Introduction
Gene therapy is thought to have great potential for
the treatment of intractable diseases, including cancer. This
approach largely depends on the effective transfection of genetic
materials such as plasmid DNA (pDNA) (1) and antisense oligonucleotides
(2) to control gene expression in
the cytoplasm. For successful gene therapy, transgenes must reach
the functional location of target cell nuclei without any form of
biodegradation (3). Therefore,
effective gene delivery systems are an essential component in the
development of gene medicines.
Among non-viral gene delivery systems, lipid
nanoparticles such as liposomes and lipoplexes are one of the most
efficient carriers for delivering genetic materials to target cells
(4). Compared to viral vectors,
lipidic nanoparticles have the advantages of low immunogenicity,
large loading capacity, biocompatibility and easy functionalization
(5). Among the lipidic
formulations, cationic liposomes have been most widely utilized as
a carrier of genetic materials for gene therapy. Their
physicochemical properties provide them with easy complexation via
charge interactions and high transfection efficiency (6,7).
However, their positive charge can be a double-edged sword, because
positively charged nanoparticles are not only easily aggregated
under aqueous conditions, but also interact with serum proteins or
non-target cells in vivo (8), resulting in rapid uptake by the
reticuloendothelial system (RES) and inefficient transfection to
target cells (9).
A great deal of effort has been spent on enhancing
the transfection efficiency of lipidic vectors in vivo.
Adding a polyethylene glycol (PEG) coating to the surface of
liposomes is a typical process for avoiding RES uptake and
increasing serum stability (10).
The hydrated PEG layer on the surface of the liposomes suppresses
their interaction with serum proteins and RES, especially in liver
Kupffer cells (11). Therefore,
PEGylated liposomes have a longer circulation in the bloodstream
and a higher likelihood of reaching the target cells (12). In addition, modification of the PEG
termini with tumor-specific ligands makes lipidic vectors a more
useful cancer-directed therapeutic gene delivery system (13).
Targeting specific cancer cells is another pivotal
process for successful cancer gene therapy. Many different types of
ligands such as antibodies (14),
aptamers (15), and peptides
(16) have been adopted for cancer
targeting. Theoretically, cancer targeting can enhance transfection
to cancer cells and reduce the off-target delivery of therapeutic
genes, thereby minimizing aberrant side effects. For example, the
epidermal growth factor receptor (EGFR) is a typical target
molecule for cancer-directed drug delivery. EGFR is known to be
overexpressed in many types of cancers such as breast, ovarian,
lung, head and neck, prostate and colorectal cancers (17). Antibodies against EGFR such as
cetuximab and panitumumab have been approved for cancer therapy via
EGFR-mediated binding and signal blocking (18). In addition to these direct
therapeutic applications, they can also be used as EGFR-specific
ligands for cancer-directed delivery systems.
In the present study, we developed two different
types of EGFR-directed lipidic nanoparticles: neutrally charged
pDNA-encapsulating immunoliposomes and cationic immunoliposome
complexes with pDNA (immunolipoplexes). Their EGFR-directed gene
delivery efficiencies were compared in target cancer cells and in a
mouse tumor model. In order to verify their clinical applicability
for cancer gene therapy, anticancer therapeutic genes encoding
salmosin and/or IL12 were encapsulated and administered to mice
carrying target tumors. Studies have shown that salmosin, a novel
disintegrin-derived snake (Gloydius saxatilis) venom, is a
single-chain polypeptide composed of 73 amino acids, including 12
cysteines and an RGD (Arg-Gly-Asp) sequence (19), and has a potent anti-angiogenic
function resulting in inhibition of tumor progression (20). IL12 is a well-known
immunostimulatory cytokine that activates NK cells and cytotoxic T
cells (21). IL12 is also known to
be anti-angiogenic (22). The
antitumoral efficacies of salmosin and/or IL12 gene delivery by
anti-EGFR immunoliposomes and immunolipoplexes were compared in
mice. This study suggests an appropriate gene delivery formulation
that can be applied to the targeted treatment of cancer.
Materials and methods
Materials
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
(POPC),
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000]
(DSPE-PEG2000),
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(
polyethyleneglycol)-2000] (DSPE-PEG2000-MAL), cholesterol, and
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine
rhodamine B sulfonyl) (Rho-DOPE) were purchased from Avanti Polar
Lipid, Inc. (Alabaster, AL, USA).
0,0′-dimyristyl-N-lysyl-glutamate (DMKE) cationic lipid was
chemically synthesized by Dr D.O. Jang (Yonsei University, Wonju,
Korea).
Cells and cell culture
Human ovarian adenocarcinoma SK-OV-3 (no. HTB-77),
lung adenocarcinoma A549 (no. CCL-185), and breast carcinoma MCF-7
cells (no. HTB-22) were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). Mouse melanoma B16BL6 cells
were developed by Dr I.J. Fidler at MD Anderson Cancer Center
(Houston, TX, USA). SK-OV-3 cells were maintained as monolayer
cultures in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco,
Carlsbad, CA, USA), A549 cells in RPMI-1640, MCF-7 cells in DMEM
and B16BL6 cells in minimum essential medium (MEM). The culture
media were supplemented with 10% heat-inactivated fetal bovine
serum (FBS; Gibco), 100 units/ml penicillin and 100 mg/ml
streptomycin (Gibco). The cancer cells were cultured in a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Preparation of immunonanoparticles
For preparation of the immunoliposomes encapsulating
pDNA, DMKE (5 mol%), POPC (91 mol%), DSPE-PEG2000 (3.8 mol%),
DSPEPEG2000-MAL (0.2 mol%), and Rho-DOPE (0.1 mol%) were dissolved
in a chloroform and methanol mixture (2:1, v/v). The organic
solvent was evaporated under a stream of N2 gas. Vacuum
desiccation for 2 h ensured removal of the residual organic
solvent. The dried films of 2 mg lipids were hydrated in 1 ml of
0.1 M phosphate buffer (pH 5.5) containing pDNA (lipid:pDNA = 10:1
weight ratio) and then vigorously mixed in a vortex mixer for 5
min. After hydration, the liposomes were subjected to 10 cycles of
freezing and thawing and extruded 10 times through a polycarbonate
membrane with a pore size from 800 to 80 nm using an extruder
(Avanti Polar Lipids). Cetuximab (Erbitux®; ImClone
Systems, Bridgewater, NJ, USA) was thiolated for 1 h at room
temperature by reacting with Traut’s reagent in degassed phosphate
buffer (0.1 M, 2 mM EDTA, pH 8.0). The thiolated antibodies were
added to the liposomes (0.2:1, molar ratio of Ab and maleimide) and
then incubated for 20 h at 4°C. Unconjugated antibodies were
removed using a Sepharose CL-4B column in phosphate buffer (0.1 M,
pH 7.2). Antibody unconjugated liposomes loading plasmid DNA were
refered to as PEG-liposomes.
For preparation of immunolipoplexes containing pDNA,
DMKE (48 mol%), cholesterol (48 mol%), DSPE-PEG2000 (3.8 mol%),
DSPE-PEG2000-MAL (0.2 mol%), and Rho-DOPE (0.1 mol%) were dissolved
in the chloroform and methanol mixture (2:1, v/v). The liposomes
were then prepared as described above. Plasmid DNA was
pre-condensed in the presence of protamine sulfate (PS) (1:1, wt
ratio of pDNA and PS) for 30 min at room temperature. Then the
pre-condensed pDNA was gently mixed with liposome solution at an
appropriate N/P ratio of DNA/lipid for 30 min at room temperature.
