Introduction
Lung cancer is the most frequent malignancy and the
prognosis is still poor (1). One
of the reasons for worse prognosis is that tumor metastasis is
often seen at the diagnosis. In addition, despite development of
some molecularly-targeted drugs that target for EGFR-mutation or
ALK-rearrangement, there are certain patients who cannot get these
benefits (2,3). Therefore, there is a strong need to
define new therapeutic targets and biomarkers.
As mentioned above, metastasis is a major cause of
death in lung cancer patients. In metastasis process, glycolipids
and glycoproteins play important roles and are involved in cancer
properties (4–7). Gangliosides, a kind of
glycosphingolipid, are widely expressed in many tissues (8), but some gangliosides are very
limitedly expressed in normal tissues, and their expression is
enhanced in tumors. GD2 is highly expressed in small cell lung
cancers, and is strongly associated with the malignant potential
(9,10). Furthermore, anti-GD2 monoclonal
antibodies induce apoptosis of small cell lung cancer cells
(11). NeuGcGM3 is overexpressed
in non-small cell lung cancer and an anti-idiotype vaccine
targeting the NeuGcGM3 is effective in the treatment of patients
with advanced non-small cell lung cancer (12). As for carbohydrates on
glycoproteins, O-glycans also play important roles in the
malignancy and survival of cancer cells (6). O-glycans are often highly sialylated
in cancer cells (13,14). Among them, Tn antigen, which is
formed by polypeptide N-acetylgalactosaminyltransferases
(ppGalNAc-T), and sialyl Tn antigen have been considered as
tumor-associated antigens, and the expression levels of them are
increased in many types of human cancers (15,16).
In the analysis of mechanisms for cancer metastasis,
we previously demonstrated that reduced levels of ganglioside GM1
resulted in the increased invasion and metastatic potentials
(17). We also found that
ppGalNAc-T13 (T13), which is a family member of ppGalNAc
transferases, was upregulated as a result of reduced GM1, leading
to enhanced metastasis by formation of trimeric Tn antigen on
Syndecan 1 in mouse Lewis lung cancer (18–20).
In human, it has been reported that GALNT13 mRNA is a strong
predictor of poor clinical outcome in neuroblastoma patients
(21). However, little is known
about the relationship of the T13 expression and the phenotypes and
prognosis in lung cancer patients. If the correlation between
GALNT13 gene expression levels in lung cancer tissues and
patient prognosis is indicated, it can be expected as a new
prognostic marker and a therapeutic target. In addition, if it is
detected in blood, it can be a useful biomarker for early detection
or minimal metastatic diseases.
In the present study, T13 expression levels in
surgical lung cancer specimens were analyzed to examine whether T13
and its product can be used as a tumor prognostic marker.
Materials and methods
Sample collection
Ninety-one patients received pulmonary resection at
Nagoya University Hospital (2008, January–2009, March). Before
operation, each patient’s serum sample was collected. The lung
cancer tissues and sera were stored at −80°C. The treatment policy
was decided according to the standard protocol, and fully informed
written consents were obtained from all patients. Our study
protocol was approved by the Institutional Review Boards of Nagoya
University Graduate School of Medicine.
Total RNA isolation and cDNA
synthesis
Total RNA was isolated from tissue using TRIzol™
reagent (Invitrogen, Carlsbad, CA, USA). We added 1000 μl of TRIzol
reagent to tissue, and the sample was homogenized by Psychotron™
(Nition Co., Ltd., Funabashi, Japan). Next, we incubated the
homogenized sample for 5 min at room temperature prior to the
addition of 0.2 ml of chloroform, and they were incubated for 3-min
at room temperature. An upper aqueous phase was collected after
centrifugation at 12,000 × g for 15 min at 4°C, and mixed with 0.5
ml of 100% isopropanol. After incubation for 10 min at room
temperature, the sample was centrifuged at 12,000 × g for 10 min at
4°C and the supernatant was removed. After washing, the pellet with
1 ml of 75% ethanol, RNA pellets were dried for 30 min, and
resuspended in 50 μl of RNase-free water and incubated at 55°C for
10 min. For serum samples and cell lines, total RNA was extracted
using Total RNA Purification™ kit (Norgen Biotek Corp., Thorold,
Ontario, Canada) according to the manufacturer’s instructions. We
synthesized cDNA from total RNA and extracted genomic DNA using
PrimeScript™ RT reagent kit with gDNA Eraser™ (Takara Bio, Kusatsu,
Japan).
Quantitative real-time RT-PCR
Real-time quantitative PCR (qPCR) was performed with
8 ng of cDNA and SsoAdvanced™ Universal SYBR-Green Supermix
(Bio-Rad Laboratories, Hercules, CA, USA) using CFX Connect™
Real-Time system (Bio-Rad Laboratories). Specific primers for
GALNT13, its variant exon usages, and GAPDH were
purchased from Sigma-Aldrich Japan (Tokyo, Japan). Each primer
sequence of wild-type and variant exons is shown in Table I. Cycling conditions were as
follows: 30 sec at 95°C, and then 40 cycles of 5 sec at 95°C and 20
sec at 62°C. Cq values were calculated with Bio-Rad CFX Manager™
version 2.1. GALNT13 mRNA expression was normalized with an
internal control, GAPDH mRNA.
 | Table ISequences of primers used in nested
and real-time RT-PCR. |
Table I
Sequences of primers used in nested
and real-time RT-PCR.
| Gene | Forward primer | Reverse primer |
|---|
| GALNT13 wild |
5′-TGGCCAGTGATTTGATTGCC-3′ |
5′-AACGTGCATTCACAGTGTGC-3′ |
| E5 |
5′-TGACTTTCCCTGCTTTCGTG-3′ |
5′-ATGACCTGCCCTTTTGAAGC-3′ |
| E13′ |
5′-GTTACTTGCTCCCATGTTGGTC-3′ |
5′-CAGGTCCATTGAGTCTGATTTC-3′ |
| E14 |
5′-TGGGAATATGATGCTGAGTCTTG-3′ |
5′-TTCATGTGCCCAAGGTCATG-3′ |
| GALNT13 nested |
| Outer |
5′-GCATTGAGGGCTGTTATTTCAAG-3′ |
5′-AGAGTAGATAGTGTGGGGAACG-3′ |
| Inner |
5′-AAGAAGGGCCAGGAGAAATGG-3′ |
5′-AGAGTGCTCCAAGCTTCATTATG-3′ |
Nested PCR
To detect expression of GALNT13 mRNA in serum
sample, nested PCR was performed. Each primer sequence of primary
PCR and nested PCR is shown in Table
I. First PCR was performed in 50 μl using 8 ng cDNA with 1 μl
of KOD FX™ (Toyobo Co., Ltd., Osaka, Japan). Nested PCR
were done using 1 μl of the first PCR product as a template. Both
first and nested PCR were done under following conditions: 2 min at
94°C, and then 40 cycles of 10 sec at 98°C and 30 sec at 62°C. PCR
product was visualized on a 1.5% agarose gel.
