Introduction
The clinical outcome of patients with advanced-stage
lung cancer remains poor due to intrinsic or acquired
chemoresistance to platinum-based chemotherapy and severe
dose-limiting organ toxicities (1). Therefore, new and effective clinical
agents are urgently needed to improve the outlook of these
patients. Traditional Chinese herbs are sources of compounds that
may have potential as therapeutic drugs for cancer (2). Oxymatrine is an alkaloid present in
Kushen, which is widely used as a traditional Chinese medicine. It
exhibits anti-inflammatory, anti-allergic, antiviral, antifibrotic
and cardioprotective properties (3–5).
Furthermore, oxymatrine has antitumor properties, including
inhibiting cancer cell proliferation, cell cycle progression, and
angiogenesis, promoting cellular apoptosis and reversing multidrug
resistance in patients with cancer (6–8). A
recent study demonstrated that oxymatrine inhibited the
proliferation and induced apoptosis in A549 cells by regulating the
expression of Bcl-2 and Bax (9);
however, a more detailed mechanism of action remains elusive.
A previous study showed that modulating DNA repair
pathways can sensitize a number of cancers to DNA damage-based
cancer treatments (10).
Consequently, targeting DNA repair systems is a promising strategy
for the development of a novel lung cancer therapy. Human
apurinic/apyrimidinic endonuclease 1 (APE1, also known as REF-1) is
an essential base-excision repair (BER) enzyme that is responsible
for the repair of DNA damage resulting from oxidative stress,
chemotherapy and radiotherapy (11). APE1 is essential, since the
deletion of both alleles (Apex−/−) results in early-stage embryonic
lethality in animals (12). Cell
lines with the complete lack of APE1 are also non-viable, further
indicating its significance in maintaining cell survival (12). APE1 has received significant
attention as an attractive target for the pharmacological treatment
of certain types of cancer.
Apurinic/apyrimidinic (AP) sites can form
spontaneously or in response to DNA damage, and early studies
showed that ~10,000 depurination/depyrimidination events occur
spontaneously in the mammalian genome per day (13). If left unrepaired AP sites can
block DNA synthesis and lead to mutation during semiconservative
replication (14). AP sites can
also occur as intermediates in BER, which is initiated by a DNA
glycosylase.
The mechanism of action of oxymatrine in human lung
cancer is largely unknown. To investigate the role and mechanism of
oxymatrine in human lung cancer we hypothesized that the cytotoxic
effects of oxymatrine may be exerted by inhibiting the function of
APE1. To test this hypothesis APE1shRNA (APE1 knockdown)
and APEOE (APE1 overexpression) cells were used to
investigate the effects of APE1 knockdown and overexpression in
oxymatrine-treated A549 cells. Several cellular parameters were
measured, including proliferation, survival and the induction of
DNA damage and apoptosis.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), and antibiotics (penicillin and streptomycin)
were purchased from Invitrogen-Life Technologies (Carlsbad, CA,
USA). Antibodies were purchased from Cell Signaling Technology
(Beverly, MA, USA). Oxymatrine was obtained from Shanghai Chemical
Technology Co., Ltd., (Shanghai, China) and dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) at a stock
concentration of 500 mg/ml; it was then diluted further in culture
medium. Each experiment was repeated at least three times and new
dilutions were prepared for each experiment. Thiazolyl blue
tetrazolium bromide (MTT) was obtained from Sigma-Aldrich.
Cell culture and transfections
Human lung carcinoma cell lines A549 and NCI-H1299
were purchased from the Shanghai Cell Institute Country Cell Bank
(Shanghai, China). The A549 and NCI-H1299 cell lines were cultured
in DMEM or RIPM-1640 and supplemented with 10% heat-inactivated
FBS, 100 U/ml penicillin G and 100 μg/ml streptomycin at 37°C in a
humidified 5% CO2 atmosphere.
Plasmid construction
To construct shRNA-expressing lentiviral vectors for
silencing of APE1, the targeting sequence 5′-TGACAAAGAGGCAGCAGGA-3′
and stable short hairpin RNA (shRNA) expression cassettes were
cloned into the RNAi-Ready pSIREN-RetroQ vector containing
puromycin as a screening marker, which was purchased from Clontech
Laboratories, Inc. (Mountain View, CA, USA). The primer sequences
were as follows: forward, 5′-GATCCGTGACAAA
GAGGCAGCAGGATTCAAGAGATCCTGCTGCCTCTTT GTCATTTTTTG-3′ and reverse,
5′-AATTCAAAAAATGA CAAAGAGGCAGCAGGATCTCTTGAATCCTGCTGCCT
CTTTGTCACG-3′. The APE1 gene was amplified from cDNA
isolated from K562 cells using polymerase chain reaction (PCR), and
was then cloned into pOZN-HA vector using the primers:
APE1-Xho1-forward, 5′-CGTTCGTACTCGAGATG
CCGAAGCGTGGGAAAAAG-3′ and APE1-Not1-reverse,
5′-CTTTTCCTTTTGCGGCCGCTCACAGTGCTAGGTAT AGGG-3′.
Generation of stable cell lines
Recombinant replicationdeficient VSV lentiviruses
were used and were propagated and purified and the titer was
determined. A549 cells were then infected by collected virus
supernatant. A549 cell infections were carried out at MOIs of 100
and 200 for 16 h, and the transfected cells with
APE1shRNA were screened with puromycin for 2–5 days. The
transfected cells with pOZN-HA-APE1 were screened by IL-2 receptor
magnetic activated cell sorting. To determine the effect of APE1
knock-down in APE1shRNA or overexpression in
APE1OE cells, western blotting was used to measure APE1
expression levels. Lentivirus production and cell infections were
performed according to the manufacturer’s instructions.
Immunofluorescence
Cells grown on coverslips were fixed in 4%
paraformaldehyde, permeabilized with PBS containing 0.1% Triton
X-100, and blocked in 3% normal donkey serum in PBS for 30 min at
room temperature. Rabbit anti-human 8-OHDG (dilution 1:1,000) and
p-H2AX (dilution 1:1,000) antibodies were diluted in 3% normal
donkey serum in PBS and applied at 4°C overnight. After rinsing
three times with PBS and incubating for 1 h with donkey anti-rabbit
secondary antibody (dilution 1:1,000), slides were washed three
times in PBS and the cell nuclei were stained with DAPI for 10 min
at room temperature. Images were acquired on a Leica TCS SP5
confocal fluorescence microscope.
