Introduction
Although biochemical and clinical studies have
resulted in significant advance of our knowledge of cancer, we have
not been able to find a cure for the disease. High invasive and
metastatic capacities are critical characteristics of fibrosarcoma,
a malignant mesenchymal tumor, that partially account for rapid
disease progression and poor prognosis. However, the molecular
mechanisms leading to the invasion and metastasis of fibrosarcoma
are still poorly understood.
Recently, the crucial role of DNA methylation
alterations in human carcinogenesis was emphasized (1). The development and progression of
cancer is caused by both, genetic mutations and epigenetic changes,
including DNA methylation and histone modifications (2). In mammals, DNA methylation primarily
involves a covalent modification of cytosine residues of CpG
dinucleotides (3). DNA methylation
changes include locus-targeted hypermethylation and global
hypomethylation (4). Covalent
methylation of DNA CpG islands is catalyzed by methyltransferases
that methylate C-5 of cytosine nucleotides. Global cytosine
methylation patterns in the mammalian genome appear to be
established by a complex interplay of at least three independently
encoded DNA methyltransferases (DNMTs): DNMT1, DNMT3A and
DNMT3B.
5-aza C is a methyltransferase inhibitor. It is
incorporated into the DNA of rapidly growing tumor cells during
replication and blocks DNA methylation by trapping DNA
methyltransferases on DNA, leading to their intracellular depletion
(5). 5-Aza C exerts diverse
effects on gene expression (6) and
cellular survival (7).
Matrix metalloproteinases (MMPs) are a class of
proteolytic enzymes that degrade all extracellular matrix (ECM)
components (8). These enzymes
regulate the migration of various cancer cells across the ECM, cell
growth, angiogenesis and apoptosis. All these processes are
essential for the dissemination of neoplastic cells. The MMP family
member MMP-9, can degrade collagen, the main component of vascular
basement membrane (9). MMP-9
activity is modulated on several levels, including gene
transcription (10), mRNA
stability (11), secretion
(12) and enzymatic activity.
MMP-9 gene expression can be regulated by a variety of stimuli,
such as interleukin (IL)-1β, tumor necrosis factor α, epidermal
growth factor and phorbol esters (13,14).
However, the effects of 5-aza C on MMP-9 expression and activity in
HT1080 cells have not been completely elucidated.
Therefore, we have studied the effects of 5-aza C on
MMP-9 activity, migration and invasion. We demonstrated that 5-aza
C enhances migration and invasion of HT1080 cells by activating
MMP-9 via phosphoinositide (PI)3-kinase/Akt and extracellular
signal-regulated kinase (ERK)1/2 signaling pathways.
Materials and methods
Reagents
5-aza C was purchased from Sigma-Aldrich (St. Louis,
MO, USA). PD 98059 (PD) was purchased from Calbiochem (San Diego,
CA, USA) and LY 294002 (LY) was purchased from Tocris Bioscience
(Bristol, UK).
Cell culture
Human fibrosarcoma HT1080 cells (KCLB no. 10121;
Korean Cell Line Bank, Seoul, Korea) were grown in RPMI-1640 medium
(Gibco/Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco-Invitrogen), penicillin (50 U/ml;
Sigma-Aldrich) and streptomycin (50 μg/ml; Sigma-Aldrich), in a
humid atmosphere, 5% CO2 and 95% air at 37°C.
MTT assay
Proliferation of HT1080 cells was determined using
colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. Cells were seeded into 96-well plates
(0.5×105 cells/well in 100 μl medium) and cultured for
24 h. After an overnight incubation, the cells were treated with
5-aza C concentration gradient (0, 5, 10, or 20 μM) for 24 h, or
with 10 μM 5-aza C for the indicated time periods, and then washed
with phosphate-buffered saline (PBS). Thereafter, the medium was
replaced by fresh medium (200 μl) containing 0.5 mg/ml MTT, and the
mixture was incubated for 4 h at 37°C. Following the incubation,
100 μl solubilization buffer (10% SDS, 0.01 N HCl) was added to
each well to terminate the MTT reaction and dissolve formazan
crystals. After agitation for 1 h at room temperature, optical
density of the solution in each well was measured at 595 nm using a
microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Proliferation inhibition rate was calculated as: [1-A595
(experimental well)/A595 (control well)] ×s 100%.
Wound-healing assay
HT1080 cell migration was determined by
wound-healing assay (15).
Briefly, HT1080 cells (5×105/well) were seeded into
35-mm culture dish and after reaching ~90% confluence, a scratch
was made in the cells with a 10-μl pipette tip. The remaining cells
were washed with medium and incubated with 5-aza C in the absence
or presence of inhibitors for 24 h. The migration distance of cells
was then captured and the images were quantitatively analyzed using
ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Distances between scratch edges were measured and statistically
analyzed (see below).
Invasion assay
HT1080 invasion was determined with Transwell
Matrigel system (16). Cells
(1×105 cells/well) were cultured in the top chambers of
24-well Transwell plates (8.0 mm-pore; Corning Costar, Corning, NY,
USA) and complete RPMI-1640 medium was added to the bottom
chambers. After 24 h, the cells on the surface of the top chamber
membrane were removed with a cotton swab. Cells that had migrated
to the bottom surface of the top chamber membranes were fixed with
95% methanol, stained with 1 mg/ml crystal violet and hematoxylin
solution, and counted (20 random fields) under a microscope
(magnification, x200). Each experiment was performed in triplicate
and repeated at least twice.
