Introduction
Magnolia bark is obtained from Magnolia
officinalis or other species of the Magnoliaceae, which
has long been used in traditional Chinese and Japanese medicines
for treatment of anxiety, depression and allergic disease (1). Growing experimental evidence suggests
that individual biologically active compounds isolated from
Magnolia bark, such as honokiol, magnolol and obovatol, have
anticancer effects against various cancer types in vitro and
in vivo (1–3). Most of their promising anticancer
effects are the induction of apoptosis through multiple signaling
(4–7), however, there is a relative lack of
information regarding their anti-metastatic activity, which is
considered responsible for >90% of cancer-related deaths
(8–11).
Renal cell carcinoma (RCC) is the most common
malignancy of the adult kidney and is known to have high risk of
metastasis. Clinically, therapeutic methods for metastatic RCC
cases are limited and efforts to exploit new treatments are still
ongoing (12). RhoA, one of the
most extensively characterized members of the Rho family small
GTPases, shuttles between inactive and active GTP-bound states
(13). In post-translational
level, the phosphorylation of RhoA at site Ser188 negatively
regulates its activity (14).
Activated RhoA interacts with its major downstream effector
Rho-associated protein kinase (ROCK) that induces the contraction
of actin fibers by directly phosphorylating the myosin light chain
(MLC) and indirectly inactivate MLC phosphatase (15). A previous study demonstrated that
excessive formation of actin stress fibers associated with
inhibited migration of RCC in vitro (16). In addition, Pu et al showed
the downregulated expression of RhoA in human conventional RCC
tissues in vivo (17),
indicating that RhoA/ROCK/MLC signaling pathway might be a suitable
target for the metastatic RCC treatment.
Our results indicated that honokiol suppressed
invasion and colony formation of RCC by targeting KISS1/KISS1R
signaling (18). In this study, we
first demonstrate that honokiol suppresses proliferation of human
RCC A-498 and 786-0 cells without affecting cell viability. In
addition, honokiol inhibits migration of highly metastatic RCC
786-0 (19,20) and stimulates RhoA activity.
Furthermore, phosphorylated MLC and excessive formation of actin
stress fibers were identified in 786-0 cells treated with honokiol.
Interestingly, the pharmacological ROCK inhibitor Y-27632
attenuated contraction of actin stress fibers induced by honokiol
and abrogated honokiol-mediated inhibition of cell migration.
Together these important findings suggest that honokiol suppresses
the migration of RCC through activation of RhoA/ROCK/MLC signaling
and warrants attention in the treatment of RCC metastasis as a
novel therapeutic approach.
Materials and methods
Cell culture and reagents
Human RCC 786-0 cells were obtained from ATCC
(Manassas, VA, USA) and maintained in RPMI-1640 medium containing
penicillin (50 U/ml), streptomycin (50 U/ml) and 10% FBS according
to the ATCC procedures. Media came from ATCC. Supplements and FBS
were obtained from Gibco (Grand Island, NY, USA). Honokiol 98%
(HonoPure®) was provided by EcoNugenics, Inc. (Santa
Rosa, CA, USA) and dissolved in DMSO at a concentration of 80 mM
then stored at −20°C. Rho-kinase inhibitor Y-27632 was purchased
from Calbiochem (Darmstadt, Germany). Rhodamine phalloidin was
purchased from Molecular Probes (Grand Island, NY, USA).
Methanol-free formaldehyde solution 16% was purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). DMSO and other reagents
were purchased from Sigma (St. Louis, MO, USA). Anti-RhoA,
anti-phospho-RhoA and anti-β-actin antibodies were obtained from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-MLC2 and
anti-phospho-MLC2 antibodies were obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA).
Cell proliferation and viability
assays
Human RCC A-498 and 786-0 cells were treated with
indicated concentrations of honokiol for 24 h and cell
proliferation was determined as described (21). Cell viability was determined after
incubation with honokiol for 24 h by staining with trypan blue as
described (22). Data are the mean
± SD from three independent experiments.
Cell migration assay
Cell migration of 786-0 cells treated with honokiol
(0–40 μM) or honokiol (20 μM) + Y-27632 (10 μM) or Y-27632 (10 μM)
was performed in Transwell chambers according to established method
(23). Briefly, 786-0 cells
(0.2×106) suspended in serum-free medium were added to
the upper chamber of an insert, and the insert was placed in a
24-well plate containing medium with 10% FBS. Migration assays were
carried out for 3 h. Data points represent the mean ± SD of three
individual filters within one representative experiment repeated at
least twice.
Rho activation assay
The Rho Activation Assay Kit (EMD Millipore,
Billerica, MA, USA) was used to determine whether honokiol could
modulate RhoA activity in 786-0 cells according to the
manufacturer’s instructions. In brief, cells were exposed to
vehicle or honokiol (20 μM) for 30 min, rinsed in ice-cold TBS and
lysed in the lysis buffer provided. For Rho pull-down assay, cell
lysates were incubated with glutathione-agarose beads bounding to a
GST-tagged Rho binding domain of Rhotekin. The precipitated
GTP-bound forms of proteins were analyzed by western blot analysis
with antibody specific for RhoA. Activated RhoA was normalized to
the total RhoA. 786-0 cell extract loaded with GTPγS was used as a
positive control.
