Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer death among females
worldwide. There were estimated 1.7 million cases and 521,900
deaths in 2012, which accounted for 25% of all cancer cases and 15%
of all cancer deaths. In more developed countries, it even accounts
for approximately one-half of all breast cancer cases and 38% of
deaths (1). It demonstrated that
one in eight women will develop breast cancer during their lifetime
(2). Also, owing to the
multimodality treatment for breast cancer, the 5-year or 10-year
survival rate was obviously improved. Additionally, radiotherapy
has been considered as a key component of local treatment to reduce
the risks of local recurrence (3).
However, we cannot ignore the fact that up to 30% of node-negative
and up to 70% of node-positive breast cancers will relapse
(4) and heterogeneity of radiation
responses, such as severe acute dermatitis, pneumonitis, cough and
dysphagia (5). This may contribute
to the diversity of radiosensitivity; therefore, many studies have
been conducted to improve the radiosensitivity of patients with
breast cancer.
The mammalian target of rapamycin (mTOR), which
belongs to the phosphoinositide 3-kinase (PI3K), protein kinase B
(Akt/PKB) and mTOR signaling pathway, is crucial for cell growth,
survival, motility, proliferation, protein synthesis and
transcription. The mTOR exists in two different complexes in cells,
mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The mTORC1 has
functions of nutrient-energy-redox sensor. It controls protein
synthesis and stimulates cell growth and proliferation and its
activity is mediated by its three major downstream targets: p70S6
ribosomal kinase 1 (p70S6K1), eukaryotic initiation factor 4E
binding protein 1 (4EBP1) that regulated protein synthesis and
cyclins that mediated cap-dependent translation initiation
(6). It showed that 4EBP1
deficiency could drive lymphoma cell resistance to active-site mTOR
inhibitors (7). The mTORC2 showed
to be involved in the regulation of cytoskeleton functions, by
stimulating of actin fibres, paxillin, RhoA, Rac1 and protein
kinase C (PKC). Importantly, mTORC2 could activate Akt. The latter
is upstream in the mTORC1 pathway and downstream in the mTORC2
pathway. The study also suggested that mTORC2 plays an important
role in the regulation of lipid synthesis (8).
INK128 is an orally-available, potent and selective
ATP-competitive mTOR inhibitor; it showed an IC50 value
of 1 nM against mTOR and more than 100-fold selectivity to PI3K
kinases. It even displayed anti-proliferative activity in cell
lines resistant to rapamycin (6).
Oral administration of INK128 inhibited angiogenesis and tumor
growth in multiple preclinical models (6), including diffuse large B-cell
lymphoma (9), neuroblastoma
(10), thyroid cancer cells
(11), prostate cancer metastasis
(12), B-cell acute lymphoblastic
leukemia (13) and breast cancer
(14). Furthermore, in
lipopolysaccharide activated RAW264.7 cells, INK128 showed
anti-inflammatory activity (15).
In addition to in vitro condition, it was discovered that
INK128 could overcome the resistance of anti-HER2 therapies in
three different animal models (16). In the human breast cancer xenograft
model of athymic nude mice, INK128 showed potently cell
proliferation inhibition and also reduced VEGF-induced lung
metastasis (17). Also, a study
reported encouraging results that INK128 enhanced in vitro
and in vivo radiosensitivity of pancreatic carcinoma cells
(18). Recently, it showed that
the activation of the PI3K-AKT-mTOR pathway is considered
clinically relevant for tamoxifen resistance and contribute to the
poor outcome in breast cancer (19,20),
which provides evidence and great expectation for the application
of PI3K/AKT/mTOR inhibitors.
Due to the strong rational and these promising
effects of INK128, we hypothesized that INK128 combined
radiotherapy could induce synergistic effect on breast cancer
cells. In the present study, we tested this hypothesis by
evaluating the effects of INK128 with clonogenic survival assay,
and exploring its potential mechanisms of radiosensitization.
Materials and methods
Cell lines and treatments
Human breast cancer cell line MCF-7 was obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and incubated in Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; HyClone Laboratories, Logan, UT, USA), penicillin (100
U/ml), and streptomycin (100 U/ml) at 37°C in humidified 5%
CO2 incubator. INK128 (Chemietek, Indianapolis, IN, USA)
was dissolved in dimethyl sulfoxide (DMSO). Cells were irradiated
using 210 kV X-ray source at 2.16 Gy/min (RS 2000 Biological
irradiator; Rad Source Technologies, Suwanee, GA, USA).
Cell viability assay
We analyzed the effect of INK128 as a single agent
treatment on clonogenic survival of the breast cancer cell lines in
our test. Breast cancer cell viability was measured by the Cell
Counting kit (CCK-8) assay. Cells were plated and grown in 96-well
clear-bottom plates at 104 cells/well. One day after the
cells were settled, media was changed and drugs or vehicle was
added at the graded concentrations in sextuplicate wells and the
cells were then incubated at 37°C for 1–5 days. Then, a mixture of
10 μl CCK-8 (Dojindo Laboratories) and 190 μl of RPMI-1640 with 10%
FBS was added into each well. After 2 h of incubation, luminescence
was measured at 450 nm using a microplate reader. Background
reading of the media was subtracted from each well to standardize
the results. Optical density (OD) was utilized as the indicator of
cell survival.
Clonogenic survival assay
Clonogenic survival assays were performed as
previously described (21). Cells
(250, 500, 1,000, 2,000 and 4,000) were seeded into 6-well tissue
culture plates and allowed to settle for 24 h. Then, the cells were
exposed to 25 nM of INK128 or vehicle. Similarly, cells were
irradiated with different doses of ionizing radiation (0, 2, 4, 6
and 8 Gy) then exposed to the INK128 (25 nM) or same amount of
drug-free culture medium for another 24 h. Subsequently, the
treated cells were cultured in a 37°C, 5% CO2 incubator
for 10–14 days. Individual colonies (>50 cells per colony) were
fixed with methanol for 10 min, stained with 0.05% crystal violet
dye for 10 min, washed twice with tap water, and air dried
overnight. The plates were then photographed and the colonies were
counted. Each result was performed in triplicate. Plating
efficiency (PE) and survival fractions (SF) were calculated.