TUNEL staining
Terminal deoxynucleotidyl dUTP nick end labelling
(TUNEL) assays were performed to measure apoptosis in cultured
cells using the DeadEnd colorimetric TUNEL system (Promega,
Madison, WI, USA) according to the manufacturer’s instructions.
Briefly, cells were grown on coverslips in 6-well plates after
oxymatrine treatment; the coverslips were then removed from the
media at the designated time points, fixed and permeabilized. The
cells were incubated in rTdT reaction mixture for 60 min at 37°C.
The slides were stained with 4′,6-diamidino-2-phenylindole (DAPI)
and mounted using anti-fade solution with a coverslip and nail
polish. Images were captured using a confocal laser microscope (TCS
SP5; Leica Microsystems, Wetzlar, Germany).
Protein extraction
Cells were treated with different concentrations of
oxymatrine (2, 4 and 6 mg/ml) for 48 h, and washed twice with cold
1X PBS. The cells were then lysed using a Teflon-glass homogenizer
in lysis buffer containing 0.25 M sucrose, 10 mM Tris-HCl (pH 7.5),
3 mM MgCl2, 0.1 mM EDTA, 0.05% NP40, and a mix of
protease and phosphatase inhibitors (Beyotime Institute of
Biotechnology, Shanghai, China). The homogenates were centrifuged
at 1,000 × g for 10 min at 4°C, and the supernatants were retained
for protein quantitation and western blotting. For localization
studies the total cellular extracts were fractionated into nuclear
and cytosolic fractions using a nuclear protein extraction kit
(Beyotime Institute of Biotechnology).
Western blotting
Cells were harvested and re-suspended in an SDS
buffer (Beyotime Institute of Biotechnology) for preparation of
total protein extracts. Western blot analysis was performed
according to the antibody (Cell Signaling Technology) and to the
manufacturer’s protocols.
MTT assays
Cell lines were seeded into 96-well plates at a
density of 3×103 cells/well, incubated overnight at 37°C
in 5% CO2, and treated with oxymatrine (0, 2, 4 and 6
mg/ml) for indicated time (24, 48 and 72 h). Cell viability was
determined using MTT assays (Sigma-Aldrich). Briefly, MTT (5 mg/ml)
was added and the plates were incubated at 37°C for 4 h in the
dark. The absorbance was measured at a wavelength of 490 nm using a
microplate reader (FluoDia T70; Photon Technology International,
Lawrenceville, NJ, USA).
Colony formation assays
A549 cells (500 cells/well) were seeded into a 60-mm
plate in triplicate. After incubation for 10 days the plates were
washed gently with PBS and stained with 0.1% crystal violet.
Colonies containing >50 cells were counted manually. The plating
efficiency was calculated by dividing the number of colonies formed
in the treated group by that in the control group.
Apoptosis analysis
Cells were seeded in 6-well plates
(1.5×105 cells/well), treated with different
concentrations (0, 2, 4 or 6 mg/ml) of oxymatrine for 48 h,
harvested and washed with cold PBS. The amount of cell surface
phosphatidylserine in apoptotic cells was estimated quantitatively
using an Annexin V-APC/7-AAD or Annexin V/PI double staining
apoptosis detection kit (Liankebio, Shanghai, China) according to
the manufacturer’s instructions. The percentage of apoptotic cells
was analyzed using flow cytometry. Triplicate experiments with
triplicate samples were performed.
AP endonuclease activity assay for
APE1/Ref-1
To assess the ability of oxymatrine to inhibit AP
endonuclease activity an oligonucleotide cleavage assay was
designed. The pair of oligonucleotides used in the
fluorescence-based AP endonuclease assay was 890-FAM
GGAAGGCCGCTGACAGTTT TTCTGTCAGCGG and its complementary
oligonucleotide. If APE1 cleaves the DNA at the abasic site at
position 7 from the 5′ end the 6mer fluorescein-containing molecule
can dissociate from the complementary strand by thermal melting. As
a result, the quenching effect of 3′-dabcyl (which absorbs the
fluorescence emitted by fluorescein when in close proximity) is
lost, and APE1 activity can be measured indirectly as an increase
in fluorescence signal.
For fluorescence-based AP endonuclease assays the
single-stranded oligonucleotides (10 M) were dissolved and annealed
in annealing buffer (25 mM Tris, pH 7.5, 1 mM EDTA and 50 mM NaCl)
at 95°C for 5 min at a 1:1 ratio and allowed to cool to room
temperature overnight. The DNA was diluted as appropriate with
assay buffer (20 mM HEPES-KOH, 0.5 mM MgCl2, 50 mM KCl,
0.1 mg/ml BSA), aliquoted, and stored at −20°C (15). The protein samples (8 ng/μl) and 20
μl/well hAPE1 standard (diluted to 0.05 ng/μl with assay buffer;
recombinant human APE1; Sino Biological, Inc., North Wales, PA,
USA) were added to each well followed by 20 μl oligonucleotide
probe (500 nM) and then mixed. Fluorescence readings were taken
continuously during 30-min incubation at 37°C using a LB-940
microporous multifunction analyzer in kinetic mode with excitation
at 495 nm and emission at 530 nm.
Abasic site determination
Genomic DNA was purified from cells treated with
oxymatrine using a Takara, MiniBest Universal Genomic DNA
Extraction kit ver. 5.0 (Takara, Shiga, Japan). The abasic (AP)
sites in genomic DNA were identified using Nucleostain-DNA Damage
Quantification kit-AP Site Counting (Dojindo Molecular
Technologies, Kumamoto, Japan) following the manufacturer’s
instructions (15).
Statistical analysis
Each experiment was repeated at least three times
and the new dilutions were prepared for each experiment. The
results are presented as means ± standard error of the mean (SEM).
Differences between means were analyzed using Student’s t-test and
were considered statistically significant at P<0.05. When more
than one group was compared with control, significance was
evaluated according to one-way analysis of variance (ANOVA). All
statistical analysis was performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA).