Western blot analysis
786-0 cells were treated with vehicle or honokiol
(20 μM) for 30, 60 or 120 min, respectively. Protein extracts
isolated from cells were prepared and western blot analysis with
anti-phospho-RhoA, anti-RhoA, anti-phospho-MLC2, anti-MLC2 or
anti-β-actin antibodies was performed as previously described
(21). Western blots were
quantified with HP-Scanjet 550c and analyzed by UN-SCAN-IT software
(Silk Scientific, Inc., Orem, UT, USA).
Visualization of actin stress fibers
786-0 cells (6.0×104) were plated on the
Millicell EZ Slide 4-well glass slide (EMD Millipore, Darmstadt,
Germany) and incubated for 24 h. After exposure to honokiol (20 μM)
or honokiol (20 μM) + Y-27632 (10 μM) or Y-27632 (10 μM) for 1 h,
cells were washed in PBS and fixed with 4% formaldehyde for 15 min.
The cells were then permeabilized with PBS containing 0.1% Triton
X-100 for 5 min and stained with rhodamine phalloidin for 20 min.
Nucleus was stained with DAPI for 2 min and further washed with
PBS. Detection of actin stress fibers was achieved using an
inverted microscope (Leica DMR type 020-525-024 fluorescence
microscope; Leica Microsystems GmbH, Wetzlar, Germany) and a
confocal microscope (Bio-Rad Radiance 2100 laser scanning system;
Bio-Rad, Hercules, CA, USA).
Statistical analysis
All statistical analysis was performed using
SigmaPlot 11.2.0 (Systat Software, Inc., San Jose, CA, USA). Data
are presented as the mean ± SD. Statistical comparisons were
carried out using ANOVA with the significance level adjusted using
the repeated t-tests with Bonferroni correction. P<0.05 was
considered to be significant.
Results
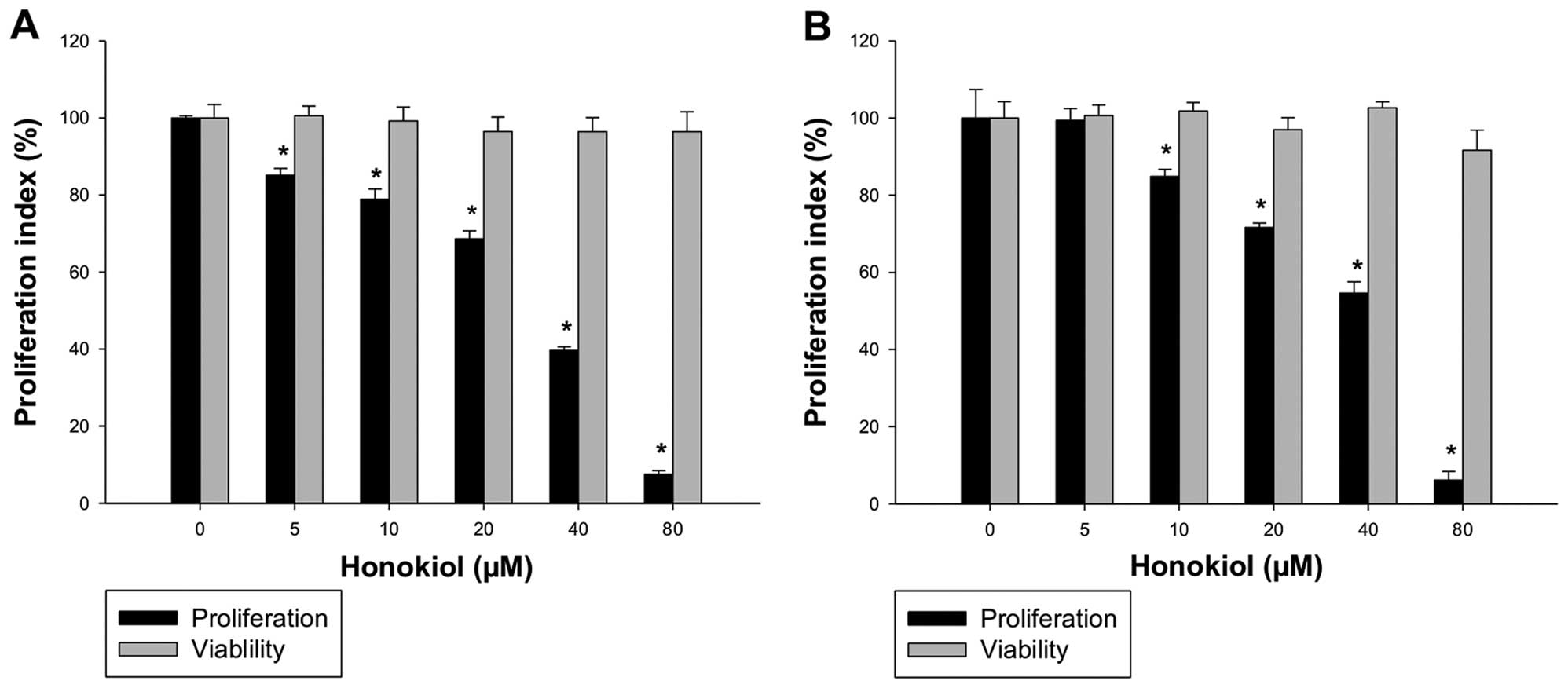
Effect of honokiol on the proliferation
and viability of RCC
As honokiol exhibits anticancer effects in different
cancer types (6,24–28),
its effect on the growth of human RCC was evaluated in this study.
