Introduction
Bladder cancer is the most prevalent tumor of the
urinary tract worldwide. The majority of bladder cancers are
low-grade non-invasive tumors that may progress to the invasive
phenotype. In contrast to non-invasive bladder cancer, muscle
invasive tumors tend to metastasize to other organs and have a very
poor prognosis (1,2). Recent epidemiological studies have
shown that the incidence of bladder cancer is highest in the
developed countries of Western Europe, North America, and Australia
and lowest in Asian countries, which may be connected with their
different diets and living habits (3,4).
Although radiation therapy, adjuvant chemotherapy, and combinations
of these modalities are standard options for managing bladder
cancer, current treatments of bladder cancer have high recurrence
rates and may cause strong side effects (5,6).
Therefore, the discovery of effective anticancer drug candidates
for the treatment of bladder cancer is still required for the
advancement of the medical treatment of bladder cancer
patients.
Recently, natural products or compounds, such as
plant-derived products, have been recognized as powerful resources
for new cancer drug discovery, because they may reduce adverse side
effects (7,8). Baicalein (5,6,7-trihydroxyflavone) is
one of the major phenolic flavonoids isolated from the rhizome of
Scutellaria baicalensis Georgi (9,10),
which has been widely used in traditional Oriental medicine for
treating various inflammatory diseases, chronic hepatitis,
bacterial and viral infections, allergies, and ischemia (11,12).
Recent research on baicalein has documented a wide spectrum of
therapeutic properties, including antimicrobial, anti-inflammatory,
antioxidative, immunomodulatory, and antiangiogenesis effects
(10,12–15).
Several studies have indicated that baicalein exhibits anticancer
activities due to its ability to inhibit cell growth and to induce
cell cycle arrest at the G1 phase and apoptotic pathways in
distinct cancer cells (16–20).
Interestingly, this agent does not exert an apoptotic effect on
normal cells and therefore can be developed as an anticancer drug
(21–23). Moreover, we recently demonstrated
that baicalein causes apoptosis in human lung carcinoma cells by
changing the apoptotic gene expressions through reactive oxygen
species (ROS) generation (24).
However, the direct molecular target and mode of the
baicalein anticancer mechanism in human bladder cancer cells have
not been fully clarified. Therefore, this study used human muscle
invasive bladder cancer 5637 cells to identify additional molecular
mechanisms supporting the antiproliferative and apoptotic effects
of baicalein.
Materials and methods
Chemicals and antibodies
RPMI-1640 medium, fetal bovine serum (FBS), and
antibiotics were purchased from Welgene (Daegu, Korea). Baicalein
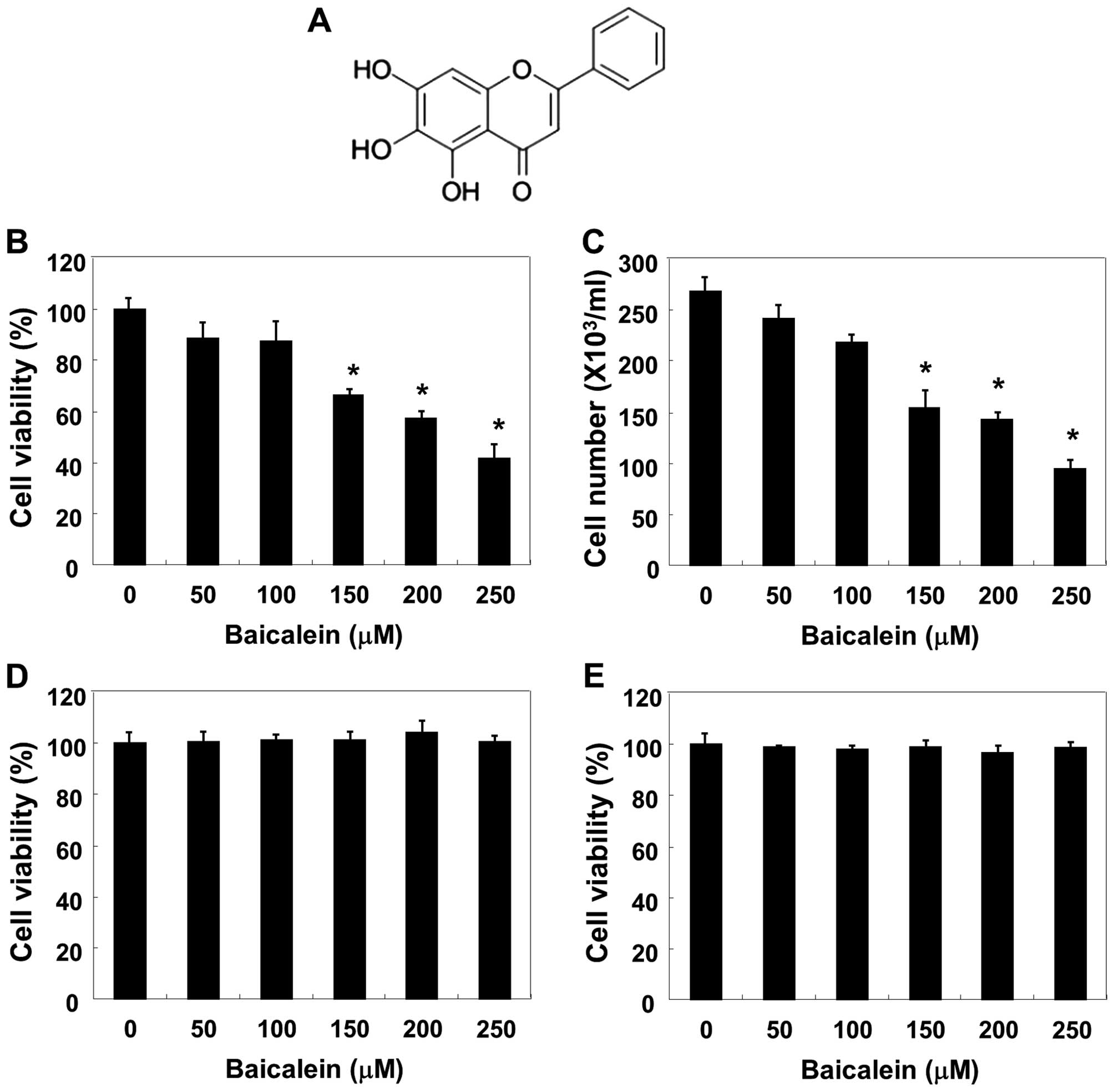
(purity 98%, Fig. 1A), 4,
6-diamidino-2-phenylindole (DAPI), 3-(4,5-dimethyl-2-thiazolyl)-2,
5-diphenyl-2H-tetrazolium (MTT), and N-acetyl-L-cysteine (NAC) were
purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone
(z-VAD-fmk), a pan-caspase inhibitor, and
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1) were purchased from Calbiochem (San Diego, CA, USA).
2′,7′-dichlorofluorescein diacetate (DCF-DA) and
fluorescein-conjugated Annexin V (Annexin V-FITC) were obtained
from Molecular Probes (Eugene, OR, USA) and BD Biosciences
Pharmingen (San Jose, CA, USA), respectively. An enhanced
chemiluminescence (ECL) detection system and in vitro
caspase colorimetric assay kits were purchased from Amersham Corp.