Cetuximab was also conjugated to the surface of lipoplexes as
described above. Antibody unconjugated lipoplexes were refered to
as PEG-lipoplexes.
Gel retardation and enzyme protection
assay
To examine the extent of pDNA encapsulation into
neutral liposomes or pDNA complexation with cationic liposomes, the
prepared immunoliposomes or immunolipoplexes were run on agarose
gel. Briefly, 2 μl of DNase I was added to the liposomes containing
pDNA (1 μg) and then incubated for 2 h at 37°C. The reaction was
stopped by the addition of 2 μl stopping solution (0.5 M EDTA) and
then further incubated for 45 min at room temperature. The reaction
solutions were treated with 2 μl of 10% Triton X-100 and incubated
for an additional 2 h at room temperature to release the pDNA from
the liposomes. Finally, the reaction mixtures were run on 1%
agarose gel and pDNA bands visualized by UV illumination.
Assay of EGFR-specific cell binding
SK-OV-3, A549, MCF-7 and B16BL6 cell lines
(4×105/well) were cultured on 6-well plates (Nunclon,
New York, NY, USA). After 24 h, the cells were treated with
Rhodamine B-labeled immunonanoparticles for 30 min at room
temperature. After being washed twice with PBS, the cells were
trypsinized, washed with PBS and fixed with 0.2% paraformaldehyde
for 5 min at room temperature in the dark. Anti-EGFR
immunonanoparticle binding to the cells was counted by a flow
cytometer (FACSCalibur; Becton-Dickinson, San Jose, CA, USA).
In vitro transfection
The SK-OV-3, A549, MCF-7 and B16BL6 cells were
treated with prepared anti-EGFR immunonanoparticles (1 μg of pDNA)
containing pDNA encoding luciferase (pLuc) in 24-well plates
(4×104/well). After transfection for 4 h, the treated
cells were additionally incubated in fresh 10% FBS-containing media
for 24 h at 37°C. The transfected cells were harvested and then
lysed with 200 μl of lysis buffer (1% Triton X-100, 1 mM
dithiothreitol and 2 mM EDTA, pH 7.8) for 2 h at room temperature
with gentle agitation. Luciferase activity in the lysates was
measured with a luciferase assay kit (Promega Bio Sciences LLC, San
Luis Obispo, CA, USA) and a MiniLumat LB 9506 luminometer (Berthold
Technologies, Bad Wildbad, Germany). The protein concentration of
the supernatant was measured with a DC Protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA). The data were expressed as
relative light units (RLU) of luciferase/mg of total proteins.
For verification of EGFR-mediated transfection, the
same cancer cells were pretreated with free cetuximab (1 μg each
well) for 30 min, and then treated with the anti-EGFR
immunonanoparticles containing pLuc (1 μg pDNA). Finally, the cells
were transfected and assayed as described above.
In vivo gene transfection
Five-week-old BALB/c nude mice (Orient, Co., Ltd.,
Seongnam, Korea) were subcutaneously injected with 1×107
SK-OV-3 cells in the right abdominal quadrant. All animal
experiments were approved by the Institutional Animal Care and Use
Committee (IACUC) of Yonsei University at Wonju (YWC-100323-1).
After the tumors grew to ~200 mm2 (length x
width2/2), the mice (n=3) were injected with the
Rhodamine B-labeled anti-EGFR immunonanoparticles containing pLuc
(40 μg DNA in 200 μl) via the tail vein. The transfected tumor
tissues were excised 24 h post-administration and were immediately
frozen. The frozen tumor tissues were transversally sectioned (3
μm) using a Leica CM1510 cryostat (Leica Microsystems, Wetzlar,
Germany). The sections were examined by fluorescence microscopy
(magnification, x100).
Two days post-administration, the major organs,
including tumors, were excised and homogenized in a lysis buffer.
Luciferase activities in the lysates were measured with a
luciferase assay kit and a luminometer as described above.
Transfection efficiency was also expressed as RLU per mg of
proteins.
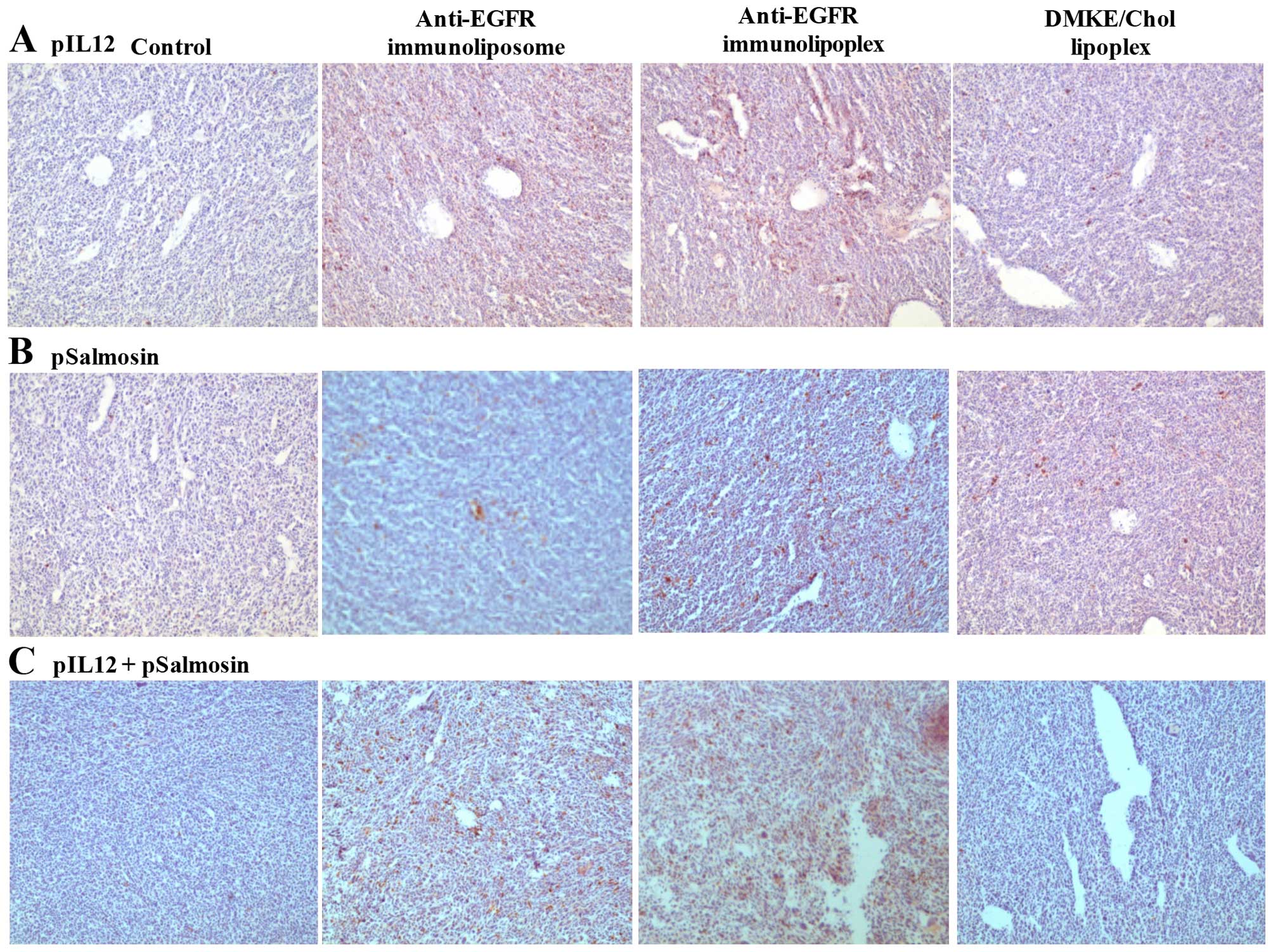
Transfection of anticancer genes by
anti-EGFR immunonanoparticles
In order to verify the expression of anticancer
genes, anti-EGFR immunonanoparticles containing pAAVCMV-mIL12
(pIL12) and/or pFLAG-Sal (pSal) were intravenously administered to
mice carrying SK-OV-3 tumors (10 μg pDNA in 200 μl PBS per mouse).
