Introduction
Cervical cancer (CC), the third most common cancer
among women worldwide (1), is
strongly associated with infection and subsequent transformation of
cervical cells by high risk-human papillomavirus (HR-HPVs)
(2), of which HPV16 the most
prevalent high-risk genotype in CC (3). The HR-HPVs contain three major viral
oncoproteins: E5, E6 and E7, which are involved in the induction
and maintenance of the transformed phenotype (4). The E7 oncoprotein is the major
transforming protein and in the context of genetically engineered
mice expressing E7 in cooperation with estradiol (E2) it
induces cervical tumors (5), in
part due to the E7 functions shown previously to dysregulate the
cell cycle including: E7 binding, destabilization and consequent
destruction of pRb (6). HPV16/18
E7 has been shown to associate and modify the normal activities of
cellular regulatory complexes altering cellular gene expression
(6,7). Moreover, E2 binds to the
estrogen receptor (ER) to regulate the transcription of many genes
through both genomic and non-genomic pathways (8,9).
Genomic pathways include the classical interactions of ligand-bound
ER dimers with estrogen-responsive elements in target gene
promoters, in which ER interacts with Sp1, AP1 and NF-κB proteins.
Non-genomic pathways involve effects through cell surface receptors
linked to the mitogen-activated protein kinase pathway (8,9) and
may indirectly regulate gene activity (8).
MicroRNAs (miRNAs or miRs) are an abundant class of
small non-coding RNA molecules that measure approximately 22
nucleotides in length. They function to regulate gene expression at
the post-transcriptional level by mRNA degradation or alternatively
by translational repression of protein-coding mRNAs (10). Moreover, miRNAs function as
oncogenes or tumor suppressor, depending on the cellular context
and the target genes (11). By
targeting mRNAs, miRNAs play critical roles in cell proliferation
(12) and apoptosis (13), as well as in the initiation and
progression of cancer (14,15).
Several studies have documented a relationship between the aberrant
expression of a class of miRNAs and the pathogenesis of many human
cancers (11,14,16),
including CC (17–22). Even though miR-21 and miR-143 are
aberrantly expressed in CC and are closely related to CC
development (23,24), the participation of the E7
oncoprotein and the hormonal environment have not been studied.
miR-21 functions as an oncogene by targeting tumor suppressor genes
including tropomyosin 1 (TPM1), programmed cell death 4 (PDCD4),
and phosphatase and tensin homolog (PTEN); increase in miR-21
levels leads to cell proliferation and inhibition of apoptosis,
inducing cancer invasion and metastasis (25). The promoter regulatory region of
miR-21 gene consists of several binding sites for transcription
factors such as activator protein 1 (AP1), and signal transducer
and activator of transcription 3 (STAT3) (26). In contrast, miR-143 through the
regulation of antiapoptotic Bcl-2 may play an important role in the
pathogenesis of CC as tumor suppressor; overexpression of miR-143
in HeLa cells results in apoptosis and suppression of both Bcl-2
expression and cell proliferation (27). Furthermore, the presence of the
Bcl-2 protein is strongly associated with the development of CC
(28) and was significantly
correlated to the grades of cervical intraepithelial neoplasia
(29).
The HR-HPVs are responsible for the upregulation of
oncogenic and/or downregulation of tumor suppressive miRNAs through
their viral oncoproteins. The HR-HPV16 E7 oncoprotein upregulates
the expression of miR-15b and miR-27b (30,31).
E2 has also been involved in regulating the expression
of microRNAs with tumor suppressor and oncogenic functions.
E2 induces 21 miRNAs and represses seven miRNAs in MCF-7
breast cancer cells that have the potential to control 420
E2-regulated mRNAs at the post-transcriptional level
(32).
In the present study we investigated if HPV16 E7
oncoprotein and 17β-estradiol deregulate the expression of miR-21
and miR-143 and their target genes, PTEN and Bcl-2, respectively.
We found that in the presence of HPV16 E7 oncoprotein and
E2, miR-21 expression was upregulated, while miR-143
expression was downregulated. In addition, we observed a negative
correlation between miR-21 and miR-143 expression and their target
genes in vitro and in vivo.
Materials and methods
Clinical samples
We analyzed cervical scrapings from 10 HPV negative
healthy women and 14 HPV16 positive cervical samples (biopsy
tissues) from patients with CC, samples were obtained in the
Molecular Biomedicine and Cytopathology Laboratories at the School
of Chemistry and Biology of the Autonomous University of Guerrero
in Chilpancingo Guerrero, and National Cancer Institute in Mexico
City, Mexico between 2012 and 2013. This study was approved by the
Bioethical Committee of both institutions. Written informed consent
was obtained from all the participants.
The scraped cells from HPV-negative healthy women
were suspended in 5 ml ice cold phosphate-buffered saline [(6.4 mM
Na2HPO4, 1.5 mM KH2PO4,
140 mM NaCl, and 2.7 mM KCl (pH 7.2)] and kept on ice until further
processing. The cell suspension was centrifuged and washed with
wash buffer [10 mM Hepes/KOH (pH 7.5), 1.5 mM MgCl2, 10
mM KCl and 1 mM dithiothreitol]; after the pellet was snap-frozen
in liquid nitrogen and then stored at −80°C until used for miRNAs
and mRNA quantification.
Cell lines
Osteosarcoma Saos2 cell line was obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). This
cell line was maintained in Dulbecco's modified Eagle's medium
(Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum
(FBS; Invitrogen, Carlsbad, CA, USA), 2 mM L-glutamine and 100 U/ml
penicillin/streptomycin (Invitrogen) and incubated at 37°C in a
humidified 5% CO2 atmosphere. Transfection of the E7
oncogene (plasmid pcDNA3E7) was performed using Lipofectamine 2000
reagent (Invitrogen) according to the manufacturer's instructions.
In order to obtain a stable cell line, transfected cells were
selected for 2 weeks in a growth media containing 1,200 mg/ml of
G418 (Invitrogen). To keep clone selection, cells were grown
continuously in a medium containing 800 mg/ml of G418.
Transgenic mice
The K14E7HPV16 transgenic (K14E7) and FvB/n control
[non-transgenic (NT)] mice have been previously described (5,33).
K14E7 mice were backcrossed in the FVB/n background, maintained and
used as heterozygotes in our experiments. The mice were housed in a
pathogen-free barrier facility, according to the Association for
Assessment and Accreditation of Laboratory Animal Care
International (AAALAC-International). All the experiments and
procedures were approved by the Research Unit for Laboratory Animal
Care Committee (UPEAL-Cinvestav-IPN, Mexico; NOM-062-ZOO-1999).
Hormone treatment
Mice were treated with 17β-estradiol (E2)
as previously reported (5).
Briefly, one month-old virgin female E7 transgenic and NT mice were
s.c. implanted in the dorsal skin with continuous release pellets
delivering 0.05 mg E2 over 60 days (Innovative Research
of America, Sarasota, FL, USA). Mice were treated with hormone for
6 months; the treatment time required the insertion of three
pellets. Groups of 5 transgenic and NT female mice were used for
this study.
Harvesting of the of specimens
After sacrifice by cervical dislocation, cervical
tissue was rapidly removed and was immediately stored in
RNAlater solution (Ambion, USA) at 4°C overnight. Tissue was
recovered from the solution with sterile forceps, quickly blotted
to remove excess RNAlater and immediately snap frozen in
liquid nitrogen.
Total RNA extraction
Total RNA (large and small size RNAs) was extracted
from cultured cells and cervical tissue. For total RNA extraction
the TRIzol method (Invitrogen) was employed according to the
manufacturer's protocol, and the quality and concentration of RNA
were spectrophotometrically assessed by measuring absorbance at
A260/280 and by agarose gels stained with ethidium bromide.
Quantification of miRNAs and mRNA levels
using real-time PCR
To detect the levels of mature miRNA in cultured
cells and cervical tissue, 1–10 ng of total RNA were reverse
transcribed to cDNA with specific RT primers using
TaqMan® MicroRNA reverse transcription kit (Applied
Biosystems, Foster City, CA, USA). Stem-loop real-time PCR [miR-21
(ID 000397) and miR-143 (002249)] was used to detect miRNAs level
by the TaqMan® MicroRNA assays (Applied Biosystems). The
PCR cycles were as follows: 94°C for 5 min, followed by 40 cycles
of 94°C for 30 sec, 60°C for 30 sec. Real-time reverse
transcription-polymerase chain reactions were performed in an
Applied Biosystems 7300 Detection system (Applied Biosystems).