Results
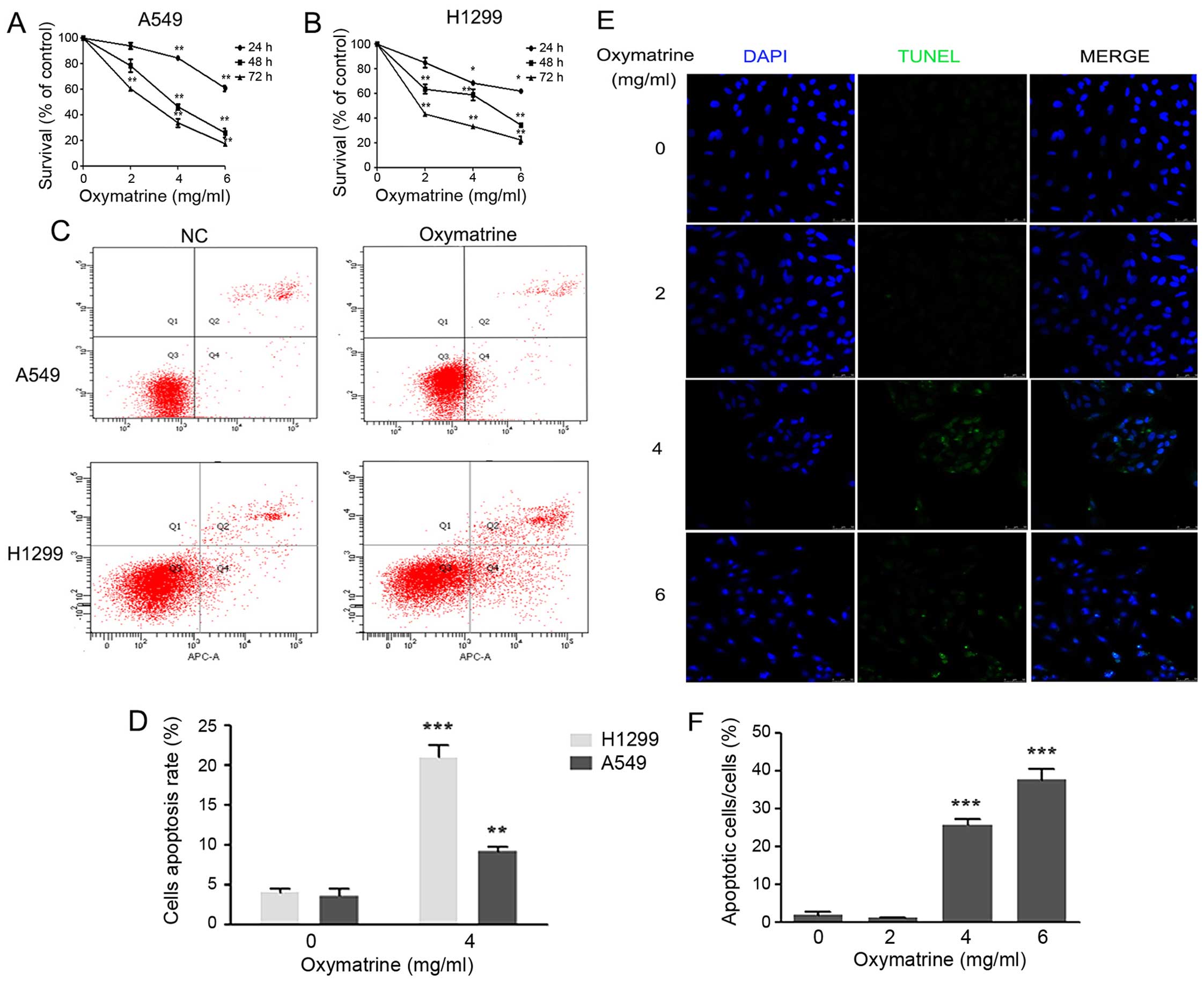
Oxymatrine induces apoptosis and inhibits
the proliferation of lung cancer cells
Oxymatrine is a promising anticancer drug that
inhibits the proliferation and growth of various cancer cells in
vitro and in vivo (16–18).
To investigate the effects of oxymatrine on the viability of lung
cancer cells, A549 and H1299 cells were treated with different
concentrations of oxymatrine (0, 2, 4 or 6 mg/ml) for the indicated
times (24, 48 or 72 h). Compared with the PBS group (0 mg/ml, 0 h),
treatment with 4 and 6 mg/ml oxymatrine inhibited the viability of
A549 and H1299 cells, significantly (P<0.05 or P<0.01;
Fig. 1A and B). Next, Annexin
V-APC/7-AAD staining and flow cytometry was performed to quantify
cellular apoptosis. Forty-eight hours after treatment, apoptosis
was significantly higher in the oxymatrine group compared with the
control group (P<0.05) (Fig. 1C and
D). These observations were confirmed by investigating whether
oxymatrine promoted A549 cell death using TUNEL staining. The
number of TUNEL-positive cells was significantly higher in the 4
and 6 mg/ml oxymatrine groups than in 0 and 2 mg/ml oxymatrine
groups (P<0.05; Fig. 1E and F).
These results suggest that oxymatrine induced apoptosis in lung
cancer cells.
