Introduction
Pancreatic cancer is a highly malignant and lethal
tumor with a 5-year survival rate of less than 5%, partially
because of the lack of early diagnosis and treatment options
(1). Although the incidence of
most other cancers have been declining, the rate of incidence for
pancreatic cancer continues to increase by 1.5% per year (1). It has been projected that pancreatic
cancer will become the leading cause of cancer-related deaths in
the USA by 2050 (2). In China, it
has been estimated that 90,100 subjects will be newly diagnosed
with pancreatic cancer and will account for 79,400 cancer-related
death in 2015 (3). However, there
are very limited therapeutic options for pancreatic cancer
currently. Therefore, it is important to gain increased
understanding of tumor risk factors to allow for early disease
detection and the development of therapeutic strategies.
Epidemiologic studies as well as many meta-analyses
have established clear evidence for the association between
diabetes mellitus (DM) and pancreatic cancer that DM is not only a
risk factor, but also a consequence of pancreatic cancer (4,5).
Approximately 85% of patients diagnosed with pancreatic cancer have
impaired glucose tolerance or even DM (6). Our previous studies demonstrated that
hyperglycemia could not only promote the proliferation of
pancreatic cancer cells, but also enhance the invasive ability in
pancreatic cancer (7–9). We proved that high glucose-induced
hydrogen peroxide (H2O2) production
contributes to the invasion in pancreatic cancer cells by
modulating the expression of the metastasis-related factor
urokinase plasminogen activator (uPA) through the activation of the
extracellular signal-regulated kinase (ERK) and p38 mitogen
activated protein kinase (MAPK) signaling pathways (10).
Reactive oxygen species (ROS), including
H2O2, generated by the mitochondrial
respiratory chain are a number of chemically reactive molecules
derived from oxygen, which play a significant role in the
initiation and progression of cancer (11). ROS may play dual roles in cancer
progression in a dose-dependent manner. On the one hand, excess ROS
production can cause oxidative damage and trigger cancer cell
death; on the other hand, mild intracellular ROS can stimulate
tumor progression by promoting cell proliferation, survival,
invasion and metastasis (12). Our
previous studies have demonstrated that both hyperglycemic
condition and superoxide dismutase (SOD)-induced mild ROS
production were able to promote the invasive and migratory
activities of pancreatic cancer (9,13).
MAPK signaling pathways are important signaling cascades downstream
of ROS that is involved in tumor migration and invasion (14).
Resveratrol (trans-3,4′,5-trihydroxystilbene), a
natural polyphenolic phytoalexin, has been found in various plants
(such as grape skin and red wine) and in many types of traditional
Chinese medicines (such as Rheum officinale Baill and
Polygonum cuspidatum) (15). In recent years, resveratrol is
gaining more and more attention for its anticancer effects and
antioxidant properties as well as the influence on glucose
metabolism (16,17). Our previous study has demonstrated
that resveratrol could inhibit the growth of pancreatic cancer
cells by inhibiting cell proliferation and promoting cell apoptosis
via inhibition of the Hh signaling pathway (18). We have also shown that resveratrol
plays an important role in suppressing the proliferation and
epithelial-mesenchymal transition of pancreatic cancer cells via
the PI-3K/Akt/NF-κB signaling pathway (19). Recently, we have also proven that
resveratrol could suppress hypoxia-driven ROS-induced pancreatic
cancer invasive and migratory abilities by inhibiting the Hh
signaling pathway (20). However,
whether resveratrol could influence hyperglycemia-induced
proliferation and migration of pancreatic cancer cells has not been
elucidated.
In the present study, we tested the hypothesis that
resveratrol is able to inhibit hyperglycemia-induced production of
ROS and H2O2 as well as the invasion and
migratory abilities of pancreatic cancer cells. We also
investigated the effect of resveratrol on hyperglycemia-induced
activation of ERK and p38 MAPK signaling pathways as well as the
transcription factor, NF-κB. Results from this study suggest that
resveratrol treatment may be a novel option for therapy of
pancreatic cancer via the inhibition of the ERK and p38 MAPK
signaling pathways.
Materials and methods
Cell culture and reagents
The human pancreatic cancer cell line, Panc-1, was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) medium containing 10% dialyzed heat-inactivated FBS,
100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified
atmosphere of 5% CO2 at 37°C. Exponentially growing
cells in complete medium were pretreated for 1 h with 50 μM
resveratrol, followed by continual incubation in normal culturing
conditions (5.5 mM glucose) or high glucose (25 mM) conditions for
indicated time intervals according to the purpose of the
experiment. DMEM and fetal bovine serum (FBS) were from Gibco
(Grand Island, NY, USA). Resveratrol (>99% pure) was acquired
from Xi'an Chongxin Natural Additive Co. (Xi'an, China).
N-acetylcysteine (NAC) was purchased from Sigma. Millicell
transwells for the invasion assays were obtained from Millipore
(Billerica, MA, USA). Matrigel was from BD (Biosciences, Bedford,
MA, USA). Primary antibodies against uPA, E-cadherin and glucose
transporter type 1 (Glut-1) were procured from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The anti-ERK, anti-phospho-ERK
(Thr202/Tyr204), anti-p38 MAPK, anti-phospho-p38 MAPK
(Thr180/Tyr182), anti-NF-κB and anti-phospho-NF-κB p65 (Ser468)
antibodies were obtained from Cell Signaling Technology (Beverly,
MA, USA). The ERK inhibitor PD 98059 and the p38 MAPK inhibitor SB
203580 were obtained from Sigma Chemical Co. Nitrocellulose
membranes were from Millipore. The BCA assay kit and the
chemiluminescence kit were from Pierce (Rockford, IL, USA). Other
reagents were purchased from common commercial sources. All drug
solutions were freshly prepared on the day of testing.
MTT proliferation assays
Panc-1 cells were seeded in 96-well plates at the
density of 1×104 cells/well and incubated overnight in
10% FBS medium. The cells were then treated with resveratrol in
normal glucose or high glucose condition. After incubation for 24,
48 and 72 h at 37°C, 15 μl of MTT solution [5 mg/ml in
phosphate-buffered saline (PBS)] was added to each well, and then
the cells were incubated for 4 h at 37°C. A total of 100 μl of DMSO
was then added to each well. The optical density (OD) value at 490
nm was determined using a spectrophotometer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Measurement of intracellular ROS
The level of intracellular ROS was measured using
the reactive oxygen species assay kit. In brief, cells were
incubated with 2,7-dichlorodihydrofluorecein diacetate (DCFDA) for
30 min, washed in PBS 3 times, and fluorescence intensity measured
using a fluorometer (Becton-Dickinson, Franklin Lakes, NJ, USA)
with excitation at 488 nm and emission at 525 nm.
Hydrogen peroxide assay
The level of intracellular
H2O2 was measured using a commercial kit
(hydrogen peroxide assay kit) according to the manufacturer's
instructions. In this kit, ferrous ions (Fe2+) were
oxidative to ferric ions (Fe3+) by
H2O2. The Fe3+ then formed a
complex with an indicator dye xylenol orange and produced a visible
purple colored complex which could be measured with a microplate
reader at a wavelength of 560–590 nm (Bio-Rad Laboratories).
