Introduction
Patients affected by PDAC have an extremely poor
prognosis, with a 5-year survival rate of only ~ 4–5% and a mean
survival of 4–6 months. Surgical resection is the only treatment
with curative intent, but patients with resectable PDAC are in the
minority (1). Upon tumor
resection, the 5-year survival rate increases to approximately 15%,
while 25% survival is attained from treatment with adjuvant
chemotherapy (2). There are many
reasons for such a poor prognosis, including rapid tumor
dissemination and late diagnosis (3), due to the lack of early,
disease-specific symptoms. One of the most important features of
PDAC is the early occurrence of immune suppression that affects the
T cell-mediated antitumor response. In general, immune suppression
is manifested in two different ways: firstly, by immune suppressive
cells, such as regulatory T cells (Tregs) and myeloid-derived
suppressor cells (MDSC) and secondly, by surface regulatory
molecules, such as CTLA-4 and PD-L1. In human PDAC, B7-H1
expression was upregulated and correlated strongly with poor
patient prognosis (4) while a
decrease in the number of CD4+ T cells expressing CTLA-4
is associated with improved and disease-free survival of PDAC
patients (5).
However, different studies report an antitumor
immune response in PDAC patients, for example, accumulation of
CD8+ T cells correlates with increased survival of
patients (5–7), supporting the protective role of the
immune system against developing tumors. In fact, usually, the
PD-L1 expression has a significant inverse correlation with
CD4+ TILs and CD8+ TILs (8,9).
In cancer immunology, the balance of co-stimulatory
or inhibitory responses is an important underlying mechanism in the
function of T cell-mediated anticancer immunity.
In particular, the PD-1/PD-L1 axis is one of the
prominent immune checkpoints, which are known as the inhibitory
mechanisms of immune responses. The linkage of PD-L1 of the tumor
cells with PD-1 expressed on the activated tumor-infiltrating
lymphocytes (TILs), induces a co-inhibitory signal and decreases
the level of cytokines, which subsequently results in the anergy
and apoptosis of the effector T lymphocytes and to a poor patient
prognosis (10). Thus, although
CD8+ T cells were thought to be the effective antitumor
T-cell subset, some studies did not find any correlation between
CD8+ T-cells and survival rate in patients with
pancreatic cancer (11).
The immune response elicited by the deregulated
expression of various tumor-associated antigens (TAA) has been
demonstrated in different cancers (12,13),
including PDAC, as shown by serological profiling that identified
29 preferentially immunogenic proteins in the serum of PDAC
patients (14,15). Numerous data have demonstrated that
PDAC patients generate T/B cells against TAA (16–22),
including α-enolase (ENO1) which we have previously described
(23,24) and that patients with anti-TAA
antibodies had a better survival rate than the antibody-negative
patients (25,26). Vaccination against ENO1, a
glycolytic enzyme that plays a role in cell migration and cancer
metastasis (27), delays tumor
progression and significantly extends survival in a mouse model of
PDAC (28). As ENO1 can be
considered as a promising candidate for immunotherapy in PDAC,
information on the antitumor function of ENO1-specific T cells from
peripheral blood lymphocytes may provide a prognostic tool.
Naive CD4+ T cells differentiate into
mature Th1, Th2, Th17 or Tregs. Th1 cells play an effective
anticancer role, while Th2, and especially Tregs, suppress immune
responses and induce tolerance at tumor sites, particularly in PDAC
(29,30). There are conflicting reports,
however, on the role of Th17 cells in cancer (31), with some studies reporting that
higher levels of Th17 cells correlate with advanced cancer
(32,33), while others suggest that Th17 may
have a potent antitumor effect (34).
The aim of the present study was to characterize the
phenotypic and functional properties of ENO1-specific T-cells
isolated from the blood of PDAC patients, evaluating their
anticancer functional role and to ascertain whether they are able
to recirculate from the tumor to the periphery and vice versa.
Finally, we correlated the presence of peripheral anti-ENO1 T cells
with patient survival.
Materials and methods
Patients
For the present study, we used PBMCs (peripheral
blood mononuclear cells) from 15 patients (8 males and 7 females)
with pancreatic ductal adenocarcinoma (according to the TNM
classification for pancreatic tumors) (35), who were already enrolled in a
previous study approved by the local Ethics Committee (24). The age of PDAC patients ranged from
36 to 92 years (mean age, 63 years). All patients underwent
surgical resection of the primary lesion but did not receive
chemotherapy. Patients with evidence of serious illness,
immunosuppression, autoimmune or infectious diseases were excluded.
Characteristics of PDAC patients and ENO1 expression of PDAC tissue
are summarized in Table I. For
comparison with healthy controls, 15 blood donors (8 males and 7
females, mean age 60) were studied.
 | Table IPatient characteristics and
ENO1-reactivity of T cell clones (Tcc) generated from blood of
patients with pancreatic ductal adenocarcinoma. |
Table I
Patient characteristics and
ENO1-reactivity of T cell clones (Tcc) generated from blood of
patients with pancreatic ductal adenocarcinoma.
| Code | Overall survival
(mo) | Tumor grade | CA19-9 (U/ml) | ENO1
expression | Anti-ENO1 IgG | ENO1-PBMC
proliferation | Peripheral Tcc | Peripheral
ENO1-specific Tcc (%) |
|---|
| 01.PCp | 11 | Well | 400±21 | + | ++ | Y | 16 | 4 (25) |
| 02.PCp | 13 | Moderate | 420±28 | ++ | ++ | Y | 20 | 5 (25) |
| 03.PCp | 8 | Poor | 570±35 | + | + | N | 0 | - |
| 04.PCp | 7 | Well | 850±62 | +++ | + | N | 10 | 2 (20) |
| 05.PCp | 9 | Well | 1100±42 | +++ | + | Y | 15 | 3 (20) |
| 06.PCp | 8 | Moderate | 1250±31 | ++ | + | N | 0 | - |
| 07.PCp | 12 | Well | 210±15 | ++ | ++ | Y | 15 | 5 (33) |
| 08.PCp | 8 | Moderate | 830±68 | +++ | + | Y | 10 | 2 (20) |
| 09.PCp | 14 | Poor | 150±45 | +++ | +++ | Y | 20 | 8 (40) |
| 10.PCp | 9 | Poor | 1350±89 | + | - | N | 0 | - |
| 11.PCp | 10 | Well | 135±24 | + | ++ | Y | 15 | 6 (40) |
| 12.PCp | 11 | Poor | 216±21 | ++ | ++ | Y | 14 | 4 (29) |
| 13.PCp | 8 | Well | 897±57 | +++ | + | N | 10 | 2 (20) |
| 14.PCp | 12 | Moderate | 235±18 | + | ++ | Y | 12 | 4 (33) |
| 15.PCp | 13 | Well | 326±35 | +++ | +++ | Y | 24 | 9 (38) |
| All PDAC
patients | 10.2 | Well (47%) | 384.6±39 | 15/15 | 14/15 (93%) | 10/15 (67%) | 181 | 54 (30%) |
Reagents
T cell cultures were carried out in RPMI-1640
culture medium (SERO-Med GmbH, Vienna, Austria) supplemented with
10% FCS HyClone Laboratories (Gibco Laboratories, Grand Island, NY,
USA) and recombinant human interleukin-2 (IL-2) (Eurocetus, Milan,
Italy). Unlabeled or fluorochrome-conjugated anti-CD3, CD4, CD8,
CD25, and isotype-matched control mAbs were purchased from BD
Biosciences (San Jose, CA, USA). Fluorochrome-conjugated
anti-IL-10, anti-TGF-β and anti-FoxP3 mAbs were purchased from
eBioscience (San Diego, CA, USA).
