Introduction
Metastasis is the main cause of morbidity and
fatality in various cancers. Breast cancer is a major public health
issue and is the most common malignancy in females (1). Expression of numerous genes has been
linked to impart metastatic potential in breast cancer. In the
clinic, breast cancer is classified mainly into four molecular
subtypes: luminal A/B, human epidermal growth factor receptor type
II (HER2) and basal-like. The HER2 subtype is overexpressed in
~25–30% of all breast cancer cases and HER2 overexpression is
strongly associated with an aggressive phenotype and poor outcomes
(2).
Chemokine receptors belong to G protein-coupled
receptor (GPCR) family, which trigger chemotactic and growth
signals following reciprocal action with their ligands. CXCR4, the
receptor of CXCL12/stromal cell-derived factor-1α (SDF-1α), has
recently been shown to play an important role in breast cancer
metastasis (3). The CXCR4/CXCL12
axis makes breast cancer cells to leave of the circulation and
traffic into specific organs with large amounts of chemokines, thus
forming angiogenesis, proliferation and metastatic tumors (4). The RTK HER2 and GPCR CXCR4 are two
structurally unrelated receptors, but a recent study indicated that
HER2 enhances CXCR4 expression and that CXCR4 is required for
HER2-induced breast cancer metastasis (5). In addition, a potential relationship
between CXCR4/CXCL12 and HER2 in breast cancer cells is not
completely understood, especially on its role in metastasis.
Therefore, targeting disease-associated proteins for HER2 and CXCR4
represents a promising alternative therapeutic strategy in breast
cancer.
Pomolic acid (PA) is a pentacyclic triterpene
isolated from Euscaphis japonica, and is highly effective in
inhibiting cell growth (6) and
induces apoptosis (7,8). In a previous study, our group showed
that PA suppressed CXCR4 expression in breast cancer cells
(9), but how CXCR4 is regulated in
these cancer cells is not understood. Although it has been
established that HER2-mediated upregulation of CXCR4 protein is
responsible for the invasiveness of HER2-overexpressing breast
cancer cells (5), no information
is available regarding the regulation of CXCR4 in
HER2-overexpressing breast cancer cells.
Therefore, the aim of this study was to examine the
effects of PA on CXCR4 and HER2 regulation in HER2 overexpressing
SKBR3 cells. We found that PA inhibited HER2 and CXCR4 expression
at both the protein and mRNA levels, with critical involvement of
the ERK pathway and suppression of NF-κB activation. Furthermore,
we investigated the PA- inhibited metastatic potential of
HER2-overexpressing breast cancer cells when the cells were
transactivated by CXCL12.
Materials and methods
Cell culture and tumor cell lines
SKBR3, MCF7 and MDA-MB-231 human breast cancer cell
lines were obtained from the American Type Culture Collection
(ATCC; Rockville, MD, USA).
The human HER2-overexpressing breast cancer cell
line SKBR3 was cultured in RPMI-1640 containing 25 mM HEPES, 10%
fetal bovine serum (FBS) and 1% antibiotics. MCF7 breast cancer
cell lines was cultured in RPMI-1640 supplemented with 10% FBS and
1% antibiotics. MDA-MB-231 breast cancer cell lines was cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS
and 1% antibiotics. Cells were maintained at 37°C in an atmosphere
of 5% CO2-95% air. All cells were passaged at 80%
confluence in 0.25% trypsin-EDTA for 3–5 min. RPMI-1640, DMEM, FBS,
antibiotic and trypsin-EDTA were purchased from Gibco (Gibco, Grand
Island, NY, USA).
Materials and reagents
Pomolic acid (PA) was received from Dr Ki Yong Lee,
a professor of the College of Pharmacy, Korea University (10). Pomolic acid was dissolved in
dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA) as a 10-mM
stock solution and stored at 4°C. Further dilution was done in cell
culture medium. Lactacystin and chloroquine were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against
CXCR4 was obtained from Abcam (Cambridge, MA, USA). HER2,
phospho-ERK, ERK, phospho-p38, p38, phospho-AKT, AKT, phospho-JNK,
JNK, β-actin were obtained from Cell Signaling Technology (Beverly,
MA, USA). β-actin was used as a loading control. CXCL12 was
purchased from R&D Systems (Minneapolis, MN, USA).
Western blot analysis
SKBR3 cells, grown under our experimental
conditions, were lysed for 30 min on ice in
radioimmunoprecipitation assay (RIPA) lysis buffer [150 mM NaCl, 10
mM Tris (pH 7.2), 0.1% sodium dodecyl sulphate (SDS), 1% Triton
X-100, 1% deoxycholate and 5 mM ethylene diamine tetra acetic acid
(EDTA)] enriched with a complete protease inhibitor cocktail tablet
(Roche Diagnostics, Mannheim, Germany). Protein concentration was
determined by using bicinchoninic acid (BCA) protein assay kit
(Pierce Biotechnology, Rockford, IL, USA).
Total proteins (25 μg) was loaded onto 10%
SDS-polyacrylamide gel, separated, and transferred onto polyvinyl
difluoride (PVDF) membrane (Roche, Penzberg, Germany). The
membranes were blocked with 5% non-fat dry milk in Tris-buffered
saline with Tris-buffered saline with Tween-20 [TBST; 10 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 0.05% Tween-20] and incubated with
primary antibody at 4°C. After three washes of 10 min each in TBST,
the membranes were incubated with hybridization with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary
antibodies for 2 h and subsequently washed again. The transferred
proteins were incubated with super-signal pico-chemiluminescent
substrate or dura-luminol substrate (Thermo Scientific, Waltham,
MA, USA) for 2 min according to the manufacturer's instructions and
visualized with ImageQuant™ LAS 4000 (Fujifilm Life Science, Roche
Diagnostics).
