Introduction
The incidence of pancreatic neuroendocrine neoplasms
(p-NENs), which originate from pancreatic neuroendocrine cells, has
been increasing over the last 20 years (1,2). The
vast majority of p-NENs are non-functioning, with symptoms stemming
from distant metastases or mass effects (3,4). In
metastatic p-NENs, liver is the most important location for
metastatic disease and LM is frequently observed in p-NEN patients
(5–7).
Having low mortality and complication rates, surgery
for local p-NENs has long been the standard treatment strategy
(8). In recent decades,
technological improvements have also improved surgical success for
liver-metastatic p-NENs, which has an overall mortality rate of
<5% (9,10).
To allow for more specific and individualized
treatment of liver-metastatic p-NENs, Frilling et al
(11) classified them into three
radiological types. Type I is defined as a single metastasis
regardless of size. Type II is defined as an isolated metastatic
bulk accompanied by smaller deposits. Type III is defined as a
disseminated metastatic spread. These three groups differ
significantly in terms of treatment strategies and clinical
outcomes (12).
Pathological classification is another key
prognostic factor in p-NENs (13).
In 2010, the WHO updated their grading system for the pathological
classification of p-NEN based on the Ki-67 index and mitotic counts
(14). A pancreatic carcinoid is
now defined as G1/G2 p-NET, while small-cell or large-cell
neuroendocrine carcinoma is defined as G3 p-NEC. Tumors with a
Ki-67 index of ≤2%, 3–20% or >20% are classified as G1, G2 and
G3, respectively. While a group of well-to-moderately
differentiated G3 p-NETs respond more poorly to chemotherapy than
poorly differentiated G3 p-NEC, they are nevertheless associated
with longer median survival (15).
This group of G3 p-NETs may be considered separate from p-NEC
(16). In 2013, 15 Chinese
pathology experts reached the consensus for NET G3 and defined it
as NET with high proliferative activity.
Despite the existence of various guidelines and
comprehensive reviews addressing surgical management for
non-functional p-NENs with synchronous LM, the best strategy is
still poorly defined. In the present study, we report the findings
of a panel of multidisciplinary experts from CSNET who assessed
available evidence with the aim of developing recommendations for
the surgical management of patients with liver-metastatic p-NENs.
Consensus statements were developed that take into consideration
the radiological type of liver metastasis, the WHO 2010 grade
classification and the resectability of the primary tumor.
Materials and methods
The PubMed database was searched for studies
relating to the treatment of p-NENs with LM by entering the terms
including (pancreatic neuroendocrine tumor OR neuroendocrine tumor
OR carcinoma), (operative OR surgical, operative surgical OR
pancreatectomies OR pancreaticoduodenectomy OR
pancreaticoduodenectomies OR duodenopancreatectomy OR
duodenopancreatectomies OR primary resection), (metastatic OR
metastasis OR metastases), (neoplasm OR neoplasms), and (liver OR
hepatic). Randomized trials, reviews and observational studies were
included. Studies published in English were reviewed and selected
for further screening analyses and for subsequent consensus
studies. Data extraction was carried out by all experts in
CSNET.
Results and Consensus statements
Pathological confirmation
Endoscopic ultrasound-guided fine-needle aspiration
(EUS-FNA) and percutaneous core needle biopsy are two common
methods for obtaining tissue samples in p-NENs (17).
EUS-FNA has become a successful approach in
establishing a definitive tissue diagnosis of p-NENs for more than
20 years (18). Several
retrospective studies have shown advantages of EUS-FNA, including
generation of high-resolution images enabling detection of small
lesions (19–21). However, EUS-FNA has an accuracy
fluctuation dependent upon the tumor type. Diagnostic accuracy is
lower for p-NENs (46.7%) than for adenocarcinomas (81.4%) (22). The accuracy in the grading of
p-NENs (the concordance rate between EUS-FNA samples and surgical
specimens) is also unsatisfactory (23), especially without adequate
cellularity (24). Only when more
than 2000 tumor cells obtained by EUS-FNA may increase the accuracy
of tumor grading (24). Therefore,
due to the small amount of tissue obtained from EUS-FNA, it remains
unclear if EUS-FNA samples truly reflect the entire tumor.
In comparison, percutaneous core needle biopsy has
relatively greater availability, lower cost, a higher success rate,
enables access to sufficient material and allows access to lesions
in any part of the pancreas (17,25–27).
Sufficient material can be extracted to determine cell type and
origin via histologic and immunohistochemical analyses, thus,
allowing for reliable differentiation of p-NENs (28). Moreover, collecting sufficient
tissue may also decrease the difference between percutaneous core
needle biopsy and surgical specimens.
There is no guideline as to whether liver-metastatic
lesions should be biopsied for tumor grading in cases of
liver-metastatic p-NENs and several studies have considered the
homogeneity of the Ki-67 staining expression between primaries and
metastases (29,30). Nevertheless, Zen et al
(31) reported that biopsy of
liver metastasis may yield a higher Ki-67 index than that in
primary tumor; moreover, increased Ki-67 index in liver metastases
is associated with a poorer prognosis. Similar results were also
reported in another two retrospective studies (32,33).
Therefore, liver biopsy is required for an accurate evaluation and
biopsies of both primary tumors and metastatic tumors was
recommended by a Canadian National Expert Group (34).
According to the ENETS 2012 guidelines, pathological
classification (either primary tumor or metastatic lesions) should
be confirmed before treatment, as only G1/G2 are recommended for
operation in liver-metastatic p-NENs (35). The updated NCCN guidelines
(8), NANETS guidelines (36), and the European Society for Medical
Oncology (ESMO) (37) guidelines
all suggest that Ki-67 assessment is required in liver-metastatic
p-NENs, no matter which method is used.
Consensus statements
Biopsy for Ki-67 assessment is required in
liver-metastatic p-NENs before a treatment decision is made. Liver
biopsy is important for more accurate grading of tumors in patients
with LM. Percutaneous core needle biopsy is more available than
EUS-FNA.
Liver-metastatic p-NENs with unresectable
primary tumor
The aim of the surgery for non-functional p-NENs
with LM is to prolong overall survival through potential curative
resection or cytoreduction. Previous reports showed that survival
benefits were observed for R0/R1 resection (38). Nevertheless, for liver-metastatic
p-NENs with an unresectable primary, surgical resection of the
metastases is always considered as less effective.
No guideline from ENETS (39), NANETS (40), or NCCN (8) reports any survival benefit associated
with cyto-reductive resection only for liver metastases per se. In
the 2010 UICC/AJCC/WHO TNM staging system (41), stage T4 was defined as unresectable
tumor with the involvement of the celiac axis or the superior
mesenteric artery. Tumor-invading adjacent organs (stomach, spleen,
colon and adrenal gland) were also grouped in T4 in the ENETS
staging system; surgery should not be performed in patients with a
T4 primary tumor (42).
Multiphasic CT or MRI are recommended to preoperative evaluation
(8).
Consensus statements
If the primary tumor is T4, surgical resection for
liver-metastatic lesions should be avoided, and this should be
carefully assessed preoperatively. Whether R2 resection to the
primary improves prognosis is not yet clear.
G1/G2 p-NET with type I LM
Unlike pancreatic adenocarcinoma, when the R0
resection is possible, surgery may also be considered in part of
patients with G1/G2 liver-metastatic p-NET. Type I metastasis is
defined as one metastatic lesion regardless of size (11). Generally, for type I metastasis,
surgical treatment should be undertaken with the goal of an R0
resection (12).
An early study of 16 patients recommended aggressive
surgery as the first choice for G1/G2 liver-metastatic p-NET
(43). If the metastasis is
unilobar, LM can be resected at the same time as the primary tumor
with little additional risk (44).
When an R0 resection can be achieved (all
macroscopic disease is removed and surgical margins are negative
for microscopic disease), G1/G2 liver-metastatic p-NET patients can
benefit from improved long-term survival compared to patients with
unresected disease, as confirmed by several retrospective studies
(45,46). Furthermore, another relatively
large-scale study prospectively reported there were no differences
in survival found between R0 and R1 resection. Both R0 and R1
resection can be beneficial (38).
In recent years, ENETS issued general guidelines for
patients with hepatic involvement of type I, with surgical
resection being the first therapeutic option (39). Despite the lack of randomized data,
the ENETS consensus statement emphasized that a curative resection
for type I liver metastasis should be the first-line treatment
option (39,47). Since then, curative surgery in
G1/G2 p-NET with type I LM has been accepted in clinical
practice.
Additional studies published in last decade have
demonstrated the advantage of surgery in type I liver-metastatic
p-NET. Patients can have 3- and 5-year overall survival rates of up
to 100% if R0/R1 resection is performed (11,45).
However, the 5- and 10-year recurrence rate was 84 and 94%,
respectively, even if an R0 resection was performed (48). This high postoperative recurrence
should be noted.
Also, laparoscopic surgery, which has a similar
surgical complication rate and short-term prognosis as compared to
open surgery, is also a practical option for the treatment of p-NET
with type I LM (49).
Consensus statements
Curative surgery is recommended for resectable
G1/G2 p-NET with type I LM. While R0 resection is the aim of the
operation, R1 resection also seems to improve the overall survival
rate.
G1/G2 p-NET with type II LM
Compared to type I metastasis, curative surgery may
not always be performed in patients with type II LM. Only for
unilobar metastatic lesions, curative surgery is the goal just like
that in type I liver-metastatic p-NETs. Therefore, regarding type
II liver-metastatic p-NETs, whether cytoreductive surgery can be of
benefit needs to be determined.
In early 1996, liver resection in patients with a
metastatic neuroendocrine tumor was recommended to provide good
long-term symptom palliation (50). In general, cytoreductive surgery
for liver-metastatic p-NET is indicated to reduce hormone levels
and improve clinical symptoms (51,52).
The effects on the prognosis are still debatable (51,53,54).
Furthermore, hepatic resection, used in
cytoreductive surgery, may come at the cost of high morbidity and
mortality (55), especially when
combined with the pancreaticoduodenectomy (56). A study of 120 p-NET patients
reported that cytoreductive surgery carried significant
perioperative mortality (6%) and complication rates (43%) without
long-term survival benefits, and should be discouraged (53).
Nevertheless, with the development of surgical
technology, improved long-term survival of patients with type II
liver-metastatic p-NET after cytoreductive surgery has also been
recently reported. In a prospective, multicenter study from Zerbi
et al (57), the 2-year
prognosis of patients undergoing cytoreductive surgery was
satisfactory and cytoreductive surgery for patients with low Ki-67
staining may achieve a statistically improved OS. In another
relatively larger retrospective study of 72 patients with
metastatic non-functional p-NETs from the Mayo Clinic, there was no
difference in overall survival in patients undergoing cytoreductive
surgery vs. those undergoing R0 resections, despite a higher
incidence of tumor recurrence in the cytoreductive surgery group
(58). Therefore, some researchers
believe cytoreductive surgery should be pursued whenever possible,
even if curative resection may not be achievable.
To identify the population that may benefit from
the operation, Mayo et al (59) compared patients who underwent liver
resection (n=339) with those who underwent intra-arterial therapy
(IAT) (n=414). Their data indicated that non-functional p-NET
patients with low (<25%) liver involvement benefited most from
surgery, while patients with a large (>25%) burden of liver
metastases benefited the least from surgery. Tumor volume with
<25% liver involvement was identified as a useful selection
criterion for patients who may benefit from cytoreductive surgery
(55,60). Other researchers also considered
the tumor volume as one of key prognostic factors (5).
In order for treatment to be successful,
cytoreductive surgery is required to remove at least 90% of the
tumor, including not only liver metastases, but the primary tumor
and lymph nodes (46,52,61).
ENETS, NCCN and NANETS guidelines have recommended 90% reduction of
all visible tumors (8,35,36).
However, securing a 90% reduction is relatively difficult, and such
a reduction may only be practical for less than 10% of patients
(5,35,36,48).
Nevertheless, a recent study of 108 pancreatic and
small bowel NET patients showed that where 70% cytoreduction was
achieved (in nearly two-thirds of p-NET cases), patients enjoyed
improved progression-free survival (median 3.0 years). Also, it is
worth noting that there was no peri-operative mortality (55). Similar results also suggested that
a 70% or greater reduction of the tumor burden was enough to
prolong survival and should be considered in the surgical strategy
(55,62–64).
According to ESMO guidelines, excision of >70% of the tumor load
is recommended to improve combined treatment (37).
Liver surgery can be performed as either a one-step
or a two-step procedure. For unilobar metastases and during a
low-risk operation, resection for LM can be performed at the same
time as the primary. If major or complex liver resection is
required, a two-stage surgery may be preferable in order to reduce
the operative risk, especially in patients with type II metastases
(65). The two-step surgery may
include a resection of the primary tumor, lymph nodes and
metastases of one lobe. Then, contralateral liver volume, enhanced
by right portal venous embolization hypertrophy, after that, right
hepatectomy or lobectomy may be performed as a second step
(66). Such an approach can
benefit patients with bilobar metastases and avoid or delay
indications for LT (67).
In an effort to achieve loco-regional control,
cytoreductive surgery in combination with liver-directed therapies
may also be considered. Combination approaches to cytoreduction are
very effective and are associated with similar survival rates as
those that use R0 resection only. Recent reports of cytoreduction
that use resection and ablation or resection combined with other
liver-directed therapy demonstrated a 5-year survival rate of ~75%
(62,68,69),
which was comparable to those undergoing R0 resection (46).
An estimate of the remnant liver parenchyma volume
is another factor in surgical decision making. Studies have
reported that up to 70% of the whole liver volume can be safely
removed by specialized liver surgeons (63,64).
A remnant functional liver parenchyma volume should be at least 30%
of the entire liver volume to ensure the safety of operation.
Therefore, more remnant functional liver parenchyma volume is
required for the patient with impaired hepatic function.
ENETS guidelines have established and updated the
essential criteria for patient selection. NCCN guidelines also
recommend non-curative debulking surgery in select cases of type II
liver-metastatic p-NET. According to guidelines and related studies
(8,47,54,70–72),
the minimum criteria for cytoreductive surgery of liver-metastatic
p-NETs are as follows (Table I):
i) G1/G2 liver-metastatic p-NET; ii) the primary is resectable;
iii) no unresectable extrahepatic disease; iv) younger patients
with an acceptable morbidity and low mortality; v) tumor volume
with <25% liver involvement; vi) up to 90% or at least >70%
of tumor load is thought to be resectable preoperatively; and vii)
treatment decision making requires a multidisciplinary
approach.
 | Table ISelection criteria and requirements
for cytoreduction in p-NETs with type II LM. |
Table I
Selection criteria and requirements
for cytoreduction in p-NETs with type II LM.
| Selection
criteria |
|---|
| G1/G2 p-NET with
LM; |
| The primary is
resectable; |
| No unresectable
extrahepatic disease; |
| Younger patients
with an acceptable morbidity and low mortality; |
| Tumor volume
<25% liver involvement; |
| Up to 90% when
possible or >70% of tumor load is thought resectable
preoperatively; |
| Treatment decision
making requires a multidisciplinary approach. |
|
| Requirements |
|
| Up to 90% when
possible or >70% tumor volume is required to be excised; |
| One-stage or
two-stage surgery may be recommended; |
| Liver-directed
therapies are complements to cytoreduction; |
| A remnant
functional liver parenchyma volume should be at least 30% of the
entire liver. |
Consensus statements
Curative surgery is the goal and cytoreduction
should also be performed in select patients (Table I). For cytoreduction, the following
requirements should also be met (Table
I): i) up to 90% or at least >70% of the tumor volume needs
to be excised; ii) either one-stage or two-stage surgery may be
recommended; iii) liver-directed therapies are complements to
cytoreduction in surgical effectiveness; and iv) a remnant
functional liver parenchyma volume should be at least 30% of the
entire liver.
G1/G2 p-NET with type III LM
Type III liver-metastatic p-NET is defined as p-NET
with disseminated metastatic spread in the liver. It is generally
believed that there is limited potential for palliative hepatic
resection due to excessive loss of functional liver parenchyma
volume.
Both the guidelines of ENETS and NANETS currently
do not make any recommendations on whether the primary tumor should
be removed in this clinical scenario (36,39).
They also currently do not recommend routine resection in patients
with type III liver-metastatic p-NET (resection of the primary
alone fails to improve survival). Furthermore, standard resection
in the presence of unresectable metastatic disease is also not
suggested in the NCCN 2015 guidelines (8).
However, recent retrospective data suggest that
resection of the pancreatic primary is associated with improved
survival rate in p-NET patients with disseminated and unresected
liver-metastases (73,74). Patients undergoing surgical
resection of the primary tumor had a better 5-year survival rate
than patients with no resection (55–82 vs. 30–50%) (73). These studies suggest that resection
of the primary tumor may be beneficial even in the case of globally
unresectable liver-metastatic lesions.
Consensus statements
Available evidence does not support standard
resection for type III liver-metastatic p-NET. Whether these
patients benefit from removal of their primary tumor remains to be
demonstrated.
G3 p-NET with LM
p-NET G3 is characterized by a Ki-67 index in the
G3 range (Ki-67 index always <55%) and a mitotic rate suggestive
of G2. p-NET G3 is significantly less aggressive than poorly
differentiated NECs and is not defined in the WHO 2010
classification (54,75). Prognostic outcomes associated with
cases of p-NET G3 are much better than that of p-NEC (16). A multicenter study that included 37
NETs G3 and 167 NECs found that the median Ki-67 index was
significantly different (30% in NET G3 vs. 80% in NEC), and that
overall survival was also significantly higher in NET G3 (99 months
in NET G3 vs. 17 months in NEC) (76).
Further details on the management of G3 p-NET have
been summarized in a recently published comprehensive review
(77), albeit without any definite
recommendations related to surgical treatment. In the 2016 ENETS
guidelines, chemotherapy and radiotherapy, rather than surgery,
were suggested in cases of p-NET G3 with distant metastases
(16).
Consensus statements
There is insufficient data relating to the value of
surgery in the treatment of G3 p-NET; therefore, studies are needed
that investigate the effect of surgery patient survival in cases of
liver-metastatic G3 p-NET.
G3 p-NEC with LM
Compared to G3 p-NET, p-NEC is highly malignant and
has a poor prognosis and this results in there being distinct
treatment strategies for p-NET vs. p-NEC. Therefore, an accurate
grade classification is also required before making a treatment
decision for liver-metastatic p-NENs (1).
p-NECs are highly malignant neoplasms that
typically invade adjacent structures or have frequently
metastasized at diagnosis (78).
Surgery is recommended, along with postoperative
chemotherapy, for p-NEC with local disease (40). Generally, p-NEC with LM is
considered not amenable for resection due to high recurrence rates
and no overall survival benefit (79–81).
ENETS guidelines (81) for the surgical treatment of p-NEC
refer to only two early studies (82,83),
and state that ‘curative surgery should be attempted only in
localized disease’. NANETS and NCCN guidelines do not mention
surgical options for the treatment of liver metastatic p-NEC
(8,80). Moreover, the ESMO guidelines also
agree that ‘it is a general agreement not to operate on G3 p-NEC’
(37).
Other recent studies report that multifocal and
even diffuse disseminated hepatic lesions are common for p-NEC,
which leads to a low rate of surgical intervention (84). Systemic therapy is recommended as
the primary course of therapy in the majority of studies (79–81).
Nevertheless, in 2015, a Chinese retrospective
study of 36 GEP-NEC with liver metastases patients suggested that
aggressive cytoreductive surgery, as well as radiofrequency
ablation and liver-directed intra-arterial intervention, may
improve clinical outcomes (84).
A study of the largest cohort of patients with
advanced p-NEC to date was recently published. The authors
demonstrated that resection of the primary tumor was an independent
prognostic factor in improved survival for patients with p-NEC at
different disease stages (85).
These results suggest that resection of localized p-NEC and p-NEC
with LM should both be considered.
However, it is worth noting that these two
retrospective studies did not distinguish p-NET G3 from p-NEC. Not
separating p-NET G3 from all G3 patients may lead to bias in the
results.
Consensus statements
Surgical strategy is not mentioned in guidelines
for the treatment of p-NEC with LM. Large randomized studies are
required to fully justify the role of surgery in liver-metastatic
p-NEC.
LT for p-NET with LM
LT for patients with metastatic neuroendocrine
tumors was first reported in 1993 (86). Later, in 1998, Lehnert (87) reported a 2- and a 5-year survival
rate of 44 and 30% in a series of 48 patients transplanted for
p-NET with LM; however, recurrence rate was found to be high. For
unresectable liver-metastatic lesions, whether LT is an option in
patients with type II or III liver metastases is still unclear.
Several retrospective and prospective studies have
shown encouraging results with a 5-year overall survival between 67
and 90% and a low 5-year recurrence-free survival rate between 20
and 48% (88–91). Recently, a relatively large-sample
study reviewed 94 patients who had undergone LT for
liver-metastatic p-NET in 35 centers between 1982 and 2009. The
overall three-month postoperative mortality was 10%. At 5 years
after LT, overall survival was 52%, while disease-free survival was
only 30% (92).
Due to the scarcity and heterogeneity of reported
cases of LT and the lack of randomized trials, controversy
continues surrounding patient selection for LT. NCCN guidelines
recommend that LT should not be included amongst routine treatment
strategies (8). In the ENETS and
NANETS guidelines, LT is also generally not recommended as a
routine treatment option in p-NEN with LM; rather, it may only be
an option in highly selected patients (36,39).
The minimal selection criteria are as follows: i)
well-differentiated p-NETs with Ki-67 <10%; ii) age <55
years; iii) absence of extrahepatic disease; iv) primary tumor
removed before transplantation; v) stable disease for at least six
months before LT; vi) <50% liver involvement (39,93).
Unfortunately, these selection criteria have not been validated in
large prospective studies.
Consensus statements
LT is generally not recommended as a routine
treatment option in liver-metastatic p-NENs; it may be an option in
strictly select patients.
Appropriate timing for aurgical
approach
It is still controversial how to determine the best
timing of a surgical approach, and we still do not have enough data
nor research regarding this topic.
In ENETS guidelines, debulking surgery may be
considered if the disease is not progressive over a 6-month period
and the patients are suffering from symptoms related to tumor
burden (55). However, the
evidence is still insufficient. In some cases the surgical approach
could be carried out first, while other patients may benefit from
pre-operative medical treatments or observations of tumor biologic
behavior. Therefore, multidisciplinary discussion is required to
determine the best choice of treatment (8) and re-evaluating for the treatment's
effectiveness of a surgical approach is also required.
Consensus statements
Due to insufficient evidence, the appropriate time
for surgical approach is still unclear.
Conclusions
Surgical management of liver-metastatic p-NENs
still lacks consensus recomendations. Slow growth of the tumor and
the availability of numerous effective treatment strategies make
determination complicated of the optimal treatment. Different
combinations of liver metastases types, localization, and
pathological classification have different outcomes and require
different treatment strategies. Surgical resection may be an
optimal treatment option for some patients with liver-metastatic
p-NENs. Based on the existing evidence, experts in CSNET have
reached an agreement regarding the following treatment aspects
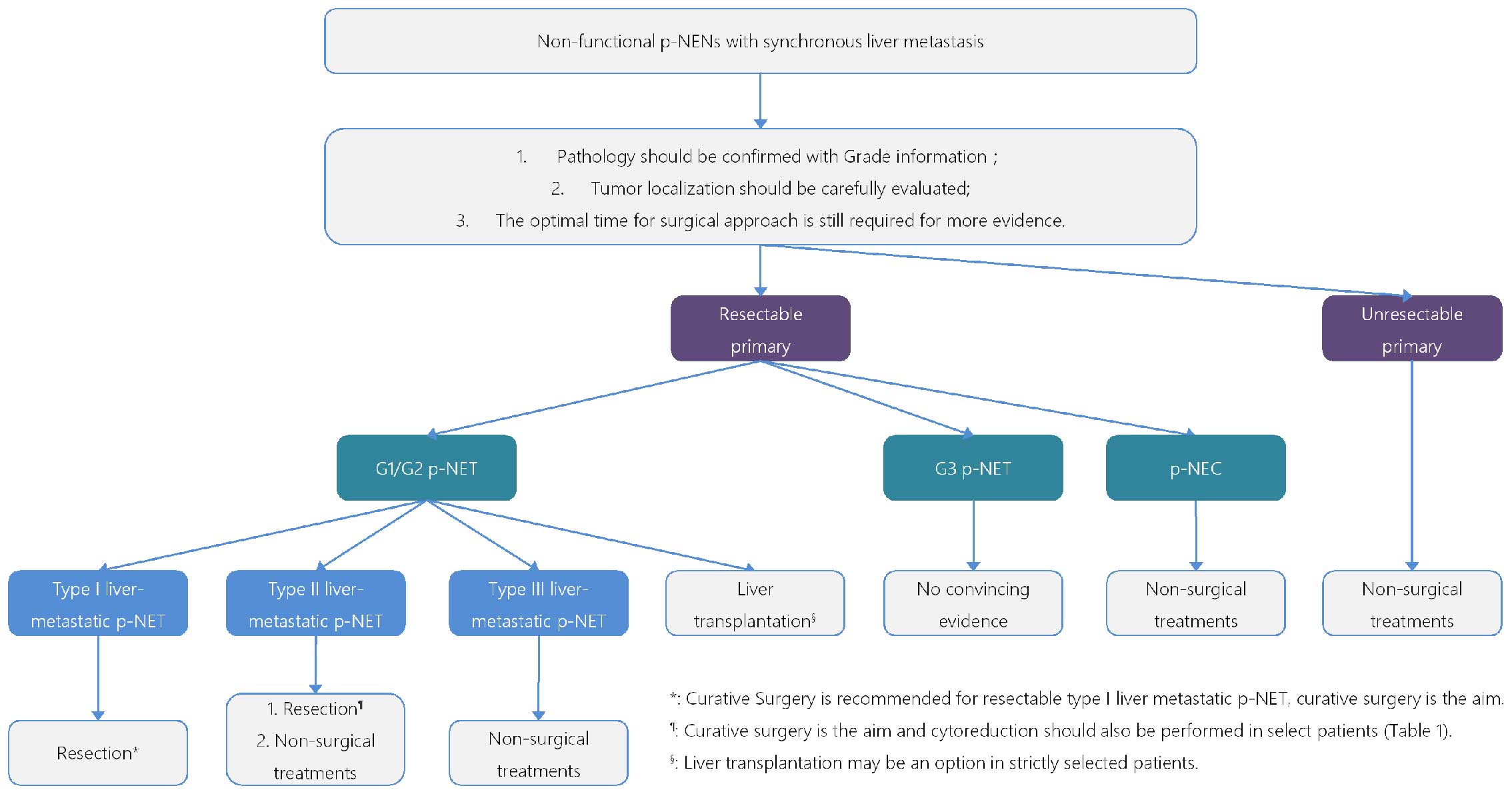
(Fig. 1):
Biopsy in p-NEN with LM: i) biopsy is essential
prior to treatment to confirm pathology; ii) liver biopsy is
important for the accuracy of tumor grading; iii) percutaneous core
needle biopsy procedure maybe more available in comparison to
EUS-FNA.
Liver-metastatic p-NEN with unresectable primary:
i) surgical resection for liver-metastatic lesions should be
avoided.
G1/G2 p-NET with type I LM: i) curative surgery is
recommended; ii) curative resection (R0 resection) is the aim of
the operation; iii) R1 resection seems to contribute to the overall
survival rate.
G1/G2 p-NET with type II LM: i) curative surgery is
also the goal; ii) cytoreductive surgery is recommended in select
patients and should meet requirements (Table I).
G1/G2 p-NET with type III LM: Surgical resection
should be avoided.
G3 p-NET with LM: There is insufficient data to
guide recommendations on surgical treatment.
p-NEC with LM: Surgical resection is not currently
recommended.
LT in liver-metastatic p-NEN: LT may be an option
in strictly selected patients for liver-metastatic p-NET.
Appropriate timing for surgical approach: The
optimal time for surgery still requires more evidence.
References
|
1
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al:
One hundred years after ‘carcinoid’: Epidemiology of and prognostic
factors for neuroendocrine tumors in 35,825 cases in the United
States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT
and Chang JS: The epidemiology of neuroendocrine tumors in Taiwan:
A nationwide cancer registry-based study. PLoS One. 8:e624872013.
View Article : Google Scholar
|
|
3
|
Modlin IM, Oberg K, Chung DC, Jensen RT,
de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA,
Krenning EP, et al: Gastroenteropancreatic neuroendocrine tumours.
Lancet Oncol. 9:61–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halfdanarson TR, Rabe KG, Rubin J and
Petersen GM: Pancreatic neuroendocrine tumors (PNETs): Incidence,
prognosis and recent trend toward improved survival. Ann Oncol.
19:1727–1733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chamberlain RS, Canes D, Brown KT, Saltz
L, Jarnagin W, Fong Y and Blumgart LH: Hepatic neuroendocrine
metastases: Does intervention alter outcomes? J Am Coll Surg.
190:432–445. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oberg K and Eriksson B: Endocrine tumours
of the pancreas. Best Pract Res Clin Gastroenterol. 19:753–781.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kulke MH, Shah MH, Benson AB III,
Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF,
Fanta P, Giordano T, et al; National Comprehensive Cancer Network.
Neuroendocrine tumors, version 1.2015. J Natl Compr Cancer Netw.
13:78–108. 2015.
|
|
9
|
Nave H, Mössinger E, Feist H, Lang H and
Raab H: Surgery as primary treatment in patients with liver
metastases from carcinoid tumors: A retrospective, unicentric study
over 13 years. Surgery. 129:170–175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Capurso G, Bettini R, Rinzivillo M,
Boninsegna L, Delle Fave G and Falconi M: Role of resection of the
primary pancreatic neuroendocrine tumour only in patients with
unresectable meta-static liver disease: A systematic review.
Neuroendocrinology. 93:223–229. 2011. View Article : Google Scholar
|
|
11
|
Frilling A, Li J, Malamutmann E, Schmid
KW, Bockisch A and Broelsch CE: Treatment of liver metastases from
neuroendocrine tumours in relation to the extent of hepatic
disease. Br J Surg. 96:175–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frilling A, Modlin IM, Kidd M, Russell C,
Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain
V, et al; Working Group on Neuroendocrine Liver Metastases.
Recommendations for management of patients with neuroendocrine
liver metastases. Lancet Oncol. 15:e8–e21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin-Perez E, Capdevila J, Castellano D,
Jimenez-Fonseca P, Salazar R, Beguiristain-Gomez A, Alonso-Orduña
V, Martinez Del Prado P, Villabona-Artero C, Diaz-Perez JA, et al:
The Large Experience of the Spanish National Tumor Registry
(RGETNE): Prognostic factors and long-term outcome of pancreatic
neuroendocrine neoplasms: Ki-67 index shows a greater impact on
survival than disease stage. The large experience of the Spanish
National Tumor Registry (RGETNE). Neuroendocrinology. 98:156–168.
2013. View Article : Google Scholar
|
|
14
|
Rindi G, Arnold R and Bosman FT:
Nomenclature and classification of neuroendocrine neoplasms of the
digestive system. WHO Classification of Tumours of the Digestive
System. Bosman FT, Carneiro F, Hruban RH and Theise ND: IARC,
Press; Lyon: 2010
|
|
15
|
Tang LH, Untch BR, Reidy DL, O'Reilly E,
Dhall D, Jih L, Basturk O, Allen PJ and Klimstra DS:
Well-differentiated neuroendocrine tumors with a morphologically
apparent high-grade component: A pathway distinct from poorly
differentiated neuroendocrine carcinomas. Clin Cancer Res.
22:1011–1017. 2016. View Article : Google Scholar
|
|
16
|
Garcia-Carbonero R, Sorbye H, Baudin E,
Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C,
Anlauf M, Cwikla JB, et al; Vienna Consensus Conference
participants. ENETS Consensus guidelines for high-grade
gastroenteropancreatic neuroendocrine Tumors and neuroendocrine
carcinomas. Neuroendocrinology. 103:186–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldin SB, Bradner MW, Zervos EE and
Rosemurgy AS II : Assessment of pancreatic neoplasms: Review of
biopsy techniques. J Gastrointest Surg. 11:783–790. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rösch T, Lightdale CJ, Botet JF, Boyce GA,
Sivak MV Jr, Yasuda K, Heyder N, Palazzo L, Dancygier H,
Schusdziarra V, et al: Localization of pancreatic endocrine tumors
by endoscopic ultrasonography. N Engl J Med. 326:1721–1726. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang KJ, Albers CG, Erickson RA, Butler
JA, Wuerker RB and Lin F: Endoscopic ultrasound-guided fine needle
aspiration of pancreatic carcinoma. Am J Gastroenterol. 89:263–266.
1994.PubMed/NCBI
|
|
20
|
Chatzipantelis P, Salla C, Konstantinou P,
Karoumpalis I, Sakellariou S and Doumani I: Endoscopic
ultrasound-guided fine-needle aspiration cytology of pancreatic
neuroendocrine tumors: A study of 48 cases. Cancer. 114:255–262.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jani N, Khalid A, Kaushik N, Brody D,
Bauer K, Schoedel K, Ohori NP, Moser AJ, Lee K and McGrath K:
EUS-guided FNA diagnosis of pancreatic endocrine tumors: New trends
identified. Gastrointest Endosc. 67:44–50. 2008. View Article : Google Scholar
|
|
22
|
Voss M, Hammel P, Molas G, Palazzo L,
Dancour A, O'Toole D, Terris B, Degott C, Bernades P and
Ruszniewski P: Value of endoscopic ultrasound guided fine needle
aspiration biopsy in the diagnosis of solid pancreatic masses. Gut.
46:244–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujimori N, Osoegawa T, Lee L, Tachibana
Y, Aso A, Kubo H, Kawabe K, Igarashi H, Nakamura K, Oda Y, et al:
Efficacy of endoscopic ultrasonography and endoscopic
ultrasonography-guided fine-needle aspiration for the diagnosis and
grading of pancreatic neuroendocrine tumors. Scand J Gastroenterol.
51:245–252. 2016. View Article : Google Scholar
|
|
24
|
Hasegawa T, Yamao K, Hijioka S, Bhatia V,
Mizuno N, Hara K, Imaoka H, Niwa Y, Tajika M, Kondo S, et al:
Evaluation of Ki-67 index in EUS-FNA specimens for the assessment
of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy.
46:32–38. 2014.
|
|
25
|
Tyng CJ, Almeida MF, Barbosa PN,
Bitencourt AG, Berg JA, Maciel MS, Coimbra FJ, Schiavon LH, Begnami
MD, Guimarães MD, et al: Computed tomography-guided percutaneous
core needle biopsy in pancreatic tumor diagnosis. World J
Gastroenterol. 21:3579–3586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang RY, Ng D, Jaskolka JD, Rogalla P and
Sreeharsha B: Evaluation of percutaneous ultrasound-guided biopsies
of solid mass lesions of the pancreas: A center's 10-year
experience. Clin Imaging. 39:62–65. 2015. View Article : Google Scholar
|
|
27
|
Yu YP, Jiang HT, Yao Z, Xia QR, Hong FM,
Zeng H and Li S: Feasibility and safety of CT-guided percutaneous
needle biopsy and subsequent iodine-125 seed interstitial
implantation for pancreatic cancer. Zhonghua Zhong Liu Za Zhi.
35:608–612. 2013.(In Chinese). PubMed/NCBI
|
|
28
|
Karlson BM, Forsman CA, Wilander E,
Skogseid B, Lindgren PG, Jacobson G and Rastad J: Efficiency of
percutaneous core biopsy in pancreatic tumor diagnosis. Surgery.
120:75–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dhall D, Mertens R, Bresee C, Parakh R,
Wang HL, Li M, Dhall G, Colquhoun SD, Ines D, Chung F, et al: Ki-67
proliferative index predicts progression-free survival of patients
with well-differentiated ileal neuroendocrine tumors. Hum Pathol.
43:489–495. 2012. View Article : Google Scholar
|
|
30
|
Couvelard A, Deschamps L, Ravaud P, Baron
G, Sauvanet A, Hentic O, Colnot N, Paradis V, Belghiti J, Bedossa
P, et al: Heterogeneity of tumor prognostic markers: A
reproducibility study applied to liver metastases of pancreatic
endocrine tumors. Mod Pathol. 22:273–281. 2009. View Article : Google Scholar
|
|
31
|
Zen Y and Heaton N: Elevated Ki-67
labeling index in ‘synchronous liver metastases’ of well
differentiated enteropancreatic neuroendocrine tumor. Pathol Int.
63:532–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh S, Hallet J, Rowsell C and Law CH:
Variability of Ki67 labeling index in multiple neuroendocrine
tumors specimens over the course of the disease. Eur J Surg Oncol.
40:1517–1522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grillo F, Albertelli M, Brisigotti MP,
Borra T, Boschetti M, Fiocca R, Ferone D and Mastracci L: Grade
increases in gastro-Enteropancreatic neuroendocrine tumor
metastases compared to the primary tumor. Neuroendocrinology.
103:452–459. 2015. View Article : Google Scholar
|
|
34
|
Singh S, Dey C, Kennecke H, Kocha W,
Maroun J, Metrakos P, Mukhtar T, Pasieka J, Rayson D, Rowsell C, et
al: Consensus recommendations for the diagnosis and management of
pancreatic neuroendocrine tumors: Guidelines from a Canadian
National Expert group. Ann Surg Oncol. 22:2685–2699. 2015.
View Article : Google Scholar
|
|
35
|
Falconi M, Bartsch DK, Eriksson B, Klöppel
G, Lopes JM, O'Connor JM, Salazar R, Taal BG, Vullierme MP and
O'Toole D; Barcelona Consensus Conference participants. ENETS
Consensus Guidelines for the management of patients with digestive
neuroendocrine neoplasms of the digestive system:
Well-differentiated pancreatic non-functioning tumors.
Neuroendocrinology. 95:120–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kulke MH, Anthony LB, Bushnell DL, de
Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier
RF, Yao JC, et al: North American Neuroendocrine Tumor Society
(NANETS): NANETS treatment guidelines: Well-differentiated
neuroendocrine tumors of the stomach and pancreas. Pancreas.
39:735–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Öberg K, Knigge U, Kwekkeboom D and Perren
A; ESMO Guidelines Working Group. Neuroendocrine
gastro-enteropancreatic tumors: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23(Suppl 7):
vii124–vii130. 2012.
|
|
38
|
Fischer L, Kleeff J, Esposito I, Hinz U,
Zimmermann A, Friess H and Büchler MW: Clinical outcome and
long-term survival in 118 consecutive patients with neuroendocrine
tumours of the pancreas. Br J Surg. 95:627–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pavel M, Baudin E, Couvelard A, Krenning
E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B and Salazar R;
Barcelona Consensus Conference participants. ENETS Consensus
Guidelines for the management of patients with liver and other
distant metastases from neuroendocrine neoplasms of foregut,
midgut, hindgut, and unknown primary. Neuroendocrinology.
95:157–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kunz PL, Reidy-Lagunes D, Anthony LB,
Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK,
Klimstra DS, et al; North American Neuroendocrine Tumor Society.
Consensus guidelines for the management and treatment of
neuroendocrine tumors. Pancreas. 42:557–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. Springer; New
York, NY: 2010
|
|
42
|
Rindi G, Klöppel G, Alhman H, Caplin M,
Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M,
Komminoth P, et al; all other Frascati Consensus Conference
participants; European Neuroendocrine Tumor Society (ENETS). TNM
staging of foregut (neuro)endocrine tumors: A consensus proposal
including a grading system. Virchows Arch. 449:395–401. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Norton JA, Warren RS, Kelly MG, Zuraek MB
and Jensen RT: Aggressive surgery for metastatic liver
neuroendocrine tumors. Surgery. 134:1057–1063; discussion
1063–1065. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Elias D, Lasser P, Ducreux M, Duvillard P,
Ouellet JF, Dromain C, Schlumberger M, Pocard M, Boige V, Miquel C,
et al: Liver resection (and associated extrahepatic resections) for
metastatic well-differentiated endocrine tumors: A 15-year single
center prospective study. Surgery. 133:375–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watzka FM, Fottner C, Miederer M, Schad A,
Weber MM, Otto G, Lang H and Musholt TJ: Surgical therapy of
neuroendocrine neoplasm with hepatic metastasis: Patient selection
and prognosis. Langenbecks Arch Surg. 400:349–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mayo SC, de Jong MC, Pulitano C, Clary BM,
Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB,
et al: Surgical management of hepatic neuroendocrine tumor
metastasis: Results from an international multi-institutional
analysis. Ann Surg Oncol. 17:3129–3136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Steinmüller T, Kianmanesh R, Falconi M,
Scarpa A, Taal B, Kwekkeboom DJ, Lopes JM, Perren A, Nikou G, Yao
J, et al; Frascati Consensus Conference participants. Consensus
guidelines for the management of patients with liver metastases
from digestive (neuro)endocrine tumors: Foregut, midgut, hindgut,
and unknown primary. Neuroendocrinology. 87:47–62. 2008. View Article : Google Scholar
|
|
48
|
Sarmiento JM, Heywood G, Rubin J, Ilstrup
DM, Nagorney DM and Que FG: Surgical treatment of neuroendocrine
metastases to the liver: A plea for resection to increase survival.
J Am Coll Surg. 197:29–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kandil E, Noureldine SI, Koffron A, Yao L,
Saggi B and Buell JF: Outcomes of laparoscopic and open resection
for neuroendocrine liver metastases. Surgery. 152:1225–1231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dousset B, Saint-Marc O, Pitre J, Soubrane
O, Houssin D and Chapuis Y: Metastatic endocrine tumors: Medical
treatment, surgical resection, or liver transplantation. World J
Surg. 20:908–914; discussion 914–915. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kimura W, Tezuka K and Hirai I: Surgical
management of pancreatic neuroendocrine tumors. Surg Today.
41:1332–1343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hanazaki K, Sakurai A, Munekage M,
Ichikawa K, Namikawa T, Okabayashi T and Imamura M: Surgery for a
gastroenteropancreatic neuroendocrine tumor (GEPNET) in multiple
endocrine neoplasia type 1. Surg Today. 43:229–236. 2013.
View Article : Google Scholar
|
|
53
|
Bloomston M, Muscarella P, Shah MH,
Frankel WL, Al-Saif O, Martin EW and Ellison EC: Cytoreduction
results in high perioperative mortality and decreased survival in
patients undergoing pancreatectomy for neuroendocrine tumors of the
pancreas. J Gastrointest Surg. 10:1361–1370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pavel M, O'Toole D, Costa F, Capdevila J,
Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, et
al; Vienna Consensus Conference participants. ENETS consensus
guidelines update for the management of distant metastatic disease
of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN)
and NEN of unknown primary site. Neuroendocrinology. 103:172–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Maxwell JE, Sherman SK, O'Dorisio TM,
Bellizzi AM and Howe JR: Liver-directed surgery of neuroendocrine
metastases: What is the optimal strategy? Surgery. 159:320–333.
2016. View Article : Google Scholar
|
|
56
|
Gaujoux S, Gonen M, Tang L, Klimstra D,
Brennan MF, D'Angelica M, Dematteo R, Allen PJ, Jarnagin W and Fong
Y: Synchronous resection of primary and liver metastases for
neuroendocrine tumors. Ann Surg Oncol. 19:4270–4277. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zerbi A, Capitanio V, Boninsegna L, Delle
Fave G, Pasquali C, Rindi G, Campana D and Falconi M; AISP-Network
Study Group. Treatment of malignant pancreatic neuroendocrine
neoplasms: Middle-term (2-year) outcomes of a prospective
observational multicentre study. HPB Oxf. 15:935–943. 2013.
View Article : Google Scholar
|
|
58
|
Cusati D, Zhang L, Harmsen WS, Hu A,
Farnell MB, Nagorney DM, Donohue JH, Que FG, Reid-Lombardo KM and
Kendrick ML: Metastatic nonfunctioning pancreatic neuroendocrine
carcinoma to liver: Surgical treatment and outcomes. J Am Coll
Surg. 215:117–124; discussion 124–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mayo SC, de Jong MC, Bloomston M, Pulitano
C, Clary BM, Reddy SK, Clark Gamblin T, Celinski SA, Kooby DA,
Staley CA, et al: Surgery versus intra-arterial therapy for
neuroendocrine liver metastasis: A multicenter international
analysis. Ann Surg Oncol. 18:3657–3665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bettini R, Mantovani W, Boninsegna L,
Crippa S, Capelli P, Bassi C, Scarpa A, Pederzoli P and Falconi M:
Primary tumour resection in metastatic nonfunctioning pancreatic
endocrine carcinomas. Dig Liver Dis. 41:49–55. 2009. View Article : Google Scholar
|
|
61
|
Sarmiento JM and Que FG: Hepatic surgery
for metastases from neuroendocrine tumors. Surg Oncol Clin N Am.
12:231–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chambers AJ, Pasieka JL, Dixon E and
Rorstad O: The palliative benefit of aggressive surgical
intervention for both hepatic and mesenteric metastases from
neuroendocrine tumors. Surgery. 144:645–651; discussion 651–653.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hemming AW, Reed AI, Howard RJ, Fujita S,
Hochwald SN, Caridi JG, Hawkins IF and Vauthey JN: Preoperative
portal vein embolization for extended hepatectomy. Ann Surg.
237:686–691; discussion 691–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yigitler C, Farges O, Kianmanesh R,
Regimbeau JM, Abdalla EK and Belghiti J: The small remnant liver
after major liver resection: How common and how relevant? Liver
Transpl. 9:S18–S25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Goessmann H, Lang SA, Fichtner-Feigl S,
Scherer MN, Schlitt HJ, Stroszczynski C, Schreyer AG and
Schnitzbauer AA: Biliodigestive anastomosis: Indications,
complications and interdisciplinary management. Chirurg.
83:1097–1108. 2012.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kianmanesh R, Sauvanet A, Hentic O,
Couvelard A, Lévy P, Vilgrain V, Ruszniewski P and Belghiti J:
Two-step surgery for synchronous bilobar liver metastases from
digestive endocrine tumors: A safe approach for radical resection.
Ann Surg. 247:659–665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schnitzbauer AA, Lang SA, Goessmann H,
Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T,
Goralcyk A, Hörbelt R, et al: Right portal vein ligation combined
with in situ splitting induces rapid left lateral liver lobe
hypertrophy enabling 2-staged extended right hepatic resection in
small-for-size settings. Ann Surg. 255:405–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Glazer ES, Tseng JF, Al-Refaie W,
Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN and Curley
SA: Long-term survival after surgical management of neuroendocrine
hepatic metastases. HPB Oxf. 12:427–433. 2010. View Article : Google Scholar
|
|
69
|
Taner T, Atwell TD, Zhang L, Oberg TN,
Harmsen WS, Slettedahl SW, Kendrick ML, Nagorney DM and Que FG:
Adjunctive radiofrequency ablation of metastatic neuroendocrine
cancer to the liver complements surgical resection. HPB Oxf.
15:190–195. 2013. View Article : Google Scholar
|
|
70
|
Frilling A and Clift AK: Therapeutic
strategies for neuroendocrine liver metastases. Cancer.
121:1172–1186. 2015. View Article : Google Scholar
|
|
71
|
Frilling A, Sotiropoulos GC, Li J,
Kornasiewicz O and Plöckinger U: Multimodal management of
neuroendocrine liver metastases. HPB Oxf. 12:361–379. 2010.
View Article : Google Scholar
|
|
72
|
Jagannath P, Chhabra D, Shrikhande S and
Shah R: Surgical treatment of liver metastases in neuroendocrine
neoplasms. Int J Hepatol. 2012:7826722012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bertani E, Fazio N, Botteri E, Chiappa A,
Falconi M, Grana C, Bodei L, Papis D, Spada F, Bazolli B, et al:
Resection of the primary pancreatic neuroendocrine tumor in
patients with unresectable liver metastases: Possible indications
for a multimodal approach. Surgery. 155:607–614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nguyen SQ, Angel LP, Divino CM, Schluender
S and Warner RR: Surgery in malignant pancreatic neuroendocrine
tumors. J Surg Oncol. 96:397–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Basturk O, Yang Z, Tang LH, Hruban RH,
Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, et
al: The high-grade (WHO G3) pancreatic neuroendocrine tumor
category is morphologically and biologically heterogenous and
includes both well differentiated and poorly differentiated
neoplasms. Am J Surg Pathol. 39:683–690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Heetfeld M, Chougnet CN, Olsen IH, Rinke
A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D and Walter
T; other Knowledge Network members. Characteristics and treatment
of patients with G3 gastroenteropancreatic neuroendocrine
neoplasms. Endocr Relat Cancer. 22:657–664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ilett EE, Langer SW, Olsen IH, Federspiel
B, Kjær A and Knigge U: Neuroendocrine carcinomas of the
gastroenteropancreatic system: A comprehensive review. Diagnostics
Basel. 5:119–176. 2015. View Article : Google Scholar
|
|
78
|
Panzuto F, Boninsegna L, Fazio N, Campana
D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L,
Tomassetti P, et al: Metastatic and locally advanced pancreatic
endocrine carcinomas: Analysis of factors associated with disease
progression. J Clin Oncol. 29:2372–2377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM,
Kim WH, Kim H, Kook MC, Park DY, Lee JH, et al; Gastrointestinal
Pathology Study Group of Korean Society of Pathologists. Current
Trends of the Incidence and Pathological Diagnosis of
Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea
2000–2009 Multicenter Study. Cancer Res Treat. 44:157–165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Strosberg JR, Coppola D, Klimstra DS, Phan
AT, Kulke MH, Wiseman GA and Kvols LK; North American
Neuroendocrine Tumor Society (NANETS). The NANETS consensus
guidelines for the diagnosis and management of poorly
differentiated (high-grade) extrapulmonary neuroendocrine
carcinomas. Pancreas. 39:799–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nilsson O, Van Cutsem E, Delle Fave G, Yao
JC, Pavel ME, McNicol AM, Sevilla Garcia MI, Knapp WH, Keleştimur
F, Sauvanet A, et al; Frascati Consensus Conference; European
Neuroendocrine Tumor Society. Poorly differentiated carcinomas of
the foregut (gastric, duodenal and pancreatic). Neuroendocrinology.
84:212–215. 2006. View Article : Google Scholar
|
|
82
|
Akerström G: Management of carcinoid
tumors of the stomach, duodenum, and pancreas. World J Surg.
20:173–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kölby L, Nilsson O and Ahlman H:
Gastroduodenal endocrine tumours. Scand J Surg. 93:317–323.
2004.
|
|
84
|
Du S, Ni J, Weng L, Ma F, Li S, Wang W,
Sang X, Lu X, Zhong S and Mao Y: Aggressive locoregional treatment
improves the outcome of liver metastases from grade 3
gastroenteropancreatic neuroendocrine tumors. Medicine (Baltimore).
94:e14292015. View Article : Google Scholar
|
|
85
|
Haugvik SP, Janson ET, Österlund P, Langer
SW, Falk RS, Labori KJ, Vestermark LW, Gr⊘nbæk H, Gladhaug IP and
Sorbye H: Surgical treatment as a principle for patients with
high-grade pancreatic neuroendocrine carcinoma: A Nordic
multicenter comparative study. Ann Surg Oncol. 23:1721–1728. 2016.
View Article : Google Scholar
|
|
86
|
Schweizer RT, Alsina AE, Rosson R and
Bartus SA: Liver transplantation for metastatic neuroendocrine
tumors. Transplant Proc. 25:19731993.PubMed/NCBI
|
|
87
|
Lehnert T: Liver transplantation for
metastatic neuroendocrine carcinoma: An analysis of 103 patients.
Transplantation. 66:1307–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Olausson M, Friman S, Herlenius G, Cahlin
C, Nilsson O, Jansson S, Wängberg B and Ahlman H: Orthotopic liver
or multivisceral transplantation as treatment of metastatic
neuroendocrine tumors. Liver Transpl. 13:327–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Frilling A, Malago M, Weber F, Paul A,
Nadalin S, Sotiropoulos GC, Cicinnati V, Beckebaum S, Bockisch A,
Mueller-Brand J, et al: Liver transplantation for patients with
metastatic endocrine tumors: Single-center experience with 15
patients. Liver Transpl. 12:1089–1096. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
van Vilsteren FG, Baskin-Bey ES, Nagorney
DM, Sanderson SO, Kremers WK, Rosen CB, Gores GJ and Hobday TJ:
Liver transplantation for gastroenteropancreatic neuroendocrine
cancers: Defining selection criteria to improve survival. Liver
Transpl. 12:448–456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rosenau J, Bahr MJ, von Wasielewski R,
Mengel M, Schmidt HH, Nashan B, Lang H, Klempnauer J, Manns MP and
Boeker KH: Ki67, E-cadherin, and p53 as prognostic indicators of
long-term outcome after liver transplantation for metastatic
neuroendocrine tumors. Transplantation. 73:386–394. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Le Treut YP, Grégoire E, Klempnauer J,
Belghiti J, Jouve E, Lerut J, Castaing D, Soubrane O, Boillot O,
Mantion G, et al; For ELITA. Liver transplantation for
neuroendocrine tumors in Europe-results and trends in patient
selection: A 213-case European liver transplant registry study. Ann
Surg. 257:807–815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Mazzaferro V, Pulvirenti A and Coppa J:
Neuroendocrine tumors metastatic to the liver: How to select
patients for liver transplantation? J Hepatol. 47:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|