Flow cytometry
Cells (5×105 cells) were grown in 35-mm
culture dish to ~80% confluence and starved in serum-free medium at
37°C for 12 h. The cells were then treated with 5-aza C for 24 h
and harvested in trypsin-EDTA (0.25%, pH 7.2) buffer. Cells were
washed with PBS prior to suspension in cold propidium iodide (PI)
solution (50 μg/ml) in PBS (pH 7.4) containing RNase A (0.1 mg/ml)
for 30 min in the dark. After incubation, cell apoptosis and cell
cycle distribution were analyzed with a flow cytometer (Partec
GmbH, Munster, Germany).
Western blot analysis
Preparation of cell lysates and western blot
analyses were performed as previously described (17). Briefly, equal amounts of proteins
from whole cell lysates (20–30 μg) were resolved by 8–12%
SDS-polyacrylamide gel electrophoresis and transferred to a
nitrocellulose membrane. The membrane was then incubated in
blocking buffer comprising 5% skimmed milk in Tris-buffered saline
with Tween-20 (TBST), at room temperature for 1 h. The blocked
membrane was next incubated with primary antibodies, at 4°C,
overnight. The following primary antibodies were used: rabbit
anti-DNMT-1 polyclonal antibody (1:1,000 dilution; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA; sc-20701); mouse
anti-phospho-Akt polyclonal antibody (1:1,000 dilution; Cell
Signaling Technology Inc., Danvers, MA, USA; #9271); rabbit
anti-Akt polyclonal antibody (1:1,000 dilution; Santa Cruz
Biotechnology; #9272); rabbit anti-phospho-p44/42 MAP kinase
(pERK1/2) polyclonal antibody (1:1,000 dilution; Cell Signaling
Technology; #9101); rabbit anti-ERK-2 poly-clonal antibody (1:1,000
dilution; Santa Cruz Biotechnology; sc-154). The membrane was
washed three times (10 min each) with TBST, and then incubated with
secondary antibodies, at room temperature, for 2 h. The following
secondary antibodies were used: anti-rabbit IgG antibody (1:3,000
dilution; Sigma-Aldrich; A0545); anti-mouse IgG antibody (1:3,000
dilution Enzo Life Sciences, Inc., Farmingdale, NY, USA;
ADI-SAB-100). The antigen-antibody complex was detected with an
enhanced chemiluminescence detection kit (Dogen, Seoul, Korea).
Protein band intensities were quantified by the ImageJ and
normalized to internal β-actin control. Normalized values were
plotted and are presented.
Zymographic assay of MMP-9 gelatinase
activity
MMP-9 gelatinase activity in conditioned media was
performed using SDS-PAGE zymography, as previously described
(18). Briefly, HT1080 cells were
split into 35-mm culture dish. After 24 h, cells were serum-starved
in RPMI-1640 medium (Gibco/Invitrogen) for additional 20 h, and
then treated with increasing concentrations of 5-aza C (0, 5, 10
and 20 μM) for 24 h. Subsequently, 30 μl conditioned medium was
mixed with reducing agent-devoid of 4X SDS sample buffer (62.5 mM
Tris, 4% SDS, 25% glycerol, 0.01% bromophenol blue, pH 6.8) and
subjected to electrophoresis in 7.5% SDS-PAGE gels containing 1
mg/ml gelatin (Sigma-Aldrich). After electrophoresis, gels were
washed twice with 2.5% (v/v) Triton X-100 for 60 min at room
temperature to remove SDS and subsequently incubated overnight at
37°C in a developing buffer containing 10 mM CaCl2, 150
mM NaCl, and 50 mM Tris-HCl, pH 8.0, for 24 h. The gels were
stained with 0.5% Coomassie brilliant blue R-250 in 10% acetic acid
(v/v) and 50% methanol, for ~1 h, and then destained in 50%
methanol and 10% acetic acid solution at room temperature to
clearly visualize the digested bands. MMP-9 proteolytic activities
were visualized as clear bands against blue background of stained
gelatin.
Statistical analysis
Data are presented as means ± SEM from three
independent experiments and compared using one-way ANOVA. For
P<0.05, differences were considered statistically significant
and are indicated by an asterisk in the figures.