Relative expression levels were normalized to the expression of
endogenous control snoRNA202 (001232; Applied Biosystems).
For specific mRNA quantification, total RNA (1 μg)
was reverse transcribed into cDNA by priming with oligo (dT) and
cDNA synthesis by the SuperScript II First-Strand Synthesis System
(Invitrogen) for RT-PCR according to the manufacturer's
instructions. Real-time PCR was performed using an Applied
Biosystems 7300 Detection system (Applied Biosystems), using
SYBR-Green (SYBR-Green PCR reagents kit; Applied Biosystems) and
the protocol provided by the manufacturer. Annealing temperatures
and MgCl2 concentrations were optimized to create a
one-peak melting curve. Additionally, the amplicons were checked by
agarose gel electrophoresis for a single band of the expected size.
PCRs were processed through 40 cycles of a 3-step PCR, including 60
sec of denaturation at 95°C, 60 sec primer dependent annealing
phase (Table I), and 60 sec
template-dependent elongation at 72°C.
 | Table IPrimer sequences used in the present
study. |
Table I
Primer sequences used in the present
study.
| Gene symbol | Forward primer | Reverse primer | Temp. (°C) | Amplicon size
(bp) |
|---|
| Mouse |
| PTEN |
GGATGTCGTCCTGAAGGGAG |
GCTTCGCTGGAGGAACCTG | 60 | 108 |
| BCL2 |
ACTTCGCAGAGATGTCCAGTCA |
TGGCAAAGCGTCCCCTC | 60 | 63 |
| HPRT |
CCAGCAAGCTTGCAACCTTAAC |
GTAATGATCAGTCAACGGGGG | 60 | 177 |
| Human |
| PTEN |
GGTCATGTGTGTGGAGAGC |
GATCCAGGTGTGCAGGTG | 60 | 78 |
| BCL2 |
CCGAAAGGTTTTGCTACCATTCT |
AAAATTATTTCCTTTCTGAGCATTCC | 60 | 105 |
| β2M |
ACCCCCACTGAAAAAGATGAG |
ATGATGCTGCTTACATGTCTCG | 60 | 100 |
The reaction (25 μl) consisted of 12.5 μl SYBR-Green
PCR Master Mix (Applied Biosystems) containing: Taq DNA polymerase,
reaction buffer, dNTP mix, 1 mM MgCl2 (final
concentration) and SYBR-Green I dye, 1 μl of each primer (0.5 μM),
500 ng template per reaction and ultrapure water. All primer
sequences and product sizes are described in Table I. All reactions were performed on
five independent mice, and relative expression levels were
normalized to the expression of HPRT mRNA. The expression of miRNA
and mRNA was determined from the threshold cycle (Ct),
and the relative expression levels were calculated by the
2−ΔΔCt method (34).
Western blot analysis
Frozen (−70°C) cervical samples were pulverized with
a mortar and pestle in liquid nitrogen. Cells and cervical samples
were lysed on ice in lysis buffer (25 mM Tris, pH 7.5, 150 mM NaCl,
1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) containing
proteinase inhibitors and incubated on ice for 30 min. Following
centrifugation at 16,000 x g for 15 min at 4°C, the supernatant
containing the total cell extract was collected and aliquots were
kept at −80°C. The protein concentrations were measured by the
Bradford assay; proteins were heat denatured and ran on 10%
SDS-PAGE gels. The proteins were transferred onto nitrocellulose
membranes and, after blocking with 10% non-fat milk in PBS containg
0.1% Tween-20 (PBST) for 1 h, the membranes were incubated
overnight with diluted (1:500) primary antibody. The following
primary antibodies were used: PTEN (Sc-7974; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and Bcl-2 (Sc-7382, Santa Cruz
Biotechnology). The next day, blots were washed three times for 5
min each with PBST and incubated with HRP-conjugated anti-mouse or
anti-rabbit secondary antibody (Sigma) for 1 h at room temperature.
The stained membranes were visualized by enhanced chemiluminescence
reaction using the SuperSignal West Pico Chemiluminescent Substrate
(Thermo Fisher Scientific).
Statistical analysis
Data were analyzed using GraphPad Prism software
(v5.0; GraphPad Software, Inc., La Jolla, CA, USA). Mann-Whitney
test was used to compare differences among miRNA, mRNA and protein
expression levels between groups and results were presented as mean
± standard deviation (SD). P-values <0.05 were considered
significantly different between data sets.
Results
The E7 oncoprotein and 17β-estradiol
affect miR-21 and miR-143 expression in cervical tissue of K14E7
transgenic mice
It is well known that high-risk HPV16 infection and
estradiol are considered as risks factors for CC. The HPV E7
oncoprotein is the primary factor responsible for blocking cell
cycle exit during differentiation (3,35).
Given that in the transgenic model of cervical cancer (K14E7 mice)
chronic exposure to 17β-estradiol (E2) is important for
neoplasia development, we investigated in mice cervical tissue the
individual and combined effect of E2 and the HPV16 E7
oncoprotein on the expression of miR-21 and miR-143, known to play
a role in the modulation of cell proliferation and survival genes.
Reverse transcription quantitative polymerase chain reaction
(RT-qPCR) analysis of miRNA from cervical tissue clearly showed
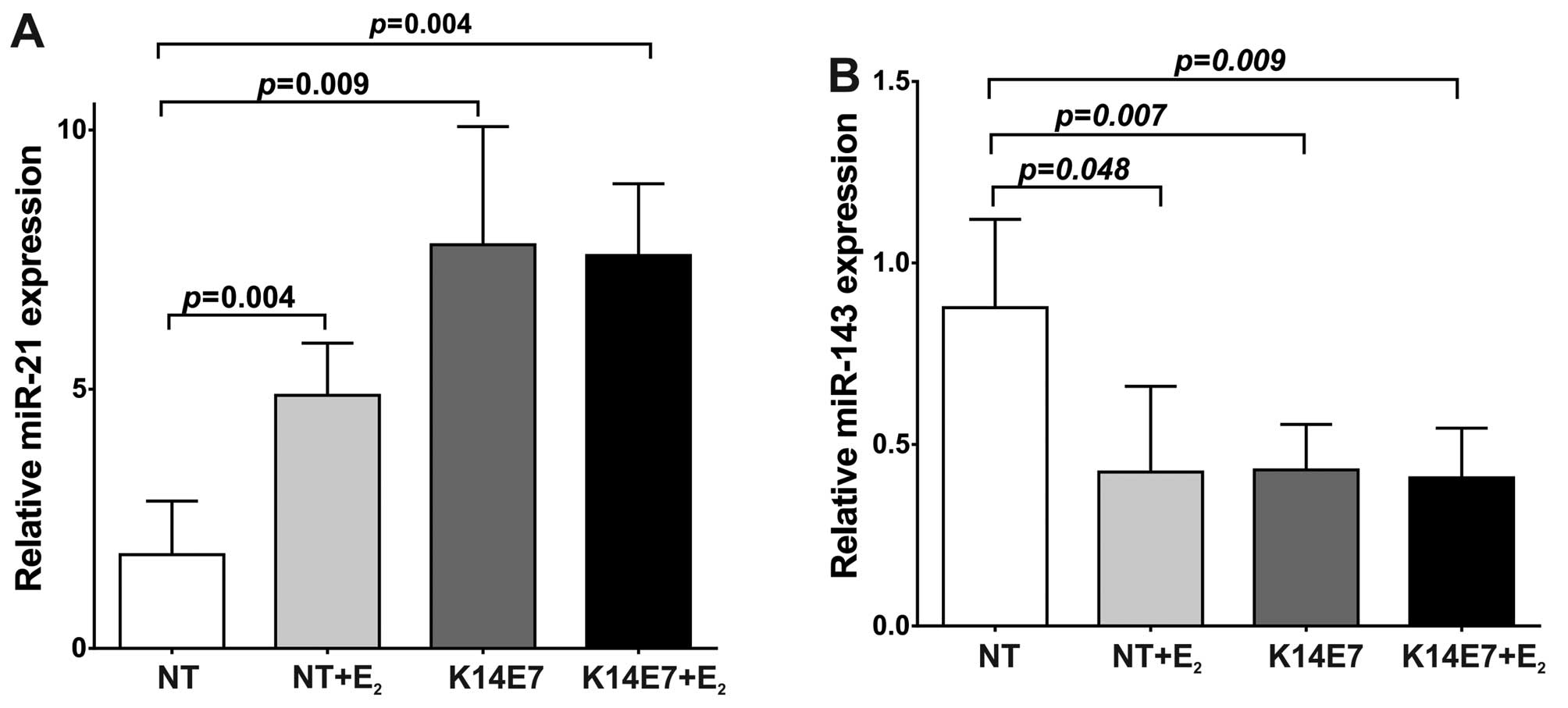
that the expression level of miR-21 was significantly higher in E7
or E2 treated mice. Compared to non-transgenic mice
(NT), miR-21 expression levels in NT+E2 mice were
increased with an average fold change of 4.88-fold (P=0.004;
Fig. 1A). In K14E7 transgenic mice
without E2 treatment miR-21 was also strongly increased
(7.77-fold) compared to NT mice (P=0.009; Fig. 1A). In K14E7+E2 mice the
average fold change of oncogenic miR-21 was 7.58-fold (P=0.004;
Fig. 1A). Thus, in 7 month-old
mice, both HPV16E7 oncoprotein and E2 clearly induce
miR-21 overexpression. In contrast to miR-21, the levels of
miR-143, considered tumor suppressor miRNA, are significantly low
in cervical tissue containing E7 or E2. When compared to
NT mice, the miR-143 expression in NT+E2 mice was
significantly decreased (the average fold change was 0.42-fold)
(Fig. 1B). In cervix of K14E7 mice
without E2 treatment the miR-143 expression level was
also significantly decreased (0.43-fold) (P=0.007; Fig. 1B). Likewise, in K14E7+E2
transgenic mice the average fold change of miR-143 was lower
(0.41-fold) compared to NT mice (P=0.009; Fig. 1B). These data show that in 7
month-old mice the presence of E2 or of the E7
oncoprotein, increased the levels of miR-21 while decreased those
of miR-143.