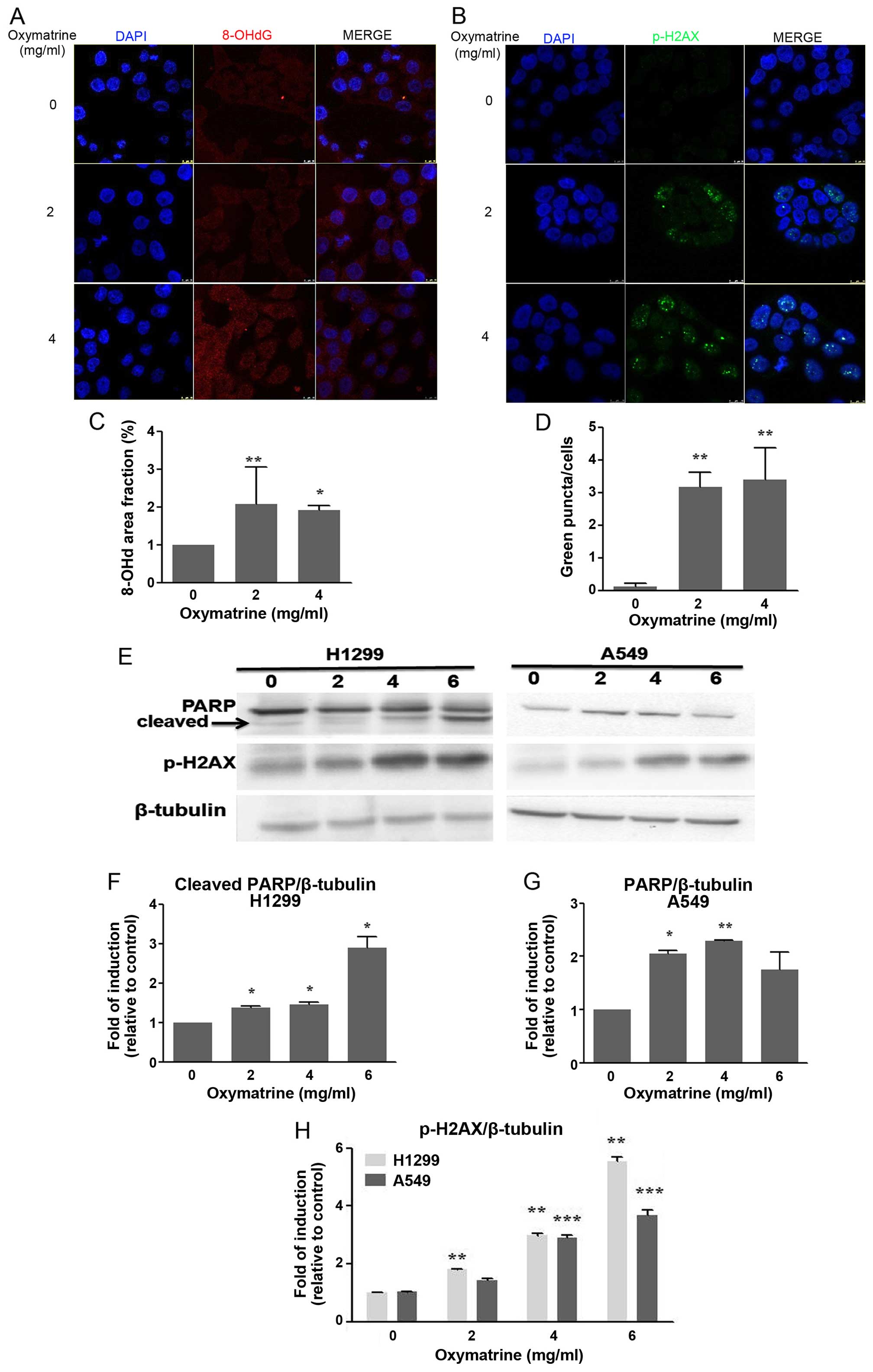
Oxymatrine induces DNA damage in lung
cancer cells
To explore the mechanism responsible for
oxymatrine-induced apoptosis, DNA damage was evaluated using 8-OHdG
and p-H2AX immunofluorescent staining and western blotting in A549
and H1299 cells that had been treated with oxymatrine for 48 h.
Fig. 2A shows markedly increased
8-OHdG-positive areas in cells treated with both 2 and 4 mg/ml
oxymatrine compared with control cells (P<0.05; Fig. 2A and C).
The H2A histone family member X (H2AX) is a key
factor in the repair of damaged DNA. The phosphorylation of H2AX is
an early event during the formation of double-stranded DNA breaks
and the response to DNA damage (19). Therefore, the expression of p-H2AX
was detected by immunofluorescence staining and western blotting.
The expression of p-H2AX was increased significantly in cells
treated with both 2 and 4 mg/ml oxymatrine compared with control
(P<0.05; Fig. 2B, D, E and
H).
PARP is a nuclear enzyme that is activated by DNA
damage. Following genotoxic stress PARP synthesizes a branched
polymer of poly(ADP-ribose) or PAR that participates in the
regulation of nuclear homeostasis (20–22).
Many different cellular insults that cause DNA damage activate PARP
and induce PARP-dependent cell death. As expected, treatment with
oxymatrine induced the activation of PARP. H1299 and A549 cells
were treated with different concentrations of oxymatrine for 48 h,
and analysis revealed that oxymatrine induced PARP activation in a
dose-dependent manner and oxymatrine can induced PARP cleavage in
H1299 (Fig. 2E–G). This suggests