A-498 and 786-0 cells were treated with honokiol (0–80 μM) for 24 h
and proliferation was determined as described in ‘Materials and
methods’. We found that honokiol suppressed the proliferation of
A-498 (IC50, 37.17 μM) and 786-0 cells (IC50,
51.28 μM) dose-dependently (Fig.
1). Moreover, honokiol does not affect the viability of A-498
and 786-0 cells after treatment of 24 h (Fig. 1), suggesting cytostatic effect of
honokiol on human RCC.
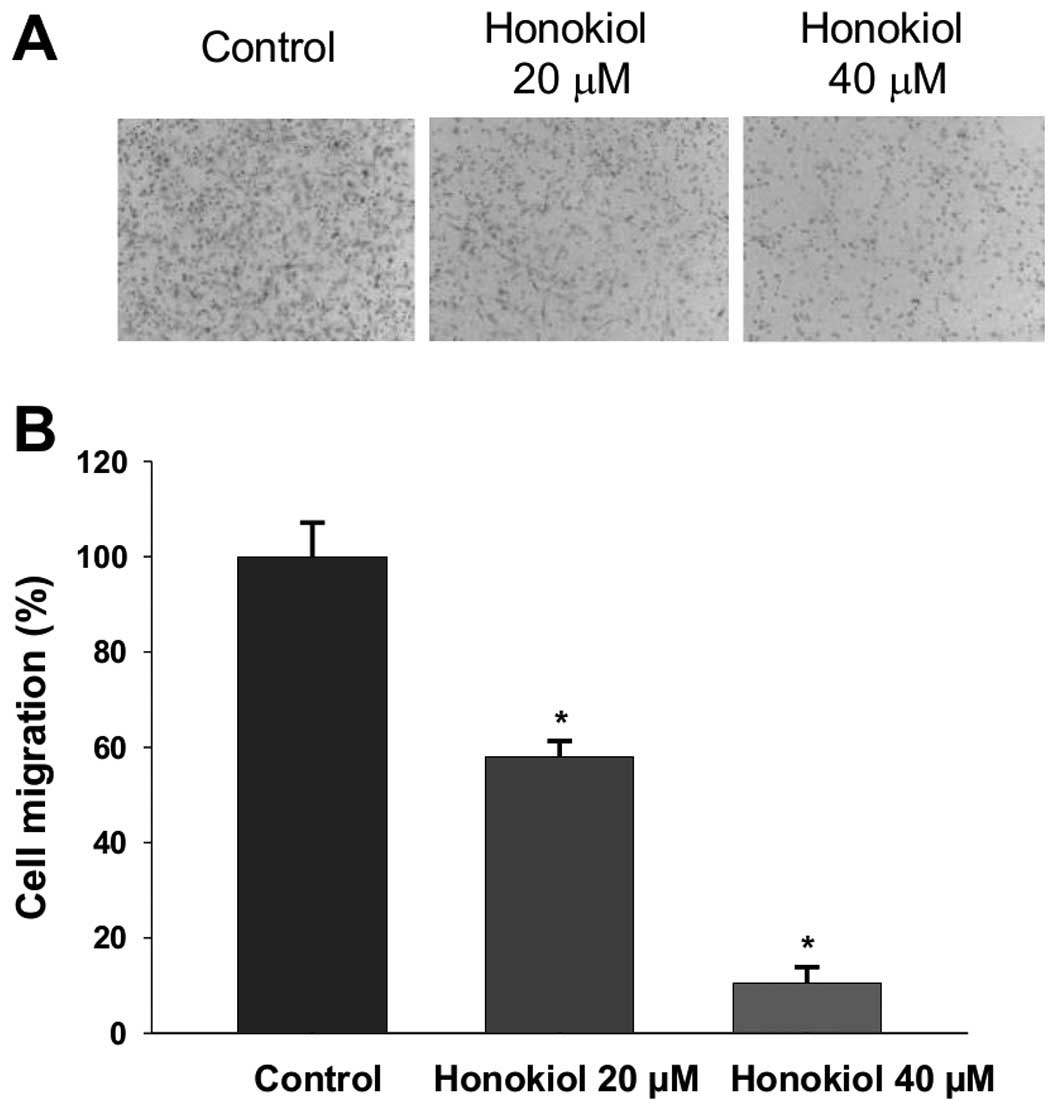
Honokiol inhibits migration of 786-0
cells
Comparing with A-498, the 786-0 cells exhibit higher
expression of LIM and SH3 protein 1 (LASP-1), which correlated with
aggressive phenotype and poor prognosis in RCC (20). Thus, the more aggressive cell line
786-0 was selected to investigate whether honokiol inhibits cell
migration under the incubation time (3 h) and concentrations (0–40
μM) that do not affect viability of 786-0 cells. As shown in
Fig. 2, honokiol significantly
inhibits cell migration in a dose-dependent manner. Taken together,
our data indicate that honokiol not only inhibits proliferation of
human RCC but also suppresses migration, an initial important step
in cancer metastasis (29).