Production of recombinant
histidine-tagged a-enolase and detection of serum anti-ENO1
immunoglobulin G (IgG)
Recombinant α-enolase was overexpressed in the E.
Coli strain BL21 (DE3)/pLysS (kindly provided by A. Giallongo,
Institute of Biomedicine and Molecular Immunology, National
Research Council, Palermo, Italy) and was produced as previously
reported (23). Anti-ENO1 IgG
levels were measured by an in-house ELISA kit (23).
Detection of CA19-9 marker
The fasting venous blood (2 ml) at the early morning
of the study objects was drawn and the serum was centrifuged and
stored at −20°C until assayed. The electrochemiluminescence
immunoassay method was used for detecting the levels of CA19-9. The
procedures were conducted strictly according to the instructions of
kits. The critical value of CA19-9 was 37 U/ml.
Immunohistochemical analysis of ENO1
Immunohistochemical analysis on 15 PDAC tissues was
performed with an anti-α-enolase mAb (1:100; clone 19/12) followed
by a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
(Santa Cruz Biotechnology, Celbio s.p.a., Milan, Italy) and DAB
detection kit (Ventana Medical Systems, Inc., Tucson, AZ, USA).
Samples were fixed with formalin and paraffin-embedded. Slides were
incubated with the primary and secondary Ab and developed in a
Ventana ES automated stainer. Stained sections were counterstained
with hematoxylin.
All images were taken with a x60 objective. Images
were acquired on a Leica DM LA upright microscope equipped with a
DC300F camera and were analyzed with IM50 software (Leica
Microsystems, Heidelberg, Germany).
Detection of ENO1-specific T cells in the
peripheral blood of patients with PDAC
To assess the presence of ENO1-specific T cells in
the peripheral blood of PDAC patients, PBMCs obtained by density
gradient centrifugation on Ficoll-Hypaque were re-suspended in
medium supplemented with 3% human serum. PBMCs (3×105)
were cultured for 96 h in the presence of medium alone or ENO1 (10
μg/ml). At 16 h before harvesting, 0.5 μCi/well
[3H]-thymidine (Amersham Pharmacia Biotech, Uppsala,
Sweden) was added, and the radionuclide uptake was measured by a
β-counter. The mitogenic index (MI) was calculated as the ratio
between mean values of cpm (counts per minute) obtained in
ENO1-stimulated cultures and those obtained in the presence of
medium alone. An MI ≥3 was considered as a positive result.
Generation of ENO1-specific T cell lines
and T cell clones (Tcc)
ENO1-specific T cell lines were generated as
previously described (25).
Briefly, 106 PBMCs in 2 ml RPMI-1640 medium supplemented
with 2 mM L-glutamine, 2×10−5 M 2-ME, and 5% human serum
(complete medium) were stimulated with ENO1 (10 mg/ml) in 24-well
flat-bottomed plates for 5 days. Then, human IL-2 (30 U/ml) was
added, and cultures were maintained for 7 days. Viable T-cell
blasts were re-suspended in complete medium and cloned under
limiting dilution, as previously described (36). Briefly, single T cell blasts were
seeded in microwell plates (0.3 cells/well) in the presence of
2×105 irradiated (9000 rad) PBMCs, phytohemagglutinin
(PHA) (0.5% vol/vol) and IL-2 (50 U/ml). At weekly intervals,
2×105 irradiated PBMC and IL-2 were added to each
microculture to maintain the expansion of growing clones.
Tcc were screened for responsiveness to ENO1 by
measuring [3H]-thymidine uptake after a 60-h co-culture
with irradiated autologous mononuclear cells in the presence of
medium or ENO1 (10 mg/ml). An MI ≥3 was considered as a positive
result.
To verify the clonality of ENO1-specific Tcc, the
repertoire of the T cell receptor (TCR) Vβ chain of single Tcc was
analyzed with the TCR Vβ Repertoire kit (Beckman Coulter,
Fullerton, CA, USA); isotype-matched non-specific IgG were used as
negative controls and the T cell blasts were gated by anti-CD3
monoclonal antibody.
Characterization of the cytokine profile
of Tcc and analysis of surface/intracytoplasmic cell markers
We analyzed surface marker (CD3, CD4 and CD8)
expression in blasts of single Tcc by flow cytometry as previously
described (37), along with the
production of the intracellular marker (FoxP3) and cytokines (IL-10
and TGF-β) of ENO1-specific Tcc (unable to secrete IFN-γ, IL-4 and
IL-17). For intracellular analysis, blasts were stimulated with PMA
(phorbol myristate acetate) (10 ng/ml) plus ionomycin (200 ng/ml),
and stained with anti-IL-10, anti-TGF-β and anti-FoxP3 mAbs. Tcc
that were negative for FoxP3, IL-10 and TGF-β were defined as T
null, and Tcc that were positive for FoxP3, IL-10 and TGF-β were
defined as Tregs.
To evaluate the amount of secreted cytokines, T cell
blasts of single Tcc were resuspended at a concentration of
106 cells/ml of medium and cultured for 36 h in the
presence of PMA (10 ng/ml) plus ionomycin (200 ng/ml). Cell-free
supernatants were collected and assayed in duplicate for IFN-γ,
IL-4 and IL-17 content by commercial ELISA kits (BioSource
International, Camarillo, CA, USA). Supernatants presenting
cytokine levels that were 5 SD above the mean levels in control
supernatants derived from irradiated APCs alone were considered
positive. Based on the cytokine profile and the CD4/CD8 expression
evaluation, we divided the clones into the following groups:
Th1/Tc1 (only IFN-γ), Th2/Tc2 (only IL-4), Th0/Tc0 (IFN-γ and IL-4)
and Th17/Tc17 (IL-17).
B-EBV cell preparation
B cells were prepared using the B cell isolation kit
II (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). To obtain
EBV-transformed lymphoblastoid B cell lines (EBV-B cells),
autologous B cells from each patient were incubated for 48 h with
EBV-producing marmoset B95.8 cell line supernatants, and
subsequently expanded in complete medium supplemented with 15%
FCS.
Perforin-mediated cytotoxicity and
Fas-Fas ligand (L)-mediated proapoptotic activity
Perforin-mediated cytolytic activity of Tcc was
assessed as previously reported (24). T cell blasts of ENO1-specific T
clones were incubated at an effector-to-target ratio of 10-, 5- and
2.5 to-1 with 51Cr-labeled autologous (EBV)-B cells
pre-incubated with ENO1 (1 μg/ml). After centrifugation,
microplates were incubated for 8 h at 37°C, and 0.1 ml supernatant
was removed from each microculture for measuring 51Cr
release. To evaluate whether Tcc-specific cytotoxicity against
target cells was ENO1/HLA-dependent, cytotoxicity tests were also
carried out under two other different conditions: i) with
ENO1-pulsed 51Cr-labeled autologous EBV-B cells in the
presence of anti-HLA-DR blocking mAbs (10 mg/ml) to evaluate the
ENO1-specific CD4+ Tcc; ii) in the presence of
anti-HLA-A-B-C to assess the ENO1-specific CD8+ Tcc.
Anti-HLA-DR and anti-HLA-A-B-C mAbs were purchased from Immunotech
(Beckman Coulter, Marseille, France).