RNA extraction and PCR analysis
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and 11 μg of total RNA was
reverse-transcribed using AccuPower® Rocketscript™ cycle
RT premix (Bioneer, Daejeon, Korea). The relative expression of
CXCR4 and HER2 was analyzed by quantitative RT-PCR with
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal
control. The following pairs of forward and reverse primer sets
were used: HER2 sense, 5′-AGC CGC GAG CAC CCA AGT-3′; antisense,
5′-TTG GTG GGC AGG TAG GTG AGT T. CXCR4, sense, 5′-CCG TGG CAA ACT
GGT ACT TT-3′; antisense, 5′-TTT CAG CCA ACA GCT TCC TT-3′. GAPDH,
sense, 5′-CAG CCT CAA GAT CAT CAG CA-3′; antisense, 5′-GTC TTC TGG
GTG GCA GTG AT-3′. The RT-PCR reaction mixture contained 2.5 μl of
10X Taq reaction buffer, 0.5 μl of each 10 mM dNTP, 1 μl each of
forward and reverse primers, and 2 μl template DNA each of in a
final volume of 25 μl. Amplification products were resolved by 1.5%
agarose gel electrophoresis stained with safe dye and photographed
by Imagequant LAS 4000.
Quantitative real-time PCR
Real-time PCR was performed on the cDNA using the
selective primers for HER2 (sense, 5′-AGC CGC GAG CAC CCsA AGT-3′;
antisense, 5′-TTG GTG GGC AGG TAG GTG AGT T) CXCR4 (5′-CCG TGG CAA
ACT GGT ACT TT-3′; antisense, 5′-TTT CAG CCA ACA GCT TCC TT-3′) and
GAPDH (sense, 5′-CAG CCT CAA GAT CAT CAG CA-3′; antisense, 5′-GTC
TTC TGG GTG GCA GTG AT-3′). PCR was performed in a Light Cycler 480
(Roche Diagnostics, Indianapolis, IN, USA) using the Light Cycler
DNA Master SYBR Green kit (Roche Diagnostics) following the
manufacturer's recommended amplification procedure. Reaction
conditions of CXCR4 consisted of 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec, 55°C for 30 sec. Reaction conditions of
HER2 consisted of 95°C for 10 min, followed by 40 cycles of 95°C
for 10 sec, 60°C for 30 sec. All reactions were triplicate
repeated, and relative mRNA expression levels for target genes were
determined using the 2−ΔΔCT method with normalization by
GAPDH (11).
MAPK inhibitor treatment and siRNA
transfection
SKBR3 cells were pre-treated with mitogen-activated
protein kinase (MAPK) inhibitors (Calbiochem, CA, USA) such as
ERK1/2-specific inhibitor: PD98059 (20 μM), JNK-specific inhibitor:
SP600125 (20 μM), p38-specific inhibitor: SB203580 (20 μM) and
PI3K-specific inhibitor: LY234002 (20 μM). After 30 min, the cells
were treated for 24 h. Cells were transfected with control siRNA
and ERK1/2-MAPK siRNA (Cell Signaling Technology) using RNAiMAX
transfection reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's instructions.
In vitro invasion assay
In vitro invasion of SKBR3 cells was measured
using Bio-Coat Matrigel invasion assay system (BD Biosciences,
Lexington, KY, USA) according to the manufacturer's instructions.
Cancer cells (5×104/ml) were suspended in medium and
seeded into the Matrigel-precoated Transwell chambers with
polycarbonate membranes of 8 μm pore size. After preincubation with
or without pomolic acid (25 μM), Transwell chambers were placed
into 24-well plates, in which was added the basal medium only or
basal medium containing 100 ng/ml CXCL12. After incubation (24 h
for SKBR3), the upper surface of Transwell chambers was wiped off
with a cotton swab and invading cells were fixed and stained with a
Diff-Quick stain. The invading cell numbers were counted in five
randomly selected microscope fields (x100). Average cell numbers in
each field were used for statistical analyses. Each experiment was
performed in triplicate. All experiments were conducted in
triplicate and the invasion index was expressed as the percentage
of invaded cell number compared with the corresponding control.
Electrophoretic mobility shift assay
A DIG Gel Shift kit (Roche, Mannheim, Germany) was
used to detect electrophoretic mobility shift assay (EMSA). The
nuclear protein was harvested and NF-κB binding with DNA was
detected according to the manufacturer's instructions. The NF-κB
sequence consensus oligonucleotide used in this study was 5′-CTT
GAA GGG ATT TCC CTG
GCT TGA AGG GAT TTC
CCT GG-3′ containing the NF-κB binding motif end-labeled
with DIG-ddUTP. For the binding reaction, 10 μg of the sample
protein was incubated at room temperature for 30 min with a
DIG-labeled probe. For supershift experiments, 4 μg of specific
anti-p65 was added to the binding reaction and incubated for 30
min, after which we added the DIG-labeled probe. The DNA-protein
complexes were separated by electrophoresis in 6% non-denatured
polyacrylamide gels using 0.5X TBE as a running buffer. After
electrophoresis, the gels were transferred to nylon membranes and
detected chemiluminescently. Signal intensity was quantified by
ImageQuant LAS 4000.
Statistical analysis
Experiments were performed at least three times,
with consistent results. The results are given as mean ± standard
deviation (SD). The P-value was assessed using ANOVA and
Student-Newman-Keul tests. Results were considered statistically
significant at P<0.05, and P<0.001.
Results
HER2 and CXCR4 expression is
downregulated in the breast cancer cells by PA
Recently, several studies have shown that the
relationship between HER2 and CXCR4 is involved in the breast
cancer metastasis to organs (lung, liver and bone) (3,12).