Results
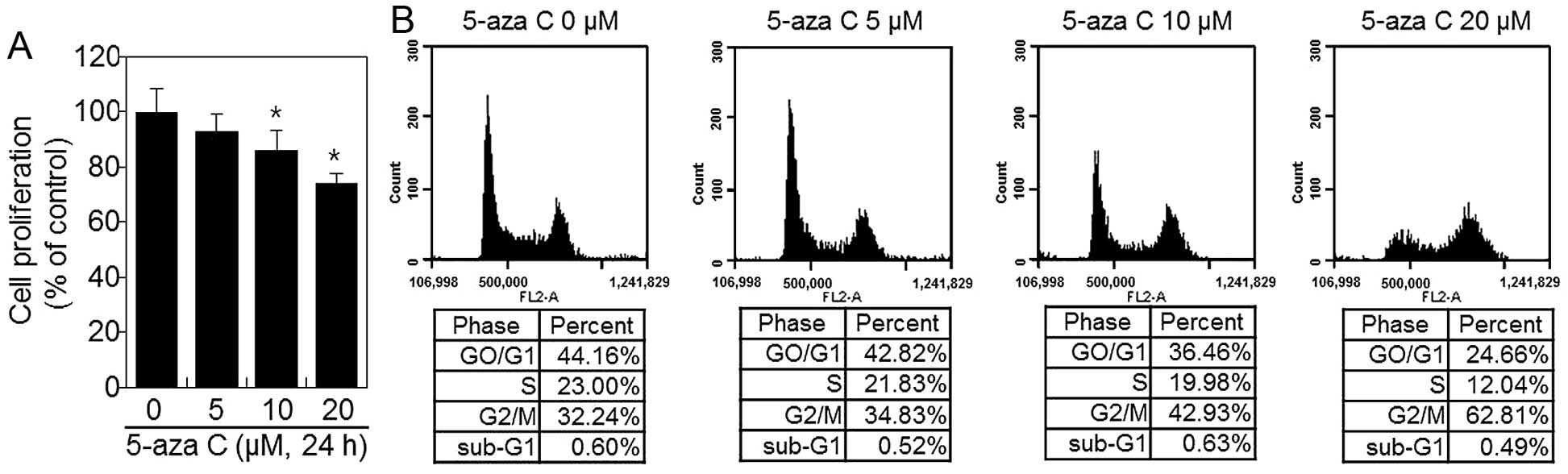
5-Aza C inhibits the proliferation of
HT1080 cells
To evaluate the effect of 5-aza C on cellular
proliferation, cells were cultured with increasing 5-aza C
concentrations (5, 10 and 20 μM), as described in Materials and
methods, and their viability was assessed by MTT assay. 5-Aza C
inhibited proliferation of cells in a dose-dependent manner
(Fig. 1A). We next performed FACS
analysis to investigate the effect of 5-aza C on the cell cycle
(Fig. 1B). FACS analysis revealed
that 5-aza C induced G2/M phase arrest (Fig. 1B). Only 32.2% untreated control
HT1080 cells were in G2/M phase. However, 24 h of
treatment with 5, 10 and 20 μM 5-aza C, the proportion of HT1080
cells arrested in the G2/M phase increased to 34.8, 42.9
and 62.8%, respectively (Fig. 1B).
These results indicated that 5-aza C inhibits proliferation in a
dose-dependent manner (Fig.
1).
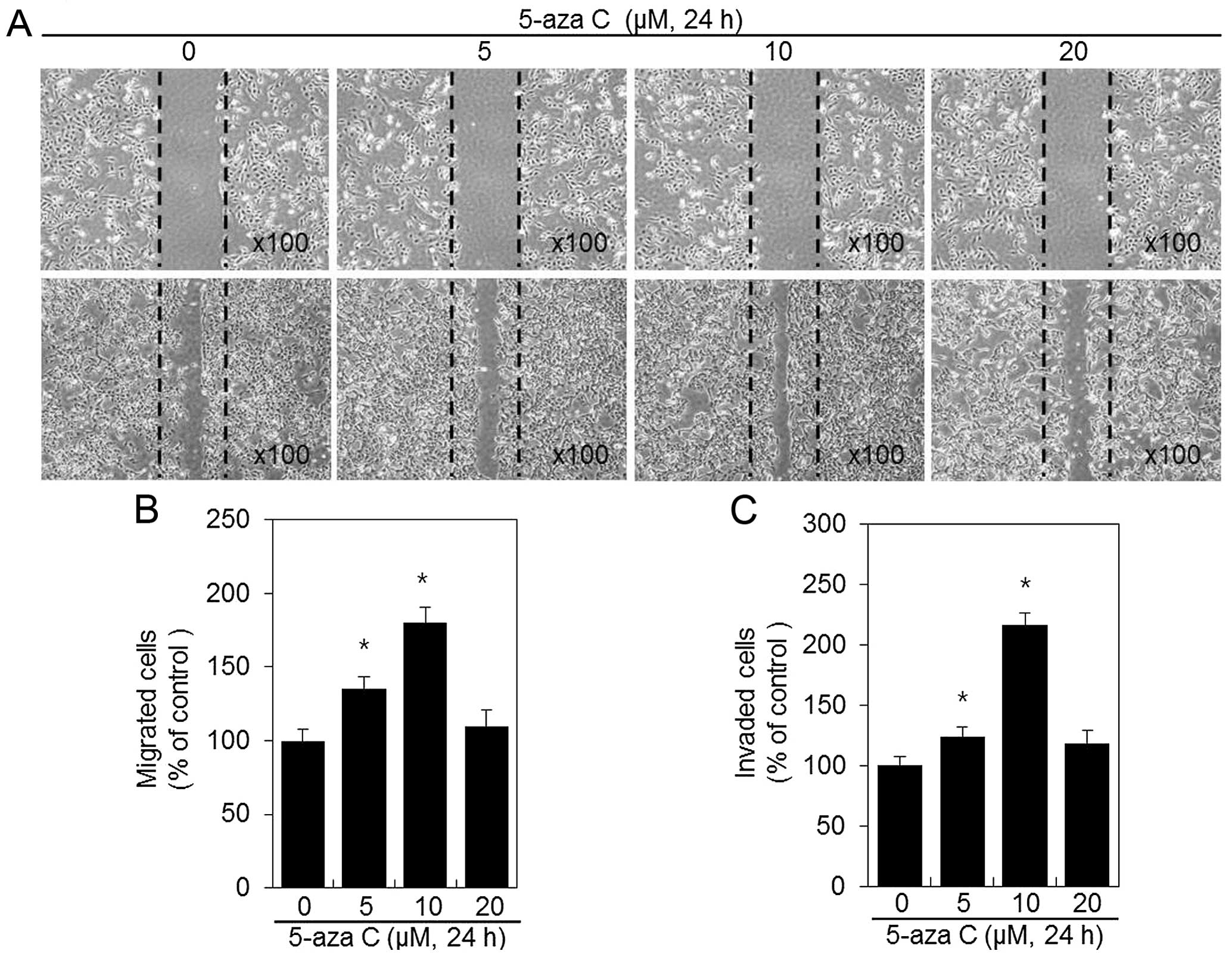
5-Aza C induces migration and invasion of
HT1080 cells
Metastatic HT1080 cells possess high migration
ability and therefore they are suitable for migration and invasion
experiments verifying the effects of 5-aza C on tumor metastasis.