Immunohistochemistry
Lung tumor samples were fixed in 4% paraformaldehyde
for 24 h, followed by a solution of 30% sucrose/PBS until the
tissues sank. After embedding in Tissue-Tek™ O.C.T Compound (Sakura
Finetek Japan, Tokyo, Japan), 5 μm-thick frozen sections were made
using Leica CM3050S™ cryostat (Leica Biosystems, Wetzlar, Germany).
The sections treated with 4% paraformaldehyde for 10 min. Samples
were then incubated with 3% H2O2 to inhibit
endogenous peroxidase activity. In T13 immunostaining, sections
were treated with Protein Block Serum-Free™ (Dako Japan, Tokyo,
Japan) at room temperature for 30 min in order to block
non-specific binding. The sections were incubated at room
temperature for 60 min in 0.4 μg/ml goat polyclonal primary
anti-ppGalNAc-T13 antibody (T18) (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), followed by applying a secondary biotinylated
rabbit anti-goat IgG. In GM1 immunostaining, sections were treated
with 3% BSA in PBS for 15 min for blocking. Sections were then
incubated with cholera toxin B subunit-biotin conjugate (List
Biological Laboratories, Campbell, CA, USA) diluted at 0.5 μg/ml
with PBS plus 3% BSA, at room temperature for 60 min. In trimeric
Tn antigen immunostaining, after treating with Protein Block
Serum-Free™, the samples were incubated at room temperature for 60
min with mouse monoclonal primary anti-trimeric Tn antibody
(MLS128) (1.6 μg/ml) (provided from Nakada, Kyoto Sangyo
University, Kyoto, Japan). Then, a secondary biotinylated horse
anti-mouse IgG was applied. The immunoreactivity of these sections
was visualized using Vectastain™ Elite ABC kit (Vector
Laboratories, Burlingame, CA, USA) and Dako Liquid DAB+ Substrate
Chromogen System™ (Dako Japan).
Cell culture
The cell line ACC-LC-94/TS1 was derived from human
lung adenocarcinoma and cultured in DMEM with 7.5% fetal bovine
serum (FBS; Sigma-Aldrich Japan).
Statistical analysis
The clinicopathological characteristics, overall
survival and recurrence-free survival of the patients were obtained
from medical records. The relationship was analyzed using the
Wilcoxon-Mann-Whitney test. The Kaplan-Meier method was conducted
to estimate survival differences and analyzed using log-rank test.
The level of significance was set at P<0.05. These data were
analyzed with IBM SPSS Statistics version 22.0 (IBM, Armonk, NY,
USA).
Results
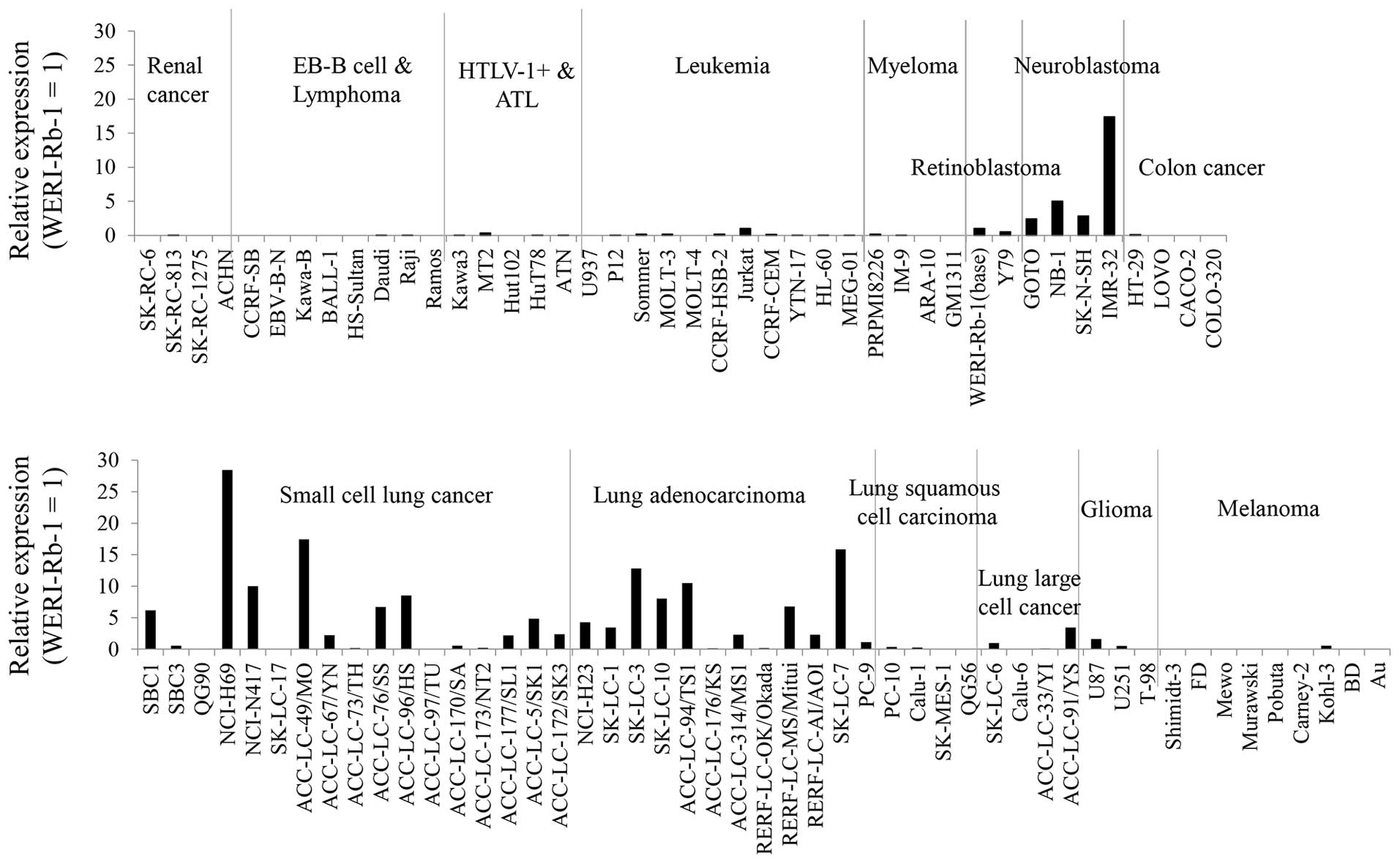
GALNT13 mRNA was highly expressed in
human lung cancer cell lines and neuroblastoma cell lines
In order to examine GALNT13 mRNA expression
among human tumors, we determined mRNA expression levels in various
human cancer cell lines (Fig. 1).