Expression of miRNA target genes in K14E7
cervical tissue
It has been reported that miR-21 is involved in the
regulation of PTEN, while miR-143 regulates the Bcl-2 expression in
CC. Employing the K14E7 model, we explored whether the
E2 and E7-induced miR-21 upregulation can repress the
levels of mRNA and protein for an important target gene, the
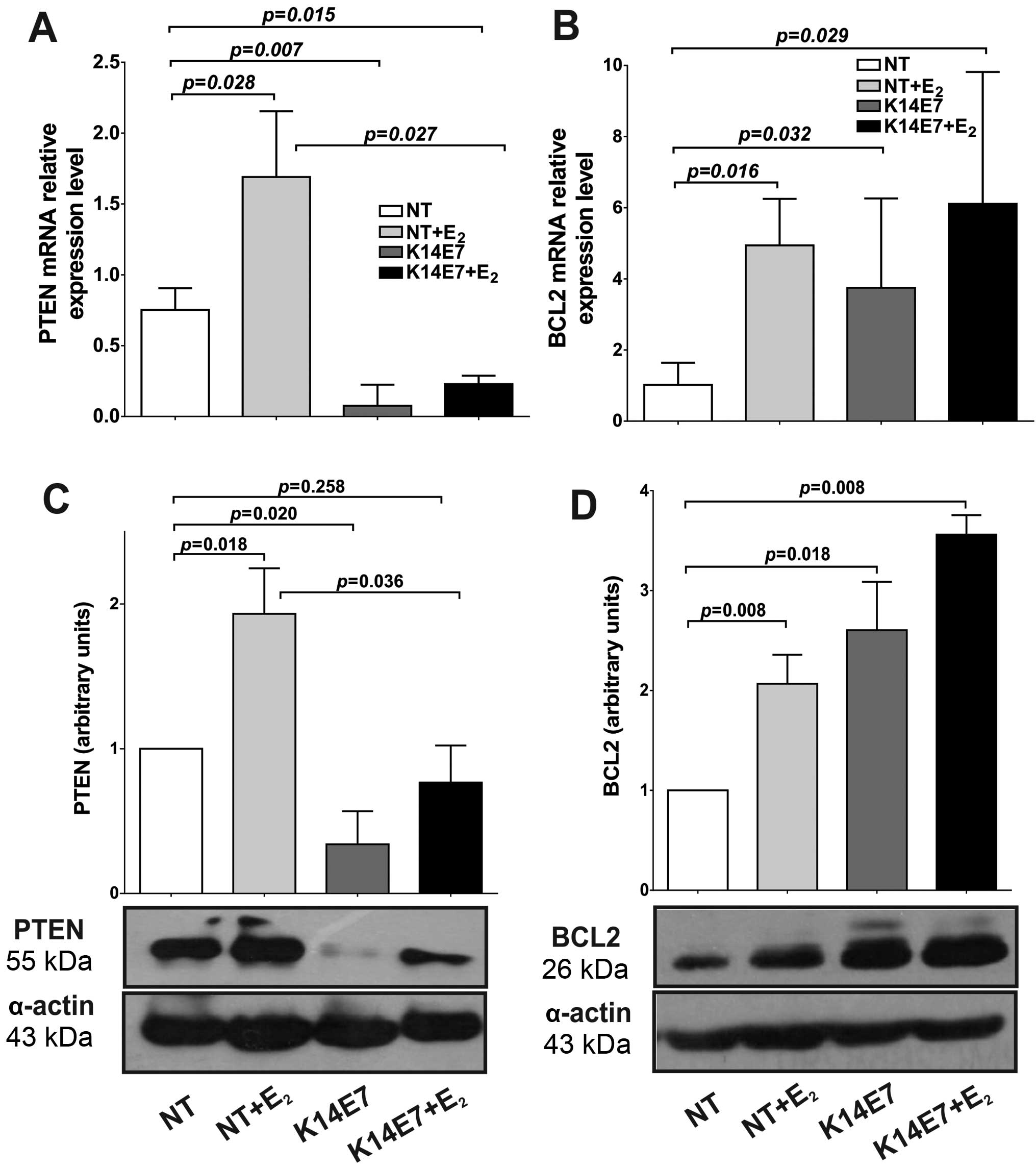
phosphatase-tensin homolog (PTEN). Surprisingly, we observed that
both PTEN mRNA and protein expression were upregulated in
NT+E2 mice as compared with NT (mRNA, 1.69-fold;
protein, 1.93-fold; Fig. 2A and
C). The cervical tissue from K14E7 transgenic mice expressed a
significantly lower level of PTEN mRNA (0.07-fold), while that in
K14E7+E2 mice expressed 0.23-fold compared to NT mice
(Fig. 2A). Similarly, PTEN protein
levels also decreased in K14E7 and K14E7+E2 mice
compared to NT mice, although the decrease in K14E7+E2
mice was not statistically significant (P=0.258). However, when
compared with NT+E2, K14E7+E2 showed a
significant decrease (P=0.036) in cervical tissue. We observed a
significant inverse correlation between miR-21 and PTEN mRNA and
protein expression in the K14E7 transgenic mice (Pearson's
correlation r=−0.881, P=0.049; r=−0.898, P=0.039, respectively),
but not in NT+E2 and K14E7+E2 (Table II).
 | Table IIPearson's correlation coefficients
between miRNA expression, mRNA levels and protein levels. |
Table II
Pearson's correlation coefficients
between miRNA expression, mRNA levels and protein levels.
|
NT+E2 | K14E7 |
K14E7+E2 |
|---|
|
|
|
|
|---|
| r | P-value | r | P-value | r | P-value |
|---|
| miR-21 |
| miR-21 expression
and PTEN mRNA expression | 0.952 | 0.048 | −0.881 | 0.049 | −0.928 | 0.071 |
| miR-21 expression
and PTEN protein levels | 0.689 | 0.199 | −0.898 | 0.039 | 0.371 | 0.539 |
| miR143 |
| miR-143 expression
and BCL2 mRNA expression | −0.916 | 0.029 | −0.939 | 0.018 | −0.960 | 0.040 |
| miR-143 expression
and BCL2 protein levels | −0.952 | 0.048 | −0.956 | 0.044 | −0.946 | 0.015 |
The expression of miR-143 was significantly
decreased by E2 and E7 in cervical tissue (Fig. 1B). To explore whether this
downregulated miR-143 may increase the mRNA and protein levels of
an important target gene, the expression of B-cell lymphoma (BCL-2)
was determined. We performed RT-qPCR and western blot analysis to
determine whether this low miR-143 level leads to increased BCL-2
mRNA and protein levels in cervical cancer. As shown in Fig. 2B downregulated miR-143 induced a
significant increase in BCL-2 mRNA (NT+E2, 4.94; K14E7,
3.75; K14E7+E2, 6.11) as compared with NT mice.