HT1080 24-h exposure to 5-aza C resulted in significant increase in
HT1080 cell migration area and induced the spread of HT1080 cells
along wound edges, compared with the untreated control cells
(Fig. 2A and B). Next, Matrigel
invasion assays were performed with 5-aza C-treated HT1080 cells
(Fig. 2C). HT1080 cell invasion
capacity was significantly induced by 5-aza C, and the number of
invasive cells increased when 5-aza C concentration was increased
to 10 μM (Fig. 2C). Approximately
29 and 54% induction in invasion was observed after treatment with
5 and 10 μM 5-aza C, respectively, compared with the control
(Fig. 2C). Both HT1080 migration
and invasion were unaffected by 20 μM 5-aza C treatment (Fig. 2B and C), most possibly due to a
cytotoxic, cell-cycle arresting effect of the compound (Fig. 1B).
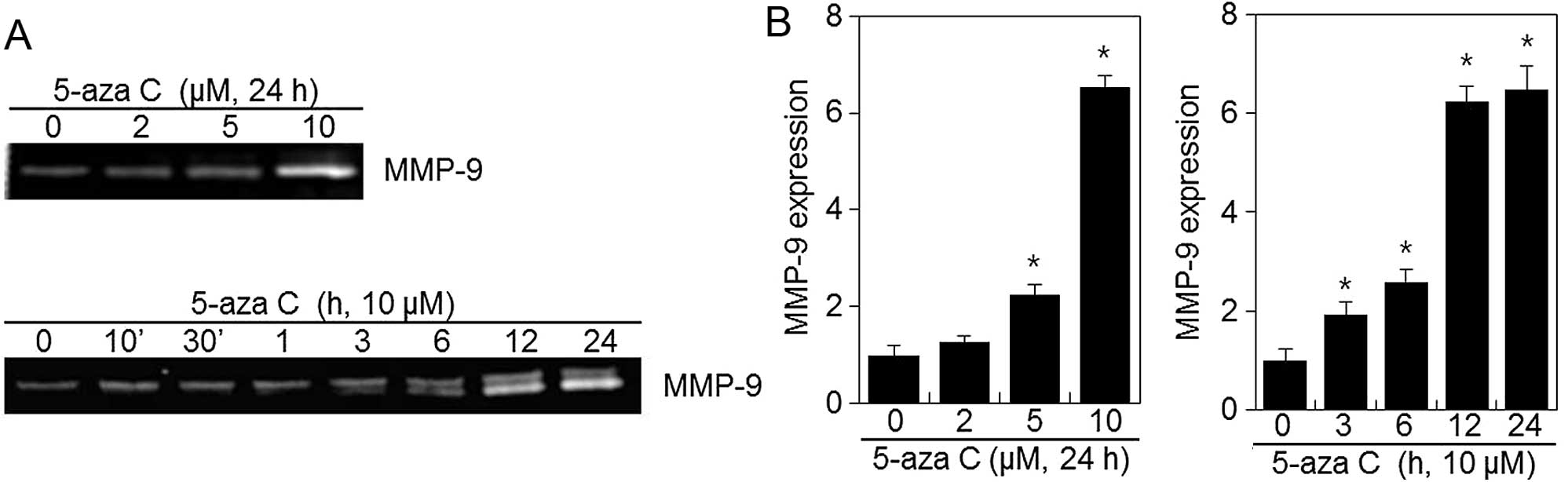
5-Aza C induces MMP-9 activity in HT1080
cells
No cytotoxic effect was observed during HT1080 cell
treatment with 5-aza C concentrations <10 μM and, therefore,
2–10 μM 5-aza C concentrations were used in subsequent experiments.
To elucidate the potential mechanisms underlying the meta-static
effects of 5-aza C on HT1080 cells, we assessed MMP-9 expression
via zymography analysis. As shown in Fig. 3, 5-aza C treatment significantly
induced MMP-9 expression in a dose- and time-dependent manner when
compared with the control (Fig.
3). These findings suggested that induction of MMP-9 expression
might be involved in the increased migration and invasion of HT1080
cells upon 5-aza C treatment.