The tumor tissues were excised 2 days post-administration and
transversally sectioned (3 μm). The tumor sections were fixed in 4%
paraformaldehyde solution for 6 h and stained with hematoxylin for
counterstaining. The tumor sections were additionally stained with
anti-FLAG antibody (1/10; Abcam, Cambridge, UK) and anti-mouse IL12
antibody (1/10; Abcam) for examination of salmosin and IL12
expression, respectively. The stained tissues were then examined
under a microscope (magnification, x100).
For cancer treatment, when the tumors reached a
volume of ~50 mm2, the mice (n=5) were intravenously
injected with anti-EGFR immunolipoplexes containing anticancer
genes at a dose of 0.5 mg/kg (10 μg DNA, 4 injections at intervals
of 3 days). For combinatorial treatment with anticancer genes and
chemical drugs, doxorubicin was also administered intravenously at
a dose of 15 mg/kg (4 times at intervals of 3 days). Tumor growth
in the treated mice was monitored until mouse sacrifice on day 43,
followed by the counting of tumor colonies in the lungs.
Statistical analysis
Statistical analysis was performed using ANOVA.
P<0.05 was considered statistically significant. P<0.05,
P<0.01 and P<0.001 vs. control or between experimental
groups, respectively. Error bars represent the standard
deviation.
Results
Characterization of anti-EGFR
immunonanoparticles containing pDNA
Two different types of anti-EGFR immunonanoparticles
(immunoliposomes and immunolipoplexes) containing pDNA were
prepared and carefully characterized. To enhance pDNA encapsulation
into liposomal vesicles, 4 mol% of DMKE was added to the neutral
lipid components, followed by freezing and thawing. According to
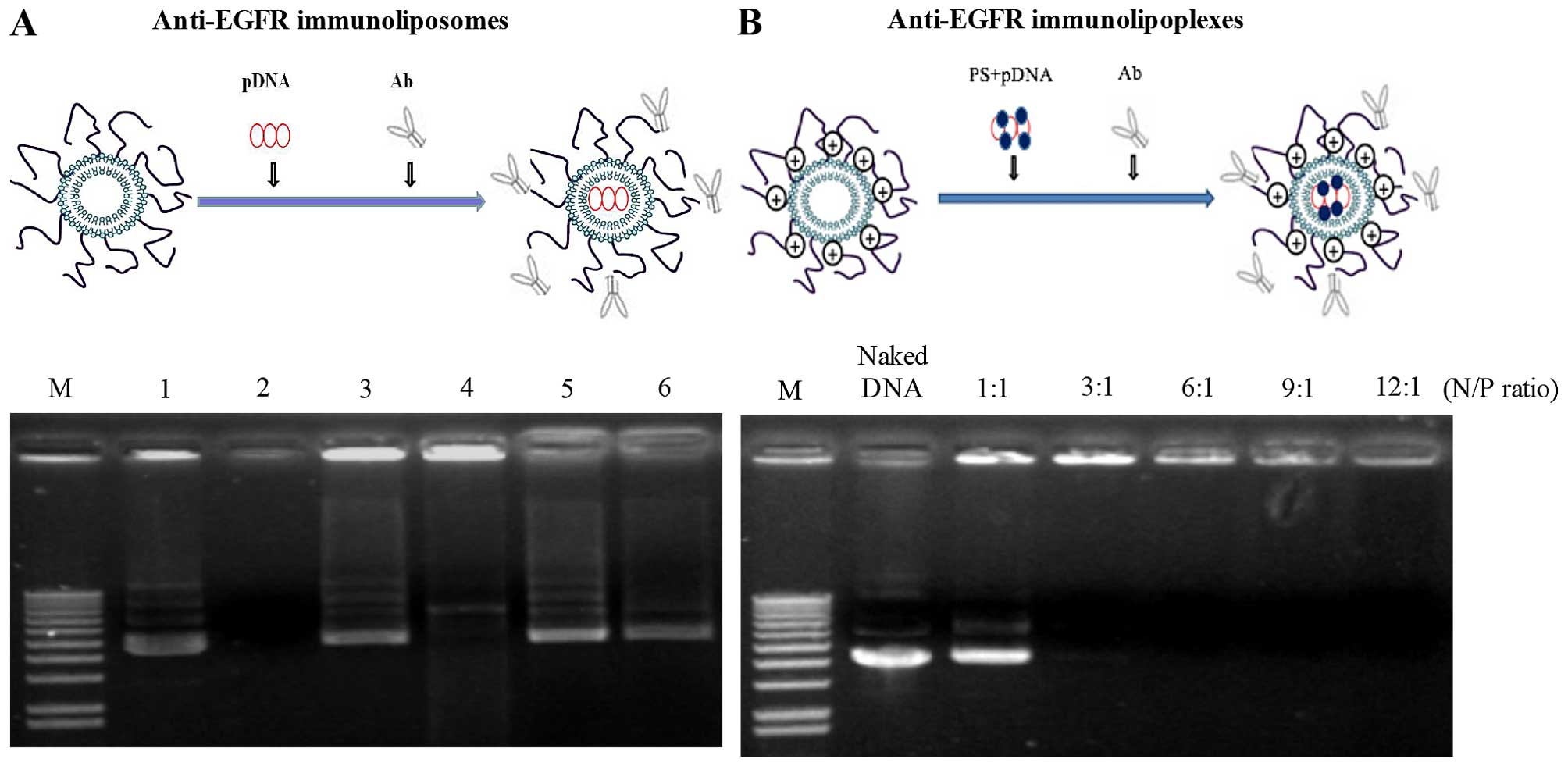
the gel retardation assay (Fig.
1A), ~70% of the pDNA was encapsulated in the liposome
formulation and well protected from DNase treatment.
Lipoplexes were also prepared by pre-condensation of
pDNA in the presence of protamine sulfate (PS), followed by
complexation with DMKE cationic liposomes via electrostatic
interaction. According to a gel retardation test (Fig. 1B), the pDNA was completely
complexed with DMKE cationic liposomes at a 3:1 N/P ratio.
Therefore, all lipoplex formulations utilized in this study were
prepared at a 3:1 N/P ratio, unless otherwise specified.
The size of the liposomes containing pDNA was
slightly larger than that of the empty liposomes (Table I). The zeta-potential of liposomes
consisting 4 mol% DMKE also shifted from weakly positive to weakly
negative due to the encapsulation of negatively charged pDNA.