Similarly, BCL-2 protein levels were increased when miR-143 was
downregulated (NT+E2, 2.07; K14E7, 2.60;
K14E7+E2, 3.56) (Fig.
2D). A strong inverse correlation between miR-143 expression
levels and BCL-2 mRNA and protein levels was observed in cervical
tissues from transgenic K14E7 mice, evaluated by Pearson's
correlation (r=−0.939, P=0.018; r=−0.956, P=0.044, respectively)
(Table II). Our results indicate
that PTEN expression was downregulated and BCL-2 expression was
upregulated in K14E7 cervical cancer correlating with miR-21 being
upregulated and miR-143 down-regulated, respectively.
The E7 oncoprotein represses PTEN
expression by upregulating miR-21 in Saos-2 cells
To confirm that E7 expression can disturb miRNA
levels, we stably transfected Saos-2 cells with the HPV16 E7 gene.
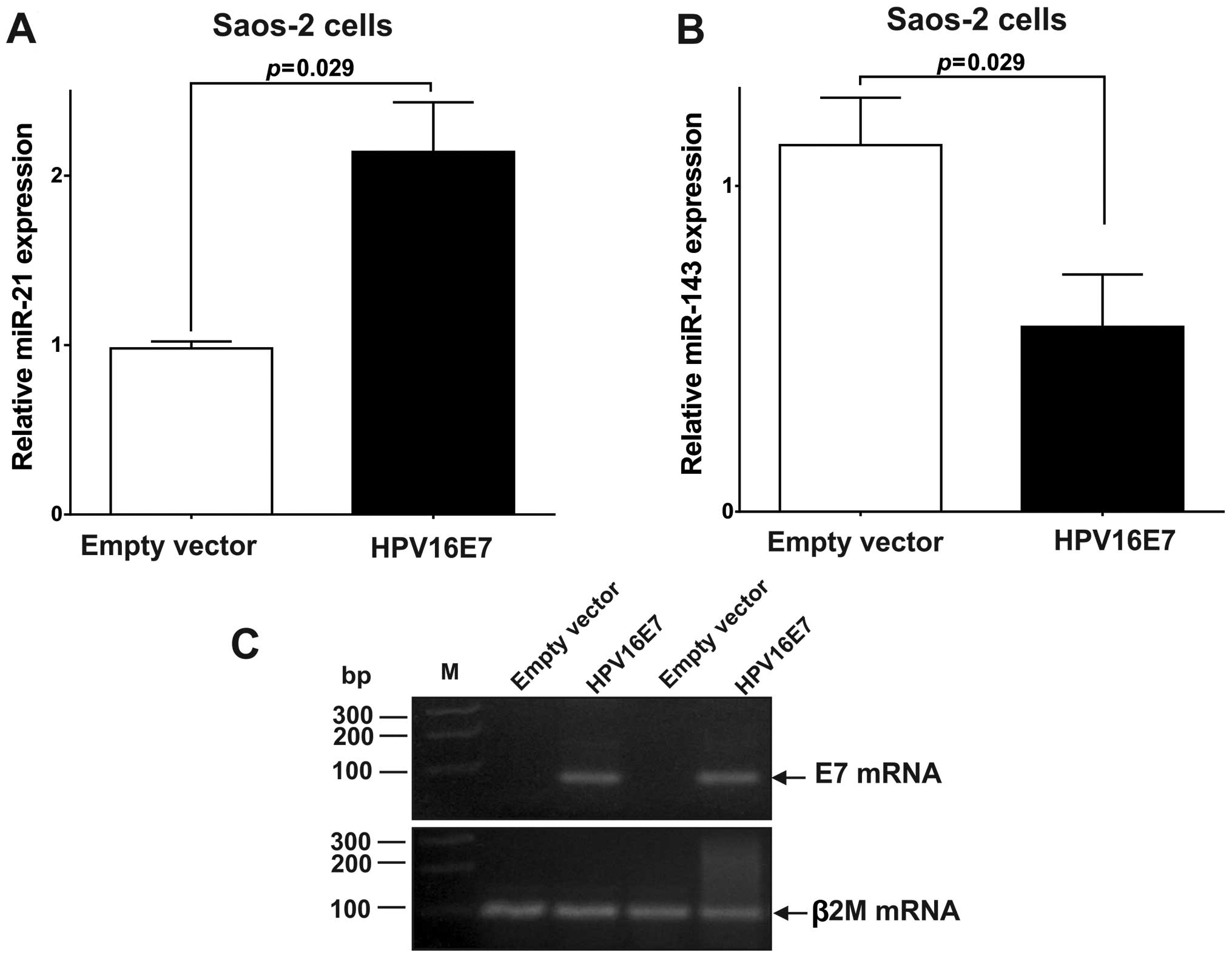
As shown in Fig. 3A, expression of
the E7 oncoprotein resulted in a 2.14-fold upregulation (P=0.029)
in the expression of miR-21 (Fig.
3A). To explore whether the upregulated expression of miR-21
caused by HPV16 E7 oncoprotein expression, can repress its target
genes, the expression of PTEN was measured by RT-qPCR and western
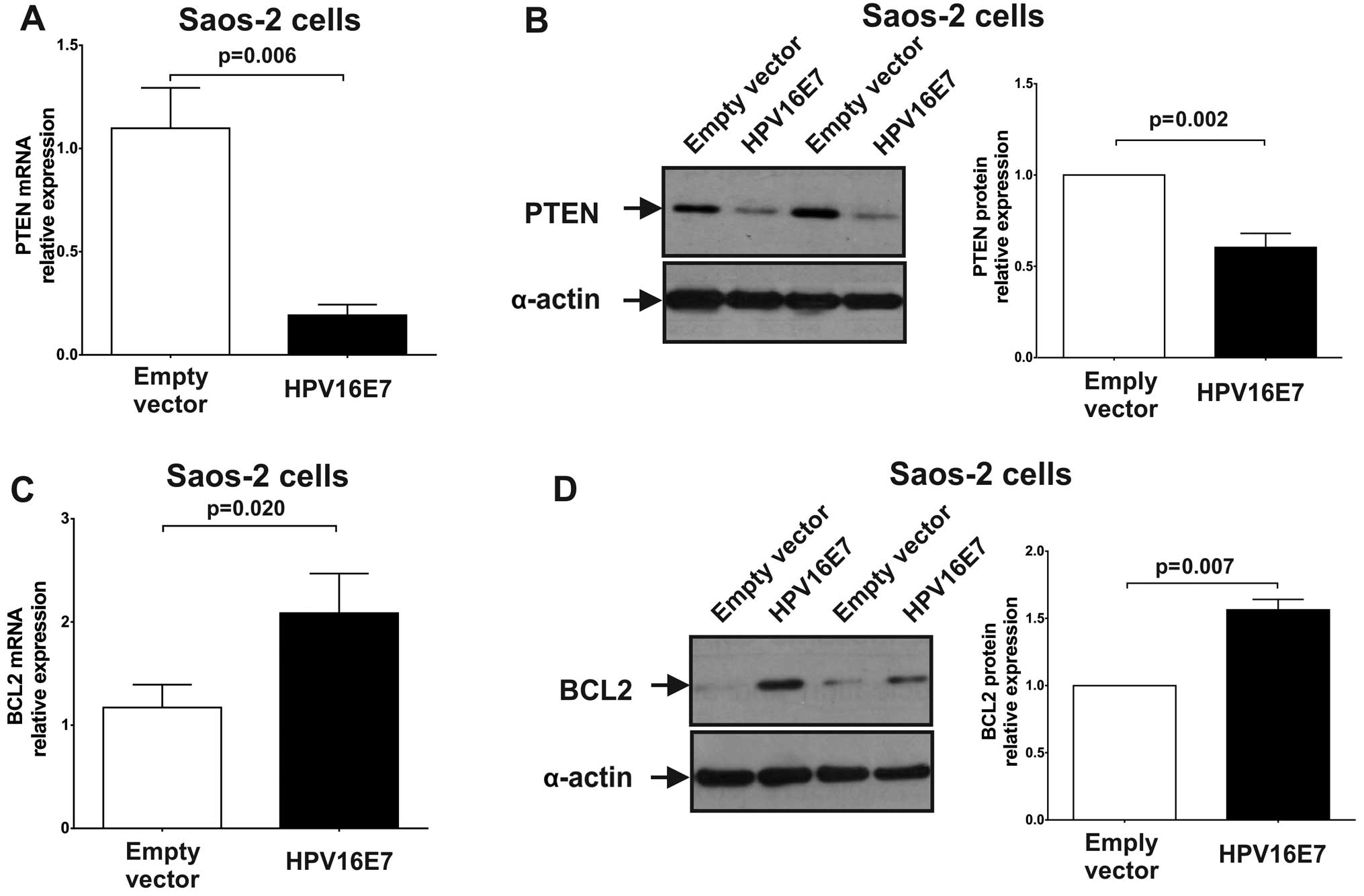
blot analysis. As shown in Fig. 4A and
B, the expression of PTEN was significantly repressed in
miR-21-upregulated Saos-2 cells compared to control (stably
transfected pcDNA3 cells) [PTEN mRNA: 0.19 (P=0.006); PTEN protein:
0.60 (P=0.002)]. The results indicated that miR-21 upregulation,
which is caused by the HPV16 E7 oncoprotein, represses PTEN
expression.