Wound healing assay
Cell migratory ability was detected by a
wound-healing assay. Panc-1 cells were seeded in 24-well plates
(1.0×105 cells/500 μl). After the cells grew to 90–100%
confluence, a sterile pipette tip was used to produce a wound line
between the cells. Cellular debris was removed by washing with PBS
and then allowed to migrate for 24 h. Images were taken at time 0
and 24 h post-wounding under a Nikon Diaphot TMD inverted
microscope (magnification, ×10). The relative distance traveled by
the leading edge from 0 to 24 h was assessed using Photoshop
software (n=5).
Transwell Matrigel invasion assay
The invasion of Panc-1 cells was performed in
Millicell invasion chambers. The 8.0 μm pore inserts were coated
with 30 μl Matrigel. After serum starvation for 24 h, the cell
suspensions (5×104) were added to the upper chambers in
DMEM containing 1% FBS. Simultaneously, 500 ml of DMEM containing
20% FBS was placed in the lower chambers. The Matrigel invasion
chamber was incubated for 48 h in a humidified tissue culture
incubator. The non-invading cells were removed from the upper
surface by scraping with a wet cotton swab. After rinsing with PBS,
the filter was fixed and stained with crystal violet. Invasion
ability was determined by counting the stained cells.
Real-time quantitative PCR (QT-PCR)
Total RNA was extracted from the pancreatic cancer
cells using the Fastgen200 RNA isolation system (Fastgen, Shanghai,
China) according to the manufacturer's protocol. Total RNA was
reverse-transcribed into cDNA using the Fermentas RevertAid™ kit
(MBI Fermentas, Canada). The primer sequences were as follows:
uPA-F, 5′-TAAGAGCTGGTGTCTGATTG-3′ and uPA-R,
5′-TTGGATGAACTAGGCTAAAA-3′; E-cadherin-F, 5′-AT
TCTGATTCTGCTGCTCTTG-3′ and E-cadherin-R, 5′-AGT
CCTGGTCCTCTTCTCC-3′; Glut-1-F, 5′-CAACCAAGTCT AAGCCGTTGCAGTGG-3′
and Glut-1-R, 5′-TGCTTGTGG ATTGAGGGTAGGA-3-3′; β-actin-F,
5′-GACTTAGTTGCG TTACACCCTTTCT-3′ and β-actin-R, 5′-GAACGGTGAAG
GTGACAGCAGT-3′.
The PCR reactions consisted of 30 sec at 95°C,
followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C
for 30 sec. After each QT-PCR experiment, a dissociation curve
analysis was conducted. The relative gene expression was calculated
using the previously described 2−ΔΔCt method (21).
Western blotting
Proteins were electrophoretically resolved on a
denaturing SDS-polyacrylamide gel and electrotransferred onto
nitrocellulose membranes. The membranes were initially blocked with
5% non-fat dry milk in Tris-buffered saline (TBS) for 2 h and then
probed with antibodies against uPA, E-cadherin, Glut-1, ERK, p-ERK,
p38, p-p38, NF-κB, p-NF-κB or β-actin (loading control). After
co-incubation with the primary antibodies at 4°C overnight,
membranes were blotted with the secondary antibody for 2 h at 37°C.
The results were visualized using the ECL Western blotting
substrate and photographed by GeneBox (SynGene).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Data are
presented as the means ± SEM of three replicate assays. Differences
between the groups were analyzed by analysis of variance (ANOVA).
Statistical significance was set at P<0.05. All experiments were
repeated independently at least three times.
Results
Resveratrol inhibits high glucose-induced
proliferation of Panc-1 cells
Our previous study proved that the 50% inhibitory
concentration (IC50) for Panc-1 cells was ~50 μM of
resveratrol, which exhibited no cytotoxic effects on the Panc-1
cells (19). Therefore, 50 μM of
resveratrol was used to treat the cells for the current
experiments.
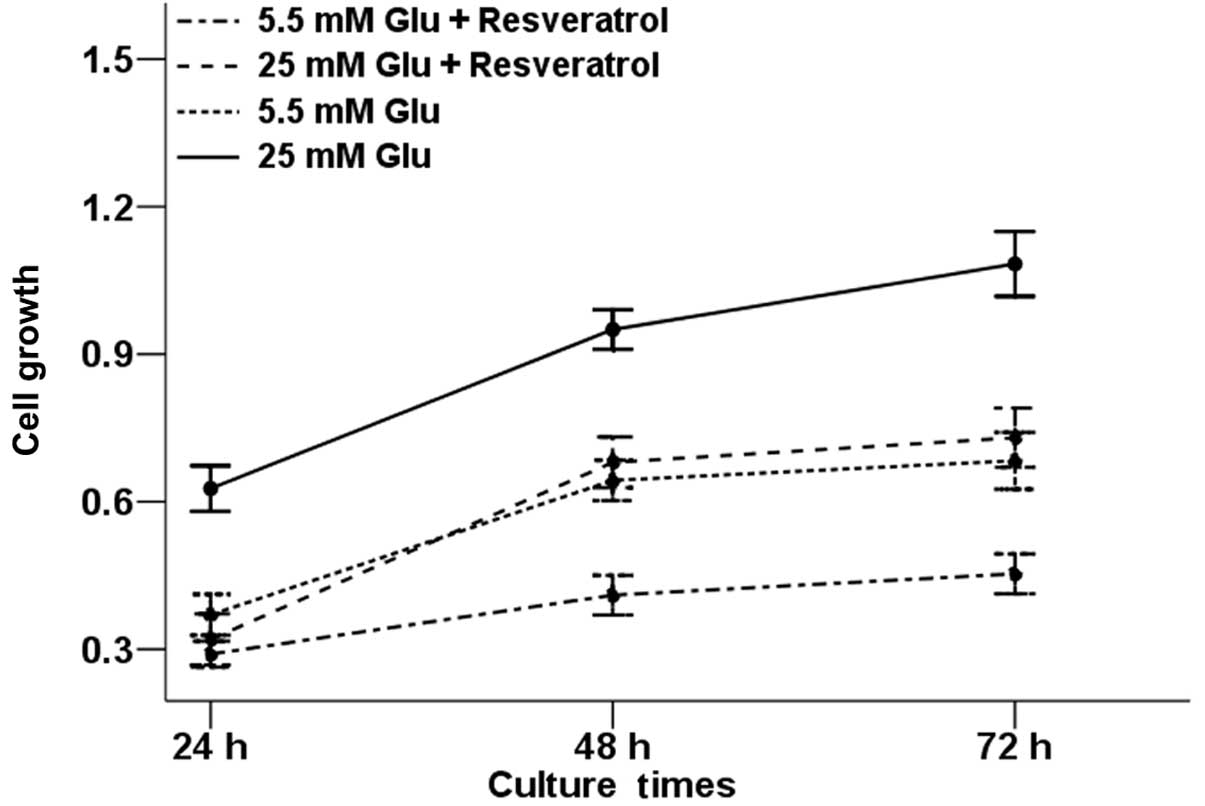
In order to explore whether resveratrol could
influence high glucose-induced proliferation of pancreatic cancer,
Panc-1 cells were treated with high glucose condition and
resveratrol alone or in combination. At the time-points indicated
in Fig. 1, the proliferative rate
of Panc-1 cells was determined by the MTT assay. The results showed
that the proliferation of Panc-1 cells increased in high glucose
condition compared with the control group and the increased rate of
cell proliferation induced by high glucose was reduced in the
presence of resveratrol. Resveratrol alone was also able to inhibit
the proliferative ability of Panc-1 cells.
