Introduction
The bombesin family contains bombesin, a
tetradecapeptide originally isolated from amphibian skin, and other
mammalian bombesin-like peptides including gastrin-releasing
peptide (GRP) and neuromedin B (NMB) (1,2).
These peptides play a role a variety of physiological and
pathological functions such as smooth muscle contraction, exocrine
and endocrine secretion, inflammation, and cancer by activating
their respective high-affinity receptors (3,4).
These receptors are members of the G protein-coupled receptor
superfamily and play critical roles in tumor development, invasion
and metastasis (5). Aberrant
expression of bombesin-like peptides and their receptors has been
found in some types of human cancers including breast cancers
(6).
Accumulating data demonstrates that GRP acts as a
mitogen, morphogen and proangiogenic factor in many types of tumors
(7–9). Therefore, blocking the GRP receptor
by antagonists, monoclonal antibodies, and antisense
oligonucleotides have become an attractive therapeutic approach for
some types of human tumors (10,11).
NMB has been reported to regulate tumor cell proliferation in
several cancer cell lines including lung, colon and glioma, and has
been shown to trigger intracellular signaling related to tumor cell
growth and proliferation through activation of the NMB receptor
(NMB-R) (12–14). In line with attempts to investigate
the function of NMB/ NMB-R in biological processes, a small
non-peptide NMB-R antagonist has been developed and has proven to
be useful in understanding the pathophysiology of NMB/NMB-R
(15,16). Previously, we have shown that NMB
and an NMB-R antagonist can regulate angiogenesis both in
vivo and in vitro (17). In addition, we have shown that
NMB-R antagonism blocks the growth of breast cancer cells (18). Here, we focused on the inhibitory
effect of an NMB-R antagonist on breast cancer cell metastasis. Our
results demonstrated that an NMB-R antagonist suppresses migration
and invasion capacity as well as EMT of MDA-MB-231 cells, thereby
inhibiting breast cancer cell metastasis.
Materials and methods
Reagents and antibodies
Neuromedin B and PD168368 were purchased from
Sigma-Aldrich (St. Louis, MO, USA) and Tocris Bioscience
(Minneapolis, MN, USA), respectively. PD168368 was dissolved in
dimethyl sulfoxide (DMSO) at a concentration of 5 mM and DMSO was
used as a solvent control for all in vitro experiments and
in vivo assays involving treatment with PD168368. Antibodies
for phospho-mTOR, mTOR, phospho-p70S6K, p70S6K, phospho-AKT, AKT,
phospho-GSK3β, GSK3β, phospho-4EBP1 and 4EBP1 were obtained from
Cell Signaling Technology (Danvers, MA, USA). Human NMB-R antibody
and α-tubulin antibody were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA) and BioGenex (Fremont, CA, USA),
respectively.
Cell culture
MDA-MB-231, MDA-MB-468 and MCF-7 cells from the
American Type Culture Collection (ATCC; Manassas, VA, USA) were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine
serum (FBS; Gibco-BRL) and 1% penicillin-streptomycin (Gibco-BRL)
at 37°C in a humidified atmosphere containing 5%
CO2.
GEO analysis
In order to compare the NMB-R mRNA expression in
normal breast tissues and invasive cancer tissues, we analyzed
publicly available gene expression datasets of human samples
(accession no. GSE10797, http://www.ncbi.nlm.nih.gov/geo/). The differential
expression of NMB-R mRNA expression was identified by web-based
application GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/) and the
Kolmogorov-Smirnov test was used to determine significant
differences.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from MDA-MB-231 cells with a
TRIZol reagent kit (Life Technologies, Carlsbad, CA, USA). cDNA
synthesis was performed using 2 μg of total RNA with a reverse
transcription kit (Promega, Madison, WI, USA). The forward and
reverse oligonucleotide primers for PCR were as follows: β-actin,
5′-GACTACCTCATGAAGATC-3′ and 5′-GATCCACATCTGCTGGAA-3′; NMB-R,
5′-CAGAAGTGGCTCGCATCAGT-3′ and 5′-GCTGTTGAAATGCCTCCTGA-3′;
E-cadherin, 5′-AACATGGTTCAGATCAAATC-3′ and
5′-AAGCTTGAAGATCGGAGGATTATCG-3′; vimentin,
5′-TGGCACGTCTTGACCTTGAA-3′ and 5′-GGTCATCGTGATGCTGAGAA-3′; Snail,
5′-CTGGGCGCCCTGAACATGCA-3′ and 5′-GGCTTCTCCCCCGTGTGAGTTCTA-3′.
Real-time PCR
Real-time PCR quantification was performed using
SYBR-Green (LightCycler; Roche Applied Science). Cycling parameters
consisted of 1 cycle of 95°C for 10 min, followed by amplification
for 30 cycles of 95°C for 10 sec, 57°C for 5 sec, and 72°C for 7
sec. Subsequently, a melting curve program was applied with
continuous fluorescence measurement. The entire cycling process
including data analysis took less than 1 h and was monitored using
the LightCycler software program (version 4.0). The forward and
reverse oligonucleotide primers for real-time PCR were designed as
follows: β-actin, 5′-ACTCTTCCAGCCTTCCTTCC-3′ and
5′-TGTTGGCGTACAGGTCTTTG-3′; NMB-R, 5′-GGGGTTTCCGTGTTCACTCT-3′ and
5′-CAGGAAGATTGTGTGCGCTT-3′.
Western blot analysis
Harvested cells were lysed in a buffer containing 40
mM Tris-HCl, 10 mM EDTA, 120 mM NaCl, 0.1% Nonidet P-40, and a
protease inhibitor cocktail (Sigma-Aldrich). Samples containing
equal amounts of protein (30 μg/lane) were separated by SDS-PAGE
and transferred to a nitrocellulose membrane (GE Healthcare Life
Sciences, Pittsburgh, PA, USA). The membrane was blocked with 5%
skim milk in PBS or TBS containing 0.1% Tween-20 for 1 h at room
temperature and probed with the appropriate antibodies. The signal
was developed using an enhanced chemiluminescence detection system
(GE Healthcare Life Sciences).