(Arlington Heights, IL, USA) and R&D Systems (Minneapolis, MN,
USA), respectively. The primary antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) used in this study were
as follows: β-actin (1:1,000, sc-7120; rabbit polyclonal), DR4
(1:1,000, sc-7863; rabbit polyclonal), DR5 (1:1,000, sc-65314;
mouse monoclonal), Fas (1:1,000, sc-715; rabbit polyclonal), FasL
(1:1,000, sc-957; rabbit polyclonal), TRAIL (1:500, sc-7877; rabbit
polyclonal), cIAP-1 (1:1,000, sc-7943; rabbit polyclonal), cIAP-2
(1:1,000, sc-7944; rabbit polyclonal), XIAP (1:1,000, sc-11426;
rabbit polyclonal), caspase-3 (1:1,000, sc-7272; mouse monoclonal),
caspase-8 (1:1,000, sc-7890; rabbit polyclonal), caspase-9
(1:1,000, sc-7885; rabbit polyclonal), PARP (1:1,000, sc-7150;
rabbit polyclonal), Bcl-2 (1:1,000, sc-509; mouse monoclonal), Bax
(1:1,000, sc-493; rabbit polyclonal), Bid (1:500, sc-11423; rabbit
polyclonal). Peroxidase-labeled donkey anti-rabbit and sheep
anti-mouse immunoglobulin were purchased from Amersham Corp. All
other chemicals were purchased from Sigma-Aldrich Chemical Co.
Cell culture
The 5637 human bladder cancer, Chang liver (an
immortalized non-tumor cell line derived from normal liver tissue),
and murine Raw 364.7 macrophage cell lines were obtained from
American Type Culture Collection (Manassas, MD, USA) and maintained
in RPMI-1640 medium supplemented with 10% FBS and antibiotics (100
μg/ml streptomycin, 100 U/ml penicillin) at 37°C in a humidified
incubator under an atmosphere of 5% CO2 in air.
Baicalein was dissolved in dimethyl sulfoxide (DMSO) as a stock
solution at 100 mM, which was then diluted with RPMI-1640 medium to
the desired concentration prior to use.
Cell viability and growth assay
The 5637 cells were seeded in 6-well plates at a
density of 4.0×105 cells per well. After a 24-h
incubation, the cells were treated with various concentrations of
baicalein for 24 h. Cell viability was determined using the MTT
assay. In brief, an MTT working solution (0.5 mg/ml) was added to
the culture plates and incubated for 3 h at 37°C. The culture
supernatant was removed from the wells, and DMSO was added to
dissolve the formazan crystals completely. The absorbance of each
well was measured at 540 nm using an enzyme-linked immunosorbent
assay (ELISA) reader (Molecular Devices, Sunnyvale, CA, USA). Cell
growth was assessed using the trypan blue dye exclusion assay.
After treatment with the indicated concentrations of the baicalein
for 24 h, the cells were trypsinized and viable cells were counted
by trypan blue dye exclusion using a hemocytometer under an
inverted microscope (Carl Zeiss, Jena, Germany). The effect of
baicalein on cell viability and growth was assessed as the
percentage of cell viability, in which the vehicle-treated cells
were considered 100% viable.
Nuclear staining with DAPI
For the assessment of apoptosis, the cells were
washed with ice-cold phosphate-buffered saline (PBS) and fixed with
4% paraformaldehyde in PBS for 10 min at room temperature. The
fixed cells were washed with PBS and stained with 2.5 μg/ml DAPI
solution for 10 min at room temperature. The cells were then washed
twice with PBS and analyzed with a fluorescence microscope (Carl
Zeiss).
DNA flow cytometric detection of
apoptosis
For the quantitative assessment of the induced cell
apoptosis rate, an Annexin V-FITC staining assay was performed
according to the manufacturer’s protocol. Briefly, the cells in
each sample were stained with 5 μl Annexin V-FITC and 5 μl
propidium iodide (PI). After incubation for 15 min at room
temperature in the dark, the degree of apoptosis was quantified by
a flow cytometer (FACSCalibur, Becton-Dickinson, San Jose, CA, USA)
as a percentage of the Annexin V-positive and PI-negative cells
(25).
Protein extraction and western blot
analysis
After removing the media, the cells were washed with
ice-cold PBS and harvested and lysed with a lysis buffer [20 mM
sucrose, 1 mM ethylendiaminetetraacetic acid, 20 μM Tris-Cl, pH
7.2, 1 mM dithiothreitol (DTT), 10 mM KCl, 1.5 mM MgCl2,
5 μg/ml pepstatin A, 10 μg/ml leupeptin, and 2 μg/ml aprotinin] for
30 min at 4°C. The supernatants were collected and protein
concentration was determined using a Bio-Rad protein assay kit
(Bio-Rad Laboratories, Hercules, CA, USA) according to the
manufacturer’s instructions. For western blotting, equal amounts of
protein extracts were denatured by boiling at 95°C for 5 min in a
sample buffer [0.5 M Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate
(SDS), 20% glycerol, 0.1% bromophenol blue, and 10%
β-mercaptoethanol] at a ratio of 1:1. Samples were stored at −80°C
or immediately used for immunoblotting. The equal cellular proteins
were separated via denaturing SDS-polyacrylamide gel
electrophoresis and transferred electrophoretically to
nitrocellulose membranes (Amersham Corp.). The membranes were then
blocked with 5% skim milk and incubated overnight at 4°C with
primary antibodies, probed with enzyme-linked secondary antibodies
for 1 h at room temperature, and detected using an ECL detection
system.
In vitro caspase activity assay
The activities of the caspases were determined using
colorimetric assay kits, which utilize synthetic tetrapeptides
[Asp-Glu-Val-Asp (DEAD) for caspase-3; Ile-Glu-Thr-Asp (IETD) for
caspase-8; and Leu-Glu-His-Asp (LEHD) for caspase-9] labeled with
p-nitroaniline (pNA), which is linked to the end of the
caspase-specific substrate. Briefly, the baicalein-treated cells
and the untreated cells were lysed in the supplied lysis buffer.
The supernatants were collected and incubated with the supplied
reaction buffer containing DTT and DEAD-pNA, IETD-pNA, or LEHD-pNA
as substrates at 37°C for 2 h in the dark. The reactions were
measured by changes in absorbance at 405 nm using an ELISA reader
(26).
Measurement of mitochondrial membrane
potential (MMP, ΔΨm)
The MMP values were determined using the
dual-emission potential-sensitive probe, JC-1. Briefly, the cells
were collected and incubated with 10 μM of JC-1 for 20 min at 37°C
in the dark. After the JC-1 was removed, the cells were washed with
PBS to remove unbound dye, and the amount of JC-1 retained by
10,000 cells per sample was measured at 488 and 575 nm using a flow
cytometer (27).
Detection of ROS generation
To assess the generated ROS, the cells were treated
with or without 10 mM NAC for 1 h before challenge with 250 μM
baicalein for 30 min, and then stained with 10 μM DCF-DA and
incubated at 37°C for 30 min in the dark to monitor ROS production.
Later, the ROS production in cells was monitored by a flow
cytometer (28).
Statistical analysis
All data are presented as mean ± standard deviation
(SD). Significant differences among the groups were determined
using the unpaired Student’s t-test. A value of p<0.05 was
accepted as an indication of statistical significance. All of the
data shown herein were obtained from at least three independent
experiments.
Results
Effects of baicalein on the cell
viability and growth in 5637 cells
To assess the effect of baicalein on 5637 cell
viability and growth, equal numbers of cells were treated with
various concentrations of baicalein (50–250 μM) for 24 h and the
cell viability and growth were detected by the MTT and trypan blue
dye exclusion assays. As shown in Fig.
1B and C, baicalein caused dose-dependent inhibition of cell
viability and growth, with a significant reduction at 150 μM and an
almost 60% reduction at 250 μM. The results of an additional
experiment using Chang liver cells and Raw 264.7 macrophages,
conducted to examine the effect of baicalein on the proliferation
of normal cells, are shown in Fig. 1D
and E. The MTT assay results indicated that baicalein
concentrations ≤250 μM/ml did not induce cytotoxicity.
