Gastric cancer (GC) is one of the most common types
of cancers worldwide. Each year, almost one million people are
newly diagnosed with GC, and over 700,000 people die of GC
(1). Due to high prevalence and
death rate, GC remains a tremendous threat to word health. An
increasing body of evidence shows that patients with GC experience
high levels of OS, which contribute to progression of GC. Markers
of OS, such as 8-hydroxy-2′-deoxyguanosine (8-OHdG), human
oxoguanine glycosylase 1 (hOGG1) and xanthine oxidase (XOD) are
aberrant in GC (2). Due to redox
imbalance, OS participates in progression of GC by affecting
expression of critical effectors, including miRs (3,4).
miRs are small non-coding RNAs that modulate gene expression by
either inhibiting mRNA translation or inducing mRNA degradation at
post-transcriptional level (5).
miRs have been relevant to promotion or suppression of
tumorigenesis in GC and are implicated in cell growth,
differentiation, invasion, metastasis, and apoptosis in GC
(6). Additionally, OS gives rise
to abnormal expression of miRs in various types of diseases,
including cancers (7–9). Although function of miRs has been
extensively investigated in GC, whether miRs may be associated with
or serve as a link between OS and GC remains unknown. In this
review, we focus on roles of OS in GC, OS-induced miRs in GC, and
roles of dysfunctional miRs in GC to gain further understanding of
the association of OS and miRs in gastric tumorigenesis.
OS, which refers to redox imbalance with excessive
production of ROS exceeding the scavenging ability of human body
(10), has been associated with
pathophysiology of both solid tumors and hematological malignant
diseases (11,12). ROS, including superoxide
(O2•−), hydroxide (•OH), and
hydrogen peroxide (H2O2), lead to damage to
cell membrane, protein, and DNA (13). Physiologically, glutathione
peroxidase (GPX), superoxide dismutase (SOD), and catalase could
scavenge ROS to preserve redox homeostasis. ROS also contribute to
cell apoptosis by regulating p38α mitogen-activated protein kinases
(MAPK) (14). More importantly,
ROS stimulate the NF-E2-related factor (NRF2)-mediated activation
of an antioxidant response element (ARE) to protect cells again OS
(15). Nevertheless, under OS,
excessive ROS in cells could damage tissues, leading to
tumorigenesis, particularly in the gastrointestinal tract.
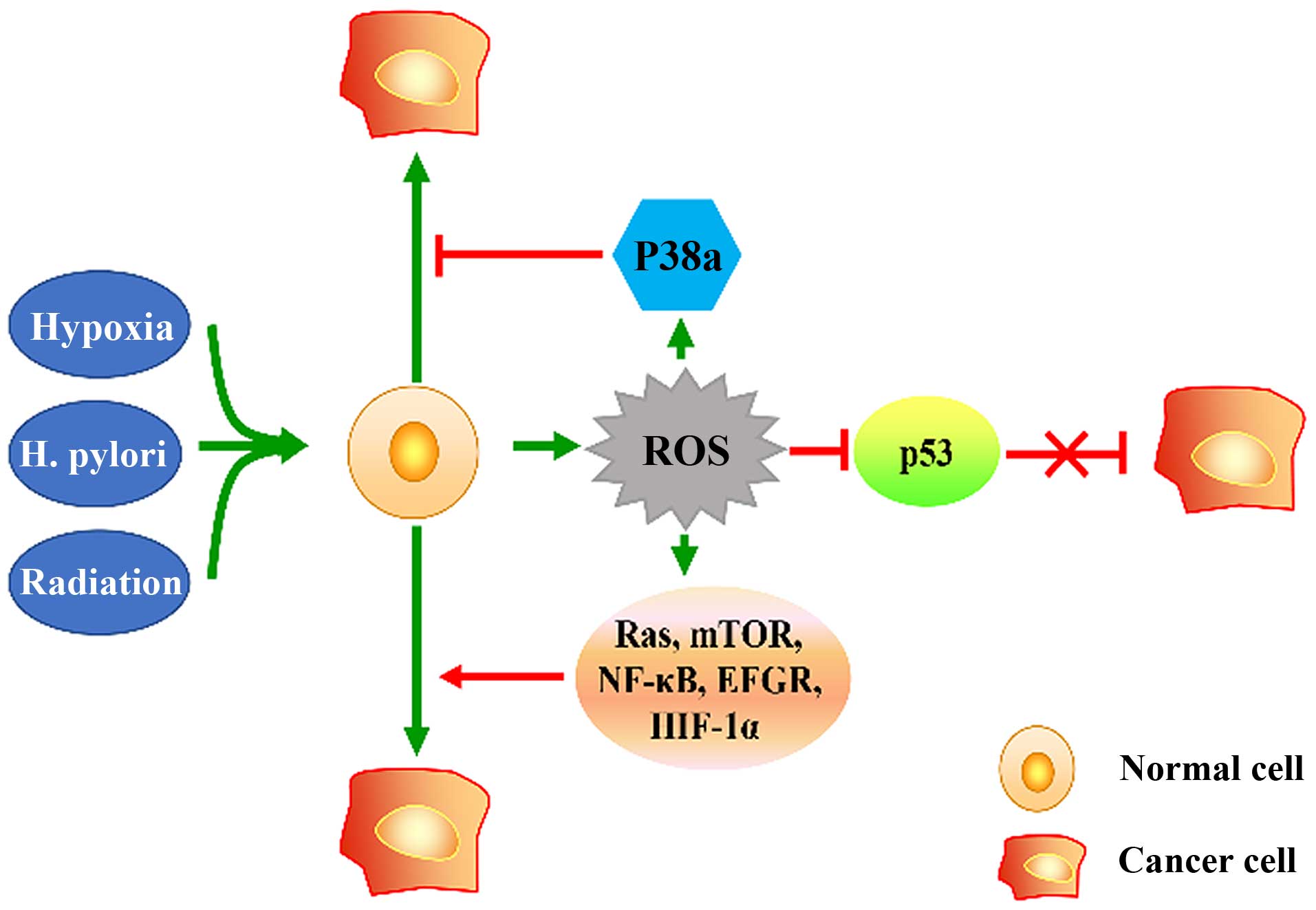
For GC, in both human and mouse, ROS are actuated
factors in gastric carcinogenesis. In human GC, ROS are
dysregulated in serum and tissue samples (16). In Helicobacter pylori (Hp)
along with N-methyl-N′-nitro-N′-nitrosoguanidine (MNNG) triggered
mouse GC models, ROS activated downstream targets including P53,
Wnt, Ras, mTOR to initiate gastric carcinogenesis (17,18).
Hypoxia leads to producing ROS, which prevent degradation of
hypoxia-inducible factor 1α (HIF-1α) (19). As a transcription factor,
accumulation of HIF-1α is able to modulate expression of various
genes, which are important in gastric tumorigenesis. For example,
HIF-1α attenuates expression of caveolin-1 (Cav-1), which can
induce the epithelial-mesenchymal transition (EMT) in GC through
regulation of E-cadherin (20).