Moreover, the complexation of pDNA with cationic DMKE liposomes
significantly increased their vesicle size, but not their surface
charge. However, the conjugation of anti-EGFR antibody to the
liposomes or lipoplexes increased their vesicle size and reduced
their surface charge. The sizes of the immunoliposomes (173.1 nm)
and immunolipoplexes (153.1 nm) containing pDNA were suitable for
in vivo tumor targeting via an enhanced permeability and
retention (EPR) effect.
 | Table ISizes and ζ-potentials of anti-EGFR
immunonanoparticles. |
Table I
Sizes and ζ-potentials of anti-EGFR
immunonanoparticles.
|
Immunonanoparticles | Sizea (nm) |
Zeta-potentiala (mV) |
|---|
| Liposome
formulation |
| Neutral
liposomes | 130.8±3.0b | 8.2±1.0b |
| Liposomes with
pDNA (PEG-liposomes) | 151.8±8.9 | −3.3±0.2 |
| Anti-EGFR
immunoliposomes | 173.1±7.5 | −4.9±0.5 |
| Lipoplex
formulation |
| Cationic DMKE
liposomes | 98.6±3.0 | 56.7±6.0 |
| Lipoplexes
(PEG-lipoplexes) | 135.6±1.0 | 50.9±0.3 |
| Anti-EGFR
immunolipoplexes | 153.1±4.2 | 25.4±1.2 |
Tumor-directed cellular binding and
transfection by anti-EGFR immunonanoparticles
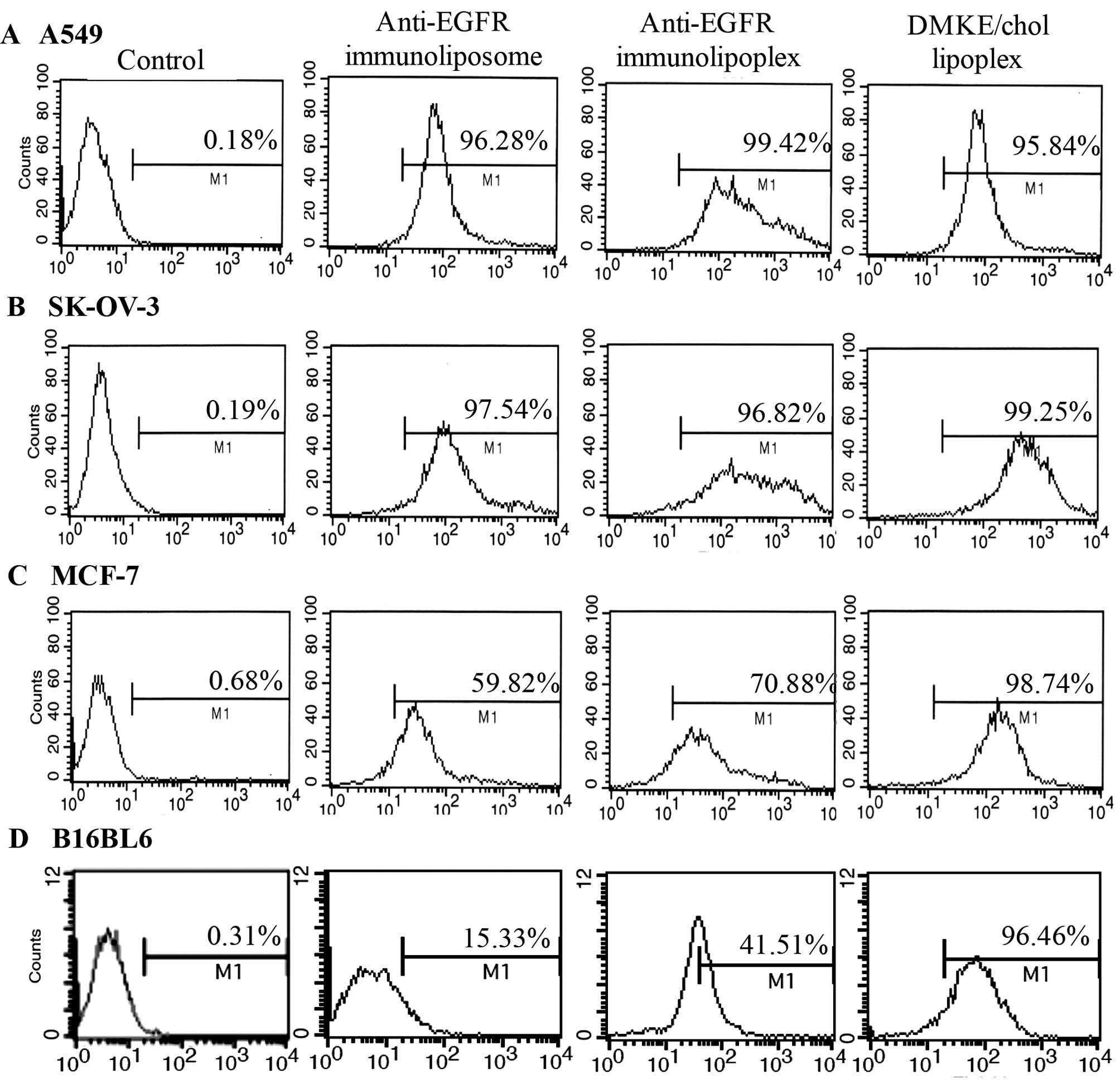
The target-specific cellular binding of the
anti-EGFR immunonanoparticles was analyzed by flow cytometry
(Fig. 2). Both the anti-EGFR
immunoliposomes and immunolipoplexes showed higher cellular binding
to EGFR-positive cells (A549 and SK-OV-3) than to EGFR-negative
cells (MCF-7 and B16BL6). The conventional DMKE lipoplexes showed a
high binding affinity to all types of cell lines, regardless of the
EGFR expression level on the cell surface.