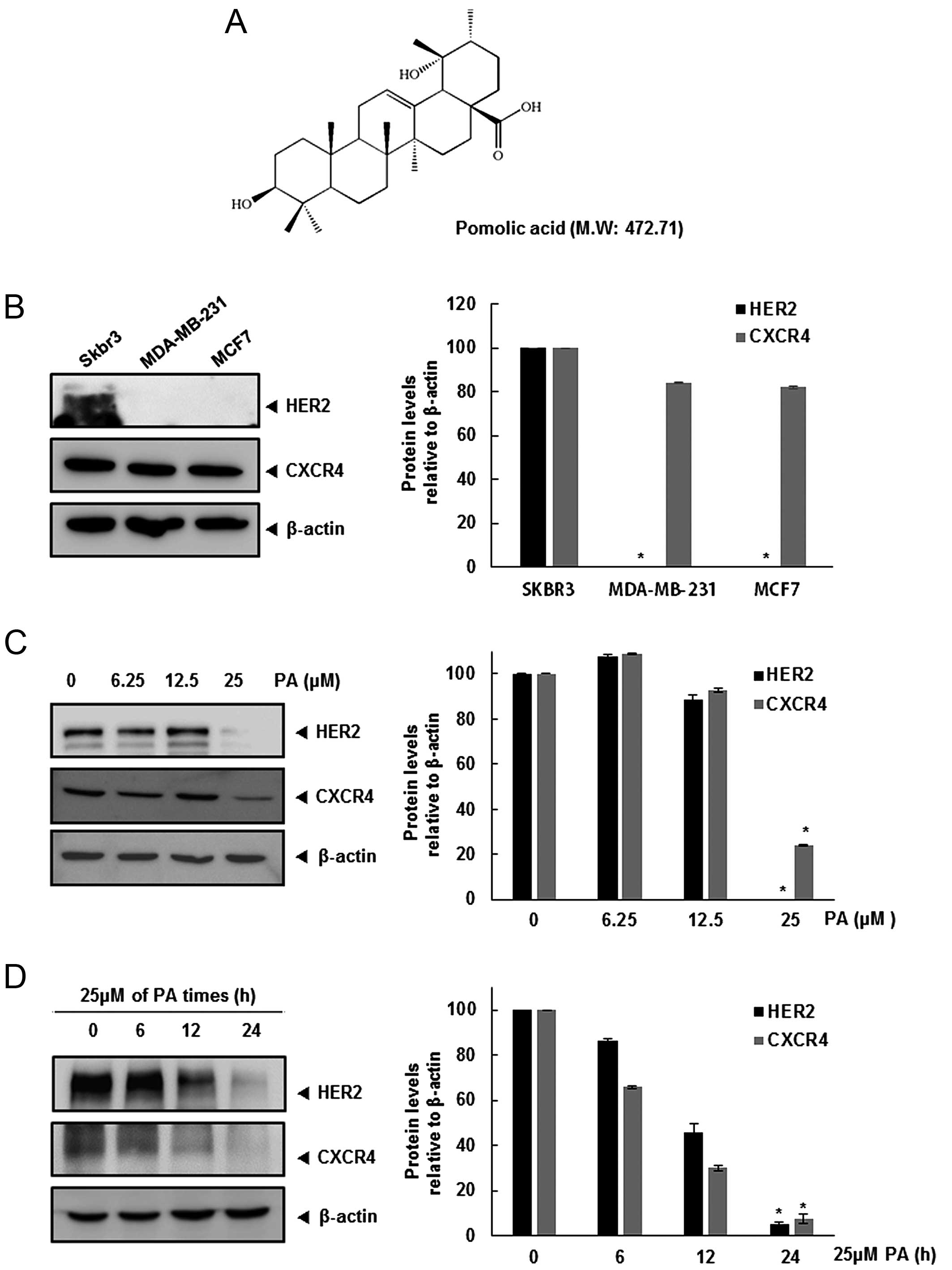
We first investigated the relative expression of CXCR4 and HER2 in
three breast cancer cell lines. Expression of CXCR4 was detected in
all three cell lines, but constitutive HER2 was found only in SKBR3
cells (Fig. 1B). Hence, we
determined whether HER2 overexpression affects CXCR4 expression,
and the pathway involved in this regulation. Furthermore, we
assessed whether PA can modulate both HER2 and CXCR4 expression in
HER2-overexpressing SKBR3 cells. When SKBR3 cells were incubated
either with different concentrations of PA for 24 h or with 25 μM
PA for different times, CXCR4 and HER2 expression was suppressed in
a dose- and time-dependent manner (Fig. 1C and D).
PA-induced downregulation of HER2 and
CXC4 is not mediated through its degradation
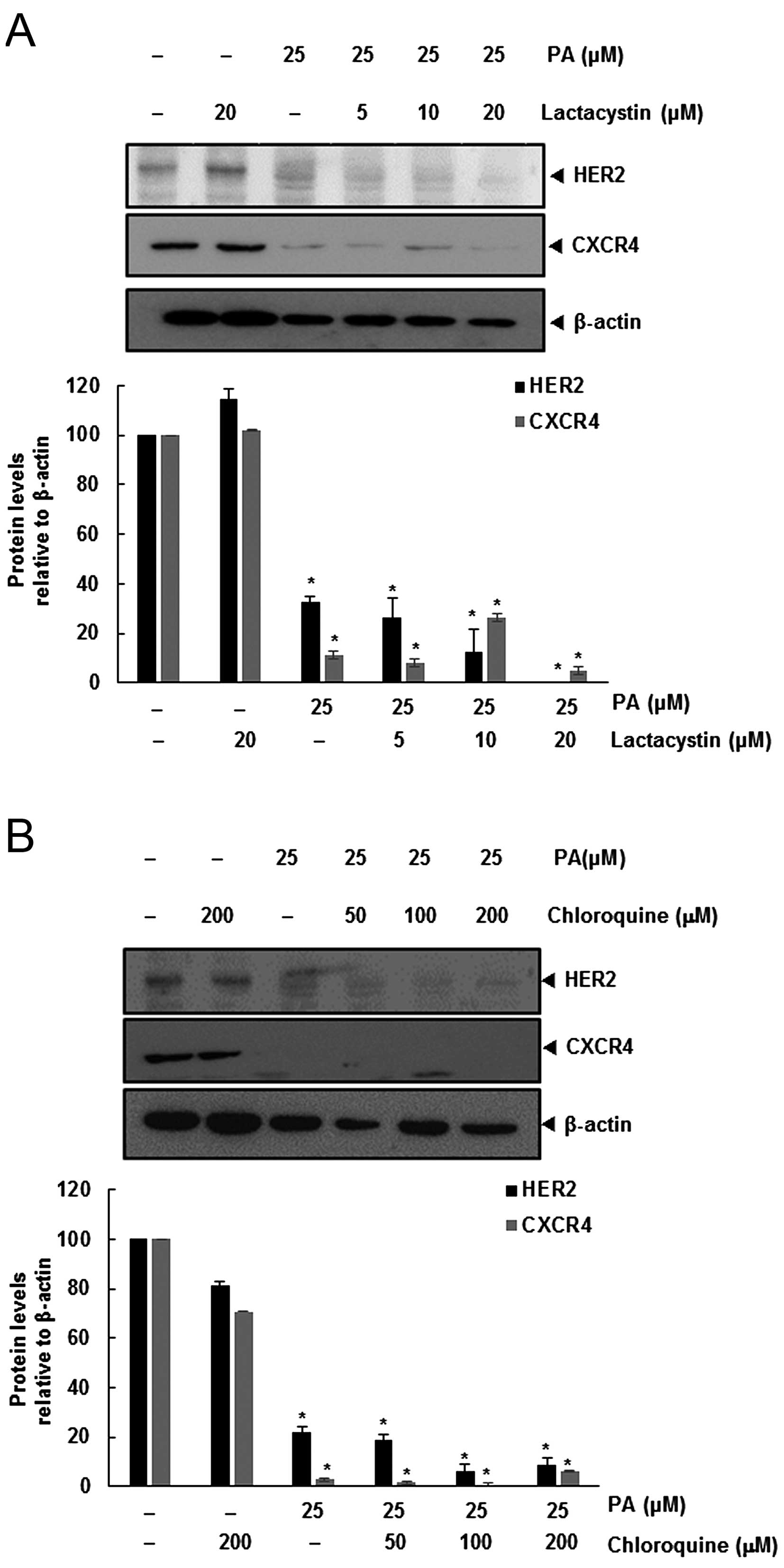
Degradation of HER2 leads to binding of Hsp90 and
ubiquitination of the receptor that targets the protein for
proteasomal degradation (13).