The ability of ENO1-specific Tcc to induce
Fas-FasL-mediated apoptosis was assessed using Fas+
Panc1 cells as targets. T cell blasts from each clone were
co-cultured with 51Cr-labeled Panc1 cells at an
effector-to-target ratio of 10-, 5- and 2.5- to-1 for 18 h in the
presence of PMA (10 ng/ml) and ionomycin (200 ng/ml), as previously
reported (24). To block the
Fas-FasL interaction, the anti-Fas antagonistic monoclonal antibody
M3 (Amgen, Thousand Oaks, CA, USA) was used, at a final
concentration of 5 mg/ml, for a 30-min pre-treatment of
51Cr-labeled Panc1 cells, as reported (24).
Suppressive assays
To assess the ability of antigen-specific Treg
clones to suppress the antigen-induced autologous cell
proliferation of ENO1-specific effector Tcc, 2×104 of
these blasts were cultured with 4×103 irradiated (9000
rad) autologous ENO1-loaded or TT (Tetanus toxoid)-loaded dendritic
cells (DCs) in the presence of 2×104 cells of
ENO1-specific Treg. DCs were obtained using the Blood Dendritic
Cell Isolation kit II (Miltenyi Biotec). At day 4, after 8 h of
pulsing with 0.5 mCi 3H-TdR/well (Amersham, Little Chalfont, UK),
cultures were harvested and the radionuclide uptake was measured by
β-counter analysis. Tetanus toxoid was used for specificity control
of T cell clones.
DNA extraction and TCR Genescan analysis
of TCRβ and TCRγ rearrangement
Genomic DNA was extracted from ENO1-specific T cell
clones obtained from three tumor samples and three peripheral blood
samples of the same patient using the Maxwell 16 Tissue DNA
Purification kit and the Maxwell 16 Instrument (Promega, Madison,
WI, USA).
TCR gene rearrangement analysis was performed using
a gene clonality assay based on the BIOMED-2/Euroclonality
multiplex-PCR protocol (38),
known as the IdentiClone TCRβ TCRγ T-Cell Clonality Assa-ABI
Fluorescence Detection (Invivoscribe, San Diego, CA, USA). The
assay consists of five tubes each containing primers that target
the TCRβ (tubes A-B-C) and the TCRγ (tubes D–E) chain locus.
Briefly, 100 ng of genomic DNA per tube was subjected to PCR
amplification according to the manufacturer's instructions. PCR
products were run on an ABI 3130 Genetic Analyzer and Genescan
profiles were obtained using GeneMapper (version 3.2) software
(Applied Biosystems, Foster City, CA, USA).
Statistical analysis
Statistical analysis of the mitogenic index was
evaluated by the unpaired two-tailed Student's t-test. A P-value
<0.05 was considered statistically significant. A two-tailed
Pearson r coefficient was used to correlate the titer of
anti-ENO1 IgG and the percentage of ENO1-specific T clones obtained
from PDAC patients. The correlation between the number of
ENO1-specific Tcc and survival of patients and hazard ratio (HR)
were evaluated by Kaplan-Meier analysis (log-rank Mentel-Cox
test).
Results
ENO1-specific T cells in the peripheral
blood of patients with PDAC
In order to assess the presence of ENO1-specific T
cells in the peripheral blood of PDAC patients, PBMCs from 15
patients, which were previously characterized for the presence of
tumor infiltrating T cells, (16)
were cultured for 5 days in the presence of medium alone or
recombinant ENO1. Notably, PBMCs from 10 of these PDAC patients
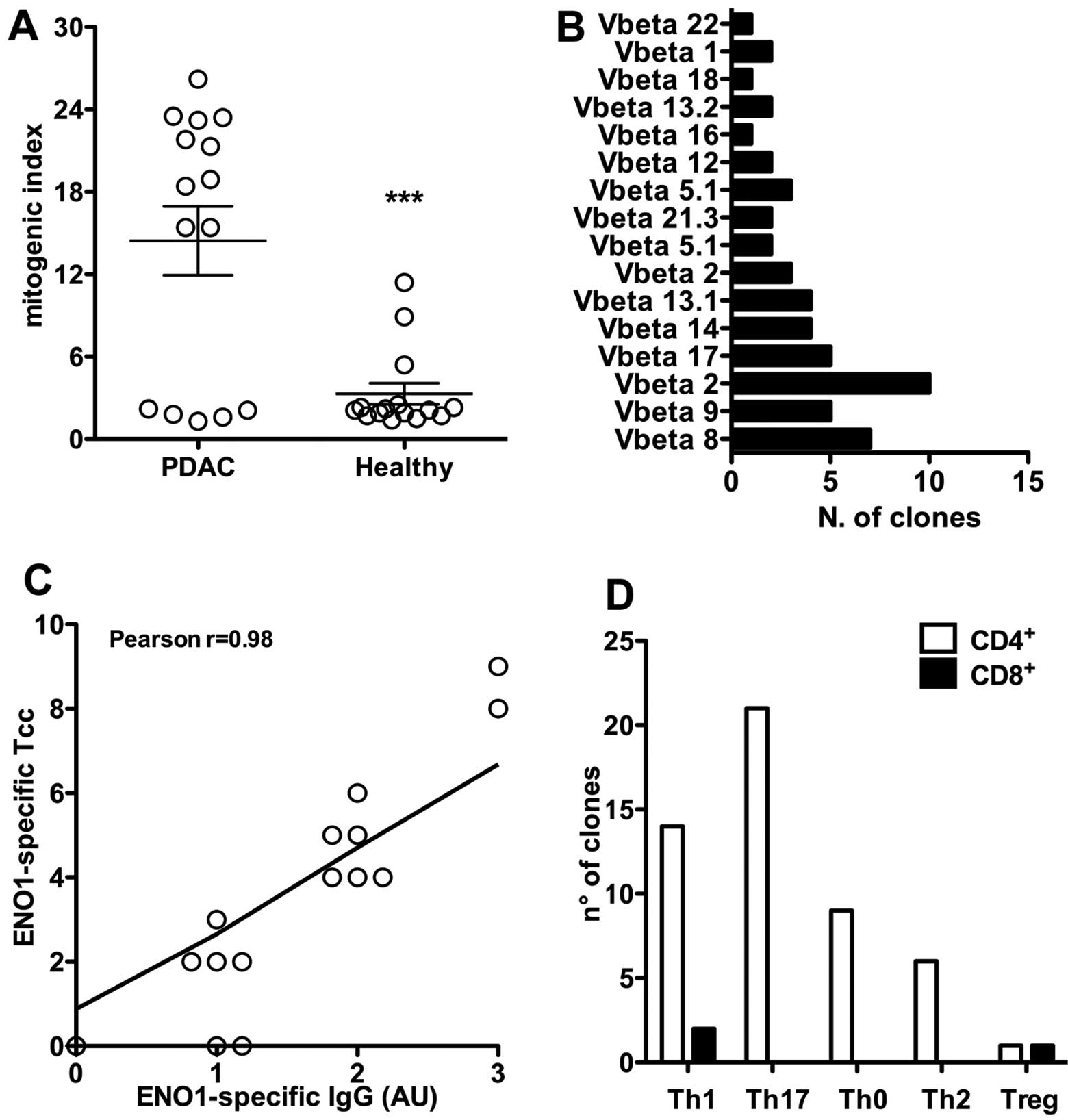
(67%) (Table I and Fig. 1A) proliferated in the presence of
ENO1. Conversely, in the healthy controls, only 3 cases out of 15
(20%) showed proliferation in response to ENO1. In addition, the
proliferation of PBMC of PDAC patients showed a significantly
greater mitogenic index (mean MI ± SEM, 14.4±2.5) with respect to
that of the three healthy controls (mean MI ± SEM, 3.3±0.8;
P=0.0002).