The E7 oncoprotein upregulates expression
of BCL-2 by downregulating miR-143
We observed that in Saos-2 cells the E7 oncoprotein
downregulated (0.57-fold, P=0.029) the expression of miR-143
(Fig. 3B). To explore whether the
downregulation of miR-143 in Saos-2 cells expressing the E7
oncoprotein can increase the expression of its target genes, BCL-2,
mRNA and protein levels for this gene were quantified. As shown in
Fig. 4C and D, the expression of
BCL-2 was significantly upregulated in E7 expressing Saos-2 cells
[BCL-2 mRNA: 2.09 (P=0.020); BCL-2 protein: 1.56 (P=0.007)]. These
results suggest that miR-143 downregulation by the HPV16 E7
oncoprotein leads to BCL-2 overexpression.
Expression of miR-21 and miR-143 is
altered by HPV16 in cervical cancer
We confirmed the pattern of miR-21 and miR-143
expression in samples obtained from HPV16-positive CC patients
compared with cervical scrapings from HPV-negative healthy
individuals, as previously reported in cell lines and HPV-positive
samples (23,24). TaqMan RT-qPCR assays showed that
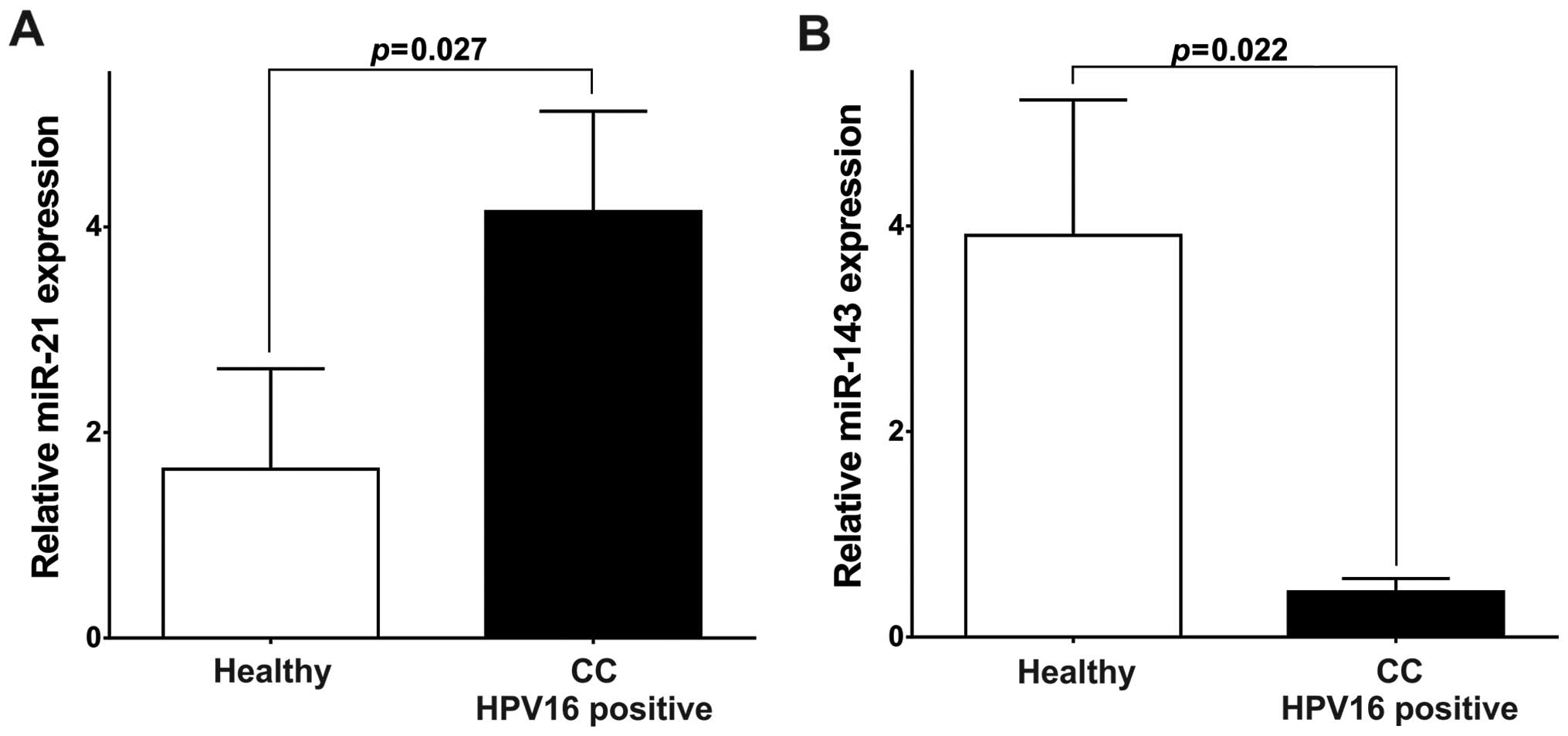
miR-21 levels were significantly higher (4.15, P=0.027; Fig. 5A) and miR-143 significantly lower
(0.44, P=0.021; Fig. 5B) in the
HPV16-positive CC patients than in HPV-negative healthy
individuals. These results support the hypothesis that HPV16
through its oncogenic oncoproteins plays a role in the deregulation
of miR-21 and miR-143 expression.