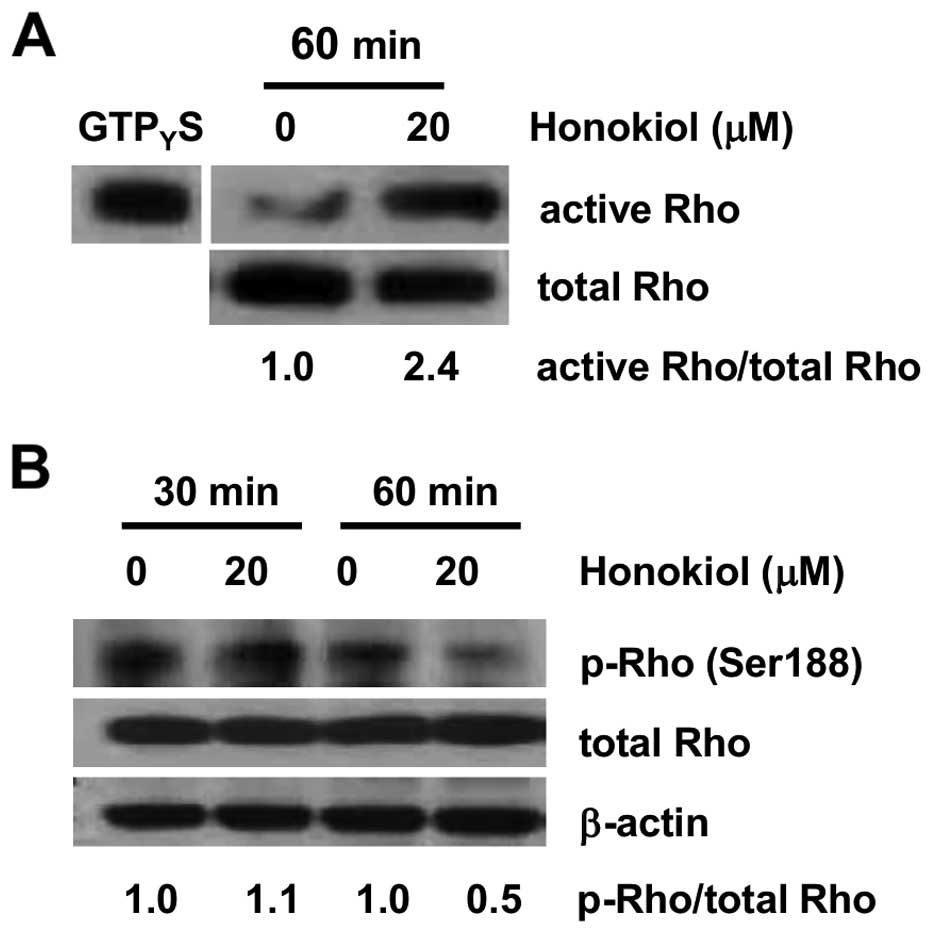
Honokiol-mediated activation of
RhoA/ROCK/MLC signaling in 786-0 cells
To investigate the effect of honokiol and its
possible mechanisms of action with regard to human RCC, we focused
on the Rho GTPases, which play key roles in coordinating the
cellular responses required for cell migration (30). Rho pull-down assay showed that
exposure of 786-0 cells to honokiol (20 μM) resulted in a strong
activation of RhoA (Fig. 3A).
GTPγS, a hydrolysis-resistant GTP analog, was used as positive
control (Fig. 3A). Because
activity of RhoA can also be negatively regulated by its
phosphorylation at Ser188 (14),
the phosphorylation status of RhoA with honokiol treatment was
determined in our study. Honokiol (20 μM) suppressed the
phosphorylation level of RhoA after 60 min without changing the
level of total RhoA (Fig. 3B).
Moreover, GTPase activation coincided with phosphorylation of MLC2,
detected by western blot analysis with antibodies specific for
phosphorylated Thr18 and Ser19 (Fig.
4). Thus, we considered that RhoA/ROCK/MLC signaling was
activated by honokiol in 786-0 cells.