Induction of apoptosis by baicalein in
5637 cells
To determine whether baicalein-mediated inhibition
of cell viability in 5637 cells is associated with induction of
apoptosis, we examined apoptotic features by measuring the
chromatin condensation of the nuclei and the amount of Annexin
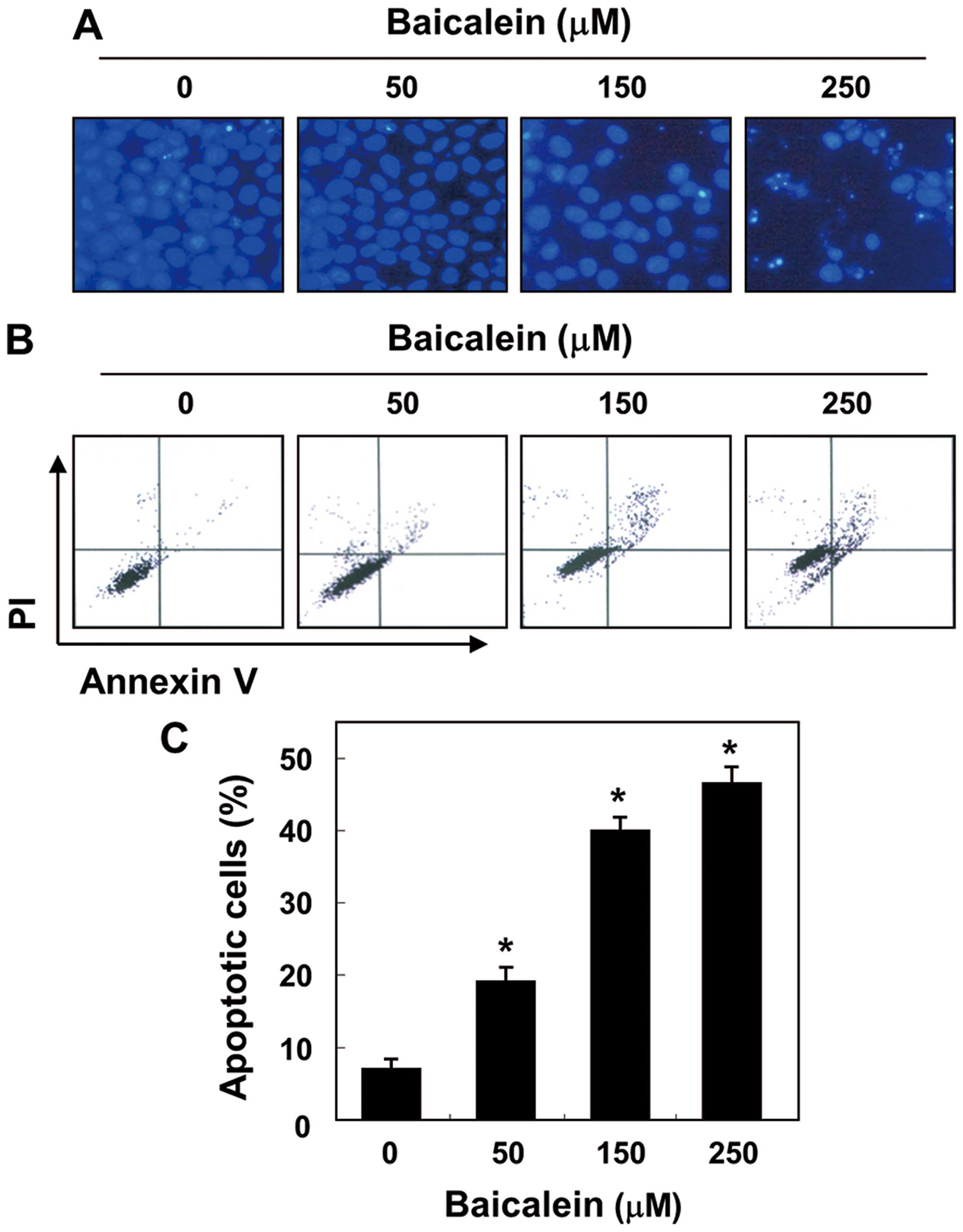
V-positive cells. As shown in Fig.
2A, the results of the DAPI reagent indicate that the nuclear
fragmentation and chromatin condensation located in apoptotic cells
were observed in baicalein-treated cells, with bright blue
fluorescence. In addition, treatment with baicalein resulted in the
increased accumulation of cells in the number of Annexin V-positive
cells in a concentration-dependent manner (Fig. 2B and C). These findings suggest
that baicalein suppresses 5637 cell viability by inducing cellular
apoptosis.
Modulation of apoptosis-related genes by
baicalein in 5637 cells
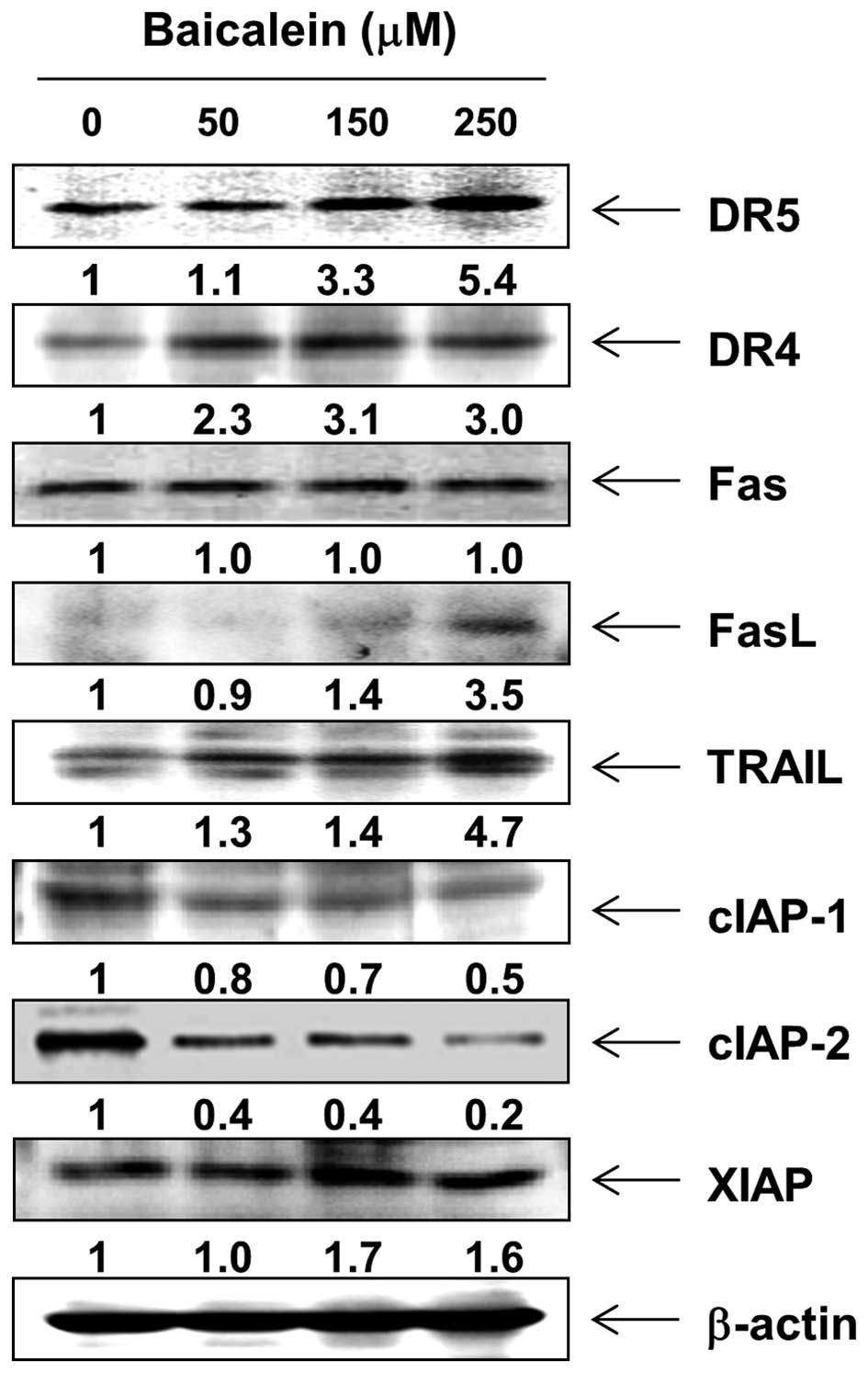
In order to determine which apoptosis pathway
contributes to baicalein-induced apoptosis, the levels of death
receptors (DRs) and corresponding proapoptotic ligands were first
examined by western blot analysis. After baicalein treatment, the
protein levels of Fas were not altered; however, the expression of
DR4, DR5, Fas ligand (FasL), and tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) were increased (Fig. 3). Next, we examined the effects of
baicalein on the levels of the inhibitor of apoptosis protein (IAP)
family of proteins. The results of western blotting showed that
baicalein treatment resulted in a concentration-dependent decrease
in the expression levels of cIAP-1 and cIAP-2, but not XIAP
(Fig. 3).