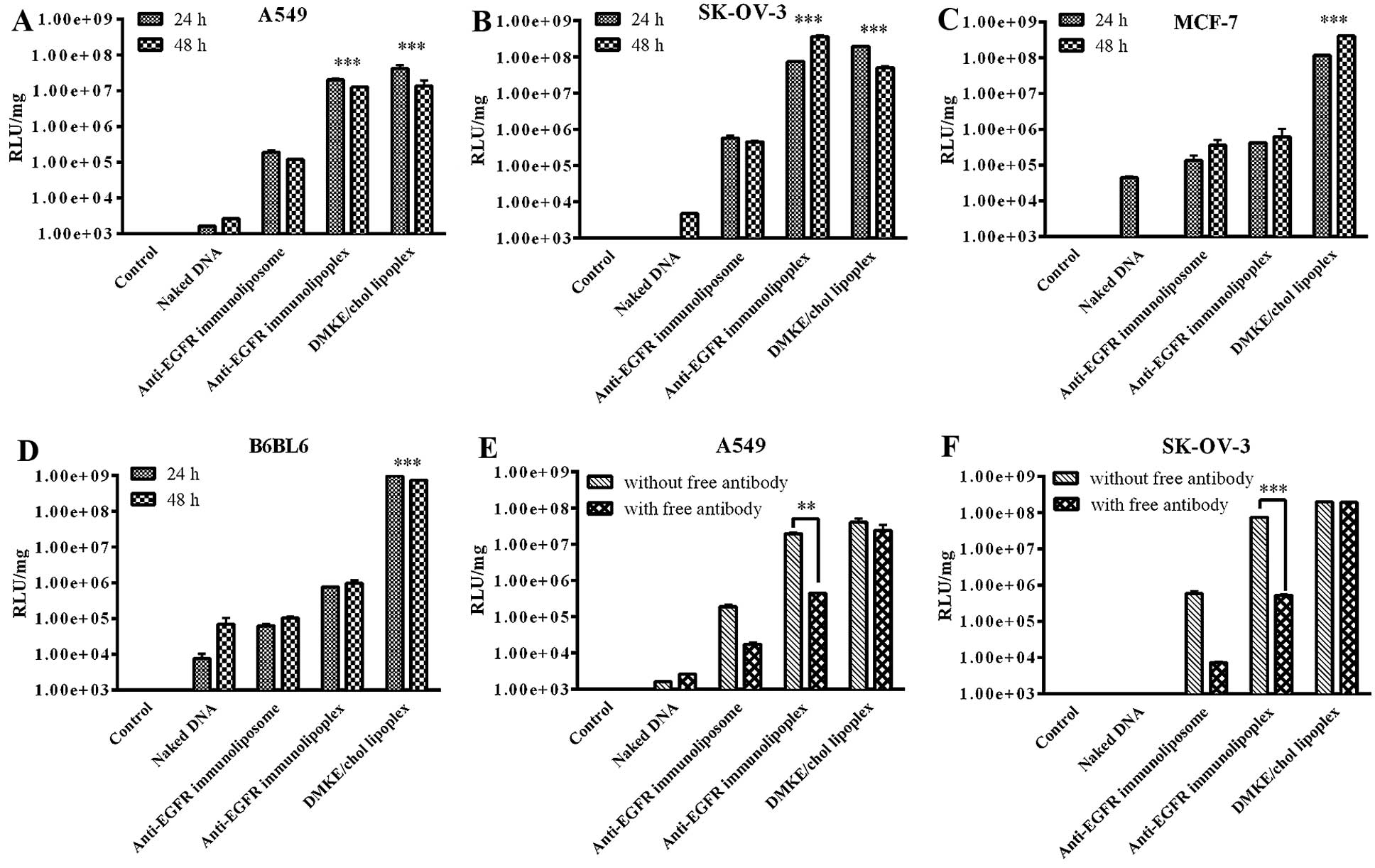
The in vitro gene transfection efficiencies
of the anti-EGFR immunonanoparticles (Fig. 3) were comparable to their cellular
binding affinities. According to the results of in vitro
pLuc transfection, EGFR-positive cells were effectively transfected
by anti-EGFR immunolipoplexes, but EGFR-negative cells were not.
Surprisingly, the neutrally charged immunoliposomes were far less
efficient than the cationic immunolipoplexes under the same
transfection conditions. Similar to the cellular binding results,
the conventional DMKE lipoplexes were effective at transfection to
all types of cells, regardless of their EGFR expression level. The
addition of free anti-EGFR antibodies significantly reduced the
levels of luciferase expression by the anti-EGFR immunolipoplexes
in EGFR-positive SK-OV-3 and A549 cells. The addition of free
cetuximab did not interfere with the transfection efficiency of
DMKE lipoplexes in the cancer cells.
In vivo gene transfection by anti-EGFR
immunonanoparticles
To investigate the in vivo tumor-targeting
capabilities and biodistribution patterns of anti-EGFR
immunonanoparticles, immunoliposomes and immunolipoplexes
containing pLuc were intravenously injected into BALB/c nude mice
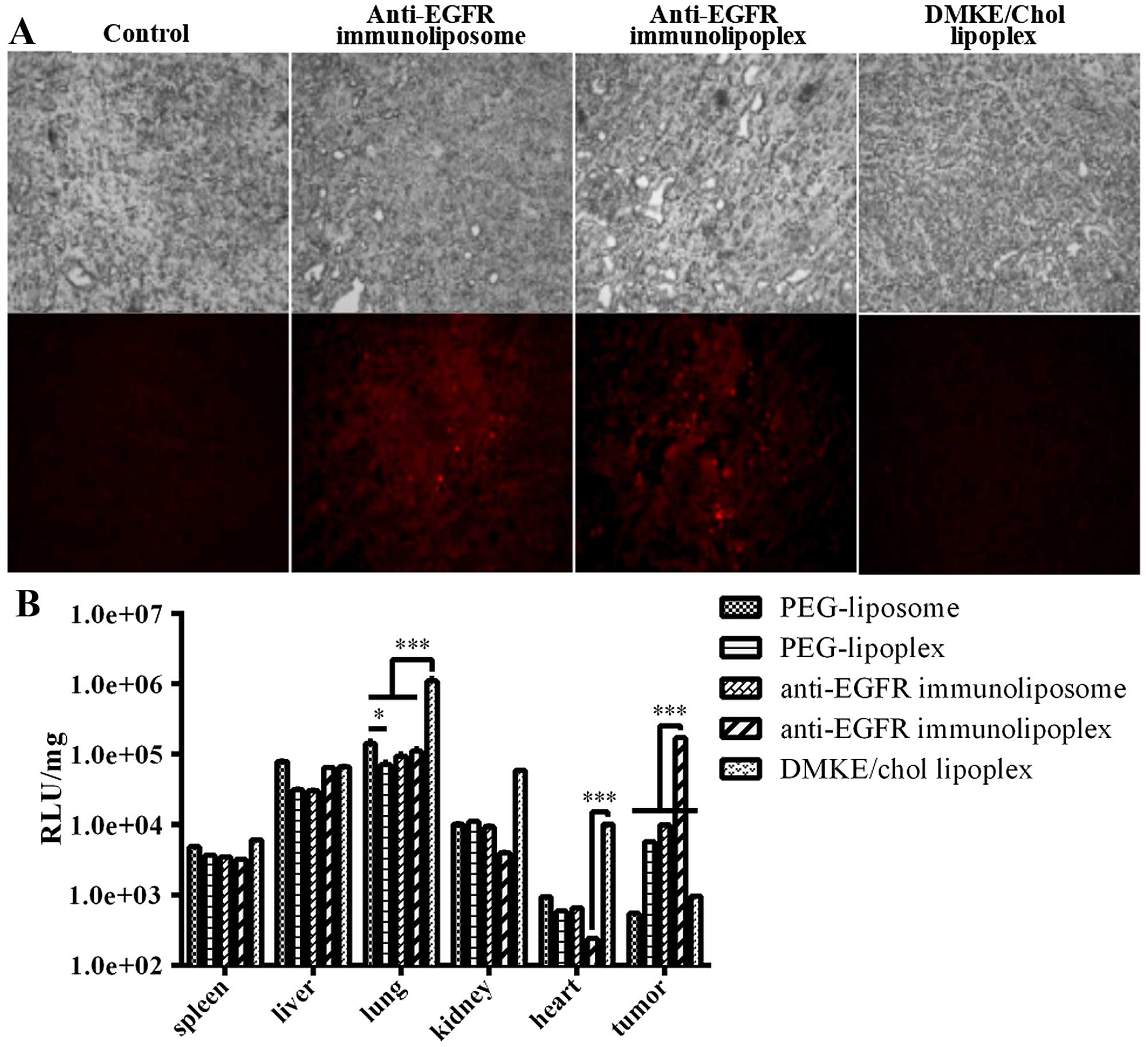
carrying SK-OV-3 tumors. Localization of the anti-EGFR
immunonanoparticles in the tumor tissues was verified by
microscopic examination, and the luciferase expression levels in
the collected internal organs (spleen, liver, lungs, kidneys, heart
and tumor) were compared. Both types of immunonanoparticles showed
relatively higher localization in the tumor tissues than did the
undirected DMKE lipoplexes (Fig.