HIF-1α also activates vascular endothelial growth factor (VEGF)
pathway to enhance angiogenesis in GC (21). In contrast, ROS act as a signaling
molecule and trigger essential signaling pathways indispensible to
promoting the occurrence and development of GC. As second
messenger, ROS are well known to activate tyrosine kinases and
MAPK, leading to cell proliferation (22), as well as trigger activation of
protein kinase-B (Akt)/mammalian target of rapamycin (mTOR)
signaling pathway to promote GC cell proliferation (23). Moreover, ROS have been found to
stimulate nuclear factor-κB (NF-κB) to promote GC invasion
(24,25).
In addition, OS exerts a crucial role in Hp-induced
diseases. Hp infection is a recognized risk factor in pathogenesis
of gastric disease, such as chronic atrophic gastritis, gastric
ulcer, and GC. Chaturvedi et al showed that Hp activation of
spermine oxidase (SMOX) in gastric epithelial cells results in OS
(26). OS causes damage to
biological structures such as proteins, carbohydrates, lipids, and
nucleic acids (27). DNA damage
promotes activation of the epidermal growth factor receptor (EFGR)
signaling pathway and mutations of tumor-suppressor genes, such as
p53 and calcium/calmodulin-dependent serine protein kinase (CASK)
(28,29), which are critical initial events in
gastric carcinogenesis. Additionally, Hp infection also induces
changes in expression of miRs in GC. For example, miR-328 is a
well-studied miR involved in Hp-induced GC. Ishimoto et al
demonstrated that miR-328 is downregulated in Hp-infected gastritis
(30). Analysis of expression of
miR-328 in GC, yielded a result similar to that obtained in
gastritis. A low level of miR-328 activates CD44, a target of
miR-328, to promote GC stem cell differentiation (4). Effect of Hp infection on miR-328
expression could be mimicked by H2O2.
Therefore, miR-328 may be a specific miR that triggers development
of Hp-induced GC by ROS. However, these researchers did not provide
an explanation for downregulation of miR-328 in Hp-infected GC.
Indeed, previous studies have demonstrated that Hp induced
expression miR-210 by regulating methylation of miR-210, thereby
increasing proliferation of gastric epithelial cells through
suppressing oncoprotein 18 or metablastin (STMN1) and
dimethyladenosine transferase 1 (DIMT1) (31). Therefore, Hp infection may alter
expression of miRs through ROS-mediated methylation of gene
promoter region. Hp-induced OS, which interferes with methylation
of miRs, may account for part of the mechanism underlying the
initiation of GC. In studies of effect of radiation on GC cells,
radiation, which acts similarly to OS, also leads to production of
ROS (32).
In conclusion, hypoxia, Hp infection, and radiation
are capable of triggering cellular OS, and excessive ROS lead to
the inhibition of anti-oncogenes such as p53, and the activation of
tumorigenesis-associated signaling pathways, which contribute to
occurrence and development of GC (Fig.
1).
As mentioned previously, under OS, ROS are produced
and play a key role under pathological conditions. Hydrogen
peroxide is widely used in experiments to simulate the effect of OS
in cell lines and results in the abnormal expression of miRs in
numerous diseases, including vitiligo, idiopathic pulmonary
fibrosis (IPF), and most cancers. ROS induced miR-25 has been shown
to enhance degeneration of melanocytes in vitiligo (35). Elevated levels of miR-9-5p has been
confirmed to be influenced by ROS both in vivo and in
vitro. In specimens of patients with IPF, expression of
miR-9-5p has been associated with ROS and is upregulated in human
fetal lung fibroblasts treated with hydrogen peroxide (36). Independently, endoplasmic reticulum
(ER) stress can be caused by hypoxia, failure of protein
maturation, or degradation (37).
Induced by ER stress, expression of miR-17, -34a, -96, and -125b
and the consequent reduction in the translation of proapoptotic
caspase-2 (38). Additionally,
exposure of cells to arsenic triggers upregulation of miR-663,
miR-222, and miR-638 (39).
ROS exhibit their characteristics in intervening in
expression of miRs in cancers. Similarly, placing cells under
hypoxic condition is a common way to test the effect of OS on
cancer cell lines. In breast and prostate cancer cells, miR-196b
could be restrained by hypoxia (40). In bladder cancer cells, hypoxia
could induce expression of miR-145 (41). Additionally, in response to
ionizing radiation, ROS were stated to upregulate miR-193a-3p and
miR-30e (42,43). miR-193a-3p has been demonstrated to
lead to apoptosis by targeting Mcl-1, and miR-30e promote glioma
cell invasion through EGFR stabilization by directly targeting
Casitas-B-lineage lymphoma (Cbl)-b.
In GC, miR-382 was upregulated by hypoxia and
participate in hypoxia-induced angiogenesis of GC (44). As discussed previously, Hp
infection alters expression of miRs, such as miR-328 and miR-210,
through ROS. Moreover, our team and other researchers focused on
activities of miR-21 in GC and concluded that ROS give rise to
progression of GC through dysfunction of miR-21 (45,46).
Both programmed cell death protein 4 (PDCD4) and phosphatase and
tension homolog deleted on chromosome 10 (PTEN) have been confirmed
to be targets of miR-21. The silencing of miR-21 with its inhibitor
AC1 MMYR2 reverse the effect of EMT accompanied by suppression of
proliferation and invasion of GC (47). We also sought to identify other
miRs affected by ROS (O2•−, •OH);
but few studies have examined the relationship of
O2•− and •OH with miRs because
H2O2 is easy to use and detect in
experiments. However, we found that enzymes catalyzing
O2•− and •OH are positively
correlated with the expression of some miRs, such as miR-200b
(48). In fact, the role of OS in
regulating the expression of miRs has been widely studied. Selected
miRs responsive to OS are shown in Table I. In summary, under OS, product of
OS in cells mediate expression of miRs by different mechanisms and
participate in progression of various types of diseases, including
GC. The detailed mechanism will be discussed in part 5.
It is well known that miRs are differentially
expressed in different types of cancers and are implicated in
processes of carcinogenesis, such as cell proliferation,
angiogenesis, invasion, and metastasis. miRs have been identified
to predict prognosis of GC patients. For example, selective
overexpression of miR-25 in advanced GC indicated poor survival
(58) and downregulation of
miR-451 was associated with worse prognosis of GC patients
(59). miRs may regulate the cell
cycle through direct interaction with key regulators, such as
cyclin, cyclin-dependent kinase (CDK), and cyclin-dependent kinase
inhibitor (CDI). Specifically, cyclins D1 and E1 are targets of
miR-16, and low levels of miR-16 contribute to development of colon
cancer (60). In leukemia, CDK2
has been found to be a target gene of miR-638, and depression of
miR-638 was found to be crucial for differentiation and
proliferation of leukemia cells (61). With regard to miR-related cancer
invasion and metastasis, miR-367 has been shown to promote invasion
by downregulating Smad7 and to stimulate the EMT through activation
of the transforming growth factor-β (TGF-β) signaling pathway
(62). Moreover, in
chondrosarcoma, miR-200b directly repress VEGF to inhibit
angiogenesis (63). In cancers,
most decreased miRs show activities as tumor-suppressor gene, and
miR mimics prevent development of cancer. Conversely, high levels
of miRs mainly act as oncogene and promote tumorigenesis. Following
this review of role of miRs in cancer, we will concentrate our
discussion on roles of miRs in GC.
