Introduction
Lung cancer is one of the most common types of
cancer and is the leading cause of cancer death worldwide (1). Approximately 80–85% of lung cancer is
non-small cell lung cancer (NSCLC) and disease is detected in
advanced stages in more than 65% of NSCLC patients (2,3).
However, owing to the development of platinum-based chemotherapy
and operation methods, the outcome of NSCLC has significantly
improved. Nevertheless, NSCLC is still an increasing threat to
human health.
Recent studies in human genetics have led to a new
strategy of cancer treatment: personalized targeted therapy based
on the status of oncogenic drivers mutated in tumor cells (4,5). To
date, small molecular drugs targeting the epidermal growth factor
receptor (EGFR) and anaplastic lymphoma kinase (ALK) have been
widely used in NCSLC patients who have EGFR or ALK mutations,
respectively. Among all of these treatments, the epidermal growth
factor receptor tyrosine kinase inhibitor (EGFR-TKI) stands out as
one of the most effective medicines for patients with NSCLC, as it
significantly prolongs overall survival (OS) and progression-free
survival (PFS) (5–7).
Recent studies have suggested that activation of
EGFR is positively associated with the activation of PD-L1 in NSCLC
cells. EGFR mutations are directly related to the upregulation of
PD-L1 expression (8–11). Programmed cell death protein 1
(PD-1) and its ligand the PD-L1, play a major role in suppressing
the immune system during cancer development and can therefore be
used as immunotherapy target. Some in vivo studies have
shown that blocking the PD-1/PD-L1 pathway can restore the
antitumor activity of T cells (12,13).
Furthermore, in clinical trials, PD-1/PD-L1 checkpoint inhibitors
showed tolerable efficacy in patients with advanced or metastatic
NSCLC (14). All of these results
indicate that highly activated EGFR is associated with
overactivation of the PD-1/PD-L1 pathway, which leads to an
increasing risk of tumor immune escape in NSCLC. However, the
potential mechanism behind this event remains unclear.
One recent study indicated that interleukin 6
(IL-6)-mediated PD-L1 expression in tolerogenic antigen-presenting
cells (APCs) was controlled by signal transducer and activator of
transcription 3 (STAT3). More specifically, activated STAT3 was
shown to bind to the PD-L1 promoter and, subsequently, promote
transcription of PD-L1 (15). To
the best of our knowledge, the IL-6/Janus kinase (JAK)/STAT3 is a
canonical signaling pathway which plays a critical role in the
initiation, development and formation of various cancers (16). One previous study suggested that
the EGFR signaling pathway is involved in the activation of the
IL-6/JAK/STAT3 pathway (17).
Taken together, it is tempting to speculate that EGFR can regulate
the expression of PD-L1 via the IL-6/JAK/STAT3 pathway.
In the present study, we investigated the
relationship between the EFGR signaling pathway and PD-L1 in NSCLC
cells and the involvement of IL-6/JAK/STAT3 pathway in this
relationship. Furthermore, we also evaluated the role of PD-L1 in
survival of NSCLC cells.
Materials and methods
Cell culture, treatment and
transfection
Human EGFR wild-type NSCLC cell lines (i.e. H1299
and SPC-A1) and EGFR-mutated NSCLC cell lines (i.e. H1975 and
HCC827) were obtained from the Shanghai Institutes of Biological
Sciences Cell Bank and were maintained in RPMI-1640 medium (Gibco)
supplemented with 10% fetal bovine serum (FBS; Gibco) and 1%
penicillin/streptomycin (Gibco). PC-9 cells were obtained from
Professor Caicun Zhou as a gift and were maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco) supplemented with 10% FBS
(Gibco) and 1% penicillin/streptomycin (Gibco). All cells were
cultured in humidified incubators at 37°C with 5%
CO2.
For gefitinib treatment, cells were seeded into
96-well plates at a density of 3×103 cells/well, grown
overnight and then induced with varying drug concentrations for 48
h: 0, 0.005, 0.01, 0.1, 1, 5, 10, 20 and 40 μM gefitinib
(AstraZeneca). For AG490 treatment, cells were seeded into 6-well
plates (3×105 cells/well), grown overnight and then
serum-starved for 24 h. Subsequently, serum-starved cells were
treated with AG490 (100 μM; Sigma-Aldrich) for 4 h.
For small interfering RNA (siRNA) transfection,
prede-signed siRNA targeting PD-L1 and STAT3 were purchased from
Shanghai GenePharama Co., Ltd., Shanghai, China. Transfections were
performed using the siRNA transfection kit (Shanghai GenePharama)
according to the manufacturer’s protocol.
EGFR mutation analysis
Genomic DNA samples were extracted by the Genomic
DNA kit (Tiangen Biotech Co., Ltd., Beijing, China) according to
the manufacturer’s instructions and sent to our collaborator in the
Central Laboratory of the First Affiliated Hospital of Soochow
University for EGFR mutation analysis (exons 18–21) by
amplification-refractory mutation system (ARMS) PCR and direct
sequencing.
Enzyme-linked immunosorbent assay
(ELISA)
Cell-free supernatant was harvested after treatment
at different time-points, i.e. 0, 2, 4, 8, 12, 24 and 48 h. IL-6
ELISA analyses were conducted by using a commercially available
ELISA kits (Multi Sciences) according to the protocols suggested by
the manufacturer. Standard dilutions were prepared as suggested.
The OD450 was read and the concentration of IL-6 was calculated
according to the standard curve.