From this result, GALNT13 mRNA was found to be highly
expressed in lung cancers and neuroblastomas. Among lung cancers,
both adenocarcinoma cell lines and small cell lung cancer cell
lines showed high expression levels.
Association of GALNT13 expression levels
with clinical data of lung cancer patients
Then, to examine whether T13 affects the intensity
and prognosis of the disease, we analyzed GALNT13 mRNA
expression levels in 91 surgical specimens by real-time RT-PCR, and
the results were evaluated by correlating with pathological and
clinical data. The GALNT13 mRNA expression levels were
normalized by GAPDH mRNA and then relative levels to that of
a control cell line (ACC-LC-94/TS1, relative expression level=1)
were calculated. We divided 91 cases into GALNT13 mRNA high
expression group (T13 high, n=16) and low expression group (T13
low, n=75) by value 0.001, cut-off point of sufficient expression
levels.
Clinicopathological parameters of 91 patients are
shown in Table II. We found no
differences in age, gender, smoking status and comorbidities. Lymph
node metastasis tended to be higher in the T13 high group than in
T13 low group, but this association was not significant (P=0.062,
Wilcoxon-Mann-Whitney test).
 | Table IIClinicopathological parameters of
patients. |
Table II
Clinicopathological parameters of
patients.
| Characteristic | T13 high (N=16) no
(%) | T13 low (N=75) no
(%) | P-valuea |
|---|
| Age (years) | | | 0.831 |
| Median | 67 | 67 | |
| Range | 54–78 | 44–84 | |
| Gender |
| Female | 4 (25) | 17 (23) | 0.841 |
| Smoking status | | | 0.343 |
| Never smoked | 3 (19) | 15 (20) | |
| Former smoker | 9 (56) | 52 (69) | |
| Current
smoker | 4 (25) | 8 (11) | |
| Comorbidity |
| COPD | 5 (31) | 2 (37) | 0.648 |
| Diabetes
mellitus | 5 (31) | 12 (16) | 0.158 |
| Hypertention | 6 (38) | 29 (39) | 0.931 |
| Tumor histologic
type |
|
Adenocarcinoma | 10 (63) | 51 (68) | 1.000 |
|
Non-adenocaricinoma | 6 (37) | 24 (32) | |
| UICC-7 stage | | | 0.236 |
| I | 7 (44) | 45 (60) | |
| II/III | 9 (56) | 30 (40) | |
| pT category | | | 0.124 |
| 0–1 | 10 (63) | 31 (41) | |
| 2–4 | 6 (37) | 44 (59) | |
| pN category | | | 0.062 |
| 0 | 9 (56) | 59 (79) | |
| 1–2 | 7 (44) | 16 (21) | |
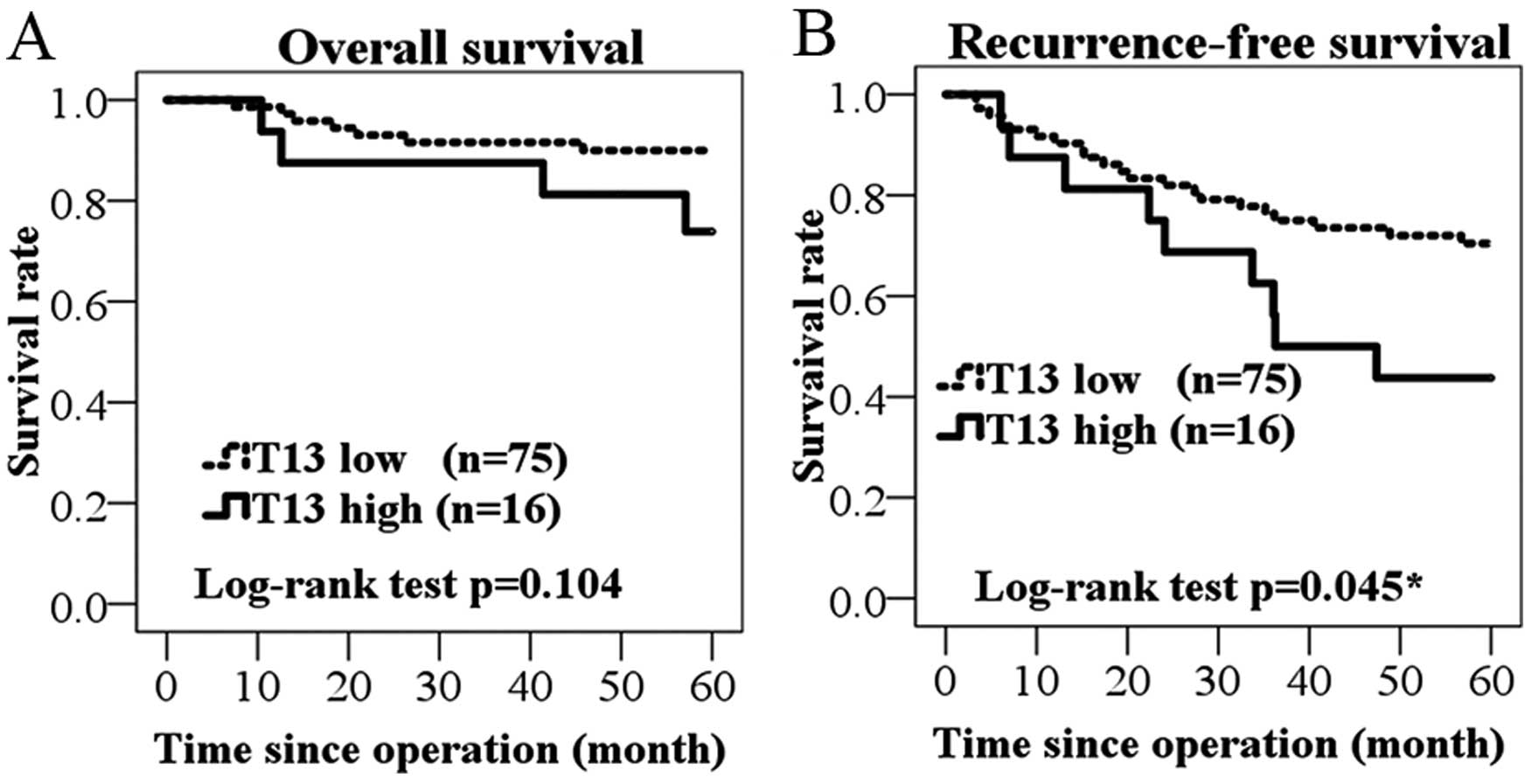
Among the 91 cases, patients with high expression
levels of GALNT13 mRNA exhibited reduced overall survival
(Fig. 2A). However, the difference
in survival rates was not significant (P=0.104; log-rank test). On
the other hand, T13 high group was significantly associated with
worse prognosis as observed in recurrence-free survival (P=0.045;
Fig. 2B).