Boyden chamber migration assay
Transwell polycarbonate membrane inserts with 8 μm
pores were coated with 10 μg gelatin. MCF-7 or MDA-MB-231 cells
were suspended in DMEM at a concentration of 1×105
cells/100 μl, and were added to the upper chamber. NMB (5 μg/ml) or
PD168368 (5 μM) in DMEM was added into the lower chamber. Migratory
MCF-7 or MDA-MB-231 cells appearing on the lower side of the
chamber were fixed by careful immersion of the filter into methanol
for 1 min, stained with hematoxylin-eosin (H&E) solution and
counted in three random fields per well. Each experiment was
performed in duplicate and three separate experiments were
performed for each group.
Scratch wound healing assay
Cells were seeded in 6-well plates at a
concentration of 1×106 cells/well in 1 ml of serum-free
DMEM for 6 h, until an adherent monolayer was obtained. A 10 μl
pipette tip was used to create a scratch in the monolayer and the
cells were washed 3 times with serum-free medium. The cells were
then placed in fresh serum-free medium and treated with either NMB
(5 μg/ml) or PD168368 (5 μM). Samples were taken at the beginning
and after 24 h of culture in 5% CO2 at 37°C. Images of
the scratch wounds were taken and measured by ImageJ software to
calculate the mean and standard deviation. Each experimental group
was compared with its respective control. The experiments were
repeated 3 times.
Invasion assay
Invasion was examined in a Corning Costar Transwell
system. The lower and upper sides of polycarbonate filters with 8
μm pores were coated with 0.5 mg/ml type I collagen and 0.5 mg/ml
Matrigel, respectively. The lower compartment contained medium with
NMB or PD168368, and MCF-7 or MDA-MB-231 cells were placed in the
upper part of the Transwell apparatus. Cell invasion was determined
by counting cells on each filter with an optical microscope at x40
magnification.
Multicellular spheroids/3-dimensional
(3D) cell culture assay
MDA-MB-231 cell culture dishes (24-well plates) were
precoated with undiluted phenol red-free Matrigel (10 mg/ml). For
each well, 1×104 cells were suspended in 200 μl PBS and
mixed with 100 μl of cold Matrigel (10 mg/ml). The cell suspension
was added dropwise over the bottom layer to cover it. After the
cell layer was completely set, culture media was added over the
top. Media was changed every 2 days without disturbing the
cell/matrix layer. Images were taken at the indicated times using
x10 magnification for an overview and x40 magnification to document
spheroid structure.
Intracardiac experimental metastasis
model
Female BALB/c-nude mice (age 8–10 weeks) were
anesthetized by intraperitoneal injection of a mixture containing
30 mg/kg zoletil and 10 mg/kg xylazine (Rompun). MDA-MB-231 cells
(2×106 cells/0.1 ml in PBS) were injected into the left
cardiac ventricle of nude mice with a 26-1G needle according to
previously described methods with modifications (19). Correct injection position in the
left ventricle was confirmed by the appearance of bright red blood
at the hub of the needle in a pulsatile fashion. The first group of
animals received 70 μl of vehicle [polyethylene glycol 400 (PEG;
Sigma-Aldrich)] by intraperitoneal injection, while the second
group of animals received 1.2 mg/kg injections of PD168368 in PEG
by intraperitoneal injection. All mice were sacrificed 4 weeks
post-tumor inoculation. Any mice showing signs of distress prior to
4 weeks were sacrificed immediately. Animals were euthanized by