Western blot analysis
Approximately 10×106 cells were harvested
and lysed in 1X RIPA buffer with proteinase and phosphatase
inhibitors. Cell lysates were cleared by centrifugation at 4°C for
20 min at 12000 × g. Protein was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a polyvinylidene fluoride (PVDF) membrane. After
blocking in 5% non-fat milk/T-BST buffer for 1 h, membranes were
incubated in primary antibodies overnight at 4°C. The following
primary antibodies were used in the dilution as suggested by the
supplier: PD-L1 (1:1,000; Cell Signal Technology), p-EGFR (1:1,000;
Cell Signal Technology), p-STAT3 (1:1,000; Cell Signal Technology),
STAT3 (1:1,000; Cell Signal Technology), GAPDH (1:4,000; Cell
Signal Technology). After primary antibody incubation, blots were
incubated in HRP-conjugated secondary antibodies for 2–3 h at room
temperature. Immunoreactive proteins were detected using the
SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher
Scientific).
RNA extraction and real-time polymerase
chain reaction (RT-PCR)
Total RNA was extracted from cells using the TRizol
method (Takara Bio). Synthesis of cDNA with reverse transcriptase
was performed with the M-MLV First Strand kit (Invitrogen). cDNA
aliquots were subjected to RT-PCR reactions using the SYBR Premix
Ex Tag II (Takara) on the ABI PRISM 7500 Sequence Detection system
(Applied Biosystems). Primers used for RT-PCR were as follows:
5′-GGTGCCGACTACAAGCGAAT-3′ (forward) and
5′-GGTGACTGGATCCACAACCAA-3′ (reverse) for PD-L1; and
5′-TGCACCACCAACTGCTTAGC-3′ (forward) and
5′-GGCATGGACTGTGGTCATGAG-3′ (reverse) for GAPDH.
Cell proliferation assay
Cells were seeded in 96-well plates at a density of
3×103 cells/well. After 24, 48 and 72 h, every well was
treated with 10 μl of cell Counting kit-8 solution (Beyotime
Institute of Biotechnology) and the OD450 was measured by a
microplate reader after 4 h (Thermo Fisher Scientific).
Colony formation assay
Cells were seeded (3×103 cells/well) in a
6-cm culture dish. After a week, cells were washed twice with PBS,
fixed with methanol for 30 min and stained with crystal violet
overnight. Images from every plate were taken and analyzed by
ImageJ software.
Cell apoptosis analyses
Cell apoptosis was detected using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (Beyotime Institute of Biotechnology) according to
the manufacturer’s instructions. Briefly, cells were washed twice
with cold PBS and then 5×104 cells were resuspended in 1
ml binding buffer containing Annexin V/FITC and PI. After
incubation at room temperature for 20 min in the dark, staining
results were detected by the fluorescence-activated cell sorting
FACSCaliber system (Beckman Coulter) within 30 min.
Statistical analysis
All numerical data were presented as the mean ±
standard error of the mean (SEM). Statistical analysis was
performed by a two-tailed Student’s t-test and the difference was
considered significant at P-value of <0.05. The statistical
analyses were carried out with SPSS 17.0 software.
Results
The expression of PD-L1 is closely
related with EGFR mutations
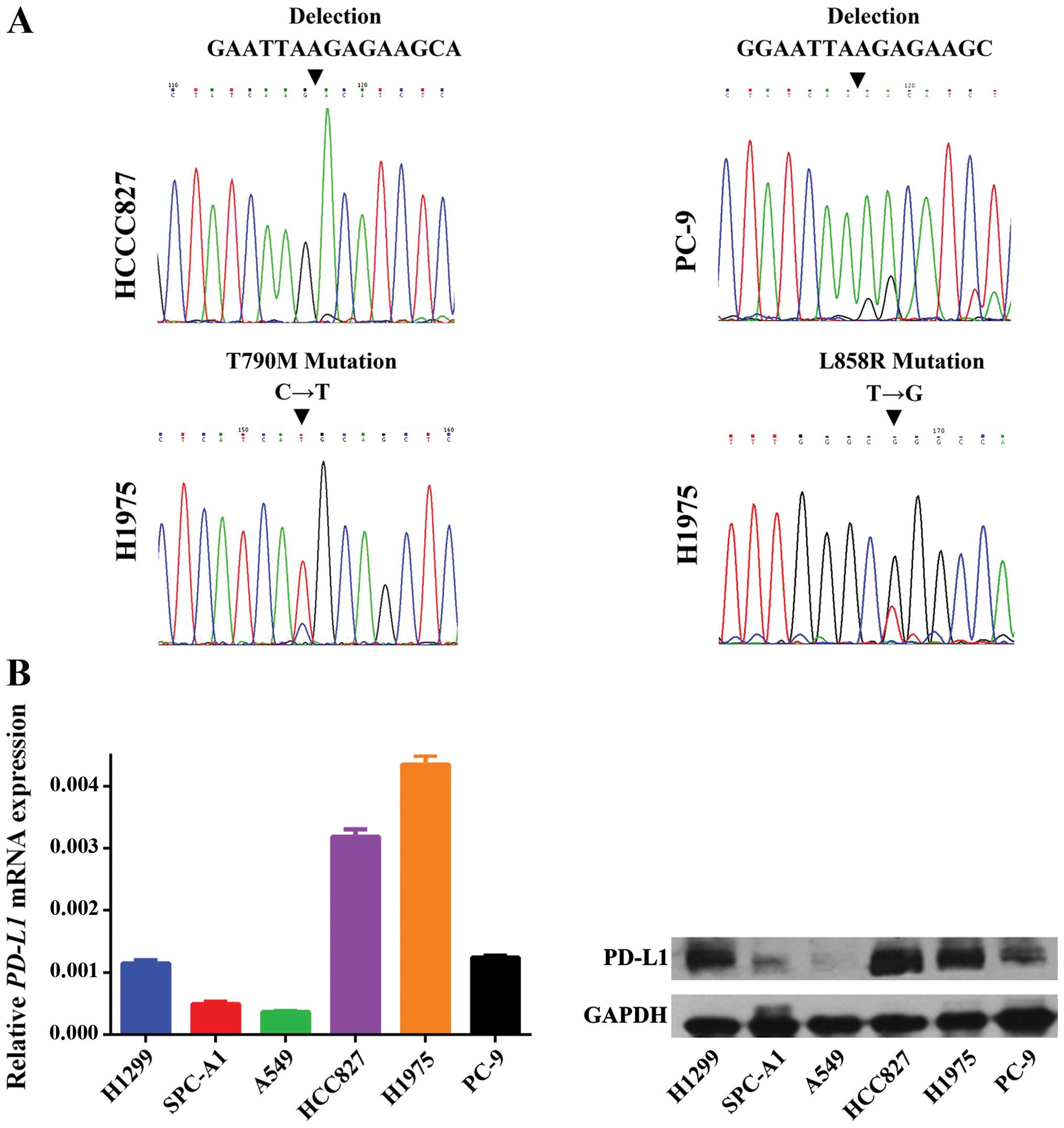
The variation in EGFR exon 18–21 was evaluated in
three EGFR-mutant cell lines, i.e. HCC827, PC-9 and H1975. An
activating deletion in exon 19 was found in the HCC827 and PC-9
cell lines and a leucine-to-arginine substitution at codon 858
(p.L858R) together with a threonine-to-methionine substitution at
codon 790 (p.T790M) were detected in the H1975 cell line (Fig. 1A).