In GC, numerous miRNAs have been identified, and
dysfunction of these miRs promotes progression of GC. Expression of
miRs has been found to be either increased or decreased in both GC
tissues and cell lines. For example, miR-133b/a-3p has been
ascertained to be reduced in primary GC tissues and two GC cell
lines, namely SGC7901 and MNK45 (64). Using TargetScan database and
luciferase assay, myeloid cell leukemia 1 (Mcl-1) and BCL2-like 1
(Bcl-xL) were shown to be targets of miR-133b/a-3p. Furthermore,
using mouse tumor-bearing model and cell lines, miR-133b/a-3p has
been ascertained to inhibit tumor growth. Similarly, miR-940 has
been shown to promote tumor cell invasion and metastasis by
interacting with zinc finger transcription factor 24 (ZNF24) in GC
(65); miR-130a and miR-495 have
been confirmed to increase cell proliferation and angiogenesis by
targeting runt-related transcription factor 3 (RUNX3) in GC
(66); miR-544a could induce the
EMT by sensitizing Wnt signaling pathway in GC (67); and a lower miR-100 level is
associated with lymphatic metastasis by targeting zinc finger and
BTB domain containing 7A (ZBTB7A) (68). In fact, miRs have been widely
investigated in GC. Studies of miRs in GC almost follow the methods
described above. A list of miRs in GC, including their expression
types, targets, and functions in gastric cancer is presented in
Table II. Similar to role of miRs
in other types of cancers, increased levels of miRs in GC act as
oncogenes and reduced levels of miRs act as tumor suppressor genes.
In general, molecules downstream of these miR targets are key
factors involved in the signaling pathways in GC. For example,
miR-141 is capable of regulating EGFR2 signaling pathway to affect
proliferation of GC cells (69),
and miR-495 can influence migration and invasion by action of
phosphatase on regenerating liver-3 (PRL-3) (70); and Kip1 (p27), acting as a CDK, is
the direct target of miR-196a, and a lower level of Kip1 due to
inhibition by miR-196a leads to the proliferation of GC cells
(71).
Few studies have evaluated screening or diagnostic
value of miRs in gastric juice and serum as a noninvasive method
for GC treatment. The miR-21 and miR-106a levels in gastric juice
were lower in GC than in benign gastric diseases, and showed a
specificity of 0.969 and 0.871, respectively, for the
identification of GC compared with benign gastric diseases
(88). In GC, serum level of
miR-203 is significantly lower compared with the control. Similar
to its value in GC tissue, lower serum levels of miR-203 reflect an
advanced clinical stage and poor overall survival, which is
consistent with well-studied activity of miR-203 in inhibiting the
EMT (89,90).
Despite the roles of these miRs, few studies have
compared levels of miR in both tissue and serum from time of
occurrence of morbidity to time of death of GC patients. This
question is challenging for several reasons, such as the fact that
we may not know when GC begins and cannot obtain adequate tissue
samples once tumor progresses. By studying changes in the levels of
miRs in body fluids, it may be possible for us to identify specific
markers for GC.
As discussed above, product of OS is involved in
development of GC, and production of OS thus leads to an abnormal
expression profile of miRs in diseases including cancers.
Importantly, miRs can either promote or suppress tumorigenesis in
GC. Whether miRs may serve as a link between OS and GC remains
unclear.
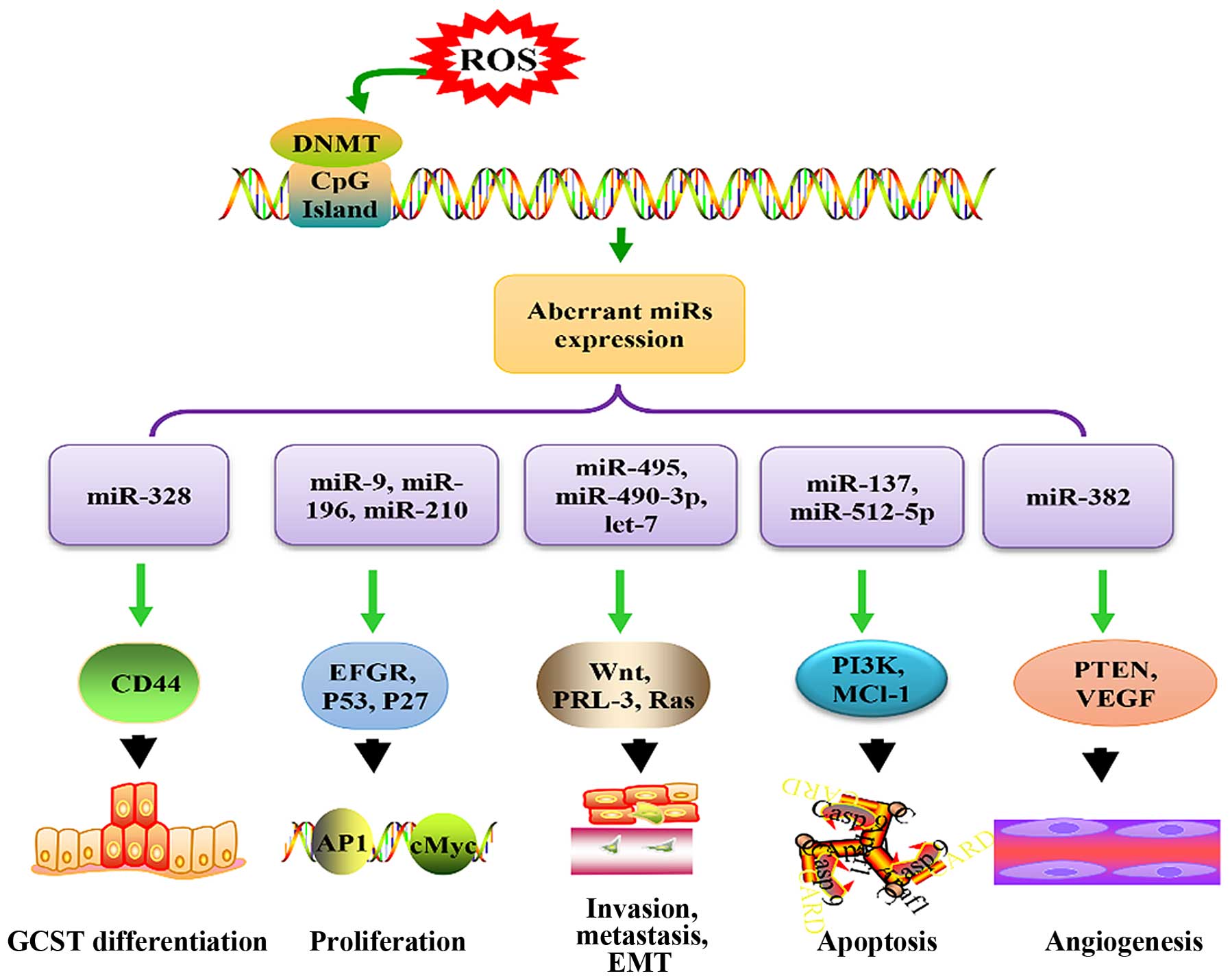
To the best of our knowledge, epigenetic factors
play a crucial role in carcinogenesis during OS. ROS contribute to
methylation of miR genes, subsequently resulting in development of
cancer by influencing expression of miRs, such as miR-145-5p,
miR-362-3p, miR-329, miR-199, and miR-125 (91–93).