Survival curves were fitted and analyzed using linear-quadratic
model [S=exp (−αD−βD2)] by GraphPad Prism software (version 4.0;
GraphPad Prism Software, San Diego, CA, USA). The radiation
sensitizing enhancement ratio (SER) by INK128 was calculated using
the following formula: SER= (SF2 of MCF-7 control)/(SF2 of INK128
MCF-7). SER=1 suggests an additive radiation effect and SER >1,
a supra-additive effect as against a sub-additive effect in the
case of SER <1.
Fluorescence activating cell sorter
(FACS) analysis of cell cycle distribution
The cell suspension was prepared by trypsinization,
and 1×106 cells/ml were washed twice with PBS. The cells
were resuspended with 10 ml of 70% ethanol (−20°C), incubated at
4°C for 4 h, washed twice in cold PBS, incubated with RNase
(Sigma-Aldrich) at a concentration of 0.25 mg/ml at 37°C for 15
min, followed by treatment with PI (10 μl/ml), and incubated for 15
min at 4°C in the dark. DNA histograms were analyzed using same
FACS machine to evaluate the cell cycle distribution.
Immunofluorescent analysis of γH2AX
foci
Cells grown in chamber slides, were fixed,
permeabilized and blocked as described as follows. The slides were
incubated with antibody to γH2AX (Cell Signaling Technology,
Danvers, MA, USA) followed by goat anti-mouse Alexa 546 (Thermo
Fisher Scientific) and mounted with ProLong Gold anti-fade reagent
containing 4′-6-diamidino-2-phenylindole (DAPI; Invitrogen) to
visualize nuclei. Slides mounted with ProLong antifade reagent with
DAPI (Molecular Probes) and examined by fluorescence microscopy
(Carl Zeiss Axioskop 2; Carl Zeiss Microscopy LLC, Thornwood, NY,
USA). Cells were judged as ‘positive’ for γH2AX foci when they
displayed 10 or more discrete dots of brightness.
Immunoblotting and antibodies
Cells were grown in 60-mm dishes, and treated with
radiation, INK128 or a combination of both INK128 and radiation for
the indicated concentrations and times. Cells were washed with
ice-cold PBS and scraped into ice-cold lysis buffer. Lysates were
cleared by centrifugation at 13,000 rpm for 10 min at 4°C, and
supernatants removed and assayed for protein concentration using
the Pierce BCA bovine serum albumin. Protein was quantified using
BCA protein assay (Thermo Fisher Scientific), separated by
SDS-PAGE, transferred to polyvinylidene difluoride (PVDF; Bio-Rad
Laboratories) and probed with the indicated antibodies. Bands were
visualized using Pierce ECL western blotting substrate (Thermo
Fisher Scientific). Anti-phospho-Akt, anti-Akt, anti-4EBP1,
anti-phospho-4EBP1, anti-phospho-S6, anti-S6, anti-cleaved PARP,
anti-LC3B-II, anti-phospho-Chk2, anti-total-Chk2 and anti-p21,
γH2AX were purchased from Cell Signaling Technology. Anti-β-actin
was obtained from Sigma-Aldrich. Donkey anti-rabbit and sheep
anti-mouse horseradish peroxidase-conjugated secondary antibodies
were purchased from GE Healthcare. Images were captured with a
Fujifilm LASS-3000 camera system.
Statistical analysis
For all experiments, the time-point was chosen based
on pre-experiment results where the most significant effect was
detected. The data were expressed as means ± SD. Statistical
differences were analyzed by one-way ANOVA followed by multiple
comparisons performed with post hoc Bonferroni test (SPSS version
16). Values of P<0.05 were considered statistically significant.
All experiments were repeated at least three times.