4A). However, luciferase expression in the tumor tissues by the
anti-EGFR immunoliposomes was far lower than that of the anti-EGFR
immunolipoplexes (Fig. 4B).
Generally, as compared to cationic DMKE lipoplexes, the PEGylated
nanoparticles exhibited lower transfection to lungs, kidney and
heart, but higher transfection to tumors. PEGylation and tumor
targeting provided the lipidic nanoparticles with at least 250-fold
higher transfection to tumors than that of the unPEGylated and
untargeted DMKE lipoplexes.
Cancer therapy with anti-EGFR
immunolipoplexes containing pIL12 and/or pSal
The expression of therapeutic genes must be verified
before the therapeutic administration of the genes to mice carrying
tumors. When prepared vehicles containing pIL12 and/or pSal were
intravenously administered to SK-OV-3-xenografted mice via the tail
vein, the immunonanoparticles were definitely more effective at
expressing the genes in the tumor tissues than the DMKE lipoplexes
were (Fig. 5). Among the
immunonanoparticles, the anti-EGFR immunolipoplexes showed the best
transgene expression in the tumors. Therefore, the anti-EGFR
immunolipoplex formulation was adopted for therapeutic transfection
of pIL12 and/or pSal to a mouse tumor model.
For cancer treatment, the anti-EGFR immunolipoplexes
containing pIL12 and/or pSal were intravenously injected into three
different sets of mice carrying SK-OV-3 tumors, four times, at
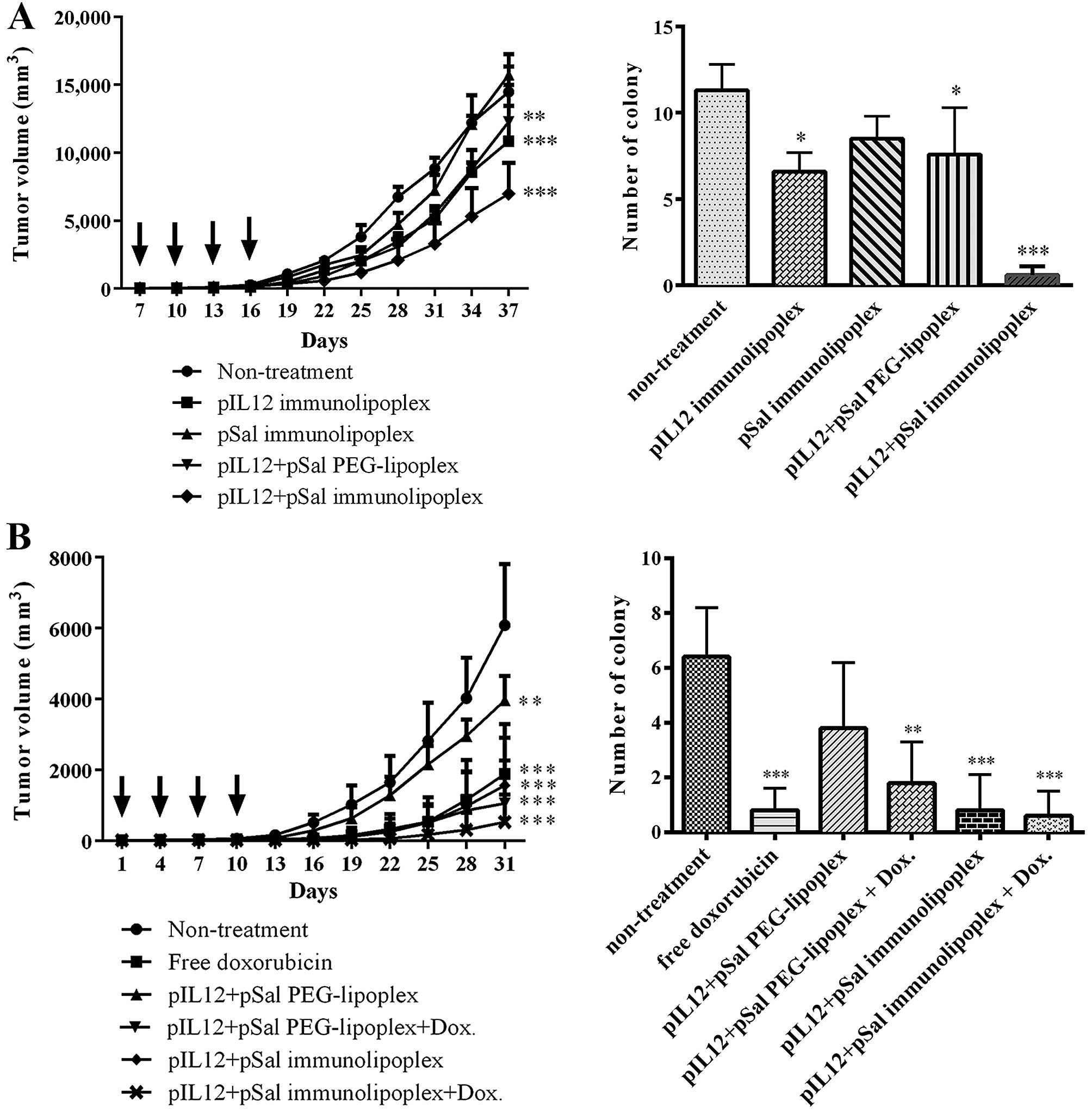
3-day intervals. According to the measurement of tumor growth,
co-transfection of pIL12 and pSal was most effective at inhibition
of tumor growth and pulmonary metastasis among the formulations
(Fig. 6A). The tumor-targeted
transfection of the therapeutic genes by the anti-EGFR
immunolipoplexes was also more effective than untargeted
transfection by the PEG-lipoplexes (Fig. 6A). Combinatorial treatment with the
anti-EGFR immunolipoplexes containing pIL12/pILSal and doxorubicin
resulted in the least tumor growth among the tested treatments
(Fig. 6B).
On 37th day, all the treated mice were sacrificed
and the number of metastatic colonies in the lungs was counted. The
pattern of metastasis inhibition by the anti-EGFR immunolipoplexes
containing pIL12 and/or pSal was similar to that of tumor growth
inhibition. Co-administration of the two genes was more effective
for the inhibition of pulmonary metastasis than treatment with
either gene alone. In addition, the mice treated with anti-EGFR
immunolipoplexes showed fewer pulmonary colonies than those treated
with untargeted PEG-lipoplexes. Combinatorial treatment with
anti-EGFR immunolipoplexes containing pIL12/pSal and doxorubicin
treatment appeared to better inhibit the pulmonary metastasis of
tumors.
Discussion
Lipidic nanoparticles such as liposomes and
lipoplexes are considered versatile delivery systems for anticancer
genes due to their biocompatibility, high loading capacity, and
convenient scale-up and functionalization. However, lipidic
delivery systems still have some limitations that need to be
overcome for wide clinical application. The in vivo
transfection efficiency of liposomal vectors, which is generally
lower than that of most viral vectors, must be improved to a
clinically meaningful level (23).