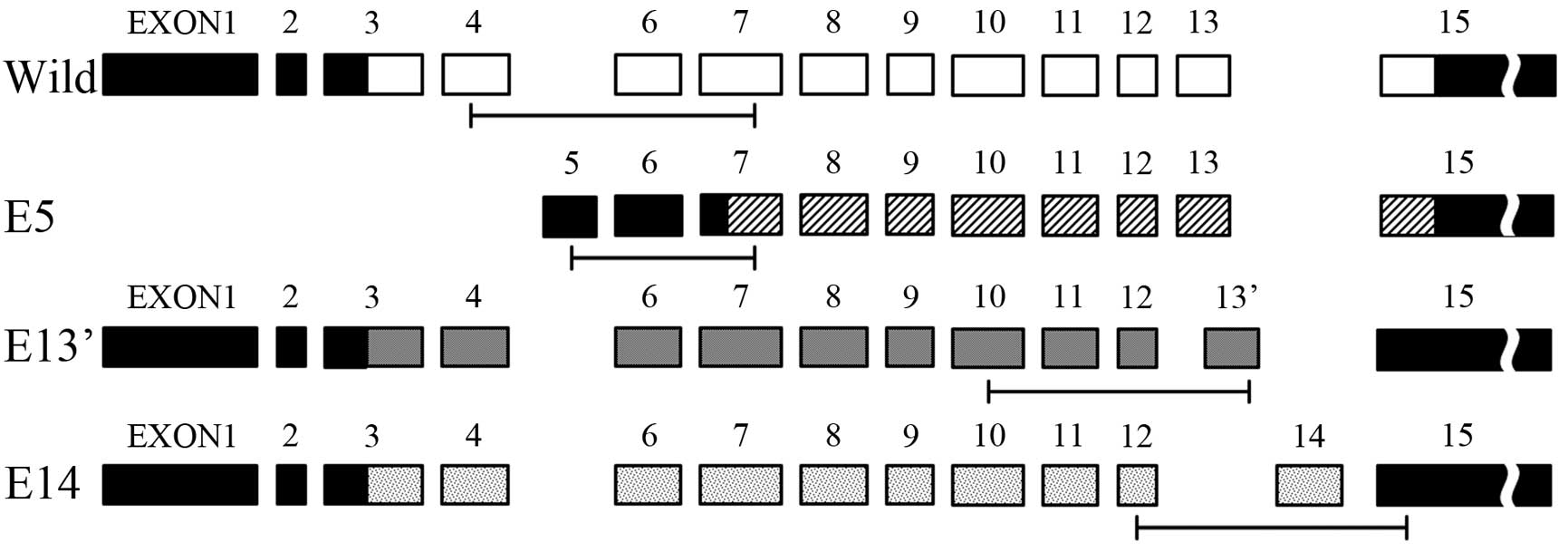
It was reported that there were differential usages
of exons in GALNT13 RNA sequence, therefore, we chose three
variant exons (named E5, E13′ E14) and analyzed their expression
levels. We obtained base sequence of GALNT13 mRNA and
variant exons from GenBank and Ensembl, and designed a specific PCR
primer of each. Their exon structures and PCR targets are shown in
Fig. 3.
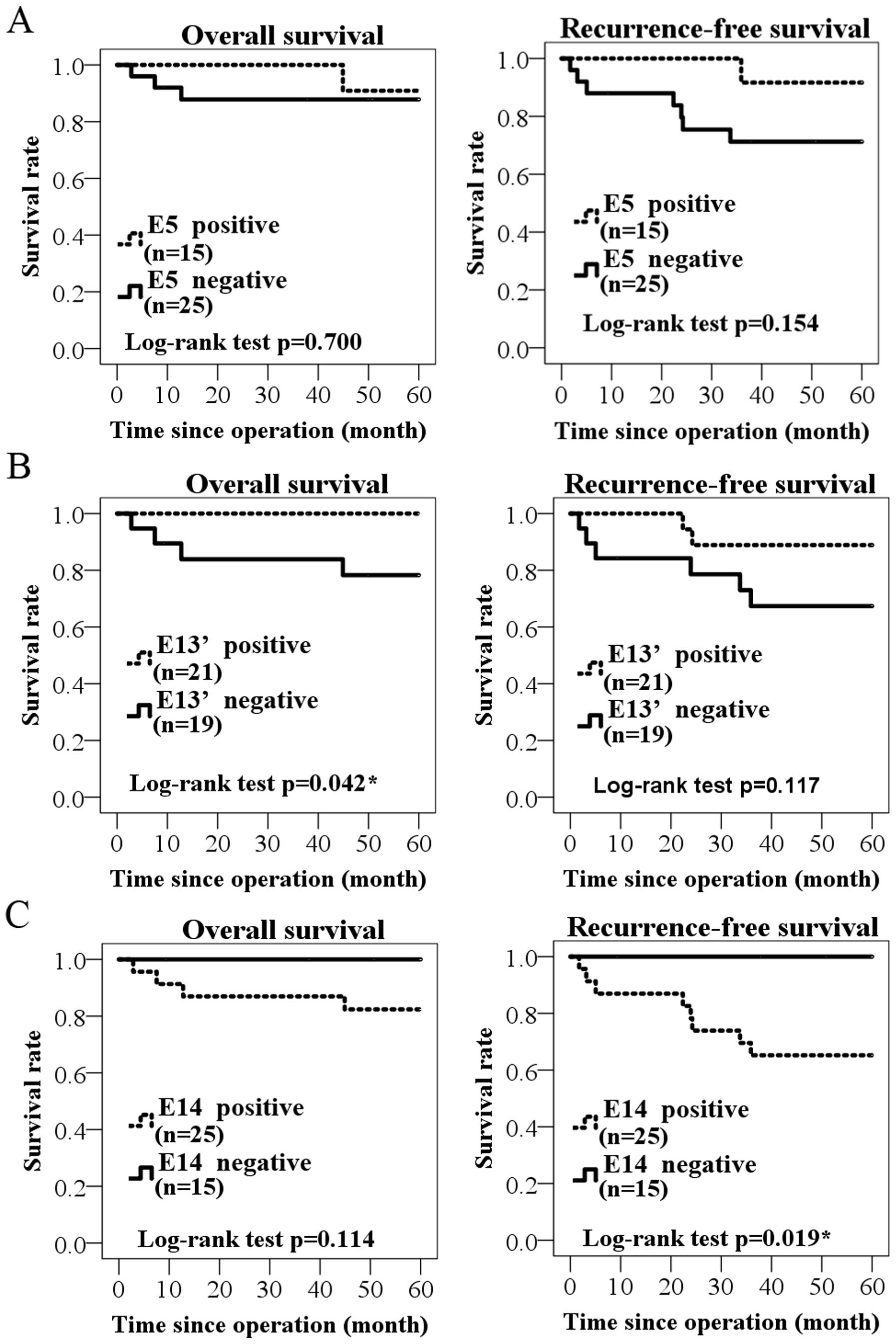
Then, the survival terms of E5, E13′ and
E14-positive cases were examined by using 40 surgical specimens
(Fig. 4). In E5, neither overall
survival nor recurrence-free survival was significantly correlated
between positive and negative groups (P=0.700, P=0.154, log-rank
test; Fig. 4A). In E13′-positive
group, significantly worse prognosis in overall survival, but not
in recurrence-free survival was found (P=0.042, P=0.117,
respectively; Fig. 4B). In turn,
E14-positive group showed rather better prognosis than negative
group, especially in the recurrence-free survival (P=0.019;
Fig. 4C).