Frequently, ROS increase methylation of miRs, which play roles of
tumor suppressor genes. For example, the hypermethylation of
miR-199a and miR-125b induced by ROS is modulated by DNA
methyltransferase (DNMT) 1, which downregulated these two miRs in
an ovarian cancer cell line (93).
Actually, ROS-mediated deregulation of DNA
methylation is quite an important aspect of biological role of ROS.
ROS-mediated elevation of 8-OHdG in CpG island is one of the major
mechanism of ROS-mediated hypomethylation, however, some form of
ROS induced DNA damage also stimulate DNA methylation of specific
loci. DNA hypermethylation of promoter region of genes is known to
result in gene silencing, whereas hypomethylation contributes to
increased gene expression. In GC, ROS induce expression of HIF-1α
(19), which increases expression
of DNMT enzymes DNMT1 and DNMT3B, resulting in gene silencing
through gene hypermethylation (108). Interestingly, HIF-1α can also
result in hypomethylation of genes by regulating MAT1A/MAT2A switch
(109). By contrast, expression
of miRs could also be modulated by histone deacetylases (HDACs).
For example, miR-466 h-5p is elevated when histone deacetylation is
inhibited by glucose deprivation-induced OS (110).
In conclusion, this review infers that in GC, ROS
lead to changes in DNMT, which results in methylation changes in
promoter region of miR genes that play roles in development of GC
through inhibition of their targets and related signaling pathways
(Fig. 2).
We summarized changes of OS as well as miRs and
analyzed their function in gastric carcinogenesis. We conclude that
ROS and parts of miRs exhibit overlapped characters in gastric
carcinogenesis. Given that ROS mediate expression of miRs through
epigenetic mechanism, we predict miRs serve as a bridge between ROS
and gastric cancer. ROS are the driving force for gastric
carcinogenesis. In both human and mouse gastric cancer models, ROS
triggered crucial signal pathways and vital molecules such as Wnt,
ERK, P53, and miRs to induce initiation and development of GC. In
Hp-positive GC patients as well as Hp evoked mouse model of GC, ROS
are also confirmed dysfunctional. While, we must accept that in
treatment of GC, most drugs function through ROS-activated
apoptosis of tumor cells, and some researchers found antioxidant
can promote progress of cancer cells. Activities of ROS levels were
dependent on cancer cells. How to define the threshold of ROS may
be an important approach to identify their function in cancer
cells. miRs are hot topics in cancer research. Diverse miRs change
in GC making them hard to be specific targets in treatment of GC.
However, detection of miRs in body fluid and tissues will be a
treatment reaction or prognostic factor. miR expression profiling
studies make it possible for us to probe miR change in different
tissues or body fluid. Despite the encouraging prospect, we are
still faced with many difficulties in the fields of OS-induced miRs
in GC, tumor-related miRs, signaling pathway network between OS and
gastric cancer and interaction between them. Expression of miRs
presents tissue, time, and space specificity and can be affected by
a variety of factors, which makes it hard to identify a consistent
miRNA signature with high specificity for diagnosis and prognosis
purpose. Furthermore, miRs exert a limited role in complex gene
regulation network and modulate expression of multiple genes,
suggesting complexity of miR function and both the potentially
limited and undeliberate effect of therapy targeted to a limited
number of miRs.
This study was supported by Natural Science
Foundation of Shaanxi Province (no. 99SM50), National Natural
Science Foundation of China (no. 81171288) for the research of
oxidative stress and depression, and the Fundamental Research Funds
for the Central Universities (no. 0601-08143036).
|
1
|
Riquelme I, Saavedra K, Espinoza JA, Weber
H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC and Bizama C:
Molecular classification of gastric cancer: Towards a
pathway-driven targeted therapy. Oncotarget. 6:24750–24779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei YC, Zhou FL, He DL, Bai JR, Ding H,
Wang XY and Nan KJ: Oxidative stress in depressive patients with
gastric adenocarcinoma. Int J Neuropsychopharmacol. 12:1089–1096.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaturvedi R, de Sablet T, Asim M,
Piazuelo MB, Barry DP, Verriere TG, Sierra JC, Hardbower DM,
Delgado AG, Schneider BG, et al: Increased Helicobacter
pylori-associated gastric cancer risk in the Andean region of
Colombia is mediated by spermine oxidase. Oncogene. 34:3429–3440.
2015. View Article : Google Scholar :
|
|
4
|
Ishimoto T, Sugihara H, Watanabe M,
Sawayama H, Iwatsuki M, Baba Y, Okabe H, Hidaka K, Yokoyama N,
Miyake K, et al: Macrophage-derived reactive oxygen species
suppress miR-328 targeting CD44 in cancer cells and promote redox
adaptation. Carcinogenesis. 35:1003–1011. 2014. View Article : Google Scholar
|
|
5
|
Benfey PN: Molecular biology: microRNA is
here to stay. Nature. 425:244–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song JH and Meltzer SJ: MicroRNAs in
pathogenesis, diagnosis, and treatment of gastroesophageal cancers.
Gastroenterology. 143:35–47.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Tang ZP, Zhao W, Cong BH, Lu JQ,
Tang XL, Li XH, Zhu XY and Ni X: MiR-22/Sp-1 links estrogens with
the up-regulation of cystathionine γ-lyase in myocardium, which
contributes to estrogenic cardioprotection against oxidative
stress. Endocrinology. 156:2124–2137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao Y, Yan W, Lu L, Wang Y, Lu W, Cao Y
and Cai W: p38/p53/miR-200a-3p feedback loop promotes oxidative
stress-mediated liver cell death. Cell Cycle. 14:1548–1558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Ziegelbauer J, Wang R, Wu WW, Shen
RF, Juhl H, Zhang Y and Rosenberg A: Distinct profiles for
mitochondrial t-RNAs and small nucleolar RNAs in locally invasive
and metastatic colorectal cancer. Clin Cancer Res. 22:773–784.