Discussion
HPV infection and 17β-estradiol (E2) are
risk factors for CC development (5,35,36);
~95% of these cancers are associated with persistent HR-HPV
infection (36). HR-HPV have been
reported to modify the expression patterns of certain miRNAs
(17–21), but the specific involvement of the
HPV16 E7 oncoprotein and E2 has not been explored. In
the present study, using a mouse model for HPV-associated cervical
carcinogenesis (K14E7 transgenic mice), we aimed to investigate
whether the high miR-21 and low miR-143 expression levels in CC are
associated with the HVP16 E7 oncoprotein and E2. In
addition, using a cell line that expressed HPV16 E7 oncoprotein we
determined if the E7 oncoprotein was involved in the deregulation
of miR-21 and miR-143 in vitro. Squamous epithelial
neoplasia in these animals progresses from low-grade squamous
intraepithelial lesion to high-grade cervical dysplasia and
ultimately invasive cervical malignancies after six months exposure
to a chronic low-dose of E2, mimicking malignant
progression in women (5). It is
widely known that miR-21 is the most highly overexpressed miRNA in
numerous cancers including cervical cancer (17–21),
and that in HPV-positive samples miR-21 expression correlates with
the progression of high grade cervical lesions to CC making it a
credible biomarker for HPV-associated cervical carcinogenesis
(37). Here, we observed in the
K14E7 murine model and cell lines expressing HPV16 E7 oncoprotein a
strong upregulation of miR-21 compared to controls. We also found
in transgenic and NT mice that E2 treatment induces an
increase in miR-21 expression levels. This is similar to the
situation observed in breast cancers where it was reported that
E2 induced expression of this important miRNA (32,38).
These results suggest that HPV16 E7 onco-protein induces miR-21
expression both in vivo and in vitro and that
E2 may cooperate in this effect.
Many different miR-21 target genes, such as TPM1,
PDCD4, CCL20 and PTEN tumor suppressor have been reported (25,39).
For example, it was observed that miR-21 overexpression was
associated with downregulation of the tumor-suppressive PTEN in
endometrial cancer (40) and
HPV-positive cervical cancers (41). Based on these results, we
investigated if the presence of the HPV16 E7 oncoprotein alone or
in conjunction with E2 induced a similar effect on the
expression of a miR-21 target gene. In the present study, we
performed matched analyses of miR-21 and PTEN mRNA and protein
expression in cervical tissue obtained from the K14E7 mouse model
in the presence or absence of E2 as well as from the
Saos-2 cell line expressing E7; we observed that PTEN mRNA and
protein levels were downregulated in both the K14E7 model and the
Saos-2 cell line expressing HPV16 E7. Likewise, our results showed
a significantly negative correlation between miR-21 levels and PTEN
protein and mRNA expression, suggesting that both in vivo
and in vitro the HPV16 E7 oncoprotein dowregulates PTEN
through the upregulation of miR-21. Notably, our results show that
miR-21 was upregulated in NT mice treated with E2, but
PTEN mRNA and protein levels were also remarkably increased in
these mice, indicating that a chronic physiological dose of
17β-estradiol induces upregulation of PTEN in cervical tissue as
previously reported in the HepG2 cell line and leiomyomas where the
increase in PTEN expression was attributed to E2
(42,43). As compared with NT+E2
mice, PTEN was strongly repressed in both K14E7 and K14E7+
E2 mice (Fig. 2A and
B), and the effect is attributed to the E7 oncoprotein.
The high expression of miR-21 in the K14E7 murine
model could be explained by a similar signaling pathway induced by
the E7 and E2 treatment. The Ras/ERK pathway plays an
important role in tumorigenesis and it is well known that the E7
expression and E2 cause activation of this pathway
(44,45). ERK signal activates AP-1 as well as
c-Jun and c-Fos, which regulate expression of genes involved in
cell proliferation, differentiation, malignant transformation and
metastasis (46). In this sense,
it has been reported that the E7 oncoprotein upregulates the
expression of AP-1 (44) and
E2 increases the signaling activity of this
transcriptional factor (47). The
activation of AP-1 could increase the miR-21 levels because it was
reported that AP-1 activates miR-21 transcription through conserved
AP-1 binding sites in its promoter (48), which would explain the increase in
miR-21 expression found by both HPV16 E7 oncoprotein and
E2 treatment.
The increased expression level of miR-21 induced by
HPV16 E7 oncoprotein and E2 might represent an important
step towards the development of CC since the majority of its
reported targets are tumor suppressors, such as PTEN, frequently
found diminished in CC (41,49).
PTEN negatively regulates cell proliferation by blocking the
PI3K/AKT signaling pathway (50),
a pathway known to play a key role in numerous cellular functions
including proliferation, adhesion, angiogenesis and migration
(51). On the other hand, it has
been reported that E7 oncoprotein and 17β-estradiol upregulate the
AKT signaling pathway (52,53).
The ability of HPV16 E7 oncoprotein to upregulate AKT activity
depends on the inactivation of the retinoblastoma (Rb) gene product
family of proteins (52), while
E2 can activate the PI3K/AKT pathway by an ER-dependent
action (53). Thus, PI3K/AKT
activation is achieved by several mechanisms, including the
upregulation of miR-21 and inactivation of PTEN in presence of the
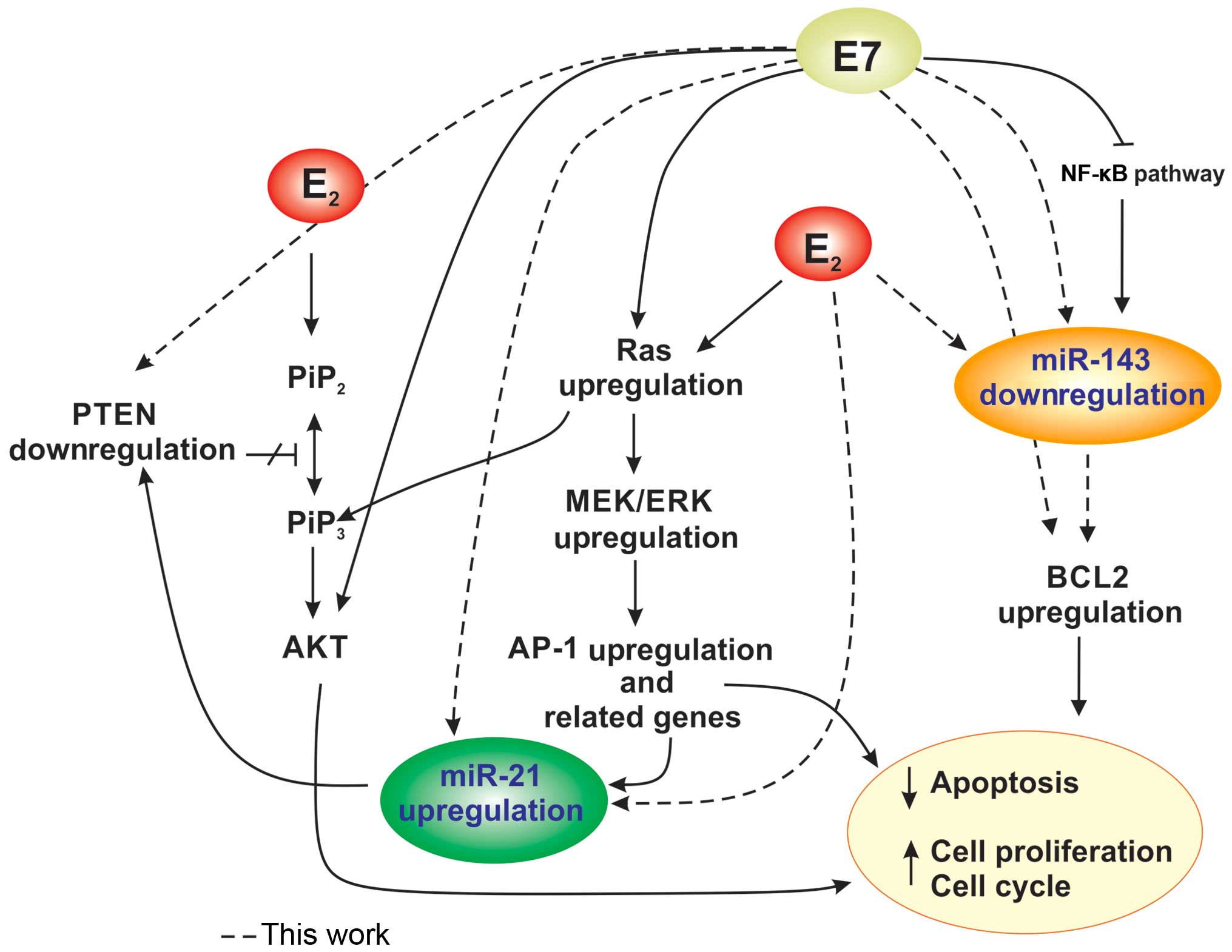
E7 oncoprotein and E2 (Fig.