CO2 asphyxiation and histological analysis was
performed. Metastatic lung tissues were prepared and thin sections
(4 μm) from selected areas were analyzed. After deparaffinization,
H&E staining was used to evaluate morphology. This study
conformed to the ethical guidelines of the Institutional Animal
Care and Use Committee at Pusan National University, Korea.
Statistical analysis
Data are represented as the mean ± standard
deviation obtained for at least 3 independent experiments.
Statistical comparisons between groups were performed by the
one-way ANOVA followed by the Student’s t-test.
Results
NMB-R is highly expressed in invasive
human breast cancer cells
We analyzed a public genomics data set deposited in
the NCBI Gene Expression Omnibus (GEO) database (accession no.
GSE10797) in which microarray data were compared between normal
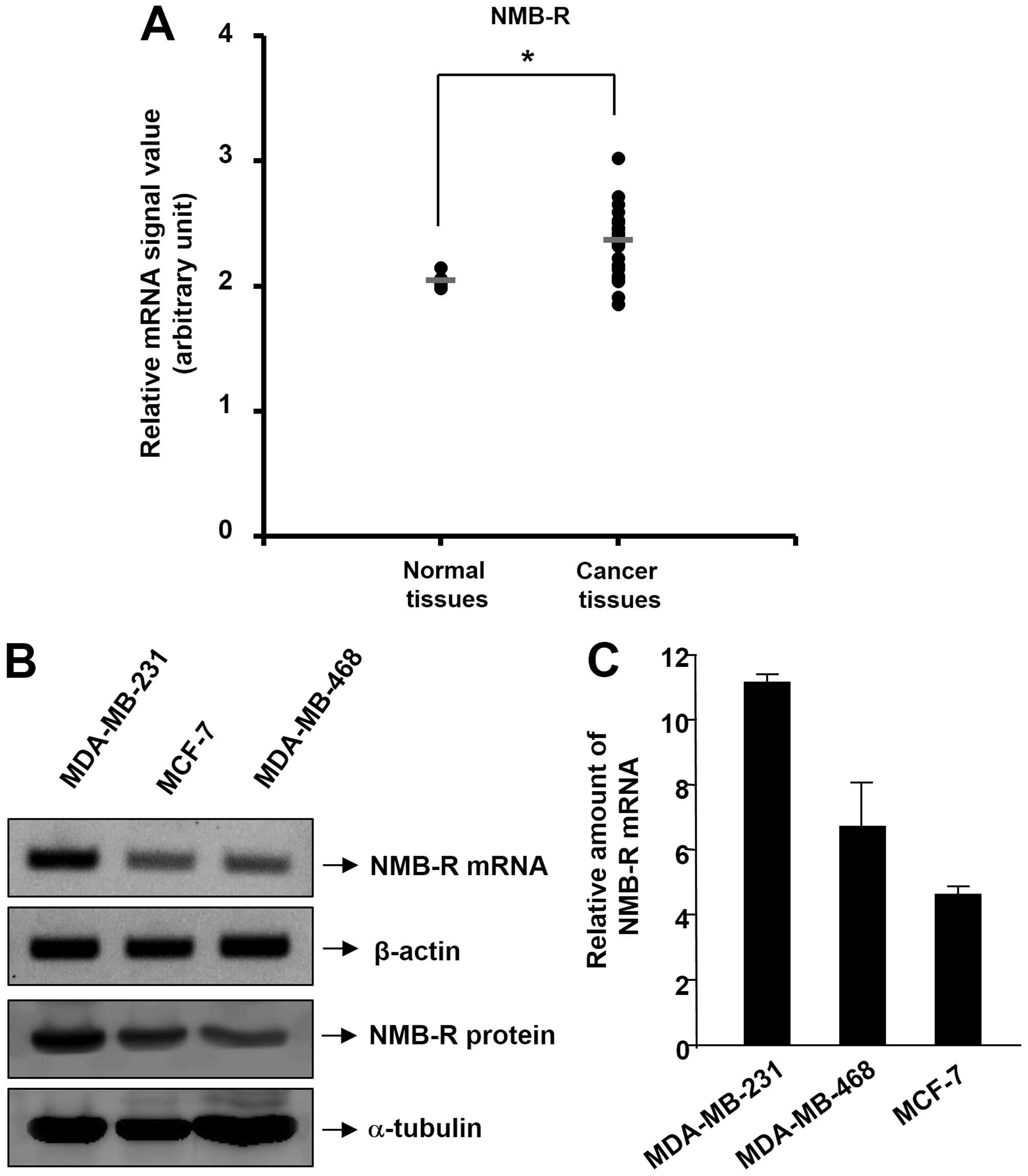
breast tissue (n=5) and invasive breast cancer (n=28) (20). This dataset showed a significant
increase in NMB-R mRNA expression in invasive breast cancer tissues
(Fig. 1A). This result is
supported by our previous findings showing NMB-R to be highly
expressed in malignant breast tissues (17,18).
Based on these results, we explored the expression levels of the
NMB-R in 3 human breast cancer cell lines (MDA-MB-231, MCF-7 and
MDB-MB-468), by employing RT-PCR, real-time PCR and western blot
analysis. As shown in Fig. 1B and
1C, examination of NMB-R gene expression in 3 breast cancer
cell lines revealed that NMB-R was expressed in all of these cancer
cell lines using RT-PCR and real-time PCR analysis. Moreover, the
expression level of the NMB-R mRNA was higher in invasive breast
cancer cells (MDA-MB-231) than in less-invasive breast cancer cells
(MCF-7 and MDB-MB-468). The pattern in NMB-R expression was
confirmed at the protein level in MDA-MB-231 and MCF-7 cells
(Fig. 1B, lower). The following
studies were designed to investigate the inhibitory role of an
NMB-R antagonist in the invasive breast cancer cell line MDA-MB-231
that highly expresses NMB-R.
PD168368 inhibits migration and
invasiveness in breast cancer cells
The effect of the NMB-R antagonist PD168368 on
cellular behavior of breast cancer cells was determined by
migration and invasion assays. Concentrations of PD168369 used in
the present study showed no cytotoxic effect on human breast cancer
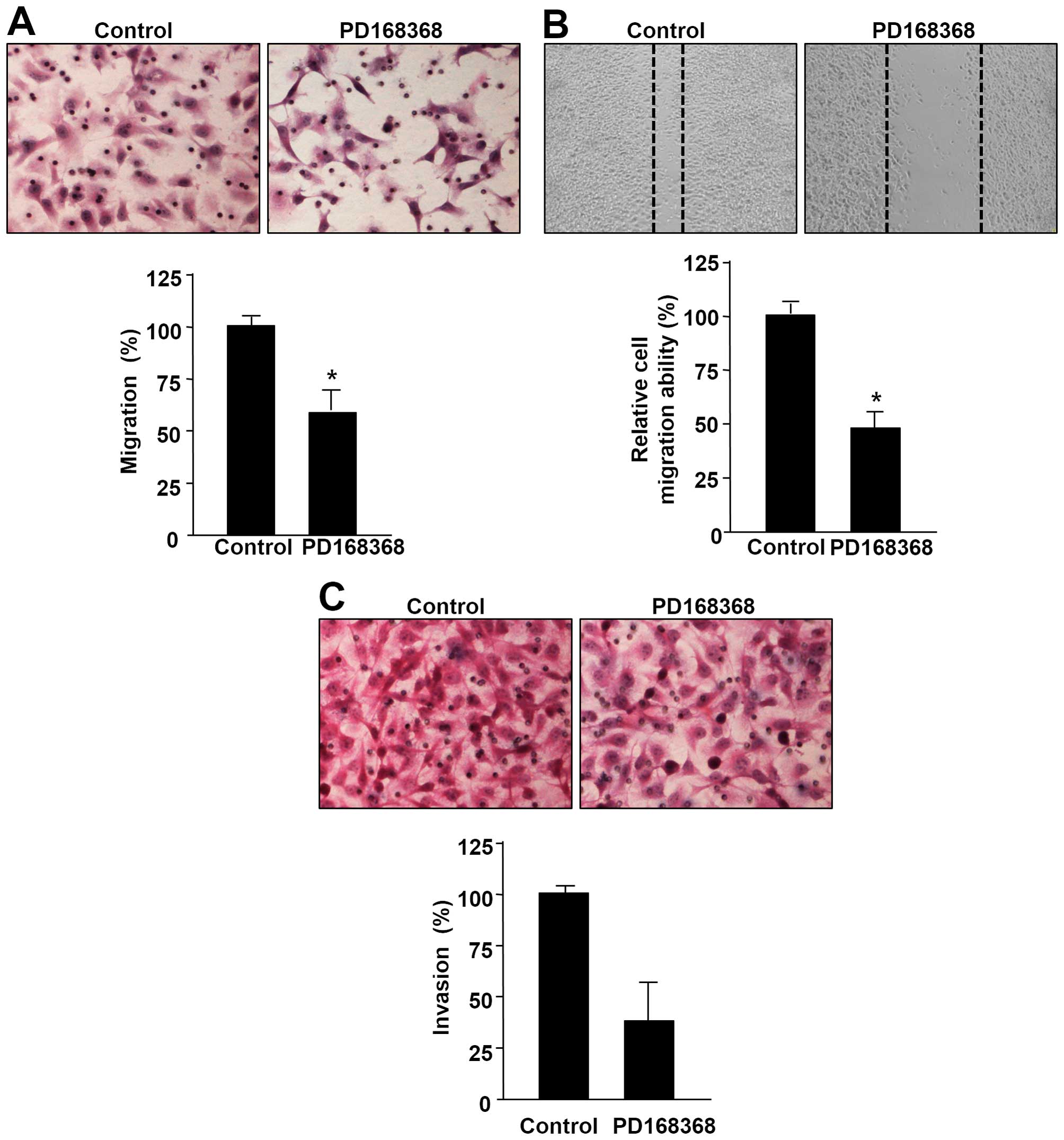
cell lines MDA-MB-231 and MCF-7. We demonstrated that the NMB-R
antagonist PD168368 clearly decreased the migratory ability of
MDA-MB-231 cells in a Boyden chamber migration assay (Fig. 2A). To complement this Boyden
chamber assay, we performed a wound healing migration assay to
qualitatively observe the inhibitory effect of PD168368 on the
motility of MDA-MB-231 cells. As shown in Fig. 2B, PD168368 treatment decreased the
number of cells that migrated into the scratch wound compared to
control MDA-MB-231 cells. Next, we examined the effect of PD168368
on invasion capacity of the breast cancer cells in a Matrigel-based
Transwell invasion assay. Treatment with PD168368 suppressed the
invasion ability of MDA-MB-231 cells (Fig. 2C). In addition, we investigated the
inhibitory effects of PD168368 on NMB-induced migration and
invasion of MCF-7 cells by utilizing a Boyden chamber assay. As
shown in Fig. 2D, PD168368
inhibited the migration and invasion ability of MCF-7 cells induced
by NMB, suggesting that PD168368 specifically inhibited NMB-induced
migration and invasion in breast cancer cells.