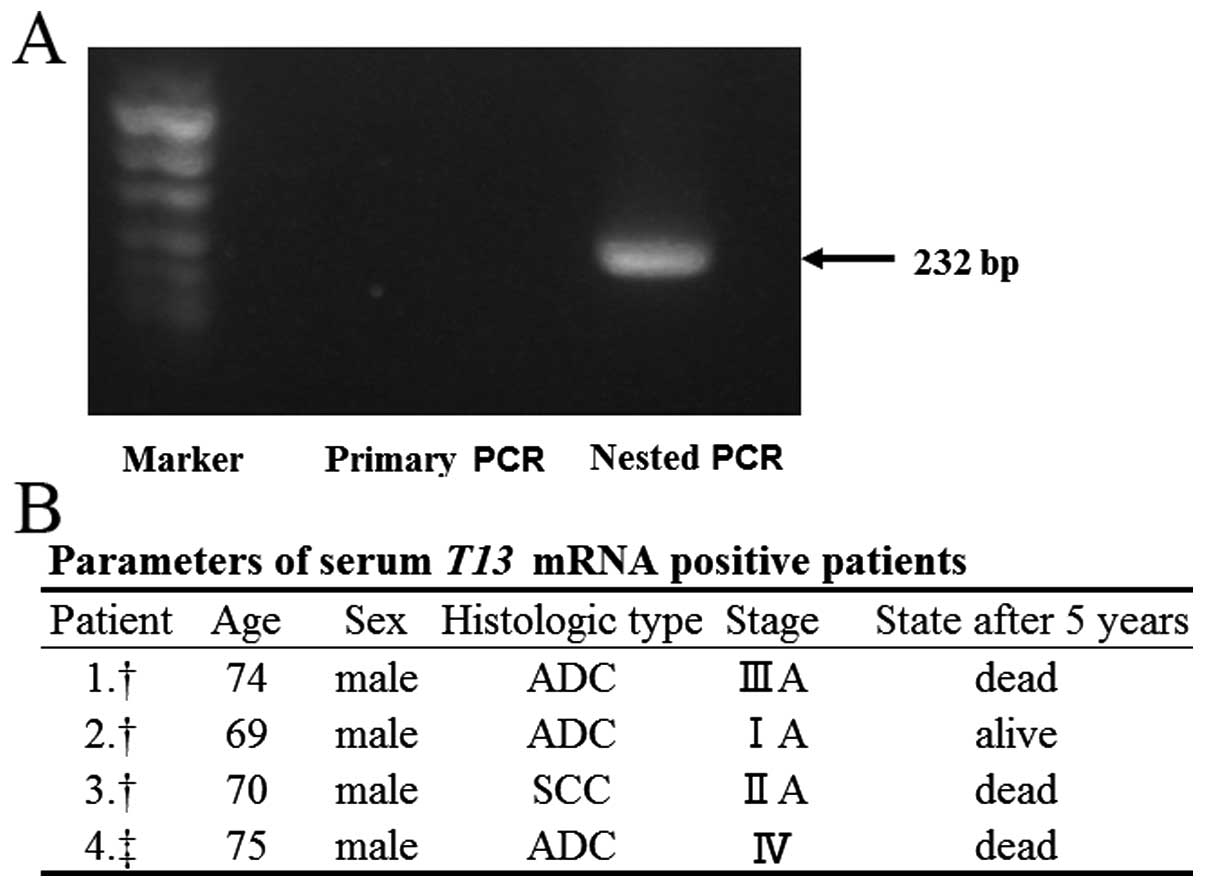
Detection of GALNT13 mRNA in sera
We examined whether T13 in sera can be used as a
tumor marker. Some frozen serum samples from surgical patients were
analyzed by PCR, but GALNT13 mRNA could not be detected by
current analytical condition. Since the expression levels in sera
were considered to be less than in tumor tissues, we tried nested
PCR, and were able to detect it as shown in Fig. 5A. Sixty-one frozen serum samples
from surgical patients and two fresh serum samples from stage IV
patients were examined. Among total of 63 serum samples examined by
nested PCR, GALNT13 mRNA could be detected in four of the
specimens. The parameters of these four patients are shown in
Fig. 5B.