Characterization of Tcc isolated from the
blood of PDAC patients and reactivity to ENO1
As five PDAC patients did not have detectable
ENO1-specific T cells in their peripheral blood, but four out of
these five patients did, however, present ENO1-specific
tumor-infiltrating T cells (16)
we expanded and cloned peripheral blood lymphocytes, by IL-2, from
all PDAC resectable patients; we obtained 181 Tcc from all PDAC
patients except patients 3, 6 and 10 (Table I).
There were 88% (159/181) of CD4+ Tcc and
12% of CD8+ Tcc (22/181). Interestingly, out of 22
CD8+ Tcc, 7 were isolated from the blood of patient 5
Pcp, who was reported as having the highest number of
CD8+ Tcc.
As ENO1 is a TAA that induces an integrated humoral
and cellular response in PDAC patients (15). Tcc obtained were assayed for
proliferation in response to ENO1, and 30% of the Tcc was (54/181,
Table I) proliferated upon
stimulation with ENO1. With the exception of three cases (Pcp
3,6,10), we obtained ENO1-specific Tcc from all patients; in
particular patients 9 and 15 were recorded as having the greatest
number of Tcc (8 and 9, respectively).
Clonality was confirmed by cytofluorimetric analysis
of TCR-Vβ expression. Each Tcc was only revealed by one of the
TCR-Vβ chain-specific monoclonal antibodies, showing a single peak
of fluorescence intensity (Fig.
1B).
We have already shown that all patients (except for
patient PCp10) had detectable serum anti-ENO1 IgG (Table I). Interestingly, we observed that
patients with higher titers of anti-ENO1 antibodies also showed the
highest percentage of ENO1-specific T clones (r=0.8745 and P-value
<0.0001) (Fig. 1C);
particularly patients 9 and 15.
Of the ENO1-specific Tcc, 51 were CD4+,
21 (41%) produced IL-17 (Th17) and among these 12 (24%) secreted
both IL-17 and IFN-γ (Fig. 1D). A
total of 15 ENO1-specific CD4+ Tcc secreted IL-4, either
alone (6 Th2, 12%) or with IFN-γ (9 Th0, 18%). Finally, 14 (27%)
ENO1-specific CD4+ Tcc produced only IFN-γ, showing a
Th1 profile. Notably, taken together, of the 14 Th1 clones and the
12 Th17 clones producing IFN-γ and the 9 Th0 Tcc, we found that the
majority of ENO1-specific CD4+ Tcc (%) were able to
produce IFN-γ. The last ENO1-specific CD4+ Tcc was
FoxP3+ and produced IL-10 and TGF-β, showing a Treg
profile. Of the remaining three ENO1-specific CD8+ Tcc,
one had a regulatory profile (defined as Tcreg) and two had a Th1
profile (defined as Tc1) (Fig.
1D).
Functional properties of ENO1-specific T
cell clones
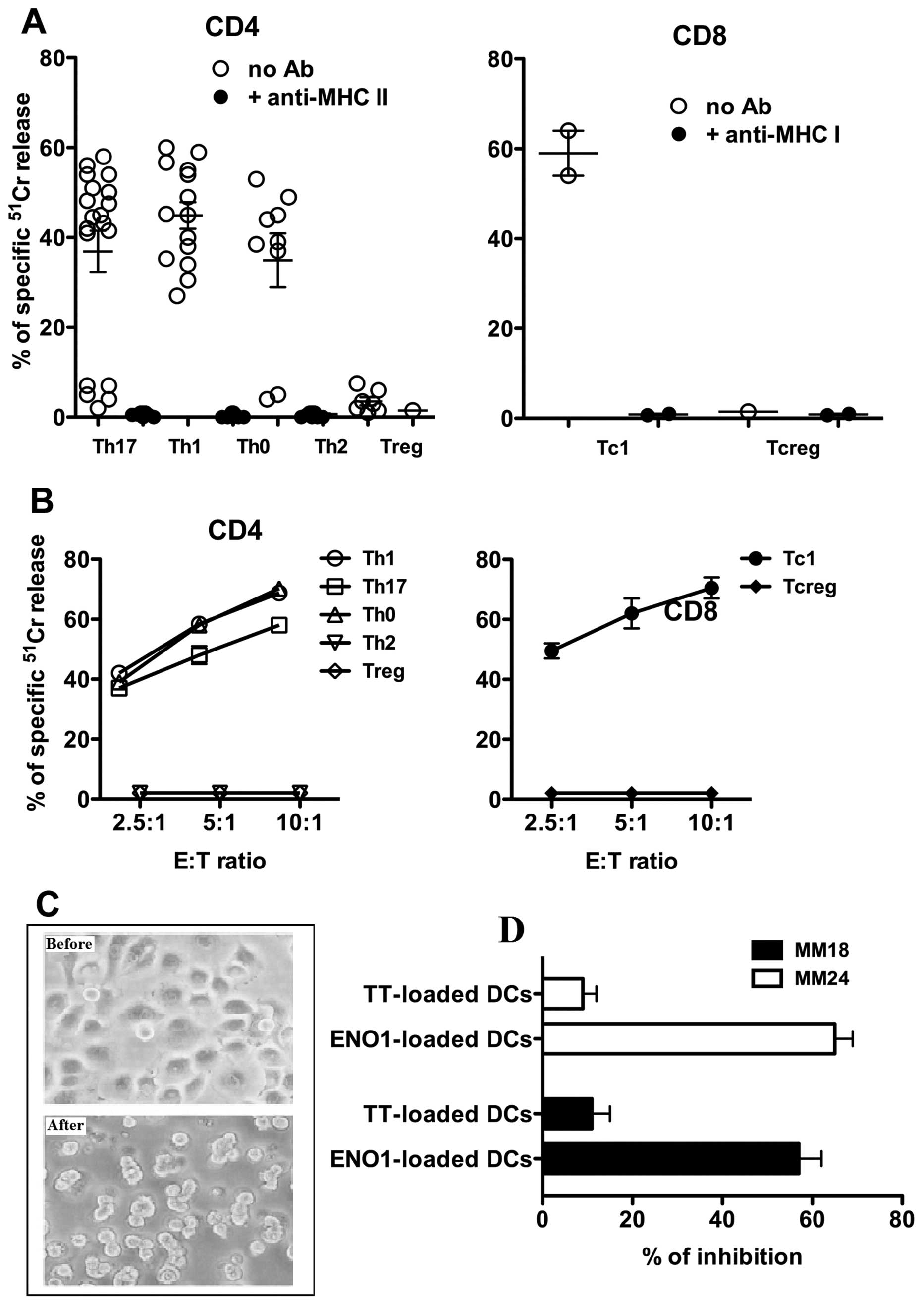
To evaluate the anticancer properties of the
ENO1-specific Tcc isolated from the peripheral blood of PDAC
patients, we assessed their cytotoxicity using ENO1-pulsed
51Cr-labeled autologous EBV-B cells as targets. At an
effector-to-target ratio of 10:1, all 14 Th1, and 7 out of 9 Th0
clones were shown to lyse their targets (Fig. 2A). Among the Th17 population, only
the 12 Tcc IL-17/IFN-γ double producers were able to kill their
target cells.
None of the six Th2 or the ENO1-specific Treg Tcc
lysed the EBV-B cells, while only the two ENO1-specific Tc1
CD8+ clones killed the EBV-B cells.
As expected, in the presence of anti-HLA class II
blocking mAbs, all the ENO1-specific CD4+ Tcc were
unable to kill the EBV-B cells, and similar results were obtained
for the two ENO1-specific Tc1 in the presence of anti-HLA class I
blocking mAbs (Fig. 2A).