The transcription and translation level of PD-L1 was
measured by RT-PCR and western blot analysis in three EGFR-mutant
cell lines as mentioned above and three EGFR wild-type cell lines,
i.e. H1299, SPC-A1 and A549. As shown in Fig. 1B, PD-L1 expression was much higher
in the three EGFR-mutant cell lines in comparison to the EGFR
wild-type cell lines, with the exception of the H1299 cell line,
which had comparable PD-L1 expression levels as seen in the PC-9
cells (Fig. 1B). The potential
reason for this could be due to the deletion of TP53 in these
cells, which has already be shown to be closely related with PD-L1
expression (18).
In summary, we were able to associate the expression
of PD-L1 with EGFR mutations in NSCLC cells. EGFR-activating
mutations can upregulate the transcription and translation of
PD-L1.
EGFR signaling pathway is involved in the
regulation of PD-L1 expression in EGFR-mutant NSCLC cells
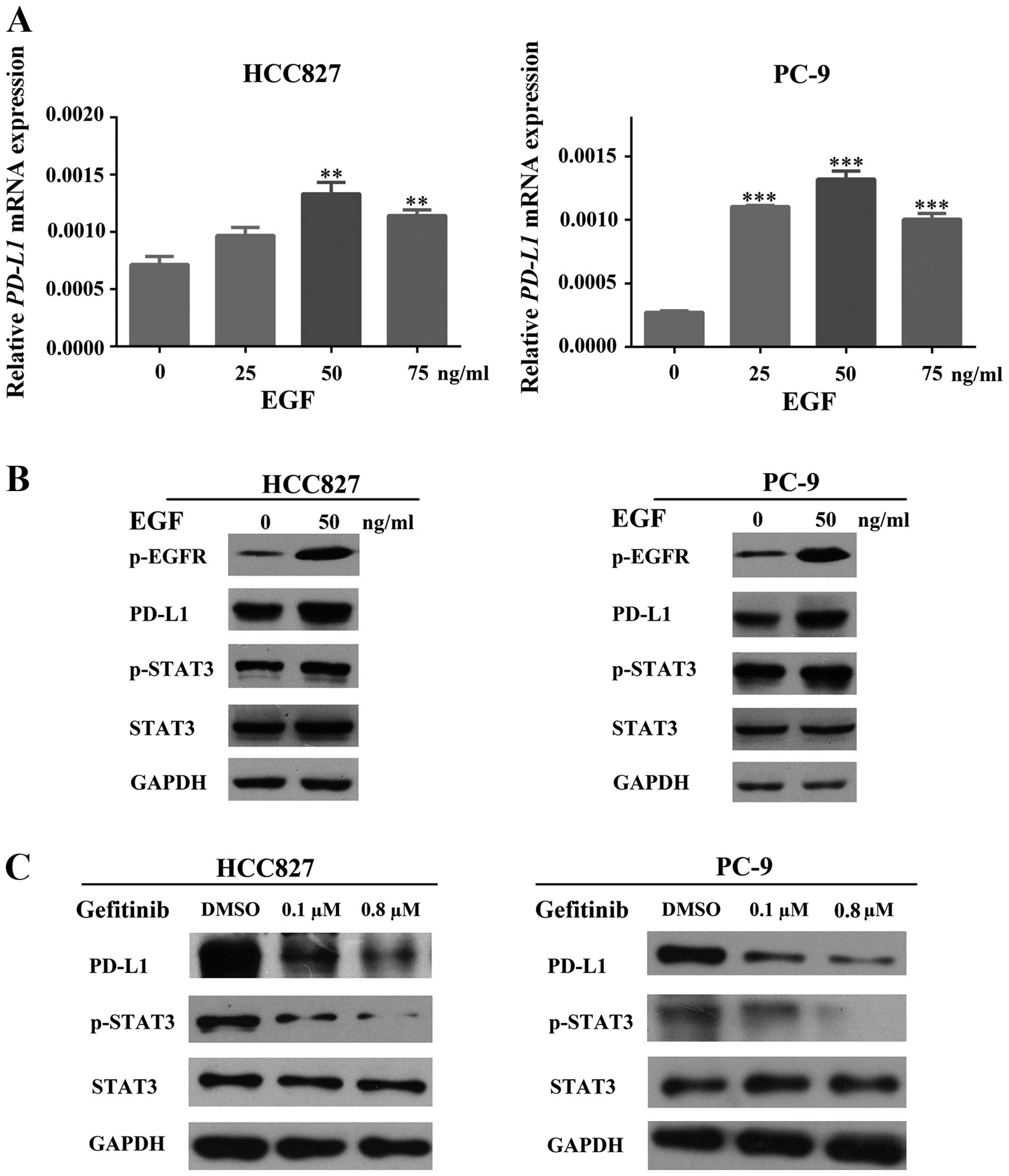
To investigate the relationship between EGFR
activation and PD-L1 expression in EGFR-mutant NSCLC cells, we
induced HCC827 and PC-9 cells with the recombined human epidermal
growth factor (EGF) in various concentrations (0, 25, 50 and 75
ng/ml) for 48 h in order to activate the EGFR pathway. The
transcriptional change of PD-L1 was measured by RT-PCR, which
showed a positive association between EGF concentration and PD-L1
mRNA levels (Fig. 2A). The PD-L1
protein expression change was compared between untreated and
EGF-treated (50 ng/ml) cells (HCC827 and PC-9) by western blot
analysis, which indicated a significant increase after treatment
(Fig. 2B). All of these results
suggested that EGFR activation led to PD-L1 upregulation.