Activation of caspases and cleavage of
PARP by baicalein in 5637 cells
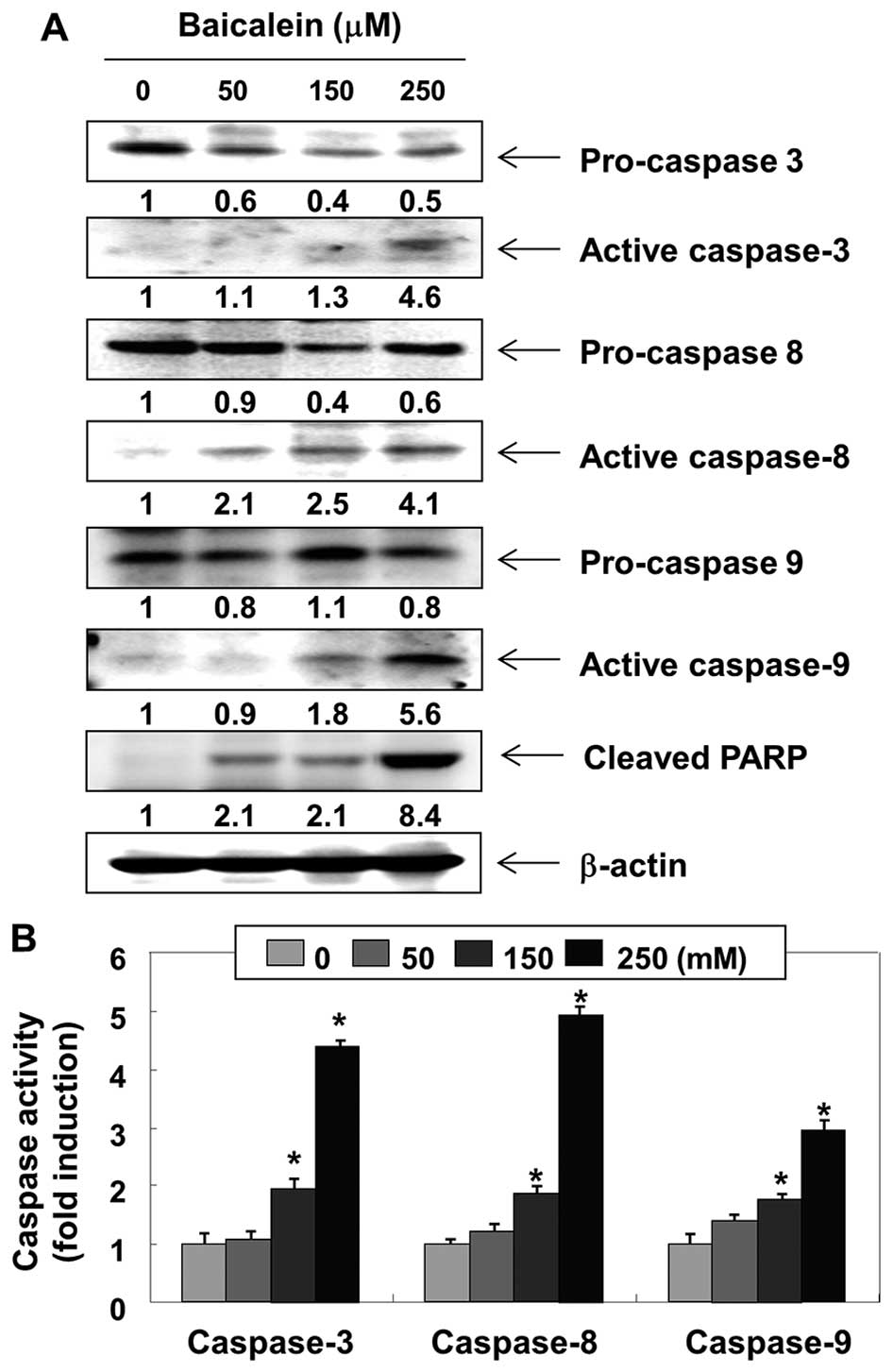
We then examined the expression levels and
activities of caspases during baicalein-induced 5637 cell
apoptosis. As shown in Fig. 4A,
western blot analyses showed that the expression levels of
pro-caspase-3 in cells treated with baicalein were
concentration-dependently downregulated and the expression levels
of active-caspase-3 were upregulated. In addition, the active forms
of caspase-8 and -9 were increased, and the levels of the pro-forms
of caspase-8 and -9, initiator caspases of extrinsic and intrinsic
apoptosis pathways, respectively, were downregulated. Under the
same conditions, the in vitro activities of these caspases
were measured using substrates specific to each caspase, and we
found that baicalein stimulates caspase-3, -8 and -9 activities in
a concentration-dependent manner (Fig.
4B). Moreover, baicalein treatment leads to progressive
proteolytic cleavage of poly(ADP-ribose)-polymerase (PARP), a
substrate protein of active caspase-3 (Fig. 4A).