Results
INK128 inhibits breast cancer cell MCF-7
viability and downstream protein
We firstly studied whether INK128 exposure was
correlated with cell viability of MCF-7 cells. We further explored
the relationship between different concentration with diverse cell
viability with the intent to find an optimal concentration. For
this purpose, we incubated MCF-7 cells with an indicated
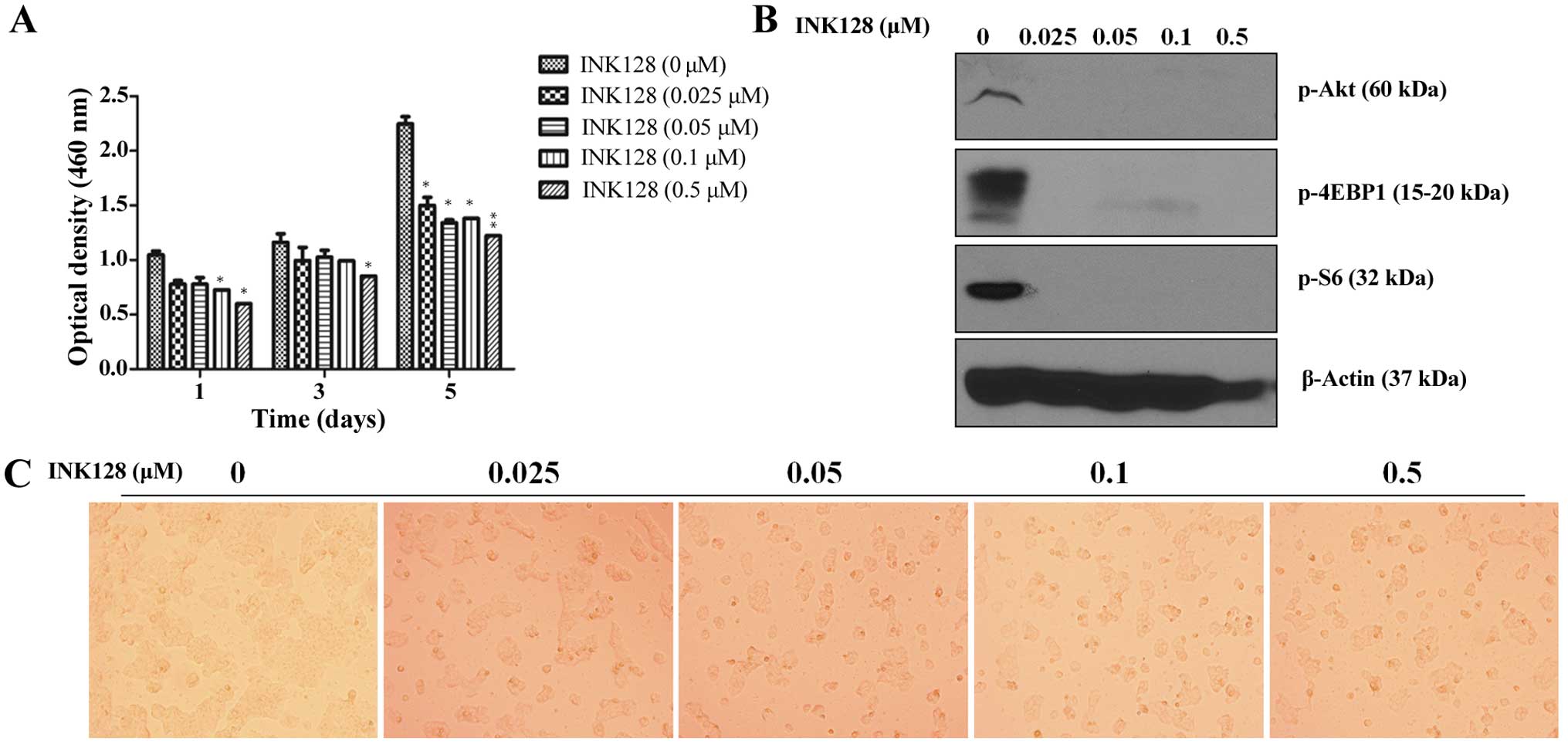
concentration. CCK-8 cell viability assay results (Fig. 1A) demonstrated that INK128 dose-
and time-dependently inhibited MCF-7 cell viability. In addition,
we noted that treatment with INK128 alone reduced cell viability
reaching a maximum inhibition by approximately day 5 as indicated
by the decrease in optical density levels at the concentration of
25 nM (Fig. 1A). It is consistent
with the clonogenic survival assay shown in Fig. 2B. Furthermore, INK128 induced
decrease of downstream signal chemicals, such as p-Akt, p-4EBP1 and
p-S6, even though at a very low concentration of 25 nM (Fig. 1B). Thus, for the following
experiment we chose INK128 at 25 nM as the experiment
concentration.
INK128 increases MCF-7 cell
radiosensitivity
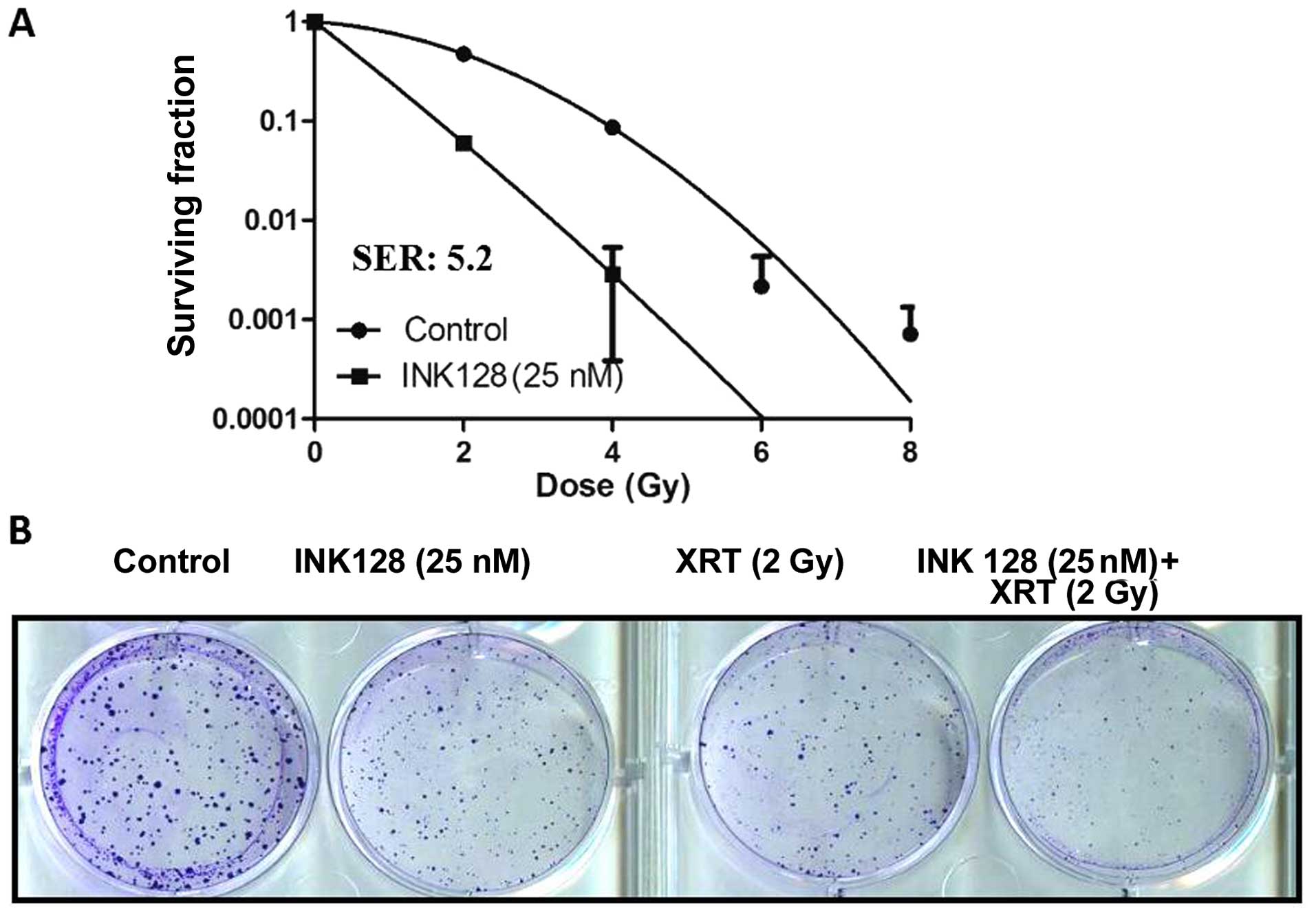
Next, we asked whether INK128 exposure was able to
increase the sensitivity of MCF-7 cells to irradiation. For this
purpose, treatment protocol was based upon a previous study
(18). INK128 exposure