Resveratrol decreased high
glucose-induced production of ROS and H2O2 in
pancreatic cancer cells
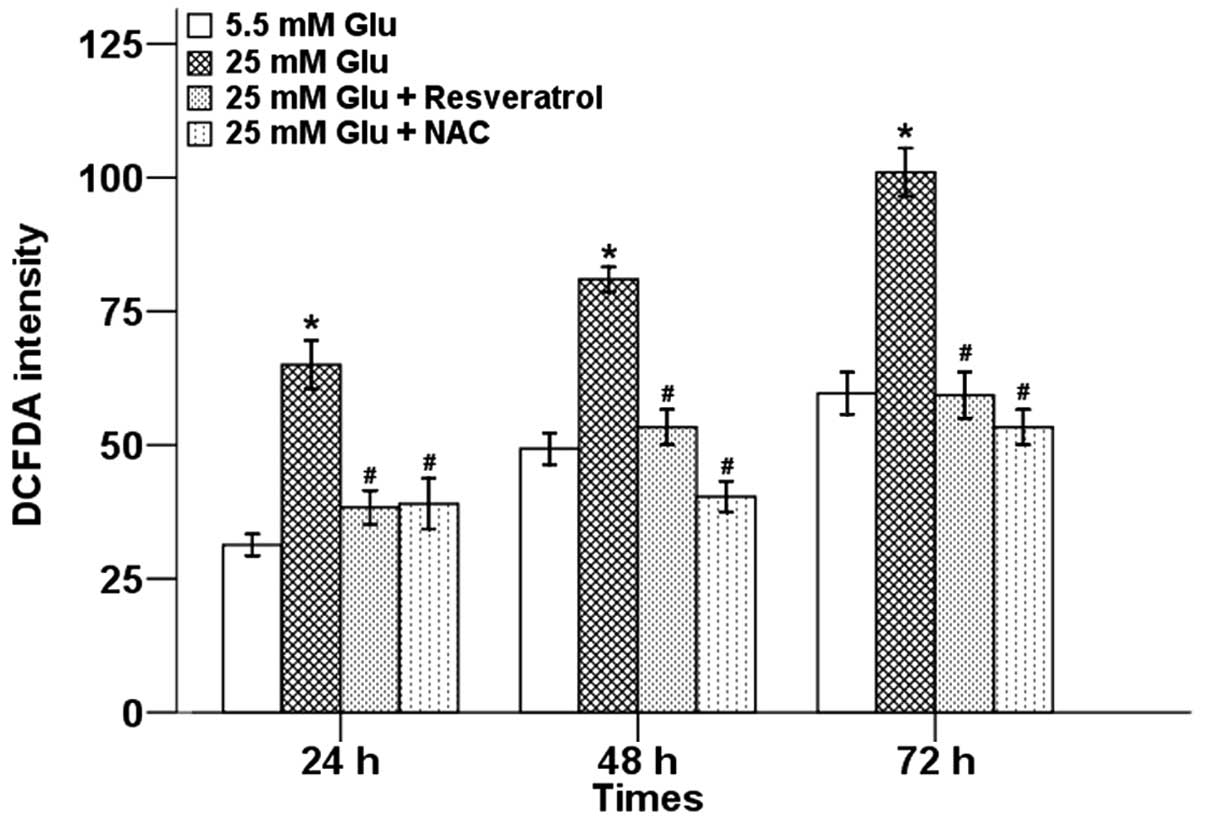
To explore the possible relationship between
resveratrol and oxidative stress, we first examined the effects of
resveratrol on high glucose-induced ROS production in Panc-1 cells
using the cell-permeable and redox-sensitive compound DCFDA by flow
cytometry. Our results showed that high glucose significantly
increased intracellular levels of ROS in a time-dependent manner,
while resveratrol suppressed high glucose-induced production of
ROS. NAC, a scavenger of free radicals, could also efficiently
reduce the high glucose-induced ROS level in Panc-1 cells (Fig. 2).
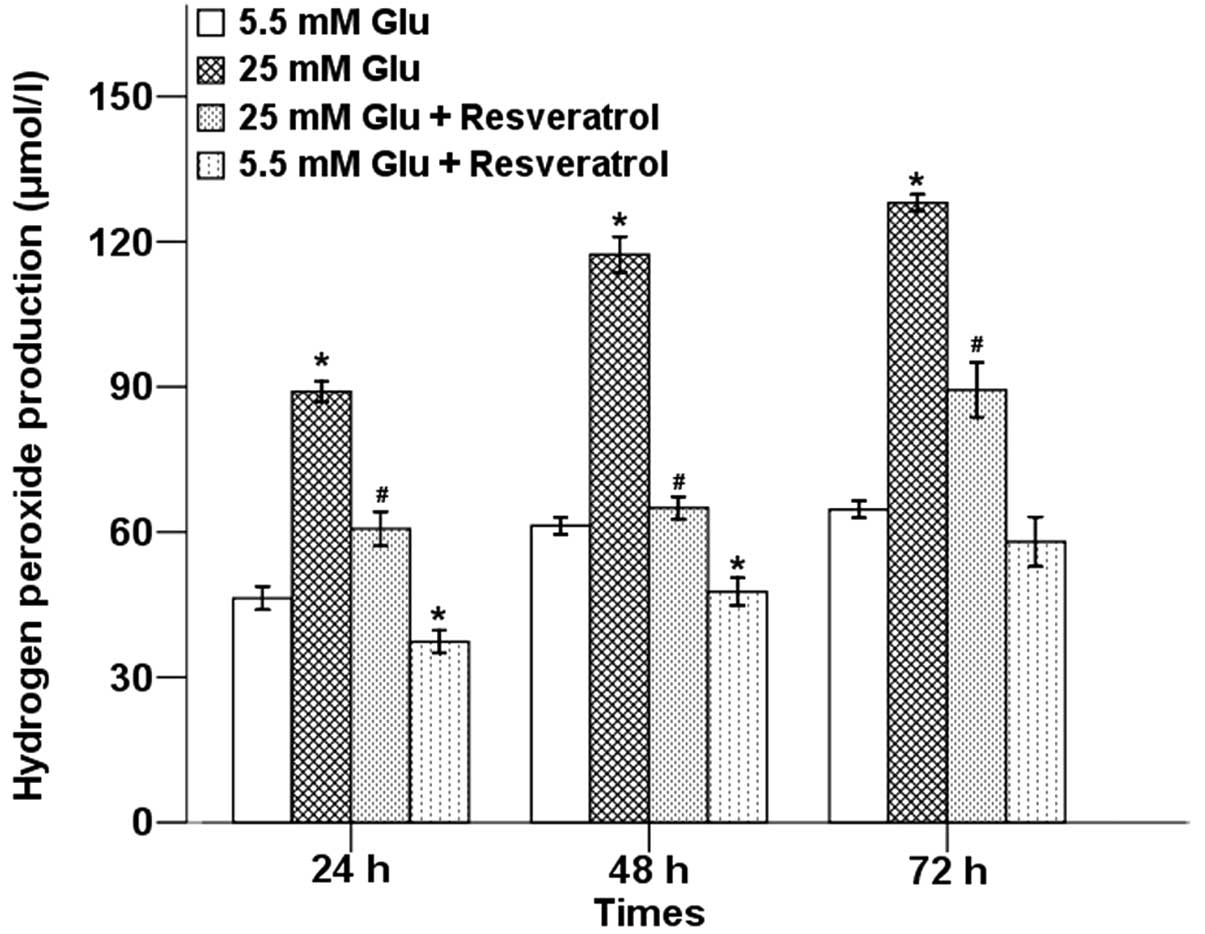
H2O2 is an important component
of ROS. We next examined the effects of hyperglycemia on
H2O2 production in Panc-1 cells using
H2O2 assay. As shown in Fig. 3, resveratrol could significantly
inhibit hyperglycemia-induced production of
H2O2 in different time-points.