Activation of the mitochondrial apoptosis
pathway by baicalein in 5637 cells
To further confirm the baicalein-induced apoptotic
pathway, this study examined the effects of baicalein on the levels
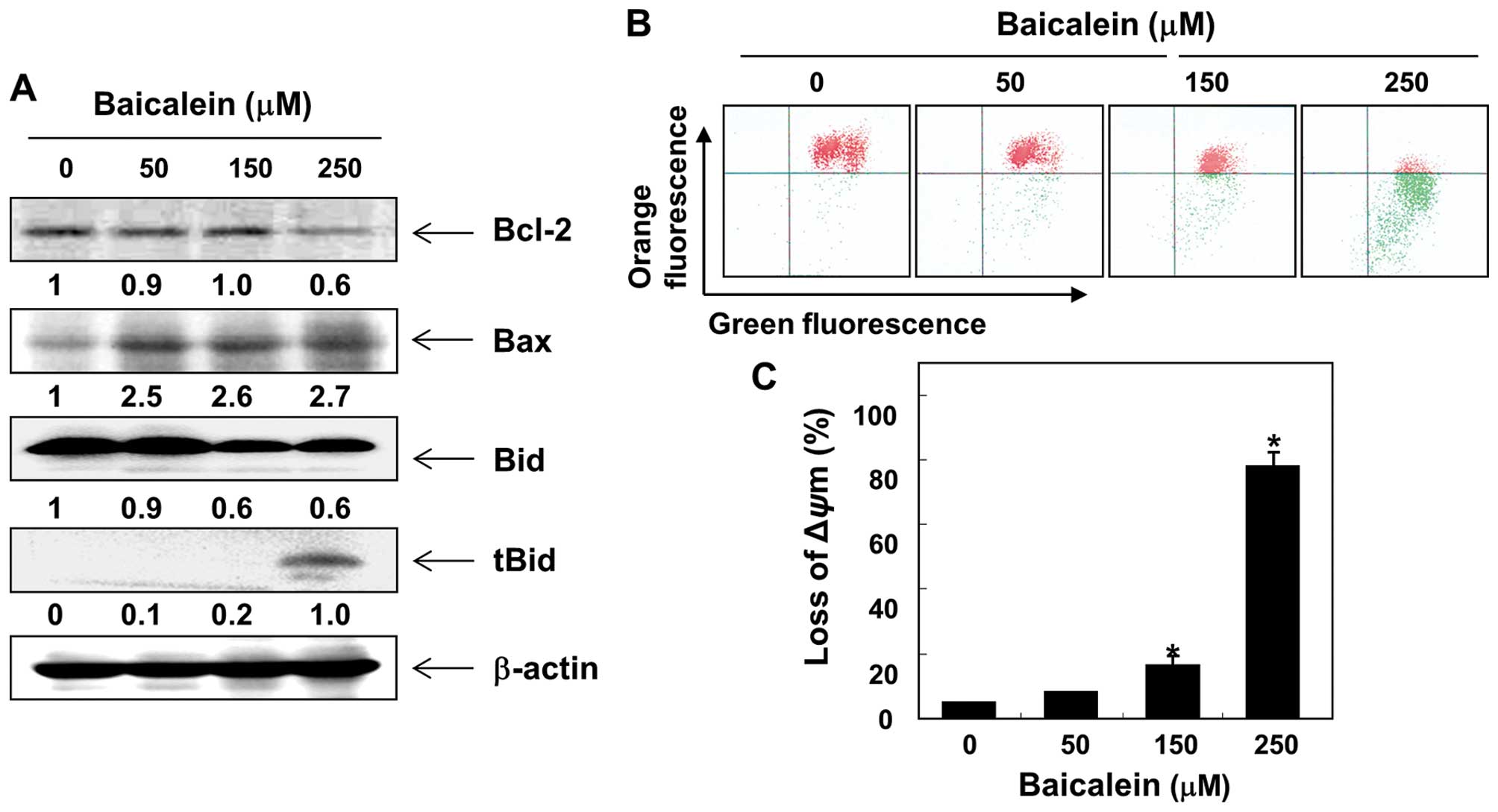
of Bcl-2 family expression and the MMP values. As shown in Fig. 5A, in response to baicalein
treatment, the levels of proapoptotic Bax were upregulated, but
those of antiapoptotic Bcl-2 were downregulated. Subsequent western
blot analyses revealed progressive downregulation of total Bid
protein and accumulation of truncated Bid (tBid). Moreover,
baicalein treatment caused a concentration-dependent loss of MMP in
comparison to the untreated control (Fig. 5B and C).
Induction of caspase-dependent apoptosis
by baicalein in 5637 cells
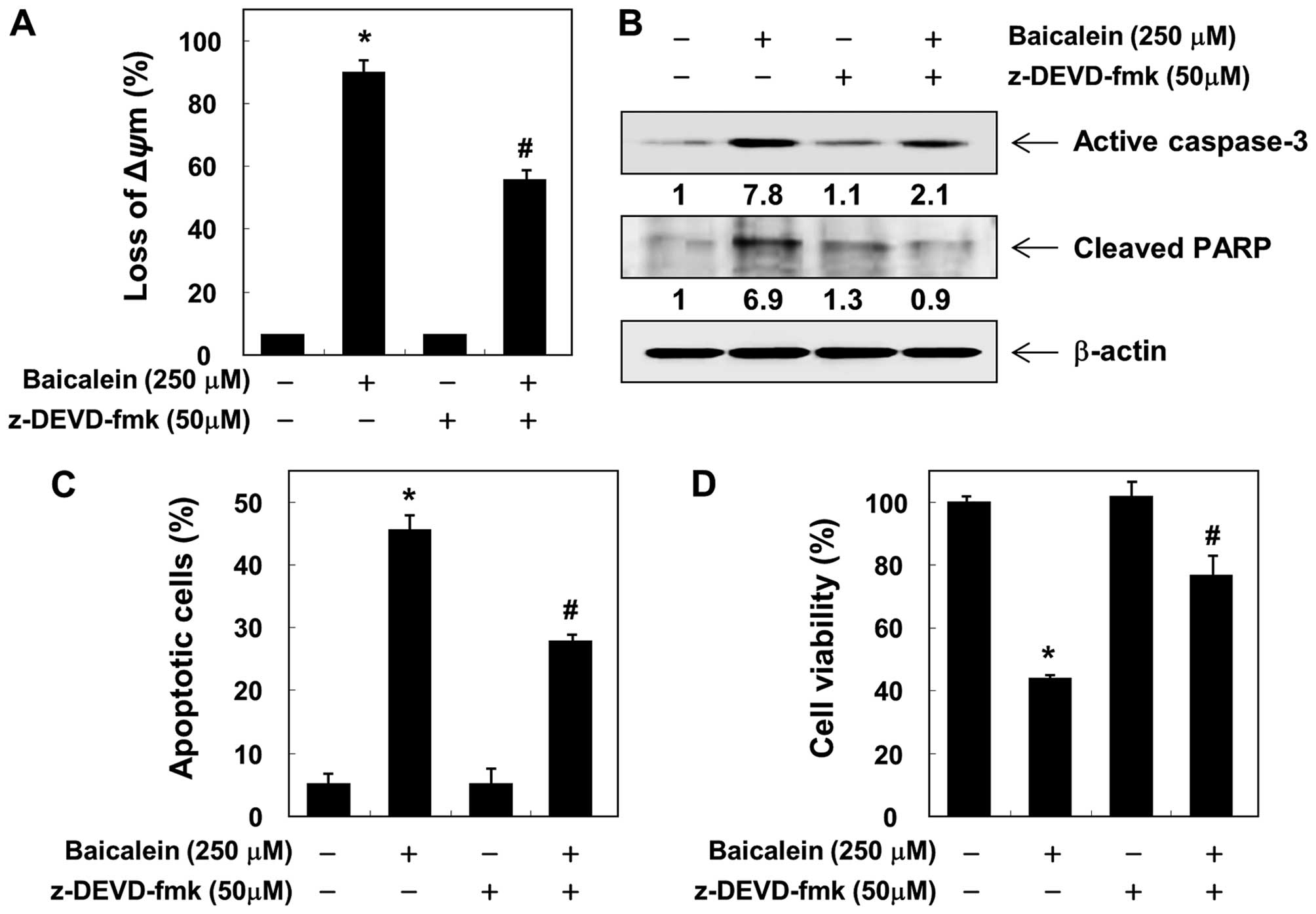
To confirm the involvement of baicalein-induced
activation of caspases, the cells were pretreated with or without
z-VAD-fmk, a pan-caspase inhibitor, for 1 h, followed by treatment
with baicalein. Pretreatment with z-VAD-fmk resulted in significant
prevention of the loss of MMP, expression of the active form of
caspase-3, and cleavage of PARP (Fig.