Effector T cells can kill their targets by
Fas-FasL-mediated apoptosis, and therefore, the ability of
ENO1-specific Tcc to induce 51Cr release by
Fas+ Panc1 cells was evaluated. Upon mitogen activation,
57% of CD4+ (29/51) and 66% of CD8+ (2/3)
ENO1-specific Tcc induced apoptosis in target cells (Fig. 2B). Among the CD4+ Tcc,
14 were Th1, 12 were Th17, and 3 were Th0, but none were Th2 or
Treg (Fig. 2B). The role of the
Fas-FasL interaction in 51Cr release was confirmed by
its inhibition (range, 38.3–57.6%) using an anti-Fas antibody (data
not shown).
Finally, we evaluated the ability of the unique
ENO1-specific Treg Tcc (Tcc 6) to inhibit the proliferation of
ENO1-effector Tcc (Th1 and Th17) obtained from the same patient
(Pcp 8). As shown in Fig. 2C, the
ENO1-specific Treg clone inhibited proliferation of both
ENO1-specific effector Tcc: the Th1 (Tcc 18) and the Th17 (Tcc
24).
TCR analysis of ENO-1 specific Tcc from
peripheral blood and tumor areas
As ENO1-specific Tcc was detected in both the
peripheral blood and tumor areas in 73% of the analyzed PDA
patients (24), we tested the
hypothesis that the same T cell clones were circulating from the
periphery to the tumor area and vice versa. We reasoned that if at
least one Tcc from the periphery and one Tcc from the tumor area of
the same PDAC patient showed the same rearrangement of their TCR,
this would provide a proof of concept that the same T lymphocyte
was able to recirculate from the tumor to the peripheral blood and
vice versa.
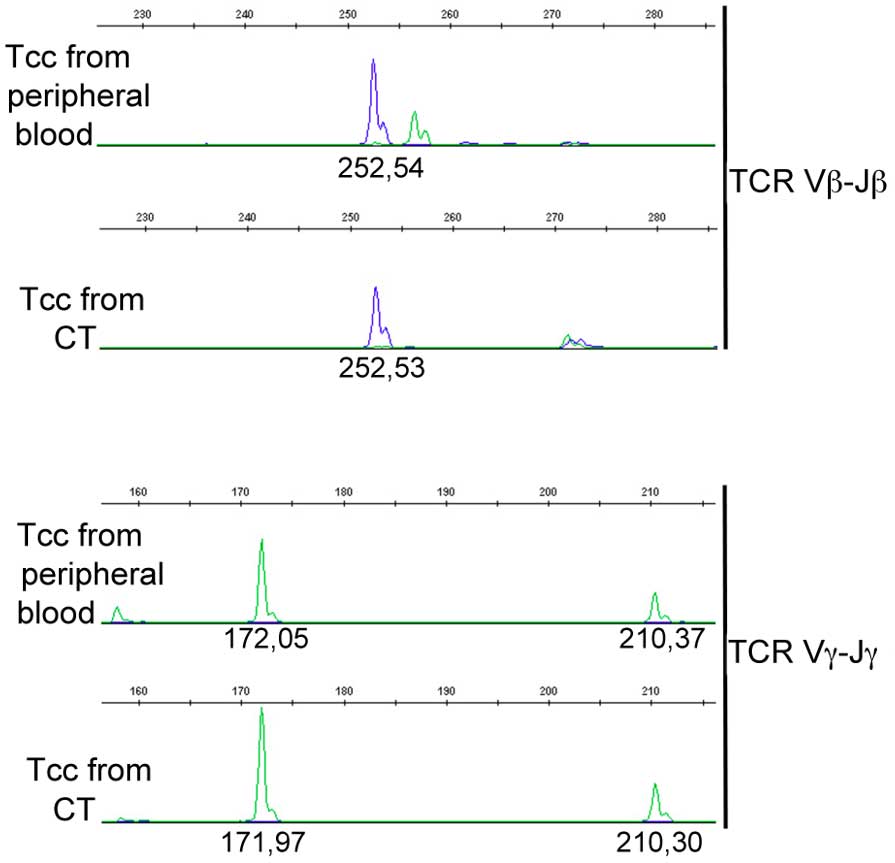
Three ENO1-specific Tcc (1Th1, 1 Th17 and 1 Tc1)
from the blood and from the central tumor area of the same PDAC
patient (Pcp9) were analyzed for TCRβ and γ chain rearrangements by
using a classical gene clonality assay (36). Results confirmed the identity of
the CD8+ Tcc with a Tc1 profile from blood and from
tumors (Fig. 3).
Prognostic role of peripheral
ENO1-specific T cell clones
Because the majority of ENO1-specific Tcc
specifically killed pancreatic cancer cells and were able to
re-circulate from the periphery to the tumor and vice versa, we
evaluated if the number of peripheral ENO1-specific Tcc correlated
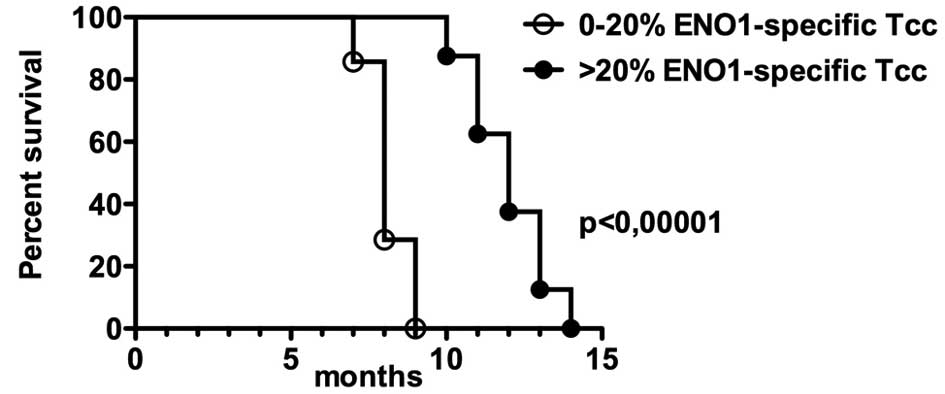
with patient survival from diagnosis.
For this purpose, we divided the patients into two
groups based on the percentage of peripheral ENO1-specific Tcc and
on serum anti-ENO1 specific antibodies: i) <20, and ii) >20
(Table I). Kaplan-Meier analysis
revealed that the patients of group ii showed a better survival
compared to group i (P<0.0001, Hazard Ratio=31) suggesting a
positive correlation between the percentage of circulating
ENO1-specific cells and a longer survival (Fig. 4). In addition, the levels of serum
CA19-9 (Table I) in patients of
group I were significantly (P<0.0001) higher than those in PDAC
patients of ii group (data not shown). On the contrary, the PDAC
expression of ENO1 is similar in the different patients of the two
groups. PDAC tissues diffusely expressed α-enolase in the cells
lining the neoplastic ducts infiltrating the stroma. In other
words, Enolase is overexpressed in most of PC tissues, and to
discriminate between high and lower overexpression is difficult.
Thus, it is not possible to achieve a correlation between the
expression of Enolase and the presence of specific ENO1-specific
Tcc.
Discussion
ENO1 is a PDAC-associated antigen, capable of
eliciting an anti-PDAC T cell response in situ, as
demonstrated in a previous study, where we characterized the
effector functions of ENO1-specific T-cells isolated from the tumor
tissue of PDAC patients (24). We
showed that ENO1-specific Th1/Th17 cells have a specific anticancer
effector function, but they accumulate in the healthy tissue
surrounding the cancer cells, where, conversely, there are elevated
levels of ENO1-specific Tregs (24). Based on these observations, we
hypothesize that the tumor microenvironment negatively influences
the cytolytic activity of T lymphocytes by promoting the
recruitment of specific Treg cells. To investigate this hypothesis,
we characterized the ENO1-specific peripheral T cell response in
the same previously-enrolled PDAC patients (24) to ascertain whether or not the T reg
pool was still maintained.