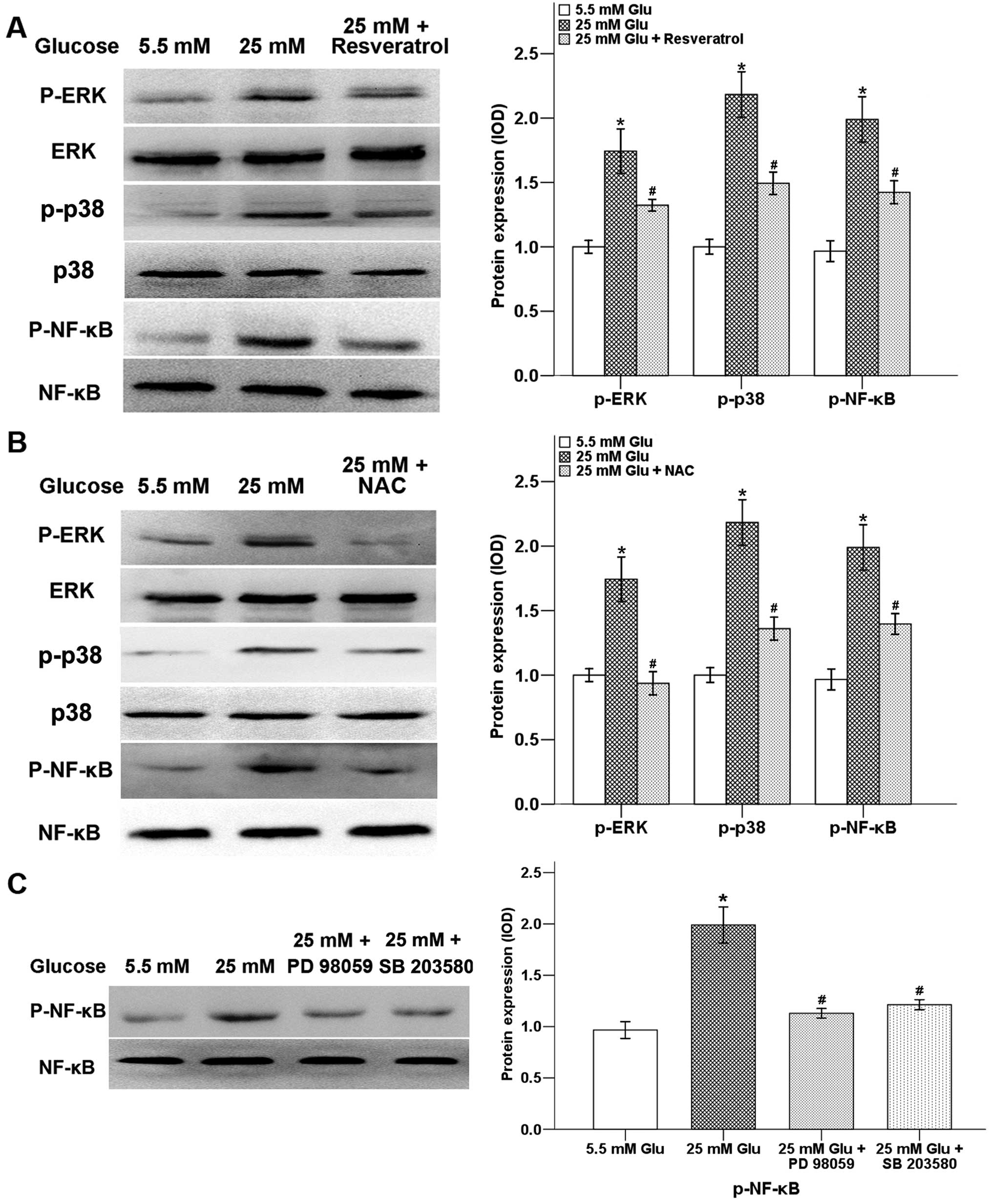
Resveratrol downregulates
hyperglycemia-induced activation of ERK and p38 MAPK pathways
ERK and p38 MAPK pathways are important signaling
cascades downstream of ROS, which are involved in tumor migration
and invasion (14). It has been
proven that ERK pathway induces activation of NF-κB transcription
factor and is associated with cell migration activity (22). Our previous study showed that high
glucose activates the ERK and p38 MAPK signaling pathways as well
as the transcription factors NF-κB and AP-1 via the production of
H2O2 (10).
In the present study, we observed that the high glucose-induced
level of p-ERK and p-p38 were inhibited after a 24-h treatment of
resveratrol. In addition, the high glucose-induced phosphorylation
of NF-κB was also decreased with the addition of resveratrol
(Fig. 4A). In order to assess
whether high glucose-induced activation of ERK and p38 MAPK
signaling pathways were ROS dependent, we treated Panc-1 cells with
NAC. As shown in Fig. 4B, NAC
could significantly decrease high glucose-induced phosphorylation
levels of ERK, p38 and NF-κB. Moreover, ERK inhibitor PD 98059 and
p38 MAPK inhibitor SB203580 could inhibit the expression of
p-NF-κB, indicating that the NF-κB transcription factor is
modulated by the ERK and p38 MAPK pathways (Fig. 4C).