ROCK inhibitor attenuates contraction of
actin stress fibers induced by honokiol
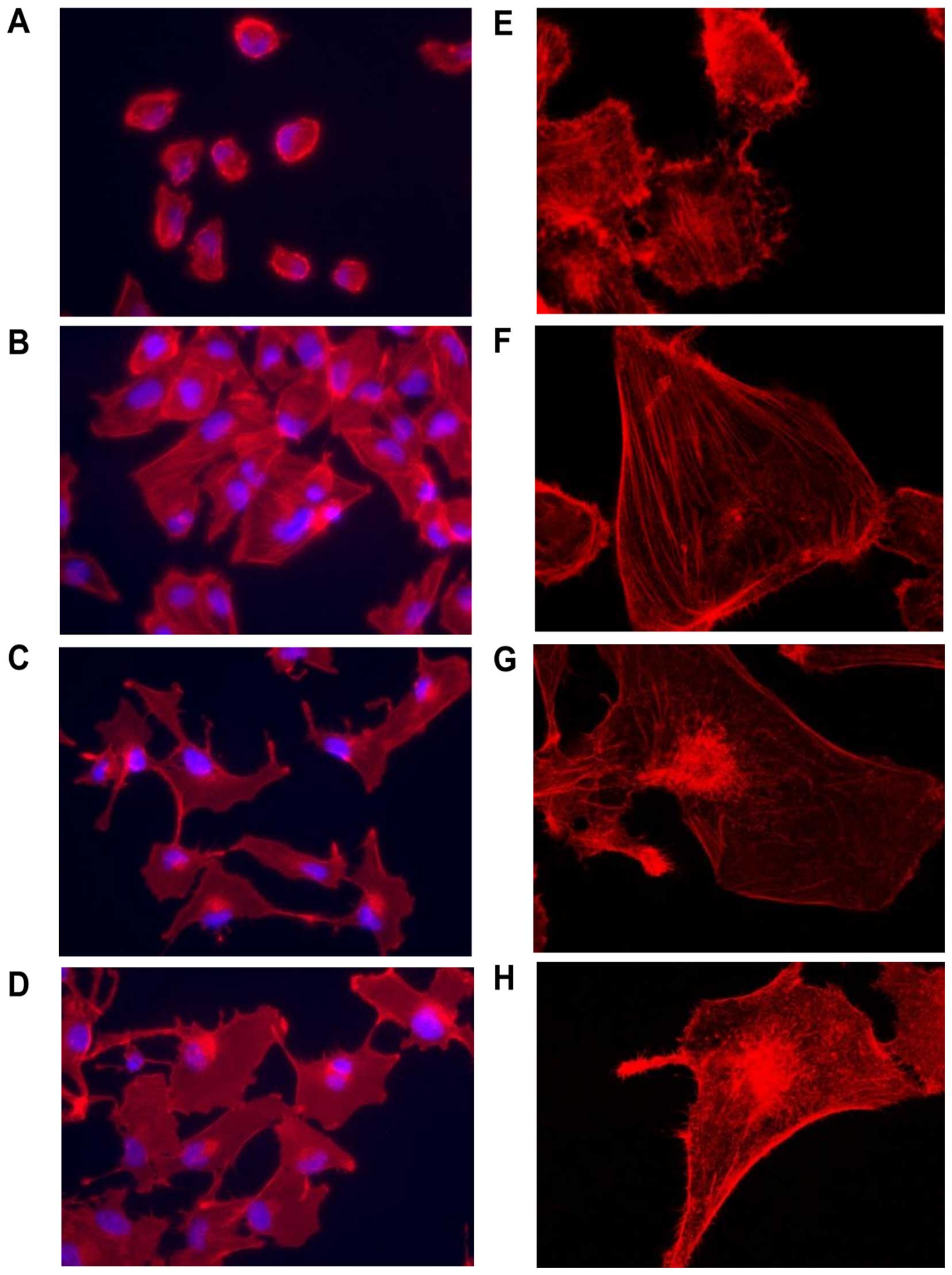
Stress fibers, which look like bundles of actin
filaments, are an actin-myosin-based contractile system regulated
by the RhoA/ROCK/MLC signaling (31). Excessive formation of actin stress
fibers (rhodamine phalloidin-positive staining of actin fibers) was
identified in 786-0 cells treated with honokiol (20 μM) compared
with vehicle control (Fig. 5A, B, E
and F). Interestingly, this phenomenon disappeared when cells
were treated with the ROCK inhibitor Y-27632 (10 μM) and honokiol
(Fig. 5C and G). This inhibition
can also be identified in 786-0 cells treated with Y-27632 only
(Fig. 5D and H).
ROCK inhibitor abrogates
honokiol-mediated inhibition of cell migration
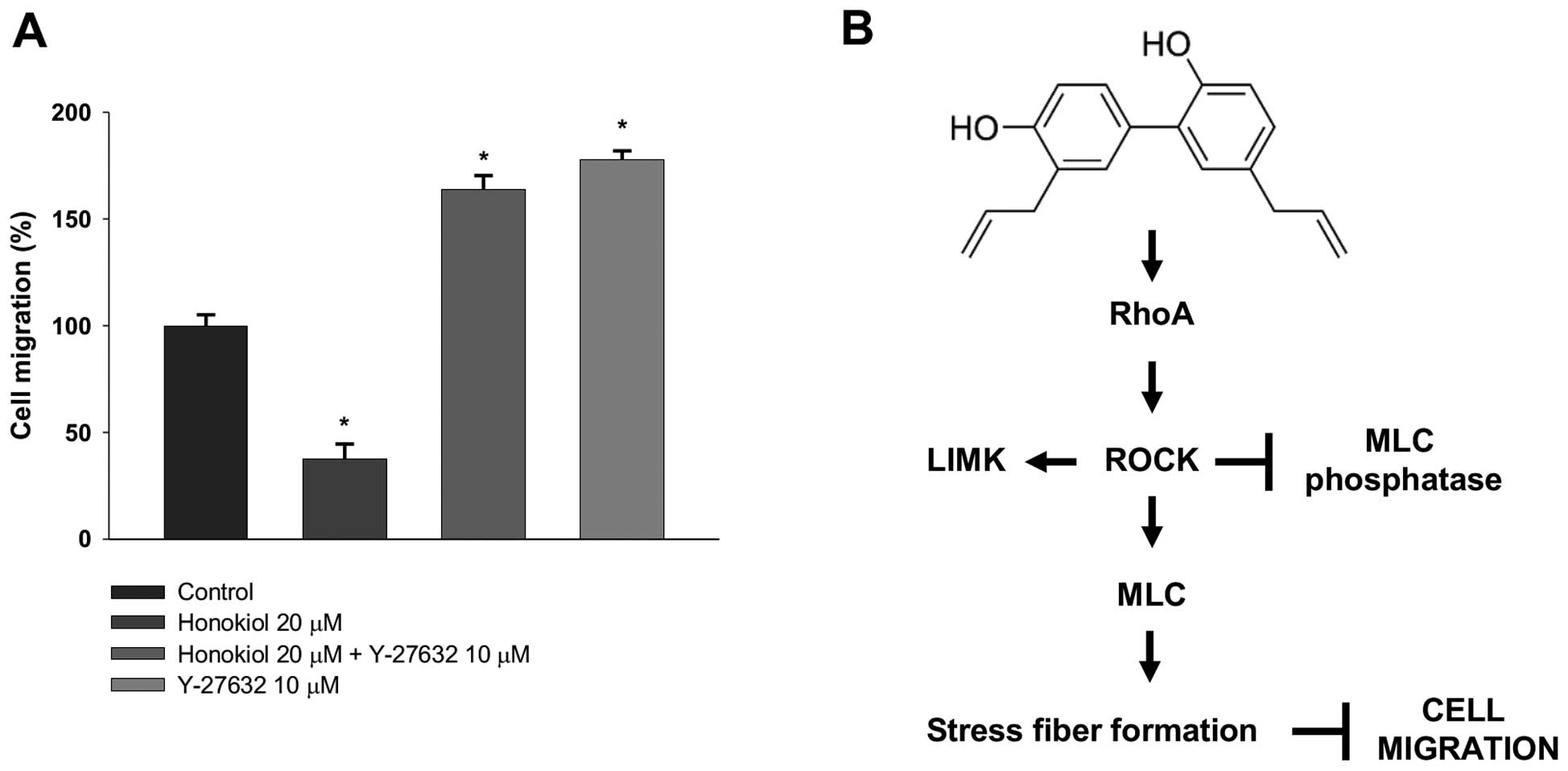
To determine whether the inhibition of cell
migration by honokiol is mediated by the activation of
RhoA/ROCK/MLC signaling in 786-0 cells, we pre-treated 786-0 cells
with Y-27632 for 60 min and then determined cell migration with
honokiol as described in ‘Materials and methods’. In accordance
with the change in actin stress fibers, the effect of honokiol on
migration of 786-0 cells was significantly abrogated by Y-27632
(Fig. 6A). These results further
proved our hypothesis that Honokiol-induced RhoA/ROCK/MLC
activation plays an integral role in honokiol-mediated inhibition
of migration potential in RCC (Fig.
6B).
Discussion
Cell migration is a key component of the tumor
metastatic process (32). Based on
a number of studies, upregulated RhoA is associated with tumor
progression in different types of cancer (33–35)
and RhoA activation promotes the migration of cervical, colon and
hepatocellular carcinoma (36–38).
However, significantly downregulated expression of RhoA was
demonstrated in human conventional RCC compared to that in normal
kidney tissues (17) and activated