Therefore, a great deal of effort has been spent on enhancing the
in vivo gene-transferring capability of lipidic
nanoparticles. Some groups have suggested novel lipidic structures
for efficient formulation (24–26),
and others have provided effective functions for longer
circulation, target recognition, cellular uptake (27–29).
Among these trials, the targeting of lipidic nanoparticles to
specific cells or tissues by conjugation of targeting ligands to
their surface has been suggested as a pivotal process for the
improvement of in vivo transfection, as well as the
reduction of off-target effect. For target cell recognition, a
variety of targeting ligands such as antibodies, aptamers, and
peptides have been adopted for the development of target-directed
lipidic nanoparticles (30–32).
In the present study, we prepared two different
types of lipidic nanoparticles, liposomes and lipoplexes,
containing anticancer genes. POPC-based liposomes (33) encapsulate pDNA, and a small amount
of cationic DMKE was added to attract anionic pDNA near the lipid
bilayers. DMKE-based lipoplexes are complex structures of cationic
liposomes and anionic pDNA (34).
Both types of lipid nanoparticles were PEGylated to increase their
blood circulation time. At the same time, tumor-recognizing
ligands, i.e., cetuximab and anti-EGF receptor antibody, were
conjugated to the surface of the lipidic nanoparticles for
tumor-targeted gene delivery. These are referred to as anti-EGFR
immunoliposomes and anti-EGFR immunolipoplexes.
When both types of tumor-directed lipidic
nanoparticles were intravenously administered to mice carrying
SK-OV-3 tumors, their localization in the tumor tissues was
apparent and similar, according to microscopic examination.
However, as expected, the untargeted DMKE lipoplexes were not
efficiently transported to the tumors, presumably due to their
non-specific deposition in the lung endothelium (35). This implies that both of the
vehicles were stably small (<200 nm) enough to travel inside
tumor tissues by the EPR effect. Even though the anti-EGFR
immunolipoplexes exhibited a certain level of cationic surface
charge, shielding by heavy PEGylation (4 mol%) may have protected
them from the binding of serum proteins and enhanced their
circulation in the blood.
Once the gene-containing lipidic nanoparticles
arrived at the cell surface, the type and extent of interaction
between the vesicular and plasma membranes may have affected gene
transfection to the tumor cells. In general, compared to the
cationic DMKE lipoplexes, the PEGylated nanoparticles showed lower
transfection efficiency in the lungs, kidney and heart, but higher
transfection efficiency in the tumors. This result implied that the
renal clearance rates of the PEGylated nanoparticles were reduced
and its blood circulation time was prolonged. PEGylation and tumor
targeting provided the lipidic nanoparticles with at least 250-fold
higher transgene expression in the tumors. This implies that
attachment of the nanoparticles to the plasma membranes of tumor
cells via EGF receptors may play a beneficial role in transfection.
In fact, transfection inhibition by the addition of free cetuximab
may explain the significance of the EGFR-mediated interactions
between the nanoparticles and cancer cells.
Even though both types of anti-EGFR
immunonanoparticles were highly accumulated in SK-OV-3 tumor
tissues, their accumulation was not directly translated into
transgene expression. The cellular binding affinity of the
anti-EGFR immunoliposomes was similar to that of the anti-EGFR
immunolipoplexes. However, interestingly, the FACS analysis was not
directly related to the luciferase expression levels in the tumors.
The in vitro and in vivo luciferase expressions by
the immunolipoplex formulations were approximately 100- and 10-fold
greater than those of the immunoliposome formulation, respectively.
These results imply that cellular recognition and endocytosis of
the nanoparticles via EGF receptors may not be able to translocate
enough nanoparticles carrying transgenes into the tumor cells. The
cationic charge on the surface of immunolipoplexes clearly aided
transgene expression, presumably due to the effective condensation
of pDNA into the carriers and enhanced cytoplasmic release of pDNA
from the endosomal vesicles (36).
Therefore, it appears that the transfection of pDNA can be
facilitated by cellular recognition via EGF receptors as well as
via electrostatic interactions between the liposomal bilayers and
plasma membranes.
In addition, the in vivo transfection results
for anticancer genes, i.e., IL12 and salmosin genes, also showed
that anti-EGFR immunolipoplexes were a better gene delivery system.
Therefore, pIL12 and/or pSal were loaded into the anti-EGFR
immunolipoplexes for the formulation of anticancer gene medicines
and then administered into mice carrying SK-OV-3 tumors. We
previously reported that local administration (37) or hydrodynamic transfection
(38) of pIL12 and pSal was
effective in the inhibition of tumor progression. There have been
few reports regarding the tumor-targeted systemic transfection of
genes. According to the cancer treatment results in this study, the
anticancer genes were efficiently transferred by intravenous
administration of the anti-EGFR lipoplexes and effectively
expressed in the target tumors grown in mice. In fact, IL12 and
salmosin genes simultaneously delivered by the anti-EGFR
immunolipoplexes were the most effective at inhibition of SK-OV-3
tumor growth and pulmonary metastasis. In addition to gene
transfection, the intravenous administration of doxorubicin
contributed an additive inhibition of tumor progression. The mice
co-treated with anti-EGFR immunolipoplexes containing pIL12/pSal
and doxorubicin showed the highest reduction in tumor growth and
metastasis. This implies that the anti-EGFR immunolipoplexes
containing pIL12/pSal could be supportively combined with
conventional chemotherapy to elicit a better cancer treatment
outcome.
In conclusion, the anti-EGFR immunonanoparticles
prepared in this study were able to specifically recognize the EGF
receptor-overexpressing cancer cells. Among the nanoparticles
examined, anti-EGFR immunolipoplexes are a better formulation for
the in vivo transfection and expression of therapeutic genes
in the target tumor tissues. Anti-EGFR immunolipoplexes containing
pIL12/pSal appear to be good candidates for anticancer gene
medicine. This formulation of anticancer genes could be clinically
adopted as a supportive combinatorial treatment with conventional
chemotherapy to elicit the best cancer treatment outcome.
Acknowledgements
The study was supported by the National Research
Foundation of Korea (2013R1A1A2009431, 2015M2B2A4031262) and the
National Cancer Center of Korea (09202002_14918).
References
|
1
|
Ferreira GN, Monteiro GA, Prazeres DM and
Cabral JM: Downstream processing of plasmid DNA for gene therapy
and DNA vaccine applications. Trends Biotechnol. 18:380–388. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knipe JM, Peters JT and Peppas NA:
Theranostic agents for intracellular gene delivery with
spatiotemporal imaging. Nano Today. 8:21–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nam HY, Park JH, Kim K, Kwon IC and Jeong
SY: Lipid-based emulsion system as non-viral gene carriers. Arch
Pharm Res. 32:639–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Miao L, Satterlee A and Huang L:
Delivery of oligonucleotides with lipid nanoparticles. Adv Drug
Deliv Rev. 87:68–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao Y and Huang L: Lipid nanoparticles
for gene delivery. Adv Genet. 88:13–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malone RW, Felgner PL and Verma IM:
Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci
USA. 86:6077–6081. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schroeder A, Levins CG, Cortez C, Langer R
and Anderson DG: Lipid-based nanotherapeutics for siRNA delivery. J
Intern Med. 267:9–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fehring V, Schaeper U, Ahrens K, Santel A,
Keil O, Eisermann M, Giese K and Kaufmann J: Delivery of
therapeutic siRNA to the lung endothelium via novel Lipoplex
formulation DACC. Mol Ther. 22:811–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergen JM, Park IK, Horner PJ and Pun SH:
Nonviral approaches for neuronal delivery of nucleic acids. Pharm
Res. 25:983–998. 2008. View Article : Google Scholar :
|
|
10
|
Jokerst JV, Lobovkina T, Zare RN and
Gambhir SS: Nanoparticle PEGylation for imaging and therapy.