6A and B). Furthermore, z-VAD-fmk also decreased the
accumulation of Annexin V-FITC stained cells and increased cell
viability in the presence of baicalein (Figs. 6C and D). These results provide
evidence of baicalein-induced apoptotic cell death in association
with the activation of caspases in 5637 cells.
Induction of ROS-dependent apoptosis by
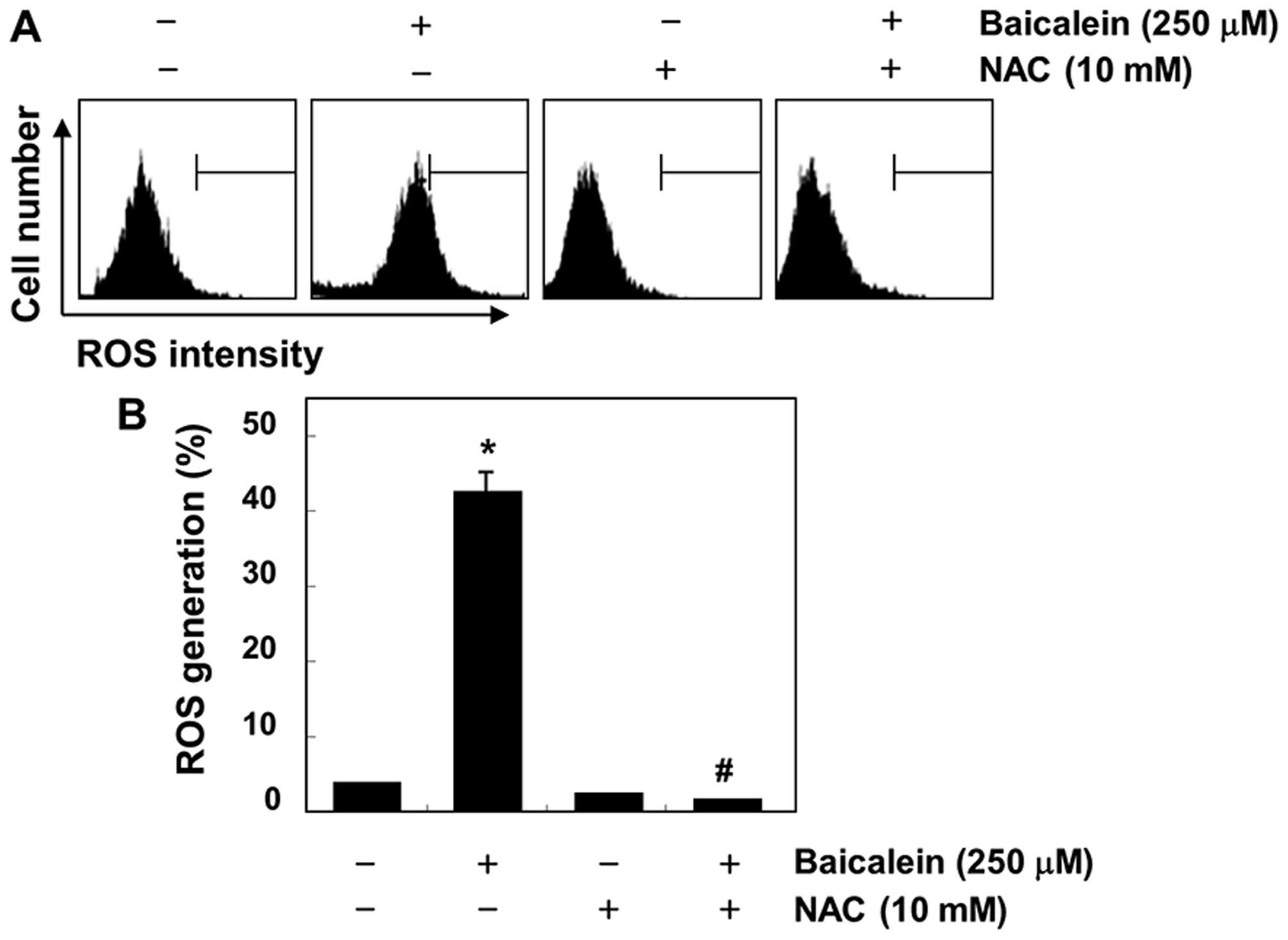
baicalein in 5637 cells
Because the generation of intracellular ROS may be
related to mitochondrial dysfunction and the induction of apoptosis
in various cell types, we further investigated whether baicalein
could stimulate ROS generation in 5637 cells. As shown in Fig. 7, the maximal generation of ROS was
observed at 30-min treatment with baicalein; however,
baicalein-induced ROS generation was effectively blocked by the
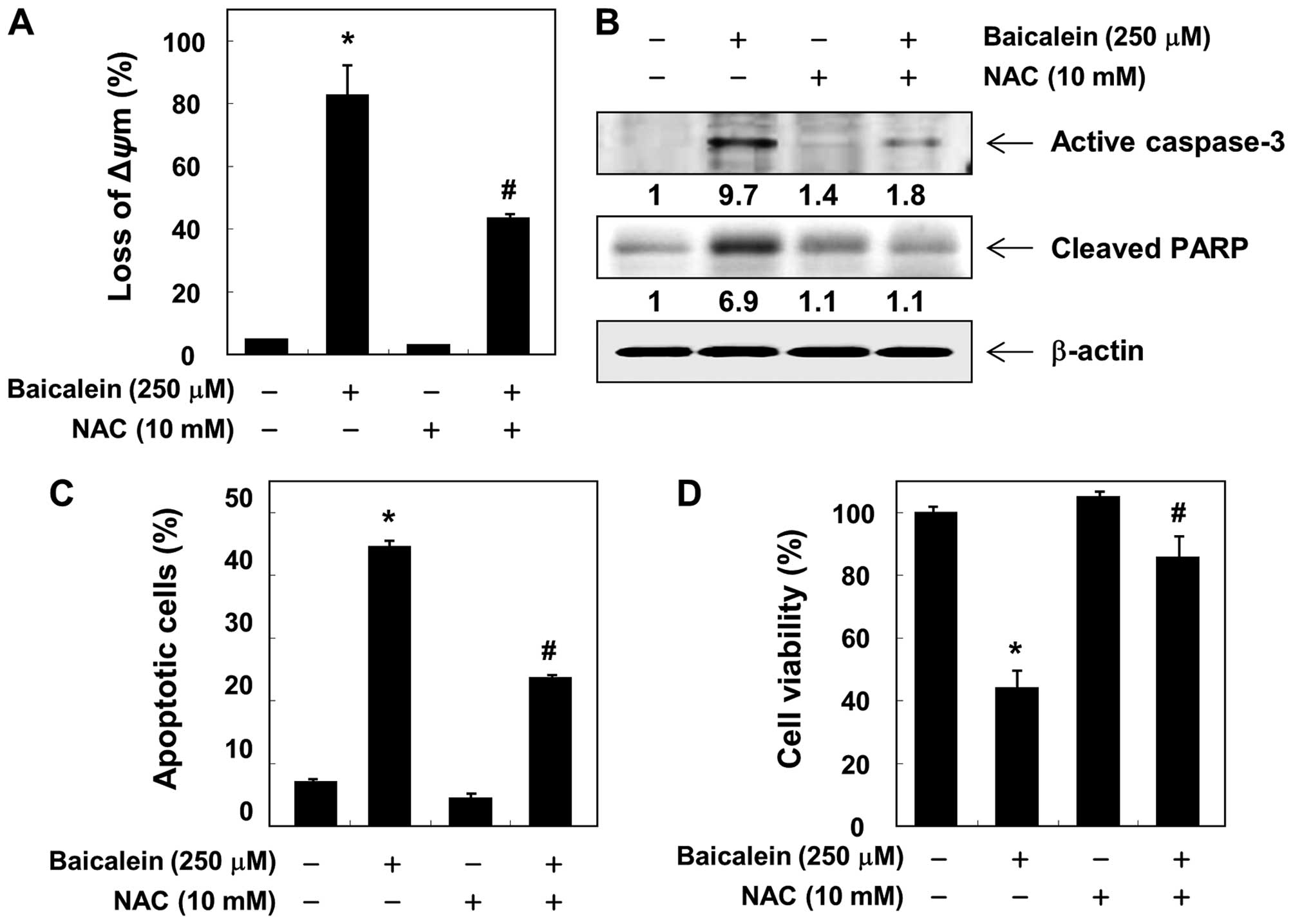
antioxidant NAC. In addition, the loss of MMP, activation of
caspase-3 and cleavage of PARP induced by baicalein were
significantly attenuated by pretreatment with NAC (Fig. 8A and B). We also observed that
baicalein-induced apoptosis and reduction of cell viability were
markedly reduced by pretreatment with NAC (Fig. 8C and D), suggesting that baicalein
induces apoptosis via ROS-dependent caspase activation mechanisms
in 5637 cells.