Also, CXCR4 undergoes ubiquitination at its lysine residue,
followed by degradation (14,15),
and so we investigated the possibility that PA enhances the rate of
both HER2 and CXCR4 degradation through proteasomal activation. We
determined if PA induced the degradation of HER2 and CXCR4, by
treating SKBR3 cells with the proteasomal inhibitor lactacystin.
SKBR3 cells were pretreated with lactacystin for 1 h before being
exposed to PA. As shown in Fig.
2A, lactacystin had no effect on PA-induced downregulation of
HER2 and CXCR4.
Several studies have shown that degradation of HER2
is dependent on both lysosomal and proteasomal proteases (16). CXCR4 undergoes ligand-dependent
lysosomal degradation (14), so we
investigated whether chloroquine, a lysosomal inhibitor, blocks
PA-induced degradation of SKBR3. Cells were pretreated with
chloroquine 1 h before exposure to PA. The lysosomal inhibitor had
no influence on the downregulation of HER2 and CXCR4 (Fig. 2B), indicating that this was not the
primary pathway for suppressing HER2 and CXCR4 expression.
PA significantly downregulates both HER2
and CXCR4 at transcriptional level
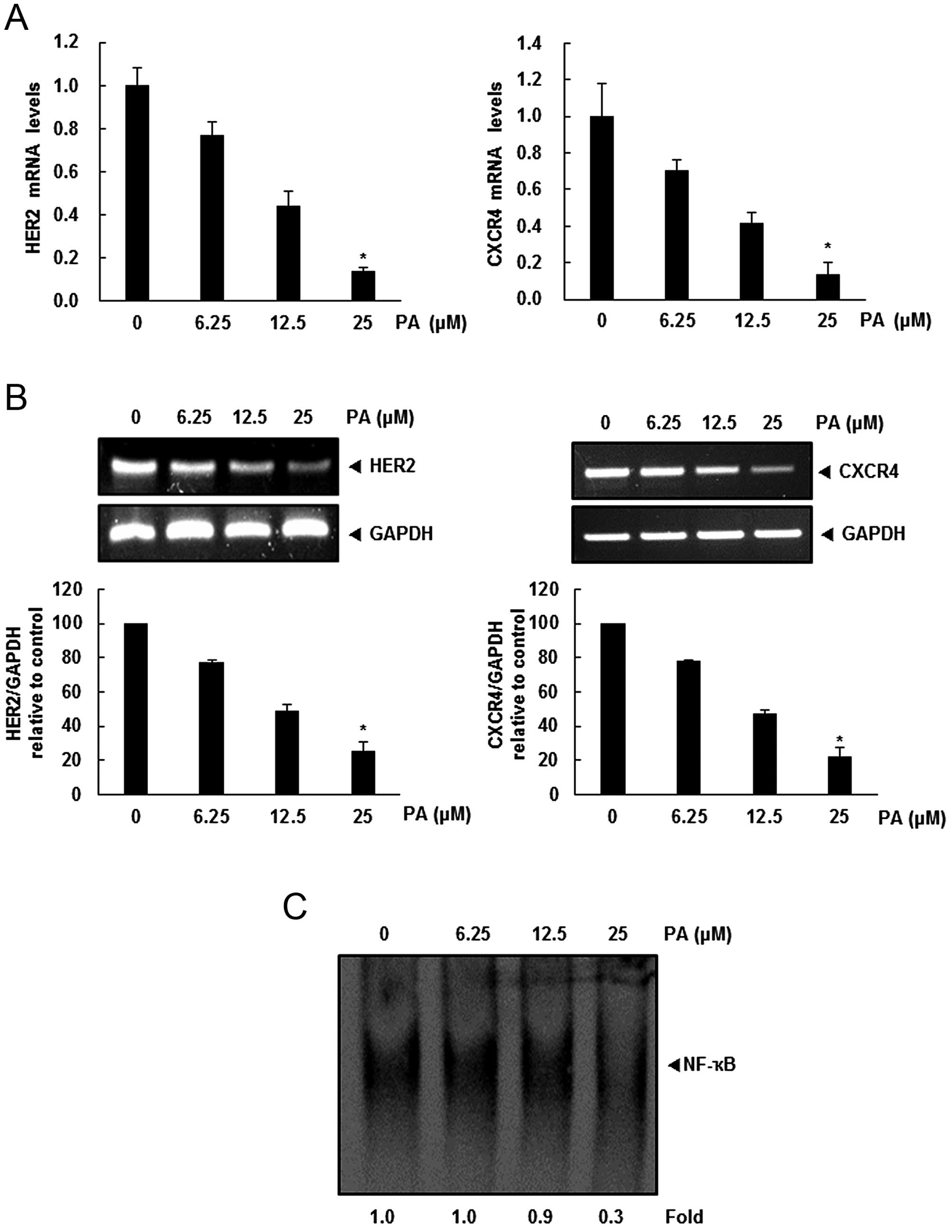
Because PA did not downregulate HER2 and CXCR4
expression by enhancing its degradation, we investigated whether
suppression occurred at transcriptional level using RT-PCR and
real-time PCR. Cells were treated with PA for the indicated
concentrations and then mRNA level of HER2 and CXCR4 was examined.