For more clarification, we treated HCC827 and PC-9
cells with gefitinib (an EGFR-TKI) in different concentrations
according to the half maximal inhibitory concentration
(IC50) which we obtained from our preliminary
experiments (data not shown) so that we could inhibit the
activation of the EGFR pathway. The expression of PD-L1 was
measured by western blot analysis, which suggested a significant
dose-dependent decrease of PD-L1 presenting after gefitinib
treatment (Fig. 2C).
As a result, activity of EGFR is positively related
with the expression of PD-L1 in EGFR-mutant NSCLC.
IL-6/JAK/STAT3 signaling pathway is
involved in regulation of EGFR-mediated PD-L1 expression in
EGFR-mutant NSCLC cells
As described previously, IL-6/JAK/STAT3 is
considered to be a potential pathway involved in the regulation of
EGFR-mediated PD-L1 expression. To confirm this, we investigated
the activation of STAT3 in samples from previous EGFR activation
and inhibition experiments. As shown in Fig. 2B and C, HCC827 and PC-9 cells
induced with EGF were able to increase the phosphorylation and
activation of EGFR and STAT3. However, inhibiting the activation of
EGFR by gefitinib was able to dephosphorylate and deactivate STAT3.
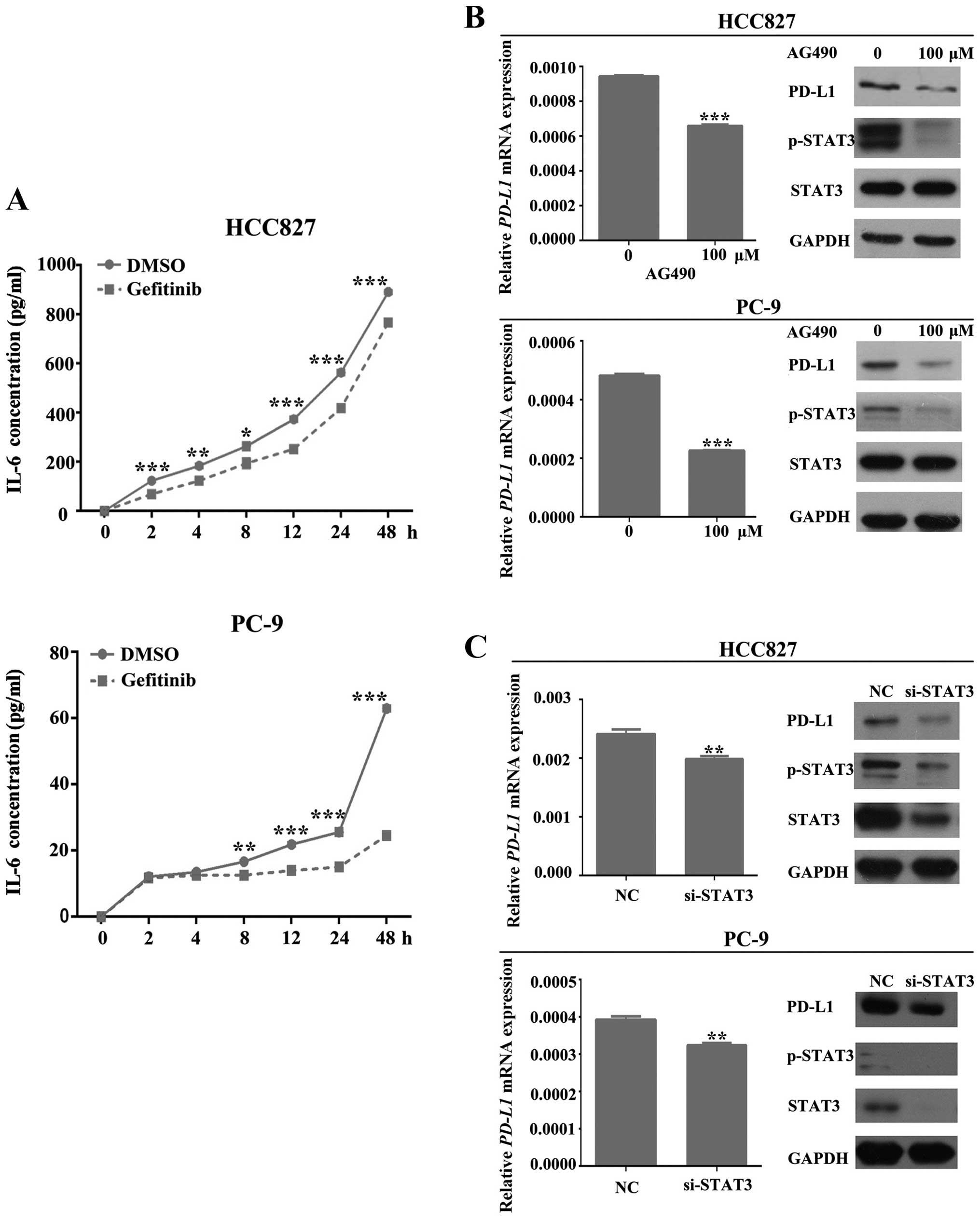
Moreover, we also checked the IL-6 levels in cell-free supernatants
from gefitinib-treated (0.8 μM) HCC827 and PC-9 cells via ELISA at
different time-points (0, 2, 4, 8, 12, 24 and 48 h). In both
untreated and gefitinib treated cells, the concentration of IL-6
increased with time. However, this increase was significantly
slower in gefitinib-treated cells (P<0.01; Fig. 3A).