Firstly, we showed that PBMCs from ten PDAC patients
were able to proliferate in vitro in the presence of ENO1,
while only three healthy controls reacted upon antigen stimulation.
This indicates that most PDAC patients have ENO1-specific T cell
precursors in their peripheral blood, which can be triggered by
in vitro ENO1 re-stimulation. The presence of the anti
ENO1-precursor appears to be PDAC-specific as very few healthy
subjects displayed peripheral blood lymphocyte proliferation in
response to ENO1 and also, a significantly lower index of
proliferation.
To gain information on the functional status of
anti-ENO1-specific peripheral T lymphocytes, we obtained
ENO1-induced T cell lines and subsequently generated Tcc from all
PDAC patients, with the exception of three patients (Pcp 3, 6 and
10). Notably, none of the PBMCs isolated from these three patients
proliferated in the presence of ENO1, even though ENO1-specific Tcc
were obtained from the tumor area in two (Pcp 3 and 10) out of the
three patients (24). The lack of
ENO1-specific T cells in the peripheral blood of these patients, is
probably due to a difference in the migratory pattern of
tumor-specific T cells (39), or
to differences in the tumor microenvironment, in cytokine/chemokine
secretion (40) or altered protein
expression (such as integrins), which circumscribe the immune
response in situ (41,42).
In addition, we did not analyze ENO1 expression in
the tumor tissue of our patients, and we therefore cannot exclude
the possibility that the absence of a T-cell response in one
patient may result from reduced levels of ENO1 expression. We have
shown, in fact, that the anti-ENO1 humoral and cellular responses
are induced as a consequence of enhanced ENO1 expression in PDAC
tissues (24).
However, the most important result is that the
peripheral anticancer response is a ‘mirror’ of the intratumoral
PDAC-specific response, clearly demonstrated as the majority of
patients displayed both immune responses with only two exceptions.
Thus, analysis of the Tcc of the peripheral immune response in PDAC
may be an adequate method to determine if a patient will have a
cancer-specific immune response in situ.
The peripheral blood of PDAC patients has been shown
to display serum anti-ENO1 IgG autoantibodies (26) and here, we provide the first
evidence that, in PDAC patients, the overall percentage of
peripheral ENO1-specific T cells, reaches one third of the total
Tcc obtained.
With the exception of one patient (Pcp10), all
patients had detectable serum anti-ENO1 IgG, and interestingly,
those with higher titers had the highest percentage of
ENO1-specific T clones, in particular patients 9 and 15.
Remarkably, out of the 54 ENO1-specific Tcc
obtained, 51 were CD4+ and the vast majority of them
(69%) displayed a Th1/Th17 profile, thus conferring to them a
potential anti-cancer role (43–46).
Of the remaining CD8+ Tcc, one displayed a Treg profile
and two were Tc1.
Previously, we have confirmed the immune-suppressive
role of the PDAC microenvironment, demonstrating that intratumoral
ENO1-specific CD4+ T cells displayed an increased number
of T null cells and a Treg phenotype (24). On the contrary, the present data
demonstrate that, within the same patients, circulating
ENO1-specific Tcc showed a different cytokine profile compared to
Tcc isolated from the tumoral tissue, with an increased IFN-γ
secretion and a decreased regulatory profile. In agreement with
these findings, we have also demonstrated that the few Tc1 and all
the ENO1-specific IFN-γ-producing CD4+ Tcc had cytotoxic
activity and most of them induced apoptosis in target cells,
showing an effective anticancer role.
Of particular relevance, the analysis of TCR
rearrangements of some ENO1-specific peripheral and intratumoral
Tcc from the same PDAC patient confirmed the ability of
tumor-infiltrating lymphocytes to circulate. Therefore, cytotoxic
ENO1-specific Tcc that reach the tumor could be subsequently
modified and switched off by different immunosuppressive mechanisms
including PD-1/PD-L1 (41,42). However, because of the retained
cytotoxic ability and potential antitumor function of peripheral
ENO1-specific Tcc, they could be very important for the elimination
of cancer-circulating cells and prevention of metastasis and
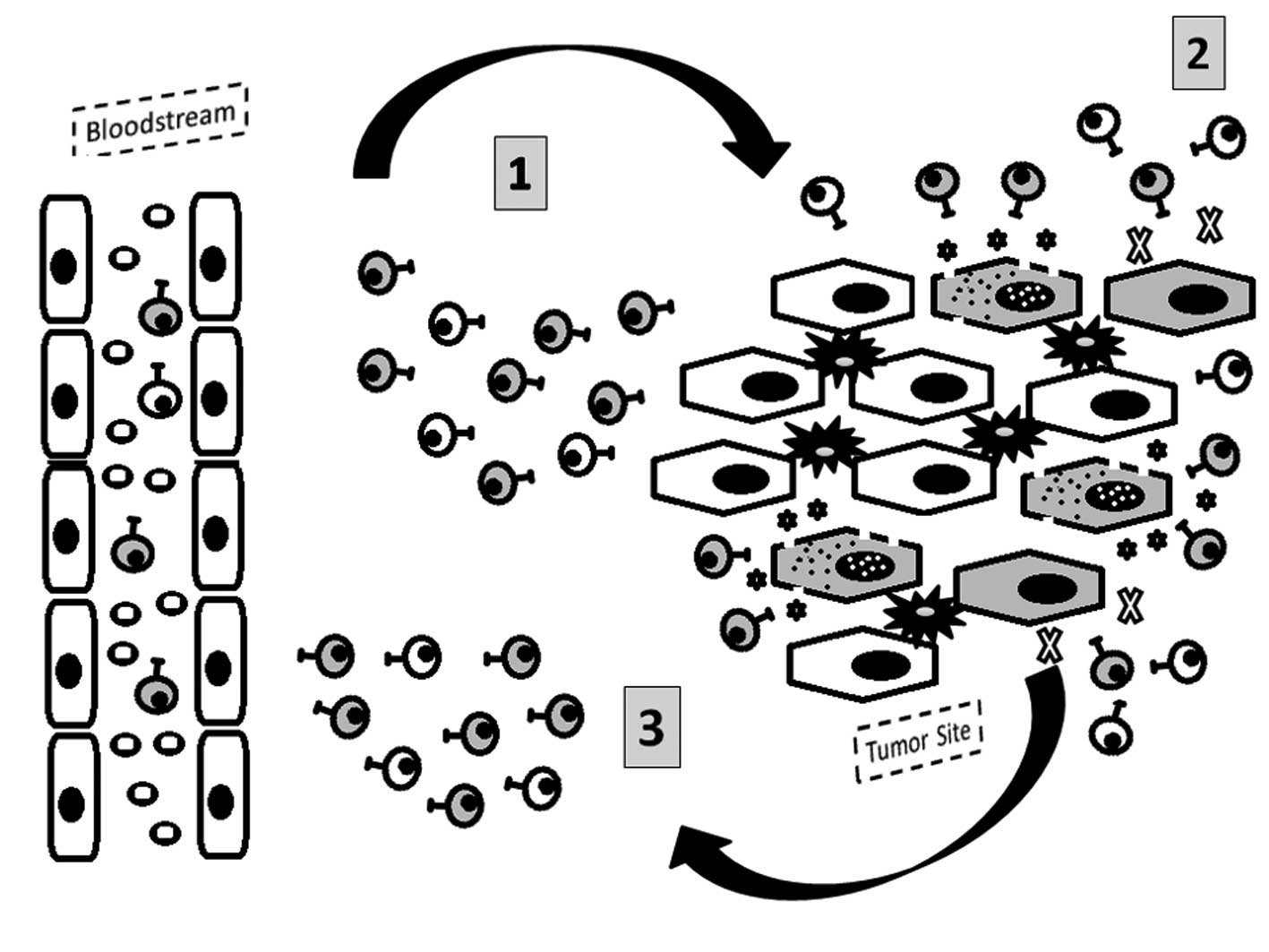
disease relapse (47,48) (Fig.