RhoA has specifically been shown to inhibit the migration of breast
and prostate cancer (39,40). Here, we indicated that activation
of RhoA/ROCK/MLC signaling by honokiol suppresses the migration of
RCC (Fig. 6B). These conflicting
results reflected that the effects of altered expression of RhoA
involved in cell migration were often cell type-specific (17).
Members of Rho-family GTPases, RhoA, Rac and Cdc42,
control cell migration by regulating the organization of actin
cytoskeleton (41). Another
striking finding presented in this study is that pharmacological
ROCK inhibitor Y-27632 not only rescued the effect of honokiol on
migration of 786-0 cells but also tended to enhance migration.
Therefore, the RhoA/ROCK/MLC signaling pathway negative regulates
the migration of 786-0 cells, which is in accordance with our
hypothesis. As ROCK-related signaling antagonizes the activity of
Rac in osteoblasts, fibroblasts and rat basophilic leukemia cells
(42–44), the involved mechanism might be
through activation of Rac. Activated Rac induces the formation of
actin-based sheet-like membrane projections from the cell periphery
named lamellipodia (45), which
plays a key role in the stimulation of cell migration (46). In accordance with this concept,
Y-27632 increases lamellipodia formation in 786-0 cells (Fig. 5C and D) and further investigation
is necessary to confirm the Rac stimulation.
In conclusion, this study demonstrated a novel
mechanism by which honokiol inhibits migration of highly metastatic
RCC, involving the activation of RhoA/ROCK/MLC signaling in
vitro. Therefore, honokiol is a biologically active component
with potential utility as an effective anti-migration agent in
treating metastatic RCC.
Acknowledgements
We thank Mr. Kevin A. Harvey (Indiana University
Health), for his technical assistance with the confocal microscope.
EcoNugenics, Inc., supported this study. One of the authors, Dr I.