MDA-MB-231 cells grown in 3D Matrigel culture form
thorn or leg shapes, which shows the migratory and invasive
properties of these cells (21).
However, treatment with PD168368 attenuated these aggressive
phenotypes in 3D Matrigel culture (Fig. 2E).
PD168368 regulates EMT in breast cancer
cells
The epithelial-mesenchymal transition (EMT) is a
crucial early event in the migration, invasion, and metastasis of
cancer cells from the primary tumor site (22,23).
To investigate whether PD168368 regulates metastatic features of
breast cancer cells via inhibiting EMT, we assessed the effect of
PD168368 on EMT marker expression in MDA-MB-231 cells by RT-PCR and
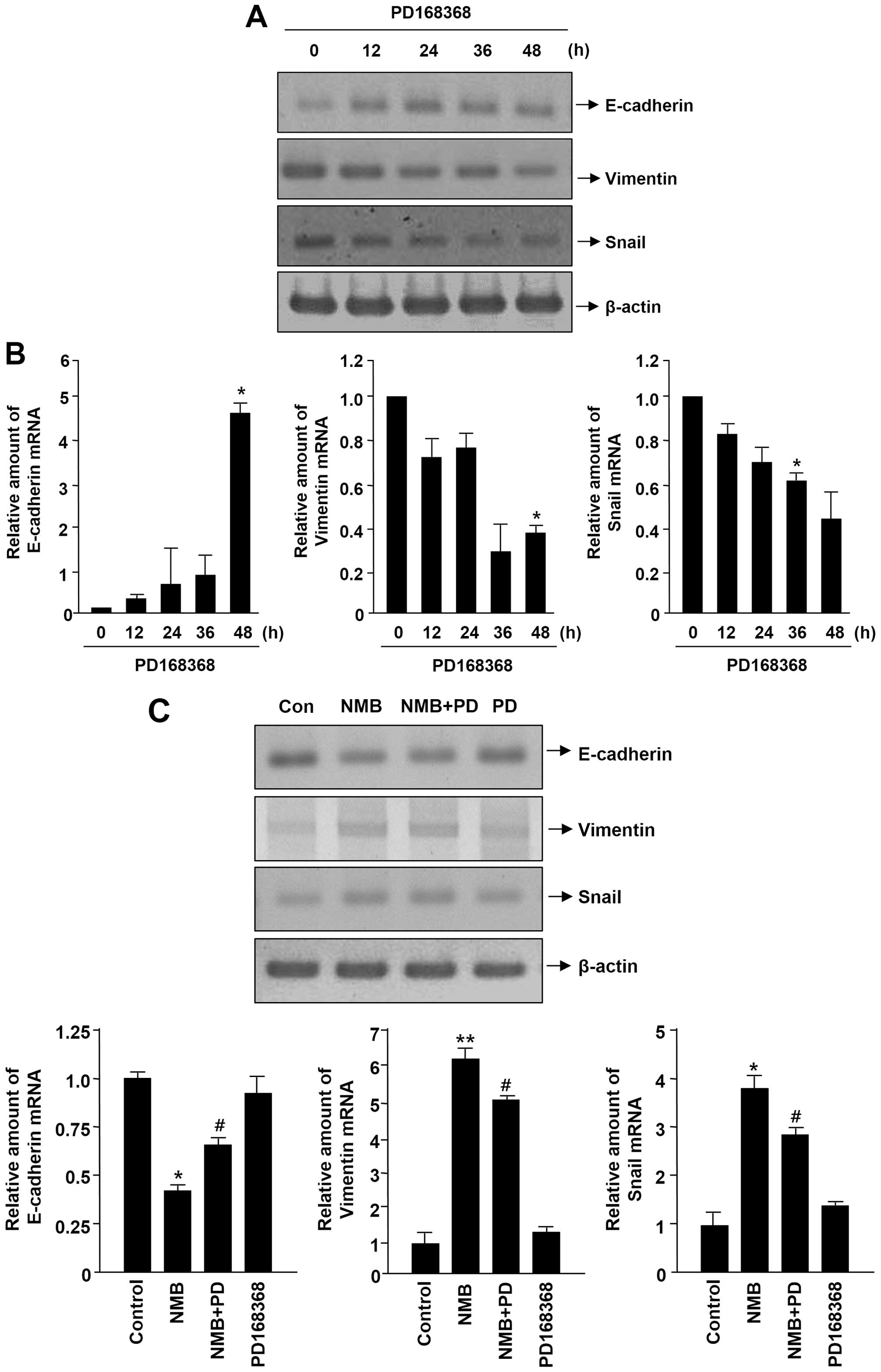
real-time PCR analysis. As shown in Fig. 3A and B, PD168368 treatment resulted
in upregulation of the epithelial marker E-cadherin and
downregulation of the mesenchymal markers vimentin and Snail in
MDA-MB-231 cells. We next investigated the inhibitory effects of
PD168368 on NMB-regulated EMT marker expression in MCF-7 cells by
adopting a RT-PCR and real-time PCR assay. As shown in Fig. 3C, PD168368 increased the levels of
E-cadherin expression reduced by NMB, whereas PD168368 diminished
the levels of vimentin and Snail expression induced by NMB. These
results suggest that PD168368 inhibits EMT in breast cancer
cells.