that the activation of these proteins occurs as a result of
apoptosis.
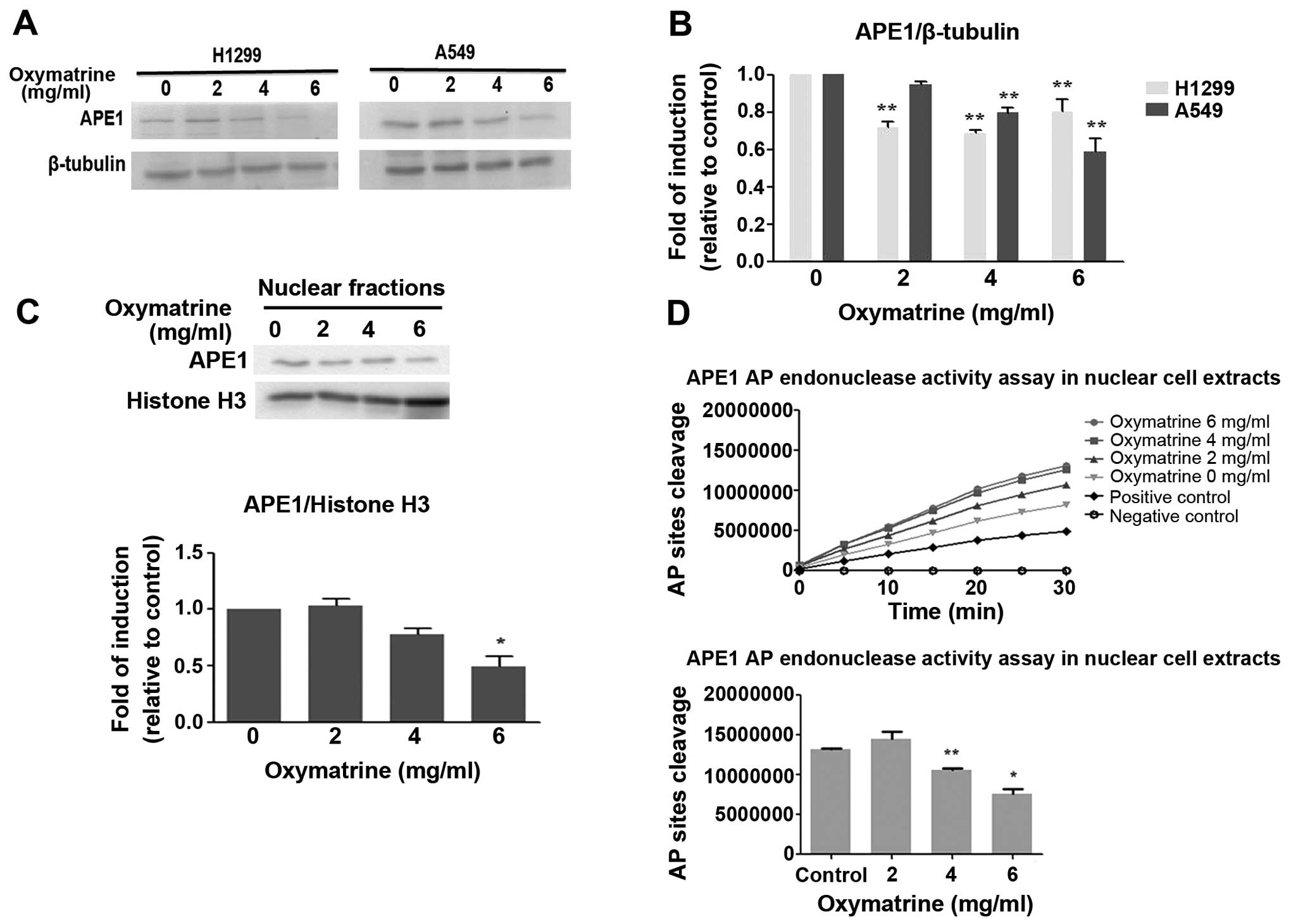
Oxymatrine inhibits the protein
expression and AP endonuclease activity of APE1
Human APE1 is an essential enzyme in the BER
pathway, where it plays a role in repairing abasic sites. APE1 is
responsible for 95% of the endonuclease activity in cells (23), and is a critical part of both the
short-patch and long-patch BER pathways (24). Western blotting revealed that
oxymatrine inhibited the expression of APE1 protein in both H1299
and A549 cells compared with control (Fig. 3A and B). The nuclear localization
of APE1/Ref-1 was also decreased significantly by oxymatrine (6
mg/ml; P<0.05) in A549 cells (Fig.
3C).
A fluorescence-based AP endonuclease assay described
by Madhusudan et al (25)
was adapted. Oxymatrine inhibited APE1 and AP endonuclease activity
in A549 cell nuclear cell extracts in a dose-dependent manner
(Fig. 3D). These results suggest
that oxymatrine is an inhibitor of the DNA repair activity of
APE1/Ref-1 in lung cancer cell lines.
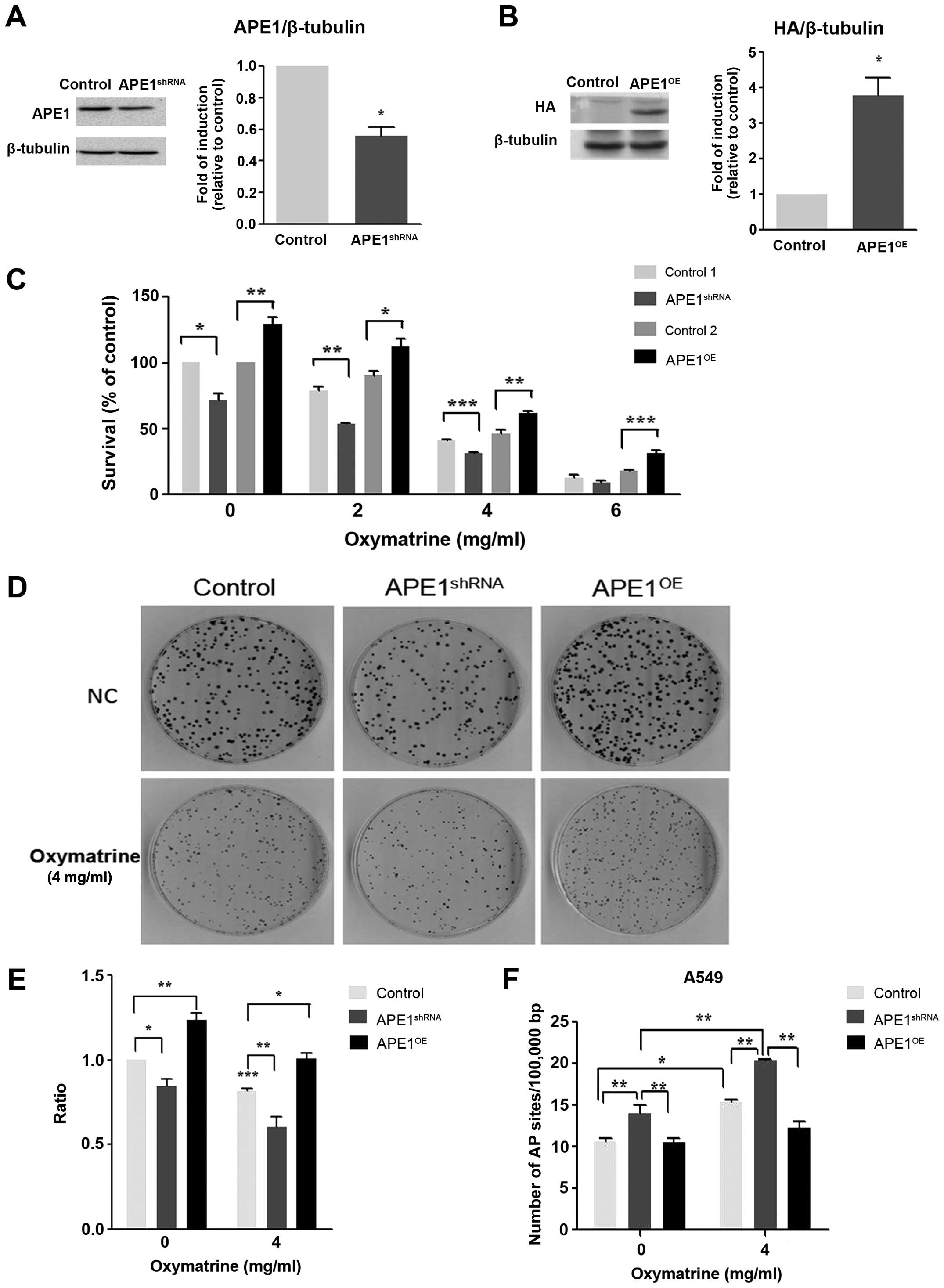
Downregulation and upregulation of APE1
expression in A549 cells
The reduction of APE1 protein expression by APE1
shRNA vector transfection in A549 cells (also called
APE1shRNA stable A549 cell lines) was confirmed by
western blotting. Transfection with APE1 shRNA reduced APE1
expression by 47% compared with vector control transfected cells
(control; Fig. 4A). In contrast,
the expression of APE1 was increased markedly in A549 cells
transfected with APE1-HA (also called APE1OE stable A549
cell lines); HA was used as a fusion tag for detection.