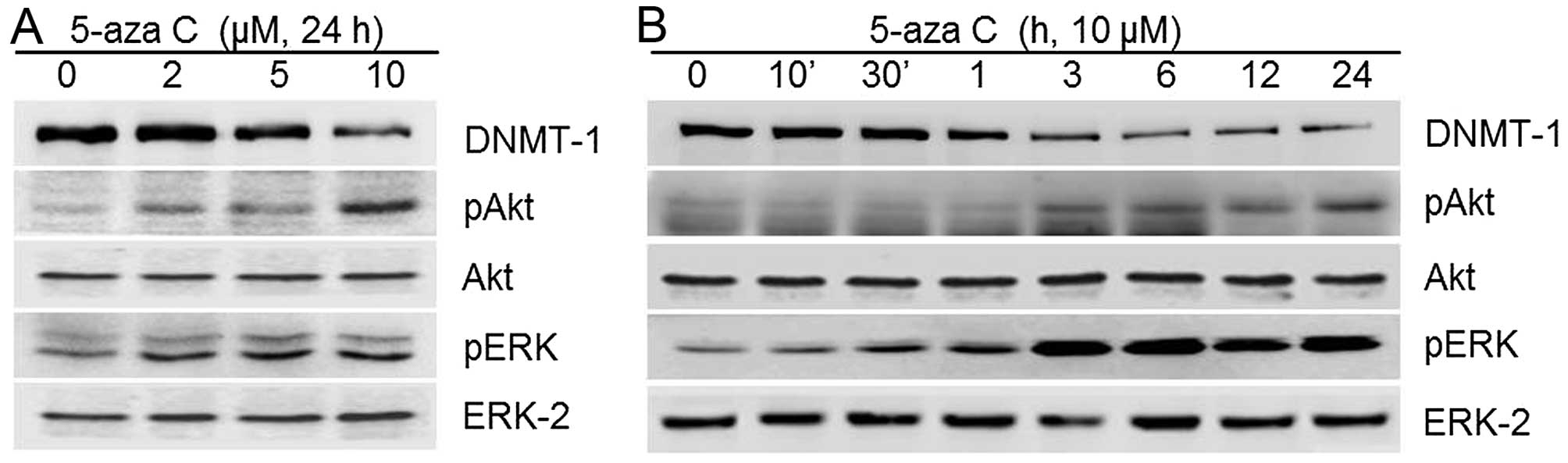
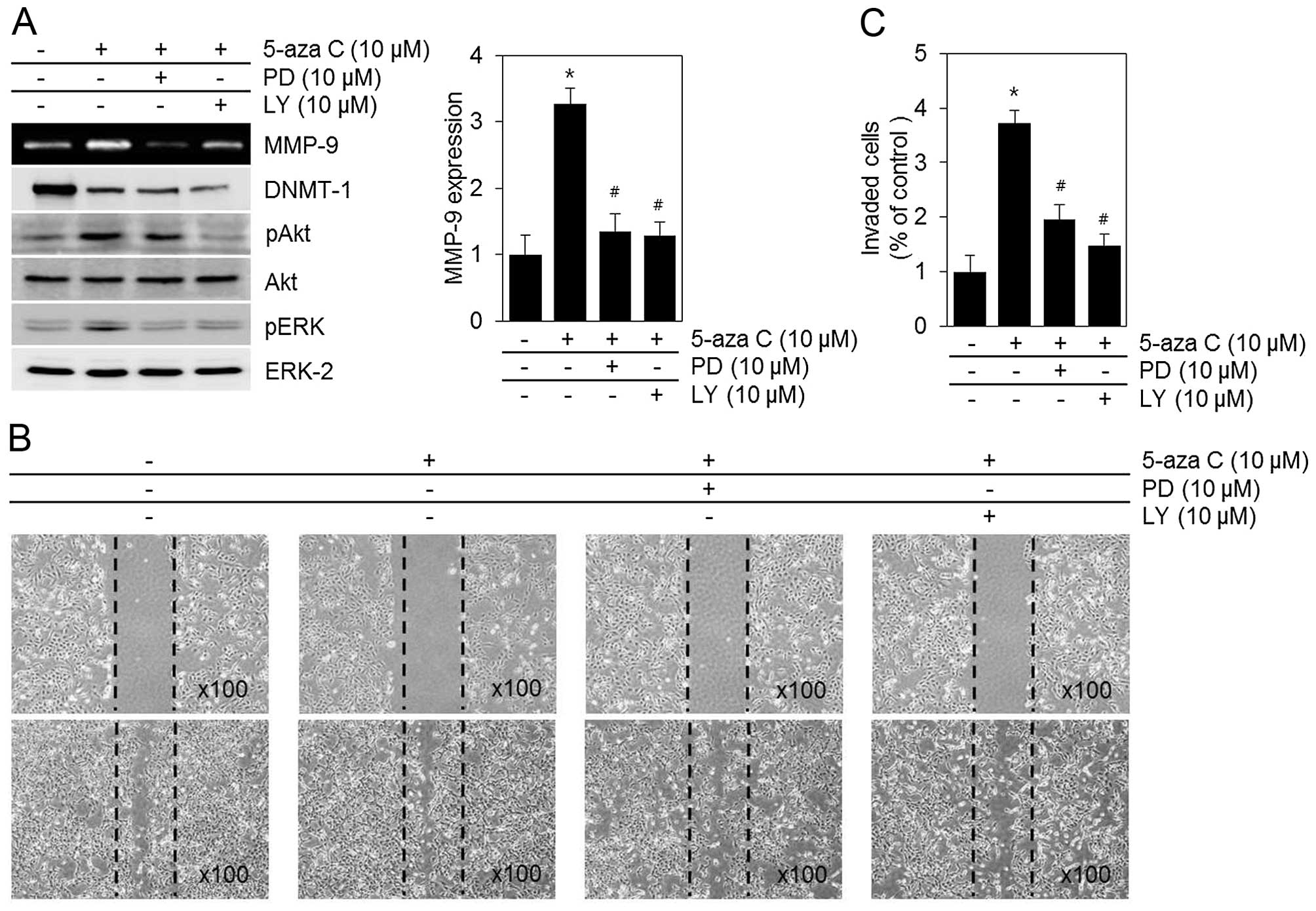
PI3-kinase/Akt and ERK1/2 MAPK signaling
pathways mediate 5-aza C-associated effects in HT1080 cells
To determine whether the MMP-9 activity and
metastatic capacities of HT1080 cells induced by 5-aza C were
associated with PI3-kinase and mitogen-activated protein kinase
(MAPK) signaling pathways, we examined Akt, ERK1/2, p38 kinase and
JNK1/2 phosphorylation. As shown in Fig. 4, 5-aza C caused a significant
increase in phosphorylated Akt and ERK1/2 levels (Fig. 4). It also activated the p38 and
JNK1/2 signaling pathways (data not shown). 5-aza C-induced MMP-9
activity and HT1080 cell metastatic capacities were significantly
attenuated after blocking of ERK1/2 and PI3-kinase/Akt with PD and
LY inhibitors, respectively (Fig.