As shown in Fig. 3A and B,
downregulation of HER2 and CXCR4 mRNA levels was detected in a
dose-dependent manner.
PA inhibits constitutive activation of
NF-κB in HER2 overexpression breast cancer cells
NF-κB activity is closely related to modulation of
critical genes involved in cancer metastasis (17). There is a positive correlation
between HER2 overexpression and constitutive activation of NF-κB in
breast cancer cells (18,19). Also, the extracellular
signal-activated transcription factor NF-κB regulates the
expression of the chemokine receptor CXCR4, which has recently been
implicated in organ-specific metastasis of breast cancer cells
(20). Therefore, we tried to
determine whether the PA exerts its effect on CXCR4 by suppressing
NF-κB activation. We performed a DNA-binding assay to explore the
effect of PA on constitutive NF-κB activation in SKBR3 cells, and
demonstrated that PA treatment suppressed NF-κB activation in a
dose-dependent manner (Fig.
3C).
PA downregulates the MAPK/ERK
pathway
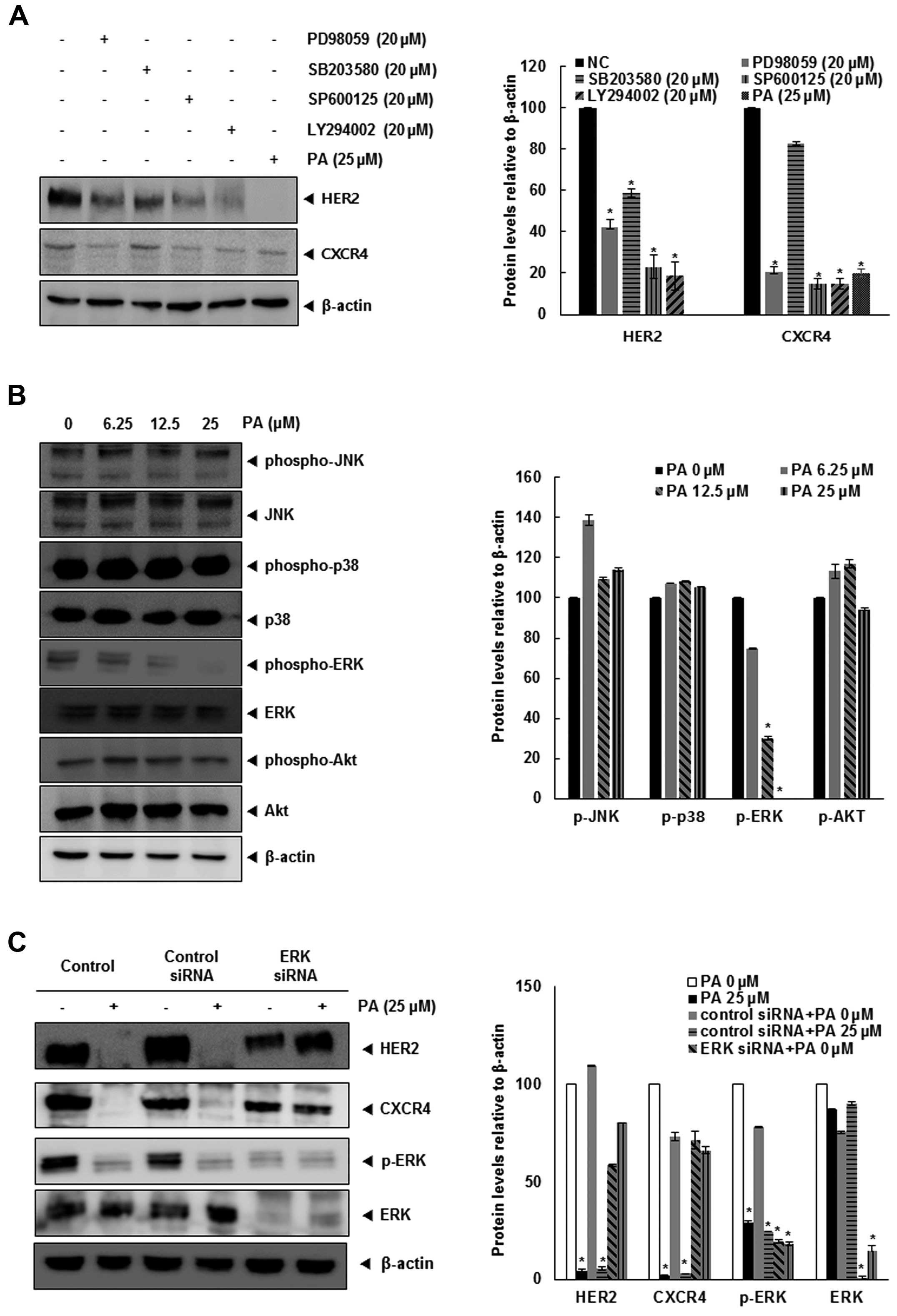
Because PA downregulates both HER2 and CXCR4, we
focused on further signaling pathways related to these receptors.
Various signaling pathways have been implicated in CXCR4 and HER2,
particularly MAPKs (21–24). Therefore, we identified the MAPK
signaling pathway involved in HER2 and CXCR4, utilizing specific
kinase inhibitors for ERK (PD98059), JNK (SP600125), p38 (SB203580)
and PI3K (LY294002). Fig. 4A shows
that PD98059, SP600125, LY294002 and PA strongly inhibited the
expression of HER2 and CXCR4 in SKBR3 cells. Moreover, PA inhibited
the phosphorylation of ERK1/2 in a dose-dependent manner (Fig. 4B).
We confirmed upregulation of ERK-MAPK-dependent HER2
and CXCR4 by treating SKBR3 cells with ERK1/2 siRNA. After
transfection with ERK1/2 siRNA for 24 h, the expression of ERK1/2
and p-ERK1/2 was abolished (Fig.
4C), and cells transfected with ERK1/2 siRNA did not show a
change in HER2 and CXCR4 expression levels. Together, these data
suggest that the underlying mechanism by which PA suppresses HER2
and CXCR4 in SKBR3 breast cancer cells is inhibition of the
ERK-MAPK pathway.