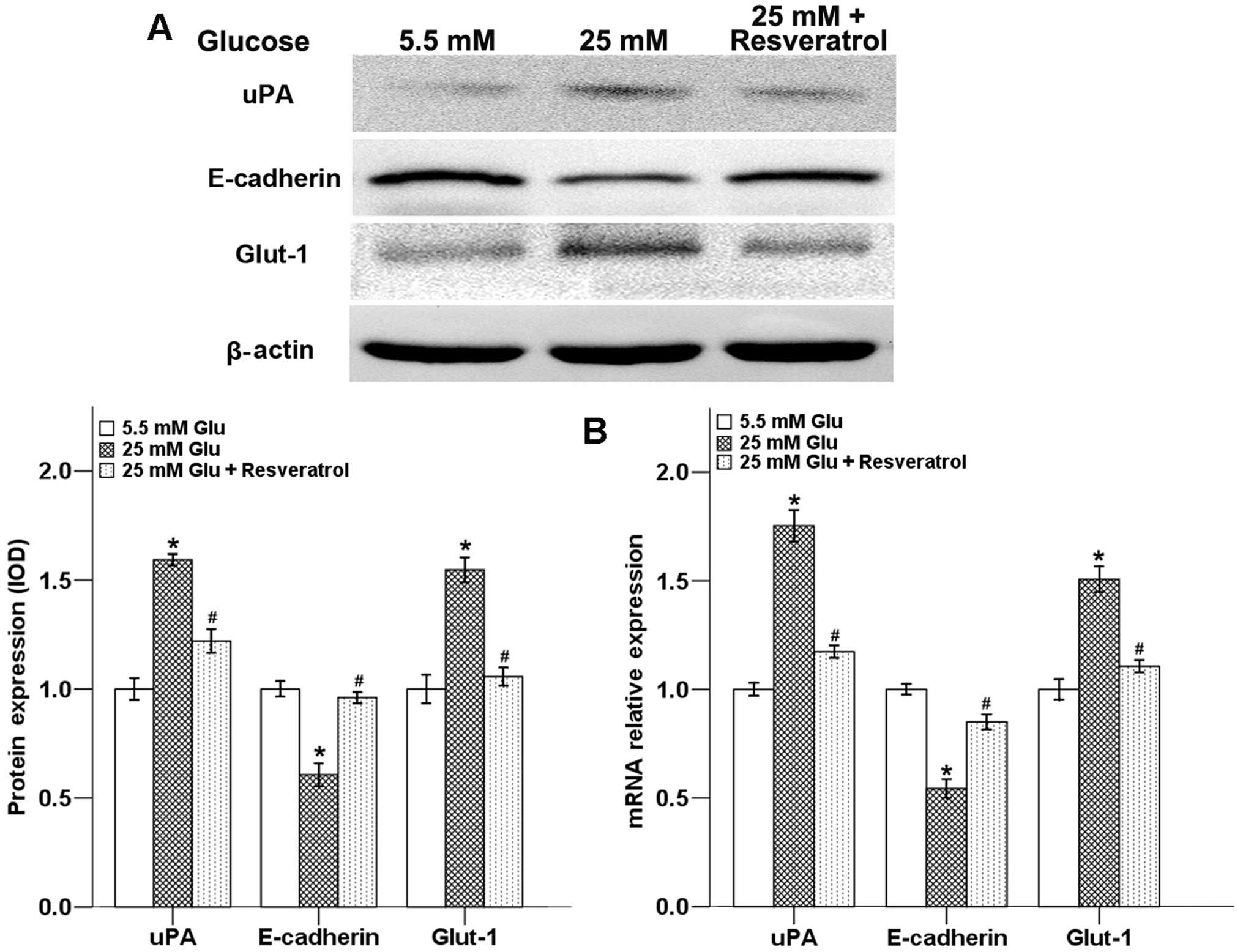
Resveratrol inhibits the expression of
hyperglycemia-modulated metastasis-related factors
Our previous study demonstrated that hyperglycemia
could promote the invasive ability of pancreatic cancer cells
through the regulation of metastatic-related factor uPA (9). Glut-1 is a member of the Glut family
of facilitative glucose transporters that mediate
Na+-independent cellular uptake of glucose. Glut-1
accounts for the high uptake of glucose by malignant cells
(23). The results of many studies
have also provided evidence that Glut-1 is intimately related with
epithelial mesenchymal transition (EMT) of tumor cells and may
contribute to cancer development by the activation of the ERK and
NF-κB pathway (24–26). In the present study, we showed that
high glucose condition downregulated the protein level of the
E-cadherin, while the expression of uPA and Glut-1 were strongly
increased. Resveratrol could significantly reverse all of these
high glucose-induced effects (Fig.
5A).
To evaluate the effects of high glucose and
resveratrol on the expression of E-cadherin, uPA and Glut-1 at mRNA
level, we determined these factors in Panc-1 cells using QT-PCR. As
shown in Fig. 5B, resveratrol
reversed the high glucose-modulated metastatic-related factors at
the mRNA level, and the trend was consistent with the protein
results.
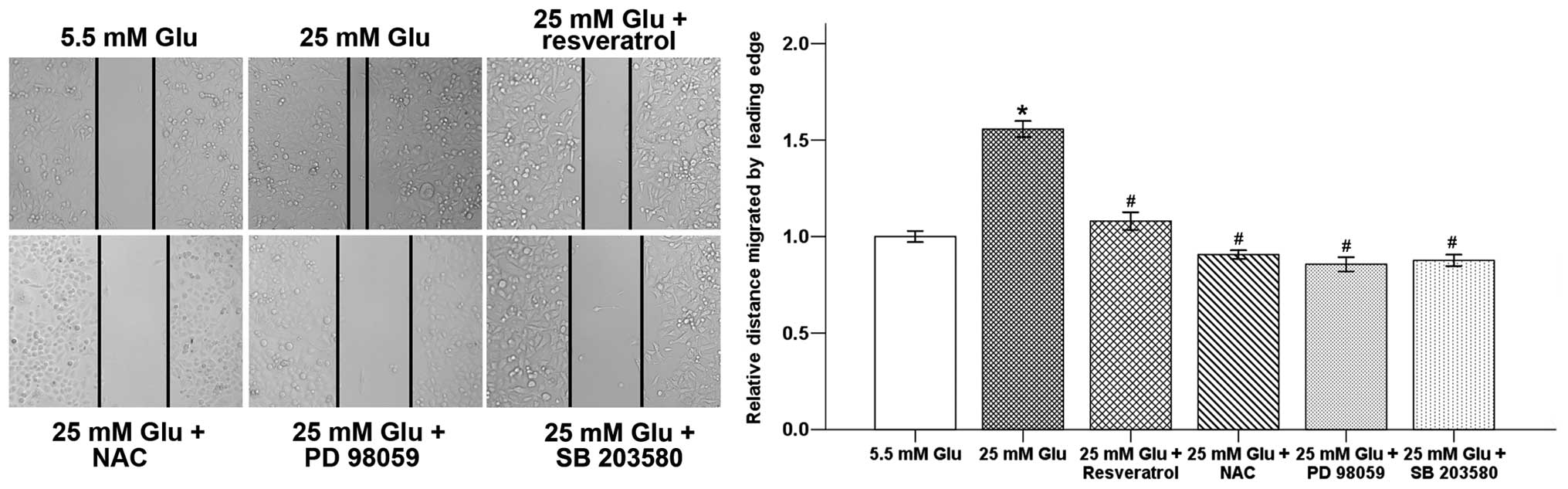
Resveratrol inhibits
hyperglycemia-induced wound closure and cell invasion of pancreatic
cancer cells
Migration and invasion are important processes that
lead to the ability of cancer cells to form metastasis. A
wound-healing assay was used to test the effect of resveratrol on
hyperglycemia-induced pancreatic cancer cell motility. Results
showed that the 24-h incubation of hyperglycemia significantly
increased the migratory ability of Panc-1 cells. Resveratrol
counter-balanced this effect of hyperglycemia (Fig. 6). In addition, NAC, PD 98059 and SB
203580 could also inhibited hyperglycemia-induced wound closure of
pancreatic cancer cells.