5).
Finally, we emphasize that patients with a high
number of peripheral blood ENO1-specific Tcc showed a significant
improved survival compared to PDAC patients without or with a low
number of ENO1-specific Tcc. However, we are aware that only a
small cohort was employed in the present study, and while the
presence of Tcc could be considered a potential prognostic marker
for PDAC, it should be validated in a larger cohort of PDAC
patients. These results, therefore, indicate the relevance of the
ENO1-specific immune response in the generation of an effective
anticancer response, which correlates with improved survival of
PDAC patients.
In conclusion, we have demonstrated for the first
time that, in PDAC patients: i) there was a correspondence between
the intratumoral and peripheral ENO1-specific immune response; ii)
the same clonal T cells recirculated from the tumor to the
periphery; and iii) the circulating ENO1-specific T cells showed a
more effective anticancer role compared to Tcc isolated from the
tumoral tissue.
These data support the idea that ENO1 could be a
good candidate antigen for immunotherapeutic treatments in PDAC
patients (49), in particular
after surgical resection to prevent relapse of the disease through
the marked anticancer activity of circulating ENO1-specific Tcc.
Additional studies should be carried out to identify the different
immunosuppressive mechanisms that are able to modify or switch off
the anticancer specific immune response in order to make this
immunotherapeutic approach feasible.
Acknowledgements
The present study was supported by grants of the
Italian Ministry of University and Research (PRIN 2009), the
University of Florence, the Associazione Italiana Ricerca sul
Cancro (AIRC IG no. 11643 and 5×1000 no. 12182), the University of
Torino-Progetti di Ateneo 2011 (grant Rethe-ORTO11RKTW), the
Seventh Framework Program of European Community (European
Pancreatic Cancer-Tumor Microenvironment-Network, EPC-TM-Net, no.
256974), the Fondazione Internazionale di Medicina Sperimentale,
and partly by the FAS-funded ‘Programma Attuativo Regionale
(Toscana)’. We thank Dr Radhika Srinivasan for English revision of
the manuscript.
Abbreviations:
|
ENO1
|
α-enolase
|
|
MDSC
|
myeloid-derived suppressor cells
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
TCR
|
T cell receptor
|
|
Th
|
T helper
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
Tregs
|
regulatory T cells
|
References
|
1
|
Hartwig W, Werner J, Jäger D, Debus J and
Büchler MW: Improvement of surgical results for pancreatic cancer.
Lancet Oncol. 14:e476–e485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh P, Srinivasan R and Wig JD: Major
molecular markers in pancreatic ductal adenocarcinoma and their
roles in screening, diagnosis, prognosis, and treatment. Pancreas.
40:644–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loos M, Giese NA, Kleeff J, Giese T, Gaida
MM, Bergmann F, Laschinger M, W Büchler M and Friess H: Clinical
significance and regulation of the costimulatory molecule B7-H1 in
pancreatic cancer. Cancer Lett. 268:98–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bazhin AV, Shevchenko I, Umansky V, Werner
J and Karakhanova S: Two immune faces of pancreatic adenocarcinoma:
Possible implication for immunotherapy. Cancer Immunol Immunother.
63:59–65. 2014. View Article : Google Scholar
|
|
6
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryschich E, Nötzel T, Hinz U, Autschbach
F, Ferguson J, Simon I, Weitz J, Fröhlich B, Klar E, Büchler MW, et
al: Control of T-cell-mediated immune response by HLA class I in
human pancreatic carcinoma. Clin Cancer Res. 11:498–504.
2005.PubMed/NCBI
|
|
8
|
Nomi T, Sho M, Akahori T, Hamada K, Kubo
A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al:
Clinical significance and therapeutic potential of the programmed
death-1 ligand/programmed death-1 pathway in human pancreatic
cancer. Clin Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park IH, Kong SY, Ro JY, Kwon Y, Kang JH,
Mo HJ, Jung SY, Lee S, Lee KS, Kang HS, et al: Prognostic
implications of tumor-infiltrating lymphocytes in association with
programmed death ligand 1 expression in early-stage breast cancer.
Clin Breast Cancer. 16:51–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blank C, Gajewski TF and Mackensen A:
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T
cells as a mechanism of immune evasion: Implications for tumor
immunotherapy. Cancer Immunol Immunother. 54:307–314. 2005.
View Article : Google Scholar
|
|
11
|
Chen Y, Sun J, Zhao H, Zhu D, Zhi Q, Song
S, Zhang L, He S, Kuang Y, Zhang Z, et al: The coexpression and
clinical significance of costimulatory molecules B7-H1, B7-H3, and
B7-H4 in human pancreatic cancer. Onco Targets Ther. 7:1465–1472.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wadle A, Kubuschok B, Imig J, Wuellner B,
Wittig C, Zwick C, Mischo A, Waetzig K, Romeike BF, Lindemann W, et
al: Serological immune response to cancer testis antigens in
patients with pancreatic cancer. Int J Cancer. 119:117–125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmitz-Winnenthal FH, Galindo-Escobedo
LV, Rimoldi D, Geng W, Romero P, Koch M, Weitz J, Krempien R,
Niethammer AG, Beckhove P, et al: Potential target antigens for
immunotherapy in human pancreatic cancer. Cancer Lett. 252:290–298.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gnjatic S, Ritter E, Büchler MW, Giese NA,
Brors B, Frei C, Murray A, Halama N, Zörnig I, Chen YT, et al:
Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad
Sci USA. 107:5088–5093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomaino B, Cappello P, Capello M,
Fredolini C, Ponzetto A, Novarino A, Ciuffreda L, Bertetto O, De
Angelis C, Gaia E, et al: Autoantibody signature in human ductal
pancreatic adenocarcinoma. J Proteome Res. 6:4025–4031. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubuschok B, Neumann F, Breit R, Sester M,
Schormann C, Wagner C, Sester U, Hartmann F, Wagner M, Remberger K,
et al: Naturally occurring T-cell response against mutated p21 ras
oncoprotein in pancreatic cancer. Clin Cancer Res. 12:1365–1372.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wenandy L, Sørensen RB, Sengeløv L, Svane
IM, thor Straten P and Andersen MH: The immunogenicity of the
hTERT540-548 peptide in cancer. Clin Cancer Res. 14:4–7. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gjertsen MK, Bakka A, Breivik J, Saeterdal
I, Solheim BG, Søreide O, Thorsby E and Gaudernack G: Vaccination
with mutant ras peptides and induction of T-cell responsiveness in
pancreatic carcinoma patients carrying the corresponding RAS
mutation. Lancet. 346:1399–1400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wobser M, Keikavoussi P, Kunzmann V,
Weininger M, Andersen MH and Becker JC: Complete remission of liver
metastasis of pancreatic cancer under vaccination with a HLA-A2
restricted peptide derived from the universal tumor antigen
survivin. Cancer Immunol Immunother. 55:1294–1298. 2006. View Article : Google Scholar
|
|
20
|
Yamaguchi K, Enjoji M and Tsuneyoshi M:
Pancreatoduodenal carcinoma: A clinicopathologic study of 304
patients and immunohistochemical observation for CEA and CA19-9. J
Surg Oncol. 47:148–154. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Komoto M, Nakata B, Amano R, Yamada N,
Yashiro M, Ohira M, Wakasa K and Hirakawa K: HER2 overexpression
correlates with survival after curative resection of pancreatic
cancer. Cancer Sci. 100:1243–1247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heller A, Zörnig I, Müller T, Giorgadze K,
Frei C, Giese T, Bergmann F, Schmidt J, Werner J, Buchler MW, et
al: Immunogenicity of SEREX-identified antigens and disease outcome
in pancreatic cancer. Cancer Immunol Immunother. 59:1389–1400.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cappello P, Tomaino B, Chiarle R, Ceruti
P, Novarino A, Castagnoli C, Migliorini P, Perconti G, Giallongo A,
Milella M, et al: An integrated humoral and cellular response is
elicited in pancreatic cancer by alpha-enolase, a novel pancreatic
ductal adenocarcinoma-associated antigen. Int J Cancer.