Discussion
In this study, we investigated whether baicalein
induces cell death in human bladder cancer 5637 cells and sought to
identify the mechanisms related to apoptosis. The findings
demonstrate that baicalein concentration-dependently inhibits cell
viability and induces apoptosis, as measured by chromatin
condensation of the nuclei and Annexin V-stained cells, which are
the hallmark features of apoptosis. The findings also suggest that
baicalein stimulates caspase-dependent extrinsic and intrinsic
apoptosis pathways in 5637 cells. Furthermore, our results show
that baicalein-induced ROS generation and activation of caspases
are significantly suppressed by pretreatment with NAC, an
antioxidant, indicating that ROS are the upstream regulators of
caspase activation during baicalein-induced apoptosis.
Apoptosis is the rigorous, active, and orderly
process of cell death, and dysregulated apoptosis is considered to
induce a number of pathological conditions, including cancer
(29,30). In general, apoptosis may be
initiated through two major pathways, the extrinsic (DR-mediated)
pathway and the intrinsic (mitochondrial-mediated) pathway, both of
which involve the activation of caspases (31,32).
The products of several genes have been demonstrated to be critical
in the regulation of these two pathways, including caspase
cascades, Bcl-2, and IAP family members. In the extrinsic pathway,
the binding of extracellular death ligands to their cell-surface
DRs leads to the activation of caspase-8 (32,33).
On the other hand, the intrinsic pathway is activated by the
release of proapoptotic factors such as cytochrome c from
the mitochondria to the cytosol, following the loss of
inner-mitochondrial-membrane integrity and activation of caspase-9.
Moreover, the extrinsic pathway can crosstalk to the intrinsic
pathway through the caspase-8-mediated cleavage of Bid, a member of
the Bcl-2 family of proteins, which ultimately amplify the
intrinsic apoptotic pathway (33,34).
Therefore, the induction of apoptosis is an important target for
cancer therapy, and agents that target the apoptosis pathway
without affecting normal cells play crucial roles as potential drug
targets in cancer treatment.
In this study, we examined various aspects of the
mechanisms of apoptosis induction by baicalein in 5637 cells, and
we found that baicalein activates initiator caspases (caspase-8 and
-9) of the extrinsic and intrinsic pathways as well as downstream
effector caspase-3 (Fig. 4), which
is associated with the degradation of PARP, a hallmark of apoptosis
and substrates of activated caspase (35). Although the levels of Fas remained
unchanged after baicalein treatment, baicalein considerably
increased the levels of DR4, DR5, FasL, and TRAIL. Baicalein also
partially downregulated the IAP family of proteins, such as cIAP-1
and cIAP-2 (Fig. 3), which
reportedly block apoptosis due to their function as direct
inhibitors by binding to and inhibiting several caspases (36,37).
In addition, baicalein increased the expression levels of
proapoptotic Bax and inhibited antiapoptotic Bcl-2, which was
associated with a dose-dependent loss of MMP and a decline in
intact Bid occurring concurrently with a pronounced increase of
tBid (Fig. 5). Therefore, our
current data suggest that baicalein induces Bid truncation, leading
to the release of proapoptotic factors to the cytosol by enhancing
mitochondrial dysfunction and ultimately to the induction of
apoptosis in 5637 cells. However, blocking caspase activity by
pretreating the cells with z-VAD-fmk, a pan-caspase inhibitor,
significantly attenuated baicalein-induced apoptosis and growth
inhibition (Fig. 6). Therefore,
the data suggest that baicalein-induced apoptosis in 5637 cells is
caspase-dependent and that both the intrinsic and the extrinsic
pathways are activated by baicalein.
Previous research has determined that the
enhancement of ROS production is associated with the apoptotic
response induced by various chemotherapeutic agents in a variety of
cell types (38,39). Mitochondria are both the source and
target of ROS generation, and damaged mitochondria can release more
ROS (40,41). Previous studies have suggested that
ROS and mitochondria may mediate apoptosis induction under both
physiological and pathological conditions (38,39).
ROS can cause the loss of MMP by activating mitochondrial
permeability transition and can induce apoptosis by releasing
apoptogenic proteins to the cytosol (42,43).
Moreover, a number of studies have noted that the proapoptotic
potential of some anticancer agents is highly correlated with the
generation of ROS from mitochondria (38,40),
indicating that the inhibition of ROS accumulation can serve as an
effective strategy for the treatment of cancers. Thus, we further
investigated whether the generation of intracellular ROS is
necessary for baicalein-induced apoptosis, and we found that ROS
levels were markedly increased in baicalein-treated 5637 cells
within 30 min (Fig. 7). However,
baicalein-induced ROS generation in cells that were co-cultured
with NAC, a commonly used reactive oxygen intermediate scavenger
(44), was effectively blocked.
These results indicate that if ROS is a crucial factor in the
induction of apoptosis in baicalein-treated 5637 cells, a ROS
scavenger must abrogate apoptosis. As shown in Fig. 8, NAC alone had no effect on the
MMP, caspase-3 expression, and PARP cleavage; however, the presence
of NAC suppressed the baicalein-induced loss of MMP, upregulation
of active-caspase-3, and degradation of PARP. Furthermore, the
blocking of ROS generation significantly prevented
baicalein-induced apoptosis as well as loss of cell viability.
Taken together, these findings suggest that an increase in ROS is
required for the occurrence of baicalein-induced apoptosis in 5637
cells.
In conclusion, the results of this study demonstrate
that baicalein triggers apoptosis of human bladder cancer 5637
cells through the activation of both the intrinsic caspase pathway
and the death-receptor-mediated extrinsic pathway and that the
activation of caspases is responsible for the mediation of
baicalein-induced apoptosis, which requires ROS generation upstream
of the disruption of MMP and activation of caspase. The data
emphasize the key role of ROS in apoptosis induced by baicalein in
5637 cells and indicate a positive correlation between ROS and
mitochondrial events leading to apoptosis. Although these findings
indicate that ROS play a pivotal role in the regulation of
baicalein-induced apoptosis in 5637 cells, further studies are
warranted to investigate the direct effect of baicalein using an
in vivo model.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean Government
(MISP) (no. 2015R1A2A2A01004633) and the Functional Districts of
the Science Belt support program, Ministry of Science, ICT and
Future Planning.
References
|
1
|
Lei AQ, Cheng L and Pan C-X: Current
treatment of metastatic bladder cancer and future directions.