5). In contrast, p38 kinase inhibition by SB203580 and JNK1/2
inhibition by SP600125 exerted no apparent effects (data not
shown). Collectively, the data indicated that PI3-kinase/Akt and
ERK1/2 may be the major pathways mediating 5-aza C-induced MMP-9
activity and metastatic capacities, migration and invasion, of
HT1080 cells.
Discussion
MMPs, a family of zinc-dependent endopeptidases,
participate in many physiological and pathological processes
(19). Imbalance between MMP
inhibition and activation is associated with various diseases,
including osteoarthritis, rheumatoid arthritis, tumor metastasis,
and cardiovascular disease (19–21).
Secretion of MMPs by structural and inflammatory cells is thought
to take part in the processes of cancer metastasis to distant sites
(22,23). MMPs are involved in this metastatic
progression by initiating turnover of ECM components and modulating
cancer cell migration (24).
Previous studies have revealed that MMP-9 expression is the
strongest in fibrosarcoma cells and has been implicated in tumor
invasion and metastasis.
The cancer epigenome is characterized by global
alterations in DNA methylation and histone modifications, as well
as altered expression profiles of chromatin-modifying enzymes
(25). Alteration of global DNA
methylation plays a significant role in tumorigenesis and occurs at
a variety of genome sequences, including repetitive elements,
retrotransposons, CpG-poor promoters, introns and gene deserts
(26).
Various epigenetic drugs have been recently
identified that can successfully reverse DNA methylation and
histone modification abnormalities that occur in cancer (27). DNA methylation inhibitors, such as
5-aza C and decitabine, promote decrease in DNA methylation,
inducing gene expression and differentiation of cultured cells.
This facilitates our understanding of the potential use of
epigenetic drugs in cancer therapy (28). In addition, DNA methylation
inhibitors are in clinical use, as part of anticancer strategy,
restoring normal function to abnormally hypermethylated genes
(29). In particular, 5-aza C
inhibits tumor cell proliferation by demethylating and reactivating
genes silenced by methylation in cervical cell lines CaSki, C-33A,
HeLa and SiHa (30). In contrast,
5-aza C treatment of acute myeloid leukemia might result in
enhanced invasiveness of tumor cells via a mechanism involving
MMP-9 (31). Previous studies
revealed that 5-aza C increased invasiveness of several pancreatic
(32) and acute myeloid leukemia
cells (31). Similarly, our
results indicated that 5-aza C-associated global DNA
hypomethylation was accompanied by an increased invasiveness of
HT1080 cells.
Considering the above, it is important to understand
epigenetic mechanisms used by cells to regulate MMP expression to
modulate their invasive properties. In the present study, we
focused on the role of DNA methylation alteration in the modulation
of MMP-9 activity in human HT1080 fibrosarcoma cells. We
demonstrated the biological properties of 5-aza C by showing that
5-aza C treatment increased migration and invasion of HT1080 cells
and induced MMP-9 activity, possibly via PI3-kinase/Akt and ERK1/2
pathway activation. We observed a significant increase of migration
with 5-aza C concentrations up to ~10 μM. This effect was abolished
in the presence of 20 μM 5-aza C, probably because of the
compound’s anti-proliferative effect (Fig. 2).
MAPKs regulate cellular responses, including gene
expression, cell proliferation, metabolism and migration (33). Various lines of evidence suggest
that MMP-9 expression in corneal epithelial cells (34), osteoblast-like MC3T3-E1 cells
(35), tracheal smooth muscle
cells (36), and breast epithelial
cells (37) is increased via
MAPKs, ERK1/2, p38 and JNK1/2 activation. The roles of individual
MAPKs in MMP expression vary with cell types and stimuli. For
example, activation of p38 kinase might play a crucial role in
transforming growth factor β-induced corneal epithelial migration
in C57BL/6J mice (38), while
ERK1/2 and JNK1/2, rather than p38 kinase, have been associated
with the regulation of IL-1β induction of MMP expression in corneal
fibroblasts (39). PI3-kinase
pathway plays a critical role in a variety of cellular processes by
phosphorylating its downstream target Akt. These processes include
mitogenic signaling, cytoskeletal remodeling, metabolic control and
cell survival (40). In the
present study, inhibiting PI3-kinase/Akt activity by LY, or ERK1/2
activity by PD, downregulated not only MMP-9 activity but also
HT1080 cell migration (Fig.