PD168368 suppresses the activation of
mTOR/p70S6K/4EBP1 and AKT/GSK-3β pathways in breast cancer
cells
Recent studies showed that the mTOR pathway is
frequently activated in breast cancer metastasis (24,25).
Activation of mTOR and the subsequent phosphorylation and
activation of its downstream targets p70S6K and eIF4E binding
protein 1 (4EBP1) play an important role in promoting cell growth
and metastasis in breast cancer (26,27).
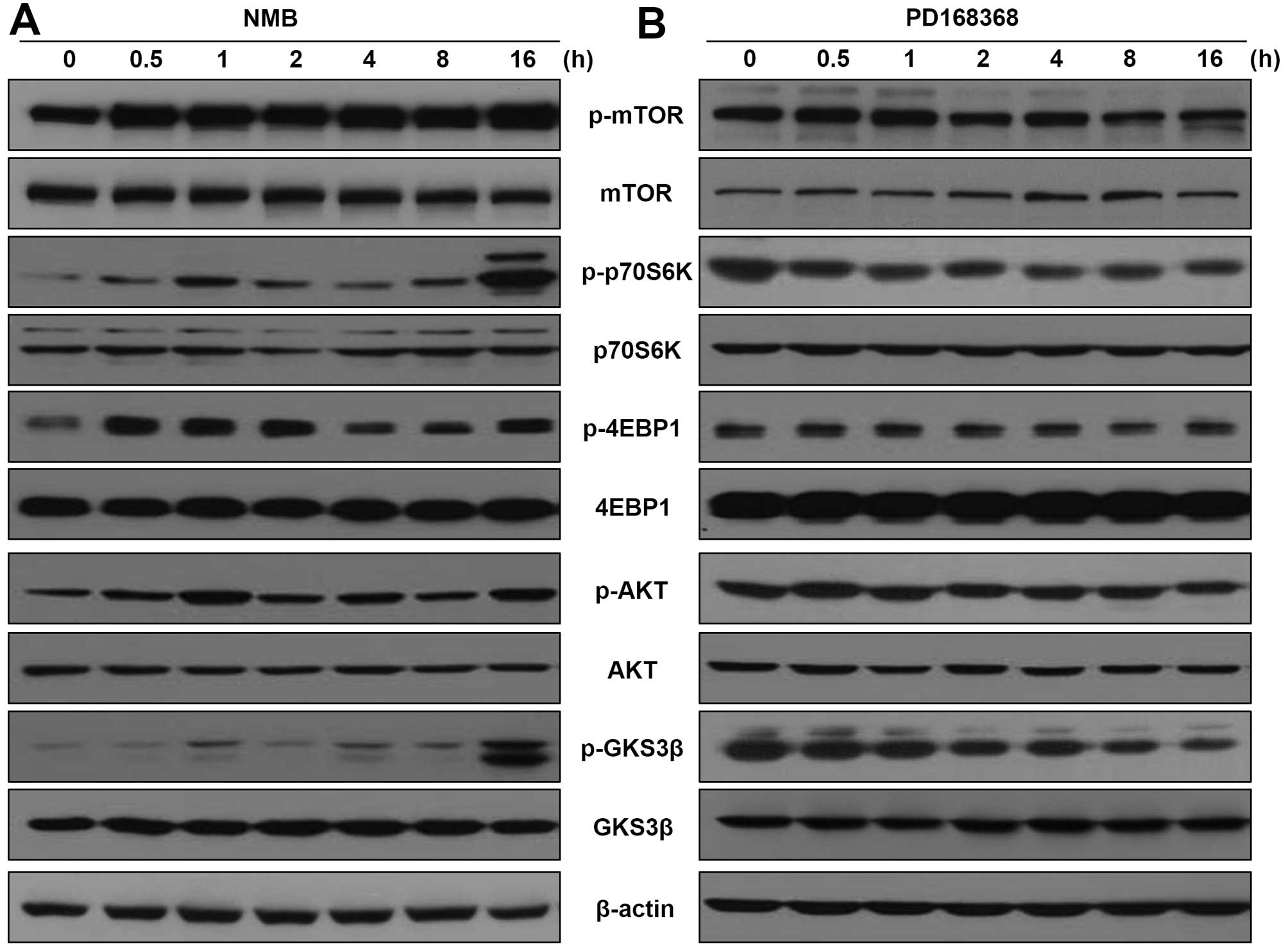
Therefore, we determined whether NMB stimulates the phosphorylation
of mTOR, p70S6K and 4EBP1 in MCF-7 cells. As shown in Fig. 4A, exogenous NMB treatment increased
the phosphorylation levels of mTOR, p70S6K and 4EBP1 in MCF-7
cells. Additionally, because activation of the AKT/GSK-3β pathway
is emerging as a central feature of EMT (21,28),
we speculated NMB regulates AKT/GSK-3β activity in breast cancer
cells. Phosphorylation of AKT and GSK-3β were also significantly
induced in MCF-7 cells treated with NMB, without obviously
influencing total AKT and GSK-3β levels. In contrast, as shown in
Fig. 4B, treatment of the
MDA-MB-231 cells with PD168368 decreased phosphorylation levels of
mTOR, p70S6K, 4EBP1, AKT and GSK-3β in a time-dependent manner.
Next, we investigated the effect of PD168368 on NMB-induced
activation of mTOR/p70S6K/4EBP1 and AKT/GSK-3β in MCF-7 cells. As
shown in Fig. 4C, PD168368
effectively reduced the NMB-induced phosphorylation of
mTOR/p70S6K/4EBP-1 and AKT/GSK-3β signaling.