significantly enhanced radiosensitivity compared with control cells
(both INK 128 alone and radiation alone) (Fig. 2), INK128 enhanced radiosensitivity
by SER=5.2 (SF2=0.47 for control cells; SF2=0.086 for INK128
exposure cells) (Fig. 2A).
According to the curve of surviving fraction we adopted radiation
dose of 3 Gy to perform the next study, these data suggested that
mTOR might be a critical regulator in radiation response in MCF-7
cells.
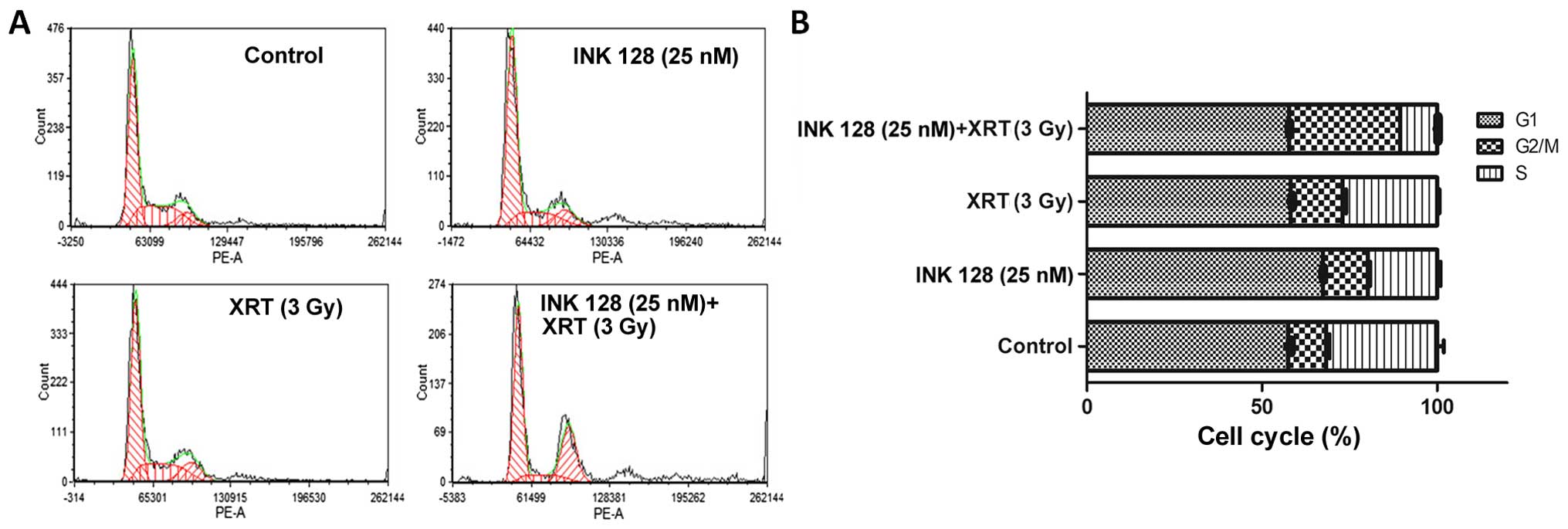
INK128 decreased the radioresistant
S-phase cells and increased G2/M blocks
It has been reported that mTORC1 is involved in the
regulation of different cyclins. To the best of our knowledge, the
different cycle distribution stands for different radiosensitivity.
Thus, we investigated the cell cycle distribution in MCF-7 cells
exposed to indicated elements (vehicle, 25 nM INK128, 3-Gy
irradiation and its combination) by FACS. The data by flow
cytometry showed 3-Gy radiation has slight effect on S-phase cell
decrease. Whereby, INK128 could obviously cause radioresistant
S-phase cell downregulation with low concentration of 25 nM. When
combining radiation and INK128, it further reduced radioresistant
S-phase cell population to 10.6±0.25 than the 31.70±1.91% of the
control (Fig. 3B). In addition,
INK128 combined radiation could obviously induce G2/M arrest as
indicated by the maximum percentage of the G2/M (31.66±0.07%).