5).
Collectively, our data suggest that 5-aza C may play
an important role in tumor invasiveness through MMP-9 modulation.
The potential adverse effects of 5-aza C treatment on tumors need
to be evaluated before future clinical applications in therapy of
other solid neoplasms.
Acknowledgements
The present was supported by the National Research
Foundation of Korea (NRF) grants funded by the Korea government
(MSIP) (nos. 2015R1C1A2A01055015 and 2014R1A1A3049653) and the
Korean Health Technology R&D Project, Ministry of Health &
Welfare, Republic of Korea (A120960-1201-0000300).
Abbreviations:
|
MMPs
|
matrix metalloproteinases
|
|
5-aza C
|
5-azacytidine
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
PI3- kinase
|
phosphoinositide 3-kinase
|
|
ECM
|
extracellular matrix
|
|
DNMTs
|
DNA methyltransferases
|
|
PD
|
PD 98059
|
|
LY
|
LY 294002
|
References
|
1
|
Avgustinova A and Benitah SA: The
epigenetics of tumour initiation: Cancer stem cells and their
chromatin. Curr Opin Genet Dev. 36:8–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miremadi A, Oestergaard MZ, Pharoah PD and
Caldas C: Cancer genetics of epigenetic genes. Hum Mol Genet.
16(R1): R28–R49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jovanovic J, Rønneberg JA, Tost J and
Kristensen V: The epigenetics of breast cancer. Mol Oncol.
4:242–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
6
|
Qiu X, Hother C, Ralfkiær UM, Søgaard A,
Lu Q, Workman CT, Liang G, Jones PA and Grønbæk K: Equitoxic doses
of 5-azacytidine and 5-aza-2′deoxycytidine induce diverse immediate
and overlapping heritable changes in the transcriptome. PLoS One.
5:e129942010. View Article : Google Scholar
|
|
7
|
Snyder RD and Lachmann PJ: Differential
effects of 5-azacyti-dine and 5-azadeoxycytidine on cytotoxicity,
DNA-strand breaking and repair of X-ray-induced DNA damage in HeLa
cells. Mutat Res. 226:185–190. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Lu H, Liu R, Chen B, Wang S, Ma J
and Fu J: The dineolignan from Saururus chinensis, manassantin B,
inhibits tumor-induced angiogenesis via downregulation of matrix
metalloproteinases 9 in human endothelial cells. Oncol Rep.
32:659–667. 2014.PubMed/NCBI
|
|
9
|
Kumta SM, Huang L, Cheng YY, Chow LT, Lee
KM and Zheng MH: Expression of VEGF and MMP-9 in giant cell tumor
of bone and other osteolytic lesions. Life Sci. 73:1427–1436. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato H and Seiki M: Regulatory mechanism
of 92 kDa type IV collagenase gene expression which is associated
with invasiveness of tumor cells. Oncogene. 8:395–405.
1993.PubMed/NCBI
|
|
11
|
Iyer V, Pumiglia K and DiPersio CM:
Alpha3beta1 integrin regulates MMP-9 mRNA stability in immortalized
keratinocytes: A novel mechanism of integrin-mediated MMP gene
expression. J Cell Sci. 118:1185–1195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramos-DeSimone N, Hahn-Dantona E, Sipley
J, Nagase H, French DL and Quigley JP: Activation of matrix
metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1
cascade enhances tumor cell invasion. J Biol Chem. 274:13066–13076.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe H, Nakanishi I, Yamashita K,
Hayakawa T and Okada Y: Matrix metalloproteinase-9 (92 kDa
gelatinase/type IV collagenase) from U937 monoblastoid cells:
Correlation with cellular invasion. J Cell Sci. 104:991–999.
1993.PubMed/NCBI
|
|
14
|
Lyons JG, Birkedal-Hansen B, Pierson MC,
Whitelock JM and Birkedal-Hansen H: Interleukin-1 beta and
transforming growth factor-alpha/epidermal growth factor induce
expression of M(r) 95,000 type IV collagenase/gelatinase and
interstitial fibroblast-type collagenase by rat mucosal
keratinocytes. J Biol Chem. 268:19143–19151. 1993.PubMed/NCBI
|
|
15
|
Ito M, Hagiyama M, Mimae T, Inoue T, Kato
T, Yoneshige A, Nakanishi J, Kondo T, Okada M and Ito A: α-Parvin,
a pseudopodial constituent, promotes cell motility and is
associated with lymph node metastasis of lobular breast carcinoma.
Breast Cancer Res Treat. 144:59–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei
X, Gao J, Zhao Z and Liu C: Oct-4 and Nanog promote the
epithelial-mesenchymal transition of breast cancer stem cells and
are associated with poor prognosis in breast cancer patients.
Oncotarget. 5:10803–10815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu SM and Kim SJ: Production of reactive
oxygen species by withaferin A causes loss of type collagen
expression and COX-2 expression through the PI3K/Akt, p38, and JNK
pathways in rabbit articular chondrocytes. Exp Cell Res.