Immunohistochemistry analysis revealed
that staining of T13 and trimeric Tn antigen significantly
correlated with worse prognosis
We evaluated the association between the prognosis
and expression of T13, GM1 and trimeric Tn antigen by
immunohistochemistry. In a mouse model, we revealed that reduced
expression level of GM1 resulted in the upregulation of
GALNT13 gene, and T13 formed trimeric Tn antigen on
Syndecan-1, leading to increased adhesion to extracellular matrix
(20). Therefore, we also examined
the correlation between T13, GM1 and trimeric Tn in lung cancer
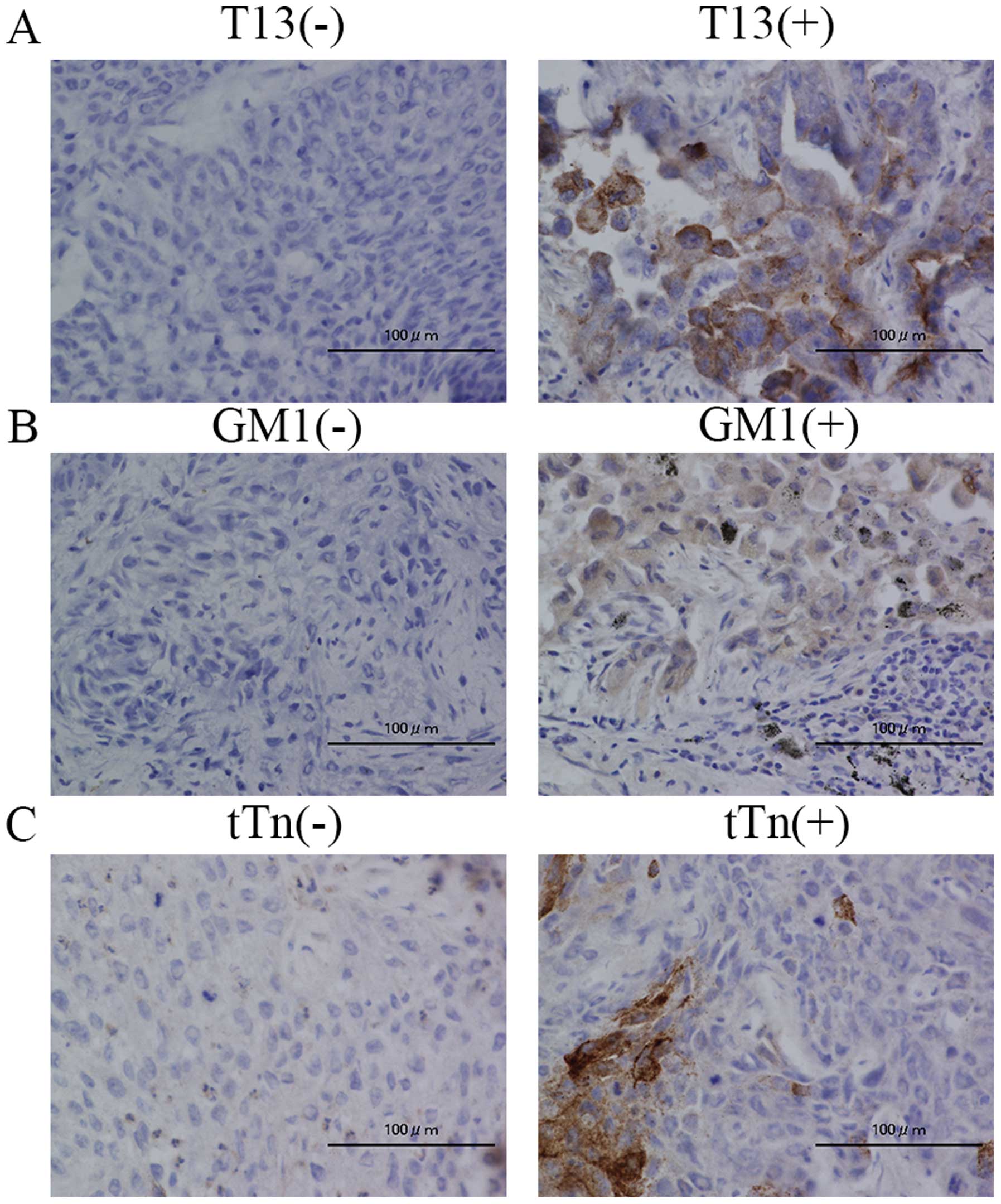
tissues. The tumor samples which showed positive staining in more
than 10% of tumor cells were defined as positive expression. Images
of the positive and negative staining of T13, GM1 and trimeric Tn
are shown in Fig. 6. We analyzed
35 samples (patients received operation in May, 2008-December,
2008) and evaluated the correlation between the staining and the
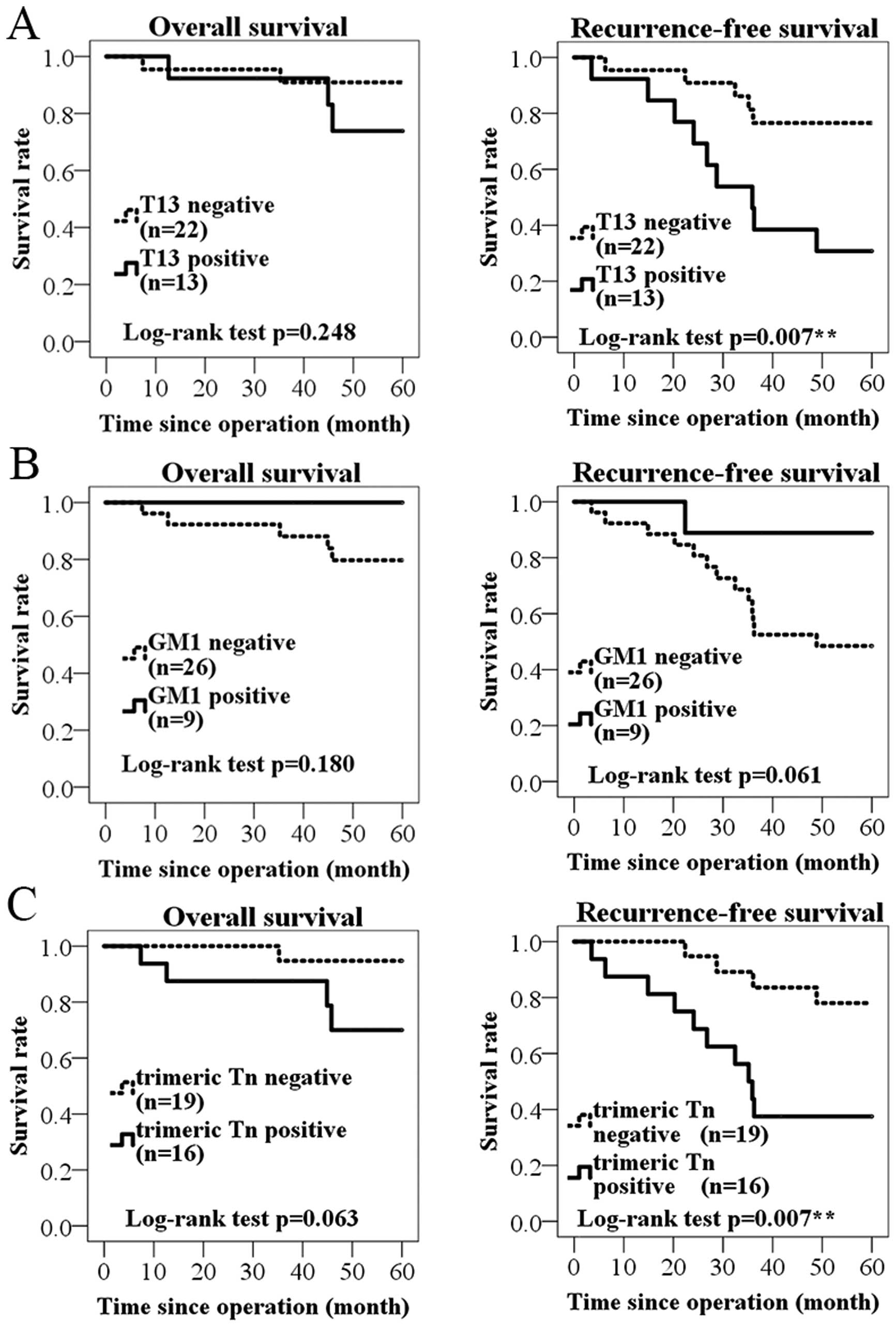
patients prognosis. We found that the T13 positive group tended to
show worse overall survival than T13-negative group without
significance (P=0.248, log-rank test; Fig. 7A). On the other hand, T13-positive
group had strongly significant association with shortened
recurrence-free survival (P=0.007). This result was similar to
results of GALNT13 mRNA expression analysis. As for GM1, GM1
positive group tended to show longer survival in overall and
recurrence-free survival, but it was not significant (P=0.180,
P=0.061; Fig. 7B). For trimeric Tn
antigen, the positive group showed a trend of short survival, and
there was a significant difference in the recurrence-free survival
(P=0.007; Fig. 7C). The
correlation between T13, GM1, and trimeric Tn expression is shown
in Table III. There was a
negative correlation between T13 and GM1 expression (P=0.007,
Fisher’s exact test). By contrast, positive correlation was
observed in T13 and trimeric Tn expression (P=0.006).