125:639–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amedei A, Niccolai E, Benagiano M, Della
Bella C, Cianchi F, Bechi P, Taddei A, Bencini L, Farsi M, Cappello
P, et al: Ex vivo analysis of pancreatic cancer-infiltrating T
lymphocytes reveals that ENO-specific Tregs accumulate in tumor
tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol
Immunother. 62:1249–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Capello M, Ferri-Borgogno S, Cappello P
and Novelli F: α-Enolase: A promising therapeutic and diagnostic
tumor target. FEBS J. 278:1064–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomaino B, Cappello P, Capello M,
Fredolini C, Sperduti I, Migliorini P, Salacone P, Novarino A,
Giacobino A, Ciuffreda L, et al: Circulating autoantibodies to
phosphorylated α-enolase are a hallmark of pancreatic cancer. J
Proteome Res. 10:105–112. 2011. View Article : Google Scholar
|
|
27
|
Cappello P, Rolla S, Chiarle R, Principe
M, Cavallo F, Perconti G, Feo S, Giovarelli M and Novelli F:
Vaccination with ENO1 DNA prolongs survival of genetically
engineered mice with pancreatic cancer. Gastroenterology.
144:1098–1106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Monte L, Reni M, Tassi E, Clavenna D,
Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C and Protti MP:
Intratumor T helper type 2 cell infiltrate correlates with
cancer-associated fibroblast thymic stromal lymphopoietin
production and reduced survival in pancreatic cancer. J Exp Med.
208:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Linehan DC and Goedegebuure PS:
CD25+ CD4+ regulatory T-cells in cancer.
Immunol Res. 32:155–168. 2005. View Article : Google Scholar
|
|
30
|
Liyanage UK, Moore TT, Joo HG, Tanaka Y,
Herrmann V, Doher ty G, Drebin JA, Strasberg SM, Eberlein TJ,
Goedegebuure PS, et al: Prevalence of regulatory T cells is
increased in peripheral blood and tumor microenvironment of
patients with pancreas or breast adenocarcinoma. J Immunol.
169:2756–2761. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bettelli E, Oukka M and Kuchroo VK:
T(H)-17 cells in the circle of immunity and autoimmunity. Nat
Immunol. 8:345–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kryczek I, Wei S, Zou L, Altuwaijri S,
Szeliga W, Kolls J, Chang A and Zou W: Cutting edge: Th17 and
regulatory T cell dynamics and the regulation by IL-2 in the tumor
microenvironment. J Immunol. 178:6730–6733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma
L, Xue X, Wei G, Liu X and Fang G: The prevalence of Th17 cells in
patients with gastric cancer. Biochem Biophys Res Commun.
374:533–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koyama K, Kagamu H, Miura S, Hiura T,
Miyabayashi T, Itoh R, Kuriyama H, Tanaka H, Tanaka J, Yoshizawa H,
et al: Reciprocal CD4+ T-cell balance of effector
CD62Llow CD4+ and CD62LhighCD25+
CD4+ regulatory T cells in small cell lung cancer
reflects disease stage. Clin Cancer Res. 14:6770–6779. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Warshaw AL and Fernández-del Castillo C:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amedei A, Della Bella C, Niccolai E,
Stanflin N, Benagiano M, Duranti R, Del Prete G, Murphy TF and
D'Elios MM: Moraxella catarrhalis-specific Th1 cells in BAL fluids
of chronic obstructive pulmonary disease patients. Int J
Immunopathol Pharmacol. 22:979–990. 2009.
|
|
37
|
Amedei A, Niccolai E, Della Bella C,
Cianchi F, Trallori G, Benagiano M, Bencini L, Bernini M, Farsi M,
Moretti R, et al: Characterization of tumor antigen
peptide-specific T cells isolated from the neoplastic tissue of
patients with gastric adenocarcinoma. Cancer Immunol Immunother.
58:1819–1830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Dongen JJ, Langerak AW, Brüggemann M,
Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E,
García-Sanz R, et al: Design and standardization of PCR primers and
protocols for detection of clonal immunoglobulin and T-cell
receptor gene recombinations in suspect lymphoproliferations:
Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia.
17:2257–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagorsen D, Scheibenbogen C, Schaller G,
Leigh B, Schmittel A, Letsch A, Thiel E and Keilholz U: Differences
in T-cell immunity toward tumor-associated antigens in colorectal
cancer and breast cancer patients. Int J Cancer. 105:221–225. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Berlin C, Berg EL, Briskin MJ, Andrew DP,
Kilshaw PJ, Holzmann B, Weissman IL, Hamann A and Butcher EC: Alpha
4 beta 7 integrin mediates lymphocyte binding to the mucosal
vascular addressin MAdCAM-1. Cell. 74:185–195. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rott LS, Briskin MJ, Andrew DP, Berg EL
and Butcher EC: A fundamental subdivision of circulating
lymphocytes defined by adhesion to mucosal addressin cell adhesion
molecule-1. Comparison with vascular cell adhesion molecule-1 and
correlation with beta 7 integrins and memory differentiation. J
Immunol. 156:3727–3736. 1996.PubMed/NCBI
|
|
43
|
Brandacher G, Winkler C, Schroecksnadel K,
Margreiter R and Fuchs D: Antitumoral activity of interferon-gamma
involved in impaired immune function in cancer patients. Curr Drug
Metab. 7:599–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zaidi MR and Merlino G: The two faces of
interferon-γ in cancer. Clin Cancer Res. 17:6118–6124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Neurath MF and Finotto S: The emerging
role of T cell cytokines in non-small cell lung cancer. Cytokine
Growth Factor Rev. 23:315–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hemdan NY: Anti-cancer versus
cancer-promoting effects of the interleukin-17-producing T helper
cells. Immunol Lett. 149:123–133. 2013. View Article : Google Scholar
|
|
47
|
Bidard FC, Huguet F, Louvet C, Mineur L,
Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C,
et al: Circulating tumor cells in locally advanced pancreatic
adenocarcinoma: The ancillary CirCe 07 study to the LAP 07 trial.
Ann Oncol. 24:2057–2061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tjensvoll K, Nordgård O and Smaaland R:
Circulating tumor cells in pancreatic cancer patients: Methods of
detection and clinical implications. Int J Cancer. 134:1–8. 2014.
View Article : Google Scholar
|
|
49
|
Niccolai E, Prisco D, D'Elios MM and
Amedei A: What is recent in pancreatic cancer immunotherapy? BioMed
Res Int. 2013:4923722013. View Article : Google Scholar : PubMed/NCBI
|