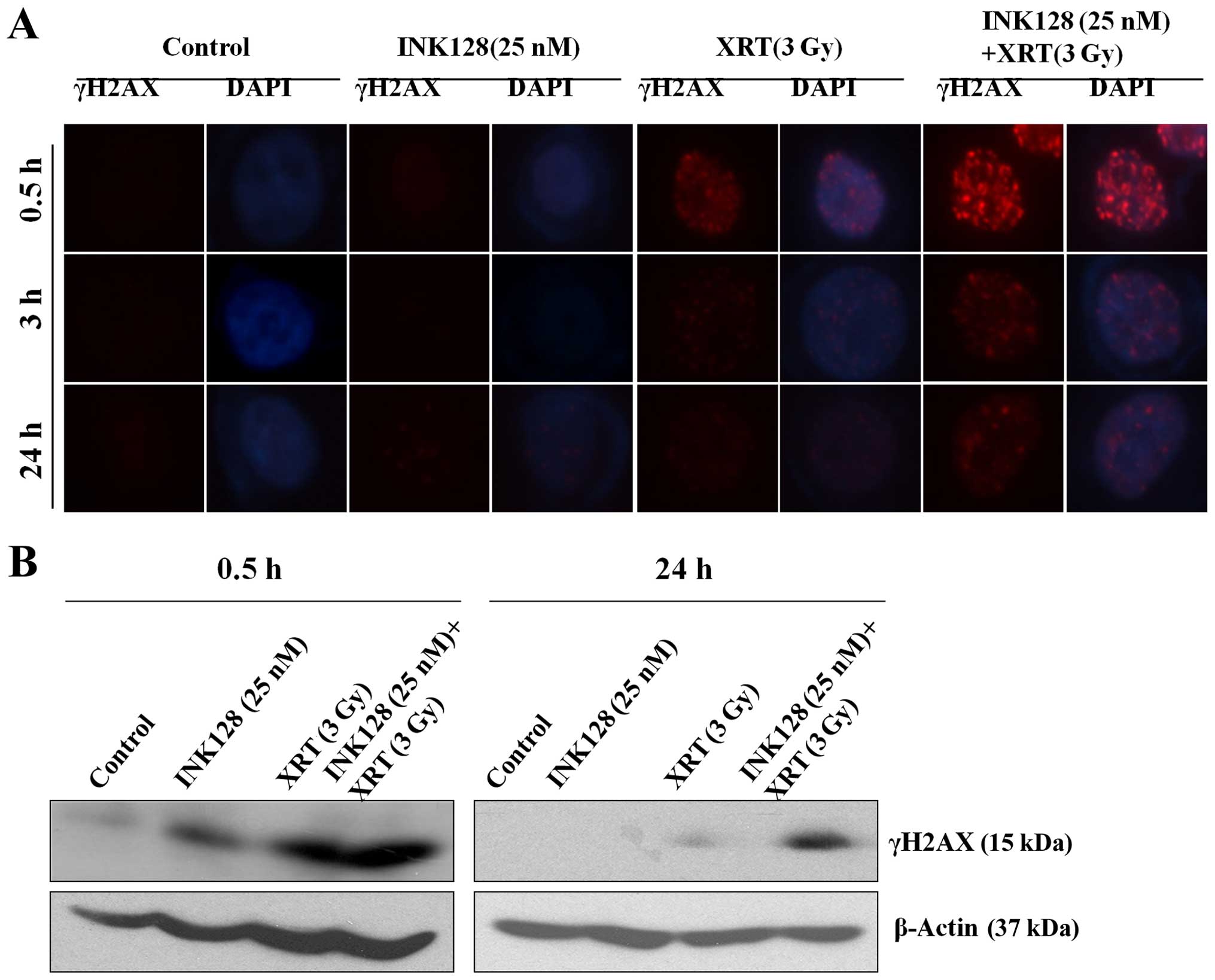
INK128 enhances radiation induced double
strand break (DSB) and suppresses its repair in MCF-7 cells
It is well known that γH2AX is a marker of DNA
double strand breaking in cells treated with radiation (22). Next, we began to investigate the
mechanisms mediating INK128-induced radiosensitization according to
the theory that γH2AX foci corresponds to radiation-induced DSB.
The treatment protocol was as follows: vehicle, 25 nM INK128, 3-Gy
irradiation, INK128 plus radiation, respectively. Then γH2AX
nuclear foci were detected in different time-points (0.5, 3 and 24
h). As shown in Fig. 4A, no
difference in foci levels was detected between control (vehicle)
and INK128 treated alone cells at 0.5 h after irradiation,
suggesting that INK128 had no effect on the initial levels of DSBs.
When combining INK128 and radiation at 0.5 h, it was significantly
higher than the other three groups. However, after 6 and 24 h of
irradiation, the residual numbers of γH2AX foci were significantly
greater in the INK128 treated cells as compared to control cells.
The bands of γH2AX from Fig. 4B
showed consistent results. It indicated that INK128 enhanced
radiation induced DNA DSB and suppressed its repair.