2016. View Article : Google Scholar
|
|
10
|
Maya-Mendoza A, Ostrakova J, Kosar M, Hall
A, Duskova P, Mistrik M, Merchut-Maya JM, Hodny Z, Bartkova J,
Christensen C, et al: Myc and Ras oncogenes engage different energy
metabolism programs and evoke distinct patterns of oxidative and
DNA replication stress. Mol Oncol. 9:601–616. 2015. View Article : Google Scholar
|
|
11
|
Zhou F, Shen Q and Claret FX: Novel roles
of reactive oxygen species in the pathogenesis of acute myeloid
leukemia. J Leukoc Biol. 94:423–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeon SM, Chandel NS and Hay N: AMPK
regulates NADPH homeostasis to promote tumour cell survival during
energy stress. Nature. 485:661–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farías JG, Herrera EA, Carrasco-Pozo C,
Sotomayor-Zárate R, Cruz G, Morales P and Castillo RL:
Pharmacological models and approaches for pathophysiological
conditions associated with hypoxia and oxidative stress. Pharmacol
Ther. 158:1–23. 2016. View Article : Google Scholar
|
|
14
|
Dolado I, Swat A, Ajenjo N, De Vita G,
Cuadrado A and Nebreda AR: p38alpha MAP kinase as a sensor of
reactive oxygen species in tumorigenesis. Cancer Cell. 11:191–205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abu-Alainin W, Gana T, Liloglou T,
Olayanju A, Barrera LN, Ferguson R, Campbell F, Andrews T, Goldring
C, Kitteringham N, et al: UHRF1 regulation of the Keap1-Nrf2
pathway in pancreatic cancer contributes to oncogenesis. J Pathol.
238:423–433. 2016. View Article : Google Scholar
|
|
16
|
Mashimo M, Nishikawa M, Higuchi K, Hirose
M, Wei Q, Haque A, Sasaki E, Shiba M, Tominaga K, Watanabe T, et
al: Production of reactive oxygen species in peripheral blood is
increased in individuals with Helicobacter pylori infection and
decreased after its eradication. Helicobacter. 11:266–271. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mikuni T and Tatsuta M: Production of
hydroxyl free radical in the xanthine oxidase system with addition
of 1-methyl-3-nitro-1-nitrosoguanidine. Free Radic Res. 36:641–647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tatsuta M, Iishi H, Baba M, Mikuni T,
Narahara H, Uedo N and Yano H: Suppression by iron chelator
phenanthroline of sodium chloride-enhanced gastric carcinogenesis
induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats.
Cancer Lett. 191:9–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JH, Kim TY, Jong HS, Kim TY, Chun YS,
Park JW, Lee CT, Jung HC, Kim NK and Bang YJ: Gastric epithelial
reactive oxygen species prevent normoxic degradation of
hypoxia-inducible factor-1alpha in gastric cancer cells. Clin
Cancer Res. 9:433–440. 2003.PubMed/NCBI
|
|
20
|
Kannan A, Krishnan A, Ali M, Subramaniam
S, Halagowder D and Sivasithamparam ND: Caveolin-1 promotes gastric
cancer progression by up-regulating epithelial to mesenchymal
transition by crosstalk of signalling mechanisms under hypoxic
condition. Eur J Cancer. 50:204–215. 2014. View Article : Google Scholar
|
|
21
|
Rath S, Das L, Kokate SB, Pratheek BM,
Chattopadhyay S, Goswami C, Chattopadhyay R, Crowe SE and
Bhattacharyya A: Regulation of Noxa-mediated apoptosis in
Helicobacter pylori-infected gastric epithelial cells. FASEB J.
29:796–806. 2015. View Article : Google Scholar
|
|
22
|
Hao W, Yuan X, Yu L, Gao C, Sun X, Wang D
and Zheng Q: Licochalcone A-induced human gastric cancer BGC-823
cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT
signaling pathways. Sci Rep. 5:103362015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen JJ, Huang WC and Chen CC:
Transcriptional regulation of cyclooxygenase-2 in response to
proteasome inhibitors involves reactive oxygen species-mediated
signaling pathway and recruitment of CCAAT/enhancer-binding protein
delta and CREB-binding protein. Mol Biol Cell. 16:5579–5591. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leone A, Roca MS, Ciardiello C,
Terranova-Barberio M, Vitagliano C, Ciliberto G, Mancini R, Di
Gennaro E, Bruzzese F and Budillon A: Vorinostat synergizes with
EGFR inhibitors in NSCLC cells by increasing ROS via up-regulation
of the major mitochondrial porin VDAC1 and modulation of the
c-Myc-NRF2-KEAP1 pathway. Free Radic Biol Med. 89:287–299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan X, Zhou Y, Wang W, Li J, Xie G, Zhao
Y, Xu D and Shen L: Activation of TLR4 signaling promotes gastric
cancer progression by inducing mitochondrial ROS production. Cell
Death Dis. 4:e7942013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaturvedi R, Asim M, Piazuelo MB, Yan F,
Barry DP, Sierra JC, Delgado AG, Hill S, Casero RA Jr, Bravo LE, et
al: Activation of EGFR and ERBB2 by Helicobacter pylori results in
survival of gastric epithelial cells with DNA damage.
Gastroenterology. 146:1739–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferreira AG, Scherer EB, da Cunha AA,
Manfredini V, Biancini GB, Vanzin CS, Vargas CR and Wyse AT:
Hyper-prolinemia induces DNA, protein and lipid damage in blood of
rats: Antioxidant protection. Int J Biochem Cell Biol. 54:20–25.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou X, Xu G, Yin C, Jin W and Zhang G:
Down-regulation of miR-203 induced by Helicobacter pylori infection
promotes the proliferation and invasion of gastric cancer by
targeting CASK. Oncotarget. 5:11631–11640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morgan C, Jenkins GJ, Ashton T, Griffiths
AP, Baxter JN, Parry EM and Parry JM: Detection of p53 mutations in
precancerous gastric tissue. Br J Cancer. 89:1314–1319. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishimoto T, Izumi D, Watanabe M, Yoshida
N, Hidaka K, Miyake K, Sugihara H, Sawayama H, Imamura Y, Iwatsuki
M, et al: Chronic inflammation with Helicobacter pylori infection
is implicated in CD44 overexpression through miR-328 suppression in
the gastric mucosa. J Gastroenterol. 50:751–757. 2015. View Article : Google Scholar
|
|
31
|
Kiga K, Mimuro H, Suzuki M,
Shinozaki-Ushiku A, Kobayashi T, Sanada T, Kim M, Ogawa M, Iwasaki
YW, Kayo H, et al: Epigenetic silencing of miR-210 increases the
proliferation of gastric epithelium during chronic Helicobacter
pylori infection. Nat Commun. 5:44972014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niu Y, DesMarais TL, Tong Z, Yao Y and
Costa M: Oxidative stress alters global histone modification and
DNA methylation. Free Radic Biol Med. 82:22–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salminen A, Haapasalo A, Kauppinen A,
Kaarniranta K, Soininen H and Hiltunen M: Impaired mitochondrial
energy metabolism in Alzheimer's disease: Impact on pathogenesis
via disturbed epigenetic regulation of chromatin landscape. Prog
Neurobiol. 131:1–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi Q, Zhang W, Guo S, Jian Z, Li S, Li K,
Ge R, Dai W, Wang G, Gao T, et al: Oxidative stress-induced
overexpression of miR-25: The mechanism underlying the degeneration
of melanocytes in vitiligo. Cell Death Differ. 23:496–508. 2016.
View Article : Google Scholar
|
|
36
|
Fierro-Fernández M, Busnadiego Ó, Sandoval
P, Espinosa-Díez C, Blanco-Ruiz E, Rodríguez M, Pian H, Ramos R,
López-Cabrera M, García-Bermejo ML, et al: miR-9-5p suppresses
pro-fibrogenic transformation of fibroblasts and prevents organ
fibrosis by targeting NOX4 and TGFBR2. EMBO Rep. 16:1358–1377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ando Y and Leung AK: Does an emergency
visit to the ER make microRNAs stronger during stress? Mol Cell.