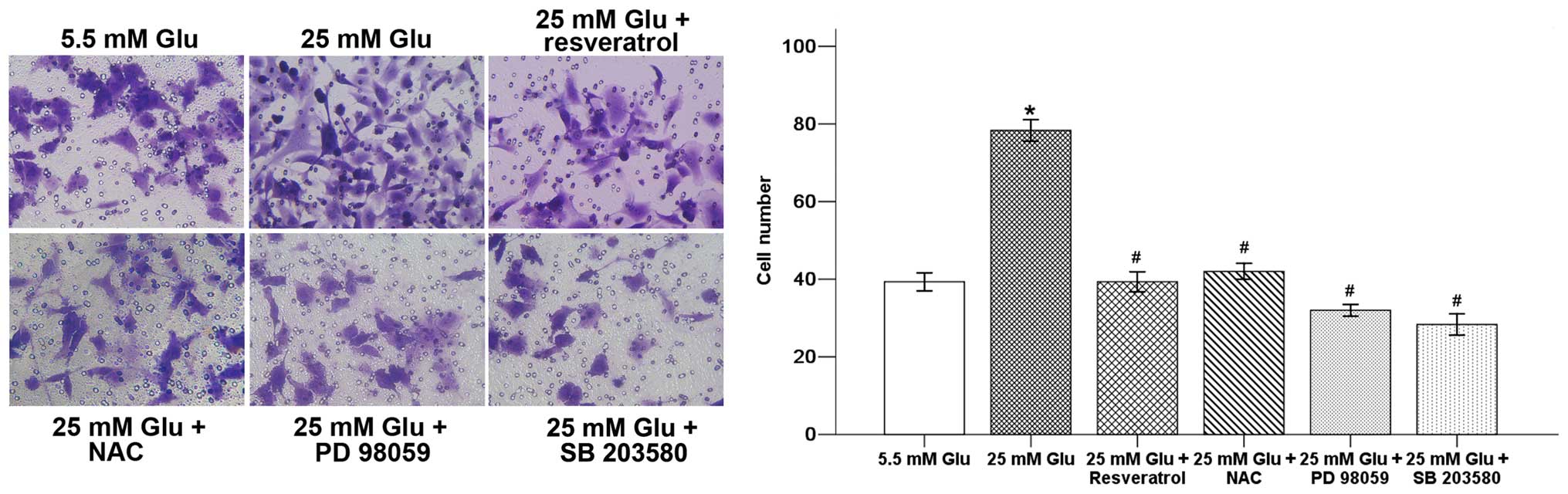
In order to confirm whether resveratrol could
influence hyperglycemia-induced cancer cell invasive ability, we
used a Transwell invasion assay. As shown in Fig. 7, hyperglycemia significantly
increased pancreatic cancer invasion, while resveratrol decreased
the average cell number that invaded into the lower chamber. NAC,
PD 98059 and SB 203580 could also inhibit hyperglycemia-induced
invasion of pancreatic cancer cells. These results indicate that
resveratrol inhibits migration and invasion of pancreatic cancer
cells under high glucose condition, which might be attributed to
the ROS/ERK/NF-κB and ROS/p38 MAPK/NF-κB pathways.
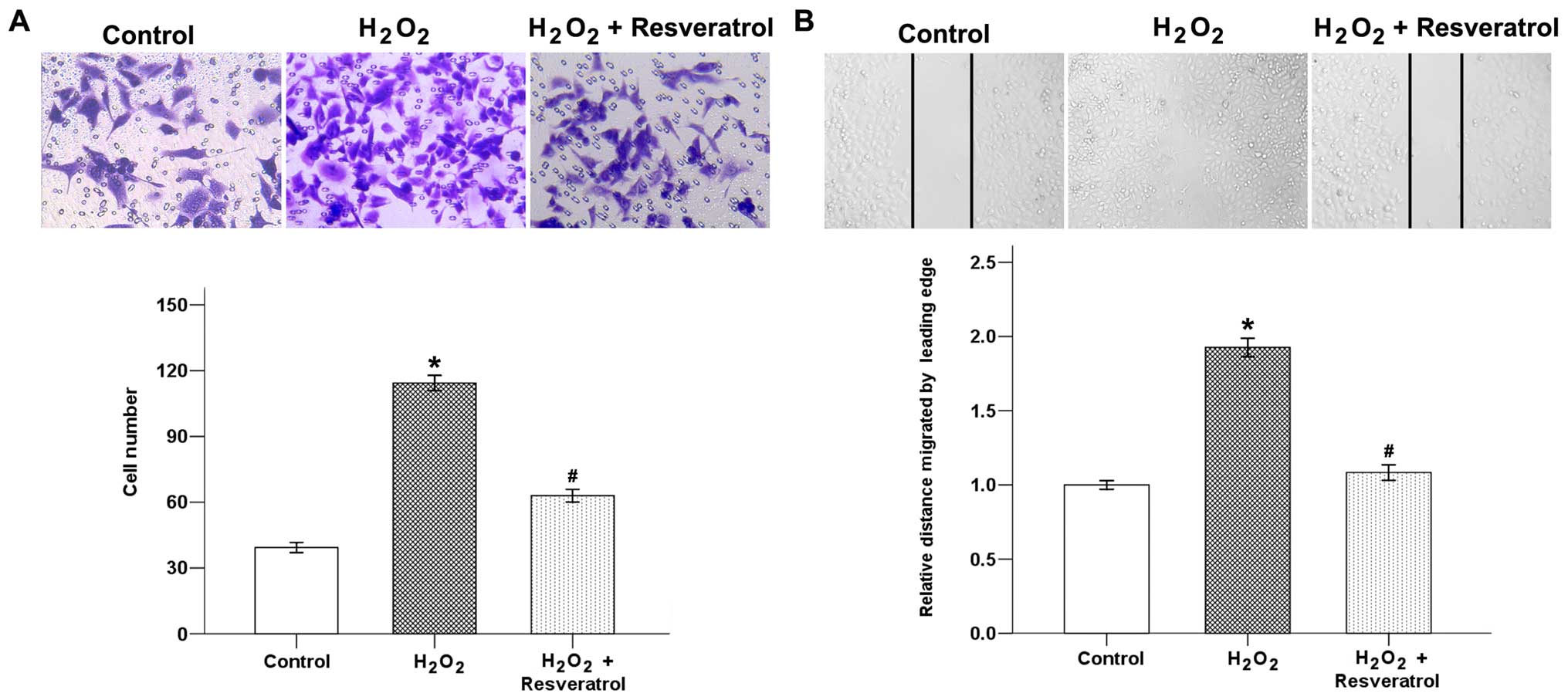
Resveratrol inhibits
H2O2-induced migration and invasion of
pancreatic cancer cells
To examine the potential anti-invasive effects of
resveratrol on H2O2, the invasion ability of
Panc-1 cells treated with resveratrol were analyzed. As shown in
Fig. 8A,
H2O2 exposure significantly increased the
average cell number that invaded into the lower chamber.
Resveratrol could efficiently reduce the
H2O2-induced invasive ability of the cancer
cells. In addition, H2O2 exposure for 24 h
caused a significant increase in the migration of Panc-1 cells,
whereas the cells treated with resveratrol showed delay in wound
closure (Fig. 8B). These findings
indicate that resveratrol might be an effective inhibitor of
H2O2-induced migration and invasion of
pancreatic cancer cells.