Specifically, the expression of APE1 was increased 4-fold in
APE1OE cells compared with control vector (Fig. 4B).
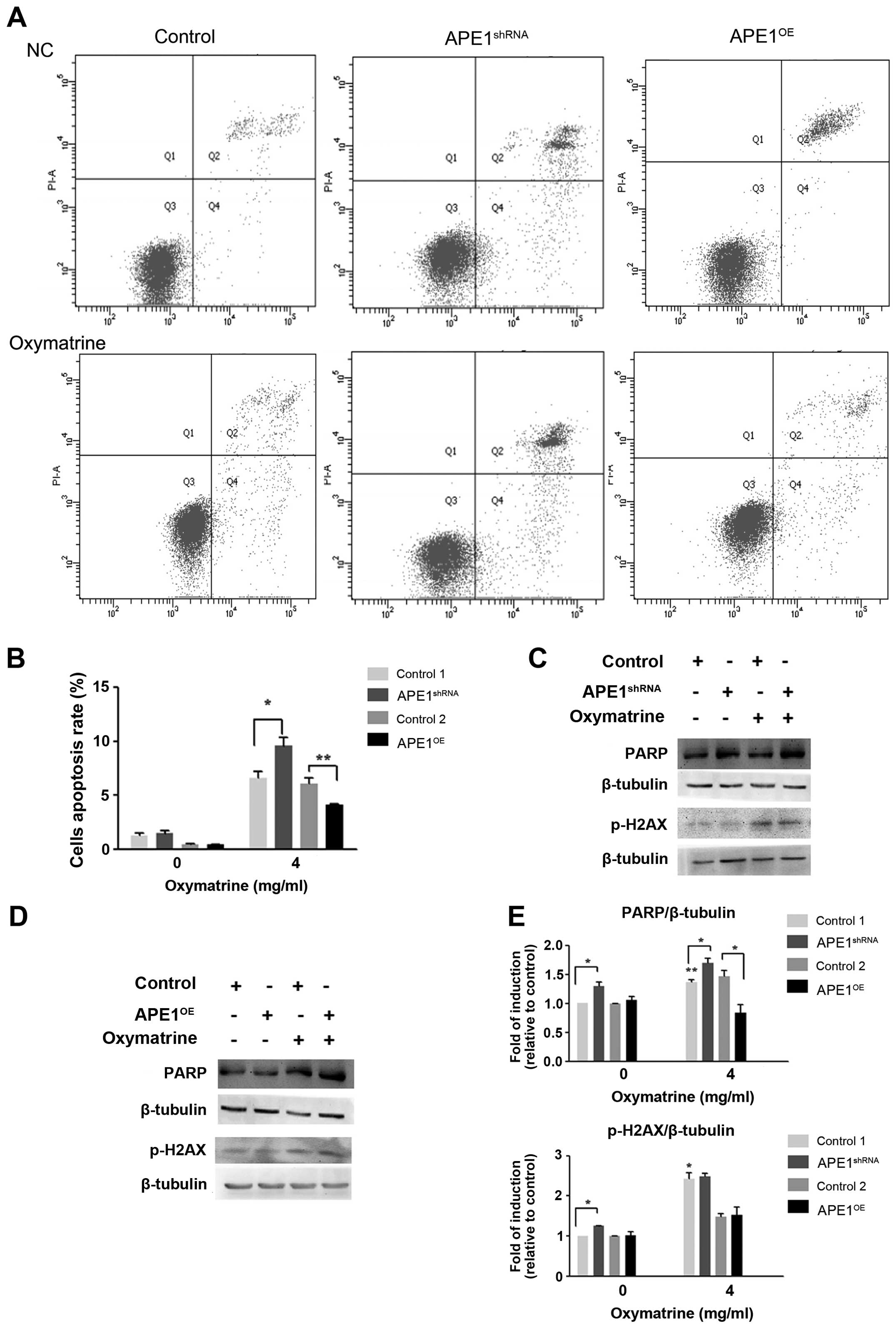
Knocking down APE1 enhances
oxymatrine-induced DNA damage and apoptosis and reduces cell
proliferation and clonogenic survival
Oxymatrine treatment significantly reduced the
proliferation of APE1shRNA cells, as measured by MTT and
colony formation assays after 48 h (P<0.001). Furthermore,
APE1shRNA alone inhibited cell growth by 20%
(P<0.05). However, oxymatrine treatment did not induce a
significant reduction in proliferation in APEOE cells,
suggesting that these cells are resistant to oxymatrine (Fig. 4C–E).
To determine if oxymatrine inhibits APE1 directly in
APE1shRNA and APEOE cells, AP site formation
was measured using ARP assays. A significant increase in the number
of AP sites in APE1shRNA cells were observed with
oxymatrine. However, there was no significant change in AP sites in
APE1OE cells treated with oxymatrine compared with
control. The assay was performed four times, each in triplicate,
and the data presented show the mean of the four experiments with
standard errors (Fig. 4F).
Compared with the APEshRNA cells,
oxymatrine treatment caused a significant increase in apoptosis
using Annexin V/PI staining and flow cytometry. In contrast,
APEOE cells presented low apoptosis induction with
statistically significant difference between the groups
APEshRNA oxymatrine-treated cells and APEOE
oxymatrine-treated cells (P<0.05) (Fig. 5A and B). Consistent with these
results there were higher levels of PARP and phosphorylated
H2AX[Ser139] in oxymatrine-treated APE1shRNA
cells compared with untreated group (Fig. 5C–E). In contrast, these changes of
phosphorylated H2AX[Ser139] were not observed in
oxymatrine-treated APE1OE cells, but PARP expression
decreased significantly. These results suggest that APE1 plays an
important role in regulating oxymatrine-induced apoptosis.