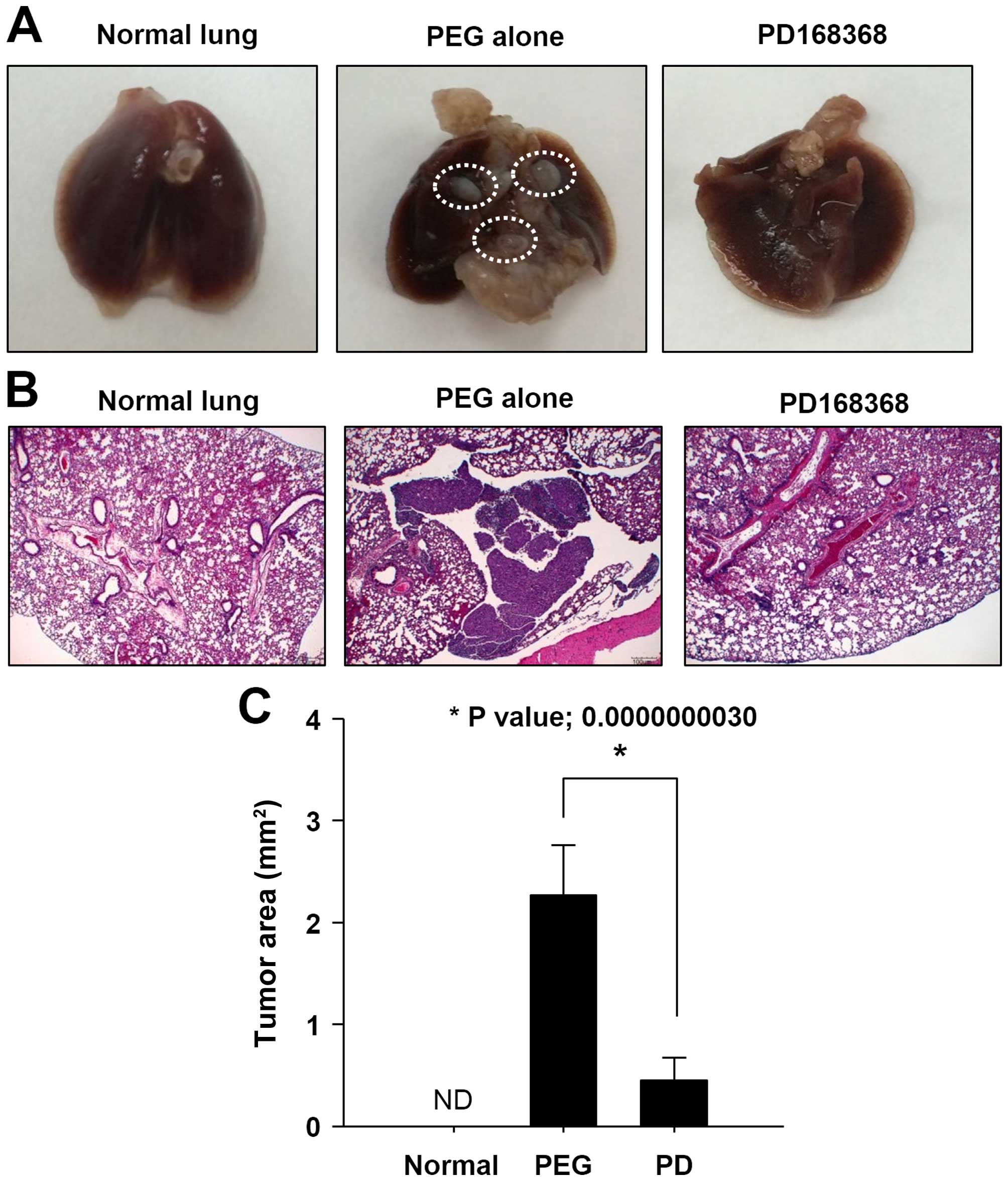
PD168368 inhibits metastasis of breast
cancer
The effect of PD168368 on breast cancer cell
metastasis was further investigated in vivo by employing
lung metastasis assays. Cardiac injection of MDA-MB-231 cells
(2×106 cells/mouse) can lead to the formation of lung
metastases in mice. The mice received intraperitoneal injections
with PD168368 (1.2 mg/kg) or PEG (vehicle) for 30 days. As shown in
Fig. 5A, no metastatic tumor
nodules were observed in lungs of PD168368-treated mice compared to
PEG-injected mice. After sacrifice, lungs were collected and
sectioned, and H&E staining showed that the PD168368-injected
mice formed much fewer metastatic lung tumors than PEG-injected
mice (Fig. 5B and C). These data
showed that PD168368 significantly reduces lung metastatic
potential of human breast cancer cells.
Discussion
Bombesin-like peptides and their receptors have been
demonstrated to be overexpressed in various types of human
malignancies (6). GRP-R is
overexpressed in many malignancies including lung (small and
non-small cell type), breast, prostate cancer, head and neck
squamous cell carcinoma, glioblastoma, pancreatic and ovarian
cancer (7,29). Compared to GRP/GRP-R, the role of
NMB/NMB-R has received considerably less attention in
tumorigenesis, yet, some studies have mentioned NMB-R expression in
tumors such as intestinal carcinoids, lung, colon cancer and
glioblastoma (30,31). GRP and NMB are often synthesized
and secreted from the tumors themselves and both peptides have been
shown to exert an autocrine effect on the growth and
differentiation of tumors that express GRP-R and NMB-R (1,32).
In the present study, we demonstrated that the NMB-R is expressed
at a significantly higher level in invasive breast cancer tissues
than in non-invasive tissues in dataset GSE10797 deposited in GEO.
These results are consistent with those of our previous studies
that show expression of NMB-R and NMB in neoplastic breast tissues.
Expression of NMB-R (54 of 63 cases, 86%), (42 of 50 cases, 84%),
and NMB (41 of 63 cases; 65%) were observed in the human breast
tumor tissues examined (17,18).