To further confirm these results, we silenced the
endogenous expression of STAT3 and assessed the expression level of
PD-L1 in HCC827 and PC-9 cells. As shown in Fig. 3C, depletion of STAT3 expression
induced a significant loss of PD-L1 at both protein and mRNA levels
(P<0.01).
All of this evidence indicated that EGFR activation
can induce the expression and secretion of IL-6 from NSCLC cells,
which conversely increases the activation of STAT3. Activated STAT3
eventually leads to the increasing expression of PD-L1.
To investigate the involvement of JAK, we treated
HCC827 and PC-9 cells with AG 490 (100 μM), which is a specific and
potent JAK-2 protein tyrosine kinase inhibitor. After 4 h of
treatment, the amount of PD-L1 protein and mRNA was measured and
results indicated a significant reduction in both levels
(P<0.001; Fig. 3B).
The above-mentioned results suggest that EGFR can
mediate PD-L1 expression in EGFR-mutant NSCLC cells by upregulating
the expression of IL-6 which, subsequently, activates the
IL-6/JAK/STAT3 signaling pathway. Activated STAT3 can eventually
increase the expression of PD-L1 in EGFR-mutant NSCLC cells.
PD-L1 plays a role in cell survival in
EGFR-mutant NSCLC cells
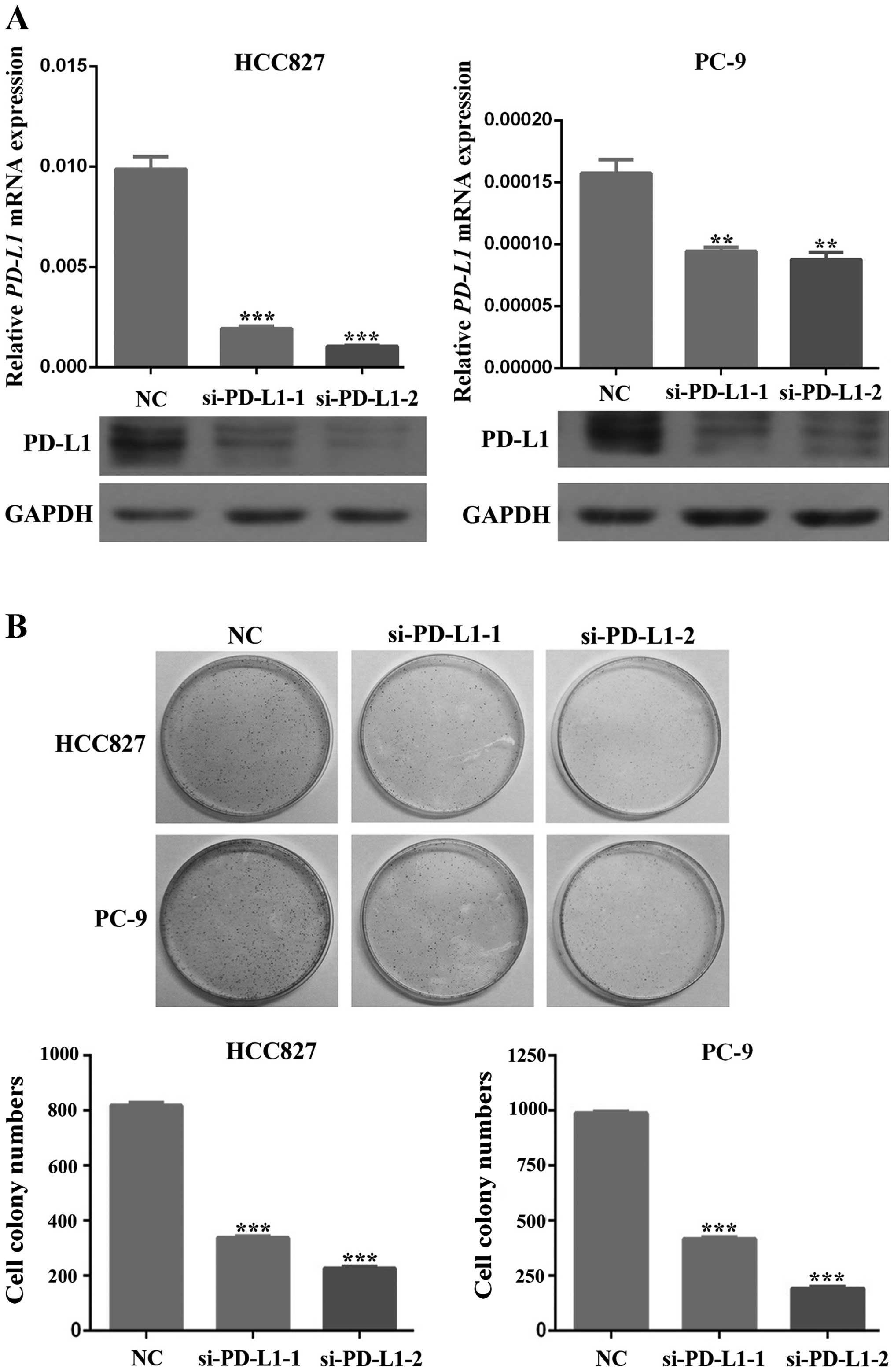
To predict the potential role of PD-L1 in cell
survival, we silenced PD-L1 expression in HCC827 and PC-9 cells and
then investigated the changes in cell proliferation and apoptosis.