 | Table IIICorrelation between T13, GM1 and
trimeric Tn. |
Table III
Correlation between T13, GM1 and
trimeric Tn.
| T13 positive
(N=13) | T13 negative
(N=22) | P-valuea |
|---|
| GM1 | | | 0.007b |
| Positive | 0 | 9 | |
| Negative | 13 | 13 | |
| Trimeric Tn | | | 0.006b |
| Positive | 10 | 6 | |
| Negative | 3 | 16 | |
Discussion
In the present study, we demonstrated that T13
expression was significantly associated with the worse prognosis of
lung cancer patients. In pathological data, lymph node metastasis,
which was a prognostic factor in resected non-small cell lung
cancer (22), tended to be higher
in the GALNT13 mRNA high expression group. From these
results, it can be considered that T13 plays an important role in
the malignancy of cancer, particularly in metastasis process in
lung cancers. This speculation is supported by our previous study,
which showed that T13 induces high metastatic potential of murine
Lewis lung cancer. In the study, we also revealed that T13 was
upregulated as a result of reduced GM1, leading to enhanced
metastasis by formation of trimeric Tn antigen on Syndecan 1 in
mouse Lewis lung cancer (19,20).
In this study, we found the negative correlation of GM1 and T13,
and the positive correlation of trimeric Tn and T13 by
immunohistochemistry. These results corresponded with our previous
data in the experimental mouse metastasis model.
In a previous study, expression levels of
GALNT13 mRNA were analyzed in various adult and fetal human
tissues. The expression level was highest in the fetal brain,
followed by the adult brain. GALNT13 mRNA was expressed at
minimal or undetectable levels in the other tissues (18). It was also shown that T13 was able
to form trimeric Tn antigen, and significant decrease in Tn antigen
expression was found in the cerebellum of the T13 knockout mouse
(18). However, roles of T13 have
not been clarified. In the analysis of GALNT13 mRNA
expression levels among human tumor cell lines, we found that the
expression levels were higher in only lung cancer and neuroblastoma
lines (Fig. 1). Combined with the
fact that GALNT13 mRNA was scarcely expressed in normal lung
tissues, T13 may be involved in the mechanism for evolution or
malignant properties of lung cancers. We also demonstrated the
association between T13 and metastatic potential and/or poor
prognosis of lung cancer patients. Considering that T13 is mainly
expressed in fetal brain, T13 and its product trimeric Tn may be
involved in the cell growth or proliferation. In fact, it was
reported that MLS128 monoclonal antibody, which binds an epitope
consisting of three consecutive Tn-antigens, inhibited colon and
breast cancer cell growth (23–25).
We also analyzed several variant exon usages in
GALNT13 mRNA sequence, and found that one variant exon
expression had significant association with worse prognosis
(Fig. 4). By contrast, another
variant exon-positive group showed better prognosis than negative
group. This was an interesting and surprising result.
GALNT13 mRNA differential usages of exons have different
sequences of lectin like domain. Therefore, the reason for opposite
result among variant exon usages may be attributed to the
difference in the recognition of the substrate during the synthesis
of O-glycans (26). Thus,
tumor-specific and malignant property-associated variant exon usage
may be important as targets for molecular therapy of cancers,
although precise mechanisms remain to be investigated.
We demonstrated that T13 and trimeric Tn antigen had
a relationship with worse prognosis of lung cancer patients by
immunohistochemistry. This result also suggests that T13 and
trimeric Tn antigen can be used as a tumor marker. Although
GALNT13 mRNA could be detected in serum sample, the
expression levels were too low to be stably quantified. If the
carrier proteins of T13 or trimeric Tn are identified, we can more
easily detect them in serum by using ELISA. Thus, they can be
expected as a tumor marker, leading to early detection of lung
cancers or minimal metastatic diseases.
The limitation of the present study is partly due to
restricted selection of specimens. GALNT13 mRNA detection
and immunohistochemistry were conducted in primary tumors obtained
in surgery. Because we did not get metastatic tumors and lymph
nodes, we could not examine the difference in the expression levels
between primary and metastatic tumors. Despite these study
limitations, we demonstrated that higher expression of T13 in
primary tumors were associated with the poor prognosis.
Consequently, it can be an indicator for whether we should perform
postoperative therapy and careful observation. That is because
postoperative therapy largely affect patient survival, while a part
of patients can obtain benefit (27,28).
Therefore, by using specific antibodies, T13 and trimeric Tn
antigen might be expected as a new target of molecular
treatment.
In conclusion, the present study showed that high
expression levels of GALNT13 mRNA is associated with poor
prognosis of lung cancer patients by using quantitative real-time
RT-PCR. Furthermore, T13 and trimeric Tn antigen expression were
strongly correlated with shortened survival in immunostaining. Our
results suggest that T13 might be a useful prognostic factor and
might be a new target for cancer treatment.
Acknowledgements
We thank T. Mizuno, Y. Nakayasu and N. Hattori for
technical assistance and K. Ushida for technical teaching of
histochemical analyses. The present study was supported by
Grants-in-Aid of the Ministry of Education, Culture, Sports,
Science and Technology of Japan (MEXT) (15H04696, 15K15080,
25670141 and 24390078).
Abbreviations:
|
PCR
|
polymerase chain reaction
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
PBS
|
phosphate-buffered saline
|
|
BSA
|
bovine serum albumin
|
|
FBS
|
fetal bovine serum
|
|
T13
|
ppGalNAc-T13
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al: Spanish Lung Cancer Group: Screening for epidermal growth
factor receptor mutations in lung cancer. N Engl J Med.
361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Solomon B, Varella-Garcia M and Camidge
DR: ALK gene rearrangements: A new therapeutic target in a
molecularly defined subset of non-small cell lung cancer. J Thorac
Oncol. 4:1450–1454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hakomori S: Cancer-associated
glycosphingolipid antigens: Their structure, organization, and
function. Acta Anat (Basel). 161:79–90. 1998. View Article : Google Scholar
|
|
5
|
Kasahara K and Sanai Y: Possible roles of
glycosphingolipids in lipid rafts. Biophys Chem. 82:121–127. 1999.