Discussion
As one of the most lethal malignant diseases,
pancreatic cancer is characterized by poor outcome, short survival
duration and resistance to therapy, due to its local recurrence,
lymph node and liver metastases and peritoneal dissemination
(27). Therefore, the exploration
of risk factors, and metastatic mechanisms might lead to more
effective therapeutic strategies for pancreatic cancer. DM, a
common metabolic disorder characterized by hyperglycemia, has been
postulated to be both an independent risk factor and a consequence
for pancreatic cancer in recent years (28). A meta-analysis of 6 case-control
studies and 3 cohort studies showed that a 2-fold higher risk of
pancreatic cancer was observed in type-1 DM patients compared with
individuals without DM (5).
Another meta-analysis from three large case-control studies
revealed a 1.8-fold increase in risk of pancreatic cancer
associated with type-2 DM (7). Our
previous study has also proven that high glucose may worsen the
prognosis of pancreatic cancer by enhancing their migratory and
invasive ability through H2O2 production via
the activation of ERK and p38 MAPK signaling pathways (9,10).
In the present study, we focus on whether resveratrol is able to
suppress hyperglycemia-induced cancer invasion and migration
abilities and its underlying mechanism.
Our data showed that resveratrol could significantly
decrease high glucose-induced production of ROS and
H2O2 in Panc-1 cells. Resveratrol was also
able to inhibit high glucose-induced proliferation, migration and
invasion abilities of pancreatic cancer cells. High
glucose-modulated expression of uPA, E-cadherin and Glut-1 were
inhibited by resveratrol. In addition, high glucose-induced
activation of ERK and p38 MAPK signaling pathways as well as the
transcription factor NF-κB could also be suppressed by resveratrol.
Furthermore, resveratrol suppressses
H2O2-induced migration and invasion of
pancreatic cancer cells. Our results indicate that resveratrol
inhibits migration and invaison of pancreatic cancer cells under
high glucose condition, which might be attributed to the
ROS/ERK/NF-κB and ROS/p38 MAPK/NF-κB pathways.
Recent studies showed that resveratrol was able to
inhibit the proliferation and induce apoptosis as well as cell
cycle arrest (20,29), moreover, it inhibited metastasis
and invasion of pancreatic cancer cells (19). In addition, resveratrol suppressed
the proliferation and viability of pancreatic cancer stem cells and
enhance the chemoradiosensitization of pancreatic cancer cells
(30,31). Resveratrol can inhibit tumor
biological behavior through multiple signaling pathways. We have
proven that resveratrol plays an important role in suppressing the
proliferation and EMT of pancreatic cancer cells via the
PI-3K/Akt/NF-κB signaling pathway (19). Recently, we have also shown that
resveratrol could suppress hypoxia-driven ROS-induced pancreatic
cancer invasive and migratory abilities by inhibiting the Hh
signaling pathway (20). Ji et
al (32) proved that
resveratrol could downregulate MALAT1 and decrease nuclear
localization of β-catenin, which in turn attenuated Wnt/β-catenin
signaling pathway and led to the inhibition of invasion and
metastasis of colorectal cancer cells. In addition, resveratrol
could also inhibit hypoxia-induced HIF-1α accumulation and VEGF
expression in both human tongue squamous cell carcinomas and
hepatoma cells via the suppression of ERK1/2 and Akt signaling
pathway (33). In the present
study, we found that resveratrol was able to inhibit high
glucose-induced invasion and migration abilities via the
suppression of the ERK and p38 MAPK signaling pathways.
Insulin resistance, hyperinsulinemia, oxidative
stress and hyperglycemia are the primary characteristics of DM, but
all of these factors potentially promote tumor progression in
various ways (27). It has been
reported that resveratrol is able to attenuate hypoxia-induced
insulin resistance in rats (34).
Brasnyó et al (35) showed
that resveratrol could improve insulin sensitivity in humans, which
might be due to a resveratrol-induced decrease in oxidative stress
that leads to a more efficient insulin signalling via the Akt
pathway. Lin et al (36)
recenly proved that resveratrol was able to inhibit glucose-induced
migration of vascular smooth muscle cells mediated by focal
adhesion kinase via AKT and ERK signaling pathways. Dai et
al (37) indicated that
resveratrol suppressed chondrosarcoma cell invasion via AKT and p38
MAPK pathways. Here, we found that resveratrol was also able to
inhibit hyperglycemia-induced invasion and migration abilities of
pancreatic cancer cells. Recently, several studies have described
an inhibitory effect of resveratrol on cellular glucose metabolism.
Jung et al (17) showed
that resveratrol could suppress cancer cell 18F-FDG
uptake and glycolytic metabolism in a manner that depends on the
capacity of resveratrol to inhibit intracellular ROS, which
downregulates HIF-1α accumulation. In a human hepatoblastoma line,
HepG2 cells, reduction of glucose utilization by resveratrol was
associated with slowed cell cycle in the S phase (38). Iqbal et al (39) showed that decreased glucose uptake
by resveratrol in several cancer cell lines was mediated by
down-regulated pyruvate kinase M2 expression through inhibition of
mTOR signaling.
The MAPK signaling pathways are important signaling
cascades downstream of ROS that are involved in tumor progression
(14). Members of the MAPK family
include ERK, c-jun NH-2 terminal kinase (JNK) and p38 MAPK. Our
previous study demonstrated that a moderate amount of
H2O2 is able to promote pancreatic cancer
invasion via the activation of the ERK and p38 MAPK signaling
pathways (10). We have also
proven that SOD could promote the EMT of pancreatic cancer cells
via the activation of the H2O2/ERK/NF-κB axis
(13). This study determined that
resveratrol could inhibit the ROS/ERK/NF-κB and ROS/p38 MAPK/NF-κB
pathways which in turn attenuates cell migration and invasion.
In conclusion, the present study demonstrates that
resveratrol plays an important role in suppressing
hyperglycemia-induced invasion and migration of pancreatic cancer
cells in vitro by inhibiting the ROS/ERK/NF-κB and ROS/p38
MAPK/NF-κB pathways. These results suggest that resveratrol might
be a potential anticancer agent for the treatment of pancreatic
cancer.
Acknowledgements
The present study is supported by the National
Natural Science Foundation of China (Grant serial nos. 81502840 and
81301846).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li D, Tang H, Hassan MM, Holly EA, Bracci
PM and Silverman DT: Diabetes and risk of pancreatic cancer: A
pooled analysis of three large case-control studies. Cancer Causes
Control. 22:189–197. 2011. View Article : Google Scholar
|
|
5
|
Stevens RJ, Roddam AW and Beral V:
Pancreatic cancer in type 1 and young-onset diabetes: Systematic
review and meta-analysis. Br J Cancer. 96:507–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pannala R, Basu A, Petersen GM and Chari
ST: New-onset diabetes: A potential clue to the early diagnosis of
pancreatic cancer. Lancet Oncol. 10:88–95. 2009. View Article : Google Scholar :
|
|
7
|
Han L, Ma Q, Li J, Liu H, Li W, Ma G, Xu
Q, Zhou S and Wu E: High glucose promotes pancreatic cancer cell
proliferation via the induction of EGF expression and
transactivation of EGFR. PLoS One. 6:e270742011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Ma Q, Liu H, Guo K, Li F, Li W, Han
L, Wang F and Wu E: Relationship between neural alteration and
perineural invasion in pancreatic cancer patients with
hyperglycemia. PLoS One. 6:e173852011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Ma Q, Li J, Guo K, Liu H, Han L and
Ma G: Hyperglycemia enhances the invasive and migratory activity of
pancreatic cancer cells via hydrogen peroxide. Oncol Rep.