The efficacy of silencing was confirmed by western blot analysis
and RT-PCR (Fig. 4A). Inhibition
of PD-L1 accumulation in HCC827 and PC-9 cells led to a remarkable
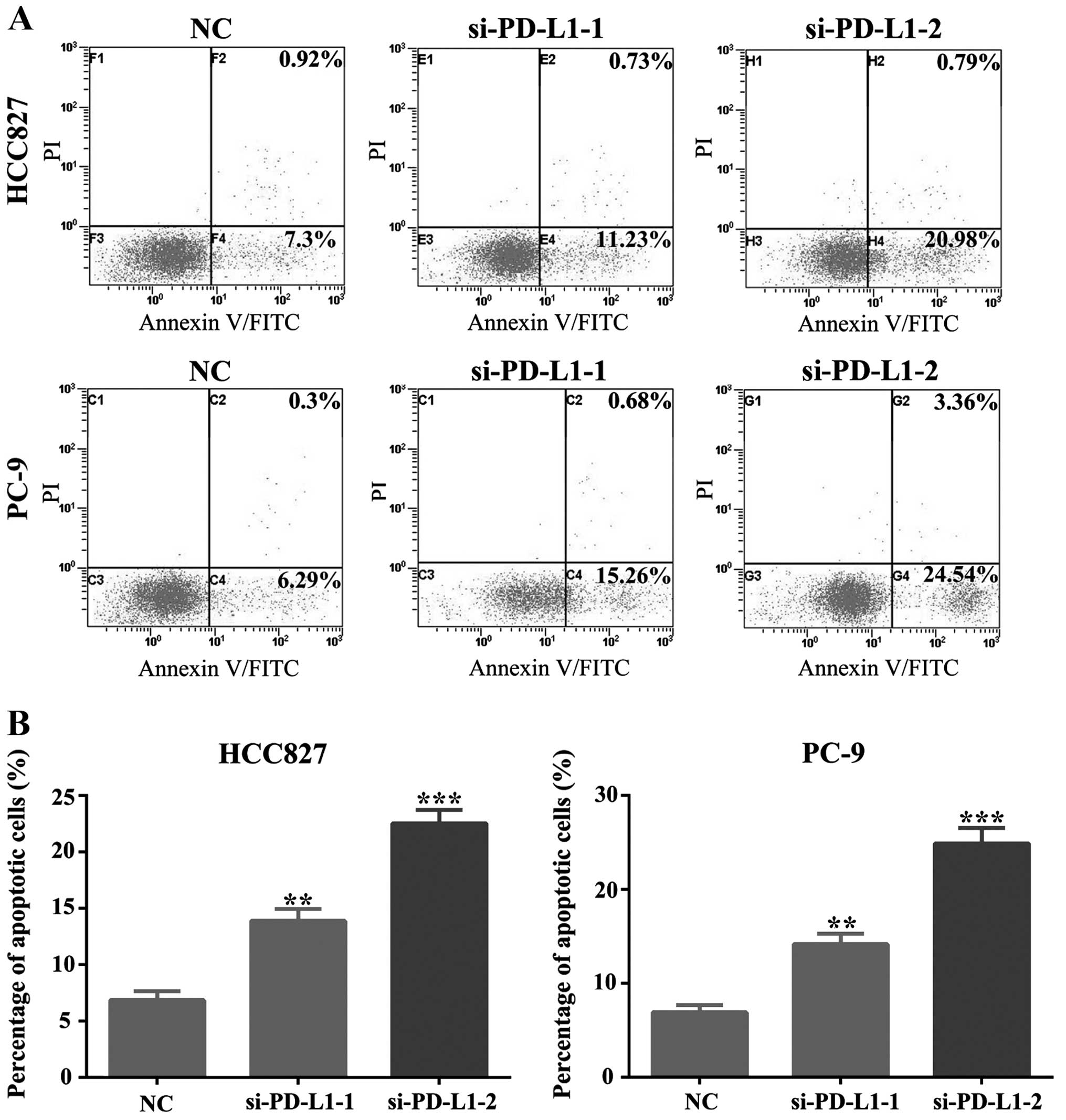
decrease in cell proliferation (P<0.01; Fig. 4B). Moreover, downregulation of
PD-L1 expression enhanced apoptosis of HCC827 and PC-9 cells
(P<0.01; Fig. 5). As shown in
Fig. 5, silencing of PD-L1 can
induce cells into early apoptosis, which suggests its potential
role in the prevention of apoptosis in these cells.
In summary, these data demonstrate that PD-L1 can
affect tumor cell survival by promoting cell proliferation and
preventing cell apoptosis, which can be reversed by downregulating
the expression of PD-L1. Taking all previous results together
indicates that EGFR activation can induce the expression of PD-L1
through the IL-6/JAK/STAT3 signaling pathway, which, in the end,
promotes the survival of cancer cells by inhibiting apoptosis and
promoting proliferation. NSCLC cells treated with EGFR-TKIs can
reverse all of these effects which in turn lead to the death of
tumor cells.
Discussion
As widely known, EFGR, which is a key molecule in
tumor cell proliferation, invasion and metastasis regulation
(19), is frequently mutated in
NSCLC. EGFR-TKIs, such as gefitinib and erlotinib, have been
approved as a first-line therapy for EGFR-mutant advanced NSCLC
patients (20). Recently,
increasing attention has been given to the association between
EGFR-targeted therapy and immunotherapy. One focus in particular is
the relationship between EGFR-TKIs and tumor cell immune
evasion.
