mTOR
pathway in esophageal cancer. Int J Oncol. 45:567–574.
2014.PubMed/NCBI
|
|
32
|
Wu X, He C, Wu Y and Chen X: Synergistic
therapeutic effects of Schiff's base cross-linked injectable
hydrogels for local co-delivery of metformin and 5-fluorouracil in
a mouse colon carcinoma model. Biomaterials. 75:148–162. 2016.
View Article : Google Scholar
|
|
33
|
Zhao Q, Wang J, Zou MJ, Hu R, Zhao L,
Qiang L, Rong JJ, You QD and Guo QL: Wogonin potentiates the
antitumor effects of low dose 5-fluorouracil against gastric cancer
through induction of apoptosis by down-regulation of NF-kappaB and
regulation of its metabolism. Toxicol Lett. 197:201–210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rattan R, Graham RP, Maguire JL, Giri S
and Shridhar V: Metformin suppresses ovarian cancer growth and
metastasis with enhancement of cisplatin cytotoxicity in vivo.
Neoplasia. 13:483–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nicolucci A: Epidemiological aspects of
neoplasms in diabetes. Acta Diabetol. 47:87–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Decensi A, Puntoni M, Goodwin P, Cazzaniga
M, Gennari A, Bonanni B and Gandini S: Metformin and cancer risk in
diabetic patients: A systematic review and meta-analysis. Cancer
Prev Res (Phila). 3:1451–1461. 2010. View Article : Google Scholar
|
|
37
|
Rizos CV and Elisaf MS: Metformin and
cancer. Eur J Pharmacol. 705:96–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Currie CJ, Poole CD, Jenkins-Jones S, Gale
EA, Johnson JA and Morgan CL: Mortality after incident cancer in
people with and without type 2 diabetes: Impact of metformin on
survival. Diabetes Care. 35:299–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Landman GW, Kleefstra N, van Hateren KJ,
Groenier KH, Gans RO and Bilo HJ: Metformin associated with lower
cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care.
33:322–326. 2010. View Article : Google Scholar :
|
|
40
|
Ruiter R, Visser LE, van Herk-Sukel MP,
Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings
RM and Stricker BH: Lower risk of cancer in patients on metformin
in comparison with those on sulfonylurea derivatives: Results from
a large population-based follow-up study. Diabetes Care.
35:119–124. 2012. View Article : Google Scholar :
|
|
41
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: A cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nair V, Pathi S, Jutooru I, Sreevalsan S,
Basha R, Abdelrahim M, Samudio I and Safe S: Metformin inhibits
pancreatic cancer cell and tumor growth and downregulates Sp
transcription factors. Carcinogenesis. 34:2870–2879. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akinyeke T, Matsumura S, Wang X, Wu Y,
Schalfer ED, Saxena A, Yan W, Logan SK and Li X: Metformin targets
c-MYC oncogene to prevent prostate cancer. Carcinogenesis.
34:2823–2832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alimova IN, Liu B, Fan Z, Edgerton SM,
Dillon T, Lind SE and Thor AD: Metformin inhibits breast cancer
cell growth, colony formation and induces cell cycle arrest in
vitro. Cell Cycle. 8:909–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The anti-diabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Petrushev B, Tomuleasa C, Soritau O, Aldea
M, Pop T, Susman S, Kacso G, Berindan I, Irimie A and Cristea V:
Metformin plus PIAF combination chemotherapy for hepatocellular
carcinoma. Exp Oncol. 34:17–24. 2012.PubMed/NCBI
|
|
47
|
Viollet B, Guigas B, Sanz Garcia N,
Leclerc J, Foretz M and Andreelli F: Cellular and molecular
mechanisms of metformin: An overview. Clin Sci (Lond). 122:253–270.
2012. View Article : Google Scholar
|
|
48
|
Martin-Castillo B, Vazquez-Martin A,
Oliveras-Ferraros C and Menendez JA: Metformin and cancer: doses,
mechanisms and the dandelion and hormetic phenomena. Cell Cycle.
9:1057–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tseng CH: Pioglitazone and oral cancer
risk in patients with type 2 diabetes. Oral Oncol. 50:98–103. 2014.
View Article : Google Scholar
|
|
50
|
Takiyama Y, Harumi T, Watanabe J, Fujita
Y, Honjo J, Shimizu N, Makino Y and Haneda M: Tubular injury in a
rat model of type 2 diabetes is prevented by metformin: A possible
role of HIF-1α expression and oxygen metabolism. Diabetes.
60:981–992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tanaka R, Tomosugi M, Horinaka M, Sowa Y
and Sakai T: Metformin causes G1-phase arrest via down-regulation
of MiR-221 and enhances TRAIL sensitivity through DR5 up-regulation
in pancreatic cancer cells. PLoS One. 10:e01257792015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ashinuma H, Takiguchi Y, Kitazono S,
Kitazono-Saitoh M, Kitamura A, Chiba T, Tada Y, Kurosu K, Sakaida
E, Sekine I, et al: Antiproliferative action of metformin in human
lung cancer cell lines. Oncol Rep. 28:8–14. 2012.PubMed/NCBI
|