Nanomedicine (Lond). 6:715–728. 2011. View Article : Google Scholar
|
|
11
|
Li SD and Huang L: Nanoparticles evading
the reticuloendothelial system: Role of the supported bilayer.
Biochim Biophys Acta. 1788:2259–2266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang L and Liu Y: In vivo delivery of
RNAi with lipid-based nanoparticles. Annu Rev Biomed Eng.
13:507–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Majzoub RN, Chan CL, Ewert KK, Silva BF,
Liang KS, Jacovetty EL, Carragher B, Potter CS and Safinya CR:
Uptake and transfection efficiency of PEGylated cationic
liposome-DNA complexes with and without RGD-tagging. Biomaterials.
35:4996–5005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YK, Lee TS, Song IH, Jeong HY, Kang
SJ, Kim MW, Ryu SH, Jung IH, Kim JS and Park YS: Inhibition of
pulmonary cancer progression by epidermal growth factor
receptor-targeted transfection with Bcl-2 and survivin siRNAs.
Cancer Gene Ther. 22:335–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek SE, Lee KH, Park YS, Oh DK, Oh S, Kim
KS and Kim DE: RNA aptamer-conjugated liposome as an efficient
anticancer drug delivery vehicle targeting cancer cells in vivo. J
Control Release. 196:234–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoo MK, Kang SK, Choi JH, Park IK, Na HS,
Lee HC, Kim EB, Lee NK, Nah JW, Choi YJ, et al: Targeted delivery
of chitosan nanoparticles to Peyer’s patch using M cell-homing
peptide selected by phage display technique. Biomaterials.
31:7738–7747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Portnoy E, Lecht S, Lazarovici P, Danino D
and Magdassi S: Cetuximab-labeled liposomes containing
near-infrared probe for in vivo imaging. Nanomedicine. 7:480–488.
2011.PubMed/NCBI
|
|
18
|
Sato JD, Kawamoto T, Le AD, Mendelsohn J,
Polikoff J and Sato GH: Biological effects in vitro of monoclonal
antibodies to human epidermal growth factor receptors. Mol Biol
Med. 1:511–529. 1983.PubMed/NCBI
|
|
19
|
Kang IC, Chung KH, Lee SJ, Yun Y, Moon HM
and Kim DS: Purification and molecular cloning of a platelet
aggregation inhibitor from the snake (Agkistrodon halys
brevicaudus) venom. Thromb Res. 91:65–73. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang IC, Lee YD and Kim DS: A novel
disintegrin salmosin inhibits tumor angiogenesis. Cancer Res.
59:3754–3760. 1999.PubMed/NCBI
|
|
21
|
Nishitani M, Sakai T, Kanayama H, Himeno K
and Kagawa S: Cytokine gene therapy for cancer with naked DNA. Mol
Urol. 4:47–50. 2000. View Article : Google Scholar
|
|
22
|
Park YS, Kim KS, Lee YK, Kim JS, Baek JY
and Huang L: Inhibition of TC-1 tumor progression by cotransfection
of Saxatilin and IL-12 genes mediated by lipofection or
electroporation. Oncol Res. 18:203–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goodwin T and Huang L: Nonviral vectors:
We have come a long way. Adv Genet. 88:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tam YY, Chen S and Cullis PR: Advances in
lipid nanoparticles for siRNA delivery. Pharmaceutics. 5:498–507.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Whitehead KA, Dorkin JR, Vegas AJ, Chang
PH, Veiseh O, Matthews J, Fenton OS, Zhang Y, Olejnik KT, Yesilyurt
V, et al: Degradable lipid nanoparticles with predictable in vivo
siRNA delivery activity. Nat Commun. 5:42772014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Zhang S, Zhang Y, Cui S, Chen H,
Zhi D, Zhen Y, Zhang S and Huang L: Tri-peptide cationic lipids for
gene delivery. J Mater Chem B Mater Biol Med. 3:119–126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Schwind S, Yu B, Santhanam R,
Wang H, Hoellerbauer P, Mims A, Klisovic R, Walker AR, Chan KK, et
al: Targeted delivery of microRNA-29b by transferrin-conjugated
anionic lipopolyplex nanoparticles: A novel therapeutic strategy in
acute myeloid leukemia. Clin Cancer Res. 19:2355–2367.
2013.PubMed/NCBI
|
|
28
|
Li SD and Huang L: Stealth nanoparticles:
High density but sheddable PEG is a key for tumor targeting. J
Control Release. 145:178–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao YN, Qureshi F, Zhang SB, Cui SH, Wang
B, Chen HY, Lv HT, Zhang SF and Huang L: Novel gemini cationic
lipids with carbamate groups for gene delivery. J Mater Chem B
Mater Biol Med. 2:2920–2928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Askarian S, Abnous K, Taghavi S, Oskuee RK
and Ramezani M: Cellular delivery of shRNA using aptamer-conjugated
PLL-alkyl-PEI nanoparticles. Colloids Surf B Biointerfaces.
136:355–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi C, Gao F, Gao X and Liu Y: A novel
anti-VEGF165 monoclonal antibody-conjugated liposomal nanocarrier
system: Physical characterization and cellular uptake evaluation in
vitro and in vivo. Biomed Pharmacother. 69:191–200. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie X, Yang Y, Yang Y and Mei X:
Photolabile-caged peptide-conjugated liposomes for siRNA delivery.
J Drug Target. 23:789–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eliaz RE and Szoka FC Jr:
Liposome-encapsulated doxorubicin targeted to CD44: A strategy to
kill CD44-overexpressing tumor cells. Cancer Res. 61:2592–2601.
2001.PubMed/NCBI
|
|
34
|
Kim HS, Song IH, Kim JC, Kim EJ, Jang DO
and Park YS: In vitro and in vivo gene-transferring characteristics
of novel cationic lipids, DMKD (O,O’-dimyristyl-N-lysyl aspartate)
and DMKE (O,O’-dimyristyl-N-lysyl glutamate). J Control Release.
115:234–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dokka S, Toledo D, Shi X, Castranova V and
Rojanasakul Y: Oxygen radical-mediated pulmonary toxicity induced
by some cationic liposomes. Pharm Res. 17:521–525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hafez IM, Maurer N and Cullis PR: On the
mechanism whereby cationic lipids promote intracellular delivery of
polynucleic acids. Gene Ther. 8:1188–1196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SI, Kim KS, Kim HS, Kim DS, Jang Y,
Chung KH and Park YS: Inhibitory effect of the salmosin gene
transferred by cationic liposomes on the progression of B16BL6
tumors. Cancer Res. 63:6458–6462. 2003.PubMed/NCBI
|
|
38
|
Kim HS, Jeong HY, Lee YK, Kim KS and Park
YS: Synergistic antitumoral effect of IL-12 gene cotransfected with
antiangiogenic genes for angiostatin, endostatin, and saxatilin.
Oncol Res. 21:209–216. 2013. View Article : Google Scholar
|