Eliaz, acknowledges his interest as the formulator and owner of
EcoNugenics, Inc.
References
|
1
|
Lee YJ, Lee YM, Lee CK, Jung JK, Han SB
and Hong JT: Therapeutic applications of compounds in the Magnolia
family. Pharmacol Ther. 130:157–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaushik G, Kwatra D, Subramaniam D, Jensen
RA, Anant S and Mammen JM: Honokiol affects melanoma cell growth by
targeting the AMP-activated protein kinase signaling pathway. Am J
Surg. 208:995–1002; discussion 1001–1002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McKeown BT and Hurta RA: Magnolol affects
expression of IGF-1 and associated binding proteins in human
prostate cancer cells in vitro. Anticancer Res. 34:6333–6338.
2014.PubMed/NCBI
|
|
4
|
Zhou Y, Bi Y, Yang C, Yang J, Jiang Y,
Meng F, Yu B, Khan M, Ma T and Yang H: Magnolol induces apoptosis
in MCF-7 human breast cancer cells through G2/M phase arrest and
caspase-independent pathway. Pharmazie. 68:755–762. 2013.PubMed/NCBI
|
|
5
|
Tsai JR, Chong IW, Chen YH, Hwang JJ, Yin
WH, Chen HL, Chou SH, Chiu CC and Liu PL: Magnolol induces
apoptosis via caspase-independent pathways in non-small cell lung
cancer cells. Arch Pharm Res. 37:548–557. 2014. View Article : Google Scholar
|
|
6
|
Lai YJ, Lin CI, Wang CL and Chao JI:
Expression of survivin and p53 modulates honokiol-induced apoptosis
in colorectal cancer cells. J Cell Biochem. 115:1888–1899.
2014.PubMed/NCBI
|
|
7
|
Liang WZ, Chou CT, Chang HT, Cheng JS, Kuo
DH, Ko KC, Chiang NN, Wu RF, Shieh P and Jan CR: The mechanism of
honokiol-induced intracellular Ca(2+) rises and apoptosis in human
glioblastoma cells. Chem Biol Interact. 221:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen DX and Massagué J: Genetic
determinants of cancer metastasis. Nat Rev Genet. 8:341–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monteiro J and Fodde R: Cancer stemness
and metastasis: Therapeutic consequences and perspectives. Eur J
Cancer. 46:1198–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deep G and Agarwal R: Antimetastatic
efficacy of silibinin: Molecular mechanisms and therapeutic
potential against cancer. Cancer Metastasis Rev. 29:447–463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ljungberg B, Hanbury DC, Kuczyk MA,
Merseburger AS, Mulders PF, Patard JJ and Sinescu IC; European
Association of Urology Guideline Group for renal cell carcinoma.
Renal cell carcinoma guideline. Eur Urol. 51:1502–1510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ellerbroek SM, Wennerberg K and Burridge
K: Serine phosphorylation negatively regulates RhoA in vivo. J Biol
Chem. 278:19023–19031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pellegrin S and Mellor H: Actin stress
fibres. J Cell Sci. 120:3491–3499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shoji S, Tang XY, Umemura S, Itoh J,
Takekoshi S, Shima M, Usui Y, Nagata Y, Uchida T, Osamura RY, et
al: Metastin inhibits migration and invasion of renal cell
carcinoma with overexpression of metastin receptor. Eur Urol.
55:441–449. 2009. View Article : Google Scholar
|
|
17
|
Pu YS, Wang CW, Liu GY, Kuo YZ, Huang CY,
Kang WY, Shun CT, Lin CC, Wu WJ and Hour TC: Down-regulated
expression of RhoA in human conventional renal cell carcinoma.
Anticancer Res. 28(4B): 2039–2043. 2008.PubMed/NCBI
|
|
18
|
Cheng S, Castillo V, Eliaz I and Sliva D:
Honokiol suppresses metastasis of renal cell carcinoma by targeting
KISS1/KISS1R signaling. Int J Oncol. 46:2293–2298. 2015.PubMed/NCBI
|
|
19
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Modulation of human renal cell carcinoma
786-0 MMP-2 and MMP-9 activity by inhibitors and inducers in vitro.
Med Oncol. 23:245–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang F, Zhou X, Du S, Zhao Y, Ren W, Deng
Q, Wang F and Yuan J: LIM and SH3 domain protein 1 (LASP-1)
overexpression was associated with aggressive phenotype and poor
prognosis in clear cell renal cell cancer. PLoS One. 9:e1005572014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang J, Slivova V, Harvey K,
Valachovicova T and Sliva D: Ganoderma lucidum suppresses growth of
breast cancer cells through the inhibition of Akt/NF-kappaB
signaling. Nutr Cancer. 49:209–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sliva D, Jedinak A, Kawasaki J, Harvey K
and Slivova V: Phellinus linteus suppresses growth, angiogenesis
and invasive behaviour of breast cancer cells through the
inhibition of AKT signalling. Br J Cancer. 98:1348–1356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lloyd FP Jr, Slivova V, Valachovicova T
and Sliva D: Aspirin inhibits highly invasive prostate cancer
cells. Int J Oncol. 23:1277–1283. 2003.PubMed/NCBI
|
|
24
|
Kim DW, Ko SM, Jeon YJ, Noh YW, Choi NJ,
Cho SD, Moon HS, Cho YS, Shin JC, Park SM, et al:
Anti-proliferative effect of honokiol in oral squamous cancer
through the regulation of specificity protein 1. Int J Oncol.