PD-L1 is a co-inhibitory molecule generally
expressed in APCs (e.g. macrophages, DCs), activated T cells, B
cells and tumor cells (21,22).
PD-L1-activated PD-1 functions as an inhibitor for the
proliferation, survival and effects of CD8+ cytotoxic T
lymphocytes (CTLs), which play a key role in tumor immune evasion
(23).
In the present study, we demonstrated that EGFR
mutations are associated with increased PD-L1 expression in NSCLC
cell lines. One exception was also detected: H1299, an EGFR
wild-type cell line, retained relatively high expression levels of
PD-L1. One potential reason for this could be the deletion of the
TP53 gene in these cells. It has been recently suggested that the
expression of PD-L1 is regulated by p53 via miR-34, and that loss
of p53 is associated with higher PD-L1 expression (18). We also found out that EGFR is
involved in the regulation of PD-L1 expression via the
IL-6/JAK/STAT3 signaling pathway in EGFR-mutant NSCLC. Cells
induced with EGF, which activates the EGFR pathway, can upregulate
the expression of PD-L1. Moreover, inhibition of EGFR by EGFR-TKIs
reduced the secretion of IL-6 from cancer cells and, subsequently,
decreased the activation of JAK/STAT3, which eventually inhibited
the expression of PD-L1 in these cells. Some investigators also
found that STAT3 promotes the expression of PD-L1 by binding to the
PD-L1 promoter (15). Recently,
research pertaining to PD-L1 discovered various signaling molecules
which are potentially involved in its expression (including IFN-γ,
NF-κB, MAPK, PI3K/AKT, mTOR and MEK/ERK/STAT1) in tumor cells
(24–31). NF-κB and MEK/ERK/STAT1 may also be
important for NSCLC, as they have both been found to be involved in
the regulation of EGFR-mediated PD-L1 expression. The cross-talk
among all of these pathways mentioned above is an interesting area
which requires further research. In the end, we were also able to
show that silencing of PD-L1 can reduce proliferation and increase
apoptosis in NSCLC cells. Some recent clinical studies have
suggested that overexpression of PD-L1 is correlated with poor
prognosis and shortened overall survival (OS) (10,32–34).
Together with our data, down-regulated PD-L1 might be a favorable
biomarker for NSCLC.
EGFR-TKIs have already been widely applied in the
clinic and are used as a first-line treatment for EGFR-mutant NSCLC
patients. However, the rapid acquired resistance is still a serious
limitation for targeted therapy. Based on our research,
immunotherapy based on targeting PD-1/PD-L1 may bring on new
approaches which are more efficient for the treatment of
EGFR-mutant patients who have developed resistance to EGFR-TKIs.
Our data suggest that both mutation and activation of EGFR can
increase the expression of PD-L1. This means that EGFR-mutant
patients who are resistant to EGFR-TKIs might also have high levels
of PD-L1 present. Furthermore, in one particular phase III clinical
trial, the anti-PD-1 antibody (Nivolumab) was shown to increase the
OS and PFS of patients with previously treated advanced or
metastatic NSCLC (14). As a
result, we assume that PD-1/PD-L1 targeted immunotherapy is a
potent solution for EGFR-TKI resistance. Recently, the potential of
combining EGFR-TKIs with immunotherapy to improve the outcome of
NSCLC patients was reported (35).
Targeted therapy usually has rapid and impressive response rates
but modest progression-free survival, while immunotherapy can
achieve durable tumor control, but is associated with lower
response rates (36). Based on the
present study, we demonstrate that combined EGFR-TKI and
PD-1/PD-L1-based immunotherapy may not be a good strategy, as NSCLC
cells treated with EGFR-TKI can inhibit the expression of PD-L1,
which in turn can significantly reduce the efficiency of the
PD-1/PD-L1 inhibitor. In this case, additional immune system check
points should be considered, such as CTLA4. Further research and
additional clinical trials are still needed.
In summary, the present study demonstrated that
PD-L1 is overexpressed in EGFR-mutant NSCLC cells. EGFR activation
can induce the expression of PD-L1 via the IL-6/JAK/STAT3 signaling
pathway in EGFR-mutant NSCLC cells, which can be inhibited by
EGFR-TKIs. In addition, downregulation of PD-L1 was associated with
inhibited proliferation and enhanced apoptosis of EGFR-mutant NSCLC
cells. This study provides us with both a new perspective regarding
the antitumor mechanism of EGFR-TKIs and novel thinking about the
combination of EGFR-TKIs with immunotherapy.
Acknowledgements
We are grateful to Professor Caicun Zhou (Shanghai
Pulmonary Hospital) for providing the PC-9 cell lines. The present
study was supported by grants from the National Natural Science
Foundation of China (31270940 to J.-A.H.), Clinical Medical Center
of Suzhou (Szzx201502) and Clinical Key Speciality Project of
China.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji M, Liu Y, Li Q, Li XD, Zhao WQ, Zhang
H, Zhang X, Jiang JT and Wu CP: PD-1/PD-L1 pathway in
non-small-cell lung cancer and its relation with EGFR mutation. J
Transl Med. 13:52015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim MY, Koh J, Kim S, Go H, Jeon YK and
Chung DH: Clinicopathological analysis of PD-L1 and PD-L2
expression in pulmonary squamous cell carcinoma: Comparison with
tumor-infiltrating T cells and the status of oncogenic drivers.
Lung Cancer. 88:24–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Spanish Lung Cancer Group in
collaboration with Groupe Français de Pneumo-Cancérologie and
Associazione Italiana Oncologia Toracica: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JK, Hahn S, Kim DW, Suh KJ, Keam B,
Kim TM, Lee SH and Heo DS: Epidermal growth factor receptor
tyrosine kinase inhibitors vs conventional chemotherapy in
non-small cell lung cancer harboring wild-type epidermal growth
factor receptor: A meta-analysis. JAMA. 311:1430–1437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cross DA, Ashton SE, Ghiorghiu S, Eberlein
C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ,
et al: AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated
resistance to EGFR inhibitors in lung cancer. Cancer Discov.