Discussion
Oxymatrine is an alkaloid that is derived from
Kushen and is a potential treatment for a number of cancers,
including pancreatic (6), gastric
(26) and breast cancer (27). However, the effects of oxymatrine
on lung cancer and the underlying molecular mechanisms of these
effects have not yet been investigated. Previous studies
demonstrated that oxymatrine markedly inhibited cell proliferation
in dose-dependent manner, induced cell apoptosis in a dose- and
time-dependent manner, upregulated cleaved caspase-3 and -9, and
downregulated Bax/Bcl-2 in human osteosarcoma MG-63 cells (17). These proteins play a pivotal role
in regulating apoptosis. In the present study, we hypothesized that
the increased apoptosis elicited by oxymatrine is associated with
the inhibition of APE1 function. To test this hypothesis, two
stable A549 cell lines (APE1shRNA and APE1OE)
were developed (Fig. 4A and B).
Oxymatrine significantly promoted lung cancer cell apoptosis, which
was associated with the reduction of APE1 AP endonuclease activity;
therefore, the association between APE1 enzymatic activity and
oxymatrine dose was examined in lung cancer cells. Oxymatrine
reduced APE1 activity in a dose-dependent manner (Fig. 3D). To the best of our knowledge,
this is the first study that clearly identifies APE1 as a potential
target for oxymatrine-induced apoptosis in lung cancer cells. This
is consistent with anticancer strategies that propose inhibiting
BER as a principle for anticancer chemotherapy (28). However, increased APE1 expression
was also reported to cause drug resistance in lung cancer patients
(29).
Cellular APE1 levels might be critical for the
repair of DNA strand breaks induced by ROS (30). This study suggests that APE1 is a
promising target for cancer treatment. This protein has been
targeted using antisense oligonucleotides, RNA interference, and
natural and chemical agents, which sensitizes tumor cells to
radiotherapy or chemotherapeutic drugs (31). For example, the present study
revealed that APE1 is a target of oxymatrine, since the protein
expression and AP endonuclease activity of APE1 were reduced by
oxymatrine (Fig. 3). Earlier
studies revealed that ~10,000 abasic sites are generated in a human
cell every day, and that these AP sites are the most commonly
generated lesions in DNA (32). AP
sites can lead to error-prone bypass synthesis and thus cause
mutagenesis (33). AP sites and
5-foU are the most common lesions in genomic DNA (34). If an AP site is present within a
double-stranded clustered lesion it would be repaired first
(35). It is assumed that
oxymatrine increased DNA damage and associated with increasing AP
site, since APE1 activity was inhibited when DNA repair is
hindered. The repair of AP sites requires an AP endonuclease or AP
lyase. In the present study, oxymatrine increased the number of AP
sites in APE1 knockdown stable cell lines (APE1shRNA)
compared with control (Fig. 5A and
B). This suggests that oxymatrine can increase DNA damage in
APE1 knockdown cells.
8-OHdG levels were significantly higher in
oxymatrine-treated cells compared with control (Fig. 2A and C). 8-OHdG is used widely as a
biomarker for measuring endogenous oxidative DNA damage; it
reflects the oxidative damage induced by free radicals to nuclear
and mitochondrial DNA (36,37).
The phosphorylation of histone H2AX on serine-139
(p-H2AX) is a sensitive marker for DNA DSBs. In this study
oxymatrine induced the phosphorylation of H2AX at the sites of DNA
DSBs, which could be observed as fluorescent foci using
immunostaining (Fig. 2B and D). In
addition, western blotting showed an increase in p-H2AX levels
after oxymatrine. Finally, phosphorylated H2AX levels were
significantly associated with the dose of oxymatrine in H1299 cells
(Fig. 2E and H). PARP cleavage was
observed in oxymatrine-treated H1299 cells in a dose-dependent
manner (Fig. 2E–G).
Although the present study revealed that oxymatrine
induced apoptosis at least in part by inhibiting APE1 activity, the
mechanisms for the proapoptotic actions of oxymatrine in cancer
cells are complex and they may include several other pathways.
Further studies are needed to determine the molecular mechanism of
APE1 in anticancer drug targets.
In conclusion, the present study revealed that
treatment with oxymatrine significantly induced apoptosis and DNA
damage in lung cancer cells. Oxymatrine also inhibited APE1
activity and protein expression significantly. The selective
inhibition of the repair activity of APE1 is a promising target for
developing novel cancer therapeutics (38). This study provides evidence
supporting the hypothesis that APE1 is a target for oxymatrine and
contributes to oxymatrine-induced apoptosis in lung cancer
cells.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. NSFC81172615, NSFC81570062
and NSFC81600049); the Guangdong Natural Science Foundation (grant
nos. S20122010008299 and 2016A030313681) and the Guangdong Medical
University Scientific Research Fund (grant nos. M2014046, M2014032
and M2015009).
References
|
1
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu YH, Li ML, Hsu MY, Pang YY, Chen IL,
Chen CK, Tang SW, Lin HY and Lin JY: Effects of a Chinese herbal
medicine, Guan-Jen-Huang (Aeginetia indica Linn.), on renal cancer
cell growth and metastasis. Evid Based Complement Alternat Med.
2012:9358602012. View Article : Google Scholar
|
|
3
|
Huang M, Hu YY, Dong XQ, Xu QP, Yu WH and
Zhang ZY: The protective role of oxymatrine on neuronal cell
apoptosis in the hemorrhagic rat brain. J Ethnopharmacol.
143:228–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP
and Wu BY: Oxymatrine liposome attenuates hepatic fibrosis via
targeting hepatic stellate cells. World J Gastroenterol.
18:4199–4206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong-Li S, Lei L, Lei S, Dan Z, De-Li D,
Guo-Fen Q, Yan L, Wen-Feng C and Bao-Feng Y: Cardioprotective
effects and underlying mechanisms of oxymatrine against ischemic
myocardial injuries of rats. Phytother Res. 22:985–989. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP
families, and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song G, Luo Q, Qin J, Wang L, Shi Y and
Sun C: Effects of oxymatrine on proliferation and apoptosis in
human hepatoma cells. Colloids Surf B Biointerfaces. 48:1–5. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang D, Xiang DB, Yang XQ, Chen LS, Li MX,
Zhong ZY and Zhang YS: APE1 overexpression is associated with
cisplatin resistance in non-small cell lung cancer and targeted
inhibition of APE1 enhances the activity of cisplatin in A549
cells. Lung Cancer. 66:298–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Helleday T, Petermann E, Lundin C, Hodgson
B and Sharma RA: DNA repair pathways as targets for cancer therapy.