319:2822–2834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu SM and Kim SJ: DNA-hypomethylating
agent, 5′-azacytidine, induces cyclooxygenase-2 expression via the
PI3-kinase/Akt and extracellular signal-regulated kinase-1/2
pathways in human HT1080 fibrosarcoma cells. Int J Oncol.
47:1469–1475. 2015.PubMed/NCBI
|
|
19
|
Yoshihara Y, Nakamura H, Obata K, Yamada
H, Hayakawa T, Fujikawa K and Okada Y: Matrix metalloproteinases
and tissue inhibitors of metalloproteinases in synovial fluids from
patients with rheumatoid arthritis or osteoarthritis. Ann Rheum
Dis. 59:455–461. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spinale FG, Coker ML, Krombach SR,
Mukherjee R, Hallak H, Houck WV, Clair MJ, Kribbs SB, Johnson LL,
Peterson JT, et al: Matrix metalloproteinase inhibition during the
development of congestive heart failure: Effects on left
ventricular dimensions and function. Circ Res. 85:364–376. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stricklin GP and Welgus HG: Human skin
fibroblast collagenase inhibitor. Purification and biochemical
characterization. J Biol Chem. 258:12252–12258. 1983.PubMed/NCBI
|
|
22
|
Gueders MM, Foidart JM, Noel A and Cataldo
DD: Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs
in the respiratory tract: Potential implications in asthma and
other lung diseases. Eur J Pharmacol. 533:133–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Offersen BV, Pfeiffer P, Andreasen P and
Overgaard J: Urokinase plasminogen activator and plasminogen
activator inhibitor type-1 in non small-cell lung cancer: Relation
to prognosis and angiogenesis. Lung Cancer. 56:43–50. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z and Zhang R: Epigenetics in
autoimmune diseases: Pathogenesis and prospects for therapy.
Autoimmun Rev. 14:854–863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodriguez J, Frigola J, Vendrell E,
Risques RA, Fraga MF, Morales C, Moreno V, Esteller M, Capellà G,
Ribas M, et al: Chromosomal instability correlates with genome-wide
DNA demethylation in human primary colorectal cancers. Cancer Res.
66:8462–9468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: Past, present and future. Nat Rev Drug Discov. 5:37–50.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Constantinides PG, Jones PA and Gevers W:
Functional striated muscle cells from non-myoblast precursors
following 5-azacytidine treatment. Nature. 267:364–366. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alexander VM, Roy M, Steffens KA,
Kunnimalaiyaan M and Chen H: Azacytidine induces cell cycle arrest
and suppression of neuroendocrine markers in carcinoids. Int J Clin
Exp Med. 3:95–102. 2010.PubMed/NCBI
|
|
30
|
Wu Y, Meng L, Wang H, Xu Q, Wang S, Wu S,
Xi L, Zhao Y, Zhou J, Xu G, et al: Regulation of DNA methylation on
the expression of the FHIT gene contributes to cervical carcinoma
cell tumorigenesis. Oncol Rep. 16:625–629. 2006.PubMed/NCBI
|
|
31
|
Bernal T, Moncada-Pazos A, Soria-Valles C
and Gutiérrez-Fernández A: Effects of azacitidine on matrix
metalloproteinase-9 in acute myeloid leukemia and myelodysplasia.
Exp Hematol. 41:172–179. 2013. View Article : Google Scholar
|
|
32
|
Sato N, Maehara N, Su GH and Goggins M:
Effects of 5-aza-2′-deoxycytidine on matrix metalloproteinase
expression and pancreatic cancer cell invasiveness. J Natl Cancer
Inst. 95:327–330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HS, Luo L, Pflugfelder SC and Li DQ:
Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK
pathways in human corneal epithelial cells. Invest Ophthalmol Vis
Sci. 46:840–848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chia-Lan T, Chen WC, Hsieh HL, Chi PL,
Hsiao LD and Yang CM: Erratum to: TNF-alpha induces MMP-9
expression and soluble ICAM-1 release via TRAF2, c-src, MAPKs and
NF-kappaB in osteoblast-like MC3T3-E1 cells. J Biomed Sci.
22:202015. View Article : Google Scholar
|
|
36
|
Liang KC, Lee CW, Lin WN, Lin CC, Wu CB,
Luo SF and Yang CM: Interleukin-1beta induces MMP-9 expression via
p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling
pathways in human tracheal smooth muscle cells. J Cell Physiol.
211:759–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reddy KB, Krueger JS, Kondapaka SB and
Diglio CA: Mitogen-activated protein kinase (MAPK) regulates the
expression of progelatinase B (MMP-9) in breast epithelial cells.
Int J Cancer. 82:268–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saika S, Okada Y, Miyamoto T, Yamanaka O,
Ohnishi Y, Ooshima A, Liu CY, Weng D and Kao WW: Role of p38 MAP
kinase in regulation of cell migration and proliferation in healing
corneal epithelium. Invest Ophthalmol Vis Sci. 45:100–109. 2004.
View Article : Google Scholar
|
|
39
|
Lu Y, Fukuda K, Liu Y, Kumagai N and
Nishida T: Dexamethasone inhibition of IL-1-induced collagen
degradation by corneal fibro-blasts in three-dimensional culture.
Invest Ophthalmol Vis Sci. 45:2998–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wymann MP and Pirola L: Structure and
function of phosphoinositide 3-kinases. Biochim Biophys Acta.
1436:127–150. 1998. View Article : Google Scholar : PubMed/NCBI
|