Breast cancer is one of the most commonly diagnosed
cancers and is the leading cause of cancer mortality in women
(33). The 5-year survival rate
for localized breast cancer is relatively high, whereas the
survival rate dramatically reduces when breast cancer metastasizes
(34). Tumor metastasis is driven
by a series of biological processes in cancer cells, including
acquisition of the ability to migrate from the primary tumor,
invade surrounding tissues and metastasize to distal organs
(35). To facilitate the early
stage of metastatic expansion, malignant cancer cells go through
EMT (36). EMT was initially known
as a developmental process in which cells lose their epithelial
characteristics (including junctions between cells and
apical-basolateral polarity) and acquire a mesenchymal phenotype
with migratory and invasive properties (37). Lately, the pathological potential
of EMT has been applied to the mechanism of tumor invasion and
metastasis in several types of cancer, including breast cancer
(38). Increasing evidence has
shown that malignant breast cancer cells undergo EMT to develop a
more motile and invasive phenotype (39,40).
Targeting key steps of EMT may serve as an efficient therapeutic
strategy for malignant and metastatic breast cancers (41). Here, we investigated the role of
PD168368 in the regulation of migration, invasion and EMT in breast
cancer cells. We showed that PD168368 regulates the expression of
canonical EMT markers, including induction of E-cadherin and loss
of vimentin. Snail, a member of the Snail transcription factor
superfamily, is known to be a master regulator of EMT by repressing
epithelial genes and inducing mesenchymal genes (42). Here, we showed that Snail
expression is downregulated after treatment with PD168368. Further
studies are necessary to investigate whether NMB/NMB-R expression
is negatively correlated with the expression of epithelial markers
and positively correlated with expression of mesenchymal markers in
breast cancer tissues.
The mTOR pathway is frequently activated in breast
cancers, and plays a significant role in the growth and metastasis
of breast cancer (43). In this
study, we demonstrated that NMB activates mTOR and subsequently
induces phosphorylation and activation of its downstream targets
p70S6K and 4EBP1 in breast cancer cells. In addition, we also found
that NMB induces the activation of AKT in breast cancer cells. The
mTOR pathway can be activated by the AKT pathway to induce
migratory and invasive properties of breast cancer cells (44), which raises the possibility that
NMB activates the mTOR pathway through AKT. Further investigations
are required to determine whether NMB drives tumorigenesis and
progression to metastasis via the AKT-mTOR signaling pathway in
breast cancer.
Collectively, we demonstrated the inhibitory effect
of the NMB-R antagonist PD168368 on migration, invasion, and
metastasis of human breast cancer cells in vitro and in
vivo. Together, our findings suggest antagonism of the NMB-R
may be an effective approach for controlling breast tumor
metastasis.
Acknowledgements
This research was supported by a grant from the
National R&D Program for Cancer Control, Ministry of Health
& Welfare, Republic of Korea (1220080), the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(2015R1A2A2A01002980) (M-K Bae), and by the 2014 Post-Doc.
Development Program of Pusan National University (H-J Park).
References
|
1
|
Majumdar ID and Weber HC: Biology of
mammalian bombesin-like peptides and their receptors. Curr Opin
Endocrinol Diabetes Obes. 18:68–74. 2011. View Article : Google Scholar
|
|
2
|
Erspamer V: Discovery, isolation, and
characterization of bombesin-like peptides. Ann NY Acad Sci. 547(1
Bombesin- Like): 3–9. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jensen RT, Battey JF, Spindel ER and Benya
RV: International Union of Pharmacology. LXVIII Mammalian bombesin
receptors: Nomenclature, distribution, pharmacology, signaling, and
functions in normal and disease states. Pharmacol Rev. 60:1–42.
2008. View Article : Google Scholar
|
|
4
|
Gonzalez N, Moody TW, Igarashi H, Ito T
and Jensen RT: Bombesin-related peptides and their receptors:
Recent advances in their role in physiology and disease states.
Curr Opin Endocrinol Diabetes Obes. 15:58–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spiegelberg BD and Hamm HE: Roles of
G-protein-coupled receptor signaling in cancer biology and gene
transcription. Curr Opin Genet Dev. 17:40–44. 2007. View Article : Google Scholar
|
|
6
|
Preston SR, Miller GV and Primrose JN:
Bombesin-like peptides and cancer. Crit Rev Oncol Hematol.
23:225–238. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel O, Shulkes A and Baldwin GS:
Gastrin-releasing peptide and cancer. Biochim Biophys Acta.
1766:23–41. 2006.PubMed/NCBI
|
|
8
|
Martínez A, Zudaire E, Julián M, Moody TW
and Cuttitta F: Gastrin-releasing peptide (GRP) induces
angiogenesis and the specific GRP blocker 77427 inhibits tumor
growth in vitro and in vivo. Oncogene. 24:4106–4113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jensen JA, Carroll RE and Benya RV: The
case for gastrin-releasing peptide acting as a morphogen when it
and its receptor are aberrantly expressed in cancer. Peptides.
22:689–699. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Chen J, Mokotoff M and Ball ED:
Targeting gastrin-releasing peptide receptors for cancer treatment.
Anticancer Drugs. 15:921–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hohla F and Schally AV: Targeting gastrin
releasing peptide receptors: New options for the therapy and
diagnosis of cancer. Cell Cycle. 9:1738–1741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moody TW, Berna MJ, Mantey S, Sancho V,
Ridnour L, Wink DA, Chan D, Giaccone G and Jensen RT: Neuromedin B
receptors regulate EGF receptor tyrosine phosphorylation in lung
cancer cells. Eur J Pharmacol. 637:38–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moody TW, Fagarasan M and Zia F:
Neuromedin B stimulates arachidonic acid release, c-fos gene
expression, and the growth of C6 glioma cells. Peptides.
16:1133–1140. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matusiak D, Glover S, Nathaniel R,
Matkowskyj K, Yang J and Benya RV: Neuromedin B and its receptor
are mitogens in both normal and malignant epithelial cells lining
the colon. Am J Physiol Gastrointest Liver Physiol. 288:G718–G728.
2005. View Article : Google Scholar
|
|
15
|
Ryan RR, Katsuno T, Mantey SA, Pradhan TK,
Weber HC, Coy DH, Battey JF and Jensen RT: Comparative pharmacology
of the nonpeptide neuromedin B receptor antagonist PD 168368. J
Pharmacol Exp Ther. 290:1202–1211. 1999.PubMed/NCBI
|
|
16
|
Tokita K, Hocart SJ, Katsuno T, Mantey SA,
Coy DH and Jensen RT: Tyrosine 220 in the 5th transmembrane domain
of the neuromedin B receptor is critical for the high selectivity
of the peptoid antagonist PD168368. J Biol Chem. 276:495–504. 2001.