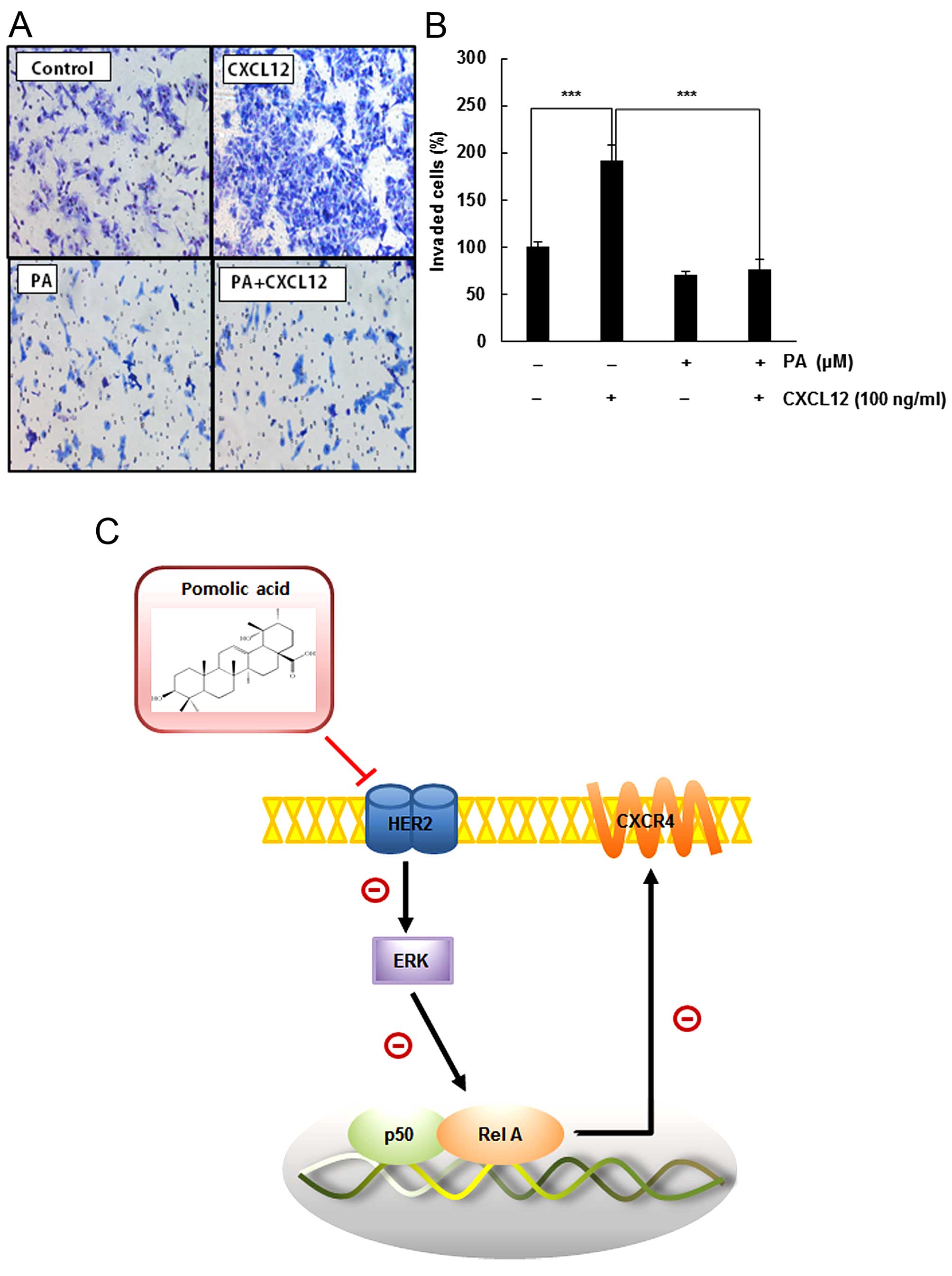
PA suppresses CXCL12-induced invasion by
HER2-positive SKBR3 breast cancer cells
The CXCL12/CXCR4 biological axis also modulates the
chemotactic motility of cancer cells. We next evaluated whether PA
inhibited this process. As shown in Fig. 5A, CXCL12 increased significantly
the number of invasive cells. On the contrary, PA abrogated the
invasiveness of SKBR3 cells induced by CXCL12.
Discussion
The mortality rate of metastatic breast cancer is
higher than that of the primary tumor. Recently several clinical
studies have demonstrated that overexpression of HER2 is associated
with increased proliferation, invasiveness and a poor prognosis
(2,25,26).
Further, CXCR4 expression is strongly correlated with the degree of
metastasis to various organs in breast cancers (27,28).
A previous study reported that expression of CXCR4 was associated
with invasiveness and migration, as well as HER2 overexpression
(29). Other researchers also
suggested that HER2 and CXCR4 expression was related to a poor
survival rate in primary breast tumor tissues (5). Furthermore, regulation of CXCR4 by
estrogens acting through ER may play an important role in
determining the metastasis of breast cancer cells.
Our previous study reported that PA inhibited CXCR4
via the NF-κB pathway in breast cancer cells (9). However, the signaling pathway
involved in CXCR4 inhibition was not fully investigated, neither
the relationship between HER2 and CXCR4. Therefore we explored the
mechanism by which PA suppresses metastasis of breast cancer cells
expressing both HER2 and CXCR4. We found that PA not only showed a
significant inhibitory effect on HER2 and CXCR4 expression, but
also regulated ERK and NF-κB activation in SKBR3 cell lines.
Recent reports have shown that degradation of CXCR4
involves atrophin-interacting protein (AIP)-4 mediated
ubiquitination and degradation (15). Moreover, degradation of HER2 is due
to ubiquitination of the receptor that targets the protein for
proteasomal and lysosomal degradation (16). However, our data indicate that PA
does not downregulate HER2 and CXCR4 through these mechanisms. This
suggests that PA downregulates the expression of HER2 and CXCR4 at
the transcriptional level.
Nonetheless, the exact mechanism of HER2 and CXCR4
by PA is not fully understood. The ERK signaling pathway has a
central action in the regulation of various biological process,
such as proliferation, survival and metastasis (30). Pharmacological inhibition of ERK
signaling has been demonstrated to reduce tumor growth in various
human cancers (31,32). One study suggested that multiple
signaling pathways, including ERK, act as downstream effectors to
promote the invasive potential of breast cancer cells (33). Also, activation of CXCR4 leads to
activation of multiple signaling pathways, including ERK, in
several cell types (34).