25:1279–1287. 2011.PubMed/NCBI
|
|
10
|
Li W, Ma Z, Ma J, Li X, Xu Q, Duan W, Chen
X, Lv Y, Zhou S, Wu E, et al: Hydrogen peroxide mediates
hyperglycemia-induced invasive activity via ERK and p38 MAPK in
human pancreatic cancer. Oncotarget. 6:31119–31133. 2015.PubMed/NCBI
|
|
11
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishikawa M, Hashida M and Takakura Y:
Catalase delivery for inhibiting ROS-mediated tissue injury and
tumor metastasis. Adv Drug Deliv Rev. 61:319–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Cao L, Han L, Xu Q and Ma Q:
Superoxide dismutase promotes the epithelial-mesenchymal transition
of pancreatic cancer cells via activation of the
H2O2/ERK/NF-κB axis. Int J Oncol.
46:2613–2620. 2015.
|
|
14
|
Wu WS, Wu JR and Hu CT: Signal cross talks
for sustained MAPK activation and cell migration: The potential
role of reactive oxygen species. Cancer Metastasis Rev. 27:303–314.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen BY, Kuo CH, Liu YC, Ye LY, Chen JH
and Shieh CJ: Ultrasonic-assisted extraction of the botanical
dietary supplement resveratrol and other constituents of Polygonum
cuspidatum. J Nat Prod. 75:1810–1813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fulda S: Resveratrol and derivatives for
the prevention and treatment of cancer. Drug Discov Today.
15:757–765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung KH, Lee JH, Thien Quach CH, Paik JY,
Oh H, Park JW, Lee EJ, Moon SH and Lee KH: Resveratrol suppresses
cancer cell glucose uptake by targeting reactive oxygen
species-mediated hypoxia-inducible factor-1α activation. J Nucl
Med. 54:2161–2167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin Y, Ma Z, Dang X, Li W and Ma Q: Effect
of resveratrol on proliferation and apoptosis of human pancreatic
cancer MIA PaCa-2 cells may involve inhibition of the Hedgehog
signaling pathway. Mol Med Rep. 10:2563–2567. 2014.PubMed/NCBI
|
|
19
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F, et al: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar
|
|
20
|
Li W, Cao L, Chen X, Lei J and Ma Q:
Resveratrol inhibits hypoxia-driven ROS-induced invasive and
migratory ability of pancreatic cancer cells via suppression of the
Hedgehog signaling pathway. Oncol Rep. 35:1718–1726. 2016.
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Hsieh HL, Wang HH, Wu WB, Chu PJ and Yang
CM: Transforming growth factor-β1 induces matrix
metalloproteinase-9 and cell migration in astrocytes: Roles of
ROS-dependent ERK- and JNK-NF-κB pathways. J Neuroinflammation.
7:882010. View Article : Google Scholar
|
|
23
|
Ito H, Duxbury M, Zinner MJ, Ashley SW and
Whang EE: Glucose transporter-1 gene expression is associated with
pancreatic cancer invasiveness and MMP-2 activity. Surgery.
136:548–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui Y, Nadiminty N, Liu C, Lou W, Schwartz
CT and Gao AC: Upregulation of glucose metabolism by NF-κB2/p52
mediates enzalutamide resistance in castration-resistant prostate
cancer cells. Endocr Relat Cancer. 21:435–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mori A, Moser C, Lang SA, Hackl C,
Gottfried E, Kreutz M, Schlitt HJ, Geissler EK and Stoeltzing O:
Up-regulation of Krüppel-like factor 5 in pancreatic cancer is
promoted by interleukin-1beta signaling and hypoxia-inducible
factor-1alpha. Mol Cancer Res. 7:1390–1398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuo J, Wen J, Lei M, Wen M, Li S, Lv X,
Luo Z and Wen G: Hypoxia promotes the invasion and metastasis of
laryngeal cancer cells via EMT. Med Oncol. 33:152016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Q, Zong L, Chen X, Jiang Z, Nan L, Li
J, Duan W, Lei J, Zhang L, Ma J, et al: Resveratrol in the
treatment of pancreatic cancer. Ann NY Acad Sci. 1348:10–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Cao G, Ma Q, Liu H, Li W and Han L:
The bidirectional interation between pancreatic cancer and
diabetes. World J Surg Oncol. 10:1712012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mo W, Xu X, Xu L, Wang F, Ke A, Wang X and
Guo C: Resveratrol inhibits proliferation and induces apoptosis
through the hedgehog signaling pathway in pancreatic cancer cell.
Pancreatology. 11:601–609. 2011. View Article : Google Scholar
|
|
30
|
Fulda S and Debatin KM: Sensitization for
anticancer drug-induced apoptosis by the chemopreventive agent
resveratrol. Oncogene. 23:6702–6711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shankar S, Nall D, Tang SN, Meeker D,
Passarini J, Sharma J and Srivastava RK: Resveratrol inhibits
pancreatic cancer stem cell characteristics in human and KrasG12D
transgenic mice by inhibiting pluripotency maintaining factors and
epithelial-mesenchymal transition. PLoS One. 6:e165302011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J, et al: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013. View Article : Google Scholar
|
|
33
|
Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J
and Le AD: Resveratrol inhibits hypoxia-induced accumulation of
hypoxia-inducible factor-1alpha and VEGF expression in human tongue
squamous cell carcinoma and hepatoma cells. Mol Cancer Ther.
4:1465–1474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carreras A, Zhang SX, Almendros I, Wang Y,
Peris E, Qiao Z and Gozal D: Resveratrol attenuates intermittent
hypoxia-induced macrophage migration to visceral white adipose
tissue and insulin resistance in male mice. Endocrinology.
156:437–443. 2015. View Article : Google Scholar
|
|
35
|
Brasnyó P, Molnár GA, Mohás M, Markó L,
Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, et al:
Resveratrol improves insulin sensitivity, reduces oxidative stress
and activates the Akt pathway in type 2 diabetic patients. Br J
Nutr. 106:383–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin YC, Chen LH, Varadharajan T, Tsai MJ,
Chia YC, Yuan TC, Sung PJ and Weng CF: Resveratrol inhibits
glucose-induced migration of vascular smooth muscle cells mediated
by focal adhesion kinase. Mol Nutr Food Res. 58:1389–1401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dai Z, Lei P, Xie J and Hu Y: Antitumor
effect of resveratrol on chondrosarcoma cells via phosphoinositide
3-kinase/AKT and p38 mitogen-activated protein kinase pathways. Mol
Med Rep. 12:3151–3155. 2015.PubMed/NCBI
|
|
38
|
Massimi M, Tomassini A, Sciubba F, Sobolev
AP, Devirgiliis LC and Miccheli A: Effects of resveratrol on HepG2
cells as revealed by 1H-NMR based metabolic profiling.
Biochim Biophys Acta. 1820:1–8. 2012. View Article : Google Scholar
|
|
39
|
Iqbal MA and Bamezai RN: Resveratrol
inhibits cancer cell metabolism by down regulating pyruvate kinase
M2 via inhibition of mammalian target of rapamycin. PLoS One.
7:e367642012. View Article : Google Scholar : PubMed/NCBI
|