View Article : Google Scholar
|
|
17
|
Park HJ, Kim SR, Bae SK, Choi YK, Bae YH,
Kim EC, Kim WJ, Jang HO, Yun I, Kim YM, et al: Neuromedin B induces
angiogenesis via activation of ERK and Akt in endothelial cells.
Exp Cell Res. 315:3359–3369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HJ, Kim SR, Kim MK, Choi KS, Jang HO,
Yun I, Bae SK and Bae MK: Neuromedin B receptor antagonist
suppresses tumor angiogenesis and tumor growth in vitro and in
vivo. Cancer Lett. 312:117–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin JJ, Selander K, Chirgwin JM, Dallas M,
Grubbs BG, Wieser R, Massagué J, Mundy GR and Guise TA: TGF-beta
signaling blockade inhibits PTHrP secretion by breast cancer cells
and bone metastases development. J Clin Invest. 103:197–206. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casey T, Bond J, Tighe S, Hunter T,
Lintault L, Patel O, Eneman J, Crocker A, White J, Tessitore J, et
al: Molecular signatures suggest a major role for stromal cells in
development of invasive breast cancer. Breast Cancer Res Treat.
114:47–62. 2009. View Article : Google Scholar
|
|
21
|
Liu J, Chen Y, Shuai S, Ding D, Li R and
Luo R: TRPM8 promotes aggressiveness of breast cancer cells by
regulating EMT via activating AKT/GSK-3β pathway. Tumour Biol.
35:8969–8977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen SC, Kung ML, Hu TH, Chen HY, Wu JC,
Kuo HM, Tsai HE, Lin YW, Wen ZH, Liu JK, et al: Hepatoma-derived
growth factor regulates breast cancer cell invasion by modulating
epithelial-mesenchymal transition. J Pathol. 228:158–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ning Q, Liu C, Hou L, Meng M, Zhang X, Luo
M, Shao S, Zuo X and Zhao X: Vascular endothelial growth factor
receptor-1 activation promotes migration and invasion of breast
cancer cells through epithelial-mesenchymal transition. PLoS One.
8:e652172013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Yang Q, Yu H, Wu L, Zhao Y, Zhang C,
Yue X, Liu Z, Wu H, Haffty BG, et al: LIF promotes tumorigenesis
and metastasis of breast cancer through the AKT-mTOR pathway.
Oncotarget. 5:788–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lauring J, Park BH and Wolff AC: The
phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target
in breast cancer. J Natl Compr Canc Netw. 11:670–678.
2013.PubMed/NCBI
|
|
26
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou X, Tan M, Stone Hawthorne V, Klos KS,
Lan KH, Yang Y, Yang W, Smith TL, Shi D and Yu D: Activation of the
Akt/ mammalian target of rapamycin/4E-BP1 pathway by ErbB2
overexpression predicts tumor progression in breast cancers. Clin
Cancer Res. 10:6779–6788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Fang R, Wang XF, Zhang F, Chen DY,
Zhou B, Wang HS, Cai SH and Du J: Stabilization of Snail through
AKT/GSK-3β signaling pathway is required for TNF-α-induced
epithelial-mesenchymal transition in prostate cancer PC3 cells. Eur
J Pharmacol. 714:48–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cornelio DB, Roesler R and Schwartsmann G:
Gastrin-releasing peptide receptor as a molecular target in
experimental anticancer therapy. Ann Oncol. 18:1457–1466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuiper P, Verspaget HW, Biemond I, de
Jonge-Muller ES, van Eeden S, van Velthuysen ML, Taal BG and Lamers
CB: Expression and ligand binding of bombesin receptors in
pulmonary and intestinal carcinoids. J Endocrinol Invest.
34:665–670. 2011.
|
|
31
|
Tsuda T, Kusui T and Jensen RT: Neuromedin
B receptor activation causes tyrosine phosphorylation of p125FAK by
a phospholipase C independent mechanism which requires p21rho and
integrity of the actin cytoskeleton. Biochemistry. 36:16328–16337.
1997. View Article : Google Scholar
|
|
32
|
Ohki-Hamazaki H and Neuromedin B:
Neuromedin B. Prog Neurobiol. 62:297–312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weigelt B, Peterse JL and van’t Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995;
discussion 5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vincent-Salomon A and Thiery JP: Host
microenvironment in breast cancer development:
Epithelial-mesenchymal transition in breast cancer development.
Breast Cancer Res. 5:101–106. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tomaskovic-Crook E, Thompson EW and Thiery
JP: Epithelial to mesenchymal transition and breast cancer. Breast
Cancer Res. 11:2132009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang T, Yuan J, Zhang J, Tian R, Ji W,
Zhou Y, Yang Y, Song W, Zhang F and Niu R: Anxa2 binds to STAT3 and
promotes epithelial to mesenchymal transition in breast cancer
cells. Oncotarget. 6:30975–30992. 2015.PubMed/NCBI
|
|
41
|
Kothari AN, Mi Z, Zapf M and Kuo PC: Novel
clinical therapeutics targeting the epithelial to mesenchymal
transition. Clin Transl Med. 3:352014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang F, Zhou X, Miao X, Zhang T, Hang X,
Tie R, Liu N, Tian F, Wang F and Yuan J: MAGEC2, an
epithelial-mesenchymal transition inducer, is associated with
breast cancer metastasis. Breast Cancer Res Treat. 145:23–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seeliger H, Guba M, Kleespies A, Jauch KW
and Bruns CJ: Role of mTOR in solid tumor systems: A therapeutical
target against primary tumor growth, metastases, and angiogenesis.
Cancer Metastasis Rev. 26:611–621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McAuliffe PF, Meric-Bernstam F, Mills GB
and Gonzalez-Angulo AM: Deciphering the role of PI3K/Akt/mTOR
pathway in breast cancer biology and pathogenesis. Clin Breast
Cancer. 10(Suppl 3): S59–S65. 2010. View Article : Google Scholar : PubMed/NCBI
|