View Article : Google Scholar
|
|
6
|
Fuster MM and Esko JD: The sweet and sour
of cancer: Glycans as novel therapeutic targets. Nat Rev Cancer.
5:526–542. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Song L and Qin X: Glycan changes:
Cancer metastasis and anti-cancer vaccines. J Biosci. 35:665–673.
2010. View Article : Google Scholar
|
|
8
|
Wiegandt H: Gangliosides. Glycolipids. 10.
Wiegandt H: Elsevier Science Publishers; Amsterdam: pp. 199–260.
1985, View Article : Google Scholar
|
|
9
|
Cheresh DA, Rosenberg J, Mujoo K,
Hirschowitz L and Reisfeld RA: Biosynthesis and expression of the
disialoganglioside GD2, a relevant target antigen on small cell
lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer
Res. 46:5112–5118. 1986.PubMed/NCBI
|
|
10
|
Yoshida S, Fukumoto S, Kawaguchi H, Sato
S, Ueda R and Furukawa K: Ganglioside GD2 in small cell
lung cancer cell lines: Enhancement of cell proliferation and
mediation of apoptosis. Cancer Res. 61:4244–4252. 2001.PubMed/NCBI
|
|
11
|
Yoshida S, Kawaguchi H, Sato S, Ueda R and
Furukawa K: An anti-GD2 monoclonal antibody enhances apoptotic
effects of anti-cancer drugs against small cell lung cancer cells
via JNK (c-Jun terminal kinase) activation. Jpn J Cancer Res.
93:816–824. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alfonso S, Valdés-Zayas A, Santiesteban
ER, Flores YI, Areces F, Hernández M, Viada CE, Mendoza IC, Guerra
PP, García E, et al: A randomized, multicenter, placebo-controlled
clinical trial of racotumomab-alum vaccine as switch maintenance
therapy in advanced non-small cell lung cancer patients. Clin
Cancer Res. 20:3660–3671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kannagi R: Molecular mechanism for
cancer-associated induction of sialyl Lewis X and sialyl Lewis A
expression-The Warburg effect revisited. Glycoconj J. 20:353–364.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brockhausen I: Mucin-type O-glycans in
human colon and breast cancer: Glycodynamics and functions. EMBO
Rep. 7:599–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Y, Stosiek P, Springer GF and Karsten
U: Thomsen-Friedenreich-related carbohydrate antigens in normal
adult human tissues: A systematic and comparative study. Histochem
Cell Biol. 106:197–207. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ju T, Otto VI and Cummings RD: The Tn
antigen-structural simplicity and biological complexity. Angew Chem
Int Ed Engl. 50:1770–1791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q and Furukawa K, Chen HH,
Sakakibara T, Urano T and Furukawa K: Metastatic potential of mouse
Lewis lung cancer cells is regulated via ganglioside GM1 by
modulating the matrix metalloprotease-9 localization in lipid
rafts. J Biol Chem. 281:18145–18155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Iwasaki H, Wang H, Kudo T, Kalka
TB, Hennet T, Kubota T, Cheng L, Inaba N, Gotoh M, et al: Cloning
and characterization of a new human
UDP-N-acetyl-α-D-galactosamine:polypeptide
N-acetylgalactosaminyltransferase, designated pp-GalNAc-T13, that
is specifically expressed in neurons and synthesizes GalNAc
α-serine/threonine antigen. J Biol Chem. 278:573–584. 2003.
View Article : Google Scholar
|
|
19
|
Matsumoto Y, Zhang Q, Akita K, Nakada H,
Hamamura K, Tokuda N, Tsuchida A, Matsubara T, Hori T, Okajima T,
et al: pp-GalNAc-T13 induces high metastatic potential of murine
Lewis lung cancer by generating trimeric Tn antigen. Biochem
Biophys Res Commun. 419:7–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto Y, Zhang Q, Akita K, Nakada H,
Hamamura K, Tsuchida A, Okajima T and Furukawa K, Urano T and
Furukawa K: Trimeric Tn antigen on syndecan 1 produced by
ppGalNAc-T13 enhances cancer metastasis via a complex formation
with integrin α5β1 and matrix metalloproteinase 9. J Biol Chem.
288:24264–24276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berois N, Blanc E, Ripoche H, Mergui X,
Trajtenberg F, Cantais S, Barrois M, Dessen P, Kågedal B, Bénard J,
et al: ppGalNAc-T13: A new molecular marker of bone marrow
involvement in neuroblastoma. Clin Chem. 52:1701–1712. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naruke T, Suemasu K and Ishikawa S: Lymph
node mapping and curability at various levels of metastasis in
resected lung cancer. J Thorac Cardiovasc Surg. 76:832–839.
1978.PubMed/NCBI
|
|
23
|
Morita N, Yajima Y, Asanuma H, Nakada H
and Fujita-Yamaguchi Y: Inhibition of cancer cell growth by anti-Tn
monoclonal antibody MLS128. Biosci Trends. 3:32–37. 2009.
|
|
24
|
Zamri N, Masuda N, Oura F, Yajima Y,
Nakada H and Fujita-Yamaguchi Y: Effects of two monoclonal
antibodies, MLS128 against Tn-antigen and 1H7 against insulin-like
growth factor-I receptor, on the growth of colon cancer cells.
Biosci Trends. 6:303–312. 2012.
|
|
25
|
Zamri N, Masuda N, Oura F, Kabayama K,
Yajima Y, Nakada H, Yamamoto K and Fujita-Yamaguchi Y:
Characterization of anti-Tn-antigen MLS128 binding proteins
involved in inhibiting the growth of human colorectal cancer cells.
Biosci Trends. 7:221–229. 2013.PubMed/NCBI
|
|
26
|
Gerken TA, Revoredo L, Thome JJ, Tabak LA,
Vester-Christensen MB, Clausen H, Gahlay GK, Jarvis DL, Johnson RW,
Moniz HA, et al: The lectin domain of the polypeptide GalNAc
transferase family of glycosyltransferases (ppGalNAc Ts) acts as a
switch directing glycopeptide substrate glycosylation in an N- or
C-terminal direction, further controlling mucin type
O-glycosylation. J Biol Chem. 288:19900–19914. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keller SM, Adak S, Wagner H, Herskovic A,
Komaki R, Brooks BJ, Perry MC, Livingston RB and Johnson DH:
Eastern Cooperative Oncology Group: A randomized trial of
postoperative adjuvant therapy in patients with completely resected
stage II or IIIA non-small-cell lung cancer. N Engl J Med.
343:1217–1222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Winton T, Livingston R, Johnson D, Rigas
J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E,
et al; National Cancer Institute of Canada Clinical Trials Group;
National Cancer Institute of the United States Intergroup JBR.10
Trial Investigators. Vinorelbine plus cisplatin vs observation in
resected non-small-cell lung cancer. N Engl J Med. 352:2589–2597.
2005. View Article : Google Scholar : PubMed/NCBI
|