INK128 downregulates signaling pathway
protein expression
INK128 is a novel mTORC1 and 2-dual inhibitor, thus,
we used western blots to verify the downregulating signal protein
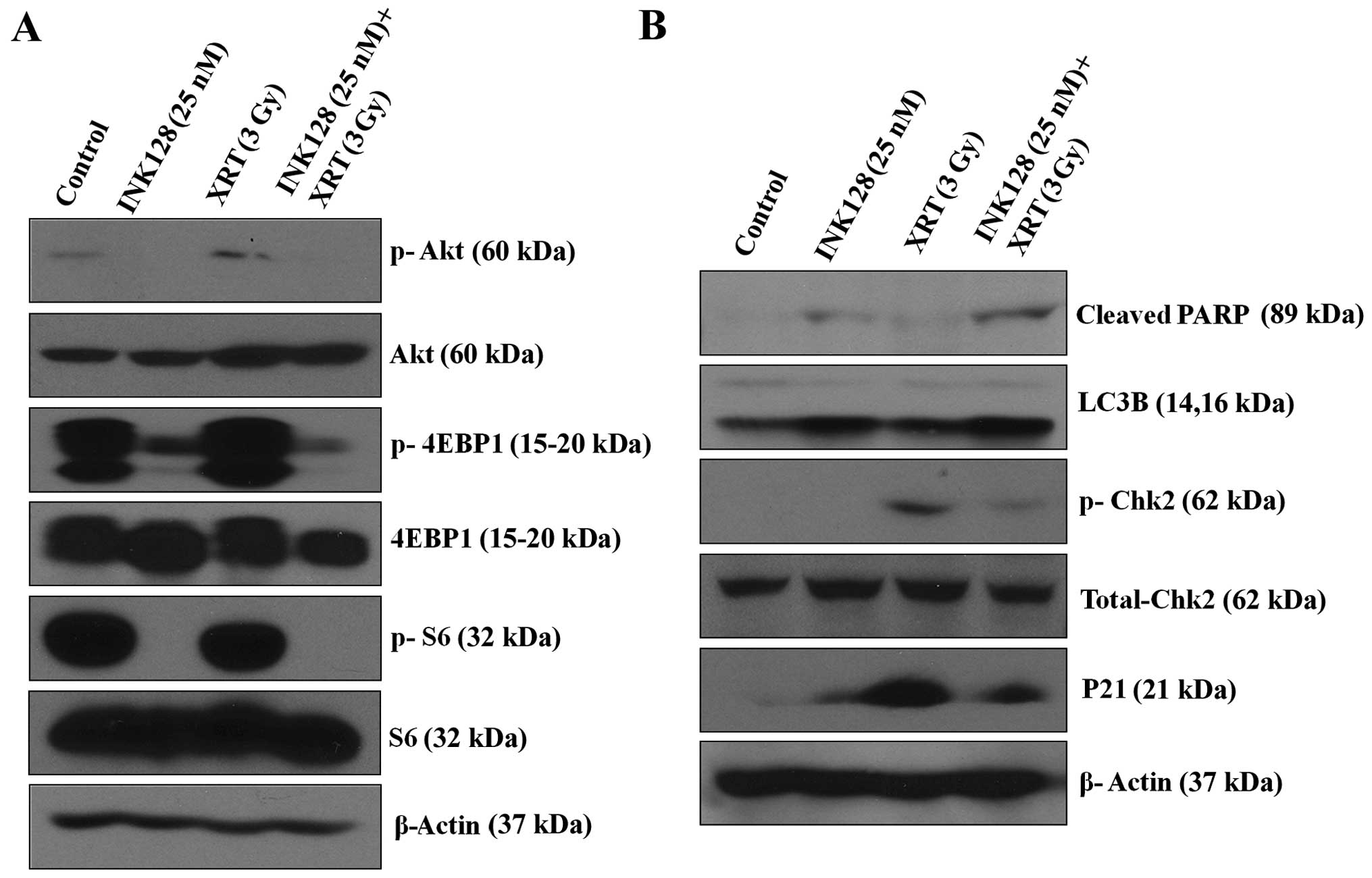
and detect the potential related protein. As indicated in Fig. 5A, INK128 alone or in combination
with ionizing radiation could obviously reduce phosphorylation of
Akt (the mTORC2 activation indicator), phosphorylation of S6
(mTORC1 activation indicators) and phosphorylation of 4EBP1 (mTORC1
activation indicators).
Next, we tested the probable protein expression,
western blot analysis was performed to analyzed the cleaved-PARP,
which is the final product of apoptotic state, that is to say
increased cleaved-PARP levels indicate apoptosis (23). As shown in Fig. 5B, INK128 alone could promote the
cleaved-PARP. When combined with radiation, cleaved-PARP was
obviously increased. Similarly, we detected the autophagy related
protein, such as LC3B-II. In MCF-7 cells, single treatments (INK128
alone) were able to increase LC3B. LC3B-II was further enhanced in
combined therapy group (Fig. 5B).
Also, we found that radiation alone could apparently increase
expression of phosphorylation of Chk2 and p21. INK128 combined with
radiation could decrease expression of phosphorylation of Chk2, an
indicator of cell cycle checkpoint kinase 2 (p-Chk2) activation and
p21.
Discussion
Breast cancer treatment includes surgery,
radiotherapy, chemotherapy and endocrine therapy. Radiotherapy is
one of most effective and critical components for local control
(24), reduced risk of local
recurrence in chest wall and regional lymph nodes (25). However, the heterogeneity of
radiation responses among breast cancer patients limits clinical
applications of radiotherapy (26). The present study focused on the
correlation of mTOR inhibition by a novel mTORC1/2 dual inhibitor
INK128 and radiosensitivity of patients with breast cancer. We
established the radiosentization effect of INK128 with a SER of 5.2
at 2 Gy. It suggested that mTOR pathway might play an important
role in the regulation of cellular response to radiation in MCF-7
cells. Also, the results underscore the importance of mTOR
targeting in combination with irradiation in tumor therapy.
In order to investigate the mechanism mediating the
INK128-induced radiosensitization, the following steps were taken.
The results showed that the combination with INK128 and radiation
could cause G2/M block with low concentration of 25 nM, that is to
say, enhance the percentage of G2 phase cells, decreased the S
phase cells. As is known, S phase cells are relatively resistant to
radiation, while G2 cell phase are relatively sensitive. Thus, our
results showed that the change of cell cycle distribution maybe the
reason to mediate INK128-induced radiosentization. A little
different to our result, another study showed that INK128 could
enhance the percentage of S and G2 phase cells, decrease the G1
phase cells by downregulating of cyclin D1 with a single agent
treatment of INK128 at the concentration of 50 nM for 48 h for
human pancreatic cancer cells (27). According to the small difference
described above, the other reason may be that single treatment or
combined strategy may hold different mechanism that regulate the
cell cycle. In terms of p21, it is well known that p21 is a potent
inhibitor of cyclin-dependent kinases capable of arresting cell
cycle progression (28), which is
the key mechanism to prevent apoptosis by promoting cell repair
(29). Our results demonstrated
that INK128 treatment could decrease the p21 expression, which
means increased cell apoptosis. It may be another cause to mediate
radiation-sensitivity.