Expert Rev Anticancer Ther. 11:1851–1862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Kessel KE, Zuiverloon TC, Alberts AR,
Boormans JL and Zwarthoff EC: Targeted therapies in bladder cancer:
An overview of in vivo research. Nat Rev Urol. 12:681–694. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donato F, Boffetta P, Fazioli R, Aulenti
V, Gelatti U and Porru S: Bladder cancer, tobacco smoking, coffee
and alcohol drinking in Brescia, northern Italy. Eur J Epidemiol.
13:795–800. 1997. View Article : Google Scholar
|
|
4
|
Wu X, Ros MM, Gu J and Kiemeney L:
Epidemiology and genetic susceptibility to bladder cancer. BJU Int.
102B:1207–1215. 2008. View Article : Google Scholar
|
|
5
|
Pal SK, Milowsky MI and Plimack ER:
Optimizing systemic therapy for bladder cancer. J Natl Compr Canc
Netw. 11:793–804. 2013.PubMed/NCBI
|
|
6
|
Feuerstein MA and Goenka A: Quality of
life outcomes for bladder cancer patients undergoing bladder
preservation with radiotherapy. Curr Urol Rep. 16:752015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fridlender M, Kapulnik Y and Koltai H:
Plant derived substances with anti-cancer activity: From folklore
to practice. Front Plant Sci. 6:7992015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagai T, Yamada H and Otsuka Y: Inhibition
of mouse liver sialidase by the root of Scutellaria baicalensis.
Planta Med. 55:27–29. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li-Weber M: New therapeutic aspects of
flavones: The anti-cancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
11
|
Huang Y, Tsang SY, Yao X and Chen ZY:
Biological properties of baicalein in cardiovascular system. Curr
Drug Targets Cardiovasc Haematol Disord. 5:177–184. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Lin G and Zuo Z: Pharmacological
effects and pharmacokinetics properties of Radix Scutellariae and
its bioactive flavones. Biopharm Drug Dispos. 32:427–445. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Firuzi O, Miri R, Tavakkoli M and Saso L:
Antioxidant therapy: Current status and future prospects. Curr Med
Chem. 18:3871–3888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Wei Z, Zhou E, Chen L, Kou J, Wang J
and Yang Z: Baicalein attenuates inflammatory responses by
suppressing TLR4 mediated NF-κB and MAPK signaling pathways in
LPS-induced mastitis in mice. Int Immunopharmacol. 28:470–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao JR, Do CW and To CH: Potential
therapeutic effects of baicalein, baicalin, and wogonin in ocular
disorders. J Ocul Pharmacol Ther. 30:605–614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng YH, Li LA, Lin P, Cheng LC, Hung CH,
Chang NW and Lin C: Baicalein induces G1 arrest in oral cancer
cells by enhancing the degradation of cyclin D1 and activating AhR
to decrease Rb phosphorylation. Toxicol Appl Pharmacol.
263:360–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng YH, Yin LH, Grahn TH, Ye AF, Zhao YR
and Zhang QY: Anticancer effects of baicalein on hepatocellular
carcinoma cells. Phytother Res. 28:1342–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25A:959–964.
2005.
|
|
19
|
Aryal P, Kim K, Park PH, Ham S, Cho J and
Song K: Baicalein induces autophagic cell death through AMPK/ULK1
activation and downregulation of mTORC1 complex components in human
cancer cells. FEBS J. 281:4644–4658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim DH, Hossain MA, Kang YJ, Jang JY, Lee
YJ, Im E, Yoon JH, Kim HS, Chung HY and Kim ND: Baicalein, an
active component of Scutellaria baicalensis Georgi, induces
apoptosis in human colon cancer cells and prevents AOM/DSS-induced
colon cancer in mice. Int J Oncol. 43:1652–1658. 2013.PubMed/NCBI
|
|
21
|
Chung H, Choi HS, Seo EK, Kang DH and Oh
ES: Baicalin and baicalein inhibit transforming growth
factor-β1-mediated epithelial-mesenchymal transition in human
breast epithelial cells. Biochem Biophys Res Commun. 458:707–713.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Li Z, Chen AY, Ye X, Luo H, Rankin
GO and Chen YC: Inhibitory effect of baicalin and baicalein on
ovarian cancer cells. Int J Mol Sci. 14:6012–6025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rushworth SA and Micheau O: Molecular
crosstalk between TRAIL and natural antioxidants in the treatment
of cancer. Br J Pharmacol. 157:1186–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HJ, Park C, Han MH, Hong SH, Kim GY,
Hoon Hong S, Deuk Kim N and Choi YH: Baicalein induces
caspase-dependent apoptosis associated with the generation of ROS
and the activation of AMPK in human lung carcinoma A549 cells. Drug
Dev Res. 77:73–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park MH and Han JS: Padina arborescens
extract protects high glucose-induced apoptosis in pancreatic β
cells by reducing oxidative stress. Nutr Res Pract. 8:494–500.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SJ, Ho Hur J, Park C, Kim HJ, Oh GS,
Lee JN, Yoo SJ, Choe SK, So HS, Lim DJ, et al: Bucillamine prevents
cisplatin-induced ototoxicity through induction of glutathione and
antioxidant genes. Exp Mol Med. 47:e1422015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim YS, Li XF, Kang KH, Ryu B and Kim SK:
Stigmasterol isolated from marine microalgae Navicula incerta
induces apoptosis in human hepatoma HepG2 cells. BMB Rep.
47:433–438. 2014. View Article : Google Scholar :
|
|
28
|
Song JL, Choi JH, Seo JH, Kil JH and Park
KY: Antioxidative effects of fermented sesame sauce against
hydrogen peroxide-induced oxidative damage in LLC-PK1 porcine renal
tubule cells. Nutr Res Pract. 8:138–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sayers TJ: Targeting the extrinsic
apoptosis signaling pathway for cancer therapy. Cancer Immunol
Immunother. 60:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
MacKenzie SH and Clark AC: Targeting cell
death in tumors by activating caspases. Curr Cancer Drug Targets.
8:98–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brenner D and Mak TW: Mitochondrial cell
death effectors. Curr Opin Cell Biol. 21:871–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hensley P, Mishra M and Kyprianou N:
Targeting caspases in cancer therapeutics. Biol Chem. 394:831–843.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaufmann SH, Desnoyers S, Ottaviano Y,
Davidson NE and Poirier GG: Specific proteolytic cleavage of
poly(ADP-ribose) polymerase: An early marker of
chemotherapy-induced apoptosis. Cancer Res. 53:3976–3985.
1993.PubMed/NCBI
|
|
36
|
Danson S, Dean E, Dive C and Ranson M:
IAPs as a target for anticancer therapy. Curr Cancer Drug Targets.
7:785–794. 2007. View Article : Google Scholar
|
|
37
|
de Graaf AO, de Witte T and Jansen JH:
Inhibitor of apoptosis proteins: New therapeutic targets in
hematological cancer? Leukemia. 18:1751–1759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kardeh S, Ashkani-Esfahani S and Alizadeh
AM: Paradoxical action of reactive oxygen species in creation and
therapy of cancer. Eur J Pharmacol. 735:150–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matés JM, Segura JA, Alonso FJ and Márquez
J: Oxidative stress in apoptosis and cancer: An update. Arch
Toxicol. 86:1649–1665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boneh A: Regulation of mitochondrial
oxidative phosphorylation by second messenger-mediated signal
transduction mechanisms. Cell Mol Life Sci. 63:1236–1248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fleury C, Mignotte B and Vayssière JL:
Mitochondrial reactive oxygen species in cell death signaling.
Biochimie. 84:131–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life
Sci. 60:6–20. 2003. View Article : Google Scholar : PubMed/NCBI
|