Therefore, it is possible that SKBR3 cells acquire invasive
capacity through induction of HER2 and CXCR4 by constitutive
activation of the ERK signaling pathway.
Clinically, NF-κB expression is strongly correlated
with expression of HER2 and CXCR4 in metastatic breast cancer cells
(20,35). Recent studies have shown that
expression of CXCR4 in cancer cells is dependent on the MEK/ERK
signaling cascade and NF-κB activation (36). Also, NF-κB is constitutively active
in breast cancer cells, and this is correlated with increased
expression of HER2 (37). This
study supports these findings in terms of showing that PA inhibits
the ERK signaling pathway and reduces NF-κB activation.
Previous studies showed that CXCL12, the ligand for
the CXCR4 receptor, is upregulated by estrogen in parental breast
cancer cells (38). Estrogen
upregulates the ligand in HER2 expressing breast cancer cells and
increases cell migration (28).
Moreover, the effect of estrogen in high HER2 expressing, estrogen
receptor (ER)-positive cells suggests that ER and HER2 affect CXCR4
levels and upregulate CXCL12, which is critical for activating the
pathway associated with invasiveness (39). Hence, we determined whether PA
suppressed the invasion of HER2-positive breast cancer cells
induced by CXCL12. Our result showed that PA suppressed
CXCL12-induced breast cancer cell invasion.
In addition, PA induced apoptosis in cells from
patients with chronic myeloid leukemia exhibiting different drug
resistance profile (8). Also, PA
may overcome multi-drug resistance mediated by overexpression of
anti-apoptotic bcl-2 proteins (40). It has been reported that bioactive
natural products such as resveratrol, quercetin and catechin will
become major sources of therapeutic agents with breast cancer
combination therapy (41).
Therefore, PA may be used as a potential anticancer drug to
facilitate earlier treatment in patients with breast cancers.
In conclusion, our data demonstrated that PA
suppressed HER2 and CXCR4 expression in HER2-positive breast cancer
cells. This result was correlated with inactivation of the ERK
pathway and suppression of constitutive NF-κB activation.
Further studies with in vivo model are needed
to manifest the relevance of these results to cancer treatment.
Acknowledgements
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea(NRF)
funded by the Ministry of Education (NRF-2016R1A6A1A03011325).
References
|
1
|
Beiki O, Hall P, Ekbom A and Moradi T:
Breast cancer incidence and case fatality among 4.7 million women
in relation to social and ethnic background: A population-based
cohort study. Breast Cancer Res. 14:R52012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nahta R and Esteva FJ: HER2 therapy:
Molecular mechanisms of trastuzumab resistance. Breast Cancer Res.
8:2152006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan
M, Zhou X, Xia W, Hortobagyi GN, Yu D, et al: Upregulation of CXCR4
is essential for HER2-mediated tumor metastasis. Cancer Cell.
6:459–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandes J, Castilho RO, da Costa MR,
Wagner-Souza K, Coelho Kaplan MA and Gattass CR: Pentacyclic
triterpenes from Chrysobalanaceae species: Cytotoxicity on
multidrug resistant and sensitive leukemia cell lines. Cancer Lett.
190:165–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandes J, Weinlich R, Castilho RO,
Kaplan MA, Amarante-Mendes GP and Gattass CR: Pomolic acid triggers
mitochondria-dependent apoptotic cell death in leukemia cell line.
Cancer Lett. 219:49–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasconcelos FC, Gattass CR, Rumjanek VM
and Maia RC: Pomolic acid-induced apoptosis in cells from patients
with chronic myeloid leukemia exhibiting different drug resistance
profile. Invest New Drugs. 25:525–533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim B, Kim JH and Park B: Pomolic acid
inhibits invasion of breast cancer cells through the suppression of
CXC chemokine receptor type 4 epression. J Cell Biochem.
117:1296–1307. 2016. View Article : Google Scholar
|
|
10
|
Lee MK, Lee KY, Jeon HY, Sung SH and Kim
YC: Antifibrotic activity of triterpenoids from the aerial parts of
Euscaphis japonica on hepatic stellate cells. J Enzyme Inhib Med
Chem. 24:1276–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Yu D and Hung MC: Overexpression of ErbB2
in cancer and ErbB2-targeting strategies. Oncogene. 19:6115–6121.
2000. View Article : Google Scholar
|
|
13
|
Yan YY, Zheng LS, Zhang X, Chen LK, Singh
S, Wang F, Zhang JY, Liang YJ, Dai CL, Gu LQ, et al: Blockade of
Her2/neu binding to Hsp90 by emodin azide methyl anthraquinone
derivative induces proteasomal degradation of Her2/neu. Mol Pharm.
8:1687–1697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marchese A and Benovic JL:
Agonist-promoted ubiquitination of the G protein-coupled receptor
CXCR4 mediates lysosomal sorting. J Biol Chem. 276:45509–45512.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhandari D, Trejo J, Benovic JL and
Marchese A: Arrestin-2 interacts with the ubiquitin-protein
isopeptide ligase atrophin-interacting protein 4 and mediates
endosomal sorting of the chemokine receptor CXCR4. J Biol Chem.
282:36971–36979. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pangburn HA, Ahnen DJ and Rice PL:
Sulindac metabolites induce proteosomal and lysosomal degradation
of the epidermal growth factor receptor. Cancer Prev Res (Phila).
3:560–572. 2010. View Article : Google Scholar
|
|
17
|
Andela VB, Schwarz EM, Puzas JE, O'Keefe
RJ and Rosier RN: Tumor metastasis and the reciprocal regulation of
prometastatic and antimetastatic factors by nuclear factor kappaB.
Cancer Res. 60:6557–6562. 2000.PubMed/NCBI
|
|
18
|
Rivas MA, Tkach M, Beguelin W, Proietti
CJ, Rosemblit C, Charreau EH, Elizalde PV and Schillaci R:
Transactivation of ErbB-2 induced by tumor necrosis factor alpha
promotes NF-kappaB activation and breast cancer cell proliferation.