It is well known that DSBs are suggestive of
critical lesions in DNA caused by ionizing radiation. DSB is the
main mechanism of tumor cell death after irradiation. The major
cause of radiotherapy failure is the success of DSB repair, which
could lead to prolonged tumor cell survival. If completion of DNA
damage repair fails, apoptosis and autophagy will be triggered for
the elimination of damaged cells (21). Our results demonstrated that INK128
exposure markedly increased DSB and significantly decreased the
rate of DNA DSB recovery. In the present study, at 0.5 h after
irradiation, the number of γH2AX foci remaining was significantly
greater when INK128 combined with radiation. Up to 24 h, the
content of γH2AX foci was gradually reduced, but it was still more
than radiation alone group. It was consistent with another study,
at 6 and 24 h after irradiation, the number of γH2AX foci remaining
was significantly greater in the INK128-treated cells than in
control cells. So the author conclude that INK128 inhibited a later
stage of DNA DSB repair (18).
There are some other reasons to explain the damaged repair ability.
Firstly, Chk2 is a kind of DNA repair proteins (30). Our results from western blot
analysis showed that combination of INK128 and radiation could
apparently decrease expression of phosphorylation of Chk2.
Secondly, a study demonstrated that another PI3K/mTOR inhibitor,
BEZ235, could block double strand break repair through attenuating
the activation of radiation-activated phosphorylation of ATM and
DNA-PKcs, the former are two major kinases for non-homologous end
joining and homologous recombination in DNA-DSB repair (22). This may be another mechanism for
INK128. Also, it showed that increased p-Akt had been linked to
decreased radiation responsiveness; therefore, inhibition of p-Akt
has radiosensitizing effect (21).
Thus, the present study showed that INK128 treatment could decrease
DNA DSB repair, p-Akt and p-Chk2, which might be important factors
to mediate INK128-induced radiosensitization.
In conclusion, we proposed that mTOR confers
radiation resistance and the strong potential of the novel mTORC1/2
dual inhibitor INK128 to enhance ionizing radiation in breast
cancer cells. Thus, effectively inhibition of mTOR by INK128 is a
potential therapeutic strategy for sensitizing resistant breast
cancer cells to radiation. Next, we need phase I and phase II
clinical trials.
Acknowledgements
The present study was partially supported by the
Hunan Administration of Foreign Experts Affairs (no. CG144300009);
the National Key Clinical Specialty (Oncology Department) (National
Health and Family Planning Commission of the PRC 2013/544); the
National Natural Science Foundation of China (nos. 81201982 and
81572500); The Specialized Research Fund for the Doctoral Program
of Higher Education (no. 20120171120110), and The Research Project
of Health and Family Planning Commission of Hunan Province (no.
B2014-112).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feys L, Descamps B, Vanhove C, Vral A,
Veldeman L, Vermeulen S, De Wagter C, Bracke M and De Wever O:
Radiation-induced lung damage promotes breast cancer
lung-metastasis through CXCR4 signaling. Oncotarget. 6:26615–26632.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cardoso F1, Harbeck N, Fallowfield L,
Kyriakides S and Senkus E; ESMO Guidelines Working Group. Locally
recurrent or metastatic breast cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
23(Suppl 7): vii11–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halyard MY, Pisansky TM, Dueck AC, Suman
V, Pierce L, Solin L, Marks L, Davidson N, Martino S, Kaufman P, et
al: Radiotherapy and adjuvant trastuzumab in operable breast
cancer: Tolerability and adverse event data from the NCCTG Phase
III Trial N9831. J Clin Oncol. 27:2638–2644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schenone S, Brullo C, Musumeci F, Radi M
and Botta M: ATP-competitive inhibitors of mTOR: An update. Curr
Med Chem. 18:2995–3014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mallya S, Fitch BA, Lee JS, So L, Janes MR
and Fruman DA: Resistance to mTOR kinase inhibitors in lymphoma
cells lacking 4EBP1. PLoS One. 9:e888652014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Oh YT, Yue P, Khuri FR and Sun SY:
Inhibition of mTOR complex 2 induces GSK3/FBXW7-dependent
degradation of sterol regulatory element-binding protein 1 (SREBP1)
and suppresses lipogenesis in cancer cells. Oncogene. 35:642–50.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazan-Mamczarz K, Peroutka RJ, Steinhardt
JJ, Gidoni M, Zhang Y, Lehrmann E, Landon AL, Dai B, Houng S,
Muniandy PA, et al: Distinct inhibitory effects on mTOR signaling
by ethanol and INK128 in diffuse large B-cell lymphoma. Cell Commun
Signal. 13:152015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Dou J, Yu Y, Zhao Y, Fan Y, Cheng
J, Xu X, Liu W, Guan S, Chen Z, et al: mTOR ATP-competitive
inhibitor INK128 inhibits neuroblastoma growth via blocking mTORC
signaling. Apoptosis. 20:50–62. 2015. View Article : Google Scholar :
|
|
11
|
Gild ML, Landa I, Ryder M, Ghossein RA,
Knauf JA and Fagin JA: Targeting mTOR in RET mutant medullary and
differentiated thyroid cancer cells. Endocr Relat Cancer.