43:1103–1110. 2013.PubMed/NCBI
|
|
25
|
Joo YN, Eun SY, Park SW, Lee JH, Chang KC
and Kim HJ: Honokiol inhibits U87MG human glioblastoma cell
invasion through endothelial cells by regulating membrane
permeability and the epithelial-mesenchymal transition. Int J
Oncol. 44:187–194. 2014.
|
|
26
|
Tian W, Deng Y, Li L, He H, Sun J and Xu
D: Honokiol synergizes chemotherapy drugs in multidrug resistant
breast cancer cells via enhanced apoptosis and additional
programmed necrotic death. Int J Oncol. 42:721–732. 2013.
|
|
27
|
Hahm ER, Sakao K and Singh SV: Honokiol
activates reactive oxygen species-mediated cytoprotective autophagy
in human prostate cancer cells. Prostate. 74:1209–1221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chilampalli C, Zhang X, Kaushik RS, Young
A, Zeman D, Hildreth MB, Fahmy H and Dwivedi C: Chemopreventive
effects of combination of honokiol and magnolol with α-santalol on
skin cancer developments. Drug Discov Ther. 7:109–115.
2013.PubMed/NCBI
|
|
29
|
Kawauchi T: Cell adhesion and its
endocytic regulation in cell migration during neural development
and cancer metastasis. Int J Mol Sci. 13:4564–4590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
31
|
Katoh K, Kano Y, Amano M, Kaibuchi K and
Fujiwara K: Stress fiber organization regulated by MLCK and
Rho-kinase in cultured human fibroblasts. Am J Physiol Cell
Physiol. 280:C1669–C1679. 2001.PubMed/NCBI
|
|
32
|
Navenot JM, Fujii N and Peiper SC:
Activation of Rho and Rho-associated kinase by GPR54 and KiSS1
metastasis suppressor gene product induces changes of cell
morphology and contributes to apoptosis. Mol Pharmacol.
75:1300–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen S, Wang J, Gou WF, Xiu YL, Zheng HC,
Zong ZH, Takano Y and Zhao Y: The involvement of RhoA and Wnt-5a in
the tumorigenesis and progression of ovarian epithelial carcinoma.
Int J Mol Sci. 14:24187–24199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horiuchi A, Imai T, Wang C, Ohira S, Feng
Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA
and RhoC, is associated with tumor progression in ovarian
carcinoma. Lab Invest. 83:861–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
Cdc42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Chen D and Liu G: Overexpression of
RhoA promotes the proliferation and migration of cervical cancer
cells. Biosci Biotechnol Biochem. 78:1895–1901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Patel M, Kawano T, Suzuki N, Hamakubo T,
Karginov AV and Kozasa T: Gα13/PDZ-RhoGEF/RhoA signaling is
essential for gastrin-releasing peptide receptor-mediated colon
cancer cell migration. Mol Pharmacol. 86:252–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin L, Yang XM, Li J, Zhang YL, Qin W and
Zhang ZG: Microfilament regulatory protein MENA increases activity
of RhoA and promotes metastasis of hepatocellular carcinoma. Exp
Cell Res. 327:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takeda S, Okajima S, Miyoshi H, Yoshida K,
Okamoto Y, Okada T, Amamoto T, Watanabe K, Omiecinski CJ and
Aramaki H: Cannabidiolic acid, a major cannabinoid in fiber-type
cannabis, is an inhibitor of MDA-MB-231 breast cancer cell
migration. Toxicol Lett. 214:314–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen X, Corbin JM, Tipton GJ, Yang LV,
Asch AS and Ruiz-Echevarría MJ: The TMEFF2 tumor suppressor
modulates integrin expression, RhoA activation and migration of
prostate cancer cells. Biochim Biophys Acta. 1843:1216–1224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Li C, Gao H, Nabeka H, Shimokawa
T, Wakisaka H, Matsuda S and Kobayashi N: Rho kinase inhibitors
stimulate the migration of human cultured osteoblastic cells by
regulating actomyosin activity. Cell Mol Biol Lett. 16:279–295.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsuji T, Ishizaki T, Okamoto M, Higashida
C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai
H, et al: ROCK and mDia1 antagonize in Rho-dependent Rac activation
in Swiss 3T3 fibroblasts. J Cell Biol. 157:819–830. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Toda M, Dawson M, Nakamura T, Munro PM,
Richardson RM, Bailly M and Ono SJ: Impact of engagement of
FcepsilonRI and CC chemokine receptor 1 on mast cell activation and
motility. J Biol Chem. 279:48443–48448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aspenström P, Fransson A and Saras J: Rho
GTPases have diverse effects on the organization of the actin
filament system. Biochem J. 377:327–337. 2004. View Article : Google Scholar
|
|
46
|
Lepley D, Paik JH, Hla T and Ferrer F: The
G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to
inhibit tumor cell migration. Cancer Res. 65:3788–3795. 2005.
View Article : Google Scholar : PubMed/NCBI
|