52:1–3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Upton JP, Wang L, Han D, Wang ES, Huskey
NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, et al: IRE1α
cleaves select microRNAs during ER stress to derepress translation
of proapoptotic Caspase-2. Science. 338:818–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sturchio E, Colombo T, Carucci N, Meconi
C, Boccia P and Macino G: Molecular biomarkers in workers and
population exposed to inorganic arsenic: Preliminary study in
vitro. G Ital Med Lav Ergon. 34(Suppl): 678–681. 2012.(In
Italian).
|
|
40
|
Rebucci M, Sermeus A, Leonard E, Delaive
E, Dieu M, Fransolet M, Arnould T and Michiels C: miRNA-196b
inhibits cell proliferation and induces apoptosis in HepG2 cells by
targeting IGF2BP1. Mol Cancer. 14:792015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blick C, Ramachandran A, McCormick R,
Wigfield S, Cranston D, Catto J and Harris AL: Identification of a
hypoxia-regulated miRNA signature in bladder cancer and a role for
miR-145 in hypoxia-dependent apoptosis. Br J Cancer. 113:634–644.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwon JE, Kim BY, Kwak SY, Bae IH and Han
YH: Ionizing radiation-inducible microRNA miR-193a-3p induces
apoptosis by directly targeting Mcl-1. Apoptosis. 18:896–909. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kwak SY, Kim BY, Ahn HJ, Yoo JO, Kim J,
Bae IH and Han YH: Ionizing radiation-inducible miR-30e promotes
glioma cell invasion through EGFR stabilization by directly
targeting CBL-B. FEBS J. 282:1512–1525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seok JK, Lee SH, Kim MJ and Lee YM:
MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the
tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res.
42:8062–8072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tu H, Sun H, Lin Y, Ding J, Nan K, Li Z,
Shen Q and Wei Y: Oxidative stress upregulates PDCD4 expression in
patients with gastric cancer via miR-21. Curr Pharm Des.
20:1917–1923. 2014. View Article : Google Scholar
|
|
46
|
Polytarchou C, Iliopoulos D,
Hatziapostolou M, Kottakis F, Maroulakou I, Struhl K and Tsichlis
PN: Akt2 regulates all Akt isoforms and promotes resistance to
hypoxia through induction of miR-21 upon oxygen deprivation. Cancer
Res. 71:4720–4731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi Z, Zhang J, Qian X, Han L, Zhang K,
Chen L, Liu J, Ren Y, Yang M, Zhang A, et al: AC1MMYR2, an
inhibitor of dicer-mediated biogenesis of Oncomir miR-21, reverses
epithelial-mesenchymal transition and suppresses tumor growth and
progression. Cancer Res. 73:5519–5531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gu H, Yu J, Dong D, Zhou Q, Wang J-Y, Fang
S and Yang P: High glucose-repressed CITED2 expression through
miR-200b triggers the unfolded protein response and endoplasmic
reticulum stress. Diabetes. 65:149–163. 2016.
|
|
49
|
Feng Y, Wang L, Zeng J, Shen L, Liang X,
Yu H, Liu S, Liu Z, Sun Y, Li W, et al: FoxM1 is overexpressed in
Helicobacter pylori-induced gastric carcinogenesis and is
negatively regulated by miR-370. Mol Cancer Res. 11:834–844. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Serguienko A, Grad I, Wennerstrøm AB,
Meza-Zepeda LA, Thiede B, Stratford EW, Myklebost O and Munthe E:
Metabolic reprogramming of metastatic breast cancer and melanoma by
let-7a microRNA. Oncotarget. 6:2451–2465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee S, Yun I, Ham O, Lee SY, Lee CY, Park
JH, Lee J, Seo HH, Choi E and Hwang KC: Suppression of miR-181a
attenuates H2O2-induced death of mesenchymal
stem cells by maintaining hexokinase II expression. Biol Res.
48:452015. View Article : Google Scholar
|
|
52
|
Chen Z, Wen L, Martin M, Hsu CY, Fang L,
Lin FM, Lin TY, Geary MJ, Geary GG, Zhao Y, et al: Oxidative stress
activates endothelial innate immunity via sterol regulatory element
binding protein 2 (SREBP2) transactivation of microRNA-92a.
Circulation. 131:805–814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meseguer S, Martínez-Zamora A,
García-Arumí E, Andreu AL and Armengod ME: The ROS-sensitive
microRNA-9/9* controls the expression of mitochondrial
tRNA-modifying enzymes and is involved in the molecular mechanism
of MELAS syndrome. Hum Mol Genet. 24:167–184. 2015. View Article : Google Scholar
|
|
54
|
Chen M, Ma G, Yue Y, Wei Y, Li Q, Tong Z,
Zhang L, Miao G and Zhang J: Downregulation of the miR-30 family
microRNAs contributes to endoplasmic reticulum stress in cardiac
muscle and vascular smooth muscle cells. Int J Cardiol. 173:65–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rahman M, Lovat F, Romano G, Calore F,
Acunzo M, Bell EH and Nana-Sinkam P: miR-15b/16-2 regulates factors
that promote p53 phosphorylation and augments the DNA damage
response following radiation in the lung. J Biol Chem.
289:26406–26416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao L, Tang M, Hu Z, Yan B, Pi W, Li Z,
Zhang J, Zhang L, Jiang W, Li G, et al: miR-504 mediated
down-regulation of nuclear respiratory factor 1 leads to
radio-resistance in nasopharyngeal carcinoma. Oncotarget.
6:15995–16018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Luo EC, Chang YC, Sher YP, Huang WY,
Chuang LL, Chiu YC, Tsai MH, Chuang EY and Lai LC: MicroRNA-769-3p
downregulates NDRG1 and enhances apoptosis in MCF-7 cells during
reoxygenation. Sci Rep. 4:59082014.
|
|
58
|
Li BS, Zuo QF, Zhao YL, Xiao B, Zhuang Y,
Mao XH, Wu C, Yang SM, Zeng H, Zou QM, et al: MicroRNA-25 promotes
gastric cancer migration, invasion and proliferation by directly
targeting transducer of ERBB2, 1 and correlates with poor survival.
Oncogene. 34:2556–2565. 2015. View Article : Google Scholar
|
|
59
|
Bandres E, Bitarte N, Arias F, Agorreta J,
Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ,
et al: microRNA-451 regulates macrophage migration inhibitory
factor production and proliferation of gastrointestinal cancer
cells. Clin Cancer Res. 15:2281–2290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Witalison EE, Cui X, Causey CP, Thompson
PR and Hofseth LJ: Molecular targeting of protein arginine
deiminases to suppress colitis and prevent colon cancer.
Oncotarget. 6:36053–36062. 2015.PubMed/NCBI
|
|
61
|
Lin Y, Li D, Liang Q, Liu S, Zuo X, Li L,
Sun X, Li W, Guo M and Huang Z: miR-638 regulates differentiation
and proliferation in leukemic cells by targeting cyclin-dependent
kinase 2. J Biol Chem. 290:1818–1828. 2015. View Article : Google Scholar :
|
|
62
|
Zhu Z, Xu Y, Zhao J, Liu Q, Feng W, Fan J
and Wang P: miR-367 promotes epithelial-to-mesenchymal transition
and invasion of pancreatic ductal adenocarcinoma cells by targeting
the Smad7-TGF-β signalling pathway. Br J Cancer. 112:1367–1375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu GT, Chen HT, Tsou HK, Tan TW, Fong YC,
Chen PC, Yang WH, Wang SW, Chen JC and Tang CH: CCL5 promotes
VEGF-dependent angiogenesis by down-regulating miR-200b through
PI3K/Akt signaling pathway in human chondrosarcoma cells.
Oncotarget. 5:10718–10731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu Y, Zhang X, Zhang Y, Hu Z, Yang D,
Wang C, Guo M and Cai Q: Identification of miRNomes in human
stomach and gastric carcinoma reveals miR-133b/a-3p as therapeutic
target for gastric cancer. Cancer Lett. 369:58–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu X, Ge X, Zhang Z, Zhang X, Chang J, Wu
Z, Tang W, Gan L, Sun M and Li J: MicroRNA-940 promotes tumor cell
invasion and metastasis by downregulating ZNF24 in gastric cancer.
Oncotarget. 6:25418–25428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lee SH, Jung YD, Choi YS and Lee YM:
Targeting of RUNX3 by miR-130a and miR-495 cooperatively increases
cell proliferation and tumor angiogenesis in gastric cancer cells.
Oncotarget. 6:33269–33278. 2015.PubMed/NCBI
|
|
67
|
Yanaka Y, Muramatsu T, Uetake H, Kozaki K
and Inazawa J: miR-544a induces epithelial-mesenchymal transition
through the activation of WNT signaling pathway in gastric cancer.
Carcinogenesis. 36:1363–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shi DB, Wang YW, Xing AY, Gao JW, Zhang H,
Guo XY and Gao P: C/EBPα-induced miR-100 expression suppresses
tumor metastasis and growth by targeting ZBTB7A in gastric cancer.
Cancer Lett. 369:376–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Du Y, Xu Y, Ding L, Yao H, Yu H, Zhou T
and Si J: Downregulation of miR-141 in gastric cancer and its
involvement in cell growth. J Gastroenterol. 44:556–561. 2009.
View Article : Google Scholar
|
|
70
|
Li Z, Cao Y, Jie Z, Liu Y, Li Y, Li J, Zhu
G, Liu Z, Tu Y, Peng G, et al: miR-495 and miR-551a inhibit the
migration and invasion of human gastric cancer cells by directly
interacting with PRL-3. Cancer Lett. 323:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun M, Liu XH, Li JH, Yang JS, Zhang EB,
Yin DD, Liu ZL, Zhou J, Ding Y, Li SQ, et al: MiR-196a is
upregulated in gastric cancer and promotes cell proliferation by
downregulating p27 (kip1). Mol Cancer Ther. 11:842–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhao XD, Lu YY, Guo H, Xie HH, He LJ, Shen
GF, Zhou JF, Li T, Hu SJ, Zhou L, et al: MicroRNA-7/NF-κB signaling
regulatory feedback circuit regulates gastric carcinogenesis. J
Cell Biol. 210:613–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kong WQ, Bai R, Liu T, Cai CL, Liu M, Li X
and Tang H: MicroRNA-182 targets cAMP-responsive element-binding
protein 1 and suppresses cell growth in human gastric
adenocarcinoma. FEBS J. 279:1252–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng L, Pu J, Qi T, Qi M, Li D, Xiang X,
Huang K and Tong Q: miRNA-145 targets v-ets erythroblastosis virus
E26 oncogene homolog 1 to suppress the invasion, metastasis, and
angiogenesis of gastric cancer cells. Mol Cancer Res. 11:182–193.
2013. View Article : Google Scholar
|
|
75
|
Yang G, Gong Y, Wang Q, Wang Y and Zhang
X: The role of miR-100-mediated Notch pathway in apoptosis of
gastric tumor cells. Cell Signal. 27:1087–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zheng B, Liang L, Huang S, Zha R, Liu L,
Jia D, Tian Q, Wang Q, Wang C, Long Z, et al: MicroRNA-409
suppresses tumour cell invasion and metastasis by directly
targeting radixin in gastric cancers. Oncogene. 31:4509–4516. 2012.
View Article : Google Scholar
|
|
77
|
Li C, Nie H, Wang M, Su L, Li J, Yu B, Wei
M, Ju J, Yu Y, Yan M, et al: MicroRNA-409-3p regulates cell
proliferation and apoptosis by targeting PHF10 in gastric cancer.
Cancer Lett. 320:189–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xia J, Wu Z, Yu C, He W, Zheng H, He Y,
Jian W, Chen L, Zhang L and Li W: miR-124 inhibits cell
proliferation in gastric cancer through down-regulation of SPHK1. J
Pathol. 227:470–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang M, Li C, Nie H, Lv X, Qu Y, Yu B, Su
L, Li J, Chen X, Ju J, et al: Down-regulated miR-625 suppresses
invasion and metastasis of gastric cancer by targeting ILK. FEBS
Lett. 586:2382–2388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gao P, Xing AY, Zhou GY, Zhang TG, Zhang
JP, Gao C, Li H and Shi DB: The molecular mechanism of microRNA-145
to suppress invasion-metastasis cascade in gastric cancer.
Oncogene. 32:491–501. 2013. View Article : Google Scholar
|
|
81
|
Zhang X, Tang J, Zhi X, Xie K, Wang W, Li
Z, Zhu Y, Yang L, Xu H and Xu Z: miR-874 functions as a tumor
suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway
in gastric cancer. Oncotarget. 6:1605–1617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang TH, Wu F, Loeb GB, Hsu R,
Heidersbach A, Brincat A, Horiuchi D, Lebbink RJ, Mo YY, Goga A, et
al: Up-regulation of miR-21 by HER2/neu signaling promotes cell
invasion. J Biol Chem. 284:18515–18524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar
|
|
84
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang M, Li C, Yu B, Su L, Li J, Ju J, Yu
Y, Gu Q, Zhu Z and Liu B: Overexpressed miR-301a promotes cell
proliferation and invasion by targeting RUNX3 in gastric cancer. J
Gastroenterol. 48:1023–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li T, Lu YY, Zhao XD, Guo HQ, Liu CH, Li
H, Zhou L, Han YN, Wu KC, Nie YZ, et al: MicroRNA-296-5p increases
proliferation in gastric cancer through repression of
Caudal-related homeobox 1. Oncogene. 33:783–793. 2014. View Article : Google Scholar
|
|
87
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cui L, Zhang X, Ye G, Zheng T, Song H,
Deng H, Xiao B, Xia T, Yu X, Le Y, et al: Gastric juice microRNAs
as potential biomarkers for the screening of gastric cancer.
Cancer. 119:1618–1626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Imaoka H, Toiyama Y, Okigami M, Yasuda H,
Saigusa S, Ohi M, Tanaka K, Inoue Y, Mohri Y and Kusunoki M:
Circulating microRNA-203 predicts metastases, early recurrence, and
poor prognosis in human gastric cancer. Gastric Cancer. 19:744–753.
2016. View Article : Google Scholar
|
|
90
|
Taube JH, Malouf GG, Lu E, Sphyris N,
Vijay V, Ramachandran PP, Ueno KR, Gaur S, Nicoloso MS, Rossi S, et
al: Epigenetic silencing of microRNA-203 is required for EMT and
cancer stem cell properties. Sci Rep. 3:26872013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Donzelli S, Mori F, Bellissimo T, Sacconi
A, Casini B, Frixa T, Roscilli G, Aurisicchio L, Facciolo F,
Pompili A, et al: Epigenetic silencing of miR-145-5p contributes to
brain metastasis. Oncotarget. 6:35183–35201. 2015.PubMed/NCBI
|
|
92
|
Kang H, Kim C, Lee H, Rho JG, Seo JW, Nam
JW, Song WK, Nam SW, Kim W and Lee EK: Downregulation of
microRNA-362-3p and microRNA-329 promotes tumor progression in
human breast cancer. Cell Death Differ. 23:484–495. 2016.
View Article : Google Scholar
|
|
93
|
He J, Xu Q, Jing Y, Agani F, Qian X,
Carpenter R, Li Q, Wang XR, Peiper SS, Lu Z, et al: Reactive oxygen
species regulate ERBB2 and ERBB3 expression via miR-199a/125b and
DNA methylation. EMBO Rep. 13:1116–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li P, Shan JX, Chen XH, Zhang D, Su LP,
Huang XY, Yu BQ, Zhi QM, Li CL, Wang YQ, et al: Epigenetic
silencing of microRNA-149 in cancer-associated fibroblasts mediates
prostaglandin E2/interleukin-6 signaling in the tumor
microenvironment. Cell Res. 25:588–603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ning X, Shi Z, Liu X, Zhang A, Han L,
Jiang K, Kang C and Zhang Q: DNMT1 and EZH2 mediated methylation
silences the microRNA-200b/a/429 gene and promotes tumor
progression. Cancer Lett. 359:198–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tsai KW, Liao YL, Wu CW, Hu LY, Li SC,
Chan WC, Ho MR, Lai CH, Kao HW, Fang WL, et al: Aberrant
hypermethylation of miR-9 genes in gastric cancer. Epigenetics.
6:1189–1197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Steponaitiene R, Kupcinskas J, Langner C,
Balaguer F, Venclauskas L, Pauzas H, Tamelis A, Skieceviciene J,
Kupcinskas L, Malfertheiner P, et al: Epigenetic silencing of
miR-137 is a frequent event in gastric carcinogenesis. Mol
Carcinog. 55:376–386. 2016. View Article : Google Scholar
|
|
98
|
Saito Y, Suzuki H, Tsugawa H, Nakagawa I,
Matsuzaki J, Kanai Y and Hibi T: Chromatin remodeling at Alu
repeats by epigenetic treatment activates silenced microRNA-512-5p
with downregulation of Mcl-1 in human gastric cancer cells.
Oncogene. 28:2738–2744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tsai KW, Hu LY, Wu CW, Li SC, Lai CH, Kao
HW, Fang WL and Lin WC: Epigenetic regulation of miR-196b
expression in gastric cancer. Genes Chromosomes Cancer. 49:969–980.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ando T, Yoshida T, Enomoto S, Asada K,
Tatematsu M, Ichinose M, Sugiyama T and Ushijima T: DNA methylation
of microRNA genes in gastric mucosae of gastric cancer patients:
Its possible involvement in the formation of epigenetic field
defect. Int J Cancer. 124:2367–2374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hayashi Y, Tsujii M, Wang J, Kondo J,
Akasaka T, Jin Y, Li W, Nakamura T, Nishida T, Iijima H, et al:
CagA mediates epigenetic regulation to attenuate let-7 expression
in Helicobacter pylori-related carcinogenesis. Gut. 62:1536–1546.
2013. View Article : Google Scholar
|
|
102
|
Zhang EB, Kong R, Yin DD, You LH, Sun M,
Han L, Xu TP, Xia R, Yang JS, De W, et al: Long noncoding RNA ANRIL
indicates a poor prognosis of gastric cancer and promotes tumor
growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget.
5:2276–2292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bao W, Fu HJ, Xie QS, Wang L, Zhang R, Guo
ZY, Zhao J, Meng YL, Ren XL, Wang T, et al: HER2 interacts with
CD44 to up-regulate CXCR4 via epigenetic silencing of microRNA-139
in gastric cancer cells. Gastroenterology. 141:2076–2087.e6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shen J, Xiao Z, Wu WK, Wang MH, To KF,
Chen Y, Yang W, Li MS, Shin VY, Tong JH, et al: Epigenetic
silencing of miR-490-3p reactivates the chromatin remodeler SMARCD1
to promote Helicobacter pylori-induced gastric carcinogenesis.
Cancer Res. 75:754–765. 2015. View Article : Google Scholar
|
|
105
|
Li Z, Li D, Zhang G, Xiong J, Jie Z, Cheng
H, Cao Y, Jiang M, Lin L, Le Z, et al: Methylation-associated
silencing of MicroRNA-335 contributes tumor cell invasion and
migration by interacting with RASA1 in gastric cancer. Am J Cancer
Res. 4:648–662. 2014.PubMed/NCBI
|
|
106
|
Suzuki H, Yamamoto E, Nojima M, Kai M,
Yamano HO, Yoshikawa K, Kimura T, Kudo T, Harada E, Sugai T, et al:
Methylation-associated silencing of microRNA-34b/c in gastric
cancer and its involvement in an epigenetic field defect.
Carcinogenesis. 31:2066–2073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kurashige J, Mima K, Sawada G, Takahashi
Y, Eguchi H, Sugimachi K, Mori M, Yanagihara K, Yashiro M, Hirakawa
K, et al: Epigenetic modulation and repression of miR-200b by
cancer-associated fibroblasts contribute to cancer invasion and
peritoneal dissemination in gastric cancer. Carcinogenesis.
36:133–141. 2015. View Article : Google Scholar
|
|
108
|
Watson CJ, Collier P, Tea I, Neary R,
Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann
A, et al: Hypoxia-induced epigenetic modifications are associated
with cardiac tissue fibrosis and the development of a
myofibroblast-like phenotype. Hum Mol Genet. 23:2176–2188. 2014.
View Article : Google Scholar
|
|
109
|
Frau M, Feo F and Pascale RM: Pleiotropic
effects of methionine adenosyltransferases deregulation as
determinants of liver cancer progression and prognosis. J Hepatol.
59:830–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Druz A, Betenbaugh M and Shiloach J:
Glucose depletion activates mmu-miR-466h-5p expression through
oxidative stress and inhibition of histone deacetylation. Nucleic
Acids Res. 40:7291–7302. 2012. View Article : Google Scholar : PubMed/NCBI
|