4:1046–1061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Azuma K, Ota K, Kawahara A, Hattori S,
Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et
al: Association of PD-L1 overexpression with activating EGFR
mutations in surgically resected nonsmall-cell lung cancer. Ann
Oncol. 25:1935–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang Y, Fang W, Zhang Y, Hong S, Kang S,
Yan Y, Chen N, Zhan J, He X, Qin T, et al: The association between
PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced
non-small cell lung cancer patients treated with EGFR-TKIs.
Oncotarget. 6:14209–14219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D’Incecco A, Andreozzi M, Ludovini V,
Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J,
Coppi E, et al: PD-1 and PD-L1 expression in molecularly selected
non-small-cell lung cancer patients. Br J Cancer. 112:95–102. 2015.
View Article : Google Scholar :
|
|
12
|
Afreen S and Dermime S: The
immunoinhibitory B7-H1 molecule as a potential target in cancer:
Killing many birds with one stone. Hematol Oncol Stem Cell Ther.
7:1–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muenst S, Soysal SD, Tzankov A and Hoeller
S: The PD-1/PD-L1 pathway: Biological background and clinical
relevance of an emerging treatment target in immunotherapy. Expert
Opin Ther Targets. 19:201–211. 2015. View Article : Google Scholar
|
|
14
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wölfle SJ, Strebovsky J, Bartz H, Sähr A,
Arnold C, Kaiser C, Dalpke AH and Heeg K: PD-L1 expression on
tolerogenic APCs is controlled by STAT-3. Eur J Immunol.
41:413–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alvarez JV, Greulich H, Sellers WR,
Meyerson M and Frank DA: Signal transducer and activator of
transcription 3 is required for the oncogenic effects of
non-small-cell lung cancer-associated mutations of the epidermal
growth factor receptor. Cancer Res. 66:3162–3168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al:
Mutations in the EGFR kinase domain mediate STAT3 activation via
IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cortez MA, Ivan C, Valdecanas D, Wang X,
Peltier HJ, Ye Y, Araujo L, Carbone DP, Shilo K, Giri DK, et al:
PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 108:1082015.
View Article : Google Scholar
|
|
19
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: A model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keedy VL, Temin S, Somerfield MR, Beasley
MB, Johnson DH, McShane LM, Milton DT, Strawn JR, Wakelee HA and
Giaccone G: American Society of Clinical Oncology provisional
clinical opinion: Epidermal growth factor receptor (EGFR) Mutation
testing for patients with advanced non-small-cell lung cancer
considering first-line EGFR tyrosine kinase inhibitor therapy. J
Clin Oncol. 29:2121–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI,
Park YM, Oh S, Shin JG, Yao S, Chen L, et al: Interferon regulatory
factor-1 is prerequisite to the constitutive expression and
IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett.
580:755–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Velcheti V, Schalper KA, Carvajal DE,
Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L
and Rimm DL: Programmed death ligand-1 expression in non-small cell
lung cancer. Lab Invest. 94:107–116. 2014. View Article : Google Scholar
|
|
23
|
Barber DL, Wherry EJ, Masopust D, Zhu B,
Allison JP, Sharpe AH, Freeman GJ and Ahmed R: Restoring function
in exhausted CD8 T cells during chronic viral infection. Nature.
439:682–687. 2006. View Article : Google Scholar
|
|
24
|
Abiko K, Matsumura N, Hamanishi J,
Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I
and Mandai M: IFN-γ from lymphocytes induces PD-L1 expression and
promotes progression of ovarian cancer. Br J Cancer. 112:1501–1509.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2015. View Article : Google Scholar
|
|
26
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL,
et al: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Hamrouni A, Wolowiec D, Coiteux V,
Kuliczkowski K, Hetuin D, Saudemont A and Quesnel B: Plasma cells
from multiple myeloma patients express B7-H1 (PD-L1) and increase
expression after stimulation with IFN-{gamma} and TLR ligands via a
MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 110:296–304.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Atefi M, Avramis E, Lassen A, Wong DJ,
Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, et
al: Effects of MAPK and PI3K pathways on PD-L1 expression in
melanoma. Clin Cancer Res. 20:3446–3457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar
|
|
30
|
Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y,
Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, et al:
Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with
elevated PD-L1 expression. Cancer Cell. 25:590–604. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin K, Cheng J, Yang T, Li Y and Zhu B:
EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through
inhibiting NF-κB. Biochem Biophys Res Commun. 463:95–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Huang S, Gong D, Qin Y and Shen
Q: Programmed death-1 upregulation is correlated with dysfunction
of tumor-infiltrating CD8+ T lymphocytes in human
non-small cell lung cancer. Cell Mol Immunol. 7:389–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YB, Mu CY and Huang JA: Clinical
significance of programmed death-1 ligand-1 expression in patients
with non-small cell lung cancer: A 5-year-follow-up study. Tumori.
98:751–755. 2012.
|
|
34
|
Mu CY, Huang JA, Chen Y, Chen C and Zhang
XG: High expression of PD-L1 in lung cancer may contribute to poor
prognosis and tumor cells immune escape through suppressing tumor
infiltrating dendritic cells maturation. Med Oncol. 28:682–688.
2011. View Article : Google Scholar
|
|
35
|
Robert L, Ribas A and Hu-Lieskovan S:
Combining targeted therapy with immunotherapy. Can 1+1 equal more
than 2? Semin Immunol. 28:73–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wargo JA, Cooper ZA and Flaherty KT:
Universes collide: Combining immunotherapy with targeted therapy
for cancer. Cancer Discov. 4:1377–1386. 2014. View Article : Google Scholar : PubMed/NCBI
|