20:659–667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh AC, Liu Y, Edlind MP, Ingolia NT,
Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et
al: The translational landscape of mTOR signalling steers cancer
initiation and metastasis. Nature. 485:55–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Janes MR, Vu C, Mallya S, Shieh MP, Limon
JJ, Li LS, Jessen KA, Martin MB, Ren P, Lilly MB, et al: Efficacy
of the investigational mTOR kinase inhibitor MLN0128/INK128 in
models of B-cell acute lymphoblastic leukemia. Leukemia.
27:586–594. 2013. View Article : Google Scholar
|
|
14
|
Wilson-Edell KA, Yevtushenko MA,
Rothschild DE, Rogers AN and Benz CC: mTORC1/C2 and pan-HDAC
inhibitors synergistically impair breast cancer growth by
convergent AKT and polysome inhibiting mechanisms. Breast Cancer
Res Treat. 144:287–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan H, Xu LH, Ouyang DY, Wang Y, Zha QB,
Hou XF and He XH: The second-generation mTOR kinase inhibitor
INK128 exhibits anti-inflammatory activity in
lipopolysaccharide-activated RAW 264.7 cells. Inflammation.
37:756–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
García-García C, Ibrahim YH, Serra V,
Calvo MT, Guzmán M, Grueso J, Aura C, Pérez J, Jessen K, Liu Y, et
al: Dual mTORC1/2 and HER2 blockade results in antitumor activity
in preclinical models of breast cancer resistant to anti-HER2
therapy. Clin Cancer Res. 18:2603–2612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gökmen-Polar Y, Liu Y, Toroni RA, Sanders
KL, Mehta R, Badve S, Rommel C and Sledge GW Jr: Investigational
drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral
antitumor activity in human breast cancer xenograft models. Breast
Cancer Res Treat. 136:673–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayman TJ, Wahba A, Rath BH, Bae H, Kramp
T, Shankavaram UT, Camphausen K and Tofilon PJ: The ATP-competitive
mTOR inhibitor INK128 enhances in vitro and in vivo
radiosensitivity of pancreatic carcinoma cells. Clin Cancer Res.
20:110–119. 2014. View Article : Google Scholar :
|
|
19
|
Woo YM, Shin Y, Lee EJ, Lee S, Jeong SH,
Kong HK, Park EY, Kim HK, Han J, Chang M, et al: Inhibition of
aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores
tamoxifen sensitivity in antiestrogen-resistant breast cancer
cells. PLoS One. 10:e01322852015. View Article : Google Scholar
|
|
20
|
Deng L, Chen J, Zhong XR, Luo T, Wang YP,
Huang HF, Yin LJ, Qiu Y, Bu H, Lv Q, et al: Correlation between
activation of PI3K/AKT/mTOR pathway and prognosis of breast cancer
in Chinese women. PLoS One. 10:e01205112015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu ZG, Liu L, Xu LH, Yi W, Tao YL, Tu ZW,
Li MZ, Zeng MS and Xia YF: Bmi-1 induces radioresistance in MCF-7
mammary carcinoma cells. Oncol Rep. 27:1116–1122. 2012.PubMed/NCBI
|
|
22
|
Chen YH, Wei MF, Wang CW, Lee HW, Pan SL,
Gao M, Kuo SH, Cheng AL and Teng CM: Dual phosphoinositide
3-kinase/mammalian target of rapamycin inhibitor is an effective
radiosensitizer for colorectal cancer. Cancer Lett. 357:582–590.
2015. View Article : Google Scholar
|
|
23
|
Li K, Cao RJ, Zhu XJ, Liu XY, Li LY and
Cui SS: Erythropoietin attenuates the apoptosis of adult neurons
after brachial plexus root avulsion by downregulating JNK
phosphorylation and c-Jun expression and inhibiting c-PARP
cleavage. J Mol Neurosci. 56:917–925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu R, Li W, Xu Y, Wan J and Zhang Z:
Upregulation of BTG1 enhances the radiation sensitivity of human
breast cancer in vitro and in vivo. Oncol Rep. 34:3017–3024.
2015.PubMed/NCBI
|
|
25
|
Warren LE, Punglia RS, Wong JS and Bellon
JR: Management of the regional lymph nodes following
breast-conservation therapy for early-stage breast cancer: An
evolving paradigm. Int J Radiat Oncol Biol Phys. 90:772–777. 2014.
View Article : Google Scholar
|
|
26
|
Zhu J, Ye Q, Chang L, Xiong W, He Q and Li
W: Upregulation of miR-195 enhances the radiosensitivity of breast
cancer cells through the inhibition of BCL-2. Int J Clin Exp Med.
8:9142–9148. 2015.PubMed/NCBI
|
|
27
|
Lou HZ, Weng XC, Pan HM, Pan Q, Sun P, Liu
LL and Chen B: The novel mTORC1/2 dual inhibitor INK-128 suppresses
survival and proliferation of primary and transformed human
pancreatic cancer cells. Biochem Biophys Res Commun. 450:973–978.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radhakrishnan SK, Feliciano CS, Najmabadi
F, Haegebarth A, Kandel ES, Tyner AL and Gartel AL: Constitutive
expression of E2F-1 leads to p21-dependent cell cycle arrest in S
phase of the cell cycle. Oncogene. 23:4173–4176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raj K, Ogston P and Beard P:
Virus-mediated killing of cells that lack p53 activity. Nature.
412:914–917. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdel-Fatah TM, Arora A, Moseley PM, Perry
C, Rakha EA, Green AR, Chan SY, Ellis IO and Madhusudan S: DNA
repair prognostic index modelling reveals an essential role for
base excision repair in influencing clinical outcomes in ER
negative and triple negative breast cancers. Oncotarget.
6:21964–21978. 2015. View Article : Google Scholar : PubMed/NCBI
|