Breast Cancer Res Treat. 122:111–124. 2010. View Article : Google Scholar
|
|
19
|
Guo G, Wang T, Gao Q, Tamae D, Wong P,
Chen T, Chen WC, Shively JE, Wong JY and Li JJ: Expression of ErbB2
enhances radiation-induced NF-kappaB activation. Oncogene.
23:535–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Helbig G, Christopherson KW II,
Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE and
Nakshatri H: NF-kappaB promotes breast cancer cell migration and
metastasis by inducing the expression of the chemokine receptor
CXCR4. J Biol Chem. 278:21631–21638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adeyinka A, Nui Y, Cherlet T, Snell L,
Watson PH and Murphy LC: Activated mitogen-activated protein kinase
expression during human breast tumorigenesis and breast cancer
progression. Clin Cancer Res. 8:1747–1753. 2002.PubMed/NCBI
|
|
22
|
Yu T, Wu Y, Helman JI, Wen Y, Wang C and
Li L: CXCR4 promotes oral squamous cell carcinoma migration and
invasion through inducing expression of MMP-9 and MMP-13 via the
ERK signaling pathway. Mol Cancer Res. 9:161–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan S, Shukla S, Sinha S, Lakra AD, Bora
HK and Meeran SM: Centchroman suppresses breast cancer metastasis
by reversing epithelial-mesenchymal transition via downregulation
of HER2/ERK1/2/MMP-9 signaling. Int J Biochem Cell Biol. 58:1–16.
2015. View Article : Google Scholar
|
|
24
|
Zhan Y, Zhang H, Li J, Zhang Y, Zhang J
and He L: A novel biphenyl urea derivate inhibits the invasion of
breast cancer through the modulation of CXCR4. J Cell Mol Med.
19:1614–1623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB Receptors in Cancer Cell Migration and
Invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berger MS, Locher GW, Saurer S, Gullick
WJ, Waterfield MD, Groner B and Hynes NE: Correlation of c-erbB-2
gene amplification and protein expression in human breast carcinoma
with nodal status and nuclear grading. Cancer Res. 48:1238–1243.
1988.PubMed/NCBI
|
|
27
|
Okuyama Kishima M, de Oliveira CE,
Banin-Hirata BK, Losi-Guembarovski R, Brajão de Oliveira K,
Amarante MK and Watanabe MA: Immunohistochemical expression of
CXCR4 on breast cancer and its clinical significance. Anal Cell
Pathol (Amst). 2015:8910202015.
|
|
28
|
Kato M, Kitayama J, Kazama S and Nagawa H:
Expression pattern of CXC chemokine receptor-4 is correlated with
lymph node metastasis in human invasive ductal carcinoma. Breast
Cancer Res. 5:R144–R150. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salvucci O, Bouchard A, Baccarelli A,
Deschênes J, Sauter G, Simon R, Bianchi R and Basik M: The role of
CXCR4 receptor expression in breast cancer: A large tissue
microarray study. Breast Cancer Res Treat. 97:275–283. 2006.
View Article : Google Scholar
|
|
30
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang Z, Tang Y, Fang J, Zhou Z, Xing Z,
Guo Z, Guo X, Wang W, Jiao W, Xu Z, et al: Simvastatin inhibits
renal cancer cell growth and metastasis via AKT/mTOR, ERK and
JAK2/STAT3 pathway. PLoS One. 8:e628232013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu S, Lao XY, Sun TT, Ren LL, Kong X, Wang
JL, Wang YC, Du W, Yu YN, Weng YR, et al: Knockdown of ZFX inhibits
gastric cancer cell growth in vitro and in vivo via downregulating
the ERK-MAPK pathway. Cancer Lett. 337:293–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ke Z, Lin H, Fan Z, Cai T-Q, Kaplan RA, Ma
C, Bower KA, Shi X and Luo J: MMP-2 mediates ethanol-induced
invasion of mammary epithelial cells over-expressing ErbB2. Int J
Cancer. 119:8–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui C, Wang P, Cui N, Song S, Liang H and
Ji A: Sulfated poly-saccharide isolated from the sea cucumber
Stichopus japonicas promotes the SDF-1α/CXCR4 axis-induced NSC
migration via the PI3K/Akt/FOXO3a, ERK/MAPK, and NF-κB signaling
pathways. Neurosci Lett. 616:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Merkhofer EC, Cogswell P and Baldwin AS:
Her2 activates NF-kappaB and induces invasion through the canonical
pathway involving IKKalpha. Oncogene. 29:1238–1248. 2010.
View Article : Google Scholar
|
|
36
|
Kukreja P, Abdel-Mageed AB, Mondal D, Liu
K and Agrawal KC: Up-regulation of CXCR4 expression in PC-3 cells
by stromal-derived factor-1alpha (CXCL12) increases endothelial
adhesion and transendothelial migration: Role of MEK/ERK signaling
pathway-dependent NF-kappaB activation. Cancer Res. 65:9891–9898.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bhat-Nakshatri P, Sweeney CJ and Nakshatri
H: Identification of signal transduction pathways involved in
constitutive NF-kappaB activation in breast cancer cells. Oncogene.
21:2066–2078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frasor J, Danes JM, Komm B, Chang KC,
Lyttle CR and Katzenellenbogen BS: Profiling of estrogen up- and
down-regulated gene expression in human breast cancer cells:
Insights into gene networks and pathways underlying estrogenic
control of proliferation and cell phenotype. Endocrinology.
144:4562–4574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sengupta S, Schiff R and Katzenellenbogen
BS: Post-transcriptional regulation of chemokine receptor CXCR4 by
estrogen in HER2 overexpressing, estrogen receptor-positive breast
cancer cells. Breast Cancer Res Treat. 117:243–251. 2009.
View Article : Google Scholar :
|
|
40
|
Fernandes J, Weinlich R, Castilho RO,
Amarante-Mendes GP and Gattass CR: Pomolic acid may overcome
multidrug resistance mediated by overexpression of anti-apoptotic
Bcl-2 proteins. Cancer Lett. 245:315–320. 2007. View Article : Google Scholar
|
|
41
|
Schlachterman A, Valle F, Wall KM, Azios
NG, Castillo L, Morell L, Washington AV, Cubano LA and
Dharmawardhane SF: Combined resveratrol, quercetin, and catechin
treatment reduces breast tumor growth in a nude mouse model. Transl
Oncol. 1:19–27. 2008. View Article : Google Scholar : PubMed/NCBI
|