6).
miR-143 acts as a tumor suppressor and it has been
reported downregulated in HPV-positive cell lines and CC (17–21,23,24,27,54).
In the present study, we found that miR-143 expression is
downregulated in the K14E7 model and cell lines expressing HPV16
E7; we also observed that miR-143 expression levels were
significantly downregulated by E2 in transgenic and NT
mice treated with E2, as reported in breast cancer
(32). Our results suggest for the
first time that the E7 oncoprotein may downregulate miR-143
expression both in vivo and in vitro and that
E2 treatment is also implicated in the downregulation of
this important miRNA in vivo.
miR-143 is involved in the negative regulation of
BCL-2 expression in CC (27), and
the suppressive effects of miR-143 on cell proliferation and
promotion of apoptosis is, at least in part, through suppression of
BCL-2 expression (27). Moreover,
it has been reported that the abnormal activation of Bcl-2 is in
agreement with a significant increase in the resistance to
apoptosis in E7-transfected cells (55). In the same manner, E2
inhibits apoptosis partially by the induction of BCL-2
transcription (56). Here, we
observed that both mRNA and protein levels of BCL-2 were remarkably
enhanced in the K14E7 mice model and cell lines expressing the
HPV16 E7 oncoprotein. Likewise, BCL-2 expression was increased in
mice treated with E2, indicating that estrogen induces
upregulation of BCL-2 in mouse cervical tissue, similarly to the
effect observed in MCF-7 cells (56). Otherwise, we determined an inverse
relationship between miR-143 expression and BCL-2 mRNA and protein
levels, comparable to that found in cervical cell lines and human
cervical tumors (27). A possible
explanation for the low expression of miR-143 in our murine model
is that this miRNA is transcribed by nuclear factor kappa B (NF-κB)
(57), which is attenuated by the
E7 oncoprotein (58); therefore,
in the presence of E7 the downregulation of miR-143 could result in
overexpression of BCL-2, thereby blocking apoptosis (Fig. 6).
We also confirmed in CC samples containing HPV16
that miR-21 expression was significantly increased, while miR-143
expression was decreased with respect to cervical scrapings
(HPV-negative), as reported in HPV-infected CC (17–21).
This is consistent with the hypothesis that the HPV16 E7
oncoprotein is responsible for the deregulation of these miRNAs in
HPV16-positive CC patients.
In conclusion, we demonstrated a role for the HPV16
E7 oncoprotein and E2 in the regulation of miR-21 and
miR-143 expression in cervical tissue. Two major trends were shown
in the presence of the HPV16 E7 oncoprotein in vivo and
in vitro: i) miR-21 overexpression and downregulation of
PTEN and ii) miR-143 expression was reduced, while BCL-2 was
overexpressed. We also showed that E2 is involved in
deregulating the expression levels of miR-21 and miR-143 in
vivo. We posit that these alterations in host gene regulation
contribute to changes in several biological processes including
cell proliferation and apoptosis that lead to CC. These findings
not only provide insight into the interplay between the HPV16 E7
oncoprotein, E2 and miRNAs in cervical tissue, but also
opens up new diagnostic perspectives in CC.
Acknowledgments
The present study was supported by the National
Council of Science and Technology of México (CONACYT), Grants
0253804 (AGC) and 00201904 (PG). Yazmín Gómez-Gómez was a recipient
of doctoral fellowships from CONACYT (46244). The study is part of
the doctoral dissertation project of Yazmín Gómez-Gómez, a student
of the Posgrado en Ciencias Biomédicas, Instituto de Fisiología
Celular, Universidad Nacional Autónoma de México (UNAM). The
authors would like to thank Gabriela Mora, Lauro Macías, Elizabeth
Alvarez Rios (CINVESTAV-IPN) and Dra. Marcela Lizano-Soberón
(Unidad de Investigación Biomédica en Cáncer, Instituto Nacional de
Cancerología) for technical support.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Münger K, Baldwin A, Edwards KM, Hayakawa
H, Nguyen CL, Owens M, Grace M and Huh K: Mechanisms of human
papillomavirus-induced oncogenesis. J Virol. 78:11451–11460. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riley RR, Duensing S, Brake T, Münger K,
Lambert PF and Arbeit JM: Dissection of human papillomavirus E6 and
E7 function in transgenic mouse models of cervical carcinogenesis.
Cancer Res. 63:4862–4871. 2003.
|
|
6
|
McLaughlin-Drubin ME and Münger K: The
human papilloma-virus E7 oncoprotein. Virology. 384:335–344. 2009.
View Article : Google Scholar
|
|
7
|
Ghittoni R, Accardi R, Hasan U, Gheit T,
Sylla B and Tommasino M: The biological properties of E6 and E7
oncoproteins from human papillomaviruses. Virus Genes. 40:1–13.
2010. View Article : Google Scholar
|
|
8
|
Nilsson S, Mäkelä S, Treuter E, Tujague M,
Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M and
Gustafsson JA: Mechanisms of estrogen action. Physiol Rev.
81:1535–1565. 2001.
|
|
9
|
Gruber CJ, Tschugguel W, Schneeberger C
and Huber JC: Production and actions of estrogens. N Engl J Med.
346:340–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar
|
|
11
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
12
|
Hayashita Y, Osada H, Tatematsu Y, Yamada
H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y and
Takahashi T: A polycistronic microRNA cluster, miR-17–92, is
overexpressed in human lung cancers and enhances cell
proliferation. Cancer Res. 65:9628–9632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M,
et al: Human microRNA genes are frequently located at fragile sites
and genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar
|
|
16
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
17
|
McBee WC, Gardiner AS, Edwards RP, Lesnock
JL and Bhargava R: MicroRNA analysis in human papillomavirus
(HPV)-associated cervical neoplasia and cancer. J Carcinog Mutagen.
1:1–9. 2011.
|
|
18
|
Pereira PM, Marques JP, Soares AR, Carreto
L and Santos MAS: MicroRNA expression variability in human cervical
tissues. PLoS One. 5:e117802010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Wang F, Xu J, Ye F, Shen Y, Zhou J,
Lu W, Wan X, Ma D and Xie X: Progressive miRNA expression profiles
in cervical carcinogenesis and identification of HPV-related target
genes for miR-29. J Pathol. 224:484–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lui WO, Pourmand N, Patterson BK and Fire
A: Patterns of known and novel small RNAs in human cervical cancer.
Cancer Res. 67:6031–6043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gómez-Gómez Y, Organista-Nava J and
Gariglio P: Deregulation of the miRNAs expression in cervical
cancer: Human papillomavirus implications. Biomed Res Int.
2013:4070522013. View Article : Google Scholar
|
|
23
|
Deftereos G, Corrie SR, Feng Q, Morihara
J, Stern J, Hawes SE and Kiviat NB: Expression of mir-21 and
mir-143 in cervical specimens ranging from histologically normal
through to invasive cervical cancer. PLoS One. 6:e284232011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Wang YL and Wang JF: Differential
expression of miR-21, miR-126, miR-143, miR-373 in normal cervical
tissue, cervical cancer tissue and HeLa cell. Sichuan Da Xue Xue
Bao Yi Xue Ban. 43:536–539. 2011.(In Chinese).
|
|
25
|
Yao Q, Xu H, Zhang QQ, Zhou H and Qu LH:
MicroRNA-21 promotes cell proliferation and down-regulates the
expression of programmed cell death 4 (PDCD4) in HeLa cervical
carcinoma cells. Biochem Biophys Res Commun. 388:539–542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk
ML and Struhl K: STAT3 activation of miR-21 and miR-181b-1 via PTEN
and CYLD are part of the epigenetic switch linking inflammation to
cancer. Mol Cell. 39:493–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Yu X, Guo X, Tian Z, Su M, Long Y,
Huang C, Zhou F, Liu M, Wu X, et al: miR-143 is downregulated in
cervical cancer and promotes apoptosis and inhibits tumor formation
by targeting Bcl-2. Mol Med Rep. 5:753–760. 2012.
|
|
28
|
Pillai MR, Halabi S, McKalip A,
Jayaprakash PG, Rajalekshmi TN, Nair MK and Herman B: The presence
of human papillomavirus-16/-18 E6, p53, and Bcl-2 protein in
cervicovaginal smears from patients with invasive cervical cancer.
Cancer Epidemiol Biomarkers Prev. 5:329–335. 1996.PubMed/NCBI
|
|
29
|
Dimitrakakis C, Kymionis G, Diakomanolis
E, Papaspyrou I, Rodolakis A, Arzimanoglou I, Leandros E and
Michalas S: The possible role of p53 and bcl-2 expression in
cervical carcinomas and their premalignant lesions. Gynecol Oncol.
77:129–136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Myklebust MP, Bruland O, Fluge Ø,
Skarstein A, Balteskard L and Dahl O: MicroRNA-15b is induced with
E2F-controlled genes in HPV-related cancer. Br J Cancer.
105:1719–1725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Liu F, Mao X, Huang J, Yang J,
Yin X, Wu L, Zheng L and Wang Q: Elevation of miR-27b by HPV16 E7
inhibits PPARγ expression and promotes proliferation and invasion
in cervical carcinoma cells. Int J Oncol. 47:1759–1766.
2015.PubMed/NCBI
|
|
32
|
Bhat-Nakshatri P, Wang G, Collins NR,
Thomson MJ, Geistlinger TR, Carroll JS, Brown M, Hammond S, Srour
EF, Liu Y, et al: Estradiol-regulated microRNAs control estradiol
response in breast cancer cells. Nucleic Acids Res. 37:4850–4861.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herber R, Liem A, Pitot H and Lambert PF:
Squamous epithelial hyperplasia and carcinoma in mice transgenic
for the human papillomavirus type 16 E7 oncogene. J Virol.
70:1873–1881. 1996.PubMed/NCBI
|
|
34
|
Nolan T, Hands RE and Bustin SA:
Quantification of mRNA using real-time RT-PCR. Nat Protoc.
1:1559–1582. 2006. View Article : Google Scholar
|
|
35
|
Arbeit JM, Howley PM and Hanahan D:
Chronic estrogen-induced cervical and vaginal squamous
carcinogenesis in human papillomavirus type 16 transgenic mice.
Proc Natl Acad Sci USA. 93:2930–2935. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
International Collaboration of
Epidemiological Studies of Cervical Cancer. Comparison of risk
factors for invasive squamous cell carcinoma and adenocarcinoma of
the cervix: Collaborative reanalysis of individual data on 8,097
women with squamous cell carcinoma and 1,374 women with
adenocarcinoma from 12 epidemiological studies. Int J Cancer.
120:885–891. 2007. View Article : Google Scholar
|
|
37
|
Gocze K, Gombos K, Kovacs K, Juhasz K,
Gocze P and Kiss I: MicroRNA expressions in HPV-induced cervical
dysplasia and cancer. Anticancer Res. 35:523–530. 2015.
|
|
38
|
Mattie MD, Benz CC, Bowers J, Sensinger K,
Wong L, Scott GK, Fedele V, Ginzinger D, Getts R and Haqq C:
Optimized high-throughput microRNA expression profiling provides
novel biomarker assessment of clinical prostate and breast cancer
biopsies. Mol Cancer. 5:242006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao T and Lin Z: MiR-21 is involved in
cervical squamous cell tumorigenesis and regulates CCL20. Biochim
Biophys Acta. 1822:248–260. 2012. View Article : Google Scholar
|
|
40
|
Qin X, Yan L, Zhao X, Li C and Fu Y:
microRNA-21 overexpression contributes to cell proliferation by
targeting PTEN in endometrioid endometrial cancer. Oncol Lett.
4:1290–1296. 2012.PubMed/NCBI
|
|
41
|
Xu J, Zhang W, Lv Q and Zhu D:
Overexpression of miR-21 promotes the proliferation and migration
of cervical cancer cells via the inhibition of PTEN. Oncol Rep.
33:3108–3116. 2015.PubMed/NCBI
|
|
42
|
Jeong YJ, Noh EM, Lee YR, Yu HN, Jang KY,
Lee SJ, Kim J and Kim JS: 17beta-estradiol induces up-regulation of
PTEN and PPARgamma in leiomyoma cells, but not in normal cells. Int
J Oncol. 36:921–927. 2010.PubMed/NCBI
|
|
43
|
Marino M, Acconcia F and Trentalance A:
Biphasic estradiol-induced AKT phosphorylation is modulated by PTEN
via MAP kinase in HepG2 cells. Mol Biol Cell. 14:2583–2591. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yuan H, Ito S, Senga T, Hyodo T, Kiyono T,
Kikkawa F and Hamaguchi M: Human papillomavirus type 16 oncoprotein
E7 suppresses cadherin-mediated cell adhesion via ERK and AP-1
signaling. Int J Oncol. 35:309–314. 2009.PubMed/NCBI
|
|
45
|
Miñano A, Xifró X, Pérez V,
Barneda-Zahonero B, Saura CA and Rodríguez-Alvarez J: Estradiol
facilitates neurite maintenance by a Src/Ras/ERK signalling
pathway. Mol Cell Neurosci. 39:143–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eferl R and Wagner EF: AP-1: A
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kakehashi A, Tago Y, Yoshida M, Sokuza Y,
Wei M, Fukushima S and Wanibuchi H: Hormonally active doses of
isoflavone aglycones promote mammary and endometrial carcinogenesis
and alter the molecular tumor environment in Donryu rats. Toxicol
Sci. 126:39–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fujita S, Ito T, Mizutani T, Minoguchi S,
Yamamichi N, Sakurai K and Iba H: miR-21 Gene expression triggered
by AP-1 is sustained through a double-negative feedback mechanism.
J Mol Biol. 378:492–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vázquez-Ulloa E, Lizano M, Avilés-Salas A,
Alfaro-Moreno E and Contreras-Paredes A: Abnormal distribution of
hDlg and PTEN in premalignant lesions and invasive cervical cancer.
Gynecol Oncol. 122:663–668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leslie NR and Downes CP: PTEN: The down
side of PI 3-kinase signalling. Cell Signal. 14:285–295. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcription and translation. Nat Rev
Cancer. 5:921–929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Menges CW, Baglia LA, Lapoint R and
McCance DJ: Human papillomavirus type 16 E7 up-regulates AKT
activity through the retinoblastoma protein. Cancer Res.
66:5555–5559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo RX, Wei LH, Tu Z, Sun PM, Wang JL,
Zhao D, Li XP and Tang JM: 17 β-estradiol activates PI3K/Akt
signaling pathway by estrogen receptor (ER)-dependent and
ER-independent mechanisms in endometrial cancer cells. J Steroid
Biochem Mol Biol. 99:9–18. 2006. View Article : Google Scholar
|
|
54
|
Martinez I, Gardiner AS, Board KF, Monzon
FA, Edwards RP and Khan SA: Human papillomavirus type 16 reduces
the expression of microRNA-218 in cervical carcinoma cells.
Oncogene. 27:2575–2582. 2008. View Article : Google Scholar :
|
|
55
|
Du J, Chen GG, Vlantis AC, Chan PKS, Tsang
RKY and van Hasselt CA: Resistance to apoptosis of HPV 16-infected
laryngeal cancer cells is associated with decreased Bak and
increased Bcl-2 expression. Cancer Lett. 205:81–88. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Perillo B, Sasso A, Abbondanza C and
Palumbo G: 17β-estradiol inhibits apoptosis in MCF-7 cells,
inducing bcl-2 expression via two estrogen-responsive elements
present in the coding sequence. Mol Cell Biol. 20:2890–2901. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang X, Liu S, Hu T, Liu S, He Y and Sun
S: Up-regulated microRNA-143 transcribed by nuclear factor kappa B
enhances hepatocarcinoma metastasis by repressing fibronectin
expression. Hepatology. 50:490–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Spitkovsky D, Hehner SP, Hofmann TG,
Möller A and Schmitz ML: The human papillomavirus oncoprotein E7
attenuates NF-κB activation by targeting the Ikappa B kinase
complex. J Biol Chem. 277:25576–25582. 2002. View Article : Google Scholar : PubMed/NCBI
|