Nat Rev Cancer. 8:193–204. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelley MR, Georgiadis MM and Fishel ML:
APE1/Ref-1 role in redox signaling: Translational applications of
targeting the redox function of the DNA repair/redox protein
APE1/Ref-1. Curr Mol Pharmacol. 5:36–53. 2012. View Article : Google Scholar :
|
|
12
|
Xanthoudakis S, Smeyne RJ, Wallace JD and
Curran T: The redox/DNA repair protein, Ref-1, is essential for
early embryonic development in mice. Proc Natl Acad Sci USA.
93:8919–8923. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindahl T: Instability and decay of the
primary structure of DNA. Nature. 362:709–715. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dianov GL, Sleeth KM, Dianova II and
Allinson SL: Repair of abasic sites in DNA. Mutat Res. 531:157–163.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bapat A, Glass LS, Luo M, Fishel ML, Long
EC, Georgiadis MM and Kelley MR: Novel small-molecule inhibitor of
apurinic/apyrimidinic endonuclease 1 blocks proliferation and
reduces viability of glioblastoma cells. J Pharmacol Exp Ther.
334:988–998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu C, Huang W, Guo Y, Xia P, Sun X, Pan X
and Hu W: Oxymatrine inhibits the proliferation of prostate cancer
cells in vitro and in vivo. Mol Med Rep. 11:4129–4134.
2015.PubMed/NCBI
|
|
17
|
Wei J, Zhu Y, Xu G, Yang F, Guan Z, Wang M
and Fang Y: Oxymatrine extracted from Sophora flavescens inhibited
cell growth and induced apoptosis in human osteosarcoma MG-63 cells
in vitro. Cell Biochem Biophys. 70:1439–1444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Hou X, Fu H, Pan X, Xu W, Tang W
and Fang H: Design, synthesis and preliminary bioactivity
evaluations of substituted quinoline hydroxamic acid derivatives as
novel histone deacetylase (HDAC) inhibitors. Bioorg Med Chem.
23:4364–4374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valdiglesias V, Giunta S, Fenech M, Neri M
and Bonassi S: γH2AX as a marker of DNA double strand breaks and
genomic instability in human population studies. Mutat Res.
753:24–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schreiber V, Dantzer F, Ame JC and de
Murcia G: Poly(ADP-ribose): Novel functions for an old molecule.
Nat Rev Mol Cell Biol. 7:517–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muñoz-Gámez JA, Rodríguez-Vargas JM,
Quiles-Pérez R, Aguilar-Quesada R, Martín-Oliva D, de Murcia G,
Menissier de Murcia J, Almendros A, Ruiz de Almodóvar M and Oliver
FJ: PARP-1 is involved in autophagy induced by DNA damage.
Autophagy. 5:61–74. 2009. View Article : Google Scholar
|
|
22
|
Krishnakumar R and Kraus WL: The PARP side
of the nucleus: Molecular actions, physiological outcomes, and
clinical targets. Mol Cell. 39:8–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scott TL, Rangaswamy S, Wicker CA and
Izumi T: Repair of oxidative DNA damage and cancer: Recent progress
in DNA base excision repair. Antioxid Redox Signal. 20:708–726.
2014. View Article : Google Scholar :
|
|
24
|
Evans AR, Limp-Foster M and Kelley MR:
Going APE over ref-1. Mutat Res. 461:83–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madhusudan S, Smart F, Shrimpton P,
Parsons JL, Gardiner L, Houlbrook S, Talbot DC, Hammonds T,
Freemont PA, Sternberg MJ, et al: Isolation of a small molecule
inhibitor of DNA base excision repair. Nucleic Acids Res.
33:4711–4724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song MQ, Zhu JS, Chen JL, Wang L, Da W,
Zhu L and Zhang WP: Synergistic effect of oxymatrine and
angiogenesis inhibitor NM-3 on modulating apoptosis in human
gastric cancer cells. World J Gastroenterol. 13:1788–1793. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu
W, Qi X, Zhu X, Pei Y and Lin H: Oxymatrine diminishes the side
population and inhibits the expression of β-catenin in MCF-7 breast
cancer cells. Med Oncol. 28(Suppl 1): S99–S107. 2011. View Article : Google Scholar
|
|
28
|
Fishel ML and Kelley MR: The DNA base
excision repair protein Ape1/Ref-1 as a therapeutic and
chemopreventive target. Mol Aspects Med. 28:375–395. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Qing Y, Guan W, Li M, Peng Y, Zhang
S, Xiong Y and Wang D: Predictive value of APE1, BRCA1, ERCC1 and
TUBB3 expression in patients with advanced non-small cell lung
cancer (NSCLC) receiving first-line platinum-paclitaxel
chemotherapy. Cancer Chemother Pharmacol. 74:777–786. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Izumi T, Hazra TK, Boldogh I, Tomkinson
AE, Park MS, Ikeda S and Mitra S: Requirement for human AP
endonuclease 1 for repair of 3′-blocking damage at DNA
single-strand breaks induced by reactive oxygen species.
Carcinogenesis. 21:1329–1334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Silber JR, Bobola MS, Blank A, Schoeler
KD, Haroldson PD, Huynh MB and Kolstoe DD: The
apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1
contributes to human glioma cell resistance to alkylating agents
and is elevated by oxidative stress. Clin Cancer Res. 8:3008–3018.
2002.PubMed/NCBI
|
|
32
|
Kunkel TA: The high cost of living. In:
American Association for Cancer Research Special Conference:
Endogenous sources of mutations; Fort Myers, Florida, USA. 11–15
November 1998; Trends Genet. 15. pp. 93–94. 1999
|
|
33
|
Loeb LA and Preston BD: Mutagenesis by
apurinic/apyrimidinic sites. Annu Rev Genet. 20:201–230. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bjelland S, Anensen H, Knaevelsrud I and
Seeberg E: Cellular effects of 5-formyluracil in DNA. Mutat Res.
486:147–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Georgakilas AG, O’Neill P and Stewart RD:
Induction and repair of clustered DNA lesions: What do we know so
far? Radiat Res. 180:100–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prabhulkar S and Li CZ: Assessment of
oxidative DNA damage and repair at single cellular level via
real-time monitoring of 8-OHdG biomarker. Biosens Bioelectron.
26:1743–1749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010. View Article : Google Scholar
|
|
38
|
Bapat A, Fishel ML and Kelley MR: Going
ape as an approach to cancer therapeutics. Antioxid Redox Signal.
11:651–668. 2